Abstract
Many strategies for controlling the fate of transplanted stem cells rely on the concurrent delivery of soluble growth factors that have the potential to produce undesirable secondary effects in surrounding tissue. Such off target effects could be eliminated by locally presenting growth factor peptide mimics from biomaterial scaffolds to control stem cell fate. Peptide mimics of bone morphogenetic protein 2 (BMP-2) were synthesized by solid phase Fmoc-peptide synthesis and covalently bound to alginate hydrogels via either carbodiimide or sulfhydryl-based coupling strategies. Successful peptide conjugation was confirmed by 1H-NMR spectroscopy and quantified by fluorescently labeling the peptides. Peptides derived from the knuckle epitope of BMP-2, presented from both 2D surfaces and 3D alginate hydrogels, were shown to increase alkaline phosphatase activity in clonally derived murine osteoblasts. Furthermore, when presented in 3D hydrogels, these peptides were shown to initiate Smad signaling, upregulate osteopontin production, and increase mineral deposition with clonally derived murine mesenchymal stem cells. These data suggest that these peptide-conjugated hydrogels may be effective alternatives to local BMP-2 release in directly and spatially eliciting osteogenesis from transplanted or host osteoprogenitors in the future.
Keywords: bone morphogenetic protein, peptide mimic, mesenchymal stem cell, osteogenesis
INTRODUCTION
Much recent work has focused on developing regenerative therapies that rely on somatic stem cells, as these cells can be obtained with relative ease, in many instances from the same patient in need of the therapy. In particular, mesenchymal stem cells (MSCs) are a promising therapeutic source for musculoskeletal regeneration 1, 2. Initial attempts to develop MSC-based therapies were met with limited success, due to the poor survival of the cells after injection and their poor engraftment into host tissues 3. Thus, several engineering challenges facing the widespread application of stem cell therapies remain. These include the need to efficiently deliver cells to damaged tissues in the host organism and to control the fate of transplanted cells in vivo. Recently, materials-based deployment systems have been developed to protect cells during implantation 4, and these materials can be modified to express chemical and mechanical cues that direct cell fate 5.
Currently, many strategies in tissue engineering and regeneration utilize soluble growth factors 6. However, because these growth factors eventually diffuse out of the scaffold, they may have to be administered in high dosage, resulting in secondary effects on surrounding host tissues and a reduced ability to locally influence the fate of cells inside a scaffold. Consequently, clinical studies have so far employed only one material basis (collagen scaffolds) with very high dosages, and new material platforms with controlled release systems have had limited transitional success into clinical studies. For instance, bone morphogenetic protein 2 (BMP-2) is a potent inducer of osteogenesis and could be used to direct the differentiation of MSCs into bone. The clinical use of BMP-2 to promote spinal fusion has led to serious complications, including ectopic bone formation 7, 8, post-operative neurological complaints 9, increased swelling at the surgery site, and incidences of airway compromise 10, 11, emphasizing the importance of appropriate delivery of growth factors. In addition to such safety concerns, growth factor therapies are very expensive, and studies have questioned the cost-effectiveness of current BMP-2 treatments 12.
In contrast to soluble growth factors, integrin-binding RGD peptides are routinely bound to hydrogel scaffolds to facilitate cell migration and adhesion 13. Binding RGD peptides to alginate has previously allowed exploration of how the valency with which RGD is presented, as well as how the mechanical properties of the hydrogel presenting the peptides, affects cell fate 14. Interestingly, it has been shown that similarly tethering epidermal growth factor to substrates to which MSCs are adherent results in improved function compared to the soluble form of the growth factor 15. Providing growth factors in a tethered form may thus affect their ability to influence cell fate, improving the utility of materials used for cell delivery, while simultaneously eliminating the complications of the growth factors diffusing into the surrounding tissue.
Two challenges in covalently attaching growth factors to substrates are that the conjugation efficiency may be very low and the factors themselves costly. However, recent efforts have produced short chain peptides that mimic the activity of growth factors by binding to their cell receptors 16–21. Covalently attaching peptide mimics of BMP-2 to alginate hydrogels is an attractive option for locally controlling the fate of transplanted MSCs, as this method will eliminate diffusion of the osteopromotive factors out of the scaffold material, while also replacing costly recombinant growth factors with chemically synthesized peptides. Alginate is an ideal substrate for these types of experiments, as it is an inert polymer that does not influence cells unless it presents specific adhesive and signaling cues. Furthermore, the mechanical properties of alginate can be varied to affect the fate of encapsulated stem cells 22.
This paper describes the synthesis of alginate hydrogels presenting peptide mimics of BMP-2 and the effect of these peptide-modified hydrogels on encapsulated osteoblasts and mesenchymal stem cells. Two distinct peptide sequences have been previously reported to mimic the bioactivity of BMP-2 16, 17, and both were investigated for their potential to induce osteogensis. The first of these peptides (DWIVA) was covalently bound to alginate using previously established carbodiimide chemistry (Fig. 1A) 23. However, the second peptide, the so-called “knuckle epitope” of BMP-2 (KIPKASSVPTELSAISTLYL), contains two lysine residues within the active sequence, and the primary amines of these residues have the potential to cross-react with the activated carboxyl groups generated using carbodiimide chemistry. This would likely result in a large fraction of peptide bound in an inactive form. Therefore, an orthogonal coupling strategy was pursued to maintain coupling specificity at the N-termini of the peptides. The alginate was functionalized with maleimide groups and the peptide sequence was modified to include an N-terminal cysteine residue. To optimize the efficiency of reaction with the carboxylate groups on the alginate, two different maleimide crosslinkers were investigated: one with an amine moiety and the other with a hydrazide moiety (Fig. 1B–C). After successful conjugation to alginate, both peptides were presented from three-dimensional hydrogels to locally influence the fate of encapsulated osteoprogenitor cells.
Figure 1. Reaction Schemes for Conjugating BMP-2 Mimicking Peptides to Alginate.
(A). DWIVA peptides were coupled to alginate using standard carbodiimide chemistry. Lysine-containing BMP-2 knuckle epitope peptides were conjugated to alginate via two-step, orthogonal coupling schemes, employing either (B) amino-maleimide or (C) hydrazide-maleimide reagents.
MATERIALS AND METHODS
Materials
Protected amino acids, pre-loaded polystyrene resins, 1-(bis(dimethylamino)methylene)-1H-benzotriazolium hexafluorophosphate 3-oxide (HBTU) for solid-phase peptide synthesis, and G4RGDSP-OH peptides, were purchased from Peptides International (Louisville, KY). N-methylpyrrolidinone (NMP), piperidine, diisopropylethylamine (DIEA), and trifluoroacetic acid (TFA) were purchased from Advanced ChemTech. Acetic anhydride, triisopropyl silane (TIS), 1,2-ethanedithiol (EDT), dichloromethane, methanol, diethyl ether, acetonitrile, isobutanol, calcium chloride, calcium sulfate, barium chloride dihydrate, sodium chloride, N-(2-aminoethyl)maleimide trifluoroacetate, (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) (HEPES), 2-(N-morpholino)ethanesulfonic acid (MES), hydroxylamine hydrochloride, rhodamine B isothiocyanate, ethylenediaminetetraacetic acid (EDTA), and 4-methylumbelliferyl phosphate (4-MUP) were purchased from Sigma (St. Louis, MO). Alginate polymers were purchased from FMC Biopolymer (Princeton, NJ). Sulfo-N-hydroxysuccinimide (Sulfo-NHS), 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC), N-β-maleimidopropionic acid hydrazide trifluoroacetate (BMPH), and sodium borate buffer were purchased from Thermo Scientific (Waltham, MA). Deuterium oxide (99.9% D), deuterated dimethyl sulfoxide (d6-DMSO) (99.9% D), and sodium 2,2,3,3-D4-3-trimethylsilylpropionate (TMSP) (98% D) were purchased from Cambridge Isotope Laboratories (Andover, MA). Dulbecco’s Phosphate Buffered Saline (dPBS), Dulbecco’s Modified Eagle Medium (DMEM), fetal bovine serum (FBS), EDTA/trypsin, and L-glutamine were purchased from Invitrogen (Carlsbad, CA). Phenol Red Free Dulbecco’s Modified Eagle Medium (SF DMEM) was purchased from MediaTech (Manassas, VA). Passive lysis buffer was purchased from Promega (Madison, WI). Collagenase P was purchased from Roche Applied Science (Indianapolis, IN). Recombinant human BMP-2 was purchased from R&D Systems (Minneapolis, MN).
