Abstract
Mycobacterial infection induces suppressor macrophages (MΦs), causing disease exacerbation. There are two major MΦ subsets (M1 and M2 MΦs) that are phenotypically and functionally different. Here, we examined which of the MΦ subsets the mycobacterial infection-induced suppressor MΦs (MIS-MΦs) belong to. MIS-MΦs down-regulated T cell production of Th1 and Th2 cytokines but markedly increased production of interleukin (IL)-17A and IL-22 through up-regulation of Th17 cell expansion. In this phenomenon, a novel MΦ population, which is functionally distinguishable from M1 and M2 MΦ subsets and possesses unique phenotypes (IL-12+, IL-1βhigh, IL-6+, tumor necrosis factor (TNF)-α+, nitric oxide synthase (NOS) 2+, CCR7high, IL-10high, arginase (Arg)-1−, mannose receptor (MR)low, Ym1high, Fizzlow, and CD163high), played central roles through the action of IL-6 and transforming growth factor (TGF)-β but not IL-21 and IL-23. This new type of MΦ population was induced in infected mice and actively supported the in vivo expansion of Th17 cells.
Various microorganisms, especially facultative intracellular bacteria, including pathogenic mycobacteria, induce the transcriptional activity of a common host response, which includes genes belonging to the M1 program, associated with macrophage (MΦ) polarization yielding classically activated MΦs (called M1 MΦs) exhibiting proinflammatory and microbicidal functions1,2. Alternatively activated MΦs (called M2 MΦs) with immunoregulatory and tissue-repairing functions play critical roles in the resolution of harmful inflammation due to the prolonged expansion and activation of M1 MΦs by producing anti-inflammatory mediators1,3,4. On the other hand, in the advanced stages of mycobacterial infection, the generation of a suppressor MΦ population is generally observed5. This mycobacterial infection-induced suppressor MΦ (designated MIS-MΦ) population suppresses T cell functions, including a proliferative response due to the down-regulation of interleukin (IL) -2 receptor expression and proinflammatory cytokine production, causing the marked suppression of cellular immunity in the advanced stages of mycobacteriosis5,6. We previously found that the immunosuppressive activity of the MIS-MΦs was mediated by reactive nitrogen intermediates, prostaglandin E2 (PGE2), transforming growth factor (TGF)-β, and phosphatidylserine produced by themselves5,7,8,9. Notably, B7-1-like molecule-mediated cell contact of MIS-MΦs with target T cells is required for the effective manifestation of their suppressor activity, and their suppressor signals cross-talk with early signalling events before the activation of protein kinase C and intracellular calcium mobilization10,11. In this context, it is of marked interest to elucidate whether the MIS-MΦ population belongs to the M1 MΦ or M2 MΦ subset. Here, we firstly examined the detailed profiles of effects of MIS-MΦs on cytokine production by T cell receptor (TCR)-stimulated T cells, and found that MIS-MΦs markedly enhanced the T cell production of Th17 cytokines, IL-17A and IL-22, while they down-regulated the generation of Th1 and Th2 cytokines by T cells. Subsequent systematic experiments revealed that a unique MΦ population, which is clearly distinguishable from M1 and M2 MΦ subsets in terms of functional and phenotypical characteristics, specifically up-regulated Th17 polarization, while it exhibited a potent suppressor function against T cell mitogenesis in vitro and in vivo3,4.
Results
MIS-MΦs up-regulate IL-17 production
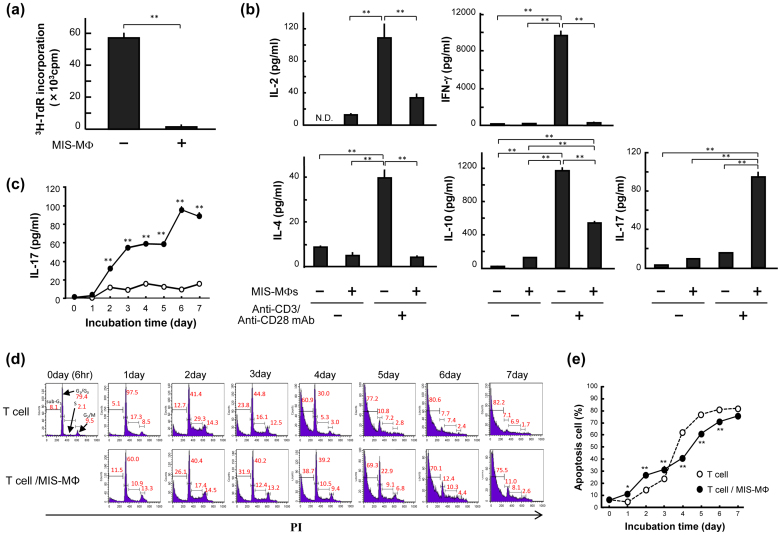
In the present study, we used splenic MΦs harvested from BALB/c mice infected 2 to 3 weeks after mycobacterial infection as MIS-MΦs and splenic T lymphocytes obtained from normal mice as the target T cells for MIS-MΦ's action, such as the suppressor function, Th17 cell-expanding activity, and cytokine production, throughout the experiments (see Methods). In this study, the ratio of MIS-MΦs to T cells was fixed at 1:14 throughout the experiments. We firstly examined the effects of MIS-MΦs exhibiting inhibitory activity against T cell mitogenesis (Fig. 1a), on cytokine production by T cells responding to TCR stimulation. T cell production of IL-2, interferon (IFN)-γ, IL-4, and IL-10 was significantly reduced when target T cells were co-cultivated with MIS-MΦs (Fig. 1b). MIS-MΦs thus down-regulated T cell production of both Th1 cytokines and Th2 cytokines. In contrast, MIS-MΦs markedly increased IL-17 production by T lymphocytes, although the amount of IL-17 generated in this experimental system was as small as 0.1 ng/ml (Fig. 1b). In this context, it was found that IL-17 production by T cells during co-cultivation with MIS-MΦs was markedly (ca. 80-fold) increased, when cultured under Th17 polarizing condition as described below (see Fig. 3d). When TCR-stimulated T cells were co-cultivated with MIS-MΦs, IL-17 secretion was initiated on day 2, and thereafter continued to increase, reaching a plateau on day 6 (Fig. 1c). Next, we examined cell-cycle distribution of T cells in terms of DNA contents by performing cytofluorometry of propidium iodide (PI)-stained cells (Fig. 1d,e). When T cells were cultured alone, the percentage of T cells that entered S and G2/M phases (S-G2/M population) markedly increased during the first 2 days and significantly decreased during day 2 to day 5 with a concomitant increase in apoptotic cells with sub-G1 peak (Fig. 1d). In contrast, when the T cells were co-cultured with MIS-MΦs, the increase in the S-G2/M population on day 2 was much smaller than with T cells alone (31.9% versus 43.6%). However, in this case, the subsequent decrease in the S-G2/M population was slower than with T cells alone (12.0% versus 35.3% decrease during day 2 to day 4) (Fig. 1d), presumably showing the proliferation of certain T cell populations, including IL-17-producing cells during this period. Next, when T cells were cultured alone, the ratio of the apoptotic cell population increased during 7-day cultivation with a sigmoidal curve, indicating that cell death was slow during the first 3 days (Fig. 1e), concurrently with the rapid growth of TCR-stimulated T cells in this period (Fig. 1d). Notably, when the T cells were co-cultivated with MIS-MΦs, the increase in apoptotic cells was more rapid during the first 3 days than with T cells alone (Fig. 1e), suggesting the partial contribution of apoptosis to the MIS-MΦ-mediated reduction of T cell proliferation during 3-day cultivation (Fig. 1a).
Figure 1. Effects of the MIS-MΦs on T cell production of various cytokines.
