Abstract
The human SLC6 family is composed of approximately 20 structurally related symporters (co-transporters) that use the transmembrane electrochemical gradient to actively import their substrates into cells. Approximately half of the substrates of these transporters are amino acids, with others transporting biogenic amines and/or closely related compounds, such as nutrients and compatible osmolytes. In this short review, five leaders in the field discuss a number of currently important research themes that involve SLC6 transporters, highlighting the integrative role they play across a wide spectrum of different functions. The first essay, by Gary Rudnick, describes the molecular mechanism of their coupled transport which is being progressively better understood based on new crystal structures, functional studies, and modeling. Next, the question of multiple levels of transporter regulation is discussed by Reinhard Krämer, in the context of osmoregulation and stress response by the related bacterial betaine transporter BetP. The role of selected members of the human SLC6 family that function as nutrient amino acid transporters is then reviewed by François Verrey. He discusses how some of these transporters mediate the active uptake of (essential) amino acids into epithelial cells of the gut and the kidney tubule to support systemic amino acid requirements, whereas others are expressed in specific cells to support their specialized metabolism and/or growth. The most extensively studied members of the human SLC6 family are neurotransmitter reuptake transporters, many of which are important drug targets for the treatment of neuropsychiatric disorders. Randy Blakely discusses the role of posttranscriptional modifications of these proteins in regulating transporter subcellular localization and activity state. Finally, Dennis Murphy reviews how natural gene variants and mouse genetic models display consistent behavioral alterations that relate to altered extracellular neurotransmitter levels.
Keywords: Nembrane transport, Amino acid transport, Neurotransmitter transporters, Compatible osmolyte uptake, Neuropsychiatric disorders, Mouse models
Introduction
The human SLC6 family contains evolutionary, and thus structurally related, molecular machines that actively translocate amino acids and related solutes into cells against their concentration gradient using, as a driving force, the energetically favorable coupled movement of ion(s) down their trans-membrane electrochemical gradients. All SLC6 members indeed transport one, two or three sodium ion(s) together with their organic substrate (symport). Many of these transporters additionally symport one chloride ion, and one transporter also exchanges a potassium ion per substrate (antiport). The SLC6 transporters are actually part of the larger neurotransmitter sodium symporter (NSS) family of structurally related transporters that includes numerous prokaryotic members, and which itself is included in the very large amino acid–poly-amine–organocation (APC) superfamily (Table 1) [26, 87]. About half of the SLC6 family members transport amino acids, of which some are neurotransmitters (glycine and GABA) and the others transport related molecules such as monoamine neurotransmitters (serotonin, norepinephrine, and dopamine), creatine, or compatible osmolytes (taurine and betaine).
Table 1.
Classification of SLC6 family transporters
| Transporter Classification Database (TCDB) (www.tcdb.org) (based on sequence homology, includes prokaryotic and eukaryotic proteins) | ||
| Superfamily | Acid–polyamine–organocation (APC) | Second largest superfamily of secondary carriers |
| Family (within APC superfamily) | Neurotransmitter sodium symporter family (NSS) | One out of currently 10 families belonging to APC superfamily |
| HGNC gene families/groupings nomenclature (www.genenames.org) (nomenclature of human genome project, now used also for transporters of other eukaryotic genomes) | ||
| SLC gene nomenclature systema | Solute carriers (SLC) | Group of human gene families originally proposed by the Human Genome Organization (HUGO), comprising currently 52 SLC families |
| SLC family (within SLC group of gene families) | Solute carrier family 6 (SLC6) | Family including transporters with >20 % sequence homology. |
| The members of SLC6 belong to the NSS family of the TCDB classification | ||
| SLC6 subfamilies [8, 9, 43, 64] | GABA transporters (= GABA, neurotransmitter, osmolyte, creatine transporters) | Subfamily includes: |
| SLC6A1, GAT1 | ||
| SLC6A6, TauT | ||
| SLC6A8, CT1 | ||
| SLC6A11, GAT3 | ||
| SLC6A12, BGT1 | ||
| SLC6A13, GAT2 | ||
| Monoamine transporters (= monoamine neurotransmitter transporters) | Subfamily includes: | |
| SLC6A2, NET | ||
| SLC6A3, DAT | ||
| SLC6A4, SERT | ||
| Amino acid transporters (I) (= neurotransmitter amino acid transporters) | Subfamily includes: | |
| SLC6A5, GlyT2 | ||
