Abstract
Objectives
Restorative yoga (RY) is a gentle type of yoga that may be beneficial for cancer patients and post-treatment survivors. Study goals were: to determine the feasibility of implementing a RY intervention for women with breast cancer; and to examine group differences in self-reported emotional, health-related quality of life, and symptom outcomes.
Methods
Women with breast cancer (n = 44; mean age 55.8 years) enrolled in this study; 34% were actively undergoing cancer treatment. Study participants were randomized to the intervention (10 weekly 75-minute RY classes) or a waitlist control group. Participants completed questionnaires at Week 0 (baseline) and Week 10 (immediately post-intervention for the yoga group).
Results
Group differences favoring the yoga group were seen for mental health, depression, positive affect, and spirituality (peace/meaning). Significant baseline * group interactions were observed for negative affect and emotional well-being. Women with higher negative affect and lower emotional well-being at baseline derived greater benefit from the yoga intervention compared to those with similar values at baseline in the control group. The yoga group demonstrated a significant within-group improvement in fatigue; no significant difference was noted for the control group.
Conclusions
Although limited by sample size, these pilot data suggest potential benefit of RY on emotional outcomes and fatigue in cancer patients. This study demonstrates that a RY intervention is feasible for women with breast cancer; implications for study design and implementation are noted with an emphasis on program adoption and participant adherence.
Keywords: yoga, mind-body, cancer, oncology, well-being
Introduction
Rates of complementary and alternative medicine (CAM) usage in women with breast cancer are high (70%–80%) [1–3]. Mind-body therapies, one type of CAM, are defined by the National Center for Complementary and Alternative Medicine as ‘interventions designed to facilitate the mind’s capacity to affect bodily functions and symptoms’ (http://nccam.nih.gov/health/whatiscam/). Meditation, imagery, and yoga are the most commonly used mind-body therapies [4,5]. Evidence from systematic reviews and meta-analyses of randomized trials is strong that mind-body therapies improve mood, quality of life, treatment-related side effects and sleep in cancer patients [6,7].
Yoga interventions have been studied both with healthy individuals and those with a variety of health conditions [8]. Benefits of yoga include increased muscular strength, flexibility, range of motion, energy, relaxation, and sense of well-being, decreased pain, improved sleep quality, stress reduction, and control over physiological parameters [9–13]. Reports of yoga for patients with cancer suggest physical and psychological benefits [14–17]. In our previous nonrandomized pilot study of Restorative Yoga (RY hereafter) in 51 women with ovarian or breast cancer, significant improvements were seen for fatigue, depression and negative affect; further, the women with breast cancer reported improved health-related quality of life [18]. Additional recent studies of yoga in persons with cancer (primarily breast cancer) suggest enhanced health-related quality of life and decreased depression, anxiety, distress, symptoms, and sleep difficulties [19–23].
Each of the previous studies of yoga for persons with cancer used a different type of yoga. For our study, yoga classes were based on the Integral Yoga tradition and used RY postures. Integral Yoga includes postures, deep relaxation, breathing practices and meditation to create a profound experience of peace and well-being. Integral Yoga has offered a longstanding yoga teacher training for persons with cancer (http://www.integralyoga-programs.org/). Further, while the physical postures of Integral Yoga are an important element, there is equal emphasis on breathing and awareness. Participants are urged to work with their bodies, notice how they feel in each posture, and respect the body’s desire to go deeper into a pose or back out of it. Specific yoga postures were drawn from RY, which has been described as ‘active relaxation.’ It is a gentle form of yoga that consists of poses supported by props, with emphasis on breathing and relaxation [24]. RY poses can be practiced when a patient is ill or recovering from illness or surgery [16,24]. Props provide a completely supportive environment for total relaxation with minimal physical effort.
The goals of this pilot study were: to determine the feasibility of a RY intervention as supportive therapy for women diagnosed with breast cancer; and to measure changes in fatigue, sleep, psychological distress (depression, negative affect) and well-being (positive affect), and health-related quality of life as compared to a randomized control group. Feasibility was assessed by examining attendance, drop-out rates, adverse events, and program satisfaction.
Methods
Study design
This was a pilot/feasibility intervention study with pre and post measures. Women were randomized to the RY group or a waitlist control group. This study was approved by the Institutional Review Board at Wake Forest University Health Sciences.
Eligibility criteria
Women were eligible if they were: (1) ≥18 years of age; (2) diagnosed with breast cancer (any stage); (3) 2 to 24 months post-primary treatment (surgery) following initial diagnosis and/or had a recurrence of breast cancer within the past 24 months (regardless of treatment status); (4) physically able to attend RY classes; (5) able to understand English; and (6) free of medical contraindications reported by their physician. The window of eligibility was intentionally broad to determine when women with breast cancer were most likely to participate.
Procedure
Recruitment
Study participants were identified by physicians from the Breast Care Center in the Comprehensive Cancer Center of Wake Forest University. Participants were recruited via mailed letters signed by each patient’s oncologist or surgeon, along with detailed study information and a consent form. The study was advertised in newsletters through local agencies that serve women with breast cancer. Women who self-referred were asked to discuss their participation with their physician. A Research Assistant made a follow-up call to determine interest and eligibility, using a standardized script.
