Abstract
Genetic mutations cause primary immunodeficiencies (PIDs), which predispose to infections. Here we describe Activated PI3K-δ Syndrome (APDS), a PID associated with a dominant gain-of-function mutation E1021K in the p110δ protein, the catalytic subunit of phosphoinositide 3-kinase δ (PI3Kδ), encoded by the PIK3CD gene. We found E1021K in 17 patients from seven unrelated families, but not among 3,346 healthy subjects. APDS was characterized by recurrent respiratory infections, progressive airway damage, lymphopenia, increased circulating transitional B cells, increased IgM and reduced IgG2 levels in serum and impaired vaccine responses. The E1021K mutation enhanced membrane association and kinase activity of p110δ. Patient-derived lymphocytes had increased levels of phosphatidylinositol 3,4,5-trisphosphate and phosphorylated AKT protein and were prone to activation-induced cell death. Selective p110δ inhibitors IC87114 and GS-1101 reduced the activity of the mutant enzyme in vitro, suggesting a therapeutic approach for patients with APDS.
Respiratory infections are the most common illnesses of people worldwide. Recurrent respiratory infections may lead to bronchiectasis, a permanent, abnormal dilation of bronchi (1). Susceptibility to recurrent respiratory infections and bronchiectasis may be conferred by an underlying primary immunodeficiency (PID) (1, 2). PIDs have variable penetrance and those that have a milder course may remain undiagnosed. Mutations in more than 200 genes are known to cause various PIDs (3). Recent improvements in DNA sequencing technology provide an opportunity to study the patient’s whole genome or its coding part, known as the exome (4). This technological advancement has significantly improved the genetic diagnostics of PIDs in patients with recurrent and severe infections and facilitated the identification of novel causative genes and mutations.
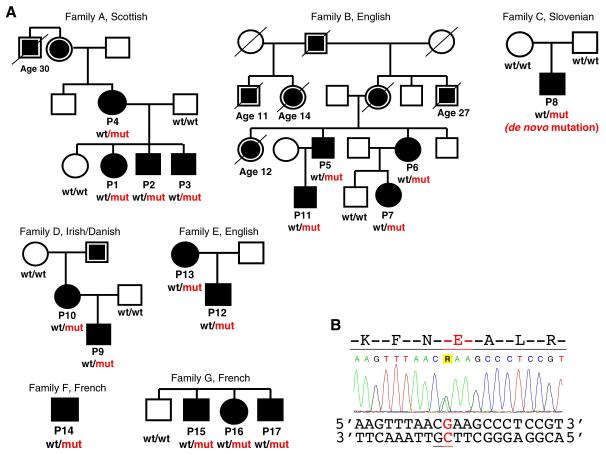
We used exome sequencing to search for causative mutations in 35 PID patients from the UK who suffered recurrent infections and had a family history of susceptibility to infections (5). Following identification of genetic variants in these patients, we excluded common polymorphisms previously detected in the 1,000 Genomes and NHLBI projects (table S1) (5). When cross-checking the remaining rare variants, we noted that three patients from one family (P1, P2 and P3 in family A) and one patient from another family (P5 in family B) had the same heterozygous G to A mutation at position 9,787,030 on chromosome 1, c.3061G>A in the PIK3CD gene (fig. 1). This mutation was not present in the other exomes and was the only rare variant shared among all patients in these two unrelated families. It encodes an amino-acid substitution, a glutamic acid for a lysine, at position 1021 (E1021K) of the p110δ protein, the catalytic subunit of phosphoinositide 3-kinase δ (PI3Kδ). Sanger sequencing confirmed the presence of the E1021K mutation in these patients and four additional affected family members. In both families the mutation co-segregated with the clinical phenotype (fig. 1).
Fig. 1. Families with the E1021K p110δ mutation.
(A) ○ and □ - unaffected; ● and ■ - affected;  and
and  - available data indicate recurrent infections. Age at the time of death is shown for patients who died ≤30 years of age. PIK3CD genotype is shown if known: wt, wild type allele encoding glutamic acid (E1021); mut, mutant allele encoding lysine (K1021). (B) Sequence chromatogram showing heterozygous mutation c.3061G>A in the PIK3CD gene leading to the E1021K amino-acid change in p110δ. CpG dinucleotide is underlined.
