Abstract
Insulin stimulated glucose uptake requires the colocalization of myosin IIA (MyoIIA) and the insulin-responsive glucose transporter 4 (GLUT4) at the plasma membrane for proper GLUT4 fusion. MyoIIA facilitates filamentous actin (F-actin) reorganization in various cell types. In adipocytes F-actin reorganization is required for insulin-stimulated glucose uptake. What is not known is whether MyoIIA interacts with F-actin to regulate insulin-induced GLUT4 fusion at the plasma membrane. To elucidate the relationship between MyoIIA and F-actin, we examined the colocalization of MyoIIA and F-actin at the plasma membrane upon insulin stimulation as well as the regulation of this interaction. Our findings demonstrated that MyoIIA and F-actin colocalized at the site of GLUT4 fusion with the plasma membrane upon insulin stimulation. Furthermore, inhibition of MyoII with blebbistatin impaired F-actin localization at the plasma membrane. Next we examined the regulatory role of calcium in MyoIIA-F-actin colocalization. Reduced calcium or calmodulin levels decreased colocalization of MyoIIA and F-actin at the plasma membrane. While calcium alone can translocate MyoIIA it did not stimulate F-actin accumulation at the plasma membrane. Taken together, we established that while MyoIIA activity is required for F-actin localization at the plasma membrane, it alone is insufficient to localize F-actin to the plasma membrane.
Keywords: Myosin IIA, Filamentous actin (F-actin), Insulin-responsive glucose transporter (GLUT4), Adipocytes, Calcium
Introduction
Insulin resistance of primarily skeletal muscle and adipose tissue is a major defect in type 2 diabetes. Insulin facilitates the translocation and fusion of insulin-responsive glucose transporter (GLUT4)-containing vesicles to the plasma membrane to stimulate glucose uptake [1,2]. The binding of insulin to its tyrosine kinase receptor stimulates several signal transduction pathways, such as the phosphatidylinositol 3-kinase (PI3K), mitogen-activated protein kinase, (MAPK), and calcium signaling pathways [3–5]. In addition to stimulating these signaling pathways, insulin also induces cytoskeletal reorganization to facilitate the translocation of GLUT4 vesicles from a perinuclear region to the plasma membrane as well as GLUT4 fusion [6–8]. Cytoskeletal reorganization, specifically F-actin reorganization is required for insulin-stimulated glucose uptake [6–9]. Since F-actin functions as a barrier at the plasma membrane, F-actin must undergo reorganization during insulin stimulated glucose uptake in order for proper GLUT4 vesicle docking and fusion [6–8]. To accomplish this function the actin cytoskeleton requires the myosin family of actin-based motor proteins. Members of the myosin family have been shown to shuttle cargo (vesicles) along actin filaments and also to contract actin filaments [10–18]. Contraction of the actomyosin cytoskeleton can lead to the localized membrane remodeling required for vesicle fusion at the plasma membrane [9,19]. Studies have shown that cortical actin remodeling must occur in order for GLUT4 fusion with the plasma membrane [7,19]. What is not known is whether MyoIIA interacts with cortical actin to facilitate GLUT4 vesicle fusion at the plasma membrane.
The myosin responsible for actin filament contraction is ‘conventional’ myosin, MyoII [20]. Much of what is known about the function and regulation of MyoII comes from studies of muscle MyoII. MyoII is a multi-subunit protein consisting of a pair of heavy chains (MHC), a pair of essential light chains, and a pair of regulatory light chains (RLC). Binding of actin and ATP to the globular head of the MHC initiates the motor activity of MyoII (reviewed in [20]). Nonmuscle cells also express MyoII isoforms that function in a manner similar to their muscle counterpart. Nonmuscle MyoII is similar to muscle MyoII, in that both are regulated by phosphorylation of the RLC by myosin light chain kinase (MLCK) [20]. Phosphorylation of the RLC induces the binding of MyoII to F-actin [21,22]. However in contrast to skeletal muscle MyoII, which is organized in a highly ordered and stable arrangement with actin filaments in sarcomeres, nonmuscle MyoII is subject to changes in localization and activation during various cellular processes [20]. Nonmuscle MyoII also differs from muscle MyoII in that it is involved in the cytoskeletal remodeling of F-actin [22,23]. Both these characteristics have implicated a role for nonmuscle MyoII in vesicle transport and fusion [9]. Previous studies have suggested that there are distinct zones at the cell cortex where myosin-dependent cytoskeletal reorganization occurs and allows for the localized membrane remodeling required for vesicle fusion with the plasma membrane. MyoII has been implicated in the regulation of exocytic processes in a variety of cells including pancreatic islets [24], chromaffin cells [25] and parietal cells [26].
We and others have demonstrated that MyoII plays a role in GLUT4-mediated glucose uptake in adipocyte [13,14,27,28]. While 3T3-L1 adipocytes express both MyoIIA and IIB isoforms, it is the IIA isoform that is regulated by insulin-stimulation [28]. Our studies show that insulin specifically stimulates the phosphorylation of the RLC associated with the MyoIIA isoform via MLCK [28] to induce its recruitment from a perinuclear region to the plasma membrane in adipocytes. We also demonstrated that GLUT4 translocates to the plasma membrane prior to MyoIIA recruitment [14]. Colocalization of MyoIIA and GLUT4 at the plasma membrane is required for proper GLUT4-vesicle fusion [14]. Studies inhibiting MyoIIA demonstrated that GLUT4-mediated glucose uptake did not occur even when GLUT4 translocated to the plasma membrane due to the lack of GLUT4 vesicle fusion. Thus, the presence of MyoIIA at the plasma membrane is necessary for GLUT4 vesicle fusion with the plasma membrane.