Data collection
Questionnaires were mailed to participants to complete and return in a postage-paid, pre-addressed envelope. If needed, the Research Assistant followed up with participants by telephone to clarify questions and obtain missed responses. All baseline questionnaires were completed at Week 0. Follow-up questionnaires (excluding demographic information) were administered in the same manner at Week 10 (within one week of intervention completion for those in the RY group). If the form was not returned within 2 weeks, the Research Assistant followed up by telephone to request the completed form and answer any questions. An incentive ($20 bookstore gift card) was offered for completing all questionnaires. Clinical data were obtained from chart reviews conducted by a research nurse.
Intervention
The intervention consisted of 10 weekly 75-minute RY classes [24] taught by a yoga instructor with cancer-specific yoga training who was registered by the National Yoga Alliance. The instructor is a cancer survivor; this information was disclosed in the recruitment packet. Classes were conducted at a local studio in a closed-group format. The average number of women in each session was 6.6 (range 3–12). No home yoga practice was required, and no home practice information was provided. Once women completed the session, they could take yoga classes on their own.
The classes combined physical postures (asanas), breathing (pranayama), and deep relaxation (****sava-sana). One guiding principle in yoga practice, ‘ahimsa’ (non-violence), was emphasized. This principle reinforces the notion of being gentle to oneself; it was made clear that participants should not practice any pose that caused or exacerbated discomfort. Yoga poses were modified based on participant needs. Individual poses were held from 20 seconds to 5 minutes, depending on the pose. The following poses (with approximate time for each) were practiced in all classes: (1) centering and meditation (conscious, deep breathing, mental inventory of body, energy, thoughts, and emotions) (15 minutes); (2) neck and shoulder series (move neck through range of motion, turning head side to side, dropping ear to shoulder, chin to chest and eyes toward ceiling, roll shoulders forward and back, then squeeze shoulders to ears and release) (5 minutes); (3) leg stretch (Janu Sirsasana variation) using a strap and circling ankles slowly in both directions (5 minutes); (4) side bend (seated Parighasana) (2 minutes); (5) seated twist (Ardha Matsyandrasana variation) (2 minutes); (6) simple supported backbend (1–5 minutes); (7) transition (resting pose to shift into another posture); (8) legs up the wall (Viparita Karani or variation) (5 minutes); and (9) supported bound-angle pose (Supta Badha Konasana variation) (5 minutes). The first five poses were done from a chair or floor mat (depending on participant’s ability), and the remaining poses were done on a floor mat. The following poses were used as time/mobility allowed: (1) mountain pose; (2) arm and shoulder stretch; (3) supported forward fold; (4) seated sun salutation (Surya Namaskar variation); and (5) reclining twist with a bolster. In all poses, the teacher helped participants adjust the props until the pose became comfortable. Participants were reminded to notice the breath and to breathe slowly and deeply.
Control group
The waitlist control group completed questionnaires at the same intervals as women in the RY group. Once their questionnaires were complete, they were offered 10 weekly 75-minute RY classes identical to the intervention group.
Measures
Demographic and clinical information
The following demographic information was collected at baseline on all study participants: age, race/ethnicity, marital/partner status, educational history, and income. The following clinical information was obtained from the patient’s medical record: diagnosis, date of diagnosis, stage of disease, previous cancer history, and prescribed treatment regimen (surgery, chemotherapy, radiation).
SF-12 health survey (SF-12) [25]
The SF-12 is an abbreviated measure of physical health status developed from the Medical Outcomes Study. It is a 12-item self-report measure of perceived health and functioning that yields summary measures of physical (PCS) and mental health (MCS). There are general population norms for this measure.
Functional Assessment of Cancer Therapy—Breast (FACT-B) [26]
Health-related quality of life was measured by the Functional Assessment of Cancer Therapy—Breast scale (FACT-B); it consists of the following subscales: physical well-being (PWB), social/family well-being (SWB), emotional well-being (EWB), functional well-being (FWB), and breast cancer-specific concerns. Total FACT-B score is calculated by summing these scores. Women rated how problematic each item had been for them during the past 7 days on a 5-point scale from ‘not at all’ problematic to ‘very much’ a problem. Overall scores range from 0–144 where a higher score indicates better health-related quality of life. This measure has established reliability and validity.
FACT-Fatigue [27,28]
The FACT-Fatigue is a 13-item instrument developed to assess fatigue in people with cancer. Responses are made on a 5-point Likert scale ranging from 0 (not at all) to 4 (very much so) and summed to yield a total score. This brief measure demonstrates excellent internal consistency (Cronbach’s α = 0.93 to 0.95) and test-retest reliability (r = 0.90). Higher scores indicate lower fatigue levels.
FACIT-Spirituality (FACIT-Sp) [29]
The 12-item FACIT-Sp spirituality scale assesses spiritual well-being. It was developed with an ethnically diverse cancer patient population and has strong psychometric properties (Cronbach’s α = 0.87). This scale taps both traditional religiosity and spirituality dimensions without assuming a belief in God. The FACIT-Sp has two domains, sense of meaning/peace (8 items) and role of faith (4 items). Responses are made on a 5-point Likert scale ranging from 0 (not at all) to 4 (very much) and are summed for total and domain scores. Higher scores indicate higher levels of spirituality. Only the sense of meaning/peace subscale was included in study analyses.