- available data indicate recurrent infections. Age at the time of death is shown for patients who died ≤30 years of age. PIK3CD genotype is shown if known: wt, wild type allele encoding glutamic acid (E1021); mut, mutant allele encoding lysine (K1021). (B) Sequence chromatogram showing heterozygous mutation c.3061G>A in the PIK3CD gene leading to the E1021K amino-acid change in p110δ. CpG dinucleotide is underlined.
We then designed a genotyping assay for this E1021K mutation and screened 3,346 healthy subjects, including 2,296 from the UK and 1,050 representing 51 different populations from around the world (5). No healthy carriers of E1021K were identified in these two large cohorts, supporting our hypothesis that this is a pathogenic mutation rather than a rare neutral polymorphism. We then studied DNA samples of an additional heterogeneous cohort of 134 PID patients from the UK and Ireland (5). In this cohort we identified five further patients from three unrelated families C, D and E who had the same heterozygous E1021K mutation (fig. 1A). The apparent high frequency of the mutation among PID patients and the fact that P8 (family C) had previously been diagnosed with hyper-IgM syndrome prompted us to study an additional cohort from France comprising 15 hyper-IgM patients from 13 families that had previously undergone exome sequencing. Among these we found three patients from two unrelated families F and G with the same mutation, indicating that E1021K may cause a typical hyper-IgM syndrome. One additional patient was identified among family members, bringing the overall number of patients with the E1021K mutation to 17.
Sequencing of the healthy parents of P8 in family C showed that both were homozygous for the normal allele (fig. 1A). Genome-wide identity-by-descent analysis in family C confirmed the relationship of both parents to P8, thus classifying this E1021K mutation as de novo. The mutation was present in DNA isolated from both fibroblast and blood samples of P8 and therefore is likely to be germline, rather than somatic. Then, in families A – E we studied genotypes of 149 markers in a 2 Mb interval on chromosome 1 flanking the mutation (5). We found no shared long-range haplotypes across any pair of families and no flanking markers that were consistently in linkage disequilibrium with the mutation across all five families. These data strongly suggest a recurrent mutation, rather than a founder effect. Nucleotide G in position 9,787,030 is part of a CpG dinucleotide (fig. 1B) that is known to be ~30 times more prone to transition mutations (e.g. G>A) than an average nucleotide in the genome (6).
Prior to our genetic analysis, patients from families A – G were not considered to have the same disease etiology. The discovery of the same causative mutation in these patients prompted us to compare their clinical and immunological histories (table S2), revealing the phenotype of this PID, which is characterized by recurrent respiratory infections and progressive airway damage (table 1, Supplementary Text and figs. S1, S2). Whereas the immunological phenotype was largely consistent between patients, the clinical presentation and disease course have been variable (e.g. mild disease in P10; table S2). Such clinical variability may be explained by differences in lifestyle, exposure to pathogens, treatment efficacy, and possibly by modifying genetic factors.
Table 1. Summary of clinical and immunological features of patients with the E1021K p110δ mutation.
