Abstract
Adenosine signalling has long been a target for drug development, with adenosine itself or its derivatives being used clinically since the 1940s. In addition, methylxanthines such as caffeine have profound biological effects as antagonists at adenosine receptors. Moreover, drugs such as dipyridamole and methotrexate act by enhancing the activation of adenosine receptors. There is strong evidence that adenosine has a functional role in many diseases, and several pharmacological compounds specifically targeting individual adenosine receptors — either directly or indirectly — have now entered the clinic. However, only one adenosine receptor-specific agent — the adenosine A2A receptor agonist regadenoson (Lexiscan; Astellas Pharma) — has so far gained approval from the US Food and Drug Administration (FDA). Here, we focus on the biology of adenosine signalling to identify hurdles in the development of additional pharmacological compounds targeting adenosine receptors and discuss strategies to overcome these challenges.
It is well known that adenosine is an important intermediary metabolite, acting as a building block for nucleic acids and a component of the biological energy currency ATP. In addition, adenosine functions as a signalling molecule through the activation of four distinct adenosine receptors — denoted A1, A2A, A2B and A3. These receptors are widely expressed and have been implicated in several biological functions, both physiological and pathological1,2. These include cardiac rhythm and circulation3,4, lipolysis5, renal blood flow6,7, immune function8, sleep regulation9,10 and angiogenesis11, as well as inflammatory diseases12,13, ischaemia-reperfusion14 and neurodegenerative disorders15 (TABLE 1,2).
Table 1. Examples of ongoing or recently completed Phase lib–III clinical trials targeting adenosine receptors.
| Type of compound | Pharmacology* Ki |
Purpose or name of study | Status | ClinicalTrials. gov identifer |
Refs |
|---|---|---|---|---|---|
|
A1: 77 nM A2A: 0.5 nM A222B: not determined A3: 45 nM |
Protection from liver ischaemia following liver surgery |
Ongoing | NCT00760708 | 2,18 |
| Postconditioning after STEMI | Ongoing | NCT00284323 | |||
| Pretreatment before stenting | Ongoing | NCT00612521 | |||
| Does intradermal adenosine release VEGF and cytokines? |
Suspended for re-evaluation |
NCT00580905 | |||
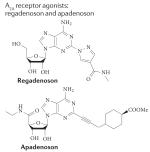
|
A1: >10,000 nM A2A: 290 nM A2B: >10,000 nM A3: >10,000 nM |
Is regadenoson superior to adenosine for myocardial perfusion imaging? |
Completed | NCT00208312 |
18,146, 191 |
| Myocardial perfusion magnetic resonance imaging using regadenoson |
Completed | NCT00881218 | |||
| ASPECT study: effectiveness of apadenoson in SPECT imaging compared to adenosine |
Completed | NCT01085201 | |||
| Regadenoson approved for treatment of sickle cell anaemia |
Recruiting | NCT01566890 | |||
| Regadenoson blood flow in type 1 diabetes (RABIT1D) |
Completed | NCT01019486 | |||
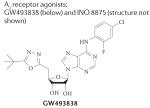
|
No data available | Analgesic effect of GW493838 in postherpetic neuralgia or peripheral nerve injury |
Discontinued | NCT00376454 | 2,18,275 |
| Tolerability and safety of INO 8875 in glaucoma and ocular hypertension |
Discontinued | NCT01123785 | |||
|
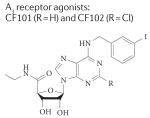
CF101: A1: 51 nM A2A: 2,900 nM A2B: 11,000 nM A3:1.8 nM C3F102: A1: 220 nM A2A: 5,360 nM A2B: >10,000 nM A3: 1.4 nM |
Safety and efficacy of CF101 in psoriasis |
Completed | NCT00428974 |
2,18,37, 146,147 |
| Safety and efficacy of CF101 in rheumatoid arthritis |
Completed | NCT00556894 | |||
| Safety and efficacy of CF102 in liver cancer |
Ongoing | NCT00790218 | |||
|
A1: 10,700 nM A2A: 23,400 nM A2B: 33,800 nM A3:13,300 nM |
Treatment of apnoea of prematurity and dose study |
Currently recruiting participants |
NCT01408173 | 2,18, 223,224 |
| Treatment of apnoea of prematurity and dose study |
Currently recruiting participants |
NCT01349205 | |||
| Cognitive long-term effects of caffeine in premature infants |
Currently recruiting participants |
NCT00809055 | |||
| Caffeine for motor manifestations of Parkinson’s disease |
Completed | NCT01190735 | |||
| Caffeine for excessive daytime somnolence in Parkinson’s disease |
Completed | NCT00459420 | |||
|
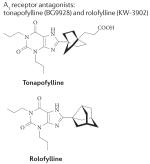
Tonapofylline: A1: 7.4 nM A2A: 6,410 nM A2B: 90 nM A3: >10,000 nM Rolofylline: A1: 0.72 nM A2A: 108 nM A2B: 296 nM A3:4390 nM |
Safety and tolerability of intravenously administered tonapofylline in individuals with acute decompensated heart failure and renal insufficiency (TRIDENT-1) |
Discontinued | NCT00709865 |
2,18, 21,22 |
| Effect of rolofylline on heart and renal function in acute heart failure |
Discontinued | NCT00328692 | |||
| Effect of rolofylline on heart and renal function in acute heart failure |
Discontinued | NCT00354458 | |||
|
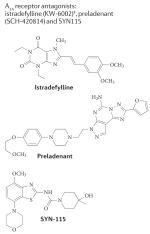
Istradefylline: A1: 841 nM A2A: 12 nM A2B: >10,000 nM A3:4470 nM Preladenant: A1: >1,000 nM A2A: 0.9 nM A2B: >1,000 nM A3: >1,000 nM SYN115: A1: 228.4 nM Am: 0.38 nM A2B: not available A3: not available |
Efficacy of istradefylline in increasing sleep in patients with advanced Parkinson’s disease |
Ongoing | NCT00955526 |
2,18,138, 142,234, 238–243,276 |
| Effect of preladenant in early Parkinson’s disease |
Ongoing | NCT01155479 | |||
| Effect of preladenant on ‘off-time’ in moderate to severe Parkinson’s disease |
Ongoing | NCT01155466 | |||
| fMRI-aided study of SYN115 on behaviour and brain activity in cocaine addicts |
Ongoing | NCT00783276 | |||
| Safety and efficacy study of SYN115 in patients with Parkinson’s disease using l-DOPA to treat end of dose wearing off |
Completed | NCT01283594 | |||
| Long-term safety study of istradefylline in patients with Parkinson’s disease‡ |
Completed | NCT00957203 | |||
| Active-controlled extension study to P04938 and P07037 (P06153 AM3) |
Recruiting participants |
NCT01215227 | |||
| Placebo-controlled study of preladenant in participants with moderate to severe Parkinsons disease (P07037 AM3) |
Recruiting participants |
NCT01227265 | |||
|
Protection after cardiac bypass surgery |
Currently recruiting participants |
NCT01295567 | 18,66 | |
| Effects on circulating adenosine levels in relation to genetics |
Currently recruiting participants |
NCT00760708 | |||
| Supplementation with prednisolone in rheumatoid arthritis |
Currently recruiting participants |
NCT01369745 |
FDA, US Food and Drug Administration; fMRI, functional magnetic resonance imaging; K1, inhibition constant; L-DOPA, L-3,4-dihydroxyphenylalanine; SPECT, single-photon emission computed tomography; STEM, ST segment elevation myocardial infarction; VEGF, vascular endothelial growth factor.
All pharmacology results are cited from REF. 18 and based on human tissue or cells expressing human receptors.
Istradefylline (KW-6002) is being investigated in a total of 17 Phase lib and Phase III clinical trials in patients with advanced Parkinson’s disease for its efficacy and safety; see Supplementary information S1 (table) for details.
Table 2. Physiological and pathological effects of adenosine receptors.
| Effects | Physiology and pathophysiology | Adaptation? | Refs |
|---|---|---|---|
| Adenosine A1 receptors | |||
| Decreased renal blood flow, tubuloglomerular feedback and inhibition of renin release |
Physiology | No (or minor) | 7,277 |
| Inhibition of lipolysis | Physiology | No (or minor) | 5 |
| Vasoconstriction | Pathophysiology? | Not examined | 278,279 |
| Bronchoconstriction | Pathophysiology? | Not examined | 280 |
| Inhibition of neurotransmitter release | Extreme physiology* | No (or minor) | 91 |
| Inhibition of insulin and glucagon release | Physiology | No | 281 |
| Reduced heart rate | Physiology | No | 282 |
| Osteoclast activation and bone resorption | Physiology? | Not clear | 283 |
| Reduced respiration | Extreme physiology* | No | 91 |
| Sleep | Physiology | Yes | 284,285 |
| Analgesia | Extreme physiology* | No | 91,286 |
| Cardiac preconditioning | Pathophysiology | No | 262,287 |
| Adenosine A2A receptors | |||
| Wakefulness and locomotion | Physiology | No | 9,56 |
| Neurodegeneration (including Parkinson’s disease, stroke, traumatic brain injury and Alzheimer’s disease) |
Pathophysiology | No |
57,61,117, 119,120 |
| Immunosuppression | Extreme physiology* or pathophysiology | Not clear | 59,288 |
| Vasodilation and hypotension | Physiology or extreme physiology* | No | 56 |
| Blood–brain barrier integrity | Pharmacology | Not clear | 289 – 291 |
| Coronary vasodilation | Physiology or extreme physiology* | Yes, but importance not clear | 292 |
| Inhibition of platelet aggregation | Extreme physiology* | Not well studied | 56 |
| Angiogenesis | Extreme physiology* | Not known | 11,293 |
| Sickle cell disease | Pathophysiology | Not known | 152,163 |
| Fibrosis | Pathophysiology | No | 169 |
| Adenosine A2B receptors | |||
| Vascular integrity | Physiology or extreme physiology* | No | 64 |
| Cardiac preconditioning | Extreme physiology* | No | 65 |
| Sickle cell disease | Pathophysiology | Not known | 153 |
| Pro-inflammation (acute injury) and anti-inflammation (some chronic disease states) |
Physiology and pathophysiology | Not known | 92 |
| Fibrosis | Pathophysiology | Not known | 169 |
| Adenosine A3 receptors | |||
| Increased mast cell activation | Extreme physiology* or pathophysiology | Some, but probably not at receptor level |
294,295 |
| Airway contraction | Pathophysiology | Not known | 295 |
| Inflammatory pain | Extreme physiology* or pathophysiology | Not known | 92 |
| White cell chemotaxis | Extreme physiology* or pathophysiology | Not clear | 43 |
| Chronic neuropathic pain relief | Pathophysiology | Not known | 149 |
| Anticancer (melanoma) | Pathophysiology | Not known | 148 |
Refers to such conditions such as heavy exercise, being at high altitude and unusually high activity in the pathways of the nervous system.
The possibility of therapeutically targeting adenosine receptors is clear12,14,16 and has been so for a long time (BOX 1). Adenosine itself is used clinically17 (in the form of the generic drugs adenocard and adenoscan) for the treatment of supraventricular tachycardia16,17, and many clinically used drugs (including dipyridamole and methotrexate) may exert their effects by altering extracellular adenosine concentrations and signalling . In addition, caffeine is used for treating premature apnoea in a clinical setting, and many people worldwide consume caffeine on a regular basis in doses that antagonize adenosine receptors. Selective adenosine receptor agonists and antagonists are available16 and several trials are currently in progress (TABLE 1) (see the ClinicalTrials.gov website and REF. 18). However, although one A2A receptor agonist — regadenoson (Lexiscan; Astellas Pharma) — is approved by the US Food and Drug Administration (FDA) for myocardial perfusion imaging in patients with suspected coronary artery disease19, in general the translation of the abundant knowledge of adenosine biology to clinical progress has been slow.
Box 1. History of adenosine receptor targeting.
More than 80 years ago, extensive studies on the actions of adenosine reported profound cardiac effects, vasodilation and a marked lowering of body temperature253,254. It was proposed that different forms of tissue trauma could release adenosine and/or AMP that could cause vasodilation253. Subsequently, the administration of adenosine derivatives was found to have a protective effect255. During the Second World War there was interest in the use of such derivatives in the clinic. A relationship was found among the severity of the trauma, the magnitude of the loss of tissue adenine nucleotides and the release of breakdown products. By the mid-1960s it was concluded that the protective effect of adenosine derivatives in the prevention of irreversible trauma may be attributed to vasodilation, energy transfer, anticoagulation or a combination of these mechanisms256. The anticoagulatory effect is now attributed to the actions of adenosine on platelet adenosine receptors to reduce their activation56,257. The second possibility — that adenine nucleotides enter cells to restore energy charge — is no longer credible. The vasodilatory effect, which was first suggested by Bennet and Drury253, was followed up in detail 30 years later258,259 when it was demonstrated that a lack of energy owing to hypoxia caused the breakdown of myocardial adenine nucleotides, and that the breakdown products were able to cause coronary vasodilation. This led to the very attractive hypothesis that adenosine is a — or the — mediator of hypoxic vasodilation. Indeed, adenosine could be increasing oxygen supply to an energy-depleted tissue, thereby subsequently limiting its own formation — a classical tenet of homeostasis and negative feedback.
The studies on the effects of adenosine on the heart were also very important in demonstrating that methylxanthines such as theophylline acted as adenosine antagonists1. They also established the use of adenosine uptake inhibitors such as dipyridamole as tools in adenosine research260. It is now believed that adenosine contributes to basal coronary tone, but has only a minor role in increasing coronary blood flow during exercise261, and appears to be a very important factor in pathophysiological conditions including ischaemia6 and ischaemic preconditioning30,65,262. The rapid breakdown of adenosine in the bloodstream was useful in the development of adenosine as a diagnostic tool or a therapeutic agent in supraventricular tachyarrhythmia17. Rapid adenosine removal was, however, a disadvantage in other potential applications; efforts to synthesize more stable analogues started in the 1960s, yielding the adenosine analogue 2-chloroadenosine, N6-phenylisopropyl adenosine (R-PIA) and N-ethyl-carboxamido adenosine (NECA). The somewhat different effects of these compounds helped to define the subtypes of adenosine receptors, but all of these compounds exhibited so many effects that their development for therapy proved difficult. In the 1970s it became clear that there are receptors for adenosine, and there was evidence that methylxanthines such as caffeine and theophylline produce many of their actions by acting as antagonists at adenosine receptors. Gradually it also became clear that some drugs with an unknown mechanism of action (for example, dipyridamole and methotrexate) probably acted, at least in part, by increasing the levels of adenosine and the stimulation of adenosine receptors. Towards the end of the twentieth century it became clear that there are subtypes of adenosine receptors, and we now recognize a family of four adenosine receptors that are present in most vertebrates. All of these developments strongly suggest that adenosine receptors are druggable.
The greatest challenge in developing adenosine recep tor ligands for specific clinical applications is that adenosine signalling is so widespread. Adenosine itself is present ubiquitously, adenosine receptors are widely distributed throughout the body and adenosine acting at these receptors exerts a broad spectrum of physiological and pathophysiological functions20. Thus, demonstrating the effects of adenosine receptor activation or inactivation on specific systems under distinct experimental settings is not sufficient to suggest that adenosine can be delivered in a manner that is clinically effective and safe. The complexity of adenosine signalling contributes to the sometimes debilitating side effects of adenosine receptor agonists and antagonists, and was responsible for the failure of one of the largest clinical trials for an A1 receptor antagonist so far21,22. Another challenge is that although the adenosine receptor antagonist caffeine is so commonly ingested in the normal diet, caffeine use has not been properly controlled in several previous clinical trials. In this article, we discuss the therapeutic potential of adenosine receptor modulators, focusing on the key biological factors limiting their clinical development and the hurdles that could and should be overcome. The important medicinal chemistry aspects have been extensively covered elsewhere in the literature (for example, see REF. 18) and are thus largely omitted here.
Overview of adenosine receptors
Based on the competitive antagonism of adenosine activity by methylxanthines, the existence of adenosine receptors was postulated more than 40 years ago; 20 years later, four receptors were cloned from several mammalian species, including humans1, and identified as members of a large G protein-coupled receptor (GPCR) family1,2 (TABLE 3).
Table 3. Human adenosine receptors.
| Receptor name |
Human gene |
Chromosome | G proteins | Localization | Potency of adenosine* |
|---|---|---|---|---|---|
| Adenosine A1 receptor |
ADORA1 | 1q32.1 | Gi,o | Broad distribution: high in nerves, heart, kidney and adipose tissue |
10−8 to 10−7 |
| Adenosine A2A receptor |
ADORA2A | 22q11.23 | Gs/olf | Broad distribution: very high in basal ganglia; high in nerves, blood vessels and immune cells |
10−8 to 10−7 |
| Adenosine A2B receptor |
ADORA2B | 17p12-p11.2 | Gs (Gq/11; G12/13) | Broad distribution, but generally low abundance |
3 × 10−7 to 10−5 |
| Adenosine A3 receptor |
ADORA3 | 1p13.2 | Gi/o | Restricted distribution, varying in different species: high in mast cells |
10−8 to 10−7 |
The potency (in mol per l) is determined by the effect of the agonist in cells expressing approximately 2 × 105 receptors per cell; see REF. 1 for details.
A1 receptors
The A1 receptor is the most conserved adenosine receptor subtype among species23, and it is widely expressed throughout the body with the highest levels found in the brain, especially at excitatory nerve endings24. Activation of the A1 receptor inhibits adenylyl cyclase activity, activates potassium channels (including KATP channels in neurons and the myocardium), blocks transient calcium channels and increases intracellular calcium and inositol-1,4,5-trisphosphate (Ins(1,4,5)P3) levels by activating phospholipase C (PLC). A1 receptors modulate neuronal activity by blocking neurotransmitter release and reducing the firing rate. A1 receptors mediate negative chronotropic and inotropic effects in the heart25 but they also exert effects in many other organs and cells, some of which are physiologically important, as discussed below (TABLE 2).
A2A receptors
High levels of the A2A receptor are found in the striatum of the brain, immune cells of the spleen, thymus, leukocytes and blood platelets, and intermediate levels are found in the heart, lung and blood vessels1,2. A2A receptor activation stimulates the cyclic AMP–protein kinase A (PKA) pathway by coupling to Gs protein23 in peripheral tissues or Golf protein26,27 in the brain. A2A receptors in the brain interact with several neurotransmitters to regulate motor activity, psychiatric behaviours, the sleep-wake cycle and neuronal cell death. In peripheral tissues, A2A receptors have a crucial role in the modulation of inflammation, myocardial oxygen consumption, coronary blood flow, angiogenesis and the control of cancer pathogenesis3.
A2B receptors
A2B receptors are widely expressed, but mostly in low abundance. Although A2B receptors stimulate mitogen-activated protein kinase (MAPK) activity at a similar affinity as A2A receptors in cultured cells28, the A2B receptor is the most adenosine-insensitive receptor among all four adenosine receptors, requiring micromolar adenosine concentrations — which are only rarely achieved under physiological conditions. During conditions in which adenosine levels are elevated, such as hypoxia, ischaemia or inflammation, functional roles of A2B receptor signalling have been described in genetic and pharmacological studies; these roles include tissue adaptation to hypoxia8,29, increased ischaemia tolerance6,30 or attenuation of acute inflammation31-33.
A3 receptors
There is considerable variation in the pharmacology and distribution — and hence function — of A3 receptors among species. In mice, A3 receptor signalling has been linked to mast cell degranulation34, but the situation may be different in humans. Despite the low level of A3 receptor expression in most cells and tissues, its expression was upregulated in blood cells from patients with rheumatoid arthritis, Crohn’s disease35 and colon cancer36 when compared to healthy individuals, correlating with the upregulation of nuclear factor-κB (NF-κB) signalling and the phosphoinositide 3-kinase (PI3K)-PKB-AKT signalling pathways. Indeed, preclinical studies have demonstrated anti-inflammatory, anticancer and cytoprotective effects of A3 receptor agonists37 (as discussed below).