Center for Epidemiologic studies–depression scale (CES-D) [30]
The CES-D is a 20-item self-report measure developed to screen for depressive symptoms. Items are rated on a 4-point scale (0 = rarely or none of the time to 3 = most or all the time), and the total score ranges from 0 to 60. The measure has excellent reliability and validity in community and cancer patient samples [30,31]. Higher scores indicate greater risk of depression, with scores ≥16 indicating potentially significant levels of depression [30].
Pittsburgh sleep quality inventory (PSQI) [32]
The PSQI is a 19-item self-report measure to assess subjective sleep quality in the past month. It yields a total global sleep score and seven subscales: subjective sleep quality, sleep latency, sleep duration, sleep efficiency, sleep disturbances, use of sleep medications, and daytime dysfunction. The PSQI has been further validated with cancer patients and demonstrated excellent construct validity and internal reliability, with Cronbach’s α = 0.80 [33].
Positive & negative affect schedule (PANAS) [34]
The PANAS is a 20-item measure to assess positive and negative affect. Respondents describe their affect over the past one-week period using a 5-point scale with responses ranging from ‘very slightly or not at all’ to ‘extremely.’ The PANAS alpha internal consistency reliabilities are high, ranging from 0.86–0.90 for positive affect and from 0.84–0.87 for negative affect [34].
Program evaluation
Following the intervention, the RY group completed a self-report questionnaire designed to elicit feedback about the RY program. They rated quality of the classes and instructor on a scale from 0 (lowest) to 4 (highest).
Feasibility
Feasibility was assessed by examining recruitment, attendance and drop-out rates, adverse events, and program satisfaction. The yoga teacher reported attendance to the Research Assistant, who recorded this information weekly.
Statistical analysis
Mean scores and standard deviations were computed for all self-report measures. Data from all participants were analyzed on an intent-to-treat basis. Analysis of covariance (ANCOVA) mixed models were used to examine differences in post-intervention scores from baseline, adjusting for baseline value of each outcome variable. Models initially tested for significant interactions between groups and baseline value of the outcome variable. If the interaction was significant, no more testing was done; the significant interaction shows that the effect of the treatment is (on average) different between groups, and that the effect differs across baseline values. If the interaction was not significant, it was removed and the model was re-run to obtain the p-value for the test of group differences. We also tested whether significant interactions or group differences differed by treatment status during the study (receipt of chemotherapy or radiation therapy). Additionally, in separate ANCOVA analyses, the relationship between yoga class attendance and each measure was assessed by including class attendance as a continuous covariate. All outcome measures were conceptualized as separate trials that were analyzed separately, with a p-value ≤0.05 indicating statistical significance (measures with p-values ≤0.10 but >0.05 are noted, as a trend towards significance may exist). SAS (version 9.1, SAS Institute, Cary, NC) was used for analyses, with PROC MIXED fitting regression models. MIXED fits random and fixed effects in modeling the repeated, correlated measures and does not delete participants with missing outcome data.
Results
Recruitment
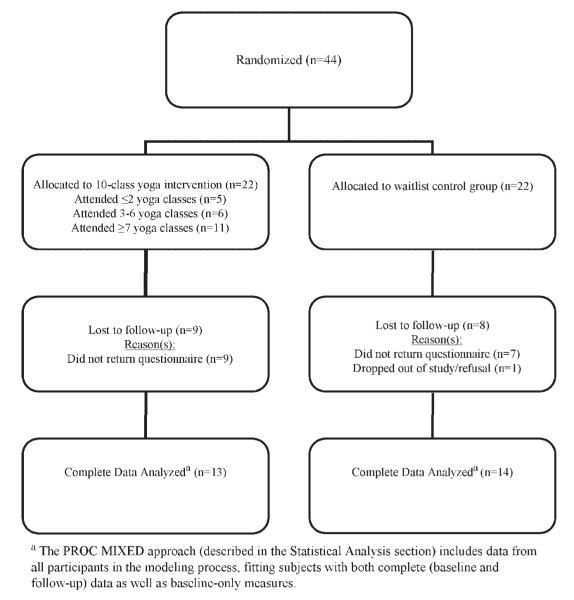
All participants were recruited between August 2005 and October 2006. We achieved a 19% recruitment rate (44 participants from 229 recruitment letters). Reasons for non-participation were not tracked initially; however, this information was systematically collected starting midway through the study. For participants who had recruitment systematically tracked, 29 participants enrolled from 109 recruitment letters (27% recruitment rate). Reasons for nonparticipation included: (1) distance (n = 24; 30%); (2) no response to recruitment letter/telephone call (n = 30; 37.5%); (3) not interested (n = 9; 11.3%); (4) health issues (too sick/not feeling well) (n = 9; 11.3%); (5) time of yoga classes not workable (n = 5; 6.3%); (6) too busy (n = 2; 2.5%); and (7) transportation issues (n = 1; 1.3%). One additional woman was not eligible since she did not speak English. The flow of participants through the study is depicted in Figure 1.
Figure 1.