| Clinical / Immunological manifestation | Patients | Frequency, n / total studied (%) |
|---|---|---|
| Recurrent respiratory and ear infections (H. influenzae, S. pneumoniae) | P1-17 | 17/17 (100) |
| CT evidence of large (bronchiectasis) or small (mosaic attenuation) airway disease | P1-7,9,11-13,17 | 12/16 (75) |
| Splenomegaly (prior to the onset of recurrent infections) | P2,3,5,6,8,9,13-16 | 10/17 (59) |
| Skin, salivary gland, lacrimal gland or dental abscess formation, orbital cellulitis | P1,3,5-8,10 | 7/17 (41) |
| Infection caused by herpes group viruses (HSV, CMV, VZV, EBV) | P3,8,12,13 (and the deceased sister of P5/P6) |
4/17 (24) |
| Marginal zone lymphoma | P13 | 1/17 (6) |
|
| ||
| Low/intermittent low serum IgG2 levels | P2-7,10-13 | 10/11 (91) |
| High/intermittent high serum IgM levels | P1-6,8-11,13-16 | 14/17 (82) |
| Low levels of anti-pneumococcal antibodies | P1-4,7,9,11-13,17 | 10/10 (100) |
| Low levels of anti-Haemophilus Influenzae type B antibodies | P1-4,8,9,12,13 | 8/10 (80) |
| Decreased circulating T cells (total CD3+) and/or CD4+ and/or CD8+ T cells | P1-9,13,14,17 | 12/17 (71) |
| Decreased circulating B cells (total CD19+) | P2-9,13,14-16 | 12/17 (71) |
| Increased circulating transitional B cells (CD19+CD38+IgM+) | P1-4,7-14,16,17 | 14/16 (88) |
| Decreased circulating class switched memory B cells (CD19+CD27+IgD−) | P1-3,8,9,12,13,16 | 8/16 (50) |
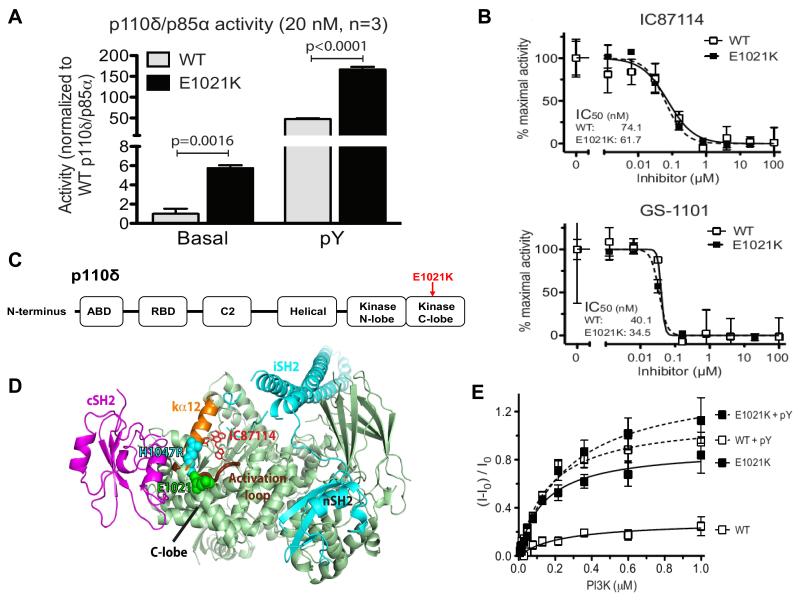
To understand how the E1021K mutation caused immunodeficiency we first studied its impact on p110δ function. The p110δ protein is a catalytic subunit that, together with a regulatory subunit, forms PI3Kδ, a heterodimeric lipid kinase. PI3Kδ phosphorylates phosphatidylinositol 4,5-bisphosphate (PIP2), generating phosphatidylinositol 3,4,5-trisphosphate (PIP3), an important second messenger molecule. We cloned the cDNA of p110δ and introduced the E1021K change by site-directed mutagenesis. Subsequently, we expressed both normal and mutant p110δ proteins, together with the regulatory subunit p85α, in baculovirus-infected insect cells and purified the proteins (fig. S3A). We measured lipid kinase activity using a modified membrane capture assay (7) and found that the basal PIP3 production by PI3Kδ containing the mutant p110δE1021K subunit was up to 6-fold higher than that produced by the wild type PI3Kδ (figs. 2A and S3B). After stimulation with a platelet-derived growth factor (PDGF) receptor’s bis-phosphorylated peptide (pY), the activity of both wild type and mutant PI3Kδ increased, but PIP3 production by the mutant PI3Kδ was still up to 3 times higher (figs. 2A and S3C). We used two structurally related isoform-selective PI3Kδ inhibitors, IC87114 and GS-1101 (8, 9), and found that both reduced the activity of the mutant PI3Kδ as efficiently as that of the wild type PI3Kδ (fig. 2B), suggesting that these compounds may be effective in patients with the E1021K mutation.
Fig. 2. In vitro activity and structure of p110δ.