Insulin signaling stimulates the phosphorylation of the RLC of MyoIIA by activating MLCK, a calcium/calmodulin [14]. Reduced intracellular calcium levels impair MLCK phosphorylation and subsequently MyoIIA activity and translocation to the plasma membrane as well as GLUT4 fusion [13,14,27]. While calcium plays an essential role in the activation of MyoIIA, the role of calmodulin is still unclear. Previous studies have shown that while calmodulin can bind MLCK without being a part of the calcium/calmodulin complex it does not activate MLCK without calcium binding [29].
In the present study we examined the insulin-induced colocalization of MyoIIA and F-actin at the site of GLUT4 vesicle fusion at the plasma membrane as well as the regulatory factors involved. Our results reveal that while MyoIIA is necessary for F-actin localization at the plasma membrane it is not sufficient to recruit F-actin to the plasma membrane. Thus our studies are the first to identify the relationship between MyoIIA and F-actin during insulin-stimulated glucose uptake in adipocytes.
Materials and methods
Materials
Tissue culture reagents were obtained from Gibco (Grand Island, NY). Dexamethasone, 3-isobytyl-1-methyl-xanthine, rhodamine-labeled phalloidin, N-(4-Aminobutyl)-5-chloro-2-naphthalenesulfonamide hydrochloride (W13), 1,2-Bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis (acetoxymethyl ester) (BAPTA) and MyoIIA antibody were from Sigma (St. Louis, MO). Insulin was purchased from Roche Diagnostics Corporation (Indianapolis, IN). A-23187 and blebbistatin were obtained from Calbiochem (San Diego, CA). GLUT4 antibody was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). The enhanced chemiluminescence (ECL) detection kit and horseradish peroxidase conjugated secondary antibodies were from Amersham Bioscience (Piscataway, NJ).
Cell culture
Confluent 3T3-L1 preadipocytes were induced to differentiate in 10% Fetal Bovine Serum (FBS)/Dulbecco’s Modified Eagle Media (DMEM) containing 0.52 mM 3-isobutyl 1-methyl-xanthine (MIX), 1.7 μM insulin, and 1 μM dexamethasone (DEX). After 2 days, media was changed to 10%FBS/DMEM plus 0.425 μM insulin. The media was replaced with 10%FBS/DMEM every other day [30].
Confocal microscopy
3T3-L1 cells were cultured and differentiated on glass coverslips. Adipocytes (Day 7–9) were serum starved for 4 h and then either left untreated (basal), treated with 100 nM insulin (INS) or 50 μM BAPTA-AM, 100 μM blebbistatin or 70 μM W-13 for 15 min followed by 100 nM insulin for an additional 15 min. Adipocytes were also treated with 0.1 μM A23187 for 15 min in the absence of insulin. Cells were then fixed with 4% formaldehyde for 15 min and permeabilized with 0.1% triton X-100 for 10 min. Cells were incubated for 1 h with either MyoIIA or GLUT4 antibodies. Cells were washed and incubated with the appropriate secondary antibodies. Rhodamine-labeled phalloidin was used to stain F-actin. Finally, coverslips were mounted and MyoIIA, GLUT4 and F-actin were visualized by confocal microscopy. Images from three independent experiments were quantified using Image-Pro Plus software (Silver Spring, MD).
Quantification of protein distribution at the plasma membrane
GLUT4, MyoIIA, and F-actin levels were quantified via intensity of fluorescence both in the cytoplasm and at the cell cortex. Intensity of fluorescence signal at the cortex and the cytoplasm were measured and the ratio of the cortical/cytoplasmic signal was averaged for individual cells (n=5) under various conditions. A percent change formula, [(Treatment−Basal)/(Basal) × 100%], was used to determine the change in protein localization at the plasma membrane.
Cytoskeletal fractionation
Cytoskeletal fractionation was performed as described previously [31]. Adipocytes (1.5 × 106 cells) were pelleted by low speed centrifugation for 3 min. Pellets were resuspended in equal volumes of Buffer A (0.1 M MES pH 6.8, 2.5 mM EGTA, 5 mM MgCl2, and 0.5 mM ATP) and Buffer B (0.5% Triton X-100, 1X protease inhibitor mix and Buffer A), mixed for 5 s and then centrifuged for 1 min. The cytoskeletal pellet fraction was retained and the proteins in the supernatant fraction (200 μL) were precipitated with acetone for 15 min on ice and pelleted by centrifugation for 15 min.
Immunoblot analysis
Supernatant and cytoskeletal pellets were resuspended in loading buffer and then subjected to SDS-PAGE. Proteins were transferred to Immobilon membranes (Millipore) and then analyzed by immunoblotting [32]. Protein bands were quantified by densitometry using ImageQuant (version 5.2 for Windows) software.
Plasma lawn assay
Day-10 adipocytes were serum-starved for 4 h in the presence of 0.1% DMSO (vehicle) or 100 μM blebbistatin. Adipocytes were rinsed in cold PBS and incubated in poly-(L-lysine) (0.5 mg/ml) for 1 min. Cells were then incubated in a hypotonic buffer (23 mM KCl, 10 mM Hepes, 2 mM MgCl2, 1 mM EDTA, pH 7.5) and sonicated. Plasma membrane sheets were incubated with rhodamine-labeled phalloidin or an anti-MyoIIA antibody and subjected to confocal microscopy. Images from three independent experiments were quantified using Image-Pro Plus software (Silver Spring, MD).