Sources of adenosine
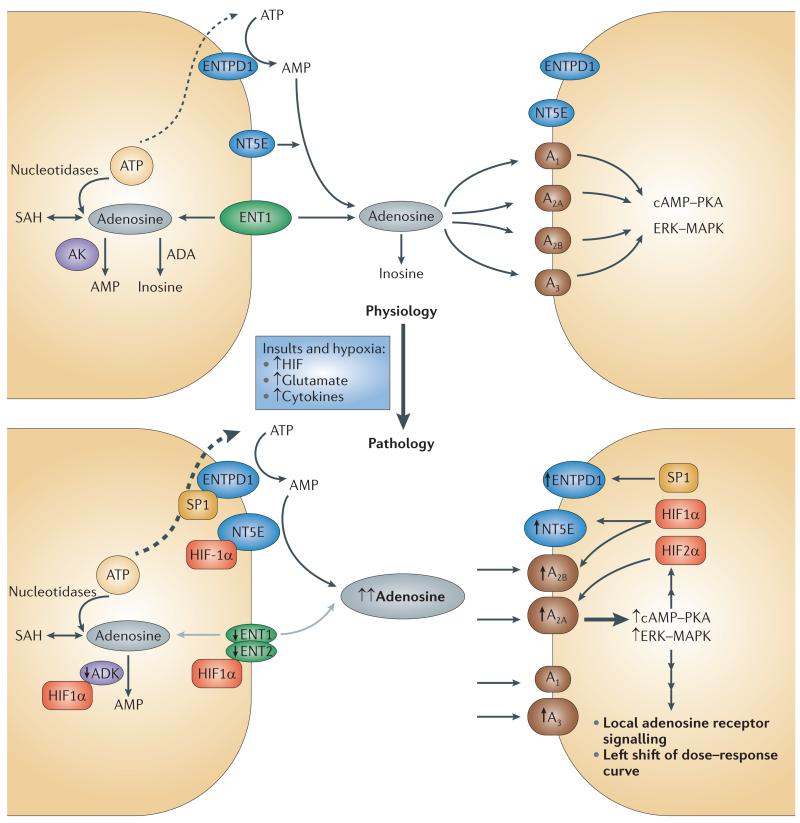
Adenosine is the only important agonist for the three key adenosine receptors — A1, A2A and A2B — and the major, full agonist ligand for the A3 receptor (for which inosine is an incomplete agonist). The concentration of adenosine in the extracellular compartment is the consequence of many biological processes, including extracellular adenosine production, adenosine transport, adenosine formation from intracellular adenosine sources (for example, via the S-adenosylhomocysteine pathway) and adenosine metabolism to inosine or AMP (see FIG. 1; upper panel).
Figure 1. Local amplification of adenosine signalling in response to insults or hypoxia.
Under physiological conditions (upper panel), the extracellular concentration of adenosine is the sum of many biological processes, excluding intracellular adenosine production. Adenosine is transported via equilibrative nucleoside transporter 1 (ENT1) and other transporters. ATP is released via multiple processes. ATP is converted to adenosine by ectonucleoside triphosphate diphosphohydrolase 1 (ENTPD1; also known as CD39) and ecto‑5′-nucleotidase (NT5E; also known as CD73). Adenosine is metabolized to inosine, AMP or S-adenosylhomocysteine (SAH). Many cell types perform all the biological processes displayed in the figure, but some cells show only a limited repertoire. Under pathological conditions (lower panel), local adenosine signalling is markedly amplified in response to insults and hypoxia by a surge in extracellular adenosine concentration from the baseline (20-300 nM) to up to 30 μM in ischaemic or hypoxic tissues. There is also a parallel marked induction of enzymes that are responsible for ATP-dependent adenosine signalling as well as adenosine receptor expression (particularly A2A and A2B adenosine receptors) and the suppression of enzymes involved in adenosine metabolism, such as adenosine kinase (AK). Adenosine signalling under pathological conditions is controlled by the following factors: increased extracellular adenosine levels by ATP release; induction of ENTPD1 expression by the transcription factor SP1 and of NT5E expression by hypoxia-inducible factor-1α (HIF1α); induction of A2A receptor expression by HIF2α and of A2B receptor expression by HIF1α; repression of AK by HIF1α and suppression of ENT1 or ENT2 activity by HIF1α. ADA, adenosine deaminase; cAMP, cyclic AMP; ERK, extracellular signal-regulated kinase; MAPK, mitogen-activated protein kinase; PKA, protein kinase A.
Extracellular adenosine comes from two sources. First, it may be derived from the external transport of intracellularly generated adenosine. However, adenosine is involved in several different metabolic pathways, and its intracellular concentration can never be zero. Therefore, most — if not all — cells possess equilibrative adenosine transporters, which allow adenosine to quickly cross the cell membrane38. Consequently, there will be — by necessity — a finite level of adenosine in the extracellular space, even under the most basal conditions. From the baseline level, adenosine concentrations can increase substantially. Notably, very minor changes in steady-state ATP levels in the cell (normally ~5 mM) will translate into major changes in intracellular adenosine concentrations (normally around 100,000 times higher). Second, extracellular adenosine may also be formed from the extracellular hydrolysis of adenine nucleotides. In many instances, extracellular adenosine is derived from the breakdown of extracellular nucleotides, particularly ATP and ADP. The generation of extracellular adenosine from ATP is predominantly controlled through a two-step enzymatic reaction: first, the conversion of ATP or ADP to AMP by ectonucleoside triphosphate diphosphohydrolase 1 (ENTPD1; also known as CD39), which is followed by AMP hydrolysis to adenosine by ecto-5′-nucleotidase (NT5E; also known as CD73).
ATP can be released from various cell types by multiple mechanisms: it can be co-released from storage vesicles together with other hormones (such as neurotransmitters), it can be released via a ‘kiss and run’ mechanism39 (a type of synaptic vesicle release where the vesicle opens and closes transiently) or it can be released from the lysosome by exocyotosis40. ATP release mechanisms include uncontrolled leakage from necrotic cells4, leakage from cells undergoing other forms of cell death, controlled release through pannexin hemichannels41,42 as well as release from inflammatory cells or vascular endothelia through connexin hemichannels and channels such as P2X purinergic receptor 7 (REFS 43-45). The basal physiological level of extracellular adenosine from both sources has been estimated to be in the range of 30-200 nM46. The processes that lead to increased intracellular adenosine formation often affect several cells or a whole tissue, thereby causing considerably widespread and enduring changes in adenosine concentration. By contrast, the release of adenine nucleotides may be limited in quantity and spatially restricted. Thus, the two modes of changes in adenosine concentration can have considerably different consequences.
Adenosine receptor functions
Adenosine receptors have been implicated in several key physiological processes, ranging from neuromodulation to immune regulation, and from vascular function to metabolic control. Adenosine has been postulated to have a role as a danger signal involved in homeostasis. One approach that has proved to be particularly effective in uncovering the normal physiological roles of adenosine receptors is genetic knockout. Genetic knockout mouse models for all four adenosine receptors (Adora1, Adora2a, Adora2b and Adora3, which encode A1, A2A, A2B and A3 receptors, respectively) have now been generated by the targeted deletion of either of the two critical exons of the adenosine receptors20,47. Although detection of the pathophysiological roles of adenosine signalling has been more difficult, as this requires models of disease in the genetically modified organism, the available genetic knockout mouse models have provided some insights. A list of some of the key physiological and pathophysiological roles of the adenosine receptors (derived mainly from studies with genetic knockout models) is given in TABLE 2, and some of these roles are discussed in more detail below.
Two lines of Adora1-knockout mice have been generated7,48, and Adora1-knockout mice exhibit decreased fertility, a significant decrease in lifespan49 and an increased risk of seizures48. In the kidney, knockout studies have confirmed the pharmacological finding that stimulating the A1 receptor on the glomerular afferent arteriole reduces renal blood flow and the glomerular filtration rate, and stimulation of the A1 receptor on the proximal tubules increases sodium and water reabsorption7. These studies provide the rationale for developing A1 receptor antagonists to control renal dysfunction in patients with acute heart failure.
A2A receptors are expressed at high levels in the dorsal striatum, a critical basal ganglia structure involved in motor control, where they are colocalized with dopamine D2 receptors; they inhibit D2 receptor binding in the striatum and immediate-early gene expression50-52. The behavioural actions of A2A receptor antagonists partly overlap with those of D2 receptor agonists, which has implications for the treatment of Parkinson’s disease (as discussed below) and also influences working memory53,54, reversal learning53 and goal-oriented behaviour55, while leaving spatial reference memory, motor function and anxiety-like behaviours intact.
Three strains of Adora2a-knockout mice have been generated (Adora2a knockout in CD1 mice56, in mixed 129sv mice crossed with C57BL/6 mice57 and in congenic C57BL/6 mice58). Adora2a-knockout mice exhibit reduced exploratory behaviour and score higher in anxiety tests, with male mice being much more aggressive towards intruders56. Because of their reduced activity they gain weight, especially in the form of fat. Their response to acute pain stimuli is slower and their blood pressure and heart rate are elevated56. Genetic Adora2a-knockout models have uncovered complex roles of the A2A receptor in tissue protection: that is, A2A receptor activation confers tissue protection in peripheral organs59,60, whereas its inactivation confers neuroprotection against brain injury57,61,62. Genetic knockout models have shown that both A1 and A2A receptors are involved in mediating the sleep-promoting properties of adenosine in the brain63. Moreover, the arousal effects of caffeine seen in wild-type animals are blunted in Adora2a-knockout mice10. It is therefore conceivable that adenosine receptor ligands could be used as normal cognitive enhancers or sleep promoters.
Several strains of Adora2b-knockout mice have also been generated64,65. Given that A2B receptors are generally only expressed at low levels and they typically exhibit low affinity for adenosine in most assays66, it is surprising that Adora2b-knockout mice have very strong phenotypes, especially in relation to the vasculature64. Adora2b-knockout mice show low-grade inflammation of the vasculature at the baseline64 and suffer from increased vascular leakiness in several organs29. A major reason for this could be that local adenine nucleotide signalling is very important in this compartment and thus adenosine levels can locally and transiently be considerably high29. Moreover, an increased susceptibility to ischaemic and inflammatory injuries is typically observed in the intestine67, liver68, kidney6,69, lung70 and heart30,65 of Adora2b-knockout mice, with conditions characterized by elevations in extracellular adenosine levels. Under such conditions, studies in Adora2b-knockout mice have shown that A2B receptor signalling is implicated in attenuating hypoxia-driven inflammation and in the adaptation of tissues to conditions of limited oxygen availability.
Conversely, activation of the A2B receptor has been shown to promote bone cell differentiation71,72, control glucose homeostasis73 and regulate hyperlipidaemia and atherosclerosis74. However, some harmful effects of A2B receptor activation have also been reported — including promotion of tumour growth in the bladder and breast75, renal fibrogenesis76 and inflammatory damage — in an experimental autoimmune encephalomyelitis (EAE) model of multiple sclerosis77. It is crucial to clarify whether these different and often opposing biological effects of the A2B receptor, as revealed by these knockout mouse models, are in part attributed to the confounding effect of different genetic backgrounds, developmental compensation or distinct biological effects under pathological conditions.
Similarly, knockout of the A3 receptor in mice was surprising, resulting in marked phenotypes even at locations where the receptors are very sparse (for example, the brain) and where antagonists (or agonists) have little effect78,79. The reason for this remains unknown, but a possible developmental role for A3 receptors has been postulated78. Mice deficient in the genes encoding ENTPD1 or NT5E (enzymes that mediate the generation of extracellular adenosine from ATP) also appear to be healthy and reproduce normally in a pathogen-free environment, but they have subtle defects in the vascular barrier function of several organs80,81. Entpd1-knockout mice have a prolonged bleeding time owing to a defect in their platelet function, which does not appear to be associated with a change in adenosine receptor signalling; rather, it appears to be related to desensitization of P2Y purinergic receptor 1 (caused by elevated ATP levels) through which adenine nucleotides signal and which is crucial for platelet activation104.
Together, these findings in Adora-knockout mice as well as Entpd1- and Nt5e-knockout mice indicate that long-term treatment with antagonists should be tolerated without having very serious consequences. The relatively minor differences between adenosine receptor knockout mice and wild-type mice under physiological conditions could theoretically be due to major compensatory changes. Although there is little evidence for the existence of such compensatory changes (TABLE 3), it should be acknowledged that this has not yet been systematically examined. For example, studies in mice with concomitant deletion of all four adenosine receptors have not been yet published; it would be interesting to see whether these mice are viable and whether they show a phenotype at the baseline.
Adenosine signalling in pathological conditions
Adenosine signalling is not very prominent under physiological conditions in most tissues, but aberrant adenosine signalling has been implicated as a common disease mechanism underlying inflammatory and ischaemic tissue damage3,59,82,83. This includes excessive inflammatory tissue damage such as that seen in acute liver injury, ischaemic kidney injury, acute lung injury, traumatic brain injury, ischaemic brain injury, epilepsy and certain neurodegenerative disorders such as Parkinson’s disease and Huntington’s disease20,61,84. As such, enhanced adenosine signalling is essential for the resolution of these pathological conditions associated with tissue inflammation and remodelling.
Adenosine levels
Intracellular adenosine formation is increased whenever ATP consumption exceeds ATP synthesis, which consequently leads to an increase in levels of AMP — the precursor of adenosine. ATP-dependent adenosine signalling occurs typically during conditions that are associated with the release of ATP from intracellular stores, and can occur during injurious conditions such as ischaemia and reperfusion14, hypoxia45 (FIG. 1) or acute inflammation3. The surge in extracellular adenosine in response to pathological conditions is accompanied by increased levels of local inflammatory cytokines such as interleukin-1β (IL-1β) and tumour necrosis factor (TNF), which leads to a delayed (~24-hour), marked and sustained increase in adenosine receptor expression on tissues and inflammatory cells32,33,85. Indeed, many studies have demonstrated that cellular responses to hypoxia are characterized by robust increases in extracellular adenosine production80,86 and signalling events through adenosine receptors3.
Several studies have indicated that extracellular adenosine levels can rise from the baseline (20-300 nM)42,46,87-89 to the low micromolar range in conditions of extreme physiology such as strenuous exercise or subsistence at high altitude and hence low ambient oxygen levels46,87,88. In ischaemic areas or after massive tissue trauma leading to cell death by necrosis, extracellular adenosine levels can increase to ~30 μM88-90. As noted above, this increase could be due to increased intracellular formation of adenosine or to increased release of nucleotides. Recent research has focused on the latter possibility.
Although the ability of adenosine to stimulate adenosine receptors is dependent on the number of adenosine receptors2,15,91, and this number can change (that is, increase) under some circumstances (see below), it is more likely that an increase in adenosine receptor signalling in pathological conditions largely depends on increased levels of adenosine. Numerous reports have indicated that increased ATP-dependent adenosine signalling (for example, as a result of hypoxia exposure, acute inflammation, and so on) can profoundly alter disease susceptibility in both animal models of disease and patients4,92.
Adenosine receptor expression and activity
Together with dramatic increases in extracellular adenosine levels, conditions of tissue hypoxia are associated with enhanced expression of adenosine receptors — particularly the A2A receptor93 and A2B receptor29,31,67,80,94,95 — and a marked induction of enzymes that are responsible for ATP-dependent adenosine signalling, such as the ectonucleotidases ENTPD1 (REFS 80,86,96-99) and NT5E31,65,81,100, as these pathways are tightly controlled on a transcriptional level.
During ischaemia, activation of A2B receptors on the organs has been shown to promote ischaemia tolerance, improve oxygen-efficient metabolism30 and protect against ischaemia-reperfusion injury of the heart30,65,94, kidneys6,69 or the intestine31,67. Activation of A2B receptors can attenuate the activation and transmigration of inflammatory cells into post-ischaemic tissues and protect against sepsis-induced mortality by dampening excessive inflammation101. Moreover, activation of A2A receptors on inflammatory cells that have invaded the tissue can be an efficient treatment for reperfusion injury102-103. For example, activation of A2A receptors expressed on T cells102 or dendritic cells296 has been implicated in the attenuation of organ injury during hepatic or renal ischaemia, respectively.
The complex interplay between adenosine and adenine nucleotide signalling is highlighted in ENTPD1-deficient mice; these mice not only have elevated circulating nucleotide levels and related pathology104,105 but also a deficiency in extracellular adenosine signalling, which is associated with increased vascular leakage during hypoxia80, or more severe tissue injury during lung inflammation or ischaemia and reperfusion96-99,106,107. In addition, protective and anti-inflammatory adenosine signalling against ischaemic and inflammatory injury is absent in the heart65, lung106, liver100, kidney108, intestine31 and blood109 of mice lacking ENTPD1.
A1 receptors are downregulated by hypoxia in C6 glioma cells110 but are upregulated in human temporal lobe epilepsy111 and in mice in which seizures were induced by pentylenetetrazole (PTZ)112. Adenosine receptor expression may also be altered in cancer cells, but the significance of this is unclear. Hypoxia in solid tumours is associated with increased levels of ENTPD1- and NT5E-dependent adenosine, causing A2A receptor-mediated attenuation of the immune response against cancer. For example, genetic deletion of NT5E113, ENTPD1 (REF. 114) or the A2A receptor115 in the host mouse is associated with the rejection of established immunogenic tumours via the regulation of T cell function and pathological angiogenesis. Conversely, A2A receptor inactivation in the brain has been consistently associated with protection against brain damage after ischaemia57, excitotoxicity116, traumatic brain injury117,118 and neurodegeneration in Parkinson’s disease119 and Alzheimer’s disease120. In some cases, both activation and inactivation of the A2A receptor have been shown to have a protective effect, including in animal models of Huntington’s disease121,122 and spinal cord injury123. Moreover, ADORA3 mRNA expression is upregulated in hepatocellular carcinoma tissues in comparison with adjacent normal tissues124 and is also upregulated in peripheral blood mononuclear cells (PBMCs) derived from patients with hepatocellular carcinoma compared to healthy individuals36; this indicates that the A3 receptor in PBMCs may be a potential biomarker of hepatocellular carcinoma, reflecting the A3 receptor status in remote tumours.
Several transcriptional mechanisms contribute to the induction of A2A receptor, A2B receptor and ectonucleotidase expression in response to stress, hypoxia and inflammation and other pathological insults as well as a local increase in adenosine levels. Hypoxia is associated with a transcriptional programme that results in the induction of ENTPD1 (REFS 80,96,97,99) and NT5E31,65,81,106, thereby elevating the capacity of different tissues for extracellular adenosine production13,14. Moreover, ENTPD1 and NT5E have been implicated in the conversion of ATP and ADP to adenosine on regulatory T cells, thereby providing an autocrine feedback loop to enhance the anti-inflammatory functions of this subset of T cells125.
Other studies have provided evidence that hypoxia also attenuates extracellular adenosine uptake126-128 and its subsequent metabolic breakdown in the intracellular compartment, through the activity of the transcription factor hypoxia-inducible factor (HIF)129,130. The mechanism underlying the transcriptional induction of the A2A receptor in inflammatory cells by IL-1β and TNF may involve activation of the transcription factor NF-κB85,131, whereas transcriptional pathways under the control of HIF have been described for the induction of the A2A receptor93 and A2B receptor31,80,94,95. Similarly, in T cells, a system involving the regulation of adenosine, haem oxygenase 1, carbon monoxide and the A2A receptor leads to resolution of the inflammatory response132. The adenosine signal is amplified through a feed-forward mechanism with both a surge in extracellular ATP and/ or adenosine and the coordinated induction of the A2A receptor by local inflammatory cytokines.
Knockout studies have revealed that IL-6 is crucial in mediating A1 receptor upregulation to amplify the A1 receptor-mediated protection against PTZ-induced seizures112. Interestingly, extracellular ATP and adenosine signalling frequently control opposing biological effects133,134 but they can also act synergistically43. Hypoxia shifts the balance from ATP towards adenosine signalling by enhancing the hydrolysis of adenosine precursor nucleotides. This shift from ATP towards adenosine signalling involves: increased extracellular adenosine levels by ATP release; induction of ENTPD1 expression by the transcription factor SP1 and of NT5E expression by HIFα; induction of A2A receptor expression by HIF2α and of A2B receptor expression by HIF1α; inhibition of adenosine kinase activity by HIF1α; and suppression of ENT1 or ENT2 activity by HIF1α3 (see FIG. 1; lower panel).
