Abstract
Background
Medical comorbidity is a confounding factor in prostate cancer (PCa) treatment selection and mortality. Large-scale comparative evaluation of PCa mortality (PCM) and overall mortality (OM) restricted to men without comorbidity at the time of treatment has not been performed.
Objective
To evaluate PCM and OM in men with no recorded comorbidity treated with radical prostatectomy (RP), external-beam radiation therapy (EBRT), or brachytherapy (BT).
Design, setting, and participants
Data from 10 361 men with localized PCa treated from 1995 to 2007 at two academic centers in the United States were prospectively obtained at diagnosis and retrospectively reviewed. We identified 6692 men with no recorded comorbidity on a validated comorbidity index. Median follow-up after treatment was 7.2 yr.
Intervention
Treatment with RP in 4459 men, EBRT in 1261 men, or BT in 972 men.
Outcome measurements and statistical analysis
Univariate and multivariate Cox proportional hazards regression analysis, including propensity score adjustment, compared PCM and OM for EBRT and BT relative to RP as reference treatment category. PCM was also evaluated by competing risks analysis.
Results and limitations
Using Cox analysis, EBRT was associated with an increase in PCM compared with RP (hazard ratio [HR]: 1.66; 95% confidence interval [CI], 1.05–2.63), while there was no statistically significant increase with BT (HR: 1.83; 95% CI, 0.88–3.82). Using competing risks analysis, the benefit of RP remained but was no longer statistically significant for EBRT (HR: 1.55; 95% CI, 0.92–2.60) or BT (HR: 1.66; 95% CI, 0.79–3.46). In comparison with RP, both EBRT (HR: 1.71; 95% CI, 1.40–2.08) and BT (HR: 1.78; 95% CI, 1.37–2.31) were associated with increased OM.
Conclusions
In a large multicenter series of men without recorded comorbidity, both forms of radiation therapy were associated with an increase in OM compared with surgery, but there were no differences in PCM when evaluated by competing risks analysis. These findings may result from an imbalance of confounders or differences in mortality related to primary or salvage therapy.
Keywords: Prostatic neoplasms, Prostatectomy, Radiation therapy, Comorbidity, Comparative effectiveness research
1. Introduction
Preexisting medical comorbidity is of paramount importance in prostate cancer (PCa) treatment decision making. A selection bias exists among cancer specialists for preferring radiation therapy (RT) for patients with significant comorbid illness who are felt not to be candidates for surgery [1,2]. In addition to influencing treatment choice, medical comorbidity influences mortality after the diagnosis of localized PCa [3,4] directly through competing causes of death or indirectly by exacerbating underlying disease states [5]. Several groups have reported that underlying medical comorbidity can influence overall mortality (OM) after PCa treatment [6–9].
In the absence of randomization, this imbalance of medical comorbidity makes valid comparisons among treatment options difficult. Several comparisons of PCa treatment options have either ignored medical comorbidity because the information was not collected [10] or have attempted to control for measured comorbidity using statistical methods [11–15]. However, concerns remain that unmeasured factors could still bias results.
Statistically adjusting for comorbidity in observational studies assumes that comorbidity severity is accurately assessed and that measurement error does not result in incomplete statistical adjustment. Experts in the methodology of comparative effectiveness literature have recommended restriction analysis as an alternative to statistical adjustment to minimize the effect of confounding bias [16]. For this reason, we sought to compare mortality among treatments for men with no medical comorbidity. Our objective was to evaluate for any differences in mortality—either PCa mortality (PCM) or OM in patients treated in the prostate-specific antigen (PSA) era with radical prostatectomy (RP), external-beam RT (EBRT), or brachytherapy (BT).
2. Patients and methods
From 1995 to 2007, 10 361 men underwent treatment (RP, EBRT, or BT) for localized PCa at the Cleveland Clinic (Cleveland, OH, USA) or Barnes-Jewish Hospital (St. Louis, MO, USA) and were prospectively entered into institutional databases. As part of each institutional database, pretreatment medical comorbidity was measured by two validated comorbidity indexes—retrospectively with the Charlson comorbidity index [17] at the Cleveland Clinic and prospectively at the time of diagnosis by the Adult Comorbidity Evaluation Index-27 (ACE-27) [18] at Barnes-Jewish Hospital—that have been reported to have similar mortality prediction [19]. The Charlson index evaluates the presence of 19 comorbid ailments, and the ACE-27 includes 26 comorbid ailments and a comorbidity score (none, mild, moderate, and severe). Of 10 361 men, 6692 (64.6%) were retrospectively identified with an ACE-27 assessment of none or a Charlson index of zero (Table 1); these patients formed the study cohort. Treatment consisted of RP, EBRT, or BT. RP was performed by way of an open retropubic or minimally invasive approach. EBRT dosage was consistent with the standard of care at the time of treatment, with doses gradually escalated from 68.4 to 79.2 Gy. BT was administered with intraoperative ultrasound guidance. Patient demographic (age at time of treatment, race) and clinical information (pretreatment PSA, clinical stage, biopsy Gleason grade) were reviewed and compared among treatments using analysis of variance for continuous data and the χ2 test for categorical data.