Results
MyoIIA and F-actin colocalize at the plasma membrane during insulin-stimulated glucose uptake
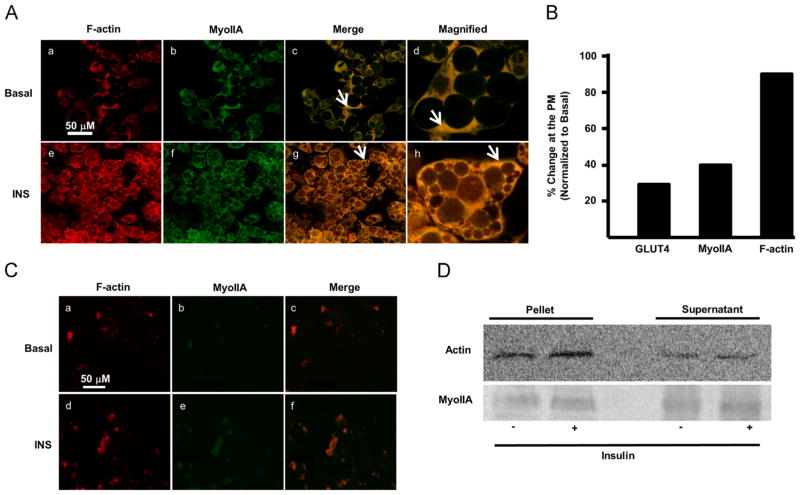
Previous studies have shown that MyoIIA colocalizes with GLUT4 at the plasma membrane to facilitate GLUT4 fusion in insulin-stimulated adipocytes. Similarly, insulin induces F-actin reorganization at the cell cortex [7]. What is not known is whether MyoIIA facilitates F-actin reorganization upon insulin stimulation. In pancreatic islets, MyoIIA and F-actin colocalize during F-actin remodeling [33]. In order to determine if MyoIIA facilitates F-actin reorganization we first had to establish whether MyoIIA and F-actin colocalize at the plasma membrane upon insulin stimulation. To this end, fully differentiated 3T3-L1 adipocytes were serum starved for 4 h and then either left untreated or stimulated with insulin. Using confocal microscopy, we observed that both MyoIIA and GLUT4 (data not shown) translocated to the plasma membrane in the presence of insulin as shown previously [28]. In the basal state, F-actin and MyoIIA colocalized primarily in the cytoplasm (Fig. 1A, panels c and d, arrow indicating colocalization). Furthermore, we observed a clear insulin-dependent co-localization of MyoIIA and F-actin at the plasma membrane (Fig. 1A, panels g and h, arrow indicating colocalization). As shown previously, insulin induced a 30% increase in the level of GLUT4 at the plasma membrane (Fig. 1B). Insulin also stimulated a 40% increase in MyoIIA and a 90% increase in F-actin at the plasma membrane (Fig. 1B). Our results show that there was no difference in the signal intensity in the cytoplasm of basal and insulin-treated adipocytes (106.5±14.06 versus 124.4±15.44, p<0.05) whereas there was a difference at the plasma membrane (43.21±8.2 versus 80.37±10.69, p<0.05). In complementary studies, plasma lawn assays also demonstrated a colocalization of F-actin and MyoIIA at the plasma membrane upon insulin stimulation (Fig. 1C). Next, we wanted to determine whether insulin increased the total levels of F-actin or reorganized the actin present. Cell fractionation assays were performed to determine the total level of F-actin in adipocytes in the presence and absence of insulin. As shown in Fig. 1D insulin stimulation did not increase the levels of F-actin or MyoIIA in the pellet fraction. The soluble fractions of both actin and MyoIIA were also detected in the supernatant fraction under both conditions. These findings suggest that there is a redistribution of F-actin and MyoIIA between the cytoplasm and the plasma membrane upon insulin stimulation such that there is no net gain in the pellet fraction. It is possible that insulin induces these changes by an assembly/disassembly mechanism of actin and MyoIIA. Furthermore since only a fraction of the total MyoIIA is recruited to the plasma membrane, there appear to be distinct pools of MyoIIA in the cell that respond differentially to insulin. These results demonstrate that MyoIIA and F-actin colocalize in proximity to the site of GLUT4 vesicle fusion with the plasma membrane upon insulin stimulation.
Fig. 1.
MyoIIA and F-actin colocalize at the plasma membrane in response to insulin. Day 7 3T3-L1 adipocytes were serum starved for 4 h and then either left untreated (basal) or treated with 100 nM insulin for 15 min. (A) Confocal microscopy was employed to determine the localization of MyoIIA and F-actin as described in Materials and methods. White arrows indicate either cytoplasmic or plasma membrane localization. (B) Distribution of GLUT4, MyoIIA, and F-actin in the cytoplasm and at the cell cortex under basal and insulin conditions were quantified as described in Material and methods. Briefly, ratios of fluorescence intensity comparing the levels of protein present in the cytoplasm to the cell cortex were determined, and then a percent difference of basal to insulin was calculated. Images are representative of 3 independent experiments. (C) Plasma lawns were prepared and MyoIIA and F-actin were visualized by confocal microscopy as described in Material and methods. (D) Adipocytes were fractionated as described in Materials and methods and pellet and supernatant proteins were subjected to SDS-PAGE, transferred and immunoblotted for actin. Images are representative of 3 independent experiments.