Targeting adenosine receptors
Numerous articles have indicated the potential of adenosine receptors as therapeutic targets3,12,16,135. The introduction of useful radioligands for adenosine receptors has aided the drug discovery process. Over the past 20 years, medicinal chemistry efforts have generated agonists and antagonists with high affinity (a dissociation constant (Kd) at low nanomolar concentrations) and high selectivity (>100- to 200-fold higher than other adenosine receptor subtypes) for the human variants of each of the four receptors1. Moreover, both agonist and antagonist ligands containing positron-emitting radio-isotopes have been developed to monitor the in vivo occupancy of adenosine receptors in humans18. As such, the lack of selective ligands is not a limiting factor for research and drug development on adenosine receptors, as has been the case for some other GPCRs. Moreover, there are continued medicinal chemistry efforts to develop novel adenosine ligands with refined structure-activity relationships, improved in vivo biodistribution and tissue selectivity, which is crucial to druggability (reviewed in REF. 18).
A bigger problem is the broad distribution of the receptors, and a possible approach for achieving tissue selectivity could be the use of partial agonists that would predominantly act where there is a high number of so-called ‘spare’ receptors. For example, it is well known that adipocytes have a large ‘receptor reserve’, and partial A1 receptor agonists might therefore selectively activate those A1 receptors136. Based on decades of preclinical studies, there have been numerous attempts to develop drugs and many clinical trials are now underway; for some recent examples, see TABLE 1. The clinical indications for drugs that are in advanced clinical trials targeting adenosine receptors include Parkinson’s disease, chronic heart failure as well as inflammatory and autoimmune disorders. Some of the most recent developments and challenges in these key therapeutic areas are discussed below.
Parkinson’s disease
Based on the concentrated striatal expression of A2A receptors, the antagonistic A2A-D2 receptor interaction and preclinical studies demonstrating motor benefit in rodent and non-human primate models of Parkinson’s disease50,52,137,138, A2A receptor antagonists have emerged as leading non-dopaminergic drugs for the treatment of Parkinson’s disease. Over the past 8 years, a total of 25 clinical trials have been conducted (see TABLE 1 and Supplementary information S1 (table)). Six double-blind, placebo-controlled clinical Phase IIb and Phase III trials of istradefylline (KW-6002), involving a total of >2,000 patients with advanced Parkinson’s disease, and one Phase IIb trial with prelad enant (SCH420814), involving 253 patients with advanced Parkinson’s disease, have been reported139. These clinical Phase IIb and Phase III trials have shown a modest but significant reduction in the average ‘off-time’ by about 1.7 hours compared to the optimal l-DOPA (l-3,4-dihydroxyphenylalanine; also known as levodopa) dose regimen138. However, another similar Phase III clinical trial with istradefylline in patients with Parkinson’s disease did not demonstrate a significant reduction in the ‘off-time’ compared to placebo140.
These relatively modest motor effects differed from the preclinical studies with MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine)-treated primates, which unambiguously demonstrated a marked motor benefit. Clearly, further clinical investigation of A2A receptor antagonism is warranted to understand its full potential as a treatment for Parkinson’s disease. An encouraging and consistent finding in the clinical trials of A2A receptor antagonists for Parkinson’s disease is that istradefylline and preladenant had an excellent safety profile138,139. In April 2007, Kyowa Hakko Kirin Pharma filed a new drug application (NDA) for istradefylline for the treatment of advanced Parkinson’s disease; however, the FDA issued a non-approval letter in 2008, citing the need for additional efficacy data. Currently, several Phase IIb and Phase III trials for A2A receptor antagonists (other than istradefylline) are still underway and these agents remain one of the leading non-dopaminergic treatment candidates for Parkinson’s disease141.
Chronic heart failure
Impaired renal function is common in patients with acute heart failure; it directly contributes to deterioration of the heart and is associated with an adverse outcome, including increased mortality. Local adenosine production in the kidney is increased in patients with heart failure as a result of hypoxia caused by reduced renal perfusion and by stimulation with diuretics142. Based on our understanding of the mechanisms associated with renal dysfunction and the demonstrated control of renal function via A1 receptors, A1 receptor antagonists were developed. These antagonists reduced the risk of persistent worsening renal failure by >50% in a Phase IIb study involving 301 patients with acute heart failure, and improved renal plasma flow in 63 ambulatory patients with chronic heart failure143.
Based on these promising results, a placebo-controlled, randomized Phase III trial involving 2,033 patients with acute heart failure (the PROTECT study) was carried out with the A1 receptor antagonist rolofylline; this was the largest study to date involving the use of A1 receptor antagonists to target renal function. Unfortunately, the results were disappointing and rolofylline did not prevent persistent worsening renal function21,144. The reason for this absence of renoprotective effects is likely to be due to an enhanced diuretic effect (as evident by more pronounced weight loss) in the rolofylline group, which may have offset the effects of rolofylline on the preservation of renal function22. Moreover, pharmacological and genetic studies have clearly demonstrated that A1 receptors mediate protective effects against ischaemic kidney injury and brain injury, which is consistent with the increased frequency of stroke and seizure activity in clinical trials of A1 receptor antagonists22. Thus, the development of A1 receptor antagonists for the treatment of disorders associated with impaired fluid retention, such as congestive heart failure, should proceed with caution.
Inflammatory diseases, autoimmune disorders and cancer
Given the high levels of expression of all four subtypes of adenosine receptors in cells of the immune system and the dynamic modification of their expression within inflammatory and tumour environments, A1, A2A, A2B and A3 receptors are being actively pursued as therapeutic targets for autoimmune diseases, chronic inflammatory disorders and cancer145. For example, based on preclinical pharmacology and encouraging safety data in Phase I studies, the A3 receptor agonists CF101 (also known as IB-MECA) and CF102 have been tested in several Phase II trials for rheumatoid arthritis146. CF101 treatment was associated with a 20% improvement in disease symptoms according to the classification of rheumatoid arthritis responses by the American College of Rheumatology. Based on anecdotal findings from this trial indicating that CF101 also improved indicators of dry eye syndrome, a follow-up Phase II trial (randomized, double-blind and placebo-controlled) was carried out, which determined that CF101 improved the clearance of corneal staining, tear break-up time and tear meniscus height147. Notably, orally administered A3 receptor agonists (given at doses that are effective for treating dry eye syndrome) do not cause cardiovascular and other side effects147.
The efficacy and safety of CF101 was also tested in a Phase II trial of moderate to severe chronic plaque-type psoriasis37; it was found to be effective and thus advanced to Phase III trials for these indications as an anti-inflammatory agent. In addition, active Phase II clinical trials are underway to test the efficacy of A3 receptor agonists for the treatment of hepatocellular carcinoma and hepatitis37. Furthermore, experimental studies in mice suggest a possible use of A3 receptor agonists in suppressing melanoma growth by inducing T cell-mediated adoptive immunity148 and in the control of chronic neuropathological pain37,149. These therapeutic effects of CF101 are believed to be mediated by its inhibition (via cAMP and calcium signalling) of the oxidative burst and its anti-inflammatory activity150. However, it should be noted that both pro-inflammatory and anti-inflammatory effects of A3 receptor activation have been demonstrated, depending on the cell type and animal species being studied151.
Sickle cell disease
Patients with sickle cell disease have periodic episodes of vaso-occlusive crisis and, in some cases, life-threatening pulmonary vaso-occlusion. Historically, microvascular occlusion was attributed to rigid sickled erythrocytes. Recently, ischaemia-reperfusion injury with resultant white cell activation has been implicated as a crucial contributor to the pathophysiology of sickle cell disease152. Like ischaemia-reperfusion injury, sickle cell disease is associated with increased levels of adenosine. An experimental study has provided strong evidence that A2B receptor activation on erythrocytes promotes sickling in patients with sickle cell disease153. However, treatment with an A2A receptor agonist has been indicated to attenuate sterile inflammation and T cell activation in this disorder152. A clinical trial in patients with sickle cell disease is currently being conducted using the FDA-approved A2A receptor agonist regadenoson (ClinicalTrials.gov identifier: NCT01085201)154,297. Thus, different adenosine receptors (A2A or A2B receptors) can mediate opposing effects in a single disease155 — a finding that may be of considerable significance when developing adenosine receptor-targeting agents.
Challenges in targeting adenosine signalling
Despite the clear potential of adenosine receptors as therapeutic targets, only one agent has so far reached the clinic. From a medicinal chemistry perspective, standard pharmacological assays of selectivity and efficacy may not provide sufficient information on the different bio-distribution and pharmacokinetics of adenosine ligands with subtle structural differences. Understanding differences in local drug distribution are important for the prediction of potential side effects, given the ubiquitous presence of adenosine and the widespread distribution of its receptors. Despite the large numbers of selective adenosine receptor agonists and antagonists reported in the literature, the clinical application of adenosine ligands is lacking. Below, we discuss the various challenges that are likely to be hampering the success of drugs targeting adenosine receptors.
Measurement of adenosine levels
As mentioned above, determining the local levels of adenosine is crucial to understanding its biology and pharmacology. Therefore, the fact that local adenosine concentrations rapidly fluctuate and are difficult to measure represents a major challenge for this field. Direct biosensor-based measurements of adenosine and microdialysis probes coupled with electrochemical detectors are commonly used to probe or sample the extracellular concentration of adenosine and various bodily fluids in different tissues under physiological and pathological conditions156. However, the microdialysis technique is known to destroy some cells when the probe is inserted into a tissue, which results in the release of ATP that is converted to adenosine, thereby elevating local adenosine levels. In addition, when the microdialysis probe is left in a tissue for an extended period of time, it is covered by cells such as glial cells, which metabolize adenosine and reduce adenosine levels before they can be measured.
Moreover, adenosine can be very rapidly formed during sampling — for example, the ATP hydrolysis that occurs when a tissue is extracted generates much higher levels of adenosine in a few seconds than were initially present. Thus, adenosine levels can only be accurately determined in tissues via the freeze-clamping technique, but this obviously precludes any finer structural resolution. When sampling blood, it is difficult to avoid platelet destruction and the subsequent release of ADP and ATP, which are rapidly broken down to adenosine. This means that the accurate determination of adenosine levels locally under physiological and pathophysiological conditions rarely occurs, and unfortunately the literature is replete with incorrect estimates.
Another major challenge relates to the difficulty in distinguishing between the various sources of extracellular adenosine under physiological and pathological conditions. For example, despite excellent evidence that ATP released from astrocytes is an important signal under numerous circumstances39, a recent study provides compelling evidence that neuronal adenosine release — and not astrocytic ATP release — mediates feedback inhibition of excitatory activity in seizure models157. Therefore, to determine the unique adenosine signals (and therefore adenosine receptors) that are associated specifically with disease status, it is crucial to develop strategies that are capable of detecting and characterizing the changes in extracellular adenosine levels in different definable extracellular domains within the brain parenchyma (that is, neuronal and/or synaptic, astrocytic, microglial or vascular domains).
Measurement of the number of adenosine receptors
Although we do not know how receptor distribution in patients varies in different diseases, recent studies indicate that this could potentially be studied by monitoring receptors using in vivo imaging methods. Indeed, two positron emission tomography (PET) ligands — the A2A receptor antagonist ligand [11C]-SCH442416 and the A2A receptor agonist ligand [11C]-TMSX — were recently developed and have been successfully used to measure the distribution of A2A receptors in the striatum of patients with Parkinson’s disease using PET imaging158,159. These studies have provided two important insights: first, that the number of A2A receptors in the putamen is increased in patients with Parkinson’s disease who have dyskinesia, indicating a possible involvement of A2A receptors in the pathogenesis of l-DOPA-induced dyskinesia; and second, that there are more A2A receptors in drug-naive patients with Parkinson’s disease than in controls, which possibly compensates for the depletion of dopamine158,159.
These A2A receptor ligands will probably also be very useful in the direct assessment of drug-A2A receptor interactions in disease pathogenesis and the development of unwanted side effects. They should also aid in improving the design of clinical trials of A2A receptor antagonists for Parkinson’s disease, as one would be able to adequately monitor receptor occupancy over time. In addition, as A2A receptor expression in human tissues has been shown to correlate with disease progression, such as in Huntington’s disease160,161, these ligands may be used as potential biomarkers to monitor the disease course during clinical trials. Furthermore, PET ligands for other adenosine receptors could be used to monitor disease progression in ischaemia and cancer by assessing the expression of adenosine receptors in patients suffering from these conditions.
Complexity of adenosine signalling during disease course
In acute injury settings, hypoxia-driven elevations in extracellular adenosine levels activate pathways that promote tissue adaptation to conditions of limited oxygen availability and dampen hypoxia-driven inflammation13,15. These pathways include those involved in restoring normal oxygen levels153, enhancing metabolic ischaemia tolerance30 and dampening hypoxia-induced inflammation3,8,82. Indeed, preclinical studies have shown that adenosine signalling is beneficial in acute injury of the lungs70,162 and ischaemic injury of the kidneys69,60, the heart65, the gastrointestinal tract163 and the liver164. However, if elevated adenosine levels are sustained beyond the acute injury phase, hypoxic adenosine responses can become detrimental owing to the activation of pathways that promote tissue injury and fibrosis165. For example, chronic elevations of adenosine levels during hypoxia can contribute to tissue fibrosis in different organs, including the lungs166,167, liver168, skin169 and penis170,171. Therefore, under conditions of chronically elevated adenosine levels, blockade of adenosine signalling appears to be beneficial.
An interesting example of the opposing effects of adenosine signalling in a single disease model comes from studies of bleomycin-induced lung injury. Studies in Nt5e-knockout mice172, which are unable to convert extracellular AMP to adenosine and thus develop a more severe degree of lung injury during the acute phase of bleomycin-induced lung disease, have shown that extracellular adenosine production is implicated in lung protection during acute lung injury. Subsequent studies in other models of lung injury — such as ventilator-induced lung injury70, lipopolysaccharide-induced lung inflammation32 or lung inflammation in adenosine deaminase-deficient mice173 — have implicated A2B receptor signalling in adenosine-dependent protection from acute lung injury92. However, there is contrasting evidence that A2B receptor signalling can be detrimental in more chronic forms of lung injury174. As such, there were substantial reductions in pulmonary fibrosis in mice following the genetic removal of Adora2b (which encodes the A2B receptor) during the chronic phase of bleomycin-induced lung injury, which indicates a profibrotic role for this receptor. These studies highlight the opposing roles of A2B receptor signalling during acute versus chronic stages of bleomycin-induced lung injury175.
Similarly, both acute activation176 or prolonged inactivation of A2A receptors can partially protect against sepsis177 via the immunosuppressive cytokine IL-10 (REF. 178). Moreover, both protective and detrimental effects of A2A receptor activation are observed at different stages of liver injury59,179, traumatic brain injury117, ischaemic spinal cord injury123 and Huntington’s disease180. Finally, as discussed above, the opposing effects of A1 receptor antagonists in renal function may have contributed to the failure of the first Phase III clinical trial of A1 receptor antagonists in acute heart failure144. The opposing effects of adenosine signalling at different stages of disease clearly represent a major challenge for drug development.
The causes of such opposing and/or time-dependent effects are one of the central questions in this field. The opposite effects of adenosine receptor activation at different stages of various disorders could reflect the complexity of adenosine receptor signalling on various cell types; each of these receptors can have a detrimental or protective effect depending on the nature of the tissue injury and associated pathological conditions. A major challenge in developing effective therapeutic strategies targeting adenosine receptors is to decipher these complex actions of adenosine receptors at the level of cellular and tissue specificity as well as disease progression, and to define the specific interactions between adenosine receptors and other neurotransmitter receptors. Moreover, other signalling molecules — such as other neurotransmitters in the brain — can further complicate this interaction. In the case of opposite effects on lung injury by Adora2b knockout and Nt5e knockout, A2B receptor-dependent regulation of IL-6 production was identified as a potential mechanism involved in the diminished pulmonary fibrosis seen in Adora2b-knockout mice175.
In the brain, a switch between a protective versus damaging effect of A2A receptors has been shown to be associated with the local interactions between adenosine and glutamate. In response to various brain injuries, extracellular levels of adenosine as well as glutamate increase rapidly (within minutes) and dramatically (up to 100-fold) owing to their presynaptic release from neurons and to the exocytosis and possible reversal of glutamate uptake from astrocytes181,182. Remarkably, increasing the local levels of glutamate redirected A2A receptor signalling from the PKA to the PKC pathway, thus switching the effect of A2A receptor activation from anti-inflammatory to pro-inflammatory117. This glutamate-A2A receptor interaction can also be demonstrated in vivo in a cortical impact model of traumatic brain injury in mice. Thus, extrasynaptic glutamate levels can control the effect of A2A receptor activation both in vivo and in vitro, switching it from anti-inflammatory and neuroprotective to pro-inflammatory and cytotoxic117. Such findings may explain — at least in part — the opposing effects of A2A receptor ligands on tissue injury by demonstrating that the effects of A2A receptors in brain injury are context-dependent as they can be influenced by local glutamate levels.
Tolerance to adenosine receptor ligands
Another finding, possibly related to the cases described above, is that repeated exposure to adenosine receptor ligands (particularly to A1 receptor ligands and caffeine) leads to the rapid development of tolerance, which is evident in both motor and cardiovascular responses183,184. In some cases, repeated or prolonged exposure even results in the development of opposite effects, as seen with caffeine exposure in various neuronal injury models; although acute caffeine exposure exacerbates tissue injury, chronic caffeine administration usually has a protective effect183,184. The mechanism underlying this desensitization is not clear.
Similar paradoxical effects of acute versus chronic treatment have been reported for A1 receptor agonists and antagonists185. Acute treatment with A1 receptor agonists has a protective effect in brain injury, whereas chronic treatment increases ischaemic brain injury in rodents185-187. A2A receptor agonists also induce desensitization after prolonged treatment in cultured cells, as a result of the binding of the carboxyl terminus of the A2A receptor to F-actin crosslinking protein (also known as α-actinin), which promotes A2A receptor internalization188,189. Conversely, unlike caffeine, selective A2A receptor antagonists do not induce the rapid development of tolerance; motor responses to A2A receptor antagonists did not decline even after 1 week of repeated treatments in an animal model of Parkinson’s disease190,191. This finding is encouraging for the current development of A2A receptor antagonists for neurodegenerative disorders, which would require chronic treatment for several years.
Distinct effects of adenosine signalling in different cellular elements
Targeting the same receptor in different cells within the same tissue can induce fundamentally different outcomes. One approach that can be applied to identify distinct functions of adenosine receptors in different cells within a tissue is to develop cell-specific conditional knockout models of individual adenosine receptor-encoding genes using the Cre-loxP system6. Brain-region-specific as well as cell-specific Adora-knockout mice are currently available (reviewed in REF. 47). Regional deletion of Adora2a genes has been achieved in the entire forebrain (that is, the striatum, cortex and hippocampus)192,193 or only in the striatum194. In addition, local deletion of the Adora1 gene in hippocampal CA1 or CA3 neurons and the Adora2a gene in the nuclear accumbens has been attained by the local injection of adeno-associated virus (AAV) vectors containing the Cre transgene into the brains of mice expressing adenosine receptor-encoding genes in which a critical exon is flanked by loxP sites195. This strategy allowed for a temporal and regional specificity that has uncovered previously under-explored or under-appreciated functions of adenosine receptors. For example, using local infection with AAV vectors carrying short hairpin RNA targeted to produce site-specific silencing of the Adora2a gene, we examined the specific role of A2A receptors in the basal ganglia in the modulation of the sleep-wake cycle and demonstrated that the arousal effect of caffeine is mediated by A2A receptors in the nuclear accumbens shell10.