(a), (b) Anti-CD3 Ab/Anti-CD28 Ab-stimulated T cells (TCR-stimulated T cells) were cultivated with or without a monolayer culture of MIS-MΦs for 72 hr and measured for proliferative response (a) and production of cytokines in culture fluids by ELISA (b). (c) TCR-stimulated T cells were cultured with or without a monolayer culture of MIS-MΦs for up to 7 days. At intervals, concentrations of IL-17 in culture fluids were measured. (d), (e) Cultured T cells harvested at the same time points were stained with PI (10 μg/ml) after RNase A (200 μg/ml) treatment, subjected to cytofluorometry (d). The ratio of apoptotic T cells was estimated based on the ratio of the sub-G1 cell population (e). Data are representative of multiple experiments; error bars, s.e.m.; **p < 0.01, *p < 0.05 (Bonferroni's multiple t-test).
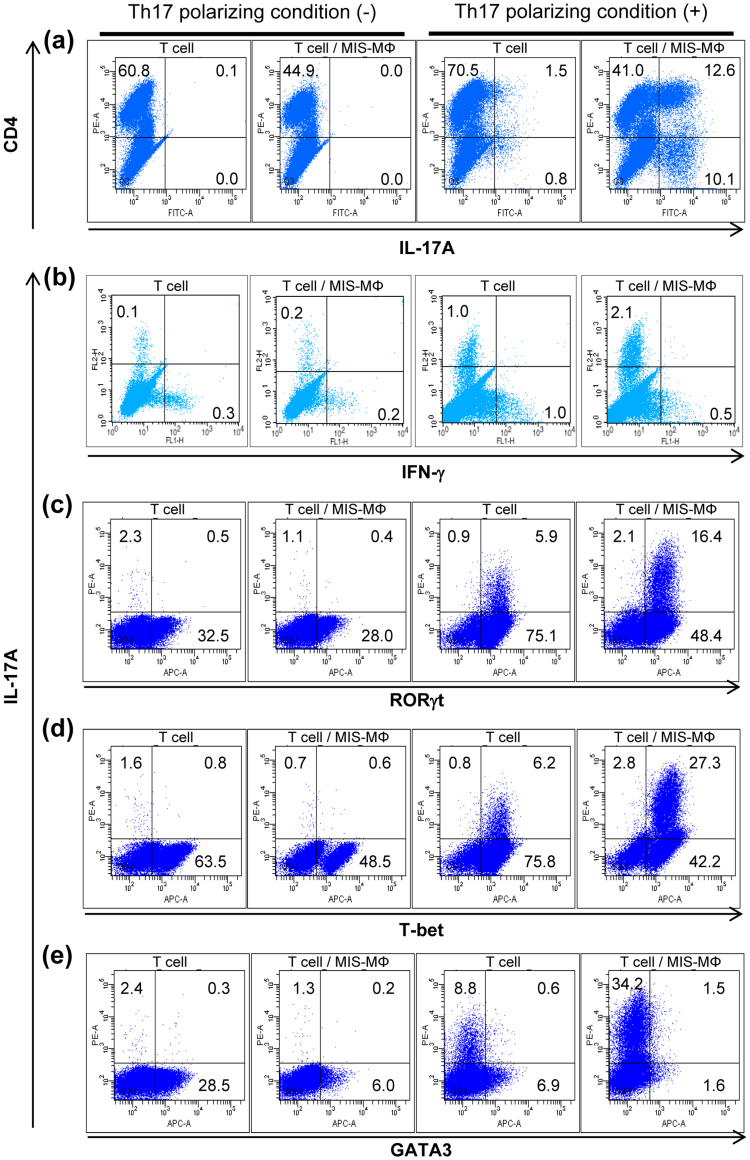
Figure 2. Effects of MIS-MΦs on intracellular expression of IL-17A (a), IFN-γ (b), and some transcription factors, including Th17-specific RORγt (c), Th1-specific T-bet (d) and Th2-specific GATA3 (e).
TCR-stimulated T cells were cultured with or without a monolayer culture of MIS-MΦs under the Th17 polarizing condition in RPMI medium containing IL-6 (20 ng/ml), TGF-β (1 ng/ml), anti-IFN-γ Ab (1 μg/ml), and anti-IL-4 Ab (1 μg/ml) (designated Th17 polarizing medium), indicated as “Th17 polarizing condition (+)”, or in the non-Th17 skewing condition in RPMI medium without these supplements, indicated as “Th17 polarizing condition (-)”. After 5-day cultivation, T cells were treated with PMA (25 ng/ml), A23187 (125 ng/ml), and Golgistop (0.33 μl/well) for 6 hr, and subjected to blocking with anti-CD16 Ab/anti-CD32 Ab (1 μg/ml each) and then paraformaldehyde fixation. After rinsing with 1% FBS-PBS, the resultant cells were treated with BD Perm for 15 min, stained with test Abs according to BD's instructions for 30 min, and subjected to cytofluorometry. Data are representative of multiple experiments.
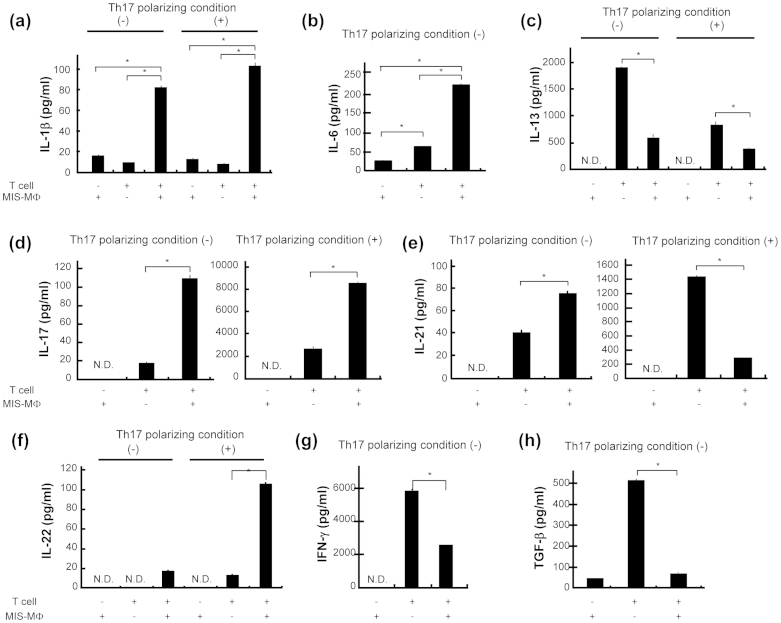
Figure 3. Production of various cytokines by T cells with or without co-cultivation with MIS-MΦs.
TCR-stimulated T cells were cultured with or without a monolayer culture of MIS-MΦs under either the Th17 polarizing condition or non-Th17 skewing condition. Culture fluids were harvested after 5-day cultivation and measured for the concentrations of test cytokines, including IL-1β (a), IL-6 (b), IL-13 (c), IL-17 (d), IL-21 (e), IL-22 (f), IFN-γ (g), and TGF-β (h) by ELISA. Data are representative of multiple experiments; error bars, s.e.m.; *p < 0.01 (Bonferroni's multiple t-test).