(A) Basal and pY-stimulated PI3K activity at 20 nM concentration. Graphs are mean ± SD of 3 independent experiments. P-values were calculated by two-tailed t-test. (B) Inhibition of mutant and wild-type p110δ/p85α as a function of IC87114 or GS-1101 concentration (data are mean ± SD, N=3). (C) Domain organization of p110δ. (D) Structural model of the p110δ/p85α heterodimer. p110δ catalytic subunit (pale green), nSH2 and iSH2 domains of the p85 regulatory subunit (cyan), cSH2 domain (magenta), p110δ activation loop (thick chocolate tube beneath kα12), residue E1021 of p110δ (green spheres) and the analogous residue in H1047R mutant of p110α (cyan spheres). The IC87114 inhibitor bound in the active site is shown in stick representation. (E) Membrane binding of p110δ. FRET between the PI3K complex and Dansyl-PS-containing membrane vesicles in the absence (solid lines) or presence (dashed lines) of the pY peptide (data are mean ± SD, N=3).
To understand the mechanism by which E1021K increases PI3Kδ activity, we first modeled the structure of the mutant p110δ protein (5). p110δ is organized similarly to other PI3K catalytic subunits (fig. 2C) (10, 11). The E1021K mutation is located in the C-lobe of the kinase domain that interacts with cellular membrane, accommodates lipid substrate and binds the cSH2 domain of the regulatory subunit (fig. 2D). Structural modeling showed that E1021K of p110δ is positioned similarly to the somatic mutation H1047R of another PI3K isoform, p110α, which is known to increase PI3K activity in cancer cells by enhancing its association with membranes (12, 13). Therefore, we used a protein-lipid fluorescence resonance energy transfer (FRET) assay to study interaction between lipid vesicles and either wild type p110δ or the mutant p110δE1021K. We found that p110δE1021K has a much higher basal affinity for lipid vesicles than the wild type p110δ (fig. 2E). After pY stimulation, the affinity of p110δE1021K was also increased, although the difference with respect to the pY-activated wild type p110δ was less striking (fig. 2E). These results suggest that stronger binding to membranes contributes to the increased activity of the mutant p110δE1021K protein. Another potential activating mechanism of E1021K may involve interaction of p110δ with the regulatory subunit p85α (14). Our structural model shows that E1021K may impair binding of p110δ to the inhibitory cSH2 domain (fig. 2D) leading to increased PI3Kδ activity. However, it is unlikely to affect binding of another inhibitory p85α domain, nSH2 (fig. 2D). This is consistent with our observation that pY stimulation further activates the mutant enzyme, probably by removing the nSH2 inhibition.
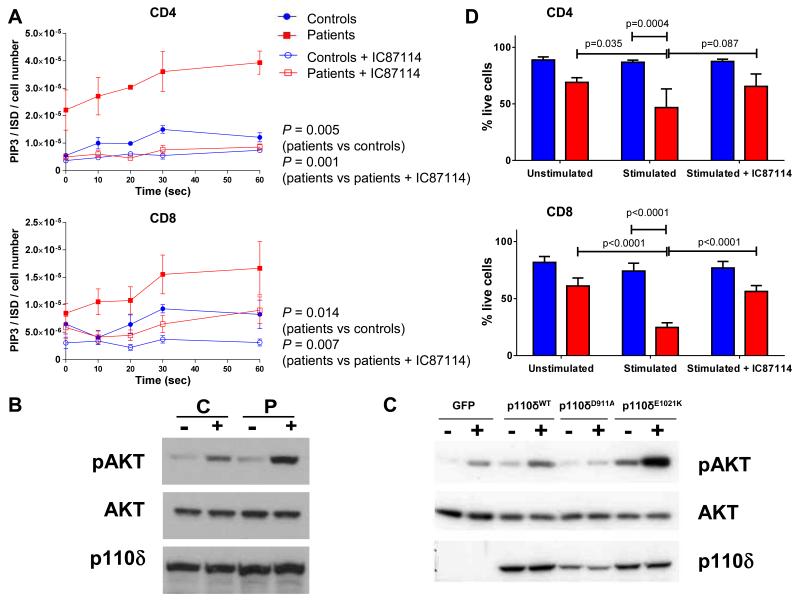
PI3Kδ is expressed predominantly in cells of hematopoietic lineage and is the major PI3K isoform signaling downstream of T and B cell antigen receptors (TCR and BCR), Toll-like receptors (TLRs), co-stimulatory molecules and cytokine receptors in T, B and myeloid cells (15). Therefore, we studied the activity of the mutant PI3Kδ ex vivo in patients’ leukocytes. We measured levels of PIP3 using a high-performance liquid chromatography – mass spectrometry-based assay (16) in CD4+ and CD8+ T cells isolated from fresh peripheral blood. In both T lymphocyte lineages we found consistently higher PIP3 levels in patients than in controls prior to stimulation and 10, 20, 30 and 60 seconds after stimulation (fig. 3A). In patient cells treated with IC87114 the levels of PIP3 were significantly reduced (fig. 3A). Furthermore, in stimulated patients’ T cells we found increased levels of phosphorylated AKT protein, a major downstream mediator of PIP3 signalling (fig. 3B). Levels of p110δ expression were normal in the patients’ T cells (fig. 3B). We then cloned in a retroviral vector the wild-type p110δ, the mutant p110δE1021K and p110δD911A with mutation D911A that inactivates the kinase domain, and transduced these constructs into T blasts isolated from the p110δ-knockout mouse (5). After stimulation cells with p110δE1021K had more phosphorylated AKT than other cells (figs. 3C and S4). Together, these results strongly suggest that the E1021K mutation increases PI3K signaling in vivo as well as in vitro.