Table 1.
Medical comorbidity of all 10 361 men treated for localized prostate cancer*
| Medical comorbidity |
Overall, n = 10361 |
RP, n = 6477 |
EBRT, n = 2204 |
BT, n = 1680 |
|---|---|---|---|---|
| None, no. (%) | 6692 (65) | 4459 (69) | 1261 (57) | 972 (58) |
| Mild, no. (%) | 2615 (25) | 1587 (25) | 583 (26) | 445 (26) |
| Moderate, no. (%) | 922 (9) | 387 (6) | 300 (14) | 235 (14) |
| Severe, no. (%) | 132 (1) | 44 (1) | 60 (3) | 28 (2) |
RP = radical prostatectomy; EBRT = external-beam radiation therapy; BT = brachytherapy.
The Adult Comorbidity Evaluation Index-27 comorbidity index groups patients into none, mild, moderate, and severe. Charlson comorbidity index: 0 = none; 1 = mild; 2–3 = moderate; ≥4 = severe.
PCM and OM were assessed by a combination of chart review, correspondence, and query of the National Death Index. Ten-year mortality estimates for PCM and OM for the entire cohort were obtained by the Kaplan-Meier method.
Analysis with the univariate Cox proportional hazards model identified variables associated with PCM or OM at a level of significance of p < 0.10. Significant covariates were incorporated into multivariate Cox proportional hazards models for PCM and OM to compare the hazard ratio (HR) for EBR and BT, with RP as the reference group with an HR of 1.0. Adjusted mortality graphs for PCM and OM were made based on the Cox models. We additionally controlled for selection bias not controlled for by multivariable methods by using propensity adjustment using logistic regression modeling similar to the method described by Mangano et al [20] for the three different treatments. A propensity score for treatment (EBRT relative to RP, BT relative to RP) was developed using clinical and disease information (age, race, PSA, biopsy Gleason grade, clinical stage), and the propensity score was then included in the model for PCM and the model for OM. We additionally evaluated PCM using a Fine and Gray competing risks analysis, which has been suggested to improve accuracy by adjusting for the competing risk of other-cause mortality [21].
A p value of <0.05 was considered statistically significant. Statistical analysis was performed with SAS 9.2 (SAS Institute Inc., Cary, NC, USA), SPSS 17 (IBM Corp., Armonk, NY, USA), and Stata (StataCorp., College Station, TX, USA). Institutional review board approval was obtained.
3. Results
In the cohort of men without measured medical comorbidity, treatment groups differed with respect to age, race, PSA, clinical stage, and biopsy Gleason grade (Table 2). Median follow-up after treatment was 7.2 yr, while 2397 of 6692 men (35.8%) had evaluation to either death or follow-up for >10 yr. Mortality occurred in 664 men (9.9%), which was classified as PCM in 123 men and other-cause mortality in 541 men. By the Kaplan-Meier method, the 10-yr PCM was 2.6% and the 10-yr OM was 13.3%.
Table 2.
Characteristics of 6692 men with no comorbidity treated for localized prostate cancer
| RP, n = 4459 | EBRT, n = 1261 |
BT, n = 972 |
p value | |
|---|---|---|---|---|
| Age, yr, median | ||||
| 60 | 68.3 | 66.8 | <0.001 | |
| Race, African American, no. (%) | 366 (8) | 260 (21) | 107 (11) | <0.001 |
| PSA, median | 6.96 | 11.11 | 6.66 | <0.001 |
| Biopsy Gleason score, no. (%) | <0.001 | |||
| 5–6 | 3316 (74) | 696 (55) | 805 (83) | |
| 7 | 976 (22) | 414 (33) | 162 (17) | |
| 8–10 | 167 (4) | 151 (12) | 5 (0.5) | |
| Clinical stage, no. (%) | <0.001 | |||
| T1 | 3480 (78) | 743 (59) | 798 (82) | |
| T2 | 951 (21) | 446 (35) | 174 (18) | |
| T3 | 28 (0.6) | 72 (6) | 0 (0) | |
| D’Amico risk group, no. (%) | <0.001 | |||
| Low | 2807 (63) | 452 (36) | 707 (73) | |
| Intermediate | 1331 (30) | 463 (37) | 248 (26) | |
| High | (321 (7) | 346 (27) | 17 (2) | |
RP = radical prostatectomy; EBRT = external-beam radiation therapy; BT = brachytherapy; PSA = prostate-specific antigen.
