Abstract
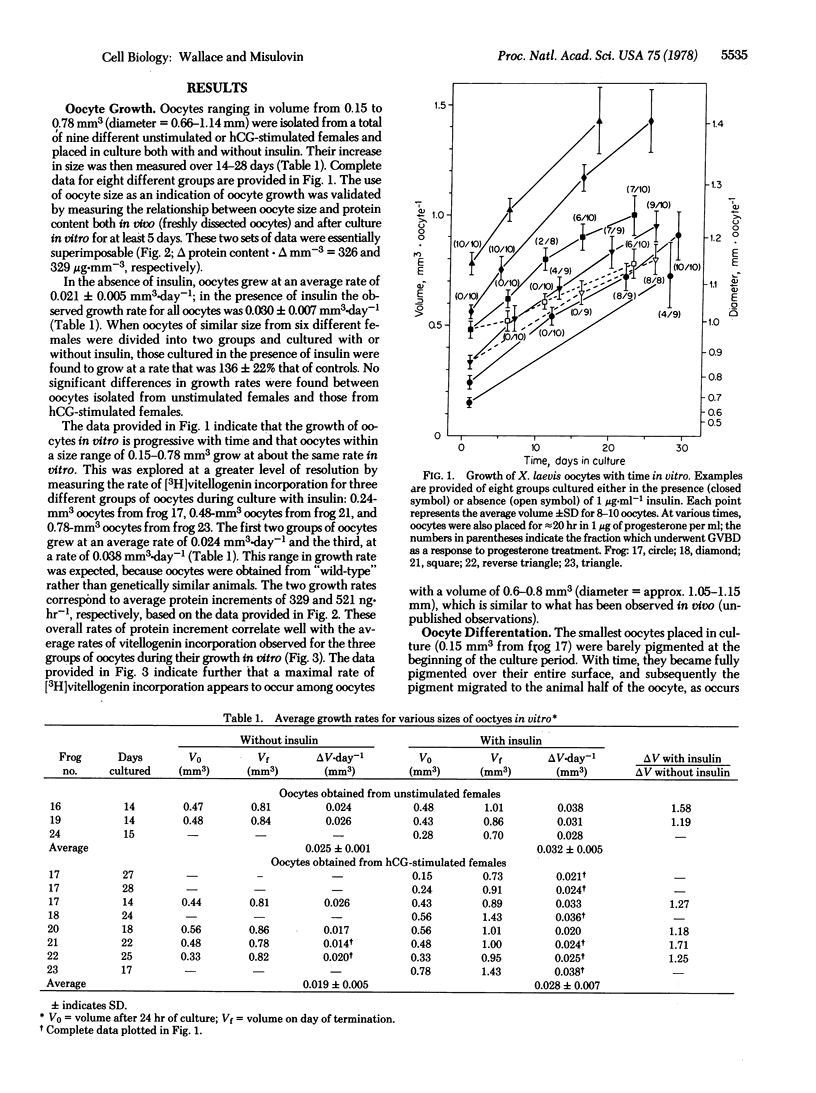
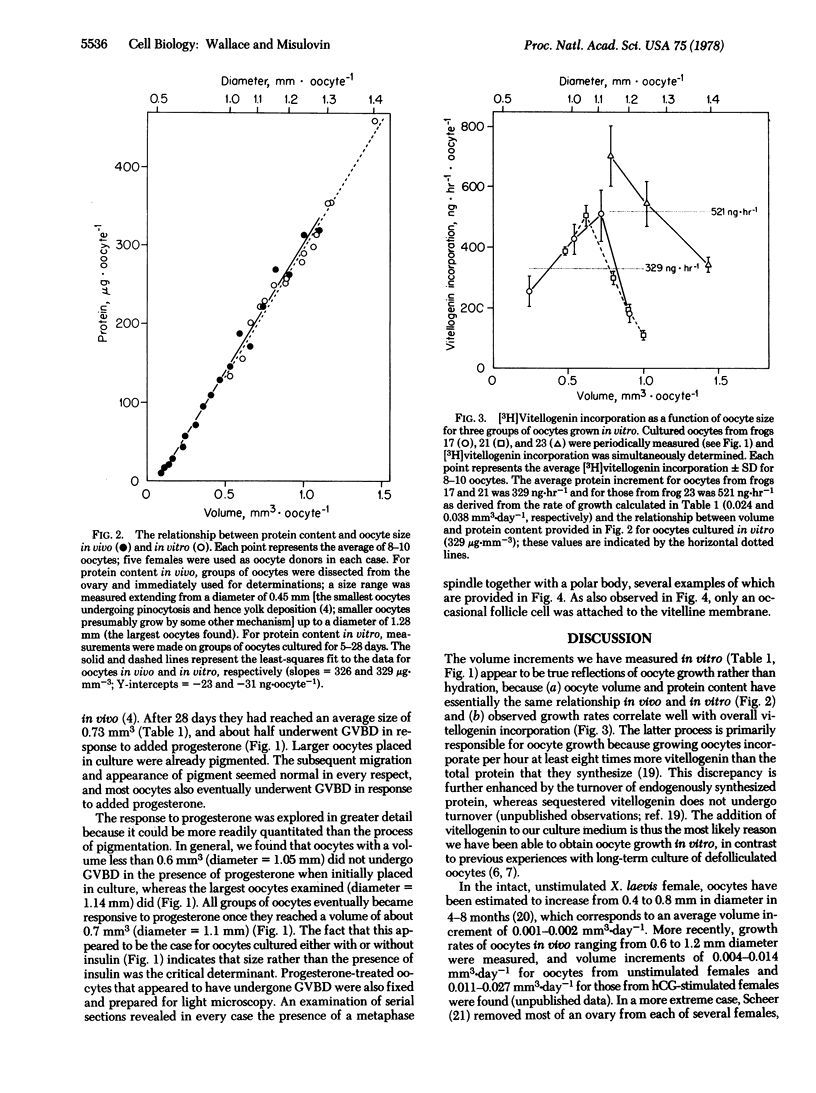
Xenpus laevis oocytes over a size range of 0.15--0.78 mm3 were dissected from their follicles and cultured in a defined medium for up to 28 days. Oocytes grew at average rates of 0.021 mm3.day-1 in the absence of insulin and 0.030 mm3.day-1 in the presence of insulin. The latter average growth rate corresponds to the fastest growth rate reported to date for oocytes in vivo. Oocytes grown in vitro can reach a size of at least 1.43 mm3, which is larger than the maximum size generally found in vivo. During growth in vitro; oocytes also acquire both a normal pigment pattern and, once they reach about 0.7 mm3, the ability to undergo complete maturation as a response to externally applied progesterone. These results show that Xenopus oocytes freed of their follicular investments are able to grow and differentiate in vitro.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bramhall S., Noack N., Wu M., Loewenberg J. R. A simple colorimetric method for determination of protein. Anal Biochem. 1969 Oct 1;31(1):146–148. doi: 10.1016/0003-2697(69)90251-6. [DOI] [PubMed] [Google Scholar]
- Dumont J. N., Brummett A. R. Oogenesis in Xenopus laevis (Daudin). V. Relationships between developing oocytes and their investing follicular tissues. J Morphol. 1978 Jan;155(1):73–97. doi: 10.1002/jmor.1051550106. [DOI] [PubMed] [Google Scholar]
- Dumont J. N. Oogenesis in Xenopus laevis (Daudin). I. Stages of oocyte development in laboratory maintained animals. J Morphol. 1972 Feb;136(2):153–179. doi: 10.1002/jmor.1051360203. [DOI] [PubMed] [Google Scholar]
- Dumont J. N. Oogenesis in Xenopus laevis (Daudin). VI. The route of injected tracer transport in the follicle and developing oocyte. J Exp Zool. 1978 May;204(2):193–217. doi: 10.1002/jez.1402040208. [DOI] [PubMed] [Google Scholar]
- Eppig J. J., Dumont J. N. Defined nutrient medium for the in vitro maintenance of Xenopus laevis oocytes. In Vitro. 1976 Jun;12(6):418–427. doi: 10.1007/BF02806021. [DOI] [PubMed] [Google Scholar]
- Eppig J. J. Mouse oocyte development in vitro with various culture systems. Dev Biol. 1977 Oct 15;60(2):371–388. doi: 10.1016/0012-1606(77)90135-x. [DOI] [PubMed] [Google Scholar]