Fig. 3. Functional analyses of T cells in patients with APDS.
(A) Intracellular PIP3 levels in CD4+ and CD8+ T lymphocytes of patients (red squares, N=6) and controls (blue circles, N=5) at indicated times after anti-CD3/anti-CD28 stimulation in the presence or absence of IC87114. The data are expressed as the ratio of the quantity of PIP3 divided by that of the internal standard (ISD) and normalized according to the cell number. The data show mean +/− SEM. P-values were calculated using two-way ANOVA with Bonferroni correction. (B) Representative (N=3) Western blot showing levels of p110δ, AKT and phospho-AKT (pAKT) proteins in CD4+ T cells isolated from fresh blood samples of a healthy control (C) and a patient (P) without stimulation (−) or after 10 min stimulation (+) with anti-CD3 and anti-CD28 antibodies. (C) Representative (N=2) Western blot showing levels of p110δ, and pAKT proteins in CD4+ T cell blasts of a p110δ knockout mouse transduced with retroviral constructs expressing either GFP or wild-type p110δ (p110δWT) or kinase dead p110δ (p110δD911A) or p110δE1021K without stimulation (−) or after stimulation (+) with anti-CD3 antibodies and anti-CD28 antibodies. (D) Quantification of surviving CD4+ and CD8+ T cells as indicated by % of cells excluding viability dye. Cells of patients (red, N=4) and controls (blue, N=7) were studied without stimulation and after stimulation with anti-CD3/anti-CD28 antibodies and in the presence of IC87114. Each subject was studied in triplicate. The data show mean +/− SEM. P-values were calculated using a two-way ANOVA with Sidak’s multiple comparisons test.
To study T cell responses we stimulated purified CD4+ and CD8+ cells with anti-CD3 and anti-CD28 antibodies. Unexpectedly, we observed that both T cell lineages from patients were prone to cell death (figs. 3D and S5A). This phenomenon was reversed by the addition of IC87114 but not IL-2 (figs. 3D and S5B), suggesting that it is caused by the increased PI3Kδ activity. Cytokine production following stimulation of T cells was profoundly reduced in the patients and was not rescued by exogenous IL-2 (fig. S6), suggesting that T cell death occurs prior to any significant cytokine response. However, stimulation with CytoStim, which did not induce T cell death, also led to reduced cytokine production by the patients-derived T cells (fig. S7). The propensity to activation-induced cell death (AICD) is consistent with T cell lymphopenia found in our patients. It may relate in part to the increased proportion of T cells with an activated/memory phenotype (table S2) (17). Moreover, given that p110δ inhibitor reduces AICD of the patient-derived T cells, the activated p110δ may increase the AICD per se, possibly by enhancing TCR signalling.
In the patients’ B lymphocytes we also found increased amounts of phosphorylated AKT, both before and after stimulation, although this analysis was complicated by enhanced protein degradation in the patient-derived cells (fig. S8). Studies in transgenic mice deficient for phosphatase and tensin homologue (PTEN), an enzyme that dephosphorylates PIP3, have shown that PI3Kδ activity, PIP3 and phosphorylated AKT suppress immunoglobulin class switch recombination (CSR) in B cells. These mice have impaired B cell function, increased IgM, decreased IgG and IgA levels and impaired antibody responses after immunization (18-21). Immunological presentation of our patients resembles this phenotype and indicates a B cell defect. However, normal total IgG and IgA levels that were found in most of our patients suggest that CSR may be only partially affected. Nevertheless, inefficient antibody production impairs responses to S. pneumoniae and H. influenzae type B vaccinations in our patients leading to recurrent infections with these pathogens. An increased population of circulating transitional B cells may reflect a block in late stages of B cell maturation or an enhanced death of mature B cells.