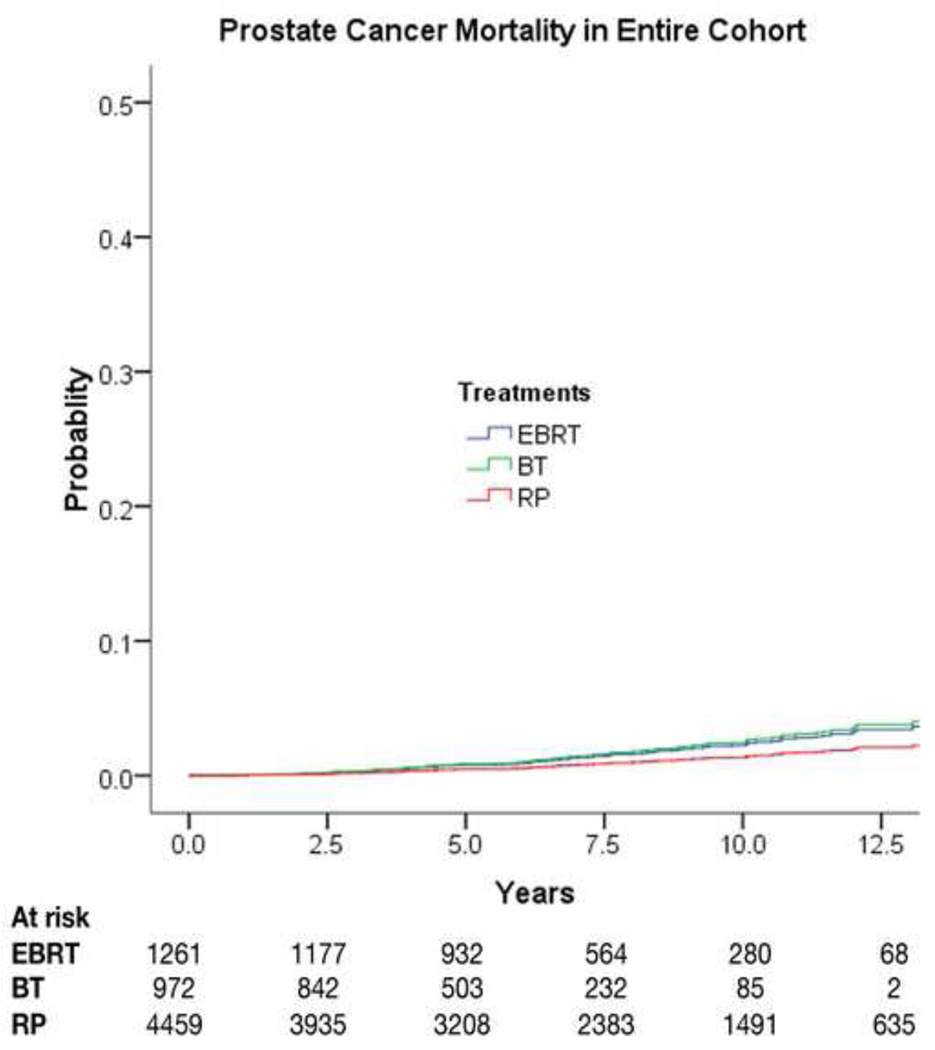
Univariate and multivariate Cox proportional hazards models are displayed for PCM in Table 3. EBRT was associated with an increase in PCM compared with RP (HR: 1.66; 95% confidence interval [CI], 1.05–2.63); for BT there was also an elevated hazard ratio (HR: 1.83) for PCM compared with RP, but it was not statistically significant (95% CI for HR, 0.88–3.82) (Fig. 1). When covariates for propensity score analysis were included in the analysis of PCM, the relationships on multivariable analysis remained similar, with EBRT associated with an increase in PCM compared with RP (HR: 1.64; 95% CI, 1.05–2.55; p = 0.03) and a similar HR for PCM with BT compared with RP (HR: 1.63; 95% CI, 0.77–3.45).
Table 3.
Univariate and multivariate models for prostate cancer mortality
| Univariate | Multivariate Cox proportional hazards model |
Multivariate competing risks model |
||||
|---|---|---|---|---|---|---|
| HR | p value | HR (95% CI) | p value | HR (95% CI) | p value | |
| Age | 1.03 | 0.03 | 1.00 (0.98–1.03) | 0.84 | 1.00 (0.97–1.02) | 0.82 |
| Race compared with non-African American | 1.55 | 0.09 | 0.95 (0.56–1.61) | 0.85 | 0.94 (0.55–1.63) | 0.83 |
| PSA | 1.08 | <0.001 | 1.03 (1.01–1.05) | 0.002 | 1.03 (1.01–1.05) | 0.002 |
| Biopsy Gleason score compared with 5–6 | ||||||
| 7 | 1.72 | 0.005 | 2.62 (1.65–4.18) | <0.001 | 2.55 (1.60–4.08) | <0.001 |
| 8–10 | 11.25 | <0.001 | 10.47 (6.42–17.08) | <0.001 | 10.11 (6.23–16.42) | <0.001 |
| Clinical stage compared with T1 | 5.79 | 0.02 | 1.29 (0.86–1.93) | 0.22 | 1.28 (0.84–1.95) | 0.25 |
| T2 | 11.52 | <0.001 | 2.63 (1.40–4.92) | 0.003 | 2.66 (1.30–5.46) | 0.008 |
| T3 | ||||||
| Treatment compared with RP | 3.27 | <0.001 | 1.66 (1.05–2.63) | 0.03 | 1.55 (0.92–2.60) | 0.10 |
| EBRT | 0.78 | 0.48 | 1.83 (0.88–3.82) | 0.11 | 1.66 (0.79–3.46) | 0.18 |
| BT | ||||||
HR = hazard ratio; CI = confidence interval; PSA = prostate-specific antigen; RP = radical prostatectomy; EBRT = external-beam radiation therapy; BT = brachytherapy.