Inhibition of MyoIIA impairs insulin induced F-actin localization at the plasma membrane
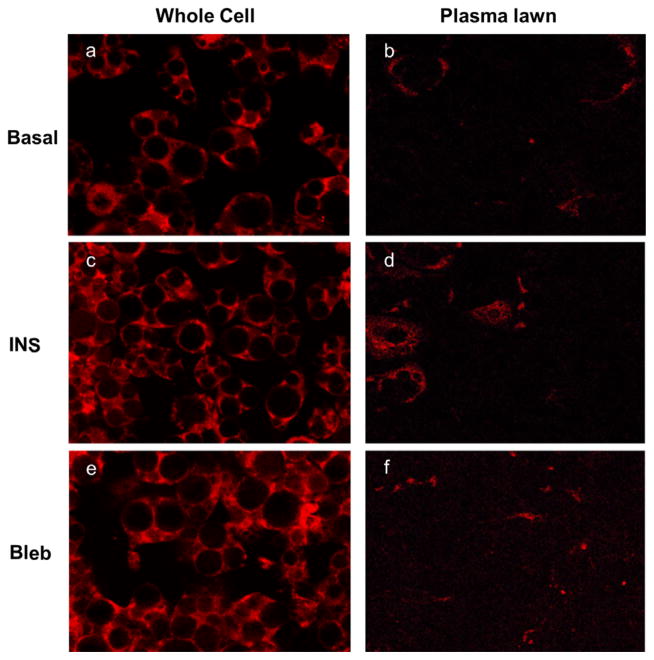
Our previous studies demonstrated that inhibition of MyoIIA with the MyoII specific inhibitor, blebbistatin, impaired insulin-induced translocation of MyoIIA to the plasma membrane as well as glucose uptake despite GLUT4 translocation to the plasma membrane [14]. While, inhibition of MyoII activity did not prevent GLUT4 translocation to the plasma membrane, it did impair the ability of GLUT4 to embed into the plasma membrane [14]. Since MyoII interacts with F-actin to organize cortical actin, we wanted to determine whether MyoIIA activity is required for F-actin localization at the plasma membrane. 3T3-L1 adipocytes were insulin stimulated in the presence or absence of 100 μM blebbistatin and assessed for F-actin localization via confocal microscopy in both whole cells as well as plasma lawns (Fig. 2). Our results show that insulin clearly induced localization of F-actin at the plasma membrane as seen in whole cells as well as plasma lawns (Fig. 2, panels c and d). We further show that inhibition of MyoII with blebbistatin impaired the insulin-stimulated localization of F-actin at the plasma membrane (Fig. 2, panels e and f). These results suggest that MyoII is required for F-actin localization at the plasma membrane upon insulin stimulation.
Fig. 2.
Blebbistatin decreased the level of F-actin at the plasma membrane upon insulin stimulation. (A) Adipocytes were serum starved for 4 h and then either left untreated (Basal) or stimulated with 100 nM insulin alone or blebbistatin (100 μM) for 15 min followed by 100 nM insulin for an additional 15 min. Whole cells and plasma lawns were prepared as described in the Material and methods section. MyoIIA and F-actin were visualized by confocal microscopy in whole cells and plasma lawns as described in Materials and methods. Images are representative of 3 independent experiments.
Calcium regulates MyoIIA and F-actin colocalization at the plasma membrane
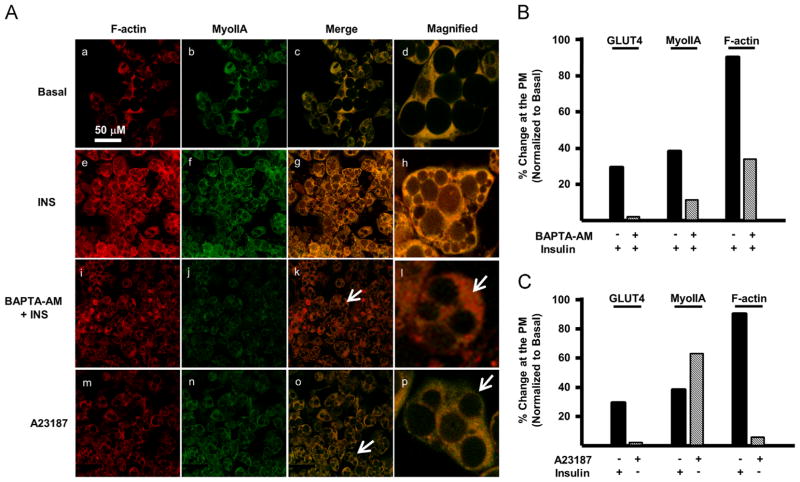
To elucidate the mechanism regulating the insulin-induced colocalization of MyoIIA and F-actin at the plasma membrane, we examined the role of calcium. Previous studies have shown that Ca2+ is required for insulin-stimulated glucose uptake in adipocytes [34,35]. Furthermore, chelation of intracellular Ca2+, by 1, 2-bis (o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetra (acetoxy-methyl) ester (BAPTA-AM) impaired insulin-stimulated glucose in adipocytes [34,35]. Our previous studies demonstrated that the Ca2+ regulated kinase, MLCK, is required for MyoIIA translocation and GLUT4-mediated glucose uptake [14]. Calcium has also been implicated in mediating the colocalization of MyoIIA and F-actin to facilitate actin reorganization and subsequent docking of secretory granules to the plasma membrane [33]. In order to determine whether calcium functions in a similar manner during insulin-stimulated glucose uptake, mature 3T3-L1 adipocytes were subjected to insulin stimulation in the presence or absence of 50 μM BAPTA-AM and assessed for MyoIIA and F-actin localization via confocal microscopy (Fig. 3A). Our results show that BAPTA-AM impaired the insulin-stimulated colocalization of MyoIIA and F-actin at the plasma membrane (Fig. 3A, panels k and l). BAPTA-AM impaired both MyoIIA and F-actin localization at the plasma membrane to less than half that observed during insulin stimulation (Fig. 3B). As shown previously, BAPTA-AM impaired the translocation of GLUT4 to the plasma membrane (Fig. 3B). These findings indicate the necessity of calcium in the colocalization of GLUT4, MyoIIA, and F-actin at the plasma membrane.