Similarly, conditional knockout of Adora2b genes using the Cre-loxP system has been developed — for example, with selective deletion of the Adora2b gene in vascular endothelial cells6. In addition, several other recent technological advances allow systems-level study of GPCR function in freely behaving animals. These include specific local modulation of neuronal activity using genetically engineered optical switches (for example, channel rhodopsin)196,197 as well as reversible silencing (for example, non-mammalian chloride channels)198 and activation (for example, stimulatory GPCRs)199,200 of neurons. Applying these technical advances to the study of adenosine receptors will provide a new level of understanding of adenosine receptor function and could facilitate the development of adenosine receptor-targeting drugs.
Different effects in developing and mature individuals
Another potential complication is the differential effects of adenosine receptor activation (or inactivation) at different stages in development. For example, studies in immature mice (7 days old) exposed to hypoxic brain injury indicate that A2A receptor signalling has a protective role201, whereas studies in adult mice in which the Adora2a gene is knocked out suggest that this receptor has a harmful effect in brain ischaemia during adult-hood57. Indeed, many studies in adult mice support the use of A2A receptor antagonists to treat brain ischaemia62,202,203. There is some evidence for similar differential effects of A1 receptors. Thus, in newborn mice, A1 receptor agonism appears to have a deleterious effect in response to hypoxia-induced brain (white matter) injury204, whereas in adult mice it has a protective effect. The underlying reason for this could be that there are differing mechanisms mediating neuronal damage in the immature and mature brain.
Adenosine receptor heterodimerization
There are reports showing that adenosine receptors — like other class A GPCRs — can undergo homo- and heterodimerization or even oligomerization. For example, a heterodimer composed of an A2A receptor and a D2 receptor has been observed in cultured cells as well as in the striatum of intact animals2,205-207. It has been suggested that some of the pharmacological differences in A2A receptor antagonists (that were tested for anti-parkinsonian effects in an animal model of Parkinson’s disease) may be correlated with differences in receptor heterodimerization (A2A-D2 versus A2A –A1) at postsynaptic and presynaptic sites208,209 . Moreover, it was recently suggested that the presence of the A2A receptor may be important for the proper targeting of A2B receptors to cell surface membranes, through the formation of A2A –A2B receptor heterodimers210.
Given that adenosine receptors can be co-immuno-precipitated with many different GPCRs even in the same cell and definitely in the same tissue, this represents a major challenge211-213. However, these results have not yet been confirmed in vivo and there are numerous reports indicating unequivocally that monomeric receptors are sufficient to induce signalling214. Therefore, until it is demonstrated that GPCR heterodimerization occurs in intact animals and that it confers major pharmacological consequences, we suggest that this additional potential complexity should not yet be taken into account for drug development.
Implications of widespread caffeine use
The methylxanthine caffeine is undoubtedly the most widely consumed psychoactive substance. It is estimated that the majority of the world’s adult population consumes caffeine in sufficient doses to influence behaviour on a daily basis184. It was realized many years ago that methylxanthines antagonize adenosine receptors and it is now accepted that many of the actions of caffeine are due to its effect of reducing the number of adenosine receptors to half the normal levels215. Nonetheless, the fact that long-term caffeine use is not associated with increased morbidity, and the fact that mice with reduced numbers of receptors fare well, strongly suggests that even the long-term use of drugs that block adenosine receptors can be safe.
Substantial adenosine receptor antagonism has already been achieved in so many people via their daily consumption of caffeine-containing beverages; this complicates the interpretation of drug trials studying adenosine receptor-targeted agents. A novel adenosine receptor antagonist must therefore be proven to confer benefit over and above that provided by readily available and low-cost caffeine. Caffeine use in humans is limited by effects that are independent of adenosine receptor blockade. Even at the receptor that is most readily blocked by caffeine — the A2A receptor — it is difficult to achieve as much as 50% occupancy. It can be calculated that the daily consumption of three to four regular cups of coffee results in approximately 50% A1 and A2A receptor occupancy for several hours184. This conclusion is supported by genetic studies in mice: heterozygous Adora1- and Adora2-knockout mice (that is, mice in which there is a 50% reduction in the expression of A1 and A2A receptors) recapitulate some of the effects associated with long-term caffeine use215. A more selective antagonist therefore has the potential of affording more complete blockade and hence — at least in theory — a larger therapeutic benefit.
A clinical trial must very carefully assess the caffeine intake of each person enrolled in the study, as a trial in which the participants do not consume caffeine would not be a representative patient sample, and individuals who have recently refrained from caffeine use would encounter other issues, including relief from some of the (albeit weak) abstinence symptoms.
Caffeine use in the general population provides important clues to the potential therapeutic indications of adenosine receptor antagonists. Recent case-control and prospective studies have linked caffeine consumption with a reduced risk of Parkinson’s disease216,217, dementia and Alzheimer’s disease218-220, type 2 diabetes221,222 and chronic liver cirrhosis221. In addition, the common use of caffeine for the treatment of apnoea in premature infants has been associated with reduced retinopathy in 2-year follow-up studies223,224. This has prompted the investigation of A2A receptor-mediated control of retinal vascularization in the developing eye and the involvement of these receptors in oxygen-induced retinopathy in an animal model of retinopathy of prematurity11. Similarly, since 2000, at least five large prospective studies have firmly established a relationship between increased caffeine consumption and a decreased risk of developing Parkinson’s disease; the initial study was from the Honolulu Heart Program217, which was followed by the Health Professionals’ Follow-Up Study and the Nurses’ Health Study (involving 47,351 men and 88,565 women)216, the Finnish Mobile Clinic Health Examination Survey225 and the study carried out by the NeuroGenetics Research Consortium226. Caffeine, A2A receptor antagonists and Adora2a knockout have also demonstrated neuroprotective effects in animal models of Parkinson’s disease119. These findings led to a clinical trial of A2A receptor antagonists as a monotherapy for de novo early-stage Parkinson’s disease227.
The main pharmacological targets of caffeine are adenosine receptors, particularly A2A receptors, as revealed by mouse knockout studies20,228. Therefore, pharmacokinetic and pharmacodynamic (PK/PD) interactions between caffeine and adenosine receptor ligands may contribute to the varying responses to an adenosine receptor-based drug in clinical trials (BOX 2). Indeed, PK/PD analyses show that recent caffeine ingestion (equivalent to two to four cups of coffee) affects A2A receptor agonist-induced myocardial perfusion imaging229 and reduces the efficacy of adenosine in the treatment of paroxysmal supraventricular tachycardia230. In this regard, it is somewhat surprising that several current clinical trials of adenosine receptor antagonists have not taken caffeine consumption into consideration in their clinical trial designs. For example, several recent Phase III clinical trials of the A2A receptor antagonist istradefylline (KW-6002) and preladenant (SCH420814) in patients with advanced Parkinson’s disease apparently did not include data on caffeine consumption139,227,231-236. We speculate that if caffeine consumption is taken into consideration in clinical trials, we may see a clearer and better clinical response to adenosine-based drugs in smaller patient populations. Thus, in our view, careful consideration of caffeine pharmacology should be incorporated into most clinical trial programmes of adenosine receptor-based therapies to address this ‘elephant in the room’ in clinical studies. Genetic studies involving caffeine may offer a unique opportunity for identifying useful pharmacogenetic markers to predict individual responses to caffeine and adenosine drugs in clinical trials (BOX 2).
Box 2. Variations in caffeine sensitivity and implications for clinical trial design.
Many studies have examined a possible genetic basis for the known human variation in the response to caffeine263. Twin studies have suggested a genetic role of the individual variability in caffeine-related traits, such as withdrawal symptoms. Recent genome-wide association studies (GWAS) have linked genetic polymorphisms of metabolic enzymes (cytochrome P450 enzyme 1A2; CYP1A2) and main target receptors (such as the gene encoding adenosine A2A receptor; ADORA2A) to individual variations in caffeine-induced insomnia264-266, anxiety and panic attack267-269. Single nucleotide polymorphisms (SNPs) in ADORA2A have been found to be associated with the age of onset of Huntington’s disease270 and with a reduced risk of Parkinson’s disease271. The strongest associations between caffeine and Parkinson’s disease were found among slow metabolizers of caffeine who were homozygous carriers of the CYP1A2 polymorphisms. In 2011, the first GWAS was carried out on coffee consumption in eight Caucasian cohorts comprising >18,000 individuals of Northern European ancestry272; the top findings were further replicated in ~8,000 additional independent individuals. Two SNPs located in the 23-kb-long commonly shared 5ʹ flanking regions between the CYP1A1 and CYP1A2 genes (rs2470893 and rs2472297) were replicated in the follow-up studies with genome-wide significance. Also, in 2011 the first genome-wide association and interaction study (GWAIS) was performed by the NeuroGenetics Research Consortium to identify genes that influence the inverse association of caffeine consumption with the risk of developing Parkinson’s disease273, and this study found that the SNP rs4998386 and neighbouring SNPs in GRIN2A (which encodes NMDA (N-methyl-d-aspartate) glutamate receptor subunit 2A) modulate the risk of developing Parkinson’s disease in individuals who drink high amounts of coffee273.
Together, these findings have provided a genetic basis for the individual variation in responses to caffeine and caffeine-related trials. This raises an exciting possibility of predicting the individual responses to caffeine (and possibly other adenosine receptor antagonists) through the identification of genetic polymorphisms of the associated alleles identified by these studies. It was recently demonstrated for the first time that the antiplatelet effect of a P2Y purinergic receptor 12 antagonist is influenced by the CYP2C19 genotype274. Given that caffeine and A2A receptor antagonists have a common target and very similar pharmacological effects in the brain, the identification of these SNPs in association with caffeine consumption (in relationship to Parkinson’s disease) led us to speculate that clinical trials might yield clearer outcomes if patients are subdivided and analysed by their genotypes for ADORA2A, neuronal cell adhesion molecule (NRCAM) GRIN2A, CYP1A1 and CYP1A2 genes. Thus, these studies not only provide proof of concept that taking caffeine consumption into consideration can help identify genes that are missed in GWAS, but they also offer a unique opportunity for personalized medicine — identifying useful pharmacogenetic markers for predicting individual responses to caffeine in Parkinson’s disease populations in clinical trials.
Alternative therapeutic approaches
Indirect receptor targeting
Adenosine receptors are found on many cells in the body, so exogenous adenosine agonists pose a substantial risk of inducing side effects. As adenosine itself is rapidly degraded and does not readily pass the blood-brain barrier, it can be given acutely — for example, to regulate cardiac rhythm. Adenosine receptor ligands that are metabolically more stable would be able to reach all receptors and, because of their generally high affinity, would provide prolonged stimulation. Alternatively, instead of directly targeting adenosine receptors, one could raise the levels of endogenous adenosine. This approach could provide some degree of tissue specificity, but this has not yet been systematically investigated.
Historically, drugs such as dipyridamole and methotrexate have been found to increase levels of endogenous adenosine. At present, clinical trials are being carried out to increase extracellular adenosine levels in humans. For example, the adenosine uptake inhibitor dipyridamole is used in patients for pharmacological stress echocardiography (as a coronary vasodilator) or as an inhibitor of platelet aggregation. However, dipyridamole could be easily used for enhancing the beneficial effects of adenosine receptors in other biomedical conditions, such as acute kidney injury, myocardial ischaemia or colitis6. An FDA-approved adenosine deaminase inhibitor, deoxycoformycin (Nipent; Astex Pharmaceuticals), can increase extracellular adenosine levels and is currently in clinical use for the treatment of haematological malignancies237. However, the production of the cytotoxic 2-deoxyadenosine owing to the inhibition of adenosine deaminase is likely to contribute to this therapeutic outcome.
Allosteric enhancers
Another possibility is to use so-called allosteric enhancers. This approach was first described for the A1 receptor238 when an allosteric enhancer was used to increase the responsiveness of this receptor to endogenous adenosine at sites of its production. At the molecular level, an allosteric enhancer amplifies the action of agonists by stabilizing the ternary complex formed by the agonist, the adenosine receptor and the G protein, thus minimizing the side effects of the drug. At least theoretically, the ability to target the receptor at two sites might increase tissue specificity because the drug would act in concert with locally increased adenosine under pathological conditions and have little effect on sites where there are low basal adenosine levels. The recent identification of allosteric regulatory sites such as sodium and water control sites by GPCR crystal structure studies239 has provided a detailed atomic structural framework that can substantially assist the biochemical identification and analysis of allosteric sites on adenosine receptors.
Multiple target drugs for adenosine signalling
The redundancy of adenosine receptor signalling, as revealed by studies in single Adora-knockout mice, indicates that targeting multiple steps and pathways involved in adenosine receptor signalling (such as adenosine generation and metabolism as well as adenosine receptors themselves) may be synergistic and more efficacious than targeting an individual step or pathway. A few multidrug target approaches involving the modulation of adenosine receptor signalling have been postulated and developed. For example, a single drug with dual actions — A2A receptor antagonism and monoamine oxidase B inhibition — is expected to offer potential synergistic effects on neuroprotection and possible motor stimulation in Parkinson’s disease240. Similarly, a new drug — T1-11 — that exhibits a dual action composed of adenosine transport (equilibrative nucleoside transporter 1; ENT1) inhibition and A2A receptor agonism has been developed and tested to show its efficacy in an animal model of Huntington’s disease241.
Another elegant use of network pharmacology is the development of a novel type of prodrug based on our understanding of the ATP-dependent adenosine signalling cascade242. In this approach, 5′-phosphate prodrugs of A2A receptor agonists are prepared that are preferably cleaved at sites of inflammation where NT5E is highly expressed to release the active A2A receptor agonists243. This prodrug approach not only allows the site-specific action of the A2A receptor within the tissues where NT5E is enriched but also avoids the potent hypotensive effect of A2A receptor agonists — a major limiting factor in their development.
Signalling pathway-biased drugs
Adenosine receptor activation can trigger alternative signalling pathways via different coupling and by functional interactions with a broad range of other GPCRs and signalling molecules244. The functional selectivity of different signalling pathways offers a new opportunity for developing signalling pathway-biased drugs that selectively activate a specific intracellular adenosine receptor signalling pathway that is essential for a particular biological function. By systematically screening compound libraries of GPCRs for this ‘biased’ form of signalling, β-arrestin-biased D2 receptor ligands were successfully identified245 and the discovery of a β-arrestin-biased A1 receptor ligand was also attempted, albeit with limited success246. New knowledge of the diversity and modularity of GPCR structures, the A2A receptor in particular, from a recent structural biology crystallization study offers opportunities to target receptor structures with different signalling functions239. The structure of the A2A receptor in several functional states was recently described247-249, revealing that the A2A receptor antagonist ZM241385 binds in an extended conformation perpendicular to the plane of the membrane bilayer249, whereas A2A receptor agonists bind in parallel to the plasma membrane, similarly to retinal rhodopsin248. Moreover, the allosteric regulatory site (such as sodium and water control sites) was recently identified239,250. It is our hope that these exciting results will help in the rational design of novel drugs targeting specific signalling pathways and functions of A2A receptors as well as other adenosine receptors251.
Conclusions
Over the past 80 years, evidence has accumulated to show that extracellular adenosine is an important modulator of physiological and pathological processes. It has emerged that adenosine receptors can be safely targeted by drugs, and it is possible to generate highly specific agonists and antagonists of adenosine receptors. As a result, increasing numbers of clinical trials testing novel adenosine-based drugs in various indications have been initiated during the past decade. Although adenosine is present in virtually all cells, levels of endogenous adenosine are sufficient to activate adenosine receptors only where they are most abundant. Activation of adenosine receptors has the potential to be beneficial in the treatment of various inflammatory and autoimmune disorders, pain, arrhythmia as well as sleep disorders and some metabolic disorders. However, given the wide-spread distribution of adenosine receptors, agonists of these receptors can have effects in most tissues and produce a variety of responses, which makes them difficult to use. Targeting adenosine receptors indirectly using drugs that enhance endogenous local adenosine production or reduce adenosine degradation may have more general applicability.
Adenosine receptor antagonists will have a larger effect where adenosine receptor activation is enhanced — for example, in various pathological processes, including several instances of local reduction of blood flow or local tissue destruction. However, several large Phase III clinical trials of A2A receptor antagonists exhibited insufficient clinical efficacy, despite convincing data from animal models and suggestive evidence from epidemiological studies of caffeine. These Phase III trials did, however, reveal a good safety profile of A2A receptor antagonists138,139, as also indicated from caffeine use. By contrast, a large Phase III trial with an A1 receptor antagonist failed because of its toxicity144,252, but it is not yet known whether this is a class effect. Activation (or blockade) of adenosine receptors can have very different effects in different cells within the same tissue. Moreover, adenosine receptor-directed pharmacotherapy can have different consequences in different stages of a disease process. A deep understanding of both the disease process being targeted and the complexity of adenosine signalling in different cellular elements and different disease courses is crucial.
There are several factors that need to be explored: examination of adenosine receptor function — globally or locally — in disease models, particularly humans; the careful dissection of the site of action of drugs through their administration in animals with cell-specific receptor deletions; careful examination of adenosine signalling and the effects of the drug over the continuum of specific disease courses (for example, acute and chronic stages of a disease); clinical studies in which individual differences are carefully monitored and related to background factors such as caffeine use; and the examination of the possibility of combining direct adenosine receptor actions with drugs targeting other pathways and/or targets. We anticipate that these considerations will move the field forward and, over the next decade, facilitate the introduction of several additional clinical applications of adenosine receptor-targeting treatments into the clinical setting.
Supplementary Material
Acknowledgements
The authors thank E. Augusto and M. Marco for help with the figure. This research was supported by: a grant from the US National Institutes of Health (NIH/NS 041083-07) and the Cogan Foundation, and grants from the Jerry McDonald Huntington’s Disease Research Fund to J.C.; grants from the US National Institutes of Health (NIH), the US National Heart, Lung, and Blood Institute (NHLBI) and the US National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (R01-HL0921, R01-DK083385 and R01-HL098294) and a grant from the Crohn’s & Colitis Foundation of America (CCFA) to H.K.E.; and a grant from the Swedish Science Research Council (grant no. 2553) to B.B.F.
Glossary
- Methylxanthines
Purine derivatives with a common xanthine core molecule and methyl group attached in various combinations to nitrogens. The most common methylxanthines include caffeine, theophylline, theobromine and paraxanthine.
- G protein-coupled receptor
(GPCR). A cell membrane protein characterized by a seven-transmembrane structure, which is coupled to trimeric G proteins; GPCRs elicit diverse sets of signalling and biological functions.
- Ectonucleoside triphosphate diphosphohydrolase 1
(ENTPD1; also known as CD39). A membrane-bound enzyme with enzymatic activity in the extracellular space; ENTPD1 catalyses the conversion of extracellular ATP and/or ADP to AMP — an important step in generating extracellular adenosine.
- Ecto-5′-nucleotidase
(NT5E; also known as CD73). A membrane-bound enzyme with enzymatic activity in the extracellular space; NT5E catalyses the conversion of extracellular AMP to adenosine, thereby functioning as a pacemaker enzyme for generating extracellular ATP-derived adenosine.
- Acute heart failure
A gradual or rapid change in the signs and symptoms of heart failure. Many pathological factors, including worsening renal function, persistent neurohormonal activation and progressive deterioration in myocardial function, all contribute to the development of acute heart failure.
- Parkinson’s disease
A neurodegenerative disease characterized by the loss of dopaminergic neurons in the substantia nigra pars compacta of the midbrain and the accumulation of proteinaceous intracellular inclusions (Lewy bodies), leading to decreased dopamine levels in the striatum and cardinal motor symptoms.
- Ischaemic preconditioning
A phenomenon in which repeated short periods of exposure to sublethal ischaemia induce tolerance and protection against subsequent lethal ischaemic injury.
- Adenosine kinase
An enzyme that converts intracellular adenosine to AMP, which is critically important in setting the basal adenosine level. Hypoxia is associated with transcriptional repression of adenosine kinase, thereby resulting in increased intracellular adenosine levels and enhanced extracellular adenosine signalling.