Fig. 1.
Prostate cancer mortality by treatment type, adjusting for covariates in multivariate Cox proportional hazards model. EBRT = external-beam radiation therapy; BT = brachytherapy; RP = radical prostatectomy.
PCM was additionally evaluated using Fine and Gray competing risks analysis (Table 3). When using a competing risks model, there was no statistically significant increase in PCM for EBRT compared with RP (HR: 1.55; 95% CI, 0.92–2.60) or for BT compared with RP (HR: 1.66; 95% CI, 0.79–3.46).
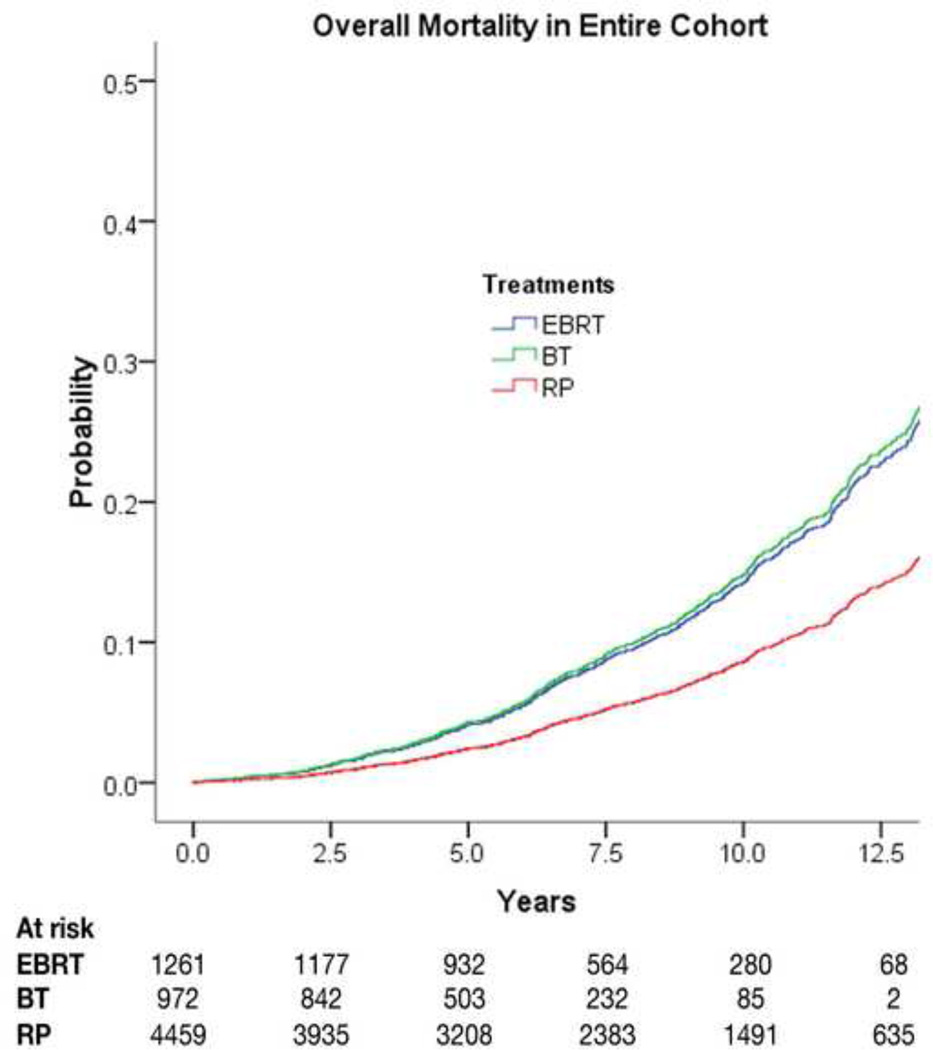
Results for OM are shown in Table 4. In comparison with RP, both EBRT (HR: 1.71; 95% CI, 1.40–2.08) and BT (HR: 1.78; 95% CI, 1.37–2.31) were associated with increased OM (Fig. 2). When using the propensity adjustment method in the multivariable analysis of OM, the relationships remained similar, with an increase in OM with EBRT (HR: 1.67; 95% CI, 1.37–2.04; p < 0.0001) and with BT (HR: 1.69; 95% CI, 1.30–2.19; p < 0.0001) compared with RP.