Peptide Synthesis
Polystyrene resins pre-loaded with the C-terminal amino acid were swelled in dichloromethane for 30 minutes before being washed with NMP and loaded into the reaction vessel of the peptide synthesizer. Peptide synthesis was performed on a CSBio CS336X peptide synthesizer, using 20% piperidine for Fmoc deprotection and working concentrations of 0.32 M HBTU and 0.2 M DIEA to facilitate amino acid coupling. Amino acids were provided in a fourfold molar excess over the amount of starting amino acids pre-loaded on the resin. A double-coupling protocol was followed to maximize coupling efficiency, and any remaining amine groups were capped by the addition of a 50-fold molar excess of acetic anhydride, activated with DIEA to a final concentration of 0.2 M, after the second coupling step.
Resins were prepared for acid-mediated peptide cleavage by washing with NMP, dichloromethane, and methanol and were allowed to dry. Peptides not containing cysteine residues were cleaved from the resin using 95% TFA with 2.5% water and 2.5% TIS as scavengers. Peptides containing cysteine residues were cleaved using 94% TFA with 2.5% water and 1% TIS as scavengers and 2.5% EDT to prevent disulfide bond formation and peptide dimerization. Cleavage solutions were concentrated in vacuo. The free peptides were collected by precipitation with cold diethyl ether and centrifugation at 10,000 rpm for 15 minutes. The supernatant was decanted, and the remaining powder was redissolved in a 1:1 mixture of water and acetonitrile and lyophilized.
Peptides were purified with an Agilent 1100 Series Purification HPLC, using an acidic mobile phase (0.1% TFA) and a gradient from 20% to 50% acetonitrile on an Agilent C18 Zorbax column. Fractions containing the desired peptides were verified by LC-MS (Agilent 1290 LC/MS System) to have a purity of >90% and then were combined and lyophilized to afford the peptide as a white powder. For NMR analysis, peptides were dissolved in deuterated dimethyl sulfoxide. 1H-NMR spectra were obtained using a Varian Mercury 400 MHz NMR Spectrometer and analyzed using ACD Labs NMR Processor software.
Preparation of Peptide-Conjugated Alginate Polymers
All alginate polymers were purified by dialyzing a 1% (w/v) solution of alginate against deionized water. The alginate was decolored with activated charcoal and filtered through a 0.22 μm membrane. If the unmodified alginate was to be used in cell culture, the polymer solution was maintained under aseptic conditions following filtration. The polymer solution was lyophilized to afford the alginate as a dry powder.
Alginate to be modified with G4RGDSP-OH (RGD) or G4DWIVA-OH (DWIVA) peptides was dissolved to 1% (w/v) in a 0.1 M MES and 0.3 M sodium chloride buffer solution at pH 6.5. After the alginate was completely dissolved, sulfo-NHS and EDC were added in a 1:2 molar ratio, followed immediately by addition of the peptide, according to a previously published procedure 23. The amount of peptide was varied to yield a theoretical degree of substitution between 2 and 10 peptides per polymer chain, based on a previous study that determined reaction efficiency using 125I labeled peptides 23. The reaction was allowed to proceed for 20 hours before being quenched by the addition of hydroxylamine. The reaction mixtures were dialyzed against a decreasing concentration of sodium chloride to remove salts and any unbound peptide. The alginate was then sterile (0.22 μm) filtered and lyophilized.
Because of the lysine residues present in the knuckle epitope of BMP-2, peptides derived from this sequence were not coupled to the alginate via carbodiimide reactions due to the possible cross-reactions with the lysine amine groups. An orthogonal coupling strategy based on maleimide-thiol chemistry was developed to remediate this problem. BMP-2 knuckle epitope peptides were synthesized to include an N-terminal cysteine for access to the sulfhydryl moiety: CGKIPKASSVPTELSAISTLYL-OH. Two separate strategies were investigated for functionalizing alginate with maleimide groups. Using an amine-carboxyl reaction strategy similar to the coupling scheme used for the RGD and DWIVA peptides, N-(2-aminoethyl)maleimide trifluoroacetate was coupled to alginate in a two-fold molar excess of the desired peptide concentration via the previously described carbodiimide reaction. The second coupling strategy employed a hydrazide-carboxyl reaction. Alginate was dissolved to a concentration of 1% (w/v) in a 0.5 M MES buffer, with the pH adjusted to 5.0–6.0. A two-fold molar excess of BMPH was added to the stirring alginate solution, followed by an 8-fold excess of EDC to initiate the coupling reaction. After 2 hours, the maleimide-modified alginate was precipitated by the addition of excess ethanol and collected by vacuum filtration. To remove excess MES, the alginate was redissolved in deionized water and precipitated for a second time by addition of excess ethanol. The maleimide-modified alginate was collected by vacuum filtration and dried overnight in a vacuum dessicator. To react with the cysteine-terminated BMP-2 knuckle epitope peptide, the maleimide-modified alginate was dissolved in phosphate buffered saline at pH 7.2 to a final concentration of 1% (w/v). The sulfhydryl-containing peptide was added, and the reaction was allowed to proceed for 24 hours. Salts and unbound peptide were removed by dialyzing against a decreasing concentration of sodium chloride. The resulting solution was sterile filtered and lyophilized.
Peptide coupling was confirmed qualitatively by 1H-NMR using a Varian Inova 500 MHz NMR Spectrometer and analyzed using ACD Labs NMR Processor software. Alginate was dissolved at 1% (w/v) in deuterium oxide with 0.04% (w/v) sodium 2,2,3,3-D4-3-trimethylsilylpropionate added as an internal standard.
Quantification of Coupling Efficiency
The efficiency of maleimide coupling to alginate was quantified using a fluorometric Maleimide Quantification Assay Kit (Abcam). Maleimide conjugated alginate with varying theoretical degrees of substitution was prepared as described above. The maleimide-modified alginate samples to be assayed were dissolved in Sample Buffer to a final concentration of 1% (w/v). Standards were prepared by making serial dilutions of BMPH plus 1% (w/v) unmodified alginate dissolved in Sample Buffer. The fluorometric assay was performed following the manufacturer’s instructions. As the presence of maleimide is observed by a secondary reaction that itself may have limited efficiency in reacting with maleimide moieties conjugated to alginate, the efficiency of maleimide conjugation is reported as relative to the alginate samples treated with sufficient BMPH to result in a theoretical degree of substitution of one maleimide per alginate polymer.