MIS-MΦs induce Th17 polarization
In order to identify T cells that generated IL-17A in our experimental condition, as a subset belonging to the Th17 cell lineage, we performed the following experiments. Splenic T cells were cultivated in medium supplemented with IL-6, TGF-β, anti-IFN-γ antibody (Ab), and anti-IL-4 Ab (Th17 polarizing condition) for 5 days after TCR stimulation. Thereafter, the cultured T cells were enhanced for intracellular accumulation of IL-17A by 6 hr incubation in the presence of phorbol 12-myristate 13-acetate (PMA), ionophore A23187, and Golgistop. The IL-17A expression by CD4+ T cells was markedly increased by co-cultivating with MIS-MΦs, compared to T cells alone (Fig. 2a). Separate experiments repeated four times indicated that the MIS-MΦ-mediated increase in the expansion of Th17 cells was significant (P = 0.021: Supplemental Table S1). Such a phenomenon was not seen when T cells were cultivated in medium without the addition of IL-6, TGF-β, anti-IFN-γ Ab, and anti-IL-4 Ab (non-Th17 skewing condition) (Fig. 2a). Interestingly, MIS-MΦs also strongly enhanced IL-17A expression of the CD4− T cell population (Fig. 2a). This CD4− T cell population may correspond to Tγδ cells, which express IL-17A in response to TCR stimulation and pathogen products13,14. However, this possibility can be excluded, because 5-day co-cultivation of TCR-stimulated T cells with MIS-MΦs under the Th17-inducing condition failed to expand γδTCR+ T cells, as described later (Supplemental Fig. S1b). Moreover, IL-17 production by Tγδ cells is substantially independent of TCR activation and promoted by signaling due to IL-23 in combination with IL-1β or IL-1814. Notably, although the Th17 polarizing condition caused the expansion of both IL-17A+ IFN-γ− T cells and IL-17A− IFN-γ+ T cells, MIS-MΦs enhanced the expansion of only the former T cell subset (Fig. 2b). In relation to these findings, it should be pointed out that our Th17 polarizing condition led to low-level expansion of Th17 cells when T cells alone were cultivated (Fig. 2a). This may be mainly due to the fact that we used whole T cells instead of naïve CD4+ T cells. A study using an unfractionated T cell preparation rather than artificially isolated T cell subsets, such as naïve CD4+ T cells, will more appropriately reflect the complicated in vivo immunological phenomena in hosts during the course of bacterial infection.
Next, we examined the expression profiles of some transcription factors, including RORγt, T-bet, and GATA3, by T cell populations responding to TCR stimulation15,16,17,18. Co-cultivation of TCR-stimulated T cells with MIS-MΦs under Th17 polarizing condition caused a marked increase in RORγt expression by IL-17A+ T cells, while such an increase was not observed under the non-Th17 skewing condition (Fig. 2c). In this case, T cell cultivation under the Th17-polarizing condition in the presence or absence of MIS-MΦs caused a marked expansion of RORγt+ IL-17A− T cells, which presumably belong to precursor populations of Th17 cells (Fig. 2c). This experiment was separately repeated seven times, and it was confirmed that the MIS-MΦ-mediated increase in RORγt expression by IL-17A+ T cells, but not by IL-17A− T cells, was significant (P < 0.01: Bonferroni's multiple t-test) (Supplemental Table S1). Next, MIS-MΦs down-regulated the expansion of T-bet+ IL-17A− T cells (Th1 cells) and GATA3+IL-17A− T cells (Th2 cells) under both Th17-polarizing and non-Th17 skewing conditions (Fig. 2d,e). In this case, the Th17 polarizing condition modestly increased the expansion of T-bet+ IL-17A− T cells when TCR-stimulated T cells were cultured alone (Fig. 2d). Notably, MIS-MΦs markedly up-regulated the expression of T-bet by IL-17A+ T cells under the Th17 polarizing condition (Fig. 2d). T-bet+ IL-17A+ cells may correspond to Th17 cell linages, since T-bet is expressed not only by Th1 cells but also by some types of Th17 cell populations15,19,20. These findings indicate that co-cultivation of T cells with MIS-MΦs potentiated Th17 cell expansion but neither Th1 nor Th2 cell expansion. In addition, MIS-MΦs did not up-regulate the expansion of Foxp3+IL-17A− T cells (iTreg cells) and γδTCR+IL-17A+ T cells (IL-17 producing Tγδ cells) under the Th17 polarizing and non-Th17 skewing conditions (Supplemental Fig. S1).
We previously found that various kinds of MΦ also exhibited inhibitory activity against T cell proliferation23. Separate experiments using murine peritoneal MΦs induced with Zymosan A (ZA-MΦs) and those induced with Mycobacterium bovis Bacille Calmette Guérin (BCG-MΦs) revealed that these MΦ populations exhibited inhibitory activity against T cell mitogenesis, and up-regulated IL-17 production by T cells under both Th17 polarizing and non-Th17 skewing conditions, followed by a concomitant increase in the expression of RORγt (data not shown). Thus, it appears that the functional characteristics of MIS-MΦs described above are shared by other MΦ populations induced with bacterial irritants.
Roles of MIS-MΦ-derived cytokines in Th17 polarization
Next, we studied the roles of MIS-MΦ- and T cell-derived cytokines in the MIS-MΦ-mediated polarization of Th17 cells. Firstly, we examined the generation of various cytokines by MIS-MΦs and TCR-stimulated T cells. As shown in Fig. 3, MIS-MΦs alone showed weak production of IL-1β, IL-6, and TGF-β (Fig. 3a,b,h), but did not generate IL-13, IL-17, IL-21, IL-22, and IFN-γ (Fig. 3c–g). Next, T cells alone produced moderate to high amounts of IL-6, IL-13, IL-21, IFN-γ, and TGF-β (Fig. 3b,c,e,g,h), and showed weak or no expression of IL-1β, IL-17, and IL-22 (Fig. 3a,d,f), under the non-Th17 skewing condition. Nevertheless, co-cultivation of T cells and MIS-MΦs significantly enhanced their production of IL-1β and IL-6 (Fig. 3a,b), leading to greatly enhanced T cell production of IL-17 and IL-22 (Fig. 3d,f). In contrast, T cell production of IL-13, IL-21 (especially in Th17 polarizing condition), IFN-γ, and TGF-β decreased markedly when co-cultured with MIS-MΦs (Fig. 3c,e,g,h). Notably, we could not detect IL-23 and IL-12 production by MIS-MΦs and T cells, even when they were co-cultivated (data not shown). Therefore, it appears that IL-1β and IL-6, especially IL-1β, act as important mediators in Th17 expansion up-regulated by MIS-MΦs. Nevertheless TGF-β, IL-21, and IL-23, which are known to play important roles in the differentiation and subsequent maintenance of murine Th17 cells16,21,22, appear to play only modest roles in Th17 polarization supported by the function of MIS-MΦs.