- Eppig J. J., Steckman M. L. Comparison of exogenous energy sources for in vitro maintenance of follicle cell-free Xenopus laevis oocytes. In Vitro. 1976 Mar;12(3):173–179. doi: 10.1007/BF02796439. [DOI] [PubMed] [Google Scholar]
- FOOTE C. L., FOOTE F. M. In vitro cultivation of gonads of larval anurans. Anat Rec. 1958 Mar;130(3):553–565. doi: 10.1002/ar.1091300307. [DOI] [PubMed] [Google Scholar]
- Fortune J. E., Concannon P. W., Hansel W. Ovarian progesterone levels during in vitro oocyte maturation and ovulation in Xenopus laevis. Biol Reprod. 1975 Dec;13(5):561–567. doi: 10.1095/biolreprod13.5.561. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B., De Robertis E. M., Partington G. Injected nuclei in frog oocytes provide a living cell system for the study of transcriptional control. Nature. 1976 Mar 11;260(5547):116–120. doi: 10.1038/260116a0. [DOI] [PubMed] [Google Scholar]
- Jared D. W., Wallace R. A. Protein uptake in vitro by amphibian oocytes. Exp Cell Res. 1969 Oct;57(2):454–457. doi: 10.1016/0014-4827(69)90175-x. [DOI] [PubMed] [Google Scholar]
- Masui Y. Relative roles of the pituitary, follicle cells, and progesterone in the induction of oocyte maturation in Rana pipiens. J Exp Zool. 1967 Dec;166(3):365–375. doi: 10.1002/jez.1401660309. [DOI] [PubMed] [Google Scholar]
- Mulner O., Thibier C., Ozon R. Steroid biosynthesis by ovarian follicles of Xenopus laevis in vitro during oogenesis. Gen Comp Endocrinol. 1978 Mar;34(3):287–295. doi: 10.1016/0016-6480(78)90250-2. [DOI] [PubMed] [Google Scholar]
- Redshaw M. R., Nicholls T. J. Oestrogen biosynthesis by ovarian tissue of the South African clawed toad, Xenopus laevis Daudin. Gen Comp Endocrinol. 1971 Feb;16(1):85–96. doi: 10.1016/0016-6480(71)90210-3. [DOI] [PubMed] [Google Scholar]
- Reynhout J. K., Taddei C., Smith L. D., LaMarca M. J. Response of large oocytes of Xenopus laevis to progesterone in vitro in relation to oocyte size and time after previous HCG-induced ovulation. Dev Biol. 1975 Jun;44(2):375–379. doi: 10.1016/0012-1606(75)90408-x. [DOI] [PubMed] [Google Scholar]
- Scheer U. Nuclear pore flow rate of ribosomal RNA and chain growth rate of its precursor during oogenesis of Xenopus laevis. Dev Biol. 1973 Jan;30(1):13–28. doi: 10.1016/0012-1606(73)90044-4. [DOI] [PubMed] [Google Scholar]