- Hypoxia-inducible factor
(HIF). Key transcription factor for hypoxia-induced responses that are critical in adapting hypoxic or ischaemic tissues to conditions of limited oxygen availability.
- Bleomycin-induced lung injury
A widely used antitumour agent causing single- and double-stranded breaks in cellular DNA, leading to genomic instability of damaged cells. Bleomycin induces apoptosis and increases the production of reactive oxygen species, resulting in oxidative stress and pulmonary fibrosis.
- Adenosine deaminase
An enzyme that converts adenosine to inosine (and deoxyadenosine to deoxyinosine). Lack of adenosine deaminase causes immune deficiency.
- Oligomerization
G protein-coupled receptors (GPCRs) can exist in a monomeric state or form dimeric, multimeric or oligomeric structures. Hetero-oligomerization of GPCRs, involving several gene products, can potentially lead to an altered biological response repertoire.
- Allosteric enhancers
Allosteric modulators do not have any activity by themselves, but as they bind to the allosteric site (which is distinct from the primary ligand binding orthosteric site) they can alter the receptor confirmation by an orthosteric ligand in such a way that the response to it is increased.
- Equilibrative nucleoside transporter1
(ENT1). A channel located in the cell membrane; along with ENT2, ENT1 speeds up the bi-directional transport of adenosine across the cell membrane along its gradient.
- Functional selectivity
Also known as biased signalling; an emerging concept of G protein-coupled receptor (GPCR) function. For example, some GPCR ligands preferentially activate signals via β-arrestins, others via G proteins.
- β-arrestin
An adaptor protein that was initially recognized as a negative regulator of G protein signalling but is now recognized to be a multifunctional adaptor that can not only mediate G protein-coupled receptor (GPCR) internalization and desensitization but also produce distinct intracellular signalling and hence functional consequences.
Footnotes
FURTHER INFORMATION
Jiang-Fan Chen’s Laboratory: http://www.bumc.bu.edu/neurology/research/molecularlab Holger K. Eltzschig’s laboratory: http://www.ucdenver.edu/academics/colleges/medicalschool/departments/Anesthesiology/anesresearch/labs/chair/Pages/chair.aspx Bertil B. Fredholm’s group: http://ki.se/ki/jsp/polopoly.jsp?l=en&d=9782 ClinicalTrials.gov website: http://clinicaltrials.gov
Competing interests statement
The authors declare no competing financial interests.
References
- 1.Fredholm BB, et al. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol. Rev. 2001;53:527–552. This is an overview of the current knowledge of adenosine receptor biology, from gene structure to expression, distribution, biochemical properties, pharmacological profiles, signalling and behavioural responses, focusing on receptor nomenclature and classification
- 2.Fredholm BB, et al. International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptors — an update. Pharmacol. Rev. 2011;63:1–34. doi: 10.1124/pr.110.003285. This is an update of reference 1, based on 10 more years of research.
- 3.Eltzschig HK, Sitkovsky MV, Robson SC. Purinergic signaling during inflammation. N. Engl. J. Med. 2012;367:2322–2333. doi: 10.1056/NEJMra1205750. This is a comprehensive review summarizing the biomedical implications of extracellular ATP, ADP and adenosine signalling in the context of a broad range of inflammatory diseases.
- 4.Eltzschig HK. Adenosine: an old drug newly discovered. Anesthesiology. 2009;111:904–915. doi: 10.1097/ALN.0b013e3181b060f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johansson SM, Yang JN, Lindgren E, Fredholm BB. Eliminating the antilipolytic adenosine A1 receptor does not lead to compensatory changes in the antilipolytic actions of PGE2 and nicotinic acid. Acta Physiol. 2007;190:87–96. doi: 10.1111/j.1365-201X.2007.01692.x. [DOI] [PubMed] [Google Scholar]
- 6.Grenz A, et al. Equilibrative nucleoside transporter 1 (ENT1) regulates postischemic blood flow during acute kidney injury in mice. J. Clin. Invest. 2012;122:693–710. doi: 10.1172/JCI60214. This report shows that a crosstalk pathway — between epithelium-expressed adenosine transporters (such as ENT1) and endothelium-expressed A2B receptors — dampens renal ischaemia and reperfusion injury by preventing a no-reflow phenomenon.
- 7.Sun D, et al. Mediation of tubuloglomerular feedback by adenosine: evidence from mice lacking adenosine 1 receptors. Proc. Natl Acad. Sci. USA. 2001;98:9983–9988. doi: 10.1073/pnas.171317998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenberger P, et al. Hypoxia-inducible factor-dependent induction of netrin-1 dampens inflammation caused by hypoxia. Nature Immunol. 2009;10:195–202. doi: 10.1038/ni.1683. This study demonstrates that the neuronal guidance molecule netrin 1 has important functions outside brain development, showing that hypoxia-dependent induction of netrin 1 dampens mucosal inflammation by enhancing a purinergic signalling event, particularly through the A2B receptor.
- 9.Huang ZL, et al. Adenosine A2A, but not A1, receptors mediate the arousal effect of caffeine. Nature Neurosci. 2005;8:858–859. doi: 10.1038/nn1491. [DOI] [PubMed] [Google Scholar]
- 10.Lazarus M, et al. Arousal effect of caffeine depends on adenosine A2A receptors in the shell of the nucleus accumbens. J. Neurosci. 2011;31:10067–10075. doi: 10.1523/JNEUROSCI.6730-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu XL, et al. Genetic inactivation of the adenosine A2A receptor attenuates pathologic but not developmental angiogenesis in the mouse retina. Invest. Ophthalmol. Vis. Sci. 2010;51:6625–6632. doi: 10.1167/iovs.09-4900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hasko G, Linden J, Cronstein B, Pacher P. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nature Rev. Drug Discov. 2008;7:759–770. doi: 10.1038/nrd2638. This is an expert and focused review on the molecular and cellular biology of adenosine receptors and their pharmacological actions, with emphasis on immune and inflammatory responses.
- 13.Eltzschig HK, Carmeliet P. Hypoxia and inflammation. N. Engl. J. Med. 2011;364:656–665. doi: 10.1056/NEJMra0910283. This review describes the relationship between hypoxia and inflammation, and demonstrates the functional roles of their interdepencence during intestinal inflammation, acute lung injury, ischaemia-reperfusion injury and cancer.
- 14.Eltzschig HK, Eckle T. Ischemia and reperfusion — from mechanism to translation. Nature Med. 2011;17:1391–1401. doi: 10.1038/nm.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fredholm BB. Adenosine, an endogenous distress signal, modulates tissue damage and repair. Cell Death Differ. 2007;14:1315–1323. doi: 10.1038/sj.cdd.4402132. [DOI] [PubMed] [Google Scholar]
- 16.Jacobson KA, Gao ZG. Adenosine receptors as therapeutic targets. Nature Rev. Drug Discov. 2006;5:247–264. doi: 10.1038/nrd1983. This is an expert and comprehensive review on the medicinal chemistry of the first and second generations of adenosine receptor agonists and antagonists as well as their possible clinical uses.
- 17.Delacretaz E. Clinical practice. Supraventricular tachycardia. N. Engl. J. Med. 2006;354:1039–1051. doi: 10.1056/NEJMcp051145. [DOI] [PubMed] [Google Scholar]
- 18.Muller CE, Jacobson KA. Recent developments in adenosine receptor ligands and their potential as novel drugs. Biochim. Biophys. Acta. 2011;1808:1290–1308. doi: 10.1016/j.bbamem.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghimire G, Hage FG, Heo J, Iskandrian AE. Regadenoson: a focused update. J. Nucl. Cardiol. 2012 Dec 11; doi: 10.1007/s12350-012-9661-3. doi:10.1007/s12350-012-9661-3. [DOI] [PubMed] [Google Scholar]
- 20.Fredholm B, Chen J-F, Masino SA, Vaugeois J-M. Actions of adeosine at its receptors in the CNS: insights from knckouts and drugs. Annu. Rev. Pharmacol. Toxicol. 2005;45:385–412. doi: 10.1146/annurev.pharmtox.45.120403.095731. This is a comprehensive review on the development and characterization of genetic knockout models for adenosine receptors (from 1997 to 2005), focusing on new insights into brain functions under physiological and pathological conditions.
- 21.Massie BM, et al. Rolofylline, an adenosine A1-receptor antagonist, in acute heart failure. N. Engl. J. Med. 2010;363:1419–1428. doi: 10.1056/NEJMoa0912613. This is a report on the clinical Phase III trial for the A1 receptor antagonist rolofylline in 2,033 patients with acute heart failure; the drug had a disappointing clinical outcome.
- 22.Teerlink JR, et al. The safety of an adenosine A1-receptor antagonist, rolofylline, in patients with acute heart failure and renal impairment: findings from PROTECT. Drug Saf. 2012;35:233–244. doi: 10.2165/11594680-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 23.Fredholm BB, et al. Structure and function of adenosine receptors and their genes. Naunyn Schmiedebergs Arch. Pharmacol. 2000;362:364–374. doi: 10.1007/s002100000313. [DOI] [PubMed] [Google Scholar]
- 24.Daly JW, Padgett WL. Agonist activity of 2- and 5′-substituted adenosine analogs and their N6-cycloalkyl derivatives at A1- and A2-adenosine receptors coupled to adenylate cyclase. Biochem. Pharmacol. 1992;43:1089–1093. doi: 10.1016/0006-2952(92)90616-q. [DOI] [PubMed] [Google Scholar]
- 25.Koeppen M, Eckle T, Eltzschig HK. Selective deletion of the A1 adenosine receptor abolishes heart-rate slowing effects of intravascular adenosine in vivo. PLoS ONE. 2009;4:e6784. doi: 10.1371/journal.pone.0006784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Corvol JC, Studler JM, Schonn JS, Girault JA, Herve D. Gαolf is necessary for coupling D1 and A2a receptors to adenylyl cyclase in the striatum. J. Neurochem. 2001;76:1585–1588. doi: 10.1046/j.1471-4159.2001.00201.x. [DOI] [PubMed] [Google Scholar]
- 27.Kull B, Svenningsson P, Fredholm BB. Adenosine A2A receptors are colocalized with and activate Golf in rat striatum. Mol. Pharmacol. 2000;58:771–777. doi: 10.1124/mol.58.4.771. [DOI] [PubMed] [Google Scholar]
- 28.Schulte G, Fredholm BB. Signalling from adenosine receptors to mitogen-activated protein kinases. Cell. Signal. 2003;15:813–827. doi: 10.1016/s0898-6568(03)00058-5. [DOI] [PubMed] [Google Scholar]
- 29.Eckle T, et al. A2B adenosine receptor dampens hypoxia-induced vascular leak. Blood. 2008;111:2024–2035. doi: 10.1182/blood-2007-10-117044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eckle T, et al. Adora2b-elicited Per2 stabilization promotes a HIF-dependent metabolic switch crucial for myocardial adaptation to ischemia. Nature Med. 2012;18:774–782. doi: 10.1038/nm.2728. This study provides a mechanism of how A2B receptor signalling can provide cardioprotection against ischaemia–reperfusion injury. The findings indicate that myocardial A2B signalling leads to the stabilization of the circadian rhythm protein PER2 (period circadian clock 2), which mediates a metabolic switch that enhances myocardial glycolytic capacity, thereby providing enhanced ischaemia tolerance.
- 31.Hart ML, et al. Hypoxia-inducible factor-1α-dependent protection from intestinal ischemia/reperfusion injury involves ecto-5′-nucleotidase (CD73) and the A2B adenosine receptor. J. Immunol. 2011;186:4367–4374. doi: 10.4049/jimmunol.0903617. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Schingnitz U, et al. Signaling through the A2B adenosine receptor dampens endotoxin-induced acute lung injury. J. Immunol. 2010;184:5271–5279. doi: 10.4049/jimmunol.0903035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frick JS, et al. Contribution of adenosine A2B receptors to inflammatory parameters of experimental colitis. J. Immunol. 2009;182:4957–4964. doi: 10.4049/jimmunol.0801324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhong H, et al. Activation of murine lung mast cells by the adenosine A3 receptor. J. Immunol. 2003;171:338–345. doi: 10.4049/jimmunol.171.1.338. [DOI] [PubMed] [Google Scholar]
- 35.Ochaion A, et al. The anti-inflammatory target A3 adenosine receptor is over-expressed in rheumatoid arthritis, psoriasis and Crohn’s disease. Cell. Immunol. 2009;258:115–122. doi: 10.1016/j.cellimm.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 36.Gessi S, et al. Elevated expression of A3 adenosine receptors in human colorectal cancer is reflected in peripheral blood cells. Clin. Cancer Res. 2004;10:5895–5901. doi: 10.1158/1078-0432.CCR-1134-03. [DOI] [PubMed] [Google Scholar]
- 37.Fishman P, Bar-Yehuda S, Liang BT, Jacobson KA. Pharmacological and therapeutic effects of A3 adenosine receptor agonists. Drug Discov. Today. 2012;17:359–366. doi: 10.1016/j.drudis.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.King AE, Ackley MA, Cass CE, Young JD, Baldwin SA. Nucleoside transporters: from scavengers to novel therapeutic targets. Trends Pharmacol. Sci. 2006;27:416–425. doi: 10.1016/j.tips.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 39.MacDonald PE, Braun M, Galvanovskis J, Rorsman P. Release of small transmitters through kiss-and-run fusion pores in rat pancreatic β cells. Cell Metab. 2006;4:283–290. doi: 10.1016/j.cmet.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Z, et al. Regulated ATP release from astrocytes through lysosome exocytosis. Nature Cell Biol. 2007;9:945–953. doi: 10.1038/ncb1620. [DOI] [PubMed] [Google Scholar]
- 41.Chekeni FB, et al. Pannexin 1 channels mediate ‘find-me’ signal release and membrane permeability during apoptosis. Nature. 2010;467:863–867. doi: 10.1038/nature09413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elliott MR, et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 2009;461:282–286. doi: 10.1038/nature08296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anselmi F, et al. ATP release through connexin hemichannels and gap junction transfer of second messengers propagate Ca2+ signals across the inner ear. Proc. Natl. Acad. Sci. USA. 2008;105:18770–18775. doi: 10.1073/pnas.0800793105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kanneganti TD, et al. Pannexin-1-mediated recognition of bacterial molecules activates the cryopyrin inflammasome independent of Toll-like receptor signaling. Immunity. 2007;26:433–443. doi: 10.1016/j.immuni.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 45.Faigle M, Seessle J, Zug S, El Kasmi KC, Eltzschig HK. ATP release from vascular endothelia occurs across Cx43 hemichannels and is attenuated during hypoxia. PLoS ONE. 2008;3:e2801. doi: 10.1371/journal.pone.0002801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ballarin M, Fredholm BB, Ambrosio S, Mahy N. Extracellular levels of adenosine and its metabolites in the striatum of awake rats: inhibition of uptake and metabolism. Acta Physiol. Scand. 1991;142:97–103. doi: 10.1111/j.1748-1716.1991.tb09133.x. [DOI] [PubMed] [Google Scholar]
- 47.Wei CJ, Li W, Chen JF. Normal and abnormal functions of adenosine receptors in the central nervous system revealed by genetic knockout studies. Biochim. Biophys. Acta. 2011;1808:1358–1379. doi: 10.1016/j.bbamem.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 48.Fedele DE, Li T, Lan JQ, Fredholm BB, Boison D. Adenosine A1 receptors are crucial in keeping an epileptic focus localized. Exp. Neurol. 2006;200:184–190. doi: 10.1016/j.expneurol.2006.02.133. [DOI] [PubMed] [Google Scholar]
- 49.Gimenez-Llort L, et al. Mice lacking the adenosine A1 receptor are anxious and aggressive, but are normal learners with reduced muscle strength and survival rate. Eur. J. Neurosci. 2002;16:547–550. doi: 10.1046/j.1460-9568.2002.02122.x. [DOI] [PubMed] [Google Scholar]
- 50.Schwarzschild MA, Agnati L, Fuxe K, Chen JF, Morelli M. Targeting adenosine A2A receptors in Parkinson’s disease. Trends Neurosci. 2006;29:647–654. doi: 10.1016/j.tins.2006.09.004. This is an expert and focused review on the rationale as well as preclinical and clinical evidence for the efficacy of an A2A receptor antagonist in Parkinson’s disease.
- 51.Ferre S, Fredholm BB, Morelli M, Popoli P, Fuxe K. Adenosine-dopamine receptor-receptor interactions as an integrative mechanism in the basal ganglia. Trends Neurosci. 1997;20:482–487. doi: 10.1016/s0166-2236(97)01096-5. [DOI] [PubMed] [Google Scholar]
- 52.Richardson PJ, Kase H, Jenner PG. Adenosine A2A receptor antagonists as new agents for the treatment of Parkinson’s disease. Trends Pharmacol. Sci. 1997;18:338–344. doi: 10.1016/s0165-6147(97)01096-1. [DOI] [PubMed] [Google Scholar]
- 53.Wei CJ, et al. Selective inactivation of adenosine A2A receptors in striatal neurons enhances working memory and reversal learning. Learn. Mem. 2011;18:459–474. doi: 10.1101/lm.2136011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou SJ, et al. Preferential enhancement of working memory in mice lacking adenosine A2A receptors. Brain Res. 2009;1303:74–83. doi: 10.1016/j.brainres.2009.09.082. [DOI] [PubMed] [Google Scholar]
- 55.Yu C, Gupta J, Chen JF, Yin HH. Genetic deletion of A2A adenosine receptors in the striatum selectively impairs habit formation. J. Neurosci. 2009;29:15100–15103. doi: 10.1523/JNEUROSCI.4215-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ledent C, et al. Aggressiveness, hypoalgesia and high blood pressure in mice lacking the adenosine A2a receptor. Nature. 1997;388:674–678. doi: 10.1038/41771. This milestone study describes the generation and initial characterization of the first genetic knockout mice for adenosine receptors — namely the Adora2a-knockout mice.
- 57.Chen JF, et al. A2A adenosine receptor deficiency attenuates brain injury induced by transient focal ischemia in mice. J. Neurosci. 1999;19:9192–9200. doi: 10.1523/JNEUROSCI.19-21-09192.1999. This study describes the generation and characterization of the second line of the Adora2a-knockout mice and provides compelling evidence that A2A receptor inactivation confers neuroprotection against ischaemia.
- 58.Xiao D, et al. Forebrain adenosine A2A receptors contribute to l -3,4-dihydroxyphenylalanine-induced dyskinesia in hemiparkinsonian mice. J. Neurosci. 2006;26:13548–13555. doi: 10.1523/JNEUROSCI.3554-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ohta A, Sitkovsky M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature. 2001;414:916–920. doi: 10.1038/414916a. This landmark study demonstrates that endogenous adenosine via the activation of A2A receptors functions to limit excessive inflammation and collateral tissue damage.
- 60.Day YJ, et al. Renal protection from ischemia mediated by A2A adenosine receptors on bone marrow-derived cells. J. Clin. Invest. 2003;112:883–891. doi: 10.1172/JCI15483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen JF, et al. Adenosine A2A receptors and brain injury: broad spectrum of neuroprotection, multifaceted actions and "fine tuning" modulation. Prog. Neurobiol. 2007;83:310–331. doi: 10.1016/j.pneurobio.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 62.Yu L, et al. Selective inactivation or reconstitution of adenosine A2A receptors in bone marrow cells reveals their significant contribution to the development of ischemic brain injury. Nature Med. 2004;10:1081–1087. doi: 10.1038/nm1103. This study demonstrates a novel role of the A2A receptor in bone marrow-derived cells: it protects against ischaemic brain injury.
- 63.Huang ZL, Urade Y, Hayaishi O. The role of adenosine in the regulation of sleep. Curr. Top. Med. Chem. 2011;11:1047–1057. doi: 10.2174/156802611795347654. [DOI] [PubMed] [Google Scholar]
- 64.Yang D, et al. The A2B adenosine receptor protects against inflammation and excessive vascular adhesion. J. Clin. Invest. 2006;116:1913–1923. doi: 10.1172/JCI27933. This is the first description of Adora2b-knockout mice, which were found to be prone to vascular inflammation — a finding that was later confirmed in many other models of vascular inflammation.