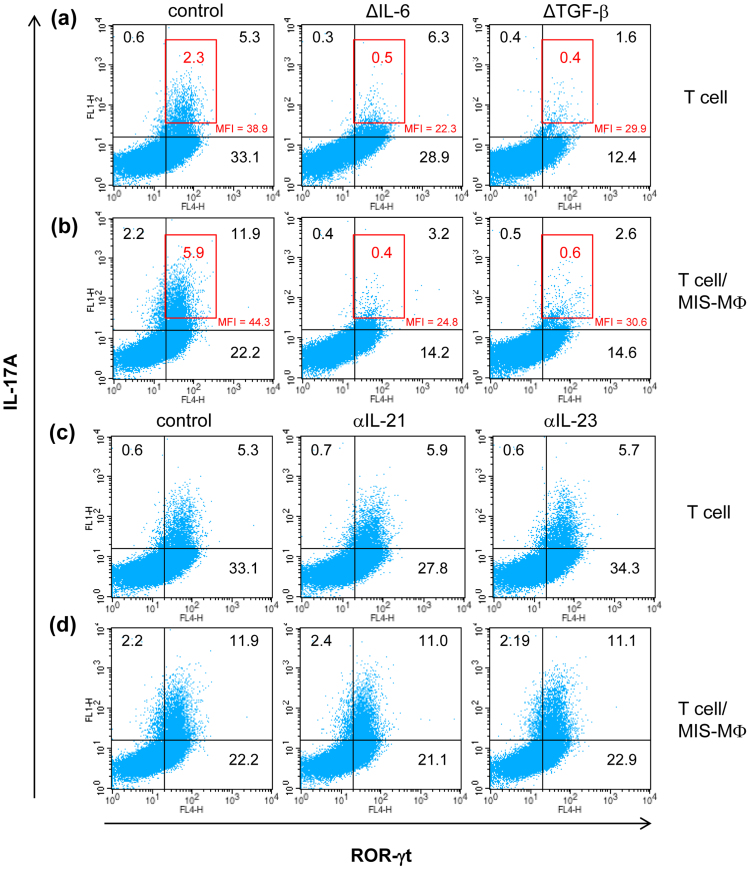
In mice, Th17 cell differentiation is initiated by IL-6 in combination with TGF-β, and IL-1β and tumor necrosis factor (TNF)-α exert an enhancing effect, while maintenance and survival of Th17 cells are dependent on IL-2318,22,24,25,26. Therefore, it is of interest to study which kinds of cytokines play a central role in the MIS-MΦ-mediated Th17 cell expansion. When TCR-stimulated T cells were cultured alone, the expansion of IL-17A+ RORγt+ T cells was greatly reduced, when TGF-β but not IL-6 was depleted from the Th17 polarizing medium (Fig. 4a). In this case, not only TGF-β but also IL-6 depletion potently decreased the expansion of T cells that strongly express IL-17A (Fig. 4a, inserted red squares), suggesting that, differing from TGF-β, endogenous IL-6 is sufficient for initiating Th17 polarization, but insufficient to support subsequent steps for full differentiation of Th17 cells. On the other hand, Th17 cell expansion during co-cultivation of T cells with MIS-MΦs was strongly restrained in Th17 polarizing medium without the addition of either IL-6 or TGF-β (Fig. 4b), indicating the important roles of both IL-6 and TGF-β in the MIS-MΦ-mediated up-regulation of Th17 expansion. This aspect is limited to the Th17 polarizing condition, because the concentrations of these cytokines, which were generated in culture fluid of T cells co-cultivated with MIS-MΦs under the non-Th17 skewing condition, were smaller by one to two orders of magnitude than those added to the Th17 polarizing medium (Fig. 3b,h, see Methods section). Next, the addition of either anti-IL-21 Ab or anti-IL-23 Ab failed to block Th17 cell expansion when T cells were cultured in the presence or absence of MIS-MΦs under the Th17 polarizing condition (Fig. 4c,d). These findings support the aspect that the MIS-MΦ population transmits triggering signals to TCR-stimulated T cells, enabling their differentiation to a Th17 cell subset by mechanisms different from those mediated by IL-21 or IL-23.
Figure 4. Determination of factors playing roles in the enhancement of Th17 polarization mediated by MIS-MΦs.
TCR-stimulated T cells were cultured in the absence (a), (c) or presence (b), (d) of monolayer culture of MIS-MΦs under the Th17 polarizing condition. In some experiments, IL-6 or TGF-β was deleted from the Th17 polarizing medium (a), (b), or anti-IL-21 Ab or anti-IL-23 Ab was added to the Th17 polarizing medium (c), (d). After 5-day cultivation, cultured T cells were harvested and subjected to the measurement of intracellular expression of IL-17A and RORγt as described in Fig. 2. The distribution profiles of cells strongly expressing IL-17A are indicated in the inserted red squares. MFI: mean fluorescence intensity of IL-17A expression by cells distributed in the upper right corner. Data are representative of multiple experiments.
A unique cell population in MIS-MΦs with M1 MΦ- and partially M2 MΦ-specific phenotypes mediates Th17 polarization
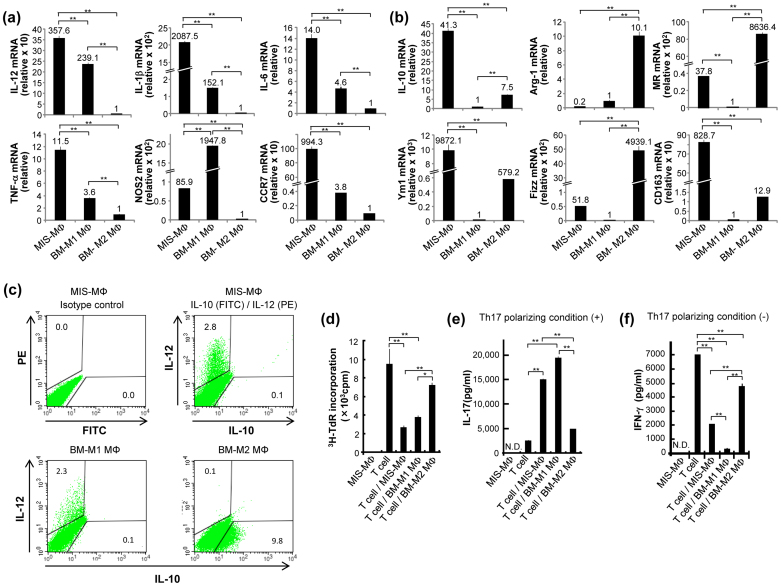
MIS-MΦs (Figs. 1, 2), ZA-MΦs, and BCG-MΦs (data not shown), which up-regulate Th17 polarization may belong to classically activated M1 MΦ population, since they are induced with microbial irritants1,2. This concept was examined by performing the following experiments. First, we prepared M1 and M2 MΦ populations from mouse bone marrow-derived MΦs (BMDMs) by treatment with IFN-γ followed by lipopolysaccharide (LPS) stimulation and by treatment with IL-4, respectively12. Real-time PCR indicated that the BMDM-derived M1 MΦs (BM-M1 MΦs) expressed significant levels of M1-specific genes encoding IL-12, IL-1β, IL-6, TNF-α, and nitric oxide synthase (NOS) 2 but not CCR7, while they showed weak expression of M2-specific genes encoding IL-10, arginase (Arg)-1, mannose receptor (MR), Ym1, Fizz, and CD163 (Fig. 5a,b)2,3,5,27. Second, the BMDM-derived M2 MΦs (BM-M2 MΦs) showed opposite profiles of M1 and M2 gene expressions from BM-M1 MΦs (Fig. 5a,b). Third, MIS-MΦs showed a similar profile of gene expression to BM-M1 MΦs, i.e., high- to moderate-level expression of M1-specific genes, including IL-12, IL-1β, IL-6, TNF-α, NOS2, and CCR7 genes (Fig. 5a); low-level or no expression of Arg-1, MR, and Fizz genes (Fig. 5b). Notably, MIS-MΦs showed high-level expression of some M2-specific genes, including IL-10, Ym1, and CD163 genes (Fig. 5b). Fourth, cytofluorometry showed that MIS-MΦs as well as BM-M1 MΦs (positive control) contained the IL-12+IL-10− population but not IL-12−IL-10+ population, whereas BM-M2 MΦs (negative control) contained the IL-12−IL-10+ population but not IL-12+IL-10− population (Fig. 5c). It thus appears that, in MIS-MΦs, the expression of IL-10, the mRNA of which is highly expressed in the same MΦ population (Fig. 5b), is severely suppressed at the protein level (Fig. 5c). These findings on the gene expression mode of MIS-MΦs may indicate that the MIS-MΦ population itself belongs to a novel type of MΦ subset that exhibits a unique gene expression profile, essentially resembling that of M1 MΦs, except for strong mRNA expression of IL-10, Ym1 and CD163 genes (Fig. 5a,b).
Figure 5. Profiles of mRNA expression of M1 MΦ- and M2 MΦ-specific marker genes by MIS-MΦs.
(a), (b) Total RNAs extracted using ISOGEN kit (Nippon Gene) from MIS-MΦs, BMDM-derived M1-type MΦs (BM-M1 MΦs), and M2-type MΦs (BM-M2 MΦs) were subjected to real-time quantitative RT-PCR for M1 MΦ-marker genes (IL-12, IL-1β, IL-6, TNF-α, NOS2, and CCR7) (a) or M2 MΦ-marker genes (IL-10, Arg-1, MR, Ym1, Fizz, and CD163 (b) as described in Experimental Procedures. (c) MIS-MΦs, BM-M1 MΦs, and BM-M2 MΦs were measured for intercellular expression of IL-12 and IL-10 by cytofluorometry, as described in Fig. 2. Control: MIS-MΦs were stained with FITC-labeled, and PE-labeled rat IgG2b isotype control antibodies. (d–f) Test MΦs were measured for their suppressor activity against T cell mitogenesis (d) and effects on T cell production of IL-17 (e) and IFN-γ (f) as described in Fig. 3. Data are representative of multiple experiments; error bars, s.e.m.; **p < 0.01, *p < 0.05 (Bonferroni's multiple t-test).