Table 4.
Univariate and multivariate models for overall mortality
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR | p value | HR (95% CI) | p value | |
| Age | 1.10 | <0.001 | 1.07 (1.06–1.08) | <0.001 |
| Race compared with non-African American | 1.80 | <0.001 | 1.34 (1.08–1.66) | 0.008 |
| PSA | 1.04 | <0.001 | 1.01 (1.00–1.02) | 0.02 |
| Biopsy Gleason score compared with 5–6 | 1.54 | <0.001 | 1.38 (1.15–1.65) | <0.001 |
| 7 | 3.10 | <0.001 | 2.52 (1.96–3.23) | <0.001 |
| 8–10 | ||||
| Clinical stage compared with T1 | 1.31 | 0.001 | 1.06 (0.89–1.26) | 0.50 |
| T2 | 3.76 | <0.001 | 1.65 (1.13–2.41) | 0.01 |
| T3 | ||||
| Treatment compared with RP | ||||
| EBRT | 3.16 | <0.001 | 1.71 (1.40–2.08) | <0.001 |
| BT | 1.58 | <0.001 | 1.78 (1.37–2.31) | <0.001 |
HR = hazard ratio; CI = confidence interval; PSA = prostate-specific antigen; RP = radical prostatectomy; EBRT = external-beam radiation therapy; BT = brachytherapy.
Fig. 2.
Overall mortality by treatment type, adjusting for covariates in multivariate Cox proportional hazards models. EBRT = external-beam radiation therapy; BT = brachytherapy; RP = radical prostatectomy.
4. Discussion
In an effort to minimize the confounding due to underlying medical comorbidity, we evaluated mortality after PCa treatment in men with no documented medical comorbidity at the time of PCa treatment. In this patient cohort, PCM was infrequent. A Cox proportional hazards statistical analysis identified that EBRT was associated with an increase in PCM compared with RP, even when adjusting for differences in patient demographics and cancer severity. However, the association of EBRT with increased PCM was no longer statistically significant when evaluated with a competing risks model. This association, of similar magnitude with either model, raises speculation that the treatment efficacy of historical RT may have been diminished compared with surgery during the same time period; however, lack of a statistically significant effect does not allow a definitive conclusion. The lack of a difference in PCM with BT may be related to the use of BT in lower-risk patients and avoidance in high-risk patients, as it is known that mortality from low-risk PCa is a relatively infrequent occurrence, to the extent that some low-risk patients may not even warrant active treatment. In addition, the number of patients treated with BT was relatively lower, which is reflected in the wider confidence interval and lower certainty of the findings.
With respect to OM, both forms of RT were associated with an increase in OM compared with surgery. The finding of increased OM after RTs could potentially be due to treatment toxicity. Speculative reasons for increased mortality after irradiation include a clinically significant increased rate of secondary malignancy after irradiation [22,23] or secondary toxicity from androgen-deprivation therapy (ADT), which is used more commonly with irradiation than surgery. However, the link between androgen deprivation and cardiovascular toxicity remains controversial [24,25].
The fact that the differences in OM with radiation treatment modalities compared with surgery were more pronounced than in PCM has several possible explanations. PCM during our cohort was relatively low, so the small number of events limits our ability to evaluate the relationship. In addition, cause-of-death data from death records can be unreliable [26]. Therefore, patients whose recorded death was from other causes may have in fact died of PCa; however, it is not known if this error would differ based on treatment modality. We attempted to minimize this error by reviewing all available records and not solely depending on death certificate data. Our evaluation relied on retrospective observational data to compare the mortality outcomes of treatment options for localized PCa. The reliance on observational data is necessary because randomized controlled trials are lacking. Other groups have attempted to use observational data to compare treatment outcomes with varying methods to evaluate and control for comorbidity. Adbollah et al [14] used Surveillance Epidemiology and End Results (SEER) data and concluded that RP provided more favorable survival rates compared with RT with median follow-up of 4.3 yr. However, a limitation of SEER data is the lack of any information on medical comorbidity. Using the SEER cancer registry data linked to Medicare, which allowed attempted statistical adjustment for comorbidity but was limited to patients >65 yr, Abdollah [14] recently reported improved outcomes with surgery compared with irradiation for PCa, as had Liu et al (median follow-up was not reported) [27]. Another population-based evaluation in a Canadian province, which included attempted statistical control for medical comorbidity, also reported increased mortality after RT compared with surgery [28].