Peptide coupling efficiency was determined by labeling BMP-2 knuckle epitope peptides with the fluorescent dye rhodamine B. BMP-2 knuckle epitope peptides with the N-terminal amines protected by Fmoc groups were dissolved in a 1:1 mixture of acetonitrile and 50 mM sodium borate buffer at pH 9.0. The temperature of the peptide solution was maintained at 4 °C. A 1.4 molar excess of rhodamine B isothiocyanate was dissolved in the buffer mixture to a final concentration of 10 mg/mL and then added dropwise to the stirring peptide solution. The reaction was allowed to proceed overnight at 4 °C. The labeled peptide was precipitated by the addition of excess acetone and collected by centrifugation. To remove the N-terminal Fmoc group, the peptide was dissolved in 20% piperidine in NMP and allowed to react for 30 minutes at room temperature. The deprotected peptide was precipitated by addition of cold diethyl ether and collected by centrifugation. The peptide was redissolved in deionized water and lyophilized to afford the labeled peptide as a pink powder. The labeled peptide was coupled to maleimide-modified alginate as described above to yield theoretical degrees of substitution of 2, 5, and 10 peptides per alginate polymer chain. Reaction efficiency was determined by dissolving labeled peptide-modified alginate in PBS at a concentration of 1% (w/v) and measuring the fluorescence emission using a FluoroMax-3 spectrofluorimeter and exciting at 560 nm. Standards were prepared by making serial dilutions of labeled peptide. Relative fluorescence quantum yield was determined by dividing the integrated fluorescence intensity from 525 to 675 nm by the absorbance at 560 nm, as measured by a Beckman DU 530 UV/Vis spectrophotometer. The calculated relative quantum yields were used to correct for changes in quantum yield after coupling to alginate.
2D Cell Culture to Assess Peptide Bioactivity
Clonally derived murine osteoblasts (7F2s; ATCC) were maintained in culture in DMEM supplemented with 10% FBS and 0.1% penicillin/streptomycin. To assess peptide bioactivity in 2D, cells were seeded into 12-well tissue culture treated plates at a density of 1,000 cells/cm2 and cultured for 5 days. Samples in which osteogenesis was to be induced were cultured in DMEM with 10% FBS and 0.1% penicillin/streptomycin supplemented with 10 mM β-glycerophosphate and 50 μM ascorbic acid, which are required for in vitro mineralization. Peptides were delivered in soluble form, with concentrations ranging from 5 nM to 50 μM, or were physically adsorbed to the surface of the plates by allowing 200 μL of a 2 mg/mL solution of peptide to evaporate in the wells. Recombinant human BMP-2 was provided at a concentration of 100 ng/mL for a positive control.
3D Cell Culture in Peptide-Presenting Hydrogels
7F2 cells or clonally derived murine mesenchymal stem cells (D1s; ATCC) were maintained in culture in DMEM supplemented with 10% FBS and 0.1% penicillin/streptomycin. Alginates were reconstituted in serum-free DMEM (SF DMEM) to a final concentration of 2% (w/v). To form the alginate hydrogels, RGD-alginate with a theoretical degree of substitution of 10 peptides per polymer chain (DS 10) was mixed with either unmodified alginate (negative control) or BMP peptide-modified alginate (DS 5) in a 1:1 ratio. A positive control was prepared by encapsulating recombinant human BMP-2 (rhBMP-2) at a concentration of 1 μg/mL in a 1:1 mixture of unmodified alginate plus DS 10 RGD alginate hydrogels. Cells were trypsinized, centrifuged at 1400 rpm for 5 minutes, and resuspended into dPBS. The PBS wash was repeated a second time to remove unbound proteins. Cells were resuspended in SF DMEM and mixed with the alginate polymer solutions, so that the final concentration of alginate was 1% (w/v) and the final concentration of cells was 2×107 per mL. Alginate hydrogels were crosslinked by addition of sterile 1.22 M calcium sulfate slurry at 2% (v/v) of total gel. Gels were cast between two glass plates separated by 1 mm for 45 minutes. Alginate discs were punched with a 9.33 mm metal die and were then transferred to multi-well plates containing DMEM with 10% FBS and 0.1% penicillin/streptomycin. For osteogenic media conditions, the media was supplemented with 10 mM β-glycerophosphate and 50 μM ascorbic acid. Media for the positive control was additionally supplemented with 100 ng/mL rhBMP-2. Cells were cultured from 4–16 days, and media was changed every 2–3 days.
Alkaline Phosphatase Assay in 7F2s
Media was removed from wells containing hydrogels, and the hydrogels were washed twice with dPBS. Hydrogels were transferred to 15-mL tubes containing 2 mL of matrix digest buffer (a 1:1 mixture of trypsin/EDTA stock solution and 5 mg/mL collagenase P in SF DMEM) and incubated for 7–10 minutes at 37° C. Eight mL of 50 mM EDTA in dPBS (pH 7.4) was added, and the mixture was incubated for an additional 25 minutes at 37° C. Cells were collected by centrifuging at 2000 rpm for 5 minutes. The cell pellet was resuspended in 1 mL dPBS and transferred to an Eppendorf tube. Cells were again pelleted and then were resuspended into 100 μL of passive lysis buffer and maintained on ice. Cell lysates were sonicated and then clarified by centrifuging at 14,000 rpm for 15 minutes at 4° C. The supernatant was transferred to a clean Eppendorf tube for alkaline phosphatase (ALP) analysis, and the DNA pellet was reserved for later analysis. ALP standards were prepared by dissolving alkaline phosphatase derived from bovine intestinal mucosa (Sigma) in passive lysis buffer. 50 μL of sample or standards were transferred to a black bottom 96-well plate. 200 μL of 4-MUP liquid substrate system were added to each well, and the fluorescence emission was read on a Biotek Synergy plate reader warmed to 37 °C and set to kinetic mode, reading every 5 minutes for 45 minutes. The time point at which the standards exhibited a linear response was chosen for analysis. ALP activity was normalized to DNA content, as determined with a PicoGreen dsDNA kit from Invitrogen. The time for which the osteoblasts were maintained in culture prior to ALP analysis was optimized by choosing the time point (3, 5, or 7 days) at which the difference in ALP activity between the positive and negative controls was largest.
Western Blot Analysis of D1s
Alginate hydrogels containing 107 D1 cells/mL were cast as described above. For PSmad 1/5/8 analysis, the samples were incubated in DMEM supplemented with 10% FBS and 0.1% penicillin/streptomycin for 30 minutes. For osteopontin (OPN) analysis, the samples were maintained in culture for 4 days. The hydrogels containing cells were removed from the culture media, washed with PBS, and transferred to clean Eppendorf tubes. Radio Immunoprecipitation Assay (RIPA) buffer (Sigma) with Minitab Protease Inhibitors (Roche) was added, and the mixture was sonicated to disrupt the gels and cell membranes. The samples were then centrifuged at 14,000 rpm to pellet the remaining alginate gel and cell membranes. The protein content of the supernatant was determined using the bicinchoninic acid (BCA) assay (Thermo Scientific), using bovine serum albumin (BSA) to generate a standard curve.
Thirty μg of protein per sample were loaded onto 16% Tris-Glycene gels, separated by SDS-PAGE, and then transferred to nitrocellulose membranes. Membranes were blocked by incubating in 3% BSA in Tris-buffered saline with Tween (TBST) for 1 hour. Membranes were then incubated with primary antibody (rabbit anti-PSmad 1/5/8 (Cell Signaling) or mouse anti-OPN (Developmental Studies Hybridoma Bank)) for 4 hours at room temperature and washed with TBST. Membranes were incubated with secondary antibody (goat anti-rabbit for PSmad 1/5/8 or rabbit anti-mouse for OPN (Cell Signaling)) conjugated to horseradish peroxidase (HRP) for 30 minutes at room temperature and then washed with TBST. Blots were developed using Bioluminescence X-ray film (Kodak) and the Enhanced Chemiluminescence Substrate System (Thermo Scientific). Actin was probed with mouse anti-actin primary antibody (Chemicon) and HRP-conjugated rabbit anti-mouse secondary antibody (Cell Signaling) to serve as a loading control.