Next, MIS-MΦs, BM-M1 MΦs, and BM-M2 MΦs were examined for their inhibitory activity against T cell mitogenesis and effects on Th17 and Th1 polarization (Fig. 5d–f). When TCR-stimulated T cells were co-cultivated with these MΦs, while BM-M1 MΦs exerted strong inhibitory activity against T cell mitogenesis comparable to MIS-MΦs, such an activity of BM-M2 MΦs was significantly weaker than those of MIS-MΦs and BM-M1 MΦs (Fig. 5d). Under the Th17 polarizing condition, BM-M1 MΦs as well as MIS-MΦs but not BM-M2 MΦs potently induced IL-17 production by T cells (Fig. 5e). Notably, MIS-MΦs and BM-M1 MΦs strongly suppressed IFN-γ production by T cells during co-cultivation under the non-Th17 skewing condition (Fig. 5f). Overall, these findings in Fig. 5 support the view that a unique MΦ subset distinguishable from M1 MΦ and M2 MΦ populations is responsible for the T cell mitogenesis-inhibiting and Th17 polarization-enhancing activities of the present MIS-MΦs.
In vivo expansion of Th17 cells in host mice with mycobacterial infection
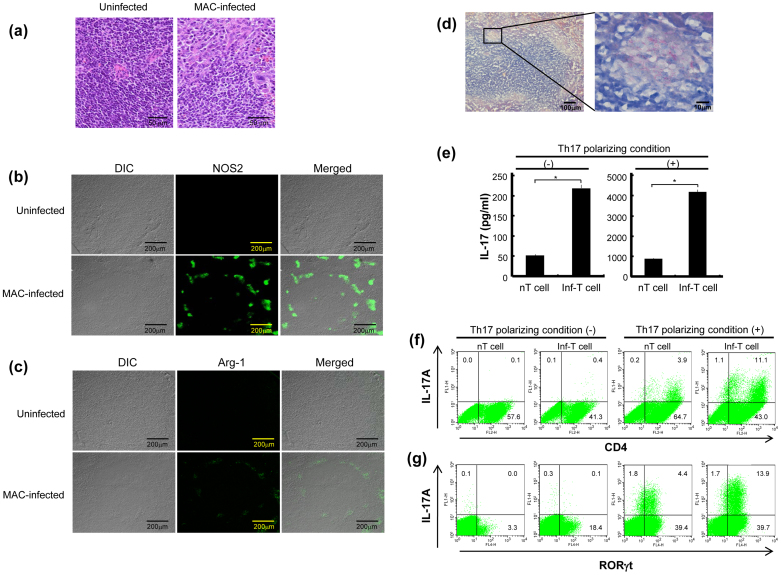
In host mice infected with Mycobacterium avium complex (MAC), one of pathogenic mycobacteria, their spleens were characterized by a marked increase in activated MΦs and epithelioid cells in peripheral regions of the white pulp, which is rich in lymphocytes (Fig. 6a), being associated with a marked increase in NOS2+ cells but not Arg-1+ cells in the same region of the white pulp (Fig. 6b,c). Acid-fast staining revealed that MΦs existing in the same region of white pulp were severely infected with MAC (Fig. 6d). These findings confirm that M1 MΦ-like cells were dominantly accumulated and/or expanded at sites of infection in the spleen of infected mice. Next, we examined the mode of Th17 cell expansion in the spleen of infected mice. When splenic T cells harvested two weeks after infection (Inf-T cells) were cultured under Th17 polarizing or non-Th17 skewing conditions for 5 days, much greater production of IL-17 was observed than in uninfected mice (normal T cells) (Fig. 6e). In this case, the Th17 polarizing condition potently increased IL-17 production by splenic T cells. This phenomenon was confirmed by flow cytometry experiments of the intracellular expression of IL-17A and RORγt by Inf-T cells cultured in Th17 polarizing medium, indicating much greater expansion of the IL-17A+RORγt+CD4+ cells than normal T cells (Fig. 6f,g). Here, Inf-T cells also showed expansion of the IL-17A−RORγt+ cell population, corresponding to precursor Th17 cells, under the non-Th17 skewing condition (Fig. 6g). These in vivo findings indicate that the Th17 cell population actually expands in the spleen of mice during the course of mycobacterial infection. Moreover, the accumulation of M1 MΦ-like cells in peripheral regions of the white pulp of the spleen during infection (Fig. 6a–c) implies that this M1 MΦ-like population plays an important role in Th17 cell expansion at sites of mycobacterial infection.
Figure 6. In vivo expansion of M1 MΦs, M2 MΦs, and Th17 cells in the spleens of host mice with mycobacterial infection.
(a–c) Histopathologic features of the spleen of MAC-infected host mice. (a) The thin sections of spleens from uninfected and infected mice (2 weeks after infection) were subjected to HE staining. (b), (c) The thin sections were stained with either rabbit anti-NOS2 Ab (b) or anti-Arg-1 Ab (c), followed by staining with Fluor 488-conjugated secondary Ab, and subjected to confocal microscopy as described in Experimental Procedures. (d) The thin sections of spleen of infected mice were stained for acid-fast bacilli by the Ziehl-Neelsen staining method. (e–g) Splenic T cells from MAC-infected mice (2 weeks after infection; Inf-T cells) and uninfected mice (nT cells) were stimulated with anti-CD3 and anti-CD28 Abs and cultured under either the Th17 polarizing condition or non-Th17 skewing condition. After 5-day cultivation, culture fluids and cultured T cells were harvested and subjected to measurement of IL-17 concentrations (e) and intracellular expressions of IL-17A (f) and RORγt (g), as described in Fig. 3 and Fig. 2, respectively. Data are representative of multiple experiments; error bars, s.e.m.; *p < 0.01 (Bonferroni's multiple t-test).
Discussion
The present findings showed that the suppressor MΦs induced by mycobacterial infection (MIS-MΦs) down-regulated T cell production of not only Th1 cytokines but also Th2 cytokines, indicating that they exert suppressor activity against both Th1 and Th2 cells (Fig. 1b). Therefore, MIS-MΦs are thought to act as nonspecific suppressor cells in the host immune response against mycobacterial infection. The most interesting finding in this study is that MIS-MΦs markedly augmented IL-17 production from T cells by up-regulating Th17 polarization (Figs. 1, 2). This suggests that MIS-MΦs drive the immune response toward a Th17 phenotype classified by the significant production of IL-17A18,24. In mice, Th17 differentiation is initiated by IL-6 in combination with TGF-β and some proinflammatory cytokines, and IL-23 plays important roles in the maintenance and survival of Th17 cells22,24,25,26. IL-21, which is produced by T cells in response to IL-6 stimulation, is known to induce T cell expression of IL-23 receptor (IL-23R) and trigger Th17 polarization in response to IL-23 generated by antigen-presenting cells28. It is also known that IL-23 up-regulates IL-23R expression by Th17 cells via the activation of the salt-sensing kinase 1-mediated signaling pathway, which subsequently causes down-regulation of Foxo1, a direct repressor of mRNA transcription of the IL-23R gene, one of the targets of RORγt29.