Kutikov et al recently reported on a nomogram using data from the Cancer of the Prostate Strategic Urologic Research Endeavor (CaPSURE) that reported on multivariate analysis that treatment with irradiation was associated with increased risk of PCa and non-PCa death [29]. Previously, Cooperberg et al had used data from CaPSURE and, after statistically adjusting for Charlson comorbidity, concluded that surgery was associated with a significant and substantial reduction in mortality relative to RT. Cooperberg et al reported that this finding was unlikely due to unmeasured confounding, as statistical adjustments and sensitivity analysis did not affect the results [15]. Our analysis differed, as we restricted our evaluation to men with no measured comorbidity in an attempt to minimize unmeasured confounding bias. In a two-institution evaluation of high-risk PCa, Boorjian et al [13] reported that PCM was similar for RP and EBRT (follow-up of 10.2 and 6–7.2 yr, respectively); however, OM was increased after EBRT if patients received ADT. In an effort to minimize confounding, Boorjian et al also reported that when restricting this analysis to the subset of patients with a Charlson comorbidity index of 0 or 1, this relationship was unchanged. Our analysis differed, as we attempted to include only healthy patients and excluded patients with Charlson comorbidity of 1, which can include myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, connective tissue disorder, ulcer disease, mild liver disease, and diabetes without end organ damage [17].
A primary concern in any evaluation comparing different treatment options for PCa is the possibility that unmeasured confounding differences exist between men who chose to receive irradiation compared with surgery. The RT group had higher stage, grade, and PSA and was older and more likely to be African American. It is likely that additional unmeasured variables exist between the two groups. We attempted to minimize the potential for confounding by restricting our analysis to men with no measured pretreatment medical comorbidity, which would be a presumptively healthy group, and statistically controlling for differences between treatment groups in age, race, PSA, biopsy Gleason grade, and clinical stage.
This nonrandomized observational evaluation has limitations. While the comorbidity indexes used in our study identified and excluded men with measured pretreatment medical comorbidities, there are other possible unmeasured treatment differences between groups. For instance, morbid obesity (body mass index ≥38) and hypertension are captured by the ACE-27 comorbidity index; however, these factors are not assessed in the Charlson comorbidity index. A history of smoking, hypercholesterolemia, or socioeconomic status are also not assessed by either comorbidity index and may have differed by treatment group. The possibility also exists that when suggesting treatment modality, clinicians may be able to identify differences in patients that are not accurately assessed by comorbidity indexes. Whether the impact of these unmeasured factors is influential enough to explain our findings remains unknown, and further research will be needed to elucidate whether and how much of a mortality difference exists in healthy patients undergoing treatment of PCa.
We restricted our analysis to men with no measured comorbidity, so the results of our analysis are best applied to that population and not generalized to all men with PCa. There is debate about who should be treated (with any modality) for PCa. Given that patients with no comorbidity have longer life expectancies, our analysis addresses the population most likely to benefit from treatment and therefore of greatest interest. In our study, median follow-up was >7 yr; further follow-up is necessary, however, as PCM can occur years after treatment.
The RT given was consistent with the standard of care at the time, but dose escalation has occurred over time, and the effect on mortality of contemporary doses of RT cannot be evaluated in this study. The use of ADT could not be controlled for in the statistical model because surgical patients did not receive ADT at the time of treatment. The administration of ADT in the context of EBRT was predicated on the standard of care at the time treatment was delivered [12]. More recently, the adoption of ADT in men with unfavorable-risk PCa has been reported to improve survival in patients treated with EBRT [30,31] and potentially could affect results in a more contemporary cohort. In addition, detailed information on the receipt of salvage therapies was not available for the entire cohort.
While our results suggested a survival benefit for prostatectomy in men with no measured comorbidity, patient choice remains important, as treatment choice incorporates not only available data on cure rates but also concerns about the adverse effects of primary treatment. Other factors such as quality of life, continence, and erectile function also affect treatment decisions [32,33] and were not evaluated in this report. Finally, our study did not include an active surveillance cohort, and active surveillance is an option for low-risk PCa [34].
5. Conclusions
In a large multicenter series of men without recorded comorbidity, both forms of RT were associated with an increase in OM compared with surgery. These findings may result from differences in cancer control, mortality related to primary or salvage therapy, or an imbalance of confounders.
Acknowledgments
Financial disclosures: Kenneth G. Nepple certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/ affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Kenneth G. Nepple was supported by a Washington University Comparative Effectiveness Research Mentored Career Development Award KM1 (NIH Grant Number 1KM1CA156708–01).
Funding/Support and role of the sponsor: This work was supported in part by the St. Louis Men’s Group Against Cancer and the David H. Nickerson Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions: Kenneth G. Nepple had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Nepple, Stephenson, Kibel.
Acquisition of data: Nepple, Stephenson, Haslag-Minoff, Kibel.
Analysis and interpretation of data: Nepple, Stephenson, Kallogjeri, Kibel.
Drafting of the manuscript: Nepple, Kibel.