| SLC6A7, PROT | ||
| SLC6A9, GlytT1 | ||
| SLC6A14, ATB0,+ | ||
| Amino acid transporters (II) (= nutrient amino acid transporters) | Subfamily includes: | |
| SLC6A15, B0AT2 | ||
| SLC6A16, NTT5 | ||
| SLC6A17, NTT4 | ||
| SLC6A18, B0AT3 | ||
| SLC6A19, B0AT1 | ||
| SLC6A20, SIT1 | ||
The SLC gene nomenclature names are often used also for the corresponding transporters
In recent years, a number of excellent, systematic reviews have presented extensive descriptions of the phylogenetic organization of the SLC6 transporter family, focusing largely on the literature published since the molecular identification of its members began more than 20 years ago [8, 9, 43, 64]. The purpose of the present review is to discuss, in a few essays, several aspects of more current research, and to highlight important questions likely to drive future studies, ranging from the mechanistic structure–function relationship of coupled transport to different aspects of transporter regulation, and finally their role in neuropsychiatric disorders.
In the first essay, Gary Rudnick provides a conceptual framework for part of the reaction cycle of SLC6 transporters and discusses open questions regarding the transport mechanism. The second essay addresses adaptation of transport activity to changes in extracellular osmolarity as an important property of a number of SLC6 family members transporting compatible osmolytes. In this context, Reinhard Krämer emphasizes the central role played by post-translational transporter processing for tuning the short term functional response to stressful conditions, using the SLC6-related betaine transporter BetP of Corynebacterium glutamicum as a model system. Next, the amino acid transporters of the SLC6 family are briefly discussed by François Verrey who describes the surprisingly broad spectrum of roles these active transporters display. Whereas some transporters expressed at epithelial surfaces mediate the first active uptake step of amino acids into the organism, others import amino acids within the organism into cells either to control the extracellular concentration of their substrates, as it is the case for neurotransmitter transporters, or to provide specific cells actively with amino acids to be used for their specialized metabolism or growth. Clearly, less information is available on amino acid transporters than on neurotransmitter transporter of the SLC6 family. This is due to their later molecular identification and to the fact that unlike neurotransmitter transporters, they have not been linked to important diseases, either by genetic association or as drug targets.
The regulation of biogenic amine neurotransmitter transporters of the SLC6 family and of their defects observed in the context of neuropsychiatric disorders are discussed by Randy Blakely, who emphasizes in his essay the role of post-transcriptional modifications on transporter subcellular localization and activity. In the last essay, Dennis Murphy discusses the role of SLC6 transporters in neuropsychiatric disorders, in particular examples of genetic variants, pharmacological targets and corresponding mouse genetic models that provide insights into the physiological and behavioral impact of SLC6 neurotransmitter transporter alterations.
Mechanisms for coupled transport by SLC6 transporters (Gary Rudnick)
Coupling of transport to ion gradients and potentials
The human SLC6 family is part of a larger NSS family of homologous transporters that includes many prokaryotic transporters. In turn, the NSS family is part of a superfamily of structurally related transporters [26]. These proteins move their substrates across the membrane using a variety of energy sources. In many cases, transmembrane ion gradients provide the driving force. Within the SLC6 family, substrates are generally amino acids, although some family members transport amines, such as the neurotransmitters serotonin (5-HT), norepinephrine (NE), and dopamine (DA).
The ability of transporters in this family to concentrate their substrates inside the cell depends on coupling the energetically favorable movement of Na+ and sometimes Cl− to the energetically unfavorable flux of substrate into the cell (symport). Because transport by these proteins is reversible, the overall direction and magnitude of substrate transport is determined by the polarity and strength of the ion and substrate gradients. If electrical charge accompanies substrate transport, the membrane potential can contribute to the driving force, and some transporters additionally couple substrate influx to the favorable efflux of K+ (antiport). By utilizing the electrochemical potential in these ion gradients, SLC6 transporters can accumulate intracellular substrate to concentrations hundreds of times higher than outside the cell. The coupling mechanisms responsible for this accumulation are well documented but poorly understood at the molecular level.