Fig. 3.
Calcium is required but not sufficient to colocalize MyoIIA and F-actin at the plasma membrane. (A) 3T3-L1 adipocytes (Day 7) were serum starved for 4 h and then either left untreated (basal), treated with 100 nM insulin for 15 min, treated with BAPTA-AM (50 μM) for 15 min followed by 100 nM insulin for an additional 15 min or with A23187 (0.1 μM) for 15 min in the absence of insulin. Confocal microscopy was used to determine the localization of MyoIIA and F-actin as described in Material and methods. White arrows indicate either cytoplasmic or cell cortex localization. Distribution of GLUT4, MyoIIA, and F-actin localized in the cytoplasm and at the cell cortex was quantified for (B) BAPTA-AM and (C) A23187 as described in Material and methods. Images are representative of 3 independent experiments.
While our studies show that calcium is necessary for MyoIIA and F-actin to colocalize at the plasma membrane upon insulin stimulation we wanted to determine if calcium alone (in the absence of insulin) was sufficient to colocalize MyoIIA and F-actin at the plasma membrane of adipocytes. To increase the intracellular concentration of calcium, serum starved adipocytes were incubated with the ionophore A23187 in the absence of insulin. Our results demonstrate that while A23187 alone was able to stimulate translocation of MyoIIA to the plasma membrane, it was unable to induce colocalization of MyoIIA and F-actin at the plasma membrane (Fig. 3A, panels o and p). While as shown previously, A23187 induced translocation of MyoIIA to the plasma membrane to the same extent as insulin (Fig. 3A, panel n and Fig. 3C), A23187 was not able to increase the levels of F-actin at the plasma membrane above basal levels (Fig. 3A, panel m and Fig. 3C). As expected, A23187 did not induce translocation of GLUT4 to the plasma membrane (Fig. 3C). Collectively, these data indicate that while calcium is required for the colocalization of MyoIIA and F-actin at the plasma membrane, calcium alone is not sufficient to increase the levels of F-actin at the plasma membrane in adipocytes. Thus while insulin stimulates increases in intracellular calcium, it must also stimulate multiple pathways to induce F-actin localization at the plasma membrane in adipocytes. Also, these studies suggest that while MyoII is required for F-actin localization at the plasma membrane upon insulin stimulation, the presence of MyoIIA at the plasma membrane in the absence of insulin stimulation is insufficient to localize F-actin at the plasma membrane.
Calmodulin is required for MyoIIA and F-actin colocalization
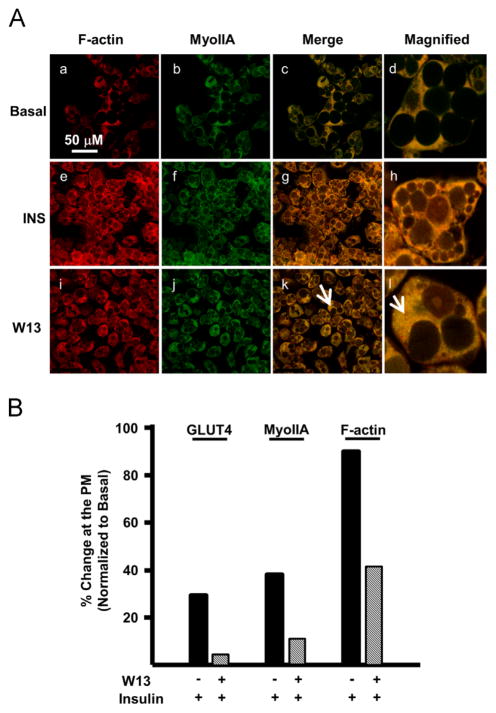
Since calcium is required for colocalization of MyoIIA and F-actin at the plasma membrane, we wanted to determine if calmodulin was also involved. We used the calmodulin antagonist, W13, to prevent the action of calcium–calmodulin complexes. A calcium–calmodulin complex has been shown to activate MLCK, which results in the phosphorylation of MyoIIA in other cell types [36]. Phosphorylation of MyoIIA is required for its translocation to the plasma membrane [14]. To determine the role of calmodulin in this process, Day 7 adipocytes were serum starved in the presence or absence of W13 and then stimulated with insulin. As expected, MyoIIA was inhibited from localizing to the plasma membrane when calmodulin was inactivated (Fig. 4A and B). Also, as shown previously, GLUT4 translocation was inhibited in W13 treated adipocytes (Fig. 4B). Finally, the presence of F-actin at the plasma membrane decreased by half when compared with insulin stimulation alone (Fig. 4B). Collectively, these studies demonstrate the role of calcium and calmodulin [30] in the colocalization of GLUT4, MyoIIA, and F-actin at the plasma membrane during insulin stimulation.
Fig. 4.
Calmodulin is required for the colocalization of MyoIIA and F-actin. (A) Mature 3T3-L1 adipocytes grown on coverslips were serum starved for 4 h and then either left untreated (basal), treated with 100 nM insulin for 15 min, or treated with W13 (70 μM) for 15 min and then stimulated with 100 nM insulin for 15 min. Confocal microscopy was used to visualize the localization of MyoIIA and F-actin. White arrows indicate either cytoplasmic or cell cortex localization. (B) Distribution of GLUT4, MyoIIA, and F-actin within the cell and at the cortex under basal, insulin or W13 conditions was quantified as described in the Materials and methods. Images are representative of 3 independent experiments.