Analysis of Matrix Embedded Calcium
After 16 days of culture in media supplemented with 10 mM β-glycerophosphate and 50 μM ascorbic acid, hydrogels with encapsulated MSCs were washed with PBS and transferred to 1 N hydrochloric acid. The embedded calcium was allowed to dissolve overnight at 4°C. The amount of calcium in solution was determined using a Calcium Assay Kit (Cayman Chemical).
Histological Analysis in 3D Hydrogels
At either day 7 (TUNEL staining) or day 16 (von Kossa), media was removed from wells containing hydrogels, and the hydrogels were washed twice with dPBS. Cells in hydrogels were fixed in a solution of 4% paraformaldehyde in SF DMEM containing 0.1% sodium azide, 0.1% Triton-X-100, and 0.1% Tween-20 for 30 minutes at room temperature. The fixation solution was removed, and the hydrogels were fixed with 100 mM barium chloride in 100 mM HEPES at pH 7.4 for 30 minutes. The hydrogels were then washed twice with 100 mM HEPES (pH 7.4) to remove excess divalent cations that would precipitate in PBS. For von Kossa staining, hydrogels were cut into small pieces using a scalpel and washed with distilled water. The gels were then covered with a 5% (w/v) solution of silver nitrate and exposed to light for 30 minutes. The gels were washed twice with distilled water and mounted on microscope slides for imaging. For TUNEL staining, the hydrogels were cryoprotected by first incubating with 5% sucrose in PBS for 15 minutes at room temperature, followed by an overnight incubation at 4 °C with 30% sucrose and a trace amount of fluorescein free acid (to help identify the gel during sectioning) in PBS. The gels were incubated in Optimal Cutting Temperature Medium (OCT) (Tissue TEK) at room temperature for one hour. Gels were then transferred to a freezing mold with fresh OCT, and the gels/OCT were frozen by immersing in isobutanol chilled in a bath of liquid nitrogen. Eight-μm sections were then prepared using a Leica cryostat. Terminal deoxynucleotidal transferase dUTP nick end labeling (TUNEL) staining was performed to identify apoptotic cells using an In Situ Cell Death Detection Kit (Roche Applied Science), following the manufacturer’s instructions. Fluorescence micrographs were captured using an upright Zeiss LSM 710 confocal microscope.
RESULTS
Peptides synthesized by solid phase Fmoc-peptide synthesis were characterized by LC-MS and 1H-NMR spectroscopy. All peptides exhibited the expected mass to charge ratios in their mass spectra (Supplemental Table S1), and following HPLC purification, LC-MS revealed purities of at least 90%. The NMR spectra of the purified peptides exhibited resonance peaks consistent with the incorporated amino acids (Supplemental Fig. S1).
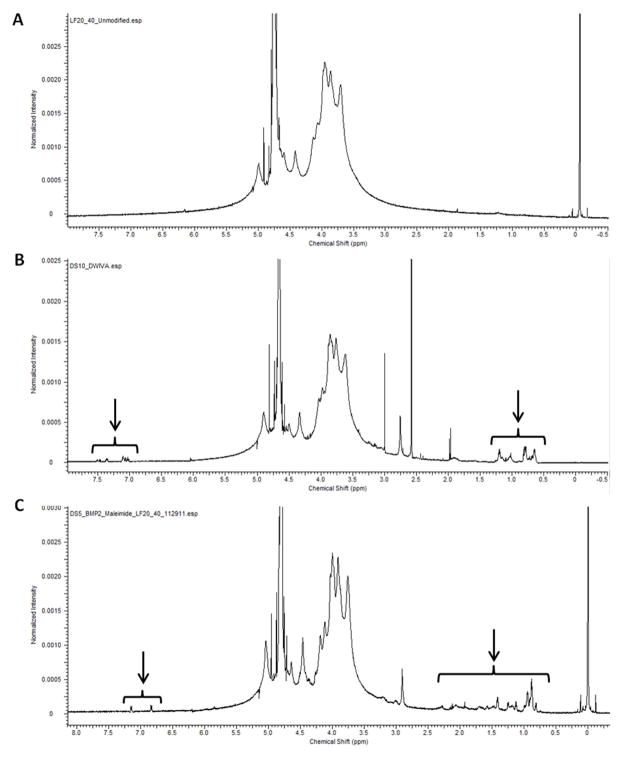
Successful peptide coupling to alginate was qualitatively confirmed via 1H-NMR spectroscopy. Both BMP-2 mimics were successfully conjugated to alginate, as evidenced by the appearance of both high field and aromatic proton resonances characteristic of the respective peptides but absent in the alginate prior to peptide addition (Fig. 2). NMR was also employed to confirm the addition of maleimide groups to alginate after the first conjugation step in the BMP-2 knuckle epitope coupling scheme, which was indicated by the appearance of a downfield singlet corresponding to the protons in the conjugated double bond of the maleimide group (Supplemental Figure S2).
Figure 2. 1H-NMR Spectra of Peptide Conjugated Alginates.
NMR spectra of (A) unmodified alginate, (B) G4DWIVA peptide conjugated alginate, and (C) CG-BMP-2 knuckle epitope peptide conjugated alginate. Arrows denote peaks corresponding to proton resonances characteristic of the respective peptides (D2O; 500 MHz).
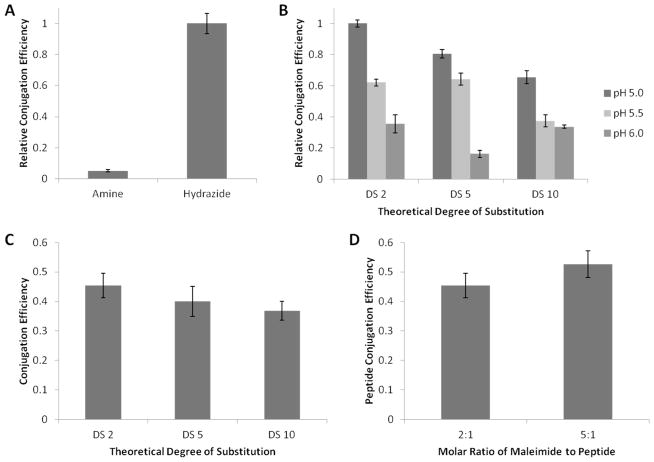
The efficiency of maleimide conjugation was quantified using a fluorometric assay (Abcam). Initial attempts to produce maleimide functionalized alginate utilized N-(2-aminoethyl)maleimide, and followed a protocol previously optimized for conjugating RGD peptides to alginate via the N-terminal amine group (Fig. 1B) 23. However, the efficiency of this coupling scheme was much lower than previously reported for peptides. Therefore, a second reaction strategy, which replaced the amine group with a hydrazide moiety, was also investigated. The hydrazide reaction was shown to be nearly 20-fold more efficient than the amine reaction (Fig. 3A). The efficiency of the maleimide reaction was further increased by decreasing the pH of the reaction buffer from pH 6.0 to pH 5.0, likely due to a decrease in the rate of EDC hydrolysis and longer lived reactive intermediates (Fig. 3B). This improvement could only be realized using the hydrazide reaction scheme, as the lower pKa of hydrazides compared to amines permits a significant proportion of the hydrazide groups to remain un-protonated at pH 5.0, allowing reaction with the activated carboxyl groups on the alginate. Furthermore, the degree of substitution of maleimide groups per alginate polymer can be quantitatively varied, although reaction efficiency does decrease for higher degrees of substitution (Fig. 3B).
Figure 3. Quantification of Peptide Conjugation Efficiency using Maleimide-Thiol Reaction.