Critical revision of the manuscript for important intellectual content: Nepple, Stephenson, Kallogjeri, Michalski, Grubb, Strope, Haslag-Minoff, Piccirilo, Ciezki, Klein, Reddy, Yu, Kattan, Kibel.
Statistical analysis: Nepple, Kallogjeri, Kibel.
Obtaining funding: Nepple, Kibel.
Administrative, technical, or material support: Kibel.
Supervision: Kibel.
Other (specify): None.
References
- 1.Konety BR, Cowan JE, Carroll PR. Patterns of primary and secondary therapy for prostate cancer in elderly men: analysis of data from CaPSURE. J Urol. 2008;179:1797–1803. doi: 10.1016/j.juro.2008.01.044. [DOI] [PubMed] [Google Scholar]
- 2.Houterman S, Janssen-Heijnen ML, Hendrikx AJ, van den Berg HA, Coebergh JW. Impact of comorbidity on treatment and prognosis of prostate cancer patients: a population-based study. Crit Rev Oncol Hematol. 2006;58:60–67. doi: 10.1016/j.critrevonc.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Guzzo TJ, Dluzniewski P, Orosco R, Platz EA, Partin AW, Han M. Prediction of mortality after radical prostatectomy by Charlson comorbidity index. Urology. 2010;76:553–557. doi: 10.1016/j.urology.2010.02.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albertsen PC, Moore DF, Shih W, Lin Y, Li H, Lu-Yao GL. Impact of comorbidity on survival among men with localized prostate cancer. J Clin Oncol. 2011;29:1335–1341. doi: 10.1200/JCO.2010.31.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nanda A, Chen MH, Braccioforte MH, Moran BJ, D’Amico AV. Hormonal therapy use for prostate cancer and mortality in men with coronary artery disease-induced congestive heart failure or myocardial infarction. JAMA. 2009;302:866–873. doi: 10.1001/jama.2009.1137. [DOI] [PubMed] [Google Scholar]
- 6.Groome PA, Rohland SL, Siemens DR, Brundage MD, Heaton J, Mackillop WJ. Assessing the impact of comorbid illnesses on death within 10 years in prostate cancer treatment candidates. Cancer. 2011;117:3943–3952. doi: 10.1002/cncr.25984. [DOI] [PubMed] [Google Scholar]
- 7.Froehner M, Koch R, Litz RJ, Hakenberg OW, Wirth MP. Which patients are at the highest risk of dying from competing causes ≤10 years after radical prostatectomy? BJU Int. 2012;110:206–210. doi: 10.1111/j.1464-410X.2011.10693.x. [DOI] [PubMed] [Google Scholar]
- 8.Daskivich TJ, Chamie K, Kwan L, et al. Overtreatment of men with low-risk prostate cancer and significant comorbidity. Cancer. 2011;117:2058–2066. doi: 10.1002/cncr.25751. [DOI] [PubMed] [Google Scholar]
- 9.Berglund A, Garmo H, Tishelman C, Holmberg L, Stattin P, Lambe M. Comorbidity, treatment and mortality: a population based cohort study of prostate cancer in PCBaSe Sweden. J Urol. 2011;185:833–839. doi: 10.1016/j.juro.2010.10.061. [DOI] [PubMed] [Google Scholar]
- 10.Abdollah F, Sun M, Thuret R, et al. A competing-risks analysis of survival after alternative treatment modalities for prostate cancer patients: 1988–2006. Eur Urol. 2011;59:88–95. doi: 10.1016/j.eururo.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Hadley J, Yabroff KR, Barrett MJ, Penson DF, Saigal CS, Potosky AL. Comparative effectiveness of prostate cancer treatments: evaluating statistical adjustments for confounding in observational data. J Natl Cancer Inst. 2010;102:1780–1793. doi: 10.1093/jnci/djq393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kibel AS, Ciezki JP, Klein EA, et al. Survival among men with clinically localized prostate cancer treated with radical prostatectomy or radiation therapy in the prostate specific antigen era. J Urol. 2012;187:1259–1265. doi: 10.1016/j.juro.2011.11.084. [DOI] [PubMed] [Google Scholar]
- 13.Boorjian SA, Karnes RJ, Viterbo R, et al. Long-term survival after radical prostatectomy versus external-beam radiotherapy for patients with high-risk prostate cancer. Cancer. 2011;117:2883–2891. doi: 10.1002/cncr.25900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdollah F, Schmitges J, Sun M, et al. Comparison of mortality outcomes after radical prostatectomy versus radiotherapy in patients with localized prostate cancer: a population-based analysis. Int J Urol. 2012;19:836–844. doi: 10.1111/j.1442-2042.2012.03052.x. [DOI] [PubMed] [Google Scholar]