Our present findings confirm the important roles of both IL-6 and TGF-β in MIS-MΦ-mediated potentiation of Th17 expansion (Fig. 4a,b), and imply that IL-1β acts as an important mediator in Th17 polarization triggered by MIS-MΦs (Fig. 3). However, the blocking of IL-21 and IL-23 using specific Abs could not inhibit the expression of the MIS-MΦ action in enhancing Th17 expansion (Fig. 4c,d), suggesting that MIS-MΦs induce Th17 cell expansion in an IL-21- and IL-23-independent fashion. From this aspect, the following observations can be made. First, it is likely that MIS-MΦs produce some unknown factors that trigger the final step of Th17 differentiation, other than IL-21 and IL-23. Indeed, LPS-stimulated bone marrow-derived dendritic cells (DCs) were also reported to produce an unidentified factor that induced Th17 development by an IL-23-independent mechanism30. Notably, Th17 polarizing activity of the factor was not mimicked by IL-21, TNF-β, IL-8, and MΦ inflammatory protein-1α30. In relation to the important role of IL-1β in the manifestation of the action of MIS-MΦs to up-regulate Th17 polarization, it has been reported that IL-26, which is overexpressed by CD68+ MΦ-like synoviocytes in rheumatoid arthritis, enhanced monocytes to generate IL-1β, and consequently augments the expansion of RORγt+ Th17 cells31. In this case, IL-26-induced IL-17A secretion by Th17 cells was inhibited by blocking IL-1β but not IL-21 using a neutralizing Ab31. Therefore, it appears that IL-1β-mediated signaling pathways leading to Th17 polarization may be different from the Th17 polarizing signaling events accomplished by the IL-21-IL-23 axis. Second, in the tumor microenvironment, tumor cell-derived PGE2 up-regulates IL-23 production by DCs via the signaling pathways of EP2 and EP4 receptors-protein kinase A axis, consequently resulting in the enhancement of Th17 polarization32. Although the T cell function inhibitory activity of MIS-MΦ is partly mediated by PGE27,8, this scheme is not applicable to the MIS-MΦ-mediated Th17 expansion, because neither MIS-MΦs or T cells produced detectable amounts of IL-23 under the culture conditions of the present study (unpublished finding). Third, MIS-MΦs transmit suppressor signals to target T cells via cell-to-cell contact through B7-1-like molecules10,11. Therefore, it is also possible that MIS-MΦs transmit Th17 polarization-potentiating signals through direct cell contact with target T cells. Fourth, certain factors are known to down-modulate Th17 polarization. For instance, APC- and Th17-derived IL-15 down-regulates the IL-17A-producing ability of Th17 cells through activation of STAT5, a repressor of the IL17 locus, to directly bind to il17a promoter33. Moreover, early growth response gene-2 (Egr-2), which participates in the maintenance of T cell homeostasis, down-regulates Th17 differentiation via the inhibition of Batf, an IL17 gene-specific transcription factor34. In this case, Egr-2 affects neither STAT3 activation nor RORγt expression. It is possible that MIS-MΦs produce some factor that deactivates IL-17 response-inhibitory factors, such as STAT5 and Egr-234.
The present findings indicate that the M1 MΦ-like population but not M2 MΦs contained in MIS-MΦs plays a central role in the MIS-MΦ-mediated enhancement of Th17 polarization and suppressor action against T cell mitogenesis (Fig. 5). Notably, the major population of MIS-MΦs, which is responsible for the up-regulation of Th17 polarization, has a unique profile of gene expression: IL-12+, IL-1βhigh, IL-6+, TNF-α+, NOS2+, CCR7high, IL-10high, Arg−, MRlow, Ym1high, Fizzlow, and CD163high (Fig. 5). This mode of gene expression is clearly distinguishable from those of typical M1 MΦs and M2 MΦs2,3,5,27. Notably, MIS-MΦs show high-level expression of CCR7 and some M2 MΦ-specific IL-10, Ym1, and CD163 genes (Fig. 5a,b). Therefore, the MΦ population having Th17 polarization-inducing activity in MIS-MΦs can be regarded as a novel type of the polarized MΦ population, provisionally named “M17 MΦ”. This aspect is supported by our present finding that MIS-MΦs enhanced Th17 polarization but conversely inhibited Th1 polarization (Figs. 1a–c, 5e,f). Differing from MIS-MΦs, M1 MΦs induced by IRF5-mediated signaling and SOCS3-deficient M1 MΦs are known to promote both Th1 and Th17 cell differentiation35,36. Therefore, the present MIS-MΦ population (M17 MΦ) is functionally distinguishable from these M1 MΦ populations.
With special reference to the induction of the suppressor MΦs in the spleen of host mice during mycobacterial infection, the following can be pointed out. The serine/threonine protein kinases called Akt, which is the primary downstream mediator of PI3K signaling, includes three isoforms, Akt1, Akt2 and Akt3, that regulate cellular functions related to cell survival, growth, proliferation, angiogenesis, metabolism, and migration37,38. Akt mediates the phosphorylation of target proteins leading to either their activation (MDM2 and eNOS) or inhibition (GSK3, FOXO, BAD, caspase 9, AS160, TCS2, PRAS40, and p27)38. Akt1 and Akt2 differentially contribute to MΦ polarization, that is, Akt1−/− MΦs are hyper-responsive to LPS and undergo M1 polarization, while Akt2−/− MΦs are hypo-responsive to LPS and undergo M2 polarization, based on the expression profiles of M1 MΦ-specific genes and M2 MΦ-specific genes3,5,39. Such distinct roles of Akt isoforms are also known in the regulation of β-integrin activity of prostate cancer cells40. Therefore, it is possible that certain modes of the expression and activation of Akt isoforms in splenic MΦs of host mice with mycobacterial infection might contribute to the differentiation of M17 MΦs.
Th17 cells play a pathogenic role in autoimmune disease18,24. Thus, it is thought that the suppressor MΦs induced by mycobacterial infection, including the present MIS-MΦs, play roles in the establishment of autoimmune symptoms, such as Crohn's disease and Type I diabetes, that are occasionally encountered in hosts with Mycobacterium avium subsp. paratuberculosis infection41,42,43. Other mycobacterial infections, including tuberculosis and leprosy, are also known evoke autoimmune diseases through molecular mimicry44. Since IL-25, belonging to the IL-17 family, down-regulates Th17 function in autoimmune inflammation45,46, it is of interest to examine the effects of IL-25 on the enhancement of Th17 polarization mediated by the present MIS-MΦs. On the other hand, Th17 cells and IL-17 are known to play important roles in host protective immunity against bacteria, such as Staphylococcus, Salmonella, Klebsiella, Bordetella, Mycobacterium, and Francisella, and some fungi, such as Candida albicans18,47. IL-17 induces inflammatory cytokines, including TNF-α, granulocyte-monocyte-colony stimulating factor, and IL-1β, which up-regulate antimicrobial function and chemokine expression of host phagocytes. IL-17 also promotes IL-12 production by DCs during BCG infection via down-regulation of IL-10 generation, consequently resulting in the potentiation of the Th1 response48. In addition, IL-17 and IL-22 are co-expressed by Th17 cells and cooperatively enhance the expression of antimicrobial peptides, including β-defensin 2, by keratinocytes49. These situations suggest that MIS-MΦs play an important role in up-regulating host defense mechanisms against pathogenic bacteria under certain conditions. Therefore, it is likely that MIS-MΦs play dual roles in the host defense against bacterial infection as follows: the potentiation of the antimicrobial function of host MΦs by enhancing their production of IL-12, proinflammatory cytokines, and antimicrobial peptides50 in the early stage of infection, and the induction of immune unresponsiveness in advanced stages of infection. Further detailed studies are currently underway to assess the validity of this view.
Methods
Microorganisms
Mycobacterium avium complex (MAC) N-260 strain (serovar 16) isolated from a patient with MAC infection was used. This organisms was identified as Mycobacterium intracellulare by a DNA probe test.
Mice
Eight- to twelve-week-old male BALB/c mice purchased from Japan Clea Co., Osaka, Japan, were used. Animal care and experimental procedures were approved by the Animal Research Committee of Shimane University and conducted according to the Regulations for Animal Experimentation at Shimane University.