PI3Kδ is also highly expressed in neutrophils. We found that patient-derived neutrophils retained their ability to undergo a respiratory burst, degranulation, chemotaxis, and apoptosis (fig. S9). We measured PIP3 accumulation in TNFα-primed neutrophils in response to fMLP stimulation at 6 seconds (a PI3Kγ-dependent response) and at 60 seconds (a predominantly PI3Kδ-mediated signal) (22) and found no significant difference between patients and controls in either response (fig. S9). Thus, the effect of the E1021K mutation on the PI3Kδ activity may be cell-type or stimulus-specific, or may be compensated for by effects of other PI3K isoforms or PTEN. Nevertheless, we cannot exclude that a subtle defect in neutrophil function may contribute to the disease pathogenesis in these patients.
Here we describe a PID caused by a recurrent autosomal dominant germline mutation E1021K in the PIK3CD gene that encodes p110δ. We found it in 17 patients from seven unrelated families, suggesting that it is frequent among PID patients and may explain a substantial fraction of patients with recurrent respiratory infections and bronchiectasis. Our rapid genotyping assay should facilitate screening for the E1021K mutation in existing PID and bronchiectasis cohorts, as well as new patients. The E1021K mutation was previously noted in one Taiwanese patient with recurrent respiratory infections and PID; however, its causative and pathogenic role has not been demonstrated (23). Here we have shown that E1021K increases PI3Kδ activity, augmenting the production of PIP3 and activating the downstream AKT protein in lymphocytes. This leads to defects in T and B cell function and inefficient immune responses to bacterial pathogens, predisposing to recurrent respiratory infections and eventually to bronchiectasis. We named this disorder Activated PI3K-Delta Syndrome (APDS).
Activation of the PI3K pathway is associated with malignant transformations and it has been shown that overexpression of p110δ can transform cells (24). To date, only one of our APDS patients, P13, has been diagnosed with lymphoma (table 1). Nonetheless, the oncogenic potential of PI3K up-regulation can be enhanced by additional mutations (25, 26). Therefore, APDS patients may be at increased risk of leukemia or lymphoma if they acquire additional somatic mutations.
The APDS patients described here had been treated with immunoglobulin replacement and antibiotics. Despite this, there is evidence of significant airway damage in most cases. Because of progressive severe disease following splenectomy, patient P8 underwent allogeneic hematopoietic stem cell transplantation (HSCT) at the age of 8 years. One year after HSCT his clinical condition had improved dramatically, suggesting that HSCT may be a long-term treatment option for young patients. Nevertheless, our results raise the possibility that selective p110δ inhibitors, such as GS-1101, may be an alternative effective therapeutic approach in APDS patients. GS-1101 (CAL-101 or Idelalisib) has been tested in phase I/II clinical trials for treatment of chronic lymphocytic leukemia (www.clinicaltrials.gov). The possibility of treating APDS patients with p110δ inhibitors should therefore be considered.
Supplementary Material
Acknowledgments
S.N. is a Wellcome Trust Senior Research Fellow in Basic Biomedical Science (095198/Z/10/Z). S.N. is also supported by the European Research Council Starting grant 260477 and the EU FP7 collaborative grant 261441 (PEVNET project). S.N., A.C., D.K., and R.D. are supported by the National Institute for Health Research (NIHR) Cambridge Biomedical Research Centre. O.V. was supported by a Swiss National Science Foundation fellowship (grant no. PA00P3_134202) and a European Commission fellowship (FP7-PEOPLE-2010-IEF, no. 275880). R.L.W was supported by the Medical Research Council (file reference U105184308). T.C. is supported by the National Children’s Research Centre, Our Lady’s Children’s Hospital, Crumlin, Dublin, Ireland. E.B.H. is supported by a Wellcome Trust Translational Medicine and Therapeutics award. A.C. is supported by the MRC and British Lung Foundation. K.O. is supported by a strategic grant from the Biotechnology and Biological Sciences Research Council and a New Investigator Award from the Wellcome Trust. P.H. and L.S. are funded by an Institute Programme grant from BBSRC (BB/J004456/1). S.K. is a Centre National de la Recherche Scientifique (CNRS) researcher. A.D., A.F. and S.K are funded by Institut National de la Santé et de la Recherche Médicale, A.D. is supported by the EU FP7 EUROPAD contract 201549, Association Contre Le Cancer and the ANR (grant: 2010-CSRD). A.F. is supported by the EU FP7 ERC PIDIMMUNE grant number 249816. The following are paid consultants for GSK (P.H., L.S., K.O., A.C., E.C.), Karus pharmaceuticals (P.H, L.S.), Roche and Novartis (E.C.). The mutation is submitted to ClinVar database; accession SCV000083058.