- 15.Cooperberg MR, Vickers AJ, Broering JM, Carroll PR. Comparative risk-adjusted mortality outcomes after primary surgery, radiotherapy, or androgen-deprivation therapy for localized prostate cancer. Cancer. 2010;116:5226–5234. doi: 10.1002/cncr.25456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Psaty BM, Siscovick DS. Minimizing bias due to confounding by indication in comparative effectiveness research: the importance of restriction. JAMA. 2010;305:897–898. doi: 10.1001/jama.2010.1205. [DOI] [PubMed] [Google Scholar]
- 17.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 18.Piccirillo JF, Tierney RM, Costas I, Grove L, Spitznagel EL., Jr Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA. 2004;291:2441–2447. doi: 10.1001/jama.291.20.2441. [DOI] [PubMed] [Google Scholar]
- 19.Hines RB, Chatla C, Bumpers HL, et al. Predictive capacity of three comorbidity indices in estimating mortality after surgery for colon cancer. J Clin Oncol. 2009;27:4339–4345. doi: 10.1200/JCO.2009.22.4758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mangano DT, Tudor IC, Dietzel C. The risk associated with aprotinin in cardiac surgery. N Engl J Med. 2006;354:353–365. doi: 10.1056/NEJMoa051379. [DOI] [PubMed] [Google Scholar]
- 21.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 22.Bhojani N, Capitanio U, Suardi N, et al. The rate of secondary malignancies after radical prostatectomy versus external beam radiation therapy for localized prostate cancer: a population-based study on 17,845 patients. Int J Radiat Oncol Biol Phys. 2010;76:342–348. doi: 10.1016/j.ijrobp.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 23.Hyde C. Rate of secondary malignancies after radical prostatectomy versus external beam radiation therapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2010;77:1607. doi: 10.1016/j.ijrobp.2010.03.025. [DOI] [PubMed] [Google Scholar]
- 24.Punnen S, Cooperberg MR, Sadetsky N, Carroll PR. Androgen deprivation therapy and cardiovascular risk. J Clin Oncol. 2011;29:3510–3516. doi: 10.1200/JCO.2011.35.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nguyen PL, Je Y, Schutz FA, et al. Association of androgen deprivation therapy with cardiovascular death in patients with prostate cancer: a meta-analysis of randomized trials. JAMA. 2011;306:2359–2366. doi: 10.1001/jama.2011.1745. [DOI] [PubMed] [Google Scholar]
- 26.Hoffman RM, Stone SN, Hunt WC, Key CR, Gilliland FD. Effects of misattribution in assigning cause of death on prostate cancer mortality rates. Ann Epidemiol. 2003;13:450–454. doi: 10.1016/s1047-2797(02)00439-8. [DOI] [PubMed] [Google Scholar]
- 27.Liu L, Coker AL, Du XL, Cormier JN, Ford CE, Fang S. Long-term survival after radical prostatectomy compared to other treatments in older men with local/regional prostate cancer. J Surg Oncol. 2008;97:583–591. doi: 10.1002/jso.21028. [DOI] [PubMed] [Google Scholar]
- 28.Jeldres C, Suardi N, Perrotte P, et al. Survival after radical prostatectomy and radiotherapy for prostate cancer: a population-based study. Can Urol Assoc J. 2009;3:13–21. [PMC free article] [PubMed] [Google Scholar]
- 29.Kutikov A, Cooperberg MR, Paciorek AT, Uzzo RG, Carroll PR, Boorjian SA. Evaluating prostate cancer mortality and competing risks of death in patients with localized prostate cancer using a comprehensive nomogram. Prostate Cancer Prostatic Dis. 2012;15:374–379. doi: 10.1038/pcan.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.D’Amico AV, Chen MH, Renshaw AA, Loffredo M, Kantoff PW. Androgen suppression and radiation vs radiation alone for prostate cancer: a randomized trial. JAMA. 2008;299:289–295. doi: 10.1001/jama.299.3.289. [DOI] [PubMed] [Google Scholar]
- 31.Bolla M, de Reijke TM, Van Tienhoven G, et al. Duration of androgen suppression in the treatment of prostate cancer. N Engl J Med. 2009;360:2516–2527. doi: 10.1056/NEJMoa0810095. [DOI] [PubMed] [Google Scholar]
- 32.Gore JL, Kwan L, Lee SP, Reiter RE, Litwin MS. Survivorship beyond convalescence: 48-month quality-of-life outcomes after treatment for localized prostate cancer. J Natl Cancer Inst. 2009;101:888–892. doi: 10.1093/jnci/djp114. [DOI] [PubMed] [Google Scholar]
- 33.Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358:1250–1261. doi: 10.1056/NEJMoa074311. [DOI] [PubMed] [Google Scholar]
- 34.Stattin P, Holmberg E, Johansson JE, Holmberg L, Adolfsson J, Hugosson J. Outcomes in localized prostate cancer: National Prostate Cancer Register of Sweden follow-up study. J Natl Cancer Inst. 2010;102:950–958. doi: 10.1093/jnci/djq154. [DOI] [PMC free article] [PubMed] [Google Scholar]