Discussion
Adipocytes possess an actin ‘barrier’ at the cell cortex that must be remodeled in order to facilitate vesicle fusion with the plasma membrane. Previous studies have demonstrated that dynamic actin reorganization is required for GLUT4-mediated glucose uptake [7]. Actin reorganization can be mediated by contraction of F-actin by MyoII in nonmuscle cells. In chromaffin cells, MyoII has been shown to remodel the actin cytoskeleton to facilitate vesicle fusion at the plasma membrane by contracting actin [25]. MyoII has also been shown to play a role in exocytic processes in pancreatic islets [24] and parietal cells [26]. Our previous studies demonstrated that inhibition of MyoII impaired GLUT4-mediated glucose uptake by preventing the proper insertion of GLUT4-containg vesicles in the plasma membrane [14]. What is not known is whether MyoIIA aids in actin remodeling by interacting with F-actin at the site of GLUT4 vesicle fusion. The studies presented here are the first to characterize a MyoIIA–F-actin localization at the site of insulin-induced GLUT4 fusion at the plasma membrane in adipocytes.
Insulin-stimulated glucose uptake requires not only the translocation of GLUT4-containing vesicles but also fusion of these vesicles with the plasma membrane, a process that requires dramatic changes in the cytoskeleton at the cell cortex [7]. Alterations in the cytoskeleton require coordinated changes in the organization and activities of both F-actin and MyoII but what is not known is whether a MyoIIA–F-actin complex is involved. Our studies are the first to reveal that insulin stimulates the colocalization of MyoIIA and F-actin at the site of GLUT4 vesicle fusion at the plasma membrane. Furthermore we demonstrate that insulin stimulation does not alter the total levels of F-actin (Fig. 1C) but instead induces a redistribution of F-actin to the plasma membrane. This finding is in agreement with previous studies which demonstrated that insulin stimulated a dynamic redistribution of actin in adipocytes [7]. Similarly, trafficking of insulin granules in β-cells requires MyoIIA mediated F-actin remodeling at the plasma membrane [33]. However this remodeling is described as a depolymerization event [33]. Since actin remodeling is a dynamic process, it may require polymerization and depolymerization at various sites to facilitate vesicle fusion.
Our previous studies demonstrated that inhibition of MyoIIA by blebbistatin impaired its translocation from a perinuclear location to the plasma membrane in adipocytes but did not inhibit GLUT4 translocation [28]. While blebbistatin inhibits the ATPase activity of MyoII, it does not inhibit the interaction between actin and MyoII [37,38]. To determine whether F-actin localization is dependent on MyoII, we treated cells with blebbistatin. In the present study we show that blebbistatin treatment inhibited the insulin induced increased levels of F-actin at the plasma membrane. This may be due to the fact that MyoII can stabilize F-actin and thus in the absence of MyoIIA at the plasma membrane F-actin is unstable and increased levels of F-actin cannot be detected [39]. Studies are in progress to determine whether MyoIIA alters the stability of F-actin upon insulin stimulation in adipocytes. Interestingly, the MyoIIB isoform which is present at the plasma membrane does not appear to facilitate insulin induced F-actin accumulation at the plasma membrane [28]. This finding is further evidence that the two MyoII isoforms have different roles in adipocytes.
Our studies are the first to show specifically that calcium plays a role in the colocalization of GLUT4, MyoIIA, and F-actin at the plasma membrane during insulin stimulated glucose uptake. Furthermore, our studies employing A23187 and BAPTA-AM provided evidence that calcium is required but not sufficient for MyoIIA and F-actin to colocalize at the plasma membrane during insulin stimulated glucose uptake. The presence of MyoIIA at the plasma membrane is required to colocalize F-actin to the plasma membrane but it alone is not sufficient. These findings suggest that insulin stimulates multiple pathways to reorganize F-actin at the plasma membrane. Our studies show that chelation of calcium reduces the levels of both MyoIIA and F-actin at the plasma membrane even in the presence of insulin. In contrast, raising intracellular calcium levels in the absence of insulin only increased levels of MyoIIA at the plasma membrane.
In summary, our studies show for the first time that insulin stimulates colocalization of MyoIIA and F-actin at the site of GLUT4 vesicle fusion at the plasma membrane of adipocytes. We also demonstrate that impairing MyoIIA translocation to the plasma membrane by either blebbistatin treatment or reduced calcium levels prevents increases in F-actin at the plasma membrane. The requirement of MyoIIA to localize F-actin may facilitate the actin reorganization required for GLUT4 vesicle fusion at the plasma membrane. The requirement for MyoIIA suggests that it may assist with actin reorganization by both stabilizing F-actin and also to contracting F-actin to facilitate GLUT4 vesicle fusion at the plasma membrane. It is also possible that MyoIIA regulates F-actin turnover by acting as a depolymerization agent [40]. Finally we demonstrated that calcium is necessary but not sufficient in the colocalization of MyoIIA and F-actin at the plasma membrane. Collectively, our results are the first to reveal that MyoIIA facilitates insulin-stimulated glucose uptake in 3T3-LI adipocytes by localizing F-actin to the site of GLUT4 vesicle fusion. Characterization of the role of MyoII in GLUT4 vesicle trafficking is critical to understanding the role of the cytoskeleton in insulin-stimulated glucose uptake and will provide new targets for regulation.
Acknowledgments
This work was supported by an NIH grant to Y.M.P. (2R15DK073180-02A1m).