(A). Conjugation efficiency at pH 6.0 of maleimide to alginate using amine or hydrazide reaction schemes. (B). Conjugation efficiency for hydrazide functionalized maleimide to alginate varying pH from 5.0 to 6.0. (C). BMP-2 knuckle epitope peptide conjugation efficiency to alginate at varying theoretical degrees of peptide substitution. (D). Effect of varying the molar ratio of maleimide to peptide on conjugation efficiency, when reacted with CG-BMP-2 knuckle epitope peptide to yield a theoretical peptide degree of substitution of 2. Error bars are ± SD, n = 3.
The conjugation efficiency of BMP-2 knuckle epitope peptides to maleimide functionalized alginate was determined using peptides labeled with rhodamine B isothiocyanate via one of the lysine residues. After correcting for fluorescence quantum yield changes after coupling to alginate (Supplemental Figures S3 and S4), the conjugation efficiency was determined to be approximately 40%. This result was consistent for degrees of peptide substitution from 2 to 10 peptides per alginate polymer (Fig. 3C). Increasing the molar ratio of maleimide to peptide by 5-fold did not result in a statistically significant increase in conjugation efficiency (Fig. 3D), suggesting that the maximal conjugation efficiency for the BMP-2 knuckle epitope peptides was reached.
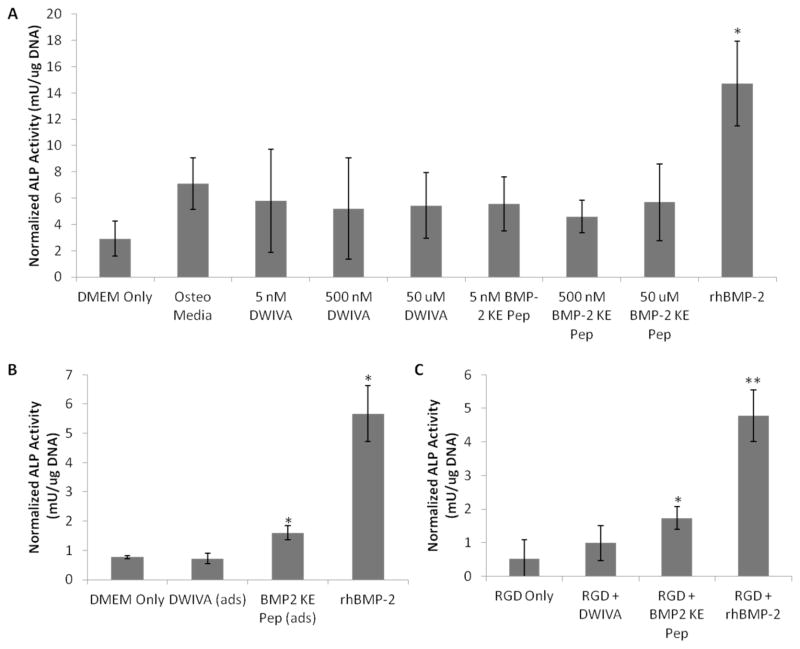
The biological activity of the peptide mimics was first assessed in 2D culture using clonally derived murine osteoblasts, as osteogenic induction in osteoblasts can be readily measured by the relatively rapid upregulation of osteogenic markers, such as alkaline phosphatase (ALP) activity, in response to treatment with BMP-2. The time point for assaying ALP activity was chosen as the earliest time point at which ALP activity in osteoblasts treated with rhBMP-2 was significantly greater than in cells treated with control media (Supplemental Figure S5). Initial studies delivered the peptides in a soluble form, the traditional delivery route for recombinant growth factors. DWIVA and BMP-2 knuckle epitope peptides were added to the culture media at concentrations ranging from those typical of recombinant growth factors (~5 nM) to those present in 3D hydrogel constructs (~50 μM). After 5 days in culture, the only conditions that exhibited increased osteogenic activity (as indicated by increased ALP activity) were those supplemented with rhBMP-2 as a positive control (Fig. 4A). The conditions supplemented with the peptide mimics showed no significant increase in ALP activity over the other control group cultured in osteogenic media.
Figure 4. Alkaline Phosphatase Activity of Osteoblasts Cultured with BMP Mimicking Peptides.
(A). ALP activity in osteoblasts maintained in 2D culture under osteogenic conditions for 5 days and treated with soluble DWIVA or BMP-2 knuckle epitope (KE) peptides at varying concentrations. Culture media alone and media with osteogenic supplements served as negative controls, and soluble recombinant human BMP-2 was provided as a positive control. *p < 0.01, two-tailed Student’s t-test. (B). ALP activity in osteoblasts cultured on 2D surfaces in standard culture media (negative control), with physically adsorbed DWIVA or BMP-2 knuckle epitope peptides, or with soluble rhBMP-2 (positive control) after 5 days in culture. *p < 0.01, two-tailed Student’s t-test. (C). ALP activity in osteoblasts cultured for 7 days in 3D alginate hydrogels presenting DWIVA or BMP-2 knuckle epitope peptides and RGD adhesion peptides. Hydrogels presenting only RGD peptides served as a negative control, and hydrogels with encapsulated rhBMP-2 served as a positive control. *p < 0.05, **p < 0.001, two-tailed Student’s t-test. Error bars are ± SD, n = 3.
In contrast to the results of studies using the soluble peptide, BMP-2 knuckle epitope peptide physically adsorbed to tissue culture plates increased ALP activity from osteoblasts after 5 days in 2D culture (Fig. 4B). However, even adsorbed DWIVA peptide was incapable of inducing osteogenesis, as measured by ALP activity. Note that the absolute level of ALP activity present in the osteoblasts may vary based on passage number, however, the relative differences between the positive and negative controls are consistent (approximately 6-fold) across all experiments. This allows for relative comparisons of peptide bioactivity within the same experiment.
The ability of the BMP-2 mimicking peptides to elicit osteogenesis in 3D culture was first assessed by encapsulating 7F2 osteoblasts in alginate hydrogels presenting these peptides. Cells remained viable within these hydrogels after seven days in culture, as indicated by low levels of TUNEL staining (Fig. 5). Osteoblasts encapsulated within alginate hydrogels presenting a combination of RGD adhesion peptides and BMP-2 knuckle epitope peptides exhibited increased ALP activity after one week in culture, as did osteoblasts cultured in RGD presenting hydrogels with soluble rhBMP-2 (Fig. 4C), which is consistent with the 2D culture results. Furthermore, cells cultured in alginate hydrogels presenting DWIVA peptides exhibited no significant increase in ALP activity.
Figure 5. TUNEL Staining of BMP-2 Mimicking Peptide Conjugated Hydrogel Sections.
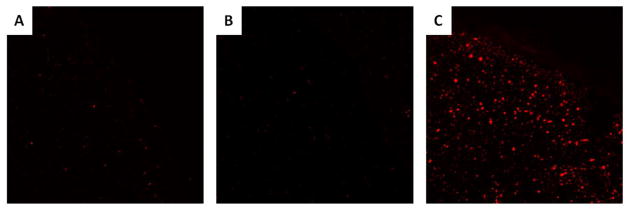
TUNEL staining for cells encapsulated in 3D hydrogels presenting both RGD adhesion peptide and either (A) BMP-2 knuckle epitope peptide or (B) DWIVA peptide after 7 days in culture. (C). Sections treated with DNAse to induce DNA fragmentation served as a positive control.