Flow of participants through study
Sample description
No significant between-group baseline differences in demographic, clinical, or yoga-related data were seen (Table 1). Mean participant age was 55.8 (SD = 9.9) years, and most were White (88.6%), married/partnered (63.6%), and well-educated. Women had minimal experience with yoga. Of the 9 women who had practiced yoga in the past 12 months, 3 had practiced regularly (≥2 times per month).
Table 1.
Demographic, clinical, and yoga-related characteristics of sample at baseline (N = 44)
| Characteristics | Yoga participants (n = 22) |
Control group (n = 22) |
|---|---|---|
| % (N) | % (N) | |
| Age [Mean (SD)] | 54.3 (9.6) | 57.2 (10.2) |
| Range | (38–76) | (41–79) |
| Race/ethnicity | ||
| Non-Hispanic White | 86.4 (19) | 90.9 (20) |
| African American | 9.1 (2) | 4.6 (1) |
| Asian/Pacific Islander | 4.6 (1) | 4.6 (1) |
| Marital Status | ||
| Married/Partnered | 54.6 (12) | 72.7 (16) |
| Never Married | 9.1 (2) | 0.0 (0) |
| Divorced/Separated | 27.3 (6) | 18.2 (4) |
| Widowed | 9.1 (2) | 9.1 (2) |
| Years of education: | ||
| H.S. Diploma/GED | 0.0 (0) | 13.6 (3) |
| Some college or vocational school |
27.3 (6) | 36.4 (8) |
| College graduate | 22.7 (5) | 13.6 (3) |
| Graduate study or degree | 50.0 (11) | 36.4 (8) |
| Income (total annual):a | ||
| <$35,000 | 30.0 (6) | 27.8 (5) |
| $35,000–$49,999 | 15.0 (3) | 5.6 (1) |
| $50,000–$99,999 | 40.0 (8) | 27.8 (5) |
| $100,000+ | 15.0 (3) | 33.3 (6) |
| Breast Cancer Stage | ||
| DCIS | 13.6 (3) | 22.7 (5) |
| Stage 1 | 22.7 (5) | 40.9 (9) |
| Stage 2 | 45.5 (10) | 13.6 (3) |
| Stage 3 | 13.6 (3) | 9.1 (2) |
| Stage 4 | 4.6 (1) | 13.6 (3) |
| Received chemotherapy during study |
36.4 (8) | 13.6 (3) |
| Received radiation therapy during study |
27.3 (6) | 13.6 (3) |
| Time since diagnosis (months) |
||
| Mean (SD) | 24.4 (39.5) | 22.8 (35.6) |
| Time since recurrence (if applicable) (months) |
||
| Mean (SD) | 7.0 (3.4) (n = 4) | 8.1 (5.5) (n = 6) |
| Mean (SD) number of yoga classes attended |
5.7 (3.4) | NA |
| ≤2 classes | 22.7 (5) | NA |
| 3–6 classes | 27.3 (6) | NA |
| ≥7 classes | 50.0 (11) | NA |
| Never had done yoga before |
90.9 (20) | 68.2 (15) |
| No yoga experience in the past year |
90.9 (20) | 81.8 (18) |
Reported family income had some missing values, so total N is less than 44.
Mental health and health-related quality of life
Mean scores (with standard deviations) and significance statistics (for baseline by group interaction and group main effects) for mental health, health-related quality of life, physical health, fatigue, and sleep are shown in Table 2. Significant baseline **** group interactions were observed for PANAS-NA (negative affect) ( p = 0.014) and the FACT EWB subscale ( p = 0.042). These interactions showed that women who started out with higher negative affect and lower emotional well-being derived greater benefit from participation in the yoga intervention compared to those with similar baseline levels in the control group. For PANAS-NA, the effect is seen at a baseline score ≥15. For the FACT EWB subscale, those with a baseline score ≤20 derived greater benefit from the yoga intervention than controls. We found significant group effects for the SF-12 MCS (p = 0.004), the CES-D ( p = 0.026), the PANAS-PA ( p = 0.01), and the FACIT-Sp peace/meaning subscale ( p = 0.0009) favoring the yoga group versus controls. Health-related quality of life (FACT-B) showed a borderline difference between the two groups ( p = 0.052).
Table 2.