Alternating access models and rules for coupling
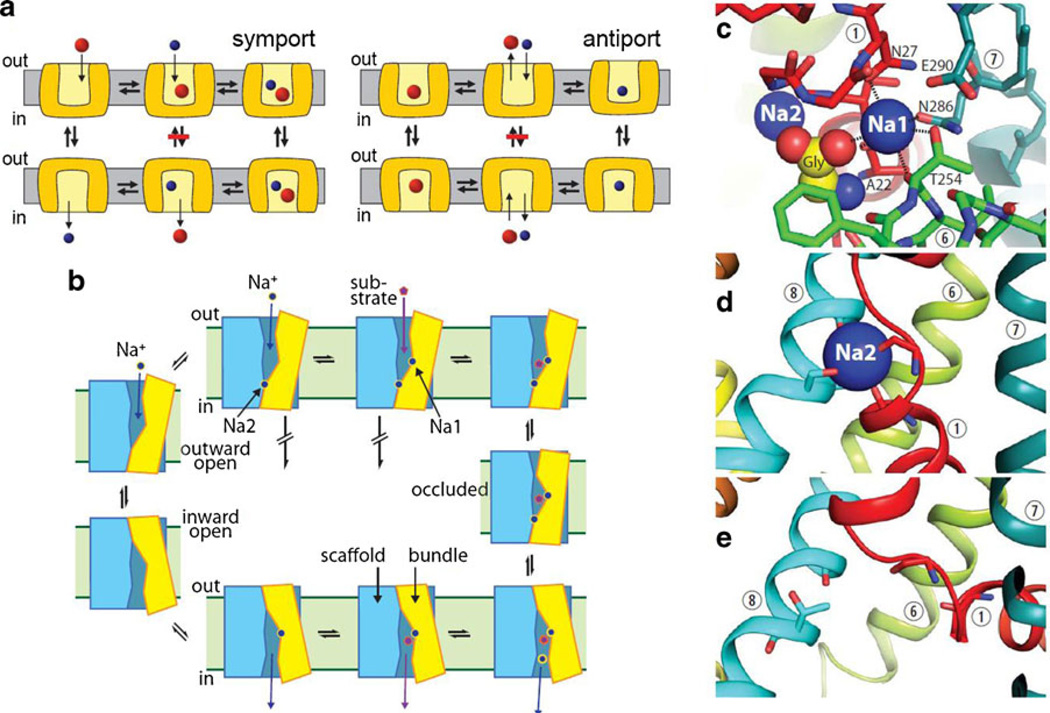
The physical model used to explain coupled transport (both symport and antiport) is the alternating access mechanism [59], which proposed two transporter conformations, each of which exposes a central binding site to one side or the other of the membrane (Fig. 1a). Interconversion of the two forms with substrate bound allows substrate transport across the membrane. However, to couple substrate and Na+ transport (for example), this step should occur only when both substrate and Na+ are bound. Then, after release of Na+ and substrate to the cytoplasm, the transporter would revert to an outward-open conformation with the binding sites empty. For strictly stoichiometric Na+-substrate coupling, this mechanism requires that the conversion between outward- and inward-open conformations should occur only when both Na+ and substrate are bound or when the binding site is empty. For antiport, however, a counter-ion such as K+ and substrate must be transported in different steps, and their binding should be mutually exclusive. Substrate would be bound during the transition from outward- to inward-open and K+ bound in the opposite direction. In its strictest form, the alternating access mechanism presumes that symported ions are bound and transported together with substrate but antiported ions are transported in the step when substrate is not bound (Fig. 1a).
Fig. 1.