References and Notes
- 1.Barker AF. Bronchiectasis. N Engl J Med. 2002;346:1383. doi: 10.1056/NEJMra012519. [DOI] [PubMed] [Google Scholar]
- 2.Durandy A, Kracker S, Fischer A. Primary antibody deficiencies. Nat Rev Immunol. 2013;13:519. doi: 10.1038/nri3466. [DOI] [PubMed] [Google Scholar]
- 3.Al-Herz W, et al. Primary immunodeficiency diseases: an update on the classification from the international union of immunological societies expert committee for primary immunodeficiency. Front Immunol. 2011;2:54. doi: 10.3389/fimmu.2011.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bamshad MJ, et al. Exome sequencing as a tool for Mendelian disease gene discovery. Nat Rev Genet. 2011;12:745. doi: 10.1038/nrg3031. [DOI] [PubMed] [Google Scholar]
- 5.Materials and methods are available as supplementary materials on Science online.
- 6.Hodgkinson A, Eyre-Walker A. Variation in the mutation rate across mammalian genomes. Nat Rev Genet. 2011;12:756. doi: 10.1038/nrg3098. [DOI] [PubMed] [Google Scholar]
- 7.Knight ZA, Feldman ME, Balla A, Balla T, Shokat KM. A membrane capture assay for lipid kinase activity. Nat Protoc. 2007;2:2459. doi: 10.1038/nprot.2007.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sadhu C, Masinovsky B, Dick K, Sowell CG, Staunton DE. Essential role of phosphoinositide 3-kinase delta in neutrophil directional movement. J Immunol. 2003;170:2647. doi: 10.4049/jimmunol.170.5.2647. [DOI] [PubMed] [Google Scholar]
- 9.Lannutti BJ, et al. CAL-101, a p110delta selective phosphatidylinositol-3-kinase inhibitor for the treatment of B-cell malignancies, inhibits PI3K signaling and cellular viability. Blood. 2011;117:591. doi: 10.1182/blood-2010-03-275305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scheeff ED, Bourne PE. Structural evolution of the protein kinase-like superfamily. PLoS Comput Biol. 2005;1:e49. doi: 10.1371/journal.pcbi.0010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vadas O, Burke JE, Zhang X, Berndt A, Williams RL. Structural basis for activation and inhibition of class I phosphoinositide 3-kinases. Sci Signal. 2011;4:re2. doi: 10.1126/scisignal.2002165. [DOI] [PubMed] [Google Scholar]
- 12.Mandelker D, et al. A frequent kinase domain mutation that changes the interaction between PI3Kalpha and the membrane. Proc Natl Acad Sci U S A. 2009;106:16996. doi: 10.1073/pnas.0908444106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burke JE, Perisic O, Masson GR, Vadas O, Williams RL. Oncogenic mutations mimic and enhance dynamic events in the natural activation of phosphoinositide 3-kinase p110alpha (PIK3CA) Proc Natl Acad Sci U S A. 2012;109:15259. doi: 10.1073/pnas.1205508109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burke JE, et al. Dynamics of the phosphoinositide 3-kinase p110delta interaction with p85alpha and membranes reveals aspects of regulation distinct from p110alpha. Structure. 2011;19:1127. doi: 10.1016/j.str.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okkenhaug K. Signaling by the Phosphoinositide 3-Kinase Family in Immune Cells. Annu Rev Immunol. 2013;31:675. doi: 10.1146/annurev-immunol-032712-095946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clark J, et al. Quantification of PtdInsP3 molecular species in cells and tissues by mass spectrometry. Nat Methods. 2011;8:267. doi: 10.1038/nmeth.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 18.Anzelon AN, Wu H, Rickert RC. Pten inactivation alters peripheral B lymphocyte fate and reconstitutes CD19 function. Nat Immunol. 2003;4:287. doi: 10.1038/ni892. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki A, et al. Critical roles of Pten in B cell homeostasis and immunoglobulin class switch recombination. J Exp Med. 2003;197:657. doi: 10.1084/jem.20021101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Omori SA, et al. Regulation of class-switch recombination and plasma cell differentiation by phosphatidylinositol 3-kinase signaling. Immunity. 2006;25:545. doi: 10.1016/j.immuni.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 21.Janas ML, et al. The effect of deleting p110delta on the phenotype and function of PTEN-deficient B cells. J Immunol. 2008;180:739. doi: 10.4049/jimmunol.180.2.739. [DOI] [PubMed] [Google Scholar]