Observed means, standard deviation, & ANCOVA analyses of baseline by group interaction and group effects between yoga and control groups
| Variable | Yoga |
Control |
BL*group interaction p-value |
Overall p-value |
||
|---|---|---|---|---|---|---|
| Baseline mean (SD) n = 22 |
Week 10 mean (SD) n = 13 |
Baseline mean (SD) n = 22 |
Week 10 mean (SD) n = 14 |
|||
| Mental Health & Health-Related Quality of Life | ||||||
| SF-12 MCS | 43.4 (10.1) | 52.2 (6.6) | 49.9 (10.2) | 47.5 (13.8) | 0.25 | 0.004 |
| CES-D | 16.3 (9.7) | 8.1 (8.9) | 16.6 (14.7) | 17.8 (16.9) | 0.14 | 0.026 |
| PANAS (negative affect) | 19.0 (8.3) | 14.0 (3.9) | 18.5 (9.4) | 19.9 (9.8) | 0.014 | – |
| PANAS (positive affect) | 32.7 (8.8) | 38.2 (6.8) | 30.6 (9.8) | 31.8 (10.8) | 0.07 | 0.01 |
| FACIT Sp (peace/meaning) | 23.3 (6.6) | 26.0 (6.7) | 23.2 (7.2) | 21.5 (9.4) | 0.31 | 0.0009 |
| FACT-B | 104.9 (19.9) | 114.8 (19.1) | 101.1 (24.4) | 98.4 (31.8) | 0.21 | 0.052 |
| FACT Social | 23.3 (4.7) | 23.1 (5.0) | 21.4 (5.4) | 20.4 (6.8) | 0.16 | 0.26 |
| FACT Functional | 18.7 (5.7) | 21.9 (4.7) | 18.0 (6.9) | 17.4 (7.5) | 0.97 | 0.14 |
| FACT Emotional | 18.1 (3.9) | 20.8 (3.2) | 18.5 (5.2) | 18.2 (6.1) | 0.042 | – |
| FACT Physical | 19.7 (7.0) | 22.5 (7.6) | 20.7 (5.1) | 21.1 (5.7) | 0.35 | 0.86 |
| Physical Health, Fatigue, & Sleep | ||||||
| SF-12 PCS | 42.7 (12.1) | 44.8 (12.4) | 40.6 (10.1) | 42.7 (11.8) | 0.73 | 0.25 |
| FACT-Fatigue | 30.1 (13.4) | 39.8 (11.5) | 32.7 (11.8) | 32.6 (15.5) | 0.95 | 0.23 |
| PSQI-Global | 8.3 (4.7) | 6.1 (4.3) | 8.6 (5.3) | 7.0 (4.2) | 0.34 | 0.97 |
All p-values are based on analyses adjusted for the baseline value of each variable.
Physical health, fatigue, & sleep
There was a trend toward a group main effect ( p = 0.078) for the PSQI sleep latency subscale; the yoga group reported a decrease in time to fall asleep, while values in the control group remained stable. There was a trend toward a baseline * group interaction for the use of sleep medications ( p = 0.10). No significant baseline * group interactions or group main effects were observed for the SF-12 PCS, FACT-Fatigue, or PSQI global score or subscales (subscale results not shown in Table 2).
Intervention adherence
Mean number of RY classes attended was 5.8 (SD = 3.4); 3 women attended 0 classes and 2 women attended all 10 classes. To test for the effect of adherence on outcome measures, an analysis of covariance was fit for each score, adjusting for baseline score and number of classes attended (fit as a continuous covariate). For the SF-12 PCS, the relationship between number of classes attended and PCS showed statistical significance ( p = 0.02); on average, for each class attended, the subject’s PCS increased by 2.5 units during the study. For the FACT-B and two of its subscales (PWB, FWB), the relationship between number of classes attended and each of these variables was statistically significant (FACT-B p = 0.04; PWB p = 0.003; FWB p = 0.01). Mean FACT-B, PWB, and FWB scores increased by 2.1 points, 1.1 points, and 0.7 points, respectively, over the study for each class attended. For the PSQI, sleep disturbance had an interaction ( p = 0.0287) with the baseline level observed, indicating that class attendance was more beneficial for those who entered the study with higher sleep disturbance scores. There was a similar interaction for use of sleep medication ( p = 0.04); those with higher use of sleep medications reported greater benefit from increased class attendance.
We examined further whether frequency of class attendance (0–6 classes versus ≥7 classes) differed at baseline on several potentially important variables (health-related quality of life, fatigue, and depression). Such differences might indicate potential difficulty with adherence. Mean baseline depression (CES-D) scores were comparable between these groups: 16.8 (SD = 9.7) for women who attended 0–6 classes and 15.7 (SD = 10.1) for women who attended ≥7 classes. Although not statistically significant, scores for health-related quality of life and fatigue showed clinically significant differences. Mean baseline FACT-B scores were 99.5 (SD = 24.2) for women who attended 0–6 classes and 109.7 (SD = 14.6) for women who attended ≥7 classes. Mean baseline fatigue (FACT-Fatigue) scores were 26.8 (SD = 15.5) for women who attended 0-6 classes and 33.5 (SD = 10.5) for women who attended ≥7 classes.
Program evaluation ratings and adverse events
Feedback provided by the yoga group at Week 10 was extremely positive; 92% of women reported that they liked the RY class ‘quite a bit’ or ‘very much.’ Mean ratings (SD) (possible range 0–4 for ‘not at all’ to ‘very much’) for various feedback items were as follows: teacher is competent 3.8 (0.4); teacher made class enjoyable 3.7 (0.8); liked RY classes 3.7 (0.6); found RY classes helpful 3.2 (1.0); and will continue to practice RY 2.5 (1.1). No adverse events were reported.