Alternating access mechanisms and LeuT. a Similar conformational changes could account for symport (left) and antiport (right) using different rules. Prohibited conformational changes are indicated by a red bar. b The reaction cycle of LeuT is initiated by binding of two Na+ ions (upper left) followed by substrate binding (upper right). Conformational changes to the occluded state and the inward-open state follow (right). Dissociation of Na+ and substrate leads to the apo-state (lower left) which can re-orient to the outward open form (left). c–e Binding sites for Na1 and substrate (c) and Na2 in outward-open (d) and inward-open (e) structures. Na1 binding site residues and transmembrane helices are numbered
Structures and models for SLC6 conformations
Any understanding of how transport is coupled to ion gradients requires that we understand the molecular details of structure and conformational change. In the NSS family, there is presently only LeuT, a prokaryotic amino acid transporter, for which we have a high-resolution atomic structure. LeuT has been crystallized in three conformations and with several substrates and inhibitors. The first structures of LeuT were with substrate bound and with the protein in an outward-occluded conformation [89]. Subsequent structures showed the protein in outward- and inward-open conformations [42]. The outward-occluded conformation is interesting and important, because it provides a way for LeuT to transform from outward- to inward-open forms without being simultaneously open to both sides of the membrane. If the transporter opened a pathway from the binding site to the cytoplasm before it closed the pathway to the cell exterior, uncoupled flux of substrate and ions could occur. The presence of an intermediate state in which the binding site is effectively sealed off from both sides prevents this uncoupled flux.
The first LeuT structure revealed an unexpected structural motif that has since become a common feature in transporter structures. In this 12-transmembrane (TM) helix protein, the structure of TMs 1–5 is repeated in the structure of TMs 6–10 except that the topological orientation of the two similar structures is inverted. If the two repeats were identical, the overall structure of TMs 1–10 would be symmetrical, but this was not found. Instead, the extracellular substrate permeation pathway in this occluded structure was open up to (but not including) the central binding sites for Na+ and substrate but the cytoplasmic pathway was totally closed. This asymmetry resulted from differences in the conformation of the two repeats that proved to be a key to understanding conformational change [27].
The structure of LeuT TMs 1–10 can be divided into two domains, a four-helix bundle (TMs 1, 2, 6, and 7) and a scaffold (TMs 3–5 and 8–10), with the two repeats contributing equally to each domain. The four-helix bundle sits at an angle to the scaffold, packing, in outward-oriented conformations, closely against the scaffold on the cytoplasmic side of the binding site and separating from the scaffold on the extracellular side to create the extracellular pathway (cartoon version in Fig. 1b). In models of the inward-open structure and structures of other members of the superfamily, the tilt axis of the bundle is reversed, suggesting a “rocking bundle” mechanism that closes the extracellular pathway and opens a pathway between the binding sites and the cytoplasm [27, 86]. All structures in the superfamily are consistent with conformational change involving movement of the bundle relative to the scaffold, and with relatively little conformational change within the scaffold [26].
Differences between inward-open structures and models raise the issue of how conformational changes in SLC6 transporters are propagated. To account for many observations where opening of the extracellular pathway was observed with reciprocal closing of the cytoplasmic pathway (and vice versa), we proposed that the long, unbroken helices of TMs 2 and 7 might serve to transmit conformational changes between the extracellular and intracellular halves within the bundle [26]. However, the LeuT inward-open structure suggests that the cytoplasmic pathway opens when the cytoplasmic half of TM1 swings away from the rest of the helical bundle [42], possibly requiring an alternate mechanism for coupling this event to closing the extracellular pathway.
Ligand-induced conformational changes
As more transporter structures are solved, the nature of conformational change in each transporter family is becoming clearer, and knowledge of how ligand binding controls conformational change is becoming more important as the next step in understanding the molecular mechanism of transport. The rules that ensure strict coupling between ion and substrate transport determine when the transporter changes conformation from inward- to outward-open. These conformational changes occur only when a specific set of conditions are satisfied. In the case of Na+-substrate symport, for example by LeuT, the coupling between Na+ and substrate movements requires that the conformational changes occur either when the transporter has bound both Na+ and substrate or when those binding sites are empty (Fig. 1a – b). Another way to view these rules is that they prevent conformational change when only Na+ or only substrate is bound. To accomplish this discrimination, the protein must use binding site occupancy to control conformational transitions.
