Abstract
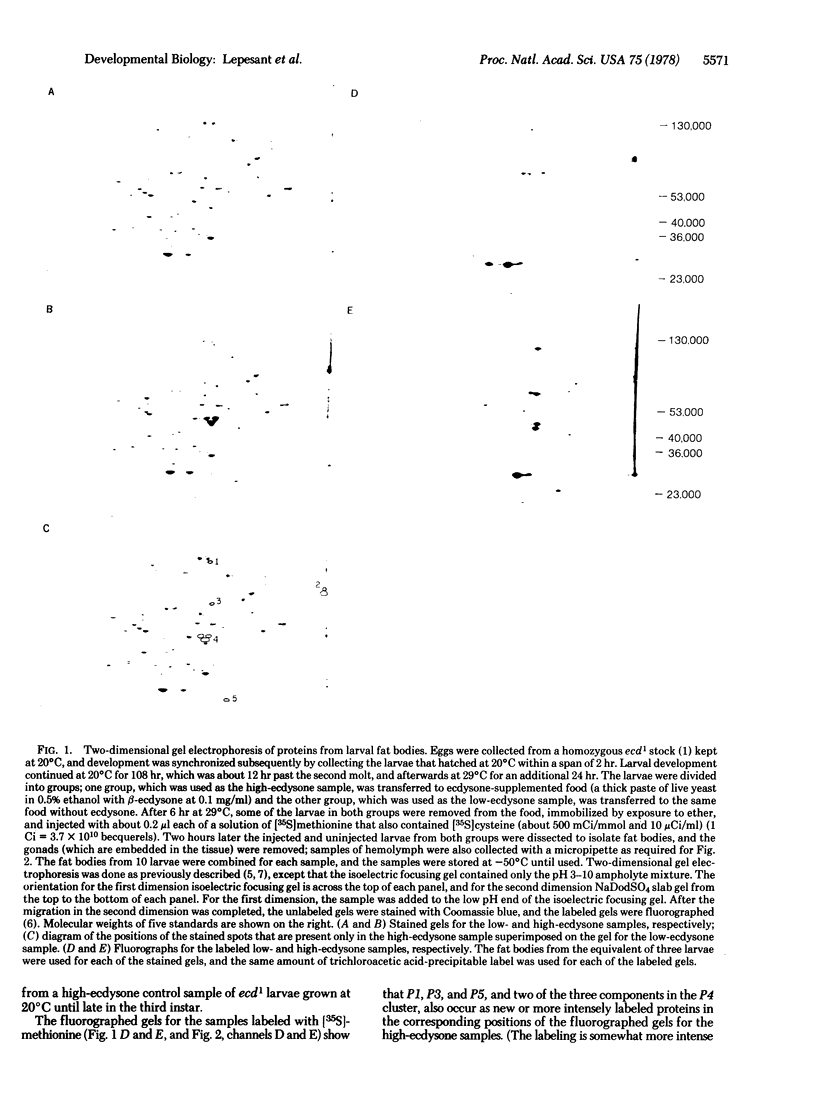
Late in the third instar larval stage of Drosophila melanogaster, the titer of the steroid hormone ecdysone increases sharply. This increase is blocked in the temperature-sensitive mutant ecd1 after a temperature shift from 20°C to 29°C. The mutant was used to prepare three samples of late third instar larvae with different titers of ecdysone; the titer was low in one sample because of an earlier temperature shift, high in a second sample because the larvae were subsequently transferred to ecdysone-supplemented food, and also high in a third sample that was kept at 20°C, providing a control for normal development. The effect of the high titer of ecdysone on proteins of the larval fat bodies was examined by comparing two-dimensional gel electrophoresis patterns of total proteins in stained gels. There were proteins at five positions in the gels for the high-ecdysone samples that were not detected at the corresponding positions in the gel for the low-ecdysone sample. The effect of ecdysone on these proteins was further studied by injecting [35S]methionine into the larvae at both early and late third instar stages, in order to label proteins synthesized before and after the increase in ecdysone titer. The results indicate that ecdysone induces two major responses in the fat bodies; certain proteins that were synthesized earlier in the fat bodies and secreted into the hemolymph are incorporated back into the fat bodies, and other proteins are newly synthesized. Attempts to induce prematurely the synthesis of the new proteins by exposing early third instar larvae to exogenous ecdysone were unsuccessful, suggesting that development must proceed further before the fat bodies can respond to ecdysone.
By in vitro translation of RNA isolated from fat bodies of low-and high-ecdysone samples of larvae, it was shown that ecdysone greatly increases the amount of translatable messenger RNA for one of the newly synthesized proteins. A clone of DNA complementary to the induced messenger RNA has been isolated from a population of λ bacteriophage carrying segments of the Drosophila genome. Using the cloned DNA to measure amounts of complementary poly(A)-RNA in the fat bodies by DNA·RNA hybridization, we detected about 50 times more complementary poly(A)-RNA in the high-ecdysone sample of larvae than in the low-ecdysone sample. This finding provides direct evidence that ecdysone induces an increase in the amount of the messenger RNA. The ecdysone-induced appearance of a major messenger RNA in late third instar larval fat bodies represents a developmental response to ecdysone that appears to be gene-specific, tissue-specific, and stage-specific, and it has exceptionally favorable features for further molecular studies of the control of gene expression by a steroid hormone.
Keywords: hormonal control of gene expression during development
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Garen A., Kauvar L., Lepesant J. A. Roles of ecdysone in Drosophila development. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5099–5103. doi: 10.1073/pnas.74.11.5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastie N. D., Held W. A. Analysis of mRNA populations by cDNA.mRNA hybrid-mediated inhibition of cell-free protein synthesis. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1217–1221. doi: 10.1073/pnas.75.3.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgetts R. B., Sage B., O'Connor J. D. Ecdysone titers during postembryonic development of Drosophila melanogaster. Dev Biol. 1977 Oct 1;60(1):310–317. doi: 10.1016/0012-1606(77)90128-2. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Roberts D. B., Wolfe J., Akam M. E. The developmental profiles of two major haemolymph proteins from Drosophila melanogaster. J Insect Physiol. 1977;23(7):871–878. doi: 10.1016/0022-1910(77)90013-0. [DOI] [PubMed] [Google Scholar]
- Thomasson W. A., Mitchell H. K. Hormonal control of protein granule accumulation in fat bodies of Drosophila melanogaster larvae. J Insect Physiol. 1972 Oct;18(10):1885–1899. doi: 10.1016/0022-1910(72)90159-x. [DOI] [PubMed] [Google Scholar]