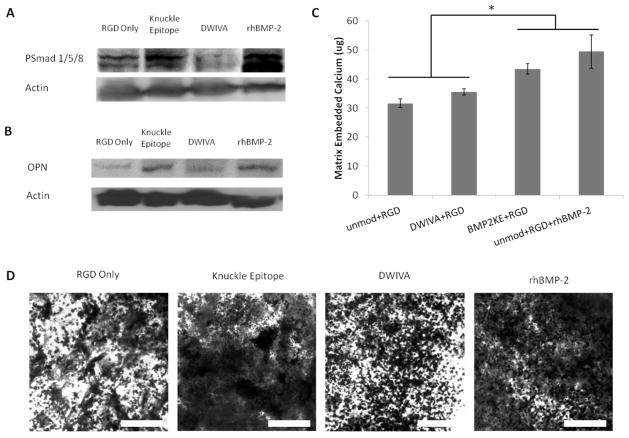
To determine the ability of 3D alginate hydrogels presenting BMP-2 mimicking peptides to elicit osteogenesis in MSCs, clonally derived murine mesenchymal stem cells were encapsulated in hydrogels presenting both RGD and either DWIVA or BMP-2 knuckle epitope peptides. The induction of Smad signaling in response to BMP-2 peptide binding was probed by Western blot analysis for PSmad 1/5/8 (Fig. 6A). Smad signaling was upregulated in response to BMP-2 knuckle epitope peptide and to rhBMP-2 within 30 minutes of encapsulation, but not in response to DWIVA peptide. The osteogenic differentiation of these cells was then assessed by probing for osteopontin (OPN) expression (Fig. 6B). Consistent with the Smad signaling results, by day 4 post-encapsulation, OPN was upregulated in response to BMP-2 kunckle epitope peptide and to rhBMP-2, but not in response to DWIVA peptide. After 16 days in culture with mineralizing supplements, MSCs encapsulated within hydrogels presenting BMP-2 knuckle epitope peptides or with co-encapsulated rhBMP-2 exhibited increased mineral deposition. The amount of matrix embedded calcium was significantly increased in both of these conditions (Fig. 6C), and von Kossa staining revealed increased phosphate deposition within the alginate gels in response to the knuckle epitope peptide and recombinant BMP-2 (Fig. 6D).
Figure 6. Osteogenic Differentiation of MSCs in Response to BMP-2 Mimicking Peptides Presented from 3D Hydrogels.
Representative Western blots for (A) PSmad 1/5/8 and (B) Osteopontin (OPN) in murine mesenchymal stem cells (D1) cultured in 3D alginate hydrogels presenting DWIVA or BMP-2 knuckle epitope peptides and RGD adhesion peptides. PSmad 1/5/8 was probed 30 minutes after encapsulation, and OPN was probed after 4 days in culture. (C) Quantification of calcium embedded within alginate hydrogels presenting BMP-2 mimicking peptides to D1 cells after 16 days in culture with mineralizing supplements. Error bars are ± SD, n = 4. *p<0.01, two-tailed Student’s t-test. (D) Von Kossa staining for mineral deposition within hydrogels presenting BMP-2 mimicking peptides to D1 cells after 16 days in culture with mineralizing supplements. Scale bar: 200 μm. In all cases, hydrogels presenting only RGD peptides served as a negative control, and hydrogels with encapsulated rhBMP-2 served as a positive control.
DISCUSSION
Covalently coupling BMP mimicking peptides to alginate hydrogels is an attractive strategy for directing the osteogenic differentiation of MSCs. A two-step, orthogonal reaction scheme for conjugating bioactive peptides to alginate was developed that permits the conjugation of peptides containing lysine residues in their active sequences that would otherwise react non-specifically in previously used carbodiimide reactions. Furthermore, an improved method for quantifying the conjugation efficiency of peptides to alginate was developed that eliminates the need for radiolabeling used in previous studies 23. The degree of peptide substitution can be quantitatively controlled, providing the ability to modulate the density of peptide presented by 3D hydrogels.
The biological activity of two peptides that have been previously reported to mimic the activity of BMP-2 was investigated in this study. The previous studies that originally identified the peptides of interest sought to recapitulate the biological activity of recombinant BMP-2 by isolating peptide sequences from two separate epitopes of the BMP-2 protein. Both studies identified target sequences and methodically reduced the size of the peptide fragments derived to obtain the shortest sequence capable of retaining the biological activity of the BMP-2 protein. Furthermore, these studies included control peptide sequences to ensure that non-specific binding interactions were not responsible for the observed biological activity. The engineered peptides were capable of inducing BMP receptor phosphorylation and upregulation of osteogenic markers, while the control peptides were not.16, 17 Therefore, the present study has focused solely on those sequences previously reported to be bioactive.
Delivering these BMP-mimicking peptides at varying concentrations to cells in 2D culture was unable to increase ALP production. A potential explanation for this apparent lack of biological activity is the monovalent nature of the peptides. Native BMPs are homodimers that bind to a heterotetrameric complex of surface receptors to initiate signaling 24, 25. Because the peptides are not divalent, it is unlikely that two peptides in solution will simultaneously bind to the receptor complex to mimic the dimeric nature of the native proteins. An earlier study delivering the BMP-2 knuckle epitope peptide in soluble form only observed significant increases in ALP activity after adding a hydroxyapatite binding domain to the peptide’s C-terminus 26. It is possible that the addition of this binding domain allowed the peptide to bind to the culture substrate, presenting the peptide at a sufficiently high density as to appear to be multivalent. Consistent with this hypothesis, and with other studies that have shown that surfaces presenting the knuckle epitope of BMP-2 are capable of inducing osteogenic differentiation 16, 27, adsorbed BMP-2 knuckle epitope peptide was capable of upregulating ALP activity in osteoblasts. Similarly, in 3D hydrogels, increased ALP activity was observed in gels presenting BMP-2 knuckle epitope peptide. Furthermore, these gels were shown to be capable of initiating Smad signaling and upregulating osteopontin production in MSCs, and significantly higher mineral deposition was observed for MSCs cultured in hydrogels either presenting BMP-2 knuckle epitope peptide or with co-encapsulated rhBMP-2. Computational models have previously revealed that the spacing of peptides within alginate hydrogels can be modulated by varying the number of peptides coupled per alginate chain and the fraction of alginate chains that possess bound peptide 28, suggesting that at sufficiently high degrees of peptide substitution, a significant fraction of the peptide may be presented in a multivalent form.
The increase in ALP activity in osteoblasts due to treatment with recombinant BMP-2 was still significantly higher than the increased ALP activity in osteoblasts treated with the BMP-2 knuckle epitope peptide. On a molar basis, the BMP-mimicking peptides were presented to the osteoblasts encapsulated in 3D gels at approximately three orders of magnitude higher concentration. The decreased activity of linear peptides compared to intact growth factors is not surprising. The linear peptides are free to adopt more conformations than the highly structured growth factor binding domains, which may contribute to a lower binding affinity of the peptides for the BMP receptors and in turn result in lower bioactivity. This effect has previously been observed with integrin binding RGD peptides, as well as platelet derived growth factor (PDGF) and nerve growth factor (NGF) mimetic peptides. Cyclic peptides that better mimic the loop structure of the native fibronectin RGD epitope were shown to bind more strongly to integrins, increase cell proliferation, and upregulate osteogenic markers in MSCs relative to linear peptides29, 30. Additionally, cyclic, but not linear, peptides derived from PDGF were recognized by antibodies derived against intact PDGF31, and bicyclic peptides that mimic the L1 and L4 loops of NGF were demonstrated to be necessary to recapitulate the full biological activity of NGF32. Furthermore, if peptide clustering is required to simulate dimerization, only some fraction of the peptides will be in the appropriate conformation to initiate signaling. However, when considering the mineralization data for encapsulated MSCs, there is no significant difference between the amount of matrix embedded calcium for MSCs presented with BMP-2 knuckle epitope peptide or recombinant BMP-2. Thus, over longer time scales, the same outcome may be reached by treatment with either immobilized BMP-2 knuckle epitope peptide or recombinant BMP-2. While on a molar basis more peptide is required, the cost of this peptide will be substantially lower than for the amount of recombinant BMP-2 required to obtain the same extent of mineralization, and treatment with immobilized peptide may also limit some of the undesirable secondary effects associated with recombinant BMP-27–12.