Cell culture
Cells were cultured in RPMI 1640 medium supplemented with 25 mM HEPES, 2 mM glutamine, 100 μg/ml streptomycin, 100 units/ml penicillin G, 50 μM 2-mercaptoethanol and containing 5% fetal bovine serum (FBS) (designated RPMI medium). The MIS-MΦs were prepared as previously described11. Briefly, 4 × 107 of spleen cells (SPCs) obtained from BALB/c mice (Japan Clea) 2 to 3 weeks after iv. infection with 1 × 108 CFUs of MAC organisms were cultured in RPMI 1640 medium in a 90-mm culture dish for 2 hr. After rinsing with 2% FBS-HBSS, adherent cells (MIS-MΦs) were harvested using a cell scraper and re-suspended in RPMI medium. BM-M1 MΦs and BM-M2 MΦs were prepared according to Edwards et al. using BMDMs, which had been flushed from the femurs of mice and cultured in 10% FBS-DMEM/F12 medium containing M-CSF (5,000 units/ml; R&D Systems)12. BM-M1 MΦs were prepared overnight by priming BMDMs with IFN-γ 100 units/ml; R&D), followed by 24-hr stimulation with LPS (10 ng/ml; Escherichia coli O111:B4; Sigma Chemical). BM-M2 MΦs were prepared by stimulating with IL-4 (10 units/ml; R&D) for 48 hr.
Suppressor activity of test MΦs
Nylon column-purified splenic T cells (4 × 105) from normal mice were cultivated in 0.2 ml RPMI medium with or without the addition of test MΦs (3 × 104: MΦ:T cell ratio = 1:14) on culture wells coated with rat anti-mouse CD3 Ab (Serotec) plus hamster anti-mouse CD28 Ab (Pharmingen) at 1 μg/ml each for 72 hr to measure T cell mitogenesis induced by TCR ligation. Proliferating T cells were pulsed with 0.5 μCi of3H-thymidine (PerkinElmer Life Science Products) for the final 6 to 8 hr, and counted for radioactivity. Suppressor activity of test MΦs was calculated as described previously11.
Apoptosis assay based on sub-G1 analysis
Test T cells after cultivation in the presence or absence of test MΦs (MΦ:T cell ratio = 1:14) were fixed with 70% ethanol, treated with RNase A for 1 hr, and then stained with propidium iodide (PI) (10 μg/ml). PI-stained cells were subjected to sub-G1 analysis by flow cytometry using a FACSCalibur cytometer (BD Biosciences).
Cytokine production by test T cells and MΦs
TCR-stimulated splenic T cells were cultured in the presence or absence of test MΦs (MΦ:T cell ratio = 1:14) for up to 7 days under the Th17 polarizing condition using RPMI medium containing mouse IL-6 (20 ng/ml; R&D Systems), human TGF-β (1 ng/ml; PeproTech), anti-mouse IFN-γ Ab (1 μg/ml; eBioscience), and anti-mouse IL-4 Ab (1 μg/ml; eBioscience) or the non-Th17 skewing condition using RPMI medium free from the above supplements. At intervals, culture fluids were harvested and measured for cytokine concentration by ELISA using the DuoSet ELISA Development System kit (R&D Systems) according to the manufacturer's instructions. In some experiments, cytokine production by test MΦs cultured alone was measured by the same ELISA system.
Intracellular expression of cytokines and transcription factors
Test T cells and MΦs (MΦ:T cell ratio = 1:14) cultured under the prescribed conditions for 5 days were harvested and re-stimulated with PMA (25 ng/ml; Sigma) and calcium ionophore A23187 (125 ng/ml; Sigma) in the presence of GolgiStop (BD Biosciences) for 6 hr. After fixation with 1% paraformaldehyde in PBS, the resultant cells were stained with individual Abs, including FITC-, PE-, APC-, eFluor-, or Alexa Fluor 647-labeled Abs specific to mouse cellular proteins, including CD4, RORγt, Foxp3, T-bet, GATA3, γδTCR, IL-17A, IL-12p40, IFN-γ, and IL-10 (eBioscience, BD Pharmingen) using the Cytofix/Cytoperm kit (BD Biosciences) according to the manufacturer's protocol. Cytofluorometry was performed using a FACSCalibur cytometer and FACSAria II Cell Sorter (BD Biosciences), followed by subsequent data analysis using CellQuest Pro software and FACSDiva software (BD Biosciences).
Real-time quantitative RT-PCR
RT-PCR analysis of mRNA expression of M1 and M2 MΦ-specific marker genes by test MΦs was performed as follows. Total RNA was extracted from test MΦs, including the MIS-MΦs, BM-M1 MΦs, and BM-M2 MΦs, using the ISOGEN kit (Nippon Gene). After DNase-I treatment, the resultant RNA samples were reverse transcribed using the PrimeScript RT reagent kit (Takara Bio) according to the manufacturer's instruction. The resultant cDNA was subjected to real-time PCR using SYBR Premix DimerEraser (Takara Bio). Primer sets for individual M1/M2 marker genes are shown in Supplemental Table S2 (available online). The comparative threshold cycle (CT) value for GAPDH was used to normalize loading variations in the real-time PCRs. The intense of mRNA expression of a given MΦ was represented by the relative value to the lower ΔΔCT between those estimated for the BM-M1 MΦ and BM-M2 MΦ: the control ΔΔCT value was fixed at 1.0.
Histology and immunohistochemistry
Spleens excised from mice 2 or 3 weeks after MAC infection were embedded in cryomolds, frozen in liquid nitrogen, cut as serial sections in 14- to 16-μm thickness, and fixed with paraformaldehyde followed by serial treatments with acetic acid/ethanol (1:2) and 1% BSA/0.05% Tween 20 in phosphate-buffered saline (PBS). The resultant sections were reacted with rabbit anti-mouse NOS2 Ab (class IgG: Santa Cruz Biotechnology) or rabbit anti-mouse Arg-1 Ab (IgG: Santa Cruz Biotechnology), followed by staining with Alexa Fluor 488- conjugated donkey anti-rabbit IgG Ab (Molecular Probes). Fluorescence microscopy was performed using an FV1000D IX81 confocal microscope (Olympus) and Eclipse 80i fluorescence microscope (Nikon). The remaining sections were subjected to HE and Ziehl-Neelsen staining.
Statistical analysis
Statistical analysis was performed using Bonferroni's multiple t-test using StatView software (Hulinks).
Author Contributions
H.T. supervised the project and wrote the manuscript. All authors designed experiments and performed experiments. H.T. and Y.T. analyzed the data and prepared all figures. All authors reviewed the manuscript.
Supplementary Material
Supplementary Information
Acknowledgments
This work was partly supported by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan (grant no. 10670255, 18590850, and 23659506). We thank R. Maruyama and T. Tsumori for advice with histopathological techniques.