References
- 1.Hirshman MF, Goodyear LJ, Wardzala LJ, Horton ED, Horton ES. Identification of an intracellular pool of glucose transporters from basal and insulin-stimulated rat skeletal muscle. J Biol Chem. 1990;265:987–991. [PubMed] [Google Scholar]
- 2.Rea S, James DE. Moving GLUT4: the biogenesis and trafficking of GLUT4 storage vesicles. Diabetes. 1997;46:1667–1677. doi: 10.2337/diab.46.11.1667. [DOI] [PubMed] [Google Scholar]
- 3.Harmon AW, Paul DS, Patel YM. MEK inhibitors impair insulin-stimulated glucose uptake in 3T3-L1 adipocytes. Am J Physiol Endocrinol Metab. 2004;287:E758–E766. doi: 10.1152/ajpendo.00581.2003. [DOI] [PubMed] [Google Scholar]
- 4.Taha C, Liu Z, Jin J, Al-Hasani H, Sonenberg N, et al. Opposite translational control of GLUT1 and GLUT4 glucose transporter mRNAs in response to insulin. Role of mammalian target of rapamycin, protein kinase b, and phosphatidylinositol 3-kinase in GLUT1 mRNA translation. J Biol Chem. 1999;274:33085–33091. doi: 10.1074/jbc.274.46.33085. [DOI] [PubMed] [Google Scholar]
- 5.Watson RT, Kanzaki M, Pessin JE. Regulated membrane trafficking of the insulin-responsive glucose transporter 4 in adipocytes. Endocr Rev. 2004;25:177–204. doi: 10.1210/er.2003-0011. [DOI] [PubMed] [Google Scholar]
- 6.Guilherme A, Emoto M, Buxton JM, Bose S, Sabini R, et al. Perinuclear localization and insulin responsiveness of GLUT4 requires cytoskeletal integrity in 3T3-L1 adipocytes. J Biol Chem. 2000;275:38151–38159. doi: 10.1074/jbc.M003432200. [DOI] [PubMed] [Google Scholar]
- 7.Kanzaki M, Pessin JE. Insulin-stimulated GLUT4 translocation in adipocytes is dependent upon cortical actin remodeling. J Biol Chem. 2001;276:42436–42444. doi: 10.1074/jbc.M108297200. [DOI] [PubMed] [Google Scholar]
- 8.Tsakiridis T, Vranic M, Klip A. Disassembly of the actin network inhibits insulin-dependent stimulation of glucose transport and prevents recruitment of glucose transporters to the plasma membrane. J Biol Chem. 1994;269:29934–29942. [PubMed] [Google Scholar]
- 9.Eitzen G. Actin remodeling to facilitate membrane fusion. Biochim Biophys Acta. 2003;1641:175–181. doi: 10.1016/s0167-4889(03)00087-9. [DOI] [PubMed] [Google Scholar]
- 10.Boguslavsky S, Chiu T, Foley KP, Osorio-Fuentealba C, Antonescu CN, et al. Myo1c binding to submembrane actin mediates insulin-induced tethering of GLUT4 vesicles. Mol Biol Cell. 2012;23:4065–4078. doi: 10.1091/mbc.E12-04-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bond LM, Brandstaetter H, Sellers JR, Kendrick-Jones J, Buss F. Myosin motor proteins are involved in the final stages of the secretory pathways. Biochem Soc Trans. 2011;39:1115–1119. doi: 10.1042/BST0391115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bose A, Robida S, Furcinitti PS, Chawla A, Fogarty K, et al. Unconventional myosin Myo1c promotes membrane fusion in a regulated exocytic pathway. Mol Cell Biol. 2004;24:5447–5458. doi: 10.1128/MCB.24.12.5447-5458.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung le TK, Hosaka T, Harada N, Jambaldorj B, Fukunaga K, et al. Myosin IIA participates in docking of Glut4 storage vesicles with the plasma membrane in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2010;391:995–999. doi: 10.1016/j.bbrc.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 14.Fulcher FK, Smith BT, Russ M, Patel YM. Dual role for myosin II in GLUT4-mediated glucose uptake in 3T3-L1 adipocytes. Exp Cell Res. 2008;314:3264–3274. doi: 10.1016/j.yexcr.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo T, Mohan K, Srivastava V, Ren Y, Iglesias PA, et al. Understanding the cooperative interaction between myosin II and actin cross-linkers mediated by actin filaments during mechanosensation. Biophys J. 2012;102:238–247. doi: 10.1016/j.bpj.2011.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Y, Wang Y, Zhang J, Deng Y, Jiang L, et al. Rab10 and myosin-Va mediate insulin-stimulated GLUT4 storage vesicle translocation in adipocytes. J Cell Biol. 2012;198:545–560. doi: 10.1083/jcb.201111091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishikura S, Klip A. Muscle cells engage Rab8A and myosin Vb in insulin-dependent GLUT4 translocation. Am J Physiol Cell Physiol. 2008;295:C1016–C1025. doi: 10.1152/ajpcell.00277.2008. [DOI] [PubMed] [Google Scholar]
- 18.Yoshizaki T, Imamura T, Babendure JL, Lu JC, Sonoda N, et al. Myosin 5a is an insulin-stimulated Akt2 (protein kinase Bbeta) substrate modulating GLUT4 vesicle translocation. Mol Cell Biol. 2007;27:5172–5183. doi: 10.1128/MCB.02298-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lopez JA, Burchfield JG, Blair DH, Mele K, Ng Y, et al. Identification of a distal GLUT4 trafficking event controlled by actin polymerization. Mol Biol Cell. 2009;20:3918–3929. doi: 10.1091/mbc.E09-03-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bresnick AR. Molecular mechanisms of nonmuscle myosin-II regulation. Curr Opin Cell Biol. 1999;11:26–33. doi: 10.1016/s0955-0674(99)80004-0. [DOI] [PubMed] [Google Scholar]