Discussion
This study examined the feasibility of a RY intervention for women with breast cancer. Collectively, our findings echo the existing literature indicating that women diagnosed with breast cancer are interested in mind-body therapies, specifically RY. Further, participants can successfully engage in a 10-week RY program without adverse effects and report satisfaction and enjoyment in the activity. However, it is important to note inherent difficulties with regard to recruitment and adherence rates. The two primary reasons for nonparticipation were distance from the intervention site and lack of response to recruitment letter or phone call. Recruitment was conducted at a large comprehensive cancer center that draws women from long distances and may partially explain why recruitment was an obstacle for this study. Given the variety of demands that women face while undergoing cancer treatment, the transition to ‘routine care’ following treatment may be another optimal time for intervention.
Our findings suggest that better intervention adherence (class attendance) was associated with higher self-reported physical health and health-related quality of life (particularly physical well-being and functional well-being). Adherence in this study was comparable to other studies of yoga in individuals with cancer that reported adherence data [19,23]. An earlier study of yoga in women with breast cancer suggested that better intervention adherence was associated with significantly improved fatigue, physical well-being, and distress [23]. Adherence is dynamic and complex; interventions designed to enhance adherence require a theoretical framework including a motivational component [35]. The integration of potential mediators into theory-based interventions would allow a true examination of treatment efficacy. At the individual level, adherence might be enhanced by having classes at varied times, make-up classes, or rolling entry, and by contacting participants immediately after missed classes to determine why they were missed. Incorporating participant perceptions about the program, as well as about their cancer and related concerns, could further impact participation.
Although not statistically significant, differences between attendance groups in baseline health-related quality of life and fatigue suggest points for intervention. FACT-B scores were 10.2 points higher for women who attended ≥7 classes versus those who attended 0–6 classes; typically, 7–8 points indicate a clinically important difference on the FACT-B [36]. Similarly, FACT-Fatigue scores at baseline were 6.7 points higher (indicating less fatigue) for women who attended ≥7 classes versus those who attended 0-6 classes; a difference of ≥3 points on the FACT-Fatigue indicates a clinically important difference [37]. These data suggest that women with lower health-related quality of life and greater fatigue at baseline may be more likely to have poor adherence to the intervention. Future research will benefit from creating strategies to decrease the number of missed classes.
The second objective of this study was to measure changes in fatigue, sleep, psychological distress (depression, negative affect), psychological well-being (positive affect), and health-related quality of life in the RY versus control groups. We found benefits in psychological outcomes for women in the RY intervention group. Women who started with higher negative affect and lower emotional well-being derived greater benefit from the RY intervention compared to the control group. Group differences were seen for mental health (SF-12 mental component score), depression (CES-D), positive affect (PANAS-PA), and spirituality (peace-meaning subscale) favoring the yoga group. Health-related quality of life (FACT B) showed a borderline difference between the two groups. Although there was not a significant group effect for fatigue, the yoga group demonstrated a significant within-group improvement in fatigue between baseline and follow-up, with no significant difference for the control group.
This study sample was a heterogeneous group of women with breast cancer, in terms of treatment status. Some of the women were in treatment (chemotherapy and/or radiation therapy) during the study (15/44, or 34%) while others had already completed their treatment upon study entry. There was a significant difference in sleep duration ( p = 0.04) based on cancer treatment status during the study, with longer sleep duration scores reported by women undergoing treatment. Also, a trend toward a group difference based on treatment status was seen for sleep efficiency ( p = 0.06), with women currently receiving cancer treatment reporting worse sleep efficiency scores. No other differences based on cancer treatment status were observed.
Because this was a pilot study of feasibility, it has inherent limitations. First, our sample size was relatively small. While we could demonstrate beneficial emotional outcomes of the RY intervention, it is not clear whether the physical health, fatigue, and sleep outcomes were truly nonsignificant or too limited by sample size to detect significant group differences. The issue of multiple statistical comparisons is another limitation; it is possible that with the number of tests done, some of the significant relationships reported are spurious rather than causal. Second, this study used a waitlist control group that did not control for time, attention from a teacher, and social contact, all factors that may contribute to the benefits we found. Future research should include an active control group that controls for these factors. Third, as stated above, the study sample was a heterogeneous group of women with breast cancer, in terms of treatment status. Finally, our study sample was not demographically diverse. While comparable to many studies of women with breast cancer, the sample was predominantly comprised of White women of a higher socioeconomic status, thus limiting generalizability.
Nonetheless, our results add to the growing literature on benefits of yoga for cancer patients. Participants enjoyed the RY intervention, and these data suggest a variety of emotional benefits of RY for women with breast cancer. The attendance and drop-out rates, however, suggest limited feasibility for an off-site 10-week RY intervention using the same design, methods and location. It will be essential for future research to examine how to address these concerns and to identify those factors significantly associated with increased interest in CAM therapies and successful participation over time. Future directions in this line of research include: narrowing the sample to determine when a RY intervention may be most useful (i.e. during or post-treatment), examining benefits of RY in patients with various types of cancers, and incorporating theoretical frameworks to enhance adherence and to strengthen participant outcomes. Some women clearly benefited from this RY intervention, suggesting this type of intervention should be considered for women undergoing multimodality treatment for breast cancer.
Acknowledgements
We are grateful to the women who participated in this study. We thank Karen Klein, MA, ELS (Research Support Core, Wake Forest University Health Sciences) for her editorial contributions to this manuscript. Funding for this project was provided by the Wake Forest University Comprehensive Cancer Center.