- 65.Eckle T, et al. Cardioprotection by ecto-5′-nucleotidase (CD73) and A2B adenosine receptors. Circulation. 2007;115:1581–1590. doi: 10.1161/CIRCULATIONAHA.106.669697. [DOI] [PubMed] [Google Scholar]
- 66.Schulte G, Fredholm BB. Human adenosine A1, A2A, A2B, and A3 receptors expressed in Chinese hamster ovary cells all mediate the phosphorylation of extracellular-regulated kinase 1/2. Mol. Pharmacol. 2000;58:477–482. [PubMed] [Google Scholar]
- 67.Hart ML, Jacobi B, Schittenhelm J, Henn M, Eltzschig HK. Cutting Edge: A2B adenosine receptor signaling provides potent protection during intestinal ischemia/reperfusion injury. J. Immunol. 2009;182:3965–3968. doi: 10.4049/jimmunol.0802193. [DOI] [PubMed] [Google Scholar]
- 68.Chouker A, et al. In vivo hypoxic preconditioning protects from warm liver ischemia-reperfusion injury through the adenosine A2B receptor. Transplantation. 2012;94:894–902. doi: 10.1097/TP.0b013e31826a9a46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grenz A, et al. The reno-vascular A2B adenosine receptor protects the kidney from ischemia. PLoS Med. 2008;5:e137. doi: 10.1371/journal.pmed.0050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Eckle T, Grenz A, Laucher S, Eltzschig HK. A2B adenosine receptor signaling attenuates acute lung injury by enhancing alveolar fluid clearance in mice. J. Clin. Invest. 2008;118:3301–3315. doi: 10.1172/JCI34203. This head-to-head comparison of all four Adora-knockout mice revealed that Adora2b-knockout mice are particularly prone to ventilator-induced lung injury. The A2B receptor agonist BAY 60-6583 enhanced alveolar fluid transport, thereby attenuating pulmonary oedema and concomittant lung inflammation during acute lung injury.
- 71.Takedachi M, et al. CD73-generated adenosine promotes osteoblast differentiation. J. Cell. Physiol. 2012;227:2622–2631. doi: 10.1002/jcp.23001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Carroll SH, et al. A2B adenosine receptor promotes mesenchymal stem cell differentiation to osteoblasts and bone formation in vivo. J. Biol. Chem. 2012;287:15718–15727. doi: 10.1074/jbc.M112.344994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Johnston-Cox H, et al. The A2b adenosine receptor modulates glucose homeostasis and obesity. PLoS ONE. 2012;7:e40584. doi: 10.1371/journal.pone.0040584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Koupenova M, et al. A2b adenosine receptor regulates hyperlipidemia and atherosclerosis. Circulation. 2012;125:354–363. doi: 10.1161/CIRCULATIONAHA.111.057596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cekic C, et al. Adenosine A2B receptor blockade slows growth of bladder and breast tumors. J. Immunol. 2012;188:198–205. doi: 10.4049/jimmunol.1101845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dai Y, et al. A2B adenosine receptor-mediated induction of IL-6 promotes CKD. J. Am. Soc. Nephrol. 2011;22:890–901. doi: 10.1681/ASN.2010080890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wei W, et al. Blocking A2B adenosine receptor alleviates pathogenesis of experimental autoimmune encephalomyelitis via inhibition of IL-6 production and Th17 differentiation. J. Immunol. 2013;190:138–146. doi: 10.4049/jimmunol.1103721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bjorklund O, et al. Decreased behavioral activation following caffeine, amphetamine and darkness in A3 adenosine receptor knock-out mice. Physiol. Behav. 2008;95:668–676. doi: 10.1016/j.physbeh.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 79.Yang JN, Wang Y, Garcia-Roves PM, Bjornholm M, Fredholm BB. Adenosine A3 receptors regulate heart rate, motor activity and body temperature. Acta Physiol. 2010;199:221–230. doi: 10.1111/j.1748-1716.2010.02091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Eltzschig HK, et al. Coordinated adenine nucleotide phosphohydrolysis and nucleoside signaling in posthypoxic endothelium: role of ectonucleotidases and adenosine A2B receptors. J. Exp. Med. 2003;198:783–796. doi: 10.1084/jem.20030891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thompson LF, et al. Crucial role for ecto-5′-nucleotidase (CD73) in vascular leakage during hypoxia. J. Exp. Med. 2004;200:1395–1405. doi: 10.1084/jem.20040915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sitkovsky MV, et al. Physiological control of immune response and inflammatory tissue damage by hypoxia-inducible factors and adenosine A2A receptors. Annu. Rev. Immunol. 2004;22:657–682. doi: 10.1146/annurev.immunol.22.012703.104731. This important review discusses the relationship between hypoxia and adenosine signalling through the A2A receptor, and implicates A2A signalling as an endogenous feedback signal to dampen collateral tissue injury.
- 83.Gomes CV, Kaster MP, Tome AR, Agostinho PM, Cunha R. A. Adenosine receptors and brain diseases: neuroprotection and neurodegeneration. Biochim. Biophys. Acta. 2011;1808:1380–1399. doi: 10.1016/j.bbamem.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 84.Boison D. Adenosine dysfunction in epilepsy. Glia. 2012;60:1234–1243. doi: 10.1002/glia.22285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Linden J. Regulation of leukocyte function by adenosine receptors. Adv. Pharmacol. 2011;61:95–114. doi: 10.1016/B978-0-12-385526-8.00004-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Synnestvedt K, et al. Ecto-5′-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J. Clin. Invest. 2002;110:993–1002. doi: 10.1172/JCI15337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zetterstrom T, et al. Purine levels in the intact rat brain. Studies with an implanted perfused hollow fibre. Neurosci. Lett. 1982;29:111–115. doi: 10.1016/0304-3940(82)90338-x. [DOI] [PubMed] [Google Scholar]
- 88.Pedata F, Corsi C, Melani A, Bordoni F, Latini S. Adenosine extracellular brain concentrations and role of A2A receptors in ischemia. Ann. NY Acad. Sci. 2001;939:74–84. doi: 10.1111/j.1749-6632.2001.tb03614.x. [DOI] [PubMed] [Google Scholar]
- 89.Andine P, Rudolphi KA, Fredholm BB, Hagberg H. Effect of propentofylline (HWA 285) on extracellular purines and excitatory amino acids in CA1 of rat hippocampus during transient ischaemia. Br. J. Pharmacol. 1990;100:814–818. doi: 10.1111/j.1476-5381.1990.tb14097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dux E, Fastbom J, Ungerstedt U, Rudolphi K, Fredholm BB. Protective effect of adenosine and a novel xanthine derivative propentofylline on the cell damage after bilateral carotid occlusion in the gerbil hippocampus. Brain Res. 1990;516:248–256. doi: 10.1016/0006-8993(90)90925-2. [DOI] [PubMed] [Google Scholar]
- 91.Johansson B, et al. Hyperalgesia, anxiety, and decreased hypoxic neuroprotection in mice lacking the adenosine A1 receptor. Proc. Natl Acad. Sci. USA. 2001;98:9407–9412. doi: 10.1073/pnas.161292398. This is the first report of the generation and charaterization of an Adora1-knockout mouse with a focus on its effects in the central nervous system.
- 92.Eckle T, Koeppen M, Eltzschig HK. Role of extracellular adenosine in acute lung injury. Physiology. 2009;24:298–306. doi: 10.1152/physiol.00022.2009. [DOI] [PubMed] [Google Scholar]
- 93.Ahmad A, et al. Adenosine A2A receptor is a unique angiogenic target of HIF-2α in pulmonary endothelial cells. Proc. Natl Acad. Sci. USA. 2009;106:10684–10689. doi: 10.1073/pnas.0901326106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Eckle T, Kohler D, Lehmann R, El Kasmi KC, Eltzschig HK. Hypoxia-inducible factor-1 is central to cardioprotection: a new paradigm for ischemic preconditioning. Circulation. 2008;118:166–175. doi: 10.1161/CIRCULATIONAHA.107.758516. These findings reveal a functional role of HIF in mediating cardioprotection during ischaemic preconditioning by enhancing extracelluluar adenosine production and A2B receptor signalling.
- 95.Kong T, Westerman KA, Faigle M, Eltzschig HK, Colgan SP. HIF-dependent induction of adenosine A2B receptor in hypoxia. FASEB J. 2006;20:2242–2250. doi: 10.1096/fj.06-6419com. [DOI] [PubMed] [Google Scholar]
- 96.Eltzschig HK, et al. Central role of Sp1-regulated CD39 in hypoxia/ischemia protection. Blood. 2009;113:224–232. doi: 10.1182/blood-2008-06-165746. In contrast to NT5E induction by hypoxia, which is mediated by HIF, this study identifies that ENTPD1 induction during conditions of limited oxygen availability is mediated by the transcription factor SP1.
- 97.Hart ML, Gorzolla IC, Schittenhelm J, Robson SC, Eltzschig HK. SP1-dependent induction of CD39 facilitates hepatic ischemic preconditioning. J. Immunol. 2010;184:4017–4024. doi: 10.4049/jimmunol.0901851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kohler D, et al. CD39/ectonucleoside triphosphate diphosphohydrolase 1 provides myocardial protection during cardiac ischemia/reperfusion injury. Circulation. 2007;116:1784–1794. doi: 10.1161/CIRCULATIONAHA.107.690180. [DOI] [PubMed] [Google Scholar]
- 99.Reutershan J, et al. Adenosine and inflammation: CD39 and CD73 are critical mediators in LPS-induced PMN trafficking into the lungs. FASEB J. 2009;23:473–482. doi: 10.1096/fj.08-119701. [DOI] [PubMed] [Google Scholar]
- 100.Hart ML, et al. Extracellular adenosine production by ecto-5′-nucleotidase protects during murine hepatic ischemic preconditioning. 2008;135:1739–1750.e3. doi: 10.1053/j.gastro.2008.07.064. [DOI] [PubMed] [Google Scholar]
- 101.Csoka B, et al. A2B adenosine receptors protect against sepsis-induced mortality by dampening excessive inflammation. J. Immunol. 2010;185:542–550. doi: 10.4049/jimmunol.0901295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lappas CM, Day YJ, Marshall MA, Engelhard VH, Linden J. Adenosine A2A receptor activation reduces hepatic ischemia reperfusion injury by inhibiting CD1d-dependent NKT cell activation. J. Exp. Med. 2006;203:2639–2648. doi: 10.1084/jem.20061097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Day YJ, et al. A2A adenosine receptors on bone marrow-derived cells protect liver from ischemia-reperfusion injury. J. Immunol. 2005;174:5040–5046. doi: 10.4049/jimmunol.174.8.5040. [DOI] [PubMed] [Google Scholar]
- 104.Enjyoji K, et al. Targeted disruption of CD39/ATP diphosphohydrolase results in disordered hemostasis and thromboregulation. Nature Med. 1999;5:1010–1017. doi: 10.1038/12447. [DOI] [PubMed] [Google Scholar]
- 105.Marcus AJ, et al. Metabolic control of excessive extracellular nucleotide accumulation by CD39/ ecto-nucleotidase-1: implications for ischemic vascular diseases. J. Pharmacol. Exp. Ther. 2003;305:9–16. doi: 10.1124/jpet.102.043729. [DOI] [PubMed] [Google Scholar]
- 106.Eckle T, et al. Identification of ectonucleotidases CD39 and CD73 in innate protection during acute lung injury. J. Immunol. 2007;178:8127–8137. doi: 10.4049/jimmunol.178.12.8127. [DOI] [PubMed] [Google Scholar]
- 107.Grenz A, et al. Contribution of E-NTPDase1 (CD39) to renal protection from ischemia-reperfusion injury. FASEB J. 2007;21:2863–2873. doi: 10.1096/fj.06-7947com. [DOI] [PubMed] [Google Scholar]
- 108.Grenz A, et al. Protective role of ecto-5′-nucleotidase (CD73) in renal ischemia. J. Am. Soc. Nephrol. 2007;18:833–845. doi: 10.1681/ASN.2006101141. [DOI] [PubMed] [Google Scholar]
- 109.Hasko G, et al. Ecto-5′-nucleotidase (CD73) decreases mortality and organ injury in sepsis. J. Immunol. 2011;187:4256–4267. doi: 10.4049/jimmunol.1003379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Castillo CA, Leon D, Ruiz MA, Albasanz JL, Martin M. Modulation of adenosine A1 and A2A receptors in C6 glioma cells during hypoxia: involvement of endogenous adenosine. J. Neurochem. 2008;105:2315–2329. doi: 10.1111/j.1471-4159.2008.05314.x. [DOI] [PubMed] [Google Scholar]
- 111.Angelatou F, et al. Upregulation of A1 adenosine receptors in human temporal lobe epilepsy: a quantitative autoradiographic study. Neurosci. Lett. 1993;163:11–14. doi: 10.1016/0304-3940(93)90217-9. [DOI] [PubMed] [Google Scholar]
- 112.Biber K, et al. Interleukin-6 upregulates neuronal adenosine A1 receptors: implications for neuromodulation and neuroprotection. Neuropsychopharmacology. 2008;33:2237–2250. doi: 10.1038/sj.npp.1301612. [DOI] [PubMed] [Google Scholar]
- 113.Jin D, et al. CD73 on tumor cells impairs antitumor T-cell responses: a novel mechanism of tumor-induced immune suppression. Cancer Res. 2010;70:2245–2255. doi: 10.1158/0008-5472.CAN-09-3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sun X, et al. CD39/ENTPD1 expression by CD4+Foxp3+ regulatory T cells promotes hepatic metastatic tumor growth in mice. Gastroenterology. 2010;139:1030–1040. doi: 10.1053/j.gastro.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ohta A, et al. A2A adenosine receptor protects tumors from antitumor T cells. Proc. Natl Acad. Sci. USA. 2006;103:13132–13137. doi: 10.1073/pnas.0605251103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Popoli P, et al. Blockade of striatal adenosine A2A receptor reduces, through a presynaptic mechanism, quinolinic acid-induced excitotoxicity: possible relevance to neuroprotective interventions in neurodegenerative diseases of the striatum. J. Neurosci. 2002;22:1967–1975. doi: 10.1523/JNEUROSCI.22-05-01967.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dai SS, et al. Local glutamate level dictates adenosine A2A receptor regulation of neuroinflammation and traumatic brain injury. J. Neurosci. 2010;30:5802–5810. doi: 10.1523/JNEUROSCI.0268-10.2010. This study demonstrates that a local increase in glutamate levels switches the anti-inflammatory effect mediated by A2A receptor activation to a pro-inflammatory effect and exacerbates traumatic brain injury, providing a partial explanation for the opposing effects on tissue damage caused by A2A receptor activation in the brain versus in peripheral tissues
- 118.Li W, et al. Genetic inactivation of adenosine A2A receptors attenuates acute traumatic brain injury in the mouse cortical impact model. Exp. Neurol. 2009;215:69–76. doi: 10.1016/j.expneurol.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 119.Chen JF, et al. Neuroprotection by caffeine and A2A adenosine receptor inactivation in a model of Parkinson’s disease. J. Neurosci. 2001;21:RC143. doi: 10.1523/JNEUROSCI.21-10-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Canas PM, et al. Adenosine A2A receptor blockade prevents synaptotoxicity and memory dysfunction caused by β-amyloid peptides via p38 mitogen-activated protein kinase pathway. J. Neurosci. 2009;29:14741–14751. doi: 10.1523/JNEUROSCI.3728-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Blum D, et al. A dual role of adenosine A2A receptors in 3-nitropropionic acid-induced striatal lesions: implications for the neuroprotective potential of A2A antagonists. J. Neurosci. 2003;23:5361–5369. doi: 10.1523/JNEUROSCI.23-12-05361.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chou SY, et al. CGS21680 attenuates symptoms of Huntington’s disease in a transgenic mouse model. J. Neurochem. 2005;93:310–320. doi: 10.1111/j.1471-4159.2005.03029.x. [DOI] [PubMed] [Google Scholar]
- 123.Li Y, et al. Mouse spinal cord compression injury is reduced by either activation of the adenosine A2A receptor on bone marrow-derived cells or deletion of the A2A receptor on non-bone marrow-derived cells. Neuroscience. 2006;141:2029–2039. doi: 10.1016/j.neuroscience.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 124.Bar-Yehuda S, et al. The A3 adenosine receptor agonist CF102 induces apoptosis of hepatocellular carcinoma via de-regulation of the Wnt and NF-κB signal transduction pathways. Int. J. Oncol. 2008;33:287–295. [PubMed] [Google Scholar]
- 125.Deaglio S, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J. Exp. Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Eltzschig HK, et al. HIF-1-dependent repression of equilibrative nucleoside transporter (ENT) in hypoxia. J. Exp. Med. 2005;202:1493–1505. doi: 10.1084/jem.20050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Loffler M, Morote-Garcia JC, Eltzschig SA, Coe IR, Eltzschig HK. Physiological roles of vascular nucleoside transporters. Arterioscler. Thromb. Vasc. Biol. 2007;27:1004–1013. doi: 10.1161/ATVBAHA.106.126714. [DOI] [PubMed] [Google Scholar]
- 128.Morote-Garcia JC, Rosenberger P, Nivillac NM, Coe IR, Eltzschig HK. Hypoxia-inducible factor-dependent repression of equilibrative nucleoside transporter 2 attenuates mucosal inflammation during intestinal hypoxia. Gastroenterology. 2009;136:607–618. doi: 10.1053/j.gastro.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 129.Sitkovsky MV. Damage control by hypoxia-inhibited AK. Blood. 2008;111:5424–5425. doi: 10.1182/blood-2008-03-143990. [DOI] [PubMed] [Google Scholar]
- 130.Morote-Garcia JC, Rosenberger P, Kuhlicke J, Eltzschig HK. HIF-1-dependent repression of adenosine kinase attenuates hypoxia-induced vascular leak. Blood. 2008;111:5571–5580. doi: 10.1182/blood-2007-11-126763. [DOI] [PubMed] [Google Scholar]
- 131.Khoa ND, et al. Inflammatory cytokines regulate function and expression of adenosine A2A receptors in human monocytic THP-1 cells. J. Immunol. 2001;167:4026–4032. doi: 10.4049/jimmunol.167.7.4026. [DOI] [PubMed] [Google Scholar]
- 132.Haschemi A, et al. Cross-regulation of carbon monoxide and the adenosine A2a receptor in macrophages. J. Immunol. 2007;178:5921–5929. doi: 10.4049/jimmunol.178.9.5921. [DOI] [PubMed] [Google Scholar]
- 133.Zhang Y, Palmblad J, Fredholm BB. Biphasic effect of ATP on neutrophil functions mediated by P2U and adenosine A2A receptors. Biochem. Pharmacol. 1996;51:957–965. doi: 10.1016/0006-2952(95)02403-4. [DOI] [PubMed] [Google Scholar]
- 134.Colgan SP, Eltzschig HK. Adenosine and hypoxia-inducible factor signaling in intestinal injury and recovery. Annu. Rev. Physiol. 2011;74:153–175. doi: 10.1146/annurev-physiol-020911-153230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Fredholm BB. Adenosine receptors as drug targets. Exp. Cell Res. 2010;316:1284–1288. doi: 10.1016/j.yexcr.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Dhalla AK, Shryock JC, Shreeniwas R, Belardinelli L. Pharmacology and therapeutic applications of A1 adenosine receptor ligands. Curr. Top. Med. Chem. 2003;3:369–385. doi: 10.2174/1568026033392246. [DOI] [PubMed] [Google Scholar]
- 137.Chen JF. The adenosine A2A receptor as an attractive target for Parkinson’s disease treatment. Drug News Perspect. 2003;16:597–604. doi: 10.1358/dnp.2003.16.9.829342. [DOI] [PubMed] [Google Scholar]
- 138.Jenner P, et al. Adenosine, adenosine A2A antagonists, and Parkinson’s disease. Parkinsonism Relat. Disord. 2009;15:406–413. doi: 10.1016/j.parkreldis.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 139.Hauser RA, et al. Preladenant in patients with Parkinson’s disease and motor fluctuations: a phase 2, double-blind, randomised trial. Lancet Neurol. 2011;10:221–229. doi: 10.1016/S1474-4422(11)70012-6. [DOI] [PubMed] [Google Scholar]
- 140.Guttman M. Efficacy of istradefylline in Parkinson’s diease patients treated with levodopa with motor response complications: results of the KW-6002 US-018 study. Mov. Disord. 2006;21(Suppl. 15):585. [Google Scholar]
- 141.Meissner WG, et al. Priorities in Parkinson’s disease research. Nature Rev. Drug Discov. 2011;10:377–393. doi: 10.1038/nrd3430. [DOI] [PubMed] [Google Scholar]
- 142.Vallon V, Muhlbauer B, Osswald H. Adenosine and kidney function. Physiol. Rev. 2006;86:901–940. doi: 10.1152/physrev.00031.2005. [DOI] [PubMed] [Google Scholar]
- 143.Cotter G, et al. The PROTECT pilot study: a randomized, placebo-controlled, dose-finding study of the adenosine A1 receptor antagonist rolofylline in patients with acute heart failure and renal impairment. J. Card. Fail. 2008;14:631–640. doi: 10.1016/j.cardfail.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 144.Voors AA, et al. Effects of the adenosine A1 receptor antagonist rolofylline on renal function in patients with acute heart failure and renal dysfunction: results from PROTECT (placebo-controlled randomized study of the selective adenosine A1 receptor antagonist rolofylline for patients hospitalized with acute decompensated heart failure and volume overload to assess treatment effect on congestion and renal function) J. Am. Coll. Cardiol. 2011;57:1899–1907. doi: 10.1016/j.jacc.2010.11.057. This paper describes the design and clinical outcome of the largest clinical Phase III trial (PROTECT) of the A1 receptor antagonist rolofylline in acute heart failure.