None of the experiments discussed above demonstrated increased osteogenic activity in response to the DWIVA peptide. These results suggest that the DWIVA sequence is not bioactive in the various methods in which it has been presented. This observation is consistent with X-ray crystallography data showing that the knuckle epitope of BMPs determines the binding of the protein to its receptors 33, not the epitope where the DWIVA motif is located. However, the study initially reporting the DWIVA peptide would contradict this assertion, as the authors presented data demonstrating BMP receptor binding and Smad phosphorylation.17 A significant difference between the present study and this earlier work is that the DWIVA peptide was previously reported as a peptide amphiphile designed to self-assemble into 3D gels. As the entire self-assembled gel was composed of these DWIVA peptides, the concentration of peptide would be significantly higher than in the alginate system developed in the current study. It is possible that the binding interaction between this relatively short peptide sequence and the BMP receptors is rather weak, and only such high concentrations would result in sufficient receptor activation for measurable biological activity.
The materials system developed in this study has implications for both basic biological research and therapeutic applications in regenerative medicine. By controlling the presentation of the peptide within the alginate hydrogels 34, 35, the roles of peptide valency 36 and crosstalk with integrin signaling 37 can be investigated in three dimensional space. Additionally, alginate hydrogel scaffolds presenting BMP mimicking peptides can be employed in bone regeneration therapies in vivo. Previous studies have shown that BMP-2 knuckle epitope peptides conjugated to hydrogels can induce bone formation when implanted into rat calf muscle 38, and nano-hydroxyapatite/poly(L-lactic acid) composite scaffolds loaded with these peptides were shown to induce healing in a rat cranial defect model 39.
CONCLUSION
Delivering peptides that mimic the bioactivity of growth factors by covalently coupling them to alginate hydrogels is an attractive strategy for controlling the fate of encapsulated cells both locally and for a significant time period post implantation. We have successfully conjugated peptides previously shown to mimic the activity of bone morphogenetic proteins to alginate. A peptide derived from the knuckle epitope of BMP-2 was shown to elicit increased alkaline phosphatase activity in murine osteoblasts, both when presented from 2D surfaces as well as from 3D hydrogel matrices. Hydrogels presenting BMP-2 knuckle epitope peptide also induced Smad signaling, upregulated osteopontin production, and increased mineralization with murine mesenchymal stem cells. Growth factor mimetic peptides tethered to alginate hydrogels have the potential to both allow for localized control over cell fate, preventing undesirable secondary effects in surrounding tissues in vivo, while also allowing for precise control over the presentation of the peptides, permitting modulation of the signaling potency for cells encapsulated within the scaffolds.
Supplementary Material
Acknowledgments
The authors would like to thank Praveen Arany for assistance with Western Blotting and Shimwoo Lee for assistance with alginate sample preparation. This project was supported by funding from the NIH (R37 DE013033) (DJM), the Harvard College Program for Research in Science and Engineering (PRISE) (CMM), NSF (DMR-0846363) (SCH), NIH (R01-DK085720) (SCH), and the award of an Einstein Visiting Fellowship by the Einstein Foundation Berlin through the Charité – Universitätsmedizin Berlin, Berlin-Brandenburg School for Regenerative Therapies GSC 203 (DJM).
Footnotes
Author Contributions
CMM, MM, and DJM designed experiments. CMM and MM performed experiments. GND, SCH, and DJM supervised research. CMM and DJM wrote the manuscript. DJM is the principal investigator.
Supplemental Table S1 and Supplemental Figures S1–S4 are provided as supporting information. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Caplan AI. Adult Mesenchymal Stem Cells for Tissue Engineering Versus Regenerative Medicine. J Cell Physiol. 2007;213:341–347. doi: 10.1002/jcp.21200. [DOI] [PubMed] [Google Scholar]
- 2.Pountos I, Corscadden D, Emery P, Giannoudis PV. Mesenchymal stem cell tissue engineering: Techniques for isolation, expansion and application. Injury. 2007;38(Supplement 4):S23–S33. doi: 10.1016/s0020-1383(08)70006-8. [DOI] [PubMed] [Google Scholar]
- 3.Mooney DJ, Vandenburgh H. Cell Delivery Mechanisms for Tissue Repair. Cell Stem Cell. 2008;2:205–211. doi: 10.1016/j.stem.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Silva EA, Kim ES, Kong HJ, Mooney DJ. Material-based deployment enhances efficacy of endothelial progenitor cells. Proc Natl Acad Sci US A. 2008;105:14347–14352. doi: 10.1073/pnas.0803873105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sittinger M, Hutmacher DW, Risbud MV. Current strategies for cell delivery in cartilage and bone regeneration. Cur Opin Biotechnol. 2004;15:411–418. doi: 10.1016/j.copbio.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 6.Mehta M, Schmidt-Bleek K, Duda GN, Mooney DJ. Biomaterial delivery of morphogens to mimic the natural healing cascade in bone. Adv Drug Delivery Rev. 2012;64:1257–1276. doi: 10.1016/j.addr.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cahill KS, Chi JH, Day A, Claus EB. Prevalence, Complications, and Hospital Charges Associated With Use of Bone-Morphogenetic Proteins in Spinal Fusion Procedures. J Am Med Assoc. 2009;302:58–66. doi: 10.1001/jama.2009.956. [DOI] [PubMed] [Google Scholar]
- 8.Haid RW, Branch CL, Alexander JT, Burkus JK. Posterior lumbar interbody fusion using recombinant human bone morphogenetic protein type 2 with cylindrical interbody cages. Spine J. 2004;4:527–539. doi: 10.1016/j.spinee.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 9.Wong DA, Kumar A, Jatana S, Ghiselli G, Wong K. Neurologic impairment from ectopic bone in the lumbar canal: a potential complication of off-label PLIF/TLIF use of bone morphogenetic protein-2 (BMP-2) Spine J. 2008;8:1011–1018. doi: 10.1016/j.spinee.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 10.Smucker J, Rhee J, Singh K, Yoon S, Heller J. Increased swelling complications associated with off-label usage of rhBMP-2 in the anterior cervical spine. Spine (Philadelphia) 2006;31:2813–2819. doi: 10.1097/01.brs.0000245863.52371.c2. [DOI] [PubMed] [Google Scholar]
- 11.Shields LB, Raque GH, Glassman SD, Campbell M, Vitaz T, Harpring J, Shields CB. Adverse effects associated with high dose rhBMP-2 use in anterior cervical spine fusion. Spine (Philadelphia) 2006;31:542–547. doi: 10.1097/01.brs.0000201424.27509.72. [DOI] [PubMed] [Google Scholar]
- 12.Garrison K, Donell S, Ryder J, Shemilt I, Mugford M, Harvey I, Song F. Clinical effectiveness and cost-effectiveness of bone morphogenetic proteins in the non-healing of fractures and spinal fusion: a systematic review. Health Technology Assessment. 2007;11 doi: 10.3310/hta11300. [DOI] [PubMed] [Google Scholar]