References
- 1.Boon HS, Olatunde F, Zick SM. Trends in complementary/alternative medicine use by breast cancer survivors: comparing survey data from 1998 and 2005. BMC Women’s Health. 2007;7(4) doi: 10.1186/1472-6874-7-4. Available at http://www.biomedcentral.com/1472-6874/7/4, http://dx.doi.org/10.1186/1472-6874-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matthews AK, Sellergren SA, Huo D, List M, Fleming G. Complementary and alternative medicine use among breast cancer survivors. J Altern Complement Med. 2007;13(5):555–562. doi: 10.1089/acm.2007.03-9040. http://dx.doi.org/10.1089/acm.2007.03-9040. [DOI] [PubMed] [Google Scholar]
- 3.Ashikaga T, Bosompra K, O’Brien P, Nelson L. Use of complementary and alternative medicine by breast cancer patients: prevalence, patterns and communication with physician. Support Care Cancer. 2002;10:542–548. doi: 10.1007/s00520-002-0356-1. http://dx.doi.org/10.1007/s00520-002-0356-1. [DOI] [PubMed] [Google Scholar]
- 4.Ni H, Simile C, Hardy AM. Utilization of complementary and alternative medicine by United States adults: Results from the 1999 National Health Interview Survey. Med Care. 2002;40:353–358. doi: 10.1097/00005650-200204000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Wolsko PM, Eisenberg DM, Davis RB, et al. Use of mind-body medical therapies. J Gen Intern Med. 2004;19:43–50. doi: 10.1111/j.1525-1497.2004.21019.x. http://dx.doi.org/10.1111/j.1525-1497.2004.21019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deng G, Cassileth BR, Yeung KS. Complementary therapies for cancer-related symptoms. J Support Oncol. 2004;2:419–426. [PubMed] [Google Scholar]
- 7.Astin JA, Shapiro SL, Eisenberg DM, et al. Mind-body medicine: state of the science, implications for practice. J Am Board Fam Pract. 2003;16:131–147. doi: 10.3122/jabfm.16.2.131. [DOI] [PubMed] [Google Scholar]
- 8.Williams KA, Petronis J, Smith D, et al. Effects of Iyengar yoga therapy for chronic low back pain. Pain. 2005;115(1–2):107–117. doi: 10.1016/j.pain.2005.02.016. http://dx.doi.org/10.1016/j.pain.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 9.Oken BS, Kishiyama S, Zajdel D, et al. Randomized controlled trial of yoga and exercise in multiple sclerosis. Neurology. 2004;62:2058–2064. doi: 10.1212/01.wnl.0000129534.88602.5c. [DOI] [PubMed] [Google Scholar]
- 10.Raub JA. Psychophysiologic effects of hatha yoga on musculoskeletal and cardiopulmonary function: a literature review. J Altern Complement Med. 2002;8:797–812. doi: 10.1089/10755530260511810. http://dx.doi.org/10.1089/10755530260511810. [DOI] [PubMed] [Google Scholar]
- 11.Tran MD, Holly RG, Lashbrook J, et al. Effects of hatha yoga practice on the health-related aspects of physical fitness. Prev Cardiol. 2001;4:165–170. doi: 10.1111/j.1520-037x.2001.00542.x. http://dx.doi.org/10.1089/10755530260511810. [DOI] [PubMed] [Google Scholar]
- 12.Vempati RP, Telles S. Yoga-based guided relaxation reduces sympathetic activity judged from baseline levels. Psychol Rep. 2002;90:487–494. doi: 10.2466/pr0.2002.90.2.487. http://dx.doi.org/10.2466/-PR0.90.2.487-494. [DOI] [PubMed] [Google Scholar]
- 13.Malathi A, Damodaran A, Shah N, et al. Effect of yogic practices on subjective well-being. Indian J Physiol Pharmacol. 2000;44:202–206. [PubMed] [Google Scholar]
- 14.Boucher S. Yoga for cancer patients. Yoga J. 1999;146:42–49. 135–138. and. [Google Scholar]
- 15.Gimbel MA. Yoga, meditation, and imagery: clinical applications. Nurse Pract Forum. 1998;9:243–255. [PubMed] [Google Scholar]
- 16.Ott MJ. Yoga as a clinical intervention. Adv Nurse Pract. 2002;10:81–83. 90. [PubMed] [Google Scholar]
- 17.Rosenbaum E, Gautier H, Fobair P, et al. Cancer supportive care, improving the quality of life for cancer patients: a program evaluation report. Support Care Cancer. 2004;12:293–301. doi: 10.1007/s00520-004-0599-0. [DOI] [PubMed] [Google Scholar]
- 18.Danhauer SC, Tooze JA, Farmer DF, Campbell CR, McQuellon RP, Barrett R, Miller BE. Restorative yoga for women with ovarian or breast cancer: findings from a pilot study. J Soc Integr Oncol. 2008;6(2):47–58. [PubMed] [Google Scholar]
- 19.Cohen L, Warneke C, Fouladi RT, et al. Psychological adjustment and sleep quality in a randomized trial of the effects of a Tibetan yoga intervention in patients with lymphoma. Cancer. 2004;100:2253–2260. doi: 10.1002/cncr.20236. http://dx.doi.org/10.1002/cncr.20236. [DOI] [PubMed] [Google Scholar]