For SLC6 transporters, the best structural model at present is LeuT, and studies with LeuT and other transporters already hint as to how SLC6 proteins accomplish the coupling of binding to conformational change. The rocking bundle hypothesis predicts that conformational changes occur by movement of the four-helix bundle relative to the scaffold domain. The point at which these two domains have the most consistent contact is a nexus of binding sites for two Na+ ions and one substrate molecule near the center of the protein (Fig. 1b – c). Thus the structure is optimized to directly couple binding site occupancy to conformational change.
In LeuT, and in the aromatic amino acid transporter Tyt1, evidence suggests that Na+ influences conformational change. Cysteine residues in the cytoplasmic pathway of Tyt1 were less accessible in the presence of Na+, indicating a shift to an inward-closed (outward-open) conformation [65]. Similar results for LeuT were observed using single molecule FRET techniques, which also suggested that the cytoplasmic pathway was closed by Na+ [90]. EPR measurements of accessibility and distance in the LeuT extracellular pathway also show changes with Na+, but because the EPR probes were in the extracellular pathway, Na+ was found to increase accessibility and distances between positions, consistent with the extracellular pathway opening as the cytoplasmic pathway closed [16]. From the results with Tyt1 and LeuT, substrate causes the extracellular pathway to close and the cytoplasmic pathway to open, but only in the presence of Na+.
These basic observations illustrate a mechanism that SLC6 transporters could use to couple Na+ and substrate transport. The rules for symport prevent conformational change when only Na+ is bound, and the ability of Na+ to stabilize one conformation could lock the transporter in that outward-open state until substrate binds. Indeed, both smFRET and EPR show apo-LeuT distributed between inward- and outward-open states in the apo-state but strongly biased by Na+ toward outward-open conformations with transitions between states largely eliminated [16, 90].
What prevents SLC6 proteins from transporting substrate in the absence of Na+? Substrate binding by some transporters in this family depends on Na+ [72]. This strong dependence means that the substrate is unlikely to bind to apo-LeuT. These observations provide a way for SLC6 transporters to prevent transport of Na+ or substrate alone, but they do not indicate how these rules ensuring strict symport are encoded in the structure.
In LeuT structures, one of the two bound Na+ ions (Na1) is directly coordinated by the substrate carboxyl group [89]. The strong ionic interaction between Na1 and substrate forms an essential part of the substrate binding site (Fig. 1c) and is likely responsible for the Na+-dependence of substrate binding. The other bound Na+ ion (Na2) is bound at a site formed by TM1 (in the four-helix bundle) and TM8 (in the scaffold) [89] (Fig. 1d). The location of Na2 at this interface provides a mechanism by which Na+ can stabilize the outward-open form of LeuT. Occupation of the Na2 site could foster interaction between the scaffold and the cytoplasmic half of the bundle, closing the cytoplasmic pathway by holding the two domains together [42]. In inward-open structures and models, by contrast, the Na2 site is not occupied and the two domains separate from each other [27, 42] (Fig. 1e). Thus, specialization of the two Na+ sites, with Na2 serving to stabilize the outward-open conformation and Na1 required for substrate binding may hold the key to coupling between ion and substrate transport.
The mechanism outlined above provides a conceptual framework for part of the reaction cycle. Na+ binding initiates the process, and the strong bias toward an outward-open conformation prevents transport of the Na+ ions before substrate binds. However, solid experimental validation is still lacking. Moreover, substrate binding must trigger conformational change by overcoming the conformational effect of Na+ binding. Substrate binding in the extracellular pathway was proposed to initiate this conformational change [72], but this proposal has encountered resistance, in part because substrate binding at the proposed site has not been observed in crystal structures. Alternative mechanisms have not been proposed, however, and the effect of substrate on conformational change remains an unresolved topic for future studies.
Although amino acid substrates cannot bind in the absence of Na+, amine neurotransmitters such as 5-HT, DA and NE do not have carboxyl groups and do not require Na+ for binding. It is still not clear how an SLC6 amine transporter would prevent substrate transport in the absence of Na+. Furthermore, transporters for 5-HT and NE are likely to symport only one Na+ ion with substrate [37, 79], despite conservation of both Na1 and Na2 sites. An aspartate in TM1 unique to these three amine transporters is now known to participate in coordinating Na1 in the Drosophila DA transporter [60]. It is likely that this aspartate replaces the missing substrate carboxyl group in SLC6 amine transporters, allowing the transporter to hold on to Na1 through the transport cycle when it releases Na2 and substrate to the cytoplasm.