- 145.Gessi S, et al. Adenosine receptor targeting in health and disease. Expert Opin. Investig. Drugs. 2011;20:1591–1609. doi: 10.1517/13543784.2011.627853. [DOI] [PubMed] [Google Scholar]
- 146.Silverman MH, et al. Clinical evidence for utilization of the A3 adenosine receptor as a target to treat rheumatoid arthritis: data from a phase II clinical trial. J. Rheumatol. 2008;35:41–48. [PubMed] [Google Scholar]
- 147.Avni I, et al. Treatment of dry eye syndrome with orally administered CF101: data from a phase 2 clinical trial. Ophthalmology. 2010;117:1287–1293. doi: 10.1016/j.ophtha.2009.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Montinaro A, et al. Adoptive immunotherapy with Cl-IB-MECA-treated CD8+ T cells reduces melanoma growth in mice. PLoS ONE. 2012;7:e45401. doi: 10.1371/journal.pone.0045401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chen Z, et al. Controlling murine and rat chronic pain through A3 adenosine receptor activation. FASEB J. 2012;26:1855–1865. doi: 10.1096/fj.11-201541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gessi S, et al. A3 adenosine receptors in human neutrophils and promyelocytic HL60 cells: a pharmacological and biochemical study. Mol. Pharmacol. 2002;61:415–424. doi: 10.1124/mol.61.2.415. [DOI] [PubMed] [Google Scholar]
- 151.Baraldi PG, Preti D, Borea PA, Varani K. Medicinal chemistry of A3 adenosine receptor modulators: pharmacological activities and therapeutic implications. J. Med. Chem. 2012;55:5676–5703. doi: 10.1021/jm300087j. [DOI] [PubMed] [Google Scholar]
- 152.Wallace KL, Linden J. Adenosine A2A receptors induced on iNKT and NK cells reduce pulmonary inflammation and injury in mice with sickle cell disease. Blood. 2010;116:5010–5020. doi: 10.1182/blood-2010-06-290643. This paper shows that adenosine signalling, through the activation of A2A receptors expressed on natural killer T cells, dampens ischaemia-reperfusion injury in models of sickle cell disease.
- 153.Zhang Y, et al. Detrimental effects of adenosine signaling in sickle cell disease. Nature Med. 2011;17:79–86. doi: 10.1038/nm.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Field JJ, Nathan DG, Linden J. Targeting iNKT cells for the treatment of sickle cell disease. Clin. Immunol. 2011;140:177–183. doi: 10.1016/j.clim.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gladwin MT. Adenosine receptor crossroads in sickle cell disease. Nature Med. 2011;17:38–40. doi: 10.1038/nm0111-38. [DOI] [PubMed] [Google Scholar]
- 156.Schmitt LI, Sims RE, Dale N, Haydon PG. Wakefulness affects synaptic and network activity by increasing extracellular astrocyte-derived adenosine. J. Neurosci. 2012;32:4417–4425. doi: 10.1523/JNEUROSCI.5689-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lovatt D, et al. Neuronal adenosine release, and not astrocytic ATP release, mediates feedback inhibition of excitatory activity. Proc. Natl Acad. Sci. USA. 2012;109:5913–5914. doi: 10.1073/pnas.1120997109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ramlackhansingh AF, et al. Adenosine 2A receptor availability in dyskinetic and nondyskinetic patients with Parkinson disease. Neurology. 2011;76:1811–1816. doi: 10.1212/WNL.0b013e31821ccce4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mishina M, et al. Adenosine A2A receptors measured with CTMSX PET in the striata of Parkinson’s disease patients. PLoS ONE. 2011;6:e17338. doi: 10.1371/journal.pone.0017338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cha JH, et al. Altered brain neurotransmitter receptors in transgenic mice expressing a portion of an abnormal human huntington disease gene. Proc. Natl Acad. Sci. USA. 1998;95:6480–6485. doi: 10.1073/pnas.95.11.6480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Popoli P, et al. Functions, dysfunctions and possible therapeutic relevance of adenosine A2A receptors in Huntington’s disease. Prog. Neurobiol. 2007;81:331–348. doi: 10.1016/j.pneurobio.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 162.Thiel M, et al. Oxygenation inhibits the physiological tissue-protecting mechanism and thereby exacerbates acute inflammatory lung injury. PLoS Biol. 2005;3:e174. doi: 10.1371/journal.pbio.0030174. This is a landmark paper showing that the therapeutic use of high inspired oxygen concentrations (mechanical ventilation with 100% oxygen) — as is frequently necessary for the treatment of patients suffering from acute lung injury to maintain adequate oxygen tissue levels — has detrimental side effects.
- 163.Eltzschig HK, Rivera-Nieves J, Colgan SP. Targeting the A2B adenosine receptor during gastrointestinal ischemia and inflammation. Expert Opin. Ther. Targets. 2009;13:1267–1277. doi: 10.1517/14728220903241666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Day YJ, et al. Protection from ischemic liver injury by activation of A2A adenosine receptors during reperfusion: inhibition of chemokine induction. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;286:G285–G293. doi: 10.1152/ajpgi.00348.2003. [DOI] [PubMed] [Google Scholar]
- 165.Zhou Y, Schneider DJ, Blackburn MR. Adenosine signaling and the regulation of chronic lung disease. Pharmacol. Ther. 2009;123:105–116. doi: 10.1016/j.pharmthera.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Chunn JL, et al. Adenosine-dependent pulmonary fibrosis in adenosine deaminase-deficient mice. J. Immunol. 2005;175:1937–1946. doi: 10.4049/jimmunol.175.3.1937. [DOI] [PubMed] [Google Scholar]
- 167.Sun CX, et al. Role of A2B receptor signaling in adenosine-dependent pulmonary inflammation and injury. J. Clin. Invest. 2006;116:1–10. doi: 10.1172/JCI27303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Peng Z, et al. Adenosine signaling contributes to ethanol-induced fatty liver in mice. J. Clin. Invest. 2009;119:582–594. doi: 10.1172/JCI37409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Chan ES, et al. Adenosine A2A receptors in diffuse dermal fibrosis: pathogenic role in human dermal fibroblasts and in a murine model of scleroderma. Arthritis Rheum. 2006;54:2632–2642. doi: 10.1002/art.21974. [DOI] [PubMed] [Google Scholar]
- 170.Wen J, et al. Increased adenosine contributes to penile fibrosis, a dangerous feature of priapism, via A2B adenosine receptor signaling. FASEB J. 2010;24:740–749. doi: 10.1096/fj.09-144147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mi T, et al. Excess adenosine in murine penile erectile tissues contributes to priapism via A2B adenosine receptor signaling. J. Clin. Invest. 2008;118:1491–1501. doi: 10.1172/JCI33467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Volmer JB, Thompson LF, Blackburn MR. Ecto-5′-nucleotidase (CD73)-mediated adenosine production is tissue protective in a model of bleomycin-induced lung injury. J. Immunol. 2006;176:4449–4458. doi: 10.4049/jimmunol.176.7.4449. [DOI] [PubMed] [Google Scholar]
- 173.Zhou Y, et al. Enhanced airway inflammation and remodeling in adenosine deaminase-deficient mice lacking the A2B adenosine receptor. J. Immunol. 2009;182:8037–8046. doi: 10.4049/jimmunol.0900515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sun CX, et al. Role of A2B adenosine receptor signaling in adenosine-dependent pulmonary inflammation and injury. J. Clin. Invest. 2006;116:2173–2182. doi: 10.1172/JCI27303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhou Y, et al. Distinct roles for the A2B adenosine receptor in acute and chronic stages of bleomycin-induced lung injury. J. Immunol. 2011;186:1097–1106. doi: 10.4049/jimmunol.1002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sullivan GW, Fang G, Linden J, Scheld WM. A2A adenosine receptor activation improves survival in mouse models of endotoxemia and sepsis. J. Infect. Dis. 2004;189:1897–1904. doi: 10.1086/386311. [DOI] [PubMed] [Google Scholar]
- 177.Nemeth ZH, et al. Adenosine A2A receptor inactivation increases survival in polymicrobial sepsis. J. Immunol. 2006;176:5616–5626. doi: 10.4049/jimmunol.176.9.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Csoka B, et al. A2A adenosine receptors and C/EBPβ are crucially required for IL-10 production by macrophages exposed to Escherichia coli. Blood. 2007;110:2685–2695. doi: 10.1182/blood-2007-01-065870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Chan ES, et al. Adenosine A2A receptors play a role in the pathogenesis of hepatic cirrhosis. Br. J. Pharmacol. 2006;148:1144–1155. doi: 10.1038/sj.bjp.0706812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Martire A, et al. Opposite effects of the A2A receptor agonist CGS21680 in the striatum of Huntington’s disease versus wild-type mice. Neurosci. Lett. 2007;417:78–83. doi: 10.1016/j.neulet.2007.02.034. [DOI] [PubMed] [Google Scholar]
- 181.Obrenovitch TP, Urenjak J. Altered glutamatergic transmission in neurological disorders: from high extracellular glutamate to excessive synaptic efficacy. Prog. Neurobiol. 1997;51:39–87. doi: 10.1016/s0301-0082(96)00049-4. [DOI] [PubMed] [Google Scholar]
- 182.Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol. Rev. 2006;86:1009–1031. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- 183.Jacobson KA, von Lubitz DK, Daly JW, Fredholm BB. Adenosine receptor ligands: differences with acute versus chronic treatment. Trends Pharmacol. Sci. 1996;17:108–113. doi: 10.1016/0165-6147(96)10002-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Fredholm BB, Battig K, Holmen J, Nehlig A, Zvartau EE. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol. Rev. 1999;51:83–133. This is an expert and comprehensive review on the complex pharmacology of the actions of caffeine, with an emphasis on the psychostimulatory effects of caffeine in the brain.
- 185.de Mendonca A, Sebastiao AM, Ribeiro JA. Adenosine: does it have a neuroprotective role after all? Brain Res. Brain Res. Rev. 2000;33:258–274. doi: 10.1016/s0165-0173(00)00033-3. [DOI] [PubMed] [Google Scholar]
- 186.Von Lubitz DK, et al. Chronic administration of selective adenosine A1 receptor agonist or antagonist in cerebral ischemia. Eur. J. Pharmacol. 1994;256:161–167. doi: 10.1016/0014-2999(94)90241-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Von Lubitz DK, et al. Reduction of postischemic brain damage and memory deficits following treatment with the selective adenosine A1 receptor agonist. Eur. J. Pharmacol. 1996;302:43–48. doi: 10.1016/0014-2999(96)00101-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Burgueno J, et al. The adenosine A2A receptor interacts with the actin-binding protein α-actinin. J. Biol. Chem. 2003;278:37545–37552. doi: 10.1074/jbc.M302809200. [DOI] [PubMed] [Google Scholar]
- 189.Chern Y, Lai HL, Fong JC, Liang Y. Multiple mechanisms for desensitization of A2a adenosine receptor-mediated cAMP elevation in rat pheochromocytoma PC12 cells. Mol. Pharmacol. 1993;44:950–958. [PubMed] [Google Scholar]
- 190.Halldner L, Lozza G, Lindstrom K, Fredholm BB. Lack of tolerance to motor stimulant effects of a selective adenosine A2A receptor antagonist. Eur. J. Pharmacol. 2000;406:345–354. doi: 10.1016/s0014-2999(00)00682-8. [DOI] [PubMed] [Google Scholar]
- 191.Pinna A, Fenu S, Morelli M. Motor stimulant effects of the adenosine A2A receptor antagonist SCH 58261 do not develop tolerance after repeated treatments in 6-hydroxydopamine-lesioned rats. Synapse. 2001;39:233–238. doi: 10.1002/1098-2396(20010301)39:3<233::AID-SYN1004>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 192.Bastia E, et al. A crucial role for forebrain adenosine A2A receptors in amphetamine sensitization. Neuropsychopharmacology. 2005;30:891–900. doi: 10.1038/sj.npp.1300630. [DOI] [PubMed] [Google Scholar]
- 193.Yu L, et al. Adenosine A2A receptor antagonists exert motor and neuroprotective effects by distinct cellular mechanisms. Ann. Neurol. 2008;63:338–346. doi: 10.1002/ana.21313. [DOI] [PubMed] [Google Scholar]
- 194.Shen HY, et al. A critical role of the adenosine A2A receptor in extrastriatal neurons in modulating psychomotor activity as revealed by opposite phenotypes of striatum and forebrain A2A receptor knock-outs. J. Neurosci. 2008;28:2970–2975. doi: 10.1523/JNEUROSCI.5255-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Scammell TE, et al. Focal deletion of the adenosine A1 receptor in adult mice using an adeno-associated viral vector. J. Neurosci. 2003;23:5762–5770. doi: 10.1523/JNEUROSCI.23-13-05762.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450:420–424. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Deisseroth K, et al. Next-generation optical technologies for illuminating genetically targeted brain circuits. J. Neurosci. 2006;26:10380–10386. doi: 10.1523/JNEUROSCI.3863-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Lerchner W, et al. Reversible silencing of neuronal excitability in behaving mice by a genetically targeted, ivermectin-gated Cl-channel. Neuron. 2007;54:35–49. doi: 10.1016/j.neuron.2007.02.030. [DOI] [PubMed] [Google Scholar]
- 199.Alexander GM, et al. Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron. 2009;63:27–39. doi: 10.1016/j.neuron.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Nawaratne V, et al. New insights into the function of M4 muscarinic acetylcholine receptors gained using a novel allosteric modulator and a DREADD (designer receptor exclusively activated by a designer drug) Mol. Pharmacol. 2008;74:1119–1131. doi: 10.1124/mol.108.049353. [DOI] [PubMed] [Google Scholar]
- 201.Aden U, et al. Aggravated brain damage after hypoxic ischemia in immature adenosine A2A knockout mice. Stroke. 2003;34:739–744. doi: 10.1161/01.STR.0000060204.67672.8B. [DOI] [PubMed] [Google Scholar]
- 202.Burnstock G, Fredholm BB, Verkhratsky A. Adenosine and ATP receptors in the brain. Curr. Top. Med. Chem. 2011;11:973–1011. doi: 10.2174/156802611795347627. [DOI] [PubMed] [Google Scholar]
- 203.Boison D, Chen JF, Fredholm BB. Adenosine signaling and function in glial cells. Cell Death Differ. 2010;17:1071–1082. doi: 10.1038/cdd.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Turner CP, et al. A1 adenosine receptors mediate hypoxia-induced ventriculomegaly. Proc. Natl Acad. Sci. USA. 2003;100:11718–11722. doi: 10.1073/pnas.1931975100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Chabre M, le Maire M. Monomeric G-protein-coupled receptor as a functional unit. Biochemistry. 2005;44:9395–9403. doi: 10.1021/bi050720o. [DOI] [PubMed] [Google Scholar]
- 206.Whorton MR, et al. A monomeric G protein-coupled receptor isolated in a high-density lipoprotein particle efficiently activates its G protein. Proc. Natl Acad. Sci. USA. 2007;104:7682–7687. doi: 10.1073/pnas.0611448104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Ferre S, et al. Building a new conceptual framework for receptor heteromers. Nature Chem. Biol. 2009;5:131–134. doi: 10.1038/nchembio0309-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Orru M, Quiroz C, Guitart X, Ferre S. Pharmacological evidence for different populations of postsynaptic adenosine A2A receptors in the rat striatum. Neuropharmacology. 2011;61:967–974. doi: 10.1016/j.neuropharm.2011.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Armentero MT, et al. Past, present and future of A2A adenosine receptor antagonists in the therapy of Parkinson’s disease. Pharmacol. Ther. 2011;132:280–299. doi: 10.1016/j.pharmthera.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Moriyama K, Sitkovsky MV. Adenosine A2A receptor is involved in cell surface expression of A2B receptor. J. Biol. Chem. 2010;285:39271–39288. doi: 10.1074/jbc.M109.098293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Canals M, et al. Adenosine A2A-dopamine D2 receptor-receptor heteromerization: qualitative and quantitative assessment by fluorescence and bioluminescence energy transfer. J. Biol. Chem. 2003;278:46741–46749. doi: 10.1074/jbc.M306451200. [DOI] [PubMed] [Google Scholar]
- 212.Ciruela F, et al. Presynaptic control of striatal glutamatergic neurotransmission by adenosine A1-A2A receptor heteromers. J. Neurosci. 2006;26:2080–2087. doi: 10.1523/JNEUROSCI.3574-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Ferre S, et al. Synergistic interaction between adenosine A2A and glutamate mGlu5 receptors: implications for striatal neuronal function. Proc. Natl Acad. Sci. USA. 2002;99:11940–11945. doi: 10.1073/pnas.172393799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Pin JP, et al. International Union of Basic and Clinical Pharmacology. LXVII. Recommendations for the recognition and nomenclature of G protein-coupled receptor heteromultimers. Pharmacol. Rev. 2007;59:5–13. doi: 10.1124/pr.59.1.5. [DOI] [PubMed] [Google Scholar]
- 215.Yang JN, et al. Mice heterozygous for both A1 and A2A adenosine receptor genes show similarities to mice given long-term caffeine. J. Appl. Physiol. 2009;106:631–639. doi: 10.1152/japplphysiol.90971.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Ascherio A, et al. Prospective study of caffeine consumption and risk of Parkinson’s disease in men and women. Ann. Neurol. 2001;50:56–63. doi: 10.1002/ana.1052. [DOI] [PubMed] [Google Scholar]
- 217.Ross GW, et al. Association of coffee and caffeine intake with the risk of Parkinson disease. JAMA. 2000;283:2674–2679. doi: 10.1001/jama.283.20.2674. References 216 and 217 provide strong epidemiological evidence — from the large, longitudinal study by the Honolulu Heart Program and the Health Professional Study — that caffeine consumption is inversely correlated with the risk of developing Parkinson’s disease.