- 13.Ruoslahti E, Pierschbacher MD. New Perspectives in Cell Adhesion: RGD and Integrins. Science. 1987;238:491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- 14.Hsiong SX, Huebsch N, Fischbach C, Kong HJ, Mooney DJ. Integrin-Adhesion Ligand Bond Formation of Preosteoblasts and Stem Cells in Three-Dimensional RGD Presenting Matrices. Biomacromolecules. 2009;9:1843–1851. doi: 10.1021/bm8000606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fan VH, Au A, Tamama K, Littrell R, Richardson LB, Wright JW, Wells A, Griffith LG. Tethered EGF Provides a Survival Advantage to Mesenchymal Stem Cells. Stem Cells. 2007;25:1241–1251. doi: 10.1634/stemcells.2006-0320. [DOI] [PubMed] [Google Scholar]
- 16.Saito A, Suzuki Y, Ogata S-i, Ohtsuki C, Tanihara M. Activation of osteo-progenitor cells by a novel synthetic peptide derived from the bone morphogenetic protein-2 knuckle epitope. Biochim Biophys Acta. 2003;1651:60–67. doi: 10.1016/s1570-9639(03)00235-8. [DOI] [PubMed] [Google Scholar]
- 17.Lee JY, Choo JE, Choi YS, Suh JS, Lee SJ, Chung CP, Park YJ. Osteoblastic differentiation of human bone marrow stromal cells in self-assembled BMP-2 receptor-binding peptide-amphiphiles. Biomaterials. 2009;30:3532–3541. doi: 10.1016/j.biomaterials.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 18.D’Andrea LD, Iaccarino G, Fattorusso R, Sorriento D, Carannante C, Capasso D, Trimarco B, Pedone C. Targeting angiogenesis: Structural characterization and biological properties of a de novo engineered VEGF mimicking peptide. Proc Natl Acad Sci US A. 2005;102:14215–14220. doi: 10.1073/pnas.0505047102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin X, Takahashi K, Campion SL, Liu Y, Gustavsen GG, Pena LA, Zamora PO. Synthetic peptide F2A4-K-NS mimics fibroblast growth factor-2 in vitro and is angiogenic in vivo. Int J Mol Med. 2006;17:833–839. doi: 10.3892/ijmm.17.5.833. [DOI] [PubMed] [Google Scholar]
- 20.Manfe V, Kochoyan A, Bock E, Berezin V. Peptides derived from specific interaction sites of the fibroblast growth factor 2 - FGF receptor complexes induce receptor activation and signaling. J Neurochem. 2010;114:74–86. doi: 10.1111/j.1471-4159.2010.06718.x. [DOI] [PubMed] [Google Scholar]
- 21.Kirkwood K, Rheude B, Kim YJ, White K, Dee KC. In Vitro Mineralization Studies with Substrate-Immobilized Bone Morphogenetic Protein Peptides. J Oral Implantol. 2003;29:57–65. doi: 10.1563/1548-1336(2003)029<0057:IVMSWS>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 22.Huebsch N, Arany PR, Mao AS, Shvartsman D, Ali OA, Bencherif SA, Rivera-Feliciano J, Mooney DJ. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat Mater. 2010;9:518–526. doi: 10.1038/nmat2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rowley JA, Madlambayan G, Mooney DJ. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials. 1999;20:45–53. doi: 10.1016/s0142-9612(98)00107-0. [DOI] [PubMed] [Google Scholar]
- 24.Senta H, Park H, Bergeron E, Drevelle O, Fong D, Leblanc E, Cabana F, Roux S, Grenier G, Faucheux N. Cell responses to bone morphogenetic proteins and peptides derived from them: Biomedical applications and limitations. Cytokine Growth Factor Rev. 2009;20:213–222. doi: 10.1016/j.cytogfr.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 25.de Gorter DJJ, van Bezooijen RL, ten Dijke P. Bone Morphogenetic Proteins and Their Receptors. Encycl Life Sci. 2009:1–9. [Google Scholar]
- 26.Lee JS, Lee JS, Murphy WL. Modular peptides promote human mesenchymal stem cell differentiation on biomaterial surfaces. Acta Biomater. 2010;6:21–28. doi: 10.1016/j.actbio.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moore NM, Lin NJ, Gallant ND, Becker ML. Synergistic enhancement of human bone marrow stromal cell proliferation and osteogenic differentiation on BMP-2-derived and RGD peptide concentration gradients. Acta Biomater. 2011;7:2091–2100. doi: 10.1016/j.actbio.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 28.Comisar WA, Hsiong SX, Kong HJ, Mooney DJ, Linderman JJ. Multi-scale modeling to predict ligand presentation within RGD nanopatterned hydrogels. Biomaterials. 2006;27:2322–2329. doi: 10.1016/j.biomaterials.2005.10.037. [DOI] [PubMed] [Google Scholar]
- 29.Kumagai H, Tajima M, Ueno Y, Giga-Hama Y, Ohba M. Effect of cyclic RGD peptide on cell adhesion and tumor metastasis. Biochem Biophys Res Commun. 1991;177:74–82. doi: 10.1016/0006-291x(91)91950-h. [DOI] [PubMed] [Google Scholar]
- 30.Hsiong SX, Boontheekul T, Huebsch N, Mooney DJ. Cyclic Arginine-Glycine-Aspartate Peptides Enhance Three-Dimensional Stem Cell Osteogenic Differentiation. Tissue Eng, Part A. 2009;15:263–272. doi: 10.1089/ten.tea.2007.0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel G, Husman W, Tehanli AM, Deadman JJ, Green D, Kakkar VV, Brennand DM. A cyclic peptide analogue of the loop III region of platelet derived growth factor-BB is a synthetic antigen for the native protein. J Pept Res. 1999;53:68–74. doi: 10.1111/j.1399-3011.1999.tb01618.x. [DOI] [PubMed] [Google Scholar]
- 32.Colangelo AM, Bianco MR, Vitagliano L, Cavaliere C, Cirillo G, Gioia LD, Diana D, Colombo D, Redaelli C, Zaccaro L, Morelli G, Papa M, Sarmientos P, Alberghina L, Martegani E. A New Nerve Growth Factor-Mimetic Peptide Active on Neuropathic Pain in Rats. J Neurosci. 2008;28:2698–2709. doi: 10.1523/JNEUROSCI.5201-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown MA, Zhao Q, Baker KA, Naik C, Chen C, Pukac L. Crystal structure of BMP-9 and functional interactions with pro-region and receptors. J Biol Chem. 2005;280:25111–25118. doi: 10.1074/jbc.M503328200. [DOI] [PubMed] [Google Scholar]
- 34.Lee KY, Alsberg E, Hsiong SX, Comisar WA, Linderman JJ, Ziff R, Mooney DJ. Nanoscale Adhesion Ligand Organization Regulates Osteoblast Proliferation and Differentiation. Nano Lett. 2004;4:1501–1506. doi: 10.1021/nl0493592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Comisar WA, Kazmers NH, Mooney DJ, Linderman JJ. Engineering RGD nanopatterned hydrogels to control preosteoblast behavior: A combined computational and experimental approach. Biomaterials. 2007;28:4409–4417. doi: 10.1016/j.biomaterials.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 36.Wall ST, Saha K, Ashton RS, Kam KR, Schaffer DV, Healy KE. Multivalency of Sonic Hedgego Conjugated to Linear Polymer Chains Modulates Protein Potency. Bioconjugate Chem. 2008;19:806–812. doi: 10.1021/bc700265k. [DOI] [PubMed] [Google Scholar]
- 37.Guo X, Wang XF. Signaling cross-talk between TGF-β/BMP and other pathways. Cell Res. 2009;19:71–88. doi: 10.1038/cr.2008.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saito A, Suzuki Y, Ogata SI, Ohtsuki C, Tanihara M. Prolonged ectopic calcification induced by BMP-2–derived synthetic peptide. J Biomed Mater Res, Part A. 2004;70:115–121. doi: 10.1002/jbm.a.30071. [DOI] [PubMed] [Google Scholar]
- 39.Li J, Hong J, Zheng Q, Guo X, Lan S, Cui F, Pan H, Zou Z, Chen C. Repair of Rat Cranial Bone Defects with nHAC/PLLA and BMP-2-Related Peptide or rhBMP-2. J Orthop Res. 2011;29:1745–1752. doi: 10.1002/jor.21439. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.