The role of SLC6 transporters in osmoregulation and stress response (Reinhard Krämer)
Stress response is a vital aspect of cellular life, both in prokaryotes and in eukaryotes. Among various types of environmental stress, a change in the external osmolality is a frequent type of challenge. The cell’s response to this challenge is called osmoregulation in prokaryotes and volume control in eukaryotic cells. Although the long-term adaptation to these conditions at the level of transcription, translation, and post-translational processing are also relevant to the response, the contribution of acute regulation of transport system activity is the main focus when considering the stress response.
The activity of several transporters in the SLC6 family has been proven or is assumed to be modulated by a change in the osmolality of their surroundings. Relevant examples are Bgt1, TauT, and SNF-12 [9, 20]. The best-studied models for osmoregulation are members of the structurally related BCCT family of transporters, named after the typical substrates betaine, carnitine, and choline [95]. One member of this family, the betaine transporter BetP from the Gram-positive soil bacterium Corynebacterium glutamicum, is a paradigm for regulated secondary transport [95]. Consequently, the major lines of interest and research perspectives will be outlined using this carrier as a basis.
BetP is a secondary transporter composed of 595 amino acids, 12 transmembrane segments, and prominent N- and C-terminal domains, which are critically involved in sensing osmotic stimuli [58, 62]. It exclusively accepts glycine betaine as a substrate. Active uptake of betaine is coupled to co-transport of two Na+ ions and thus driven by the electrochemical Na+ potential [23]. As a particular feature, BetP comprises two independent functions: It catalyzes active transport of betaine, and it senses physical stimuli related to hyperosmotic stress which lead to activation of the transporter. Detailed biochemical and structural information on BetP is available, covering both the functional analysis of catalytic activity (transport) as well as regulation (stimulus sensing and signal transduction), and in depth structural analysis on the level of 2D and 3D crystals [61, 68, 95]. Consequently, BetP is a prominent example where the integration of biochemistry and structural biology has led to deep insight into the mechanism of both transport catalysis and transport regulation. A detailed picture for the catalytic cycle of BetP is available based on the observation of several different crystal forms of this protein representing different conformational states within the transport cycle [61]. Stimulus analysis has shown that BetP activity is modulated by two different types of physical stimuli. A rise in internal K+ is the immediate cellular response to a hyperosmotic shift and has been proven to be a primary stimulus mainly based on results using purified reconstituted BetP [69]. Very recently, by detailed analysis under in vivo conditions, a second type of stimulus was discovered to be required, in addition to K+, for full activation of BetP. This stimulus is a change in the physical state of the membrane directly surrounding BetP (unpublished results).
Despite the fact that a wealth of functional and structural information regarding its response to osmotic stress is already available for BetP, there are a number of urgent and interesting questions, based on the rather advanced state of our knowledge of mechanistic aspects available for this transporter. Since BetP is a molecular machine integrating transport catalysis, stimulus perception, signal transduction and stress adaptation all in one single polypeptide chain, it is compelling to obtain a precise description of the sequence of these events at the level of domain function and peptide chain movements. On the basis of detailed structural work, we have a comparatively good insight into the conformational events in the core domain of BetP according to the mechanism of alternative access [61]. This is, unfortunately, not the case for stimulus sensing and intramolecular signal transduction. Moreover, BetP is a rare example of a transporter, for which the physical stimuli modulating its catalytic action are at least partly understood. However, the mechanistic picture is far from complete, and I would like to illustrate this for a physiologically relevant situation.