References
- Benoit M., Desnues B. & Mege J. L. Macrophage polarization in bacterial infections. J. Immunol. 181, 3733–3739 (2008). [DOI] [PubMed] [Google Scholar]
- Lugo-Villarino G., Verollet C., Maridonneau-Parini I. & Neyrolles O. Macrophage polarization: convergence point targeted by Mycobacterium tuberculosis and HIV. Front. Immunol. 2, 43 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray P. J. & Wynn T. A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 11, 723–737 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sica A. & Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J. Clin. Invest. 122, 787–795 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomioka H. et al. Characteristics of suppressor macrophages induced by mycobacterial and protozoal infections in relation to alternatively activated M2 macrophages. Clin. Dev. Immunol. 2012, 635451 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanHeyningen T. K., Collins H. L. & Russell D. G. IL-6 produced by macrophages infected with Mycobacterium species suppresses T cell responses. J. Immunol. 158, 330–337 (1997). [PubMed] [Google Scholar]
- Maw W. W., Shimizu T., Sato K. & Tomioka H. Further study on the roles of the effector molecules of immunosuppressive macrophages induced by mycobacterial infection in expression of their suppressor function against mitogen-stimulated T cell proliferation. Clin. Exp. Immunol. 108, 26–33 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomioka H., Saito H. & Yamada Y. Characteristics of immunosuppressive macrophages induced in spleen cells by Mycobacterium avium complex infections in mice. J. Gen. Microbiol. 136, 965–973 (1990). [DOI] [PubMed] [Google Scholar]
- Tomioka H., Sato K., Maw W. W. & Saito H. The role of tumor necrosis factor, interferon-γ, transforming growth factor-β, and nitric oxide in the expression of immunosuppressive functions of splenic macrophages induced by Mycobacterium avium complex infection. J. Leukoc. Biol. 58, 704–712 (1995). [DOI] [PubMed] [Google Scholar]
- Ogasawara K. et al. Profiles of cell-to-cell interaction of Mycobacterium intracellulare induced immunosuppressive macrophages with target T cells in terms of suppressor signal transmission. Clin. Exp. Immunol. 129, 272–280 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T., Sano C. & Tomioka H. The role of B7 molecules in the cell contact-mediated suppression of T cell mitogenesis by immunosuppressive macrophages induced with mycobacterial infection. Clin. Exp. Immunol. 135, 373–379 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards J. P., Zhang X., Frauwirth K. A. & Mosser D. M. Biochemical and functional characterization of three activated macrophage populations. J. Leukoc. Biol. 80, 1298–1307 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B., Hirota K., Cua D. J., Stockinger B. & Veldhoen M. Interleukin-17-producing γδT cells selectively expand in response to pathogen products and environmental signals. Immunity 31, 321–330 (2009). [DOI] [PubMed] [Google Scholar]
- Sutton C. E., Mielke L. A. & Mills K. H. IL-17-producing γδ T cells and innate lymphoid cells. Eur. J. Immunol. 42, 2221–2231 (2012). [DOI] [PubMed] [Google Scholar]
- Gocke A. R. et al. T-bet regulates the fate of Th1 and Th17 lymphocytes in autoimmunity. J. Immunol. 178, 1341–1348 (2007). [DOI] [PubMed] [Google Scholar]
- Hosoy T., Maillard I. & Engel J. D. From the cradle to the grave: activities of GATA-3 throughout T-cell development and differentiation. Immunol. Rev. 238, 110–125 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov I. I. et al. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126, 1121–1133 (2006). [DOI] [PubMed] [Google Scholar]
- Muranski P. & Restifo N. P. Essentials of Th17 cell commitment and plasticity. Blood 121, 2402–2414 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y. et al. T-bet is essential for encephalitogenicity of both Th1 and Th17 cells. J. Exp. Med. 206, 1549–1564 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoreschi K. et al. Generation of pathogenic TH17 cells in the absence of TGF-β signalling. Nature 467, 967–971 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn T. et al. IL-21 initiates an alternative pathway to induce proinflammatory TH17 cells. Nature 448, 484–487 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan P. R. et al. Transforming growth factor-β induces development of the TH17 lineage. Nature 441, 231–234 (2006). [DOI] [PubMed] [Google Scholar]
- Tomioka H. & Saito H. Characterization of immunosuppressive functions of murine peritoneal macrophages induced with various agents. J. Leukoc. Biol. 51, 24–31 (1992). [DOI] [PubMed] [Google Scholar]
- Bettelli E., Korn T., Oukka M. & Kuchroo V. K. Induction and effector functions of TH17 cells. Nature 453, 1051–1057 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeachy M. J. et al. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat. Immunol. 10, 314–324 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver C. T., Harrington L. E., Mangan P. R., Gavrieli M. & Murphy K. M. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity 24, 677–688 (2006). [DOI] [PubMed] [Google Scholar]
- Mantovani A., Sozzani S., Locati M., Allavena P. & Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 23, 549–555 (2002). [DOI] [PubMed] [Google Scholar]
- Zhou L. et al. IL-6 programs TH-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat. Immunol. 8, 967–974 (2007). [DOI] [PubMed] [Google Scholar]
- Wu C. et al. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature 496, 513–517 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura A., Naka T. & Kishimoto T. IL-6-dependent and -independent pathways in the development of interleukin 17-producing T helper cells. Proc. Natl. Acad. Sci. U.S.A. 104, 12099–12104 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corvaisier M. et al. IL-26 is overexpressed in rheumatoid arthritis and induces proinflammatory cytokine production and Th17 cell generation. PLoS Biol. 10, e1001395 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X. et al. Increased Th17 cells in the tumor microenvironment is mediated by IL-23 via tumor-secreted prostaglandin E2. J. Immunol. 190, 5894–5902 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandiyan P. et al. The role of IL-15 in activating STAT5 and fine-tuning IL-17A production in CD4 T lymphocytes. J. Immunol. 189, 4237–4246 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao T. et al. Early growth response gene-2 controls IL-17 expression and Th17 differentiation by negatively regulating Batf. J. Immunol. 190, 58–65 (2013). [DOI] [PubMed] [Google Scholar]
- Krausgruber T. et al. IRF5 promotes inflammatory macrophage polarization and TH1-TH17 responses. Nat. Immunol. 12, 231–238 (2011). [DOI] [PubMed] [Google Scholar]
- Qin H. et al. SOCS3 deficiency promotes M1 macrophage polarization and inflammation. J. Immunol. 189, 3439–3448 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hers I., Vincent E. E. & Tavaré J. M. Akt signalling in health and disease. Cell. Signal. 23, 1515–1527 (2011). [DOI] [PubMed] [Google Scholar]
- Manning B. D. & Cantley L. C. AKT/PKB signaling: navigating downstream. Cell 129, 1261–1274 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arranz A. et al. Akt1 and Akt2 protein kinases differentially contribute to macrophage polarization. Proc. Natl. Acad. Sci. USA. 109, 9517–9522 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virtakoivu R., Pellinen T., Rantala J. K., Perälä M. & Ivaska J. Distinct roles of AKT isoforms in regulating β1-integrin activity, migration, and invasion in prostate cancer. Mol. Biol. Cell 23, 3357–3369 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand S. Crohn's disease: Th1, Th17 or both? The change of a paradigm: new immunological and genetic insights implicate Th17 cells in the pathogenesis of Crohn's disease. Gut 58, 1152–1167 (2009). [DOI] [PubMed] [Google Scholar]
- Dow C. T. Paratuberculosis and Type I diabetes: is this the trigger? Med. Hypotheses 67, 782–785 (2006). [DOI] [PubMed] [Google Scholar]
- Greenstein R. J. Is Crohn's disease caused by a mycobacterium? Comparisons with leprosy, tuberculosis, and Johne's disease. Lancet Infect. Dis. 3, 507–514 (2003). [DOI] [PubMed] [Google Scholar]
- Rook G. A. W. & Stanford J. L. Slow bacterial infections or autoimmunity? Immunol. Today 13, 160–164 (1992). [DOI] [PubMed] [Google Scholar]
- Fort M. M. et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity 15, 985–995 (2001). [DOI] [PubMed] [Google Scholar]
- Kleinschek M. A. et al. IL-25 regulates Th17 function in autoimmune inflammation. J. Exp. Med. 204, 161–170 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeachy M. J. & McSorley S. J. Microbial-induced Th17: superhero or supervillain? J. Immunol. 189, 3285–3291 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopal R. et al. IL-23-dependent IL-17 drives Th1-cell responses following Mycobacterium bovis BCG vaccination. Eur. J. Immunol. 42, 364–373 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang S. C. et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 203, 2271–2279 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agerberth B. & Gudmundsson G. H. Host antimicrobial defence peptides in human disease. Curr. Top. Microbiol. Immunol. 306, 67–90 (2006). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information