- 20.Culos-Reed SN, Carlson LE, Daroux LM, Hately-Aldous S. A pilot study of yoga for breast cancer survivors: physical and psychological benefits. Psycho-Oncology. 2006;15:891–897. doi: 10.1002/pon.1021. [DOI] [PubMed] [Google Scholar]
- 21.Banerjee B, Vadiraj HS, Ram A, et al. Effects of an integreated yoga program in modulating psychological stress and radiation-induced genotoxic stress in breast cancer patients undergoing radiation. Integr Cancer Ther. 2007;6(3):242–251. doi: 10.1177/1534735407306214. http://dx.doi.org/10.1177/1534735407306214. [DOI] [PubMed] [Google Scholar]
- 22.Raghavendra RM, Nagarathna R, Nagendra HR, et al. Effects of an integrated yoga programme on chemotherapy-induced nausea and emesis in breast cancer patients. Eur J Cancer Care (Engl) 2007;16(6):462–474. doi: 10.1111/j.1365-2354.2006.00739.x. http://dx.doi.org/10.1111/j.1365-2354.2006.00739.x. [DOI] [PubMed] [Google Scholar]
- 23.Moadel AB, Shah C, Wylie-Rosett J, et al. Randomized controlled trial of yoga among a multi-ethnic sample of breast cancer patients: Effects on quality of life. J Clin Oncol. 2007;25(28):1–9. doi: 10.1200/JCO.2006.06.6027. [DOI] [PubMed] [Google Scholar]
- 24.Lasater J. Relax and Renew: Restful Yoga for Stressful Times. Rodmell Press; Berkeley, CA: 1995. [Google Scholar]
- 25.Ware JE, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Brady MJ, Cella DF, Mo F, et al. Reliability and validity of the Functional Assessment of Cancer Therapy-Breast quality-of-life instrument. J Clin Oncol. 1997;15:974–986. doi: 10.1200/JCO.1997.15.3.974. [DOI] [PubMed] [Google Scholar]
- 27.Cella D. The Functional Assessment of Cancer Therapy–Anemia (FACT-An) Scale: a new tool for the assessment of outcomes in cancer anemia and fatigue. Semin Hematol. 1997;34:13–19. [PubMed] [Google Scholar]
- 28.Yellen SB, Cella DF, Webster K, et al. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage. 1997;13:63–74. doi: 10.1016/s0885-3924(96)00274-6. http://dx.doi.org/10.1016/S0885-3924%2896%2900274-6. [DOI] [PubMed] [Google Scholar]
- 29.Peterman AH, Fitchett G, Brady MJ, et al. Measuring spiritual well-being in people with cancer: the functional assessment of chronic illness therapy—spiritual well-being scale (FACIT-Sp) Ann Behav Med. 2002;24:49–58. doi: 10.1207/S15324796ABM2401_06. http://dx.doi.org/10.1207/S15324796ABM2401_06. [DOI] [PubMed] [Google Scholar]
- 30.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. http://dx.doi.org/10.1177/014662167700100306. [Google Scholar]
- 31.Hann D, Winter K, Jacobsen P. Measurement of depressive symptoms in cancer patients: evaluation of the Center for Epidemiological Studies Depression Scale (CES-D) J Psychosom Res. 1999;46:437–443. doi: 10.1016/s0022-3999(99)00004-5. http://dx.doi.org/10.1016/S0022-3999%2899%2900004-5. [DOI] [PubMed] [Google Scholar]
- 32.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. http://dx.doi.org/10.1016/0165-1781%2889%2990047-4. [DOI] [PubMed] [Google Scholar]
- 33.Beck SL, Schwartz AL, Towsley G, Dudley W, Barsevick A. Psychometric evaluation of the Pittsburgh Sleep Quality Index in cancer patients. J Pain Symptom Manage. 2004;27(2):140–148. doi: 10.1016/j.jpainsymman.2003.12.002. http://dx.doi.org/10.1016/j.jpainsymman.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 34.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. http://dx.doi.org/10.1037/0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 35.Mihalko SL, Brenes GA, Farmer DF, Katula JA, Balkrishnan R, Bowen DJ. Challenges and innovations in enhancing adherence. Contro Clin Trials. 2004;25:447–457. doi: 10.1016/j.cct.2004.07.003. http://dx.doi.org/10.1016/j.cct.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 36.Eton DT, Cella D, Yost KJ, et al. A combination of distribution and anchor-based approaches determined minimally important differences (MIDs) for four endpoints in a breast cancer scale. J Clin Epidemiol. 2004;57:898–910. doi: 10.1016/j.jclinepi.2004.01.012. http://dx.doi.org/10.1016/j.jclinepi.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 37.Cella D, Eton DT, Lai J-S, et al. Combining anchor and distribution-based methods to derive minimal clinically important differences on the functional assessment of cancer therapy (FACT) anemia and fatigue scales. J Pain Symptom Manage. 2002;24 doi: 10.1016/s0885-3924(02)00529-8. http://dx.doi.org/10.1016/S0885-3924%2802%2900529-8. [DOI] [PubMed] [Google Scholar]