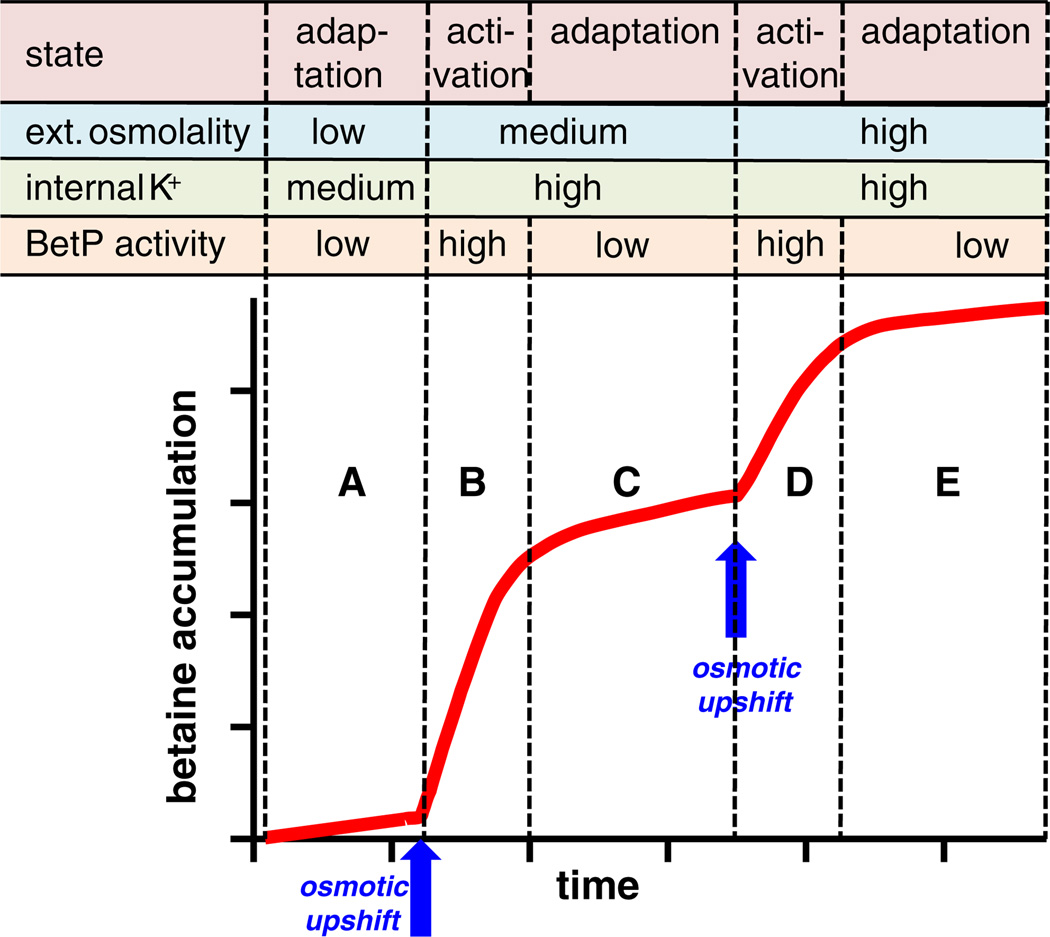
Stress response is not a simple sequence caused by the action of a stimulus and resulting in an immediate reaction of the target protein. In order to guarantee proper survival, the cell needs to adapt its response in a graduated manner, a higher extent of stress leading to a stronger reaction and a lower extent leading to a more moderate response. In fact, BetP was shown to behave exactly like this [7]. Its action, betaine uptake, ceases at an appropriate level of betaine accumulation when osmotic compensation is reached (Fig. 2). This physiologically meaningful behavior raises at least two questions. How does BetP accurately sense when betaine uptake should stop, in spite of continuing high osmolality of the external medium and, in particular, in spite of the fact that the stimulus for activation, internal K+, continues to be high for prolonged periods under these conditions? Notably a graduated response to an external stress-related stimulus is more complex than a graduated response in transport activity due to substrate availability, for example, which is simply mediated by variable saturation of the substrate binding site. The second question does not directly refer to molecular details of regulating protein activity by external stimuli, but rather asks more fundamentally: Is regulation on the level of the individual BetP molecule or the population? A perfectly adapted BetP, fine-tuned to the actual extent of osmotic stress can be achieved either by a sophisticated molecular mechanism leading to a graduated response of every single BetP molecule or by a balanced steady state of individual BetP molecules oscillating between active and inactive states. Although we intuitively tend towards the latter explanation, the answer to this question is by no means clear and has not been achieved for any regulated transport system.