- 21.Hartshorne DJ. Myosin phosphatase: subunits and interactions. Acta Physiol Scand. 1998;164:483–493. doi: 10.1046/j.1365-201X.1998.00447.x. [DOI] [PubMed] [Google Scholar]
- 22.Xia D, Stull JT, Kamm KE. Myosin phosphatase targeting subunit 1 affects cell migration by regulating myosin phosphorylation and actin assembly. Exp Cell Res. 2005;304:506–517. doi: 10.1016/j.yexcr.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 23.Krits I, Wysolmerski RB, Holliday LS, Lee BS. Differential localization of myosin II isoforms in resting and activated osteoclasts. Calcif Tissue Int. 2002;71:530–538. doi: 10.1007/s00223-001-1112-0. [DOI] [PubMed] [Google Scholar]
- 24.Wilson JR, Ludowyke RI, Biden TJ. Nutrient stimulation results in a rapid Ca2+-dependent threonine phosphorylation of myosin heavy chain in rat pancreatic islets and RINm5F cells. J Biol Chem. 1998;273:22729–22737. doi: 10.1074/jbc.273.35.22729. [DOI] [PubMed] [Google Scholar]
- 25.Neco P, Giner D, Viniegra S, Borges R, Villarroel A, et al. New roles of myosin II during vesicle transport and fusion in chromaffin cells. J Biol Chem. 2004;279:27450–27457. doi: 10.1074/jbc.M311462200. [DOI] [PubMed] [Google Scholar]
- 26.Zhou R, Watson C, Fu C, Yao X, Forte JG. Myosin II is present in gastric parietal cells and required for lamellipodial dynamics associated with cell activation. Am J Physiol Cell Physiol. 2003;285:C662–C673. doi: 10.1152/ajpcell.00085.2003. [DOI] [PubMed] [Google Scholar]
- 27.Choi YO, Ryu HJ, Kim HR, Song YS, Kim C, et al. Implication of phosphorylation of the myosin II regulatory light chain in insulin-stimulated GLUT4 translocation in 3T3-F442A adipocytes. Exp Mol Med. 2006;38:180–189. doi: 10.1038/emm.2006.22. [DOI] [PubMed] [Google Scholar]
- 28.Steimle PA, Fulcher FK, Patel YM. A novel role for myosin II in insulin-stimulated glucose uptake in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2005;331:1560–1565. doi: 10.1016/j.bbrc.2005.04.082. [DOI] [PubMed] [Google Scholar]
- 29.Takashima S. Phosphorylation of myosin regulatory light chain by myosin light chain kinase, and muscle contraction. Circ J. 2009;73:208–213. doi: 10.1253/circj.cj-08-1041. [DOI] [PubMed] [Google Scholar]
- 30.Student AK, Hsu RY, Lane MD. Induction of fatty acid synthetase synthesis in differentiating 3T3-L1 preadipocytes. J Biol Chem. 1980;255:4745–4750. [PubMed] [Google Scholar]
- 31.Steimle PA, Yumura S, Cote GP, Medley QG, Polyakov MV, et al. Recruitment of a myosin heavy chain kinase to actin-rich protrusions in Dictyostelium. Curr Biol. 2001;11:708–713. doi: 10.1016/s0960-9822(01)00182-8. [DOI] [PubMed] [Google Scholar]
- 32.Paul DS, Harmon AW, Winston CP, Patel YM. Calpain facilitates GLUT4 vesicle translocation during insulin-stimulated glucose uptake in adipocytes. Biochem J. 2003;376:625–632. doi: 10.1042/BJ20030681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilson JR, Ludowyke RI, Biden TJ. A redistribution of actin and myosin IIA accompanies Ca(2+)-dependent insulin secretion. FEBS Lett. 2001;492:101–106. doi: 10.1016/s0014-5793(01)02241-4. [DOI] [PubMed] [Google Scholar]
- 34.Whitehead JP, Molero JC, Clark S, Martin S, Meneilly G, et al. The role of Ca2+ in insulin-stimulated glucose transport in 3T3-L1 cells. J Biol Chem. 2001;276:27816–27824. doi: 10.1074/jbc.M011590200. [DOI] [PubMed] [Google Scholar]
- 35.Worrall DS, Olefsky JM. The effects of intracellular calcium depletion on insulin signaling in 3T3-L1 adipocytes. Mol Endocrinol. 2002;16:378–389. doi: 10.1210/mend.16.2.0776. [DOI] [PubMed] [Google Scholar]
- 36.Wilmann M, Gautel M, Mayans O. Activation of calcium/calmodulin regulated kinases. Cell Mol Biol (Noisyle-grand) 2000;46:883–894. [PubMed] [Google Scholar]
- 37.Kovacs M, Toth J, Hetenyi C, Malnasi-Csizmadia A, Sellers JR. Mechanism of blebbistatin inhibition of myosin II. J Biol Chem. 2004;279:35557–35563. doi: 10.1074/jbc.M405319200. [DOI] [PubMed] [Google Scholar]
- 38.Limouze J, Straight AF, Mitchison T, Sellers JR. Specificity of blebbistatin, an inhibitor of myosin II. J Muscle Res Cell Motil. 2004;25:337–341. doi: 10.1007/s10974-004-6060-7. [DOI] [PubMed] [Google Scholar]
- 39.Bosgraaf L, van Haastert PJ. The regulation of myosin II in Dictyostelium. Eur J Cell Biol. 2006;85:969–979. doi: 10.1016/j.ejcb.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 40.Haviv L, Gillo D, Backouche F, Bernheim-Groswasser A. A cytoskeletal demolition worker: myosin II acts as an actin depolymerization agent. J Mol Biol. 2008;375:325–330. doi: 10.1016/j.jmb.2007.09.066. [DOI] [PubMed] [Google Scholar]