- Schuetz A. W. Effect of steroids on germinal vesicle of oocytes of the frog (Rana pipiens) in vitro. Proc Soc Exp Biol Med. 1967 Apr;124(4):1307–1310. doi: 10.3181/00379727-124-31993. [DOI] [PubMed] [Google Scholar]
- Smith L. D., Ecker R. E., Subtelny S. In vitro induction of physiological maturation in Rana pipiens oocytes removed from their ovarian follicles. Dev Biol. 1968 Jun;17(6):627–643. doi: 10.1016/0012-1606(68)90010-9. [DOI] [PubMed] [Google Scholar]
- WALLACE R. A., KARASAKI S. Studies on amphibian yolk. 2. The isolation of yolk platelets from the eggs of Rana pipiens. J Cell Biol. 1963 Jul;18:153–166. doi: 10.1083/jcb.18.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. A., Ho T., Salter D. W., Jared D. W. Protein incorporation by isolated amphibian oocytes. IV. The role of follicle cells and calcium during protein uptake. Exp Cell Res. 1973 Dec;82(2):287–295. doi: 10.1016/0014-4827(73)90343-1. [DOI] [PubMed] [Google Scholar]
- Wallace R. A., Jared D. W., Dumont J. N., Sega M. W. Protein incorporation by isolated amphibian oocytes. 3. Optimum incubation conditions. J Exp Zool. 1973 Jun;184(3):321–333. doi: 10.1002/jez.1401840305. [DOI] [PubMed] [Google Scholar]
- Wallace R. A., Jared D. W., Nelson B. L. Protein incorporation by isolated amphibian oocytes. I. Preliminary studies. J Exp Zool. 1970 Nov;175(3):259–269. doi: 10.1002/jez.1401750302. [DOI] [PubMed] [Google Scholar]
- Wallace R. A., Jared D. W. Protein incorporation by isolated amphibian oocytes. V. Specificity for vitellogenin incorporation. J Cell Biol. 1976 May;69(2):345–351. doi: 10.1083/jcb.69.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. A., Nickol J. M., Ho T., Jared D. W. Studies on amphibian yolk. X. The relative roles of autosynthetic and heterosynthetic processes during yolk protein assembly by isolated oocytes. Dev Biol. 1972 Nov;29(3):255–272. doi: 10.1016/0012-1606(72)90066-8. [DOI] [PubMed] [Google Scholar]
- Wallace R. A., Steinhardt R. A. Maturation of Xenopus oocytes. II. Observations on membrane potential. Dev Biol. 1977 Jun;57(2):305–316. doi: 10.1016/0012-1606(77)90217-2. [DOI] [PubMed] [Google Scholar]
- Wiley H. S., Dumont J. N. Stimulation of vitellogenin uptake in stage IV xenopus oocytes by treatment with chorionic gonadotropin in vitro. Biol Reprod. 1978 Jun;18(5):762–771. doi: 10.1095/biolreprod18.5.762. [DOI] [PubMed] [Google Scholar]