- 218.Lindsay J, et al. Risk factors for Alzheimer’s disease: a prospective analysis from the Canadian Study of Health and Aging. Am. J. Epidemiol. 2002;156:445–453. doi: 10.1093/aje/kwf074. [DOI] [PubMed] [Google Scholar]
- 219.van Boxtel MP, Schmitt JA, Bosma H, Jolles J. The effects of habitual caffeine use on cognitive change: a longitudinal perspective. Pharmacol. Biochem. Behav. 2003;75:921–927. doi: 10.1016/s0091-3057(03)00171-0. [DOI] [PubMed] [Google Scholar]
- 220.Ritchie K, et al. The neuro-protective effects of caffeine: a prospective population study (the Three City Study) Neurology. 2007;69:536–545. doi: 10.1212/01.wnl.0000266670.35219.0c. [DOI] [PubMed] [Google Scholar]
- 221.Higdon JV, Frei B. Coffee and health: a review of recent human research. Crit. Rev. Food Sci. Nutr. 2006;46:101–123. doi: 10.1080/10408390500400009. [DOI] [PubMed] [Google Scholar]
- 222.Salazar-Martinez E, et al. Coffee consumption and risk for type 2 diabetes mellitus. Ann. Intern. Med. 2004;140:1–8. doi: 10.7326/0003-4819-140-1-200401060-00005. [DOI] [PubMed] [Google Scholar]
- 223.Schmidt B, et al. Caffeine therapy for apnea of prematurity. N. Engl. J. Med. 2006;354:2112–2121. doi: 10.1056/NEJMoa054065. [DOI] [PubMed] [Google Scholar]
- 224.Schmidt B, et al. Long-term effects of caffeine therapy for apnea of prematurity. N. Engl. J. Med. 2007;357:1893–1902. doi: 10.1056/NEJMoa073679. [DOI] [PubMed] [Google Scholar]
- 225.Saaksjarvi K, et al. Prospective study of coffee consumption and risk of Parkinson’s disease. Eur. J. Clin. Nutr. 2008;62:908–915. doi: 10.1038/sj.ejcn.1602788. [DOI] [PubMed] [Google Scholar]
- 226.Powers KM, et al. Combined effects of smoking, coffee, and NSAIDs on Parkinson’s disease risk. Mov. Disord. 2008;23:88–95. doi: 10.1002/mds.21782. [DOI] [PubMed] [Google Scholar]
- 227.Fernandez HH, et al. Istradefylline as monotherapy for Parkinson disease: results of the 6002-US-051 trial. Parkinsonism Relat. Disord. 2010;16:16–20. doi: 10.1016/j.parkreldis.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 228.Chen JF, et al. What knock-out animals tell us about the effects of caffeine. J. Alzheimers Dis. 2010;20(Suppl. 1):17–24. doi: 10.3233/JAD-2010-1403. [DOI] [PubMed] [Google Scholar]
- 229.Tejani FH, Thompson RC, Iskandrian AE, McNutt BE, Franks B. Effect of caffeine on SPECT myocardial perfusion imaging during regadenoson pharmacologic stress: rationale and design of a prospective, randomized, multicenter study. J. Nucl. Cardiol. 2011;18:73–81. doi: 10.1007/s12350-010-9311-6. [DOI] [PubMed] [Google Scholar]
- 230.Cabalag MS, et al. Recent caffeine ingestion reduces adenosine efficacy in the treatment of paroxysmal supraventricular tachycardia. Acad. Emerg. Med. 2010;17:44–49. doi: 10.1111/j.1553-2712.2009.00616.x. [DOI] [PubMed] [Google Scholar]
- 231.Pourcher E, et al. Istradefylline for Parkinson’s disease patients experiencing motor fluctuations: results of the KW-6002-US-018 study. Parkinsonism Relat. Disord. 2012;18:178–184. doi: 10.1016/j.parkreldis.2011.09.023. [DOI] [PubMed] [Google Scholar]
- 232.Factor S, et al. A long-term study of istradefylline in subjects with fluctuating Parkinson’s disease. Parkinsonism Relat. Disord. 2010;16:423–426. doi: 10.1016/j.parkreldis.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 233.Hauser RA, et al. Study of istradefylline in patients with Parkinson’s disease on levodopa with motor fluctuations. Mov. Disord. 2008;23:2177–2185. doi: 10.1002/mds.22095. [DOI] [PubMed] [Google Scholar]
- 234.Rao N, et al. A study of the pharmacokinetic interaction of istradefylline, a novel therapeutic for Parkinson’s disease, and atorvastatin. J. Clin. Pharmacol. 2008;48:1092–1098. doi: 10.1177/0091270008320924. [DOI] [PubMed] [Google Scholar]
- 235.Stacy M, et al. A 12-week, placebo-controlled study (6002-US-006) of istradefylline in Parkinson disease. Neurology. 2008;70:2233–2240. doi: 10.1212/01.wnl.0000313834.22171.17. [DOI] [PubMed] [Google Scholar]
- 236.LeWitt PA, et al. Adenosine A2A receptor antagonist istradefylline (KW-6002) reduces "off" time in Parkinson’s disease: a double-blind, randomized, multicenter clinical trial (6002-US-005) Ann. Neurol. 2008;63:295–302. doi: 10.1002/ana.21315. This paper describes the design and clinical outcome of one of six of the randomized, placebo-controlled, Phase III trials of the A2A receptor antagonist istradefylline in patients with advanced Parkinson’s disease.
- 237.Robak T, Robak P. Purine nucleoside analogs in the treatment of rarer chronic lymphoid leukemias. Curr. Pharm. Des. 2012;18:3373–3388. doi: 10.2174/138161212801227005. [DOI] [PubMed] [Google Scholar]
- 238.Bruns RF, Fergus JH. Allosteric enhancement of adenosine A1 receptor binding and function by 2-amino-3-benzoylthiophenes. Mol. Pharmacol. 1990;38:939–949. [PubMed] [Google Scholar]
- 239.Katritch V, Cherezov V, Stevens RC. Diversity and modularity of G protein-coupled receptor structures. Trends Pharmacol. Sci. 2012;33:17–27. doi: 10.1016/j.tips.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Chen JF, et al. 8-(3-chlorostyryl)caffeine may attenuate MPTP neurotoxicity through dual actions of monoamine oxidase inhibition and A2A receptor antagonism. J. Biol. Chem. 2002;277:36040–36044. doi: 10.1074/jbc.M206830200. [DOI] [PubMed] [Google Scholar]
- 241.Huang NK, et al. A new drug design targeting the adenosinergic system for Huntington’s disease. PLoS ONE. 2011;6:e20934. doi: 10.1371/journal.pone.0020934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.El-Tayeb A, et al. Nucleoside-5′-monophosphates as prodrugs of adenosine A2A receptor agonists activated by ecto-5′-nucleotidase. J. Med. Chem. 2009;52:7669–7677. doi: 10.1021/jm900538v. [DOI] [PubMed] [Google Scholar]
- 243.Flogel U, et al. Selective activation of adenosine A2A receptors on immune cells by a CD73-dependent prodrug suppresses joint inflammation in experimental rheumatoid arthritis. Sci. Transl. Med. 2012;4:146ra108. doi: 10.1126/scitranslmed.3003717. [DOI] [PubMed] [Google Scholar]
- 244.Fredholm BB, Chern Y, Franco R, Sitkovsky M. Aspects of the general biology of adenosine A2A signaling. Prog. Neurobiol. 2007;83:263–276. doi: 10.1016/j.pneurobio.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 245.Allen JA, et al. Discovery of β-arrestin-biased dopamine D2 ligands for probing signal transduction pathways essential for antipsychotic efficacy. Proc. Natl Acad. Sci. USA. 2011;108:18488–18493. doi: 10.1073/pnas.1104807108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Langemeijer EV, Verzijl D, Dekker SJ, Ijzerman AP. Functional selectivity of adenosine A1 receptor ligands? Purinergic Signal. 2012;7:171–192. doi: 10.1007/s11302-012-9334-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Lebon G, et al. Agonist-bound adenosine A2A receptor structures reveal common features of GPCR activation. Nature. 2011;474:521–525. doi: 10.1038/nature10136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Xu F, et al. Structure of an agonist-bound human A2A adenosine receptor. Science. 2011;332:322–327. doi: 10.1126/science.1202793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Jaakola VP, et al. The 2.6 angstrom crystal structure of a human A2A adenosine receptor bound to an antagonist. Science. 2008;322:1211–1217. doi: 10.1126/science.1164772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Liu W, et al. Structural basis for allosteric regulation of GPCRs by sodium ions. Science. 2012;337:232–236. doi: 10.1126/science.1219218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Kobilka BK. Structural insights into adrenergic receptor function and pharmacology. Trends Pharmacol. Sci. 2011;32:213–218. doi: 10.1016/j.tips.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Hocher B. Adenosine A1 receptor antagonists in clinical research and development. Kidney Int. 2010;78:438–445. doi: 10.1038/ki.2010.204. [DOI] [PubMed] [Google Scholar]
- 253.Bennet DW, Drury AN. Further observations relating to the physiological activity of adenine compounds. J. Physiol. 1931;72:288–320. doi: 10.1113/jphysiol.1931.sp002775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Drury AN, Szent-Gyorgyi A. The physiological activity of adenine compounds with especial reference to their action upon the mammalian heart. J. Physiol. 1929;68:213–237. doi: 10.1113/jphysiol.1929.sp002608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Stoner HB, Green HN. Experimental limb ischaemia in man with especial reference to the role of adenosine triphosphate. Clin. Sci. 1945;5:159–175. [PubMed] [Google Scholar]
- 256.Massion WH. Value of high energy compounds in the treatment of shock. Am. J. Surg. 1965;110:342–347. doi: 10.1016/0002-9610(65)90069-3. [DOI] [PubMed] [Google Scholar]
- 257.Yang D, et al. A new role for the A2b adenosine receptor in regulating platelet function. J. Thromb. Haemost. 2010;8:817–827. doi: 10.1111/j.1538-7836.2010.03769.x. [DOI] [PubMed] [Google Scholar]
- 258.Berne RM. Cardiac nucleotides in hypoxia: possible role in regulation of coronary blood flow. Am. J. Physiol. 1963;204:317–322. doi: 10.1152/ajplegacy.1963.204.2.317. [DOI] [PubMed] [Google Scholar]
- 259.Gerlach E, Deuticke B, Dreisbach RH. Der Nucleotid-Abbau im Herzmuskel bei Sauerstoffmangel und seine mögliche Bedeutung für die Coronardurchblutung. Naturwissenschaften. 1963;50:228–229. in German. [Google Scholar]
- 260.Mustafa SJ, Berne RM, Rubio R. Adenosine metabolism in cultured chick-embryo heart cells. Am. J. Physiol. 1975;228:1474–1478. doi: 10.1152/ajplegacy.1975.228.5.1474. [DOI] [PubMed] [Google Scholar]
- 261.Duncker DJ, Merkus D. Exercise hyperaemia in the heart: the search for the dilator mechanism. J. Physiol. 2007;583:847–854. doi: 10.1113/jphysiol.2007.135525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Lankford AR, et al. Effect of modulating cardiac A1 adenosine receptor expression on protection with ischemic preconditioning. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H1469–H1473. doi: 10.1152/ajpheart.00181.2005. [DOI] [PubMed] [Google Scholar]
- 263.Yang A, Palmer AA, de Wit H. Genetics of caffeine consumption and responses to caffeine. Psychopharmacology. 2010;211:245–257. doi: 10.1007/s00213-010-1900-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Retey JV, et al. A functional genetic variation of adenosine deaminase affects the duration and intensity of deep sleep in humans. Proc. Natl Acad. Sci. USA. 2005;102:15676–15681. doi: 10.1073/pnas.0505414102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Landolt HP. Sleep homeostasis: a role for adenosine in humans? Biochem. Pharmacol. 2008;75:2070–2079. doi: 10.1016/j.bcp.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 266.Retey JV, et al. A genetic variation in the adenosine A2A receptor gene (ADORA2A) contributes to individual sensitivity to caffeine effects on sleep. Clin. Pharmacol. Ther. 2007;81:692–698. doi: 10.1038/sj.clpt.6100102. [DOI] [PubMed] [Google Scholar]
- 267.Childs E, et al. Association between ADORA2A and DRD2 polymorphisms and caffeine-induced anxiety. Neuropsychopharmacology. 2008;33:2791–2800. doi: 10.1038/npp.2008.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Domschke K, et al. ADORA2A gene variation, caffeine, and emotional processing: a multi-level interaction on startle reflex. Neuropsychopharmacology. 2012;37:759–769. doi: 10.1038/npp.2011.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Alsene K, Deckert J, Sand P, de Wit H. Association between A2a receptor gene polymorphisms and caffeine-induced anxiety. Neuropsychopharmacology. 2003;28:1694–1702. doi: 10.1038/sj.npp.1300232. [DOI] [PubMed] [Google Scholar]
- 270.Taherzadeh-Fard E, Saft C, Wieczorek S, Epplen JT, Arning L. Age at onset in Huntington’s disease: replication study on the associations of ADORA2A, HAP1 and OGG1. Neurogenetics. 2010;11:435–439. doi: 10.1007/s10048-010-0248-3. [DOI] [PubMed] [Google Scholar]
- 271.Popat RA, et al. ADORA2A, and CYP1A2: the caffeine connection in Parkinson’s disease. Eur. J. Neurol. 2011;18:756–765. doi: 10.1111/j.1468-1331.2011.03353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Amin N, et al. Genome-wide association analysis of coffee drinking suggests association with CYP1A1/CYP1A2 and NRCAM. Mol. Psychiatry. 2011;17:1116–1129. doi: 10.1038/mp.2011.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Hamza TH, et al. Genome-wide gene-environment study identifies glutamate receptor gene GRIN2A as a Parkinson’s disease modifier gene via interaction with coffee. PLoS Genet. 2011;7:e1002237. doi: 10.1371/journal.pgen.1002237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Tantry US, et al. First analysis of the relation between CYP2C19 genotype and pharmacodynamics in patients treated with ticagrelor versus clopidogrel: the ONSET/OFFSET and RESPOND genotype studies. Circ. Cardiovasc. Genet. 2010;3:556–566. doi: 10.1161/CIRCGENETICS.110.958561. [DOI] [PubMed] [Google Scholar]
- 275.Elzein E, Zablocki J. A1 adenosine receptor agonists and their potential therapeutic applications. Expert Opin. Investig. Drugs. 2008;17:1901–1910. doi: 10.1517/13543780802497284. [DOI] [PubMed] [Google Scholar]
- 276.Pinna A. Novel investigational adenosine A2A receptor antagonists for Parkinson’s disease. Expert Opin. Investig. Drugs. 2009;18:1619–1631. doi: 10.1517/13543780903241615. [DOI] [PubMed] [Google Scholar]
- 277.Brown R, et al. Abolished tubuloglomerular feedback and increased plasma renin in adenosine A1 receptor-deficient mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;281:R1362–R1367. doi: 10.1152/ajpregu.2001.281.5.R1362. [DOI] [PubMed] [Google Scholar]
- 278.Tawfik HE, Schnermann J, Oldenburg PJ, Mustafa SJ. Role of A1 adenosine receptors in regulation of vascular tone. Am. J. Physiol. Heart Circ. Physiol. 2005;288:H1411–H1416. doi: 10.1152/ajpheart.00684.2004. [DOI] [PubMed] [Google Scholar]
- 279.Wang Y, Yang JN, Arner A, Boels PJ, Fredholm BB. Adenosine A1 receptors and vascular reactivity. Acta Physiol. 2010;199:211–220. doi: 10.1111/j.1748-1716.2010.02093.x. [DOI] [PubMed] [Google Scholar]
- 280.Hua X, et al. Involvement of A1 adenosine receptors and neural pathways in adenosine-induced bronchoconstriction in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007;293:L25–L32. doi: 10.1152/ajplung.00058.2007. [DOI] [PubMed] [Google Scholar]
- 281.Johansson SM, et al. A1 receptor deficiency causes increased insulin and glucagon secretion in mice. Biochem. Pharmacol. 2007;74:1628–1635. doi: 10.1016/j.bcp.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 282.Yang JN, et al. Sex differences in mouse heart rate and body temperature and in their regulation by adenosine A1 receptors. Acta Physiol. 2007;190:63–75. doi: 10.1111/j.1365-201X.2007.01690.x. [DOI] [PubMed] [Google Scholar]
- 283.Kara FM, et al. Adenosine A1 receptors regulate bone resorption in mice: adenosine A1 receptor blockade or deletion increases bone density and prevents ovariectomy-induced bone loss in adenosine A1 receptor-knockout mice. Arthritis Rheum. 2010;62:534–541. doi: 10.1002/art.27219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Stenberg D, et al. Sleep and its homeostatic regulation in mice lacking the adenosine A1 receptor. J. Sleep Res. 2003;12:283–290. doi: 10.1046/j.0962-1105.2003.00367.x. [DOI] [PubMed] [Google Scholar]
- 285.Oishi Y, Huang ZL, Fredholm BB, Urade Y, Hayaishi O. Adenosine in the tuberomammillary nucleus inhibits the histaminergic system via A1 receptors and promotes non-rapid eye movement sleep. Proc. Natl Acad. Sci. USA. 2008;105:19992–19997. doi: 10.1073/pnas.0810926105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Wu WP, et al. Increased nociceptive response in mice lacking the adenosine A1 receptor. Pain. 2005;113:395–404. doi: 10.1016/j.pain.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 287.Schulte G, et al. Adenosine A receptors are necessary for protection of the murine heart by remote, delayed adaptation to ischaemia. Acta Physiol. Scand. 2004;182:133–143. doi: 10.1111/j.1365-201X.2004.01350.x. [DOI] [PubMed] [Google Scholar]
- 288.Lappas CM, Rieger JM, Linden J. A2A adenosine receptor induction inhibits IFN-γ production in murine CD4+ T cells. J. Immunol. 2005;174:1073–1080. doi: 10.4049/jimmunol.174.2.1073. [DOI] [PubMed] [Google Scholar]
- 289.Carman AJ, Mills JH, Krenz A, Kim DG, Bynoe MS. Adenosine receptor signaling modulates permeability of the blood-brain barrier. J. Neurosci. 2011;31:13272–13280. doi: 10.1523/JNEUROSCI.3337-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Chen X, Lan X, Roche I, Liu R, Geiger JD. Caffeine protects against MPTP-induced blood-brain barrier dysfunction in mouse striatum. J. Neurochem. 2008;107:1147–1157. doi: 10.1111/j.1471-4159.2008.05697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Chen X, Gawryluk JW, Wagener JF, Ghribi O, Geiger JD. Caffeine blocks disruption of blood brain barrier in a rabbit model of Alzheimer’s disease. J. Neuroinflamm. 2008;5:12. doi: 10.1186/1742-2094-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Morrison RR, Talukder MA, Ledent C, Mustafa SJ. Cardiac effects of adenosine in A2A receptor knockout hearts: uncovering A2B receptors. Am. J. Physiol. Heart Circ. Physiol. 2002;282:H437–H444. doi: 10.1152/ajpheart.00723.2001. [DOI] [PubMed] [Google Scholar]
- 293.Montesinos MC, et al. Adenosine promotes wound healing and mediates angiogenesis in response to tissue injury via occupancy of A2A receptors. Am. J. Pathol. 2002;160:2009–2018. doi: 10.1016/S0002-9440(10)61151-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Tilley SL, Wagoner VA, Salvatore CA, Jacobson MA, Koller BH. Adenosine and inosine increase cutaneous vasopermeability by activating A3 receptors on mast cells. J. Clin. Invest. 2000;105:361–367. doi: 10.1172/JCI8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Hua X, et al. Adenosine induces airway hyperresponsiveness through activation of A3 receptors on mast cells. J. Allergy Clin. Immunol. 2008;122:107–113.e7. doi: 10.1016/j.jaci.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Li L, et al. Dendritic cells tolerized with adenosine A2AR agonist attenuate acute kidney injury. J. Clin. Invest. 2012;122:3931–3942. doi: 10.1172/JCI63170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Field JJ, et al. Sickle cell vaso-occlusion causes activation of iNKT cells that is decreased by the adenosine A2A receptor agonist regadenoson. Blood. 2013 Feb 1; doi: 10.1182/blood-2012-11-465963. doi:10.1182/blood-2012-11-465963. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.