- 22.Condliffe AM, et al. Sequential activation of class IB and class IA PI3K is important for the primed respiratory burst of human but not murine neutrophils. Blood. 2005;106:1432. doi: 10.1182/blood-2005-03-0944. [DOI] [PubMed] [Google Scholar]
- 23.Jou ST, et al. Identification of variations in the human phosphoinositide 3-kinase p110delta gene in children with primary B-cell immunodeficiency of unknown aetiology. Int J Immunogenet. 2006;33:361. doi: 10.1111/j.1744-313X.2006.00627.x. [DOI] [PubMed] [Google Scholar]
- 24.Kang S, Denley A, Vanhaesebroeck B, Vogt PK. Oncogenic transformation induced by the p110beta, -gamma, and -delta isoforms of class I phosphoinositide 3-kinase. Proc Natl Acad Sci U S A. 2006;103:1289. doi: 10.1073/pnas.0510772103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009;9:550. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- 26.Kinross KM, et al. An activating Pik3ca mutation coupled with Pten loss is sufficient to initiate ovarian tumorigenesis in mice. J Clin Invest. 2012;122:553. doi: 10.1172/JCI59309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wellcome Trust Case Control Consortium Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Power C, Elliott J. Cohort profile: 1958 British birth cohort (National Child Development Study) Int J Epidemiol. 2006;35:34. doi: 10.1093/ije/dyi183. [DOI] [PubMed] [Google Scholar]
- 29.Cann HM, et al. A human genome diversity cell line panel. Science. 2002;296:261. doi: 10.1126/science.296.5566.261b. [DOI] [PubMed] [Google Scholar]
- 30.The 1000 Genomes Project Consortium An integrated map of genetic variation from 1,092 human genomes. Nature. 2012;491:56. doi: 10.1038/nature11632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fu W, et al. Analysis of 6,515 exomes reveals the recent origin of most human protein-coding variants. Nature. 2013;493:216. doi: 10.1038/nature11690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berndt A, et al. The p110delta structure: mechanisms for selectivity and potency of new PI(3)K inhibitors. Nat Chem Biol. 2010;6:244. doi: 10.1038/nchembio0310-244b. [DOI] [PubMed] [Google Scholar]
- 33.Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallogr D Biol Crystallogr. 2010;66:486. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wehr C, et al. The EUROclass trial: defining subgroups in common variable immunodeficiency. Blood. 2008;111:77. doi: 10.1182/blood-2007-06-091744. [DOI] [PubMed] [Google Scholar]
- 35.Clayton E, et al. A crucial role for the p110delta subunit of phosphatidylinositol 3-kinase in B cell development and activation. J Exp Med. 2002;196:753. doi: 10.1084/jem.20020805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Revy P, et al. Activation-induced cytidine deaminase (AID) deficiency causes the autosomal recessive form of the Hyper-IgM syndrome (HIGM2) Cell. 2000;102:565. doi: 10.1016/s0092-8674(00)00079-9. [DOI] [PubMed] [Google Scholar]
- 37.Hayward AR, Mowla R, Harvey B. Defect of neutrophil mobility with dominant inheritance in a family with Waardenberg’s syndrome. Arch Dis Child. 1981;56:279. doi: 10.1136/adc.56.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.