Fig. 2.
Fine-tuning of BetP-mediated betaine uptake. Upon a hyperosmotic upshift (A > B) which leads to an increase in the cytoplasmic K+ concentration, BetP switches into the active state and actively accumulates betaine in the cell. When osmotic adaptation is reached, BetP activity ceases (B >C). Upon application of a second upshift to higher external osmolality (C>D), the cycle of activation and adaptation (C>D, D >E) is initiated again
The aim of the question(s) raised is to explain fine-tuned, physiologically relevant responses of transporters on a mechanistic level or, in other words, to gain full mechanistic understanding of the regulatory and catalytic action of a transporter in a physiologically relevant context. What are the tools required to reach this aim? On a macroscopic level, we need to challenge results obtained by biochemical and/or structural biology approaches, frequently in a simplified experimental setup, by checking their validity under in vivo conditions in intact cells to the extent possible. In the case of BetP, as an example, the specific stimulus of high luminal K+ concentrations was identified. However, the fact that BetP activity in intact cells down-regulates during osmotic adaptation despite continuing high internal K+ led us to propose and finally identify a second type of stimulus, which seems to be relevant for this mechanism of adaptive fine-tuning. On a microscopic level, we need a couple of additional tools in order to be able to solve questions of the quality raised above. High resolution 3D structures are truly a tremendous help for interpreting transporter function, however, they probably will not provide appropriate answers to some of the functional questions described above. Various spectroscopic techniques, particularly fluorescence spectroscopy, as well as EPR and NMR, are instrumental for responding to the challenges in understanding the dynamic properties of membrane proteins. Only with successful application of single molecule techniques, combined, for example, with FRET analysis, we will be able to resolve questions regarding the mechanistic basis of the perfectly fine-tuned response by this type of proteins to external stress conditions in physiologically relevant surroundings.
Concentrative amino acid uptake transporters: cellular and systemic roles (Francois Verrey)
SLC6 amino acid transporters
Approximately half of the SLC6 family genes encode amino acid transporters that are phylogenetically grouped in two subfamilies (I and II). Their common function is the concentrative cellular uptake of proteinogenic amino acids which is driven by the symport of at least one Na+ ion down its electrochemical gradient. However, these transporters differ in terms of amino acid substrates, localization, co-transport stoichiometry and functional roles. As discussed below, these SLC6 amino acid transporters are involved in three different functions each requiring concentrative cellular uptake: (1) control of the extracellular concentration of neurotransmitter amino acids in the context of synaptic transmission (transmitter reuptake); (2) active uptake of amino acids into specific cells to support their specialized metabolism or growth and/or (3) active epithelial uptake of amino acids from the lumen of the gut and kidney tubule to support systemic amino acid requirements.
The first of the two SLC6 amino acid transporter subfamilies is called amino acid transporters (I) or amino acid neuro-transmitter transporters and includes GLYT1 and GLYT2 (SLC6A9 and SLC6A5), PROT (SLC6A7), and ATB0+ (SLC6A14) [9, 64]. The first three members of this subfamily appear indeed to play a role in the modulation of synaptic transmission, whereas ATB0+ (SLC6A14) has quite different functions. The GLYTs function as high affinity transporters (KM in 10–100-µM range) for glycine. GLYT2 (SLC6A5) was suggested to function mostly as neuronal reuptake transporter for the inhibitory neurotransmitter glycine with a high driving force (3 Na+/1Cl–/glycine co-transport). It localizes to glycinergic nerve terminals where it keeps extracellular glycine levels low and displays highest expression in the brain stem, cerebellum and spinal cord [48]. GLYT1 (SLC6A9) is expressed as several isoforms with different localizations and functions and its driving force for glycine uptake is somewhat lower with a co-transport stoichiometry of 2 Na+/1Cl−/glycine. In the central nervous system, it has been suggested to localize to glial cells and to modulate the effect of glycine on NMDA receptor-mediated glutamatergic transmission. In addition, it appears to play a role in cellular glycine uptake in cells of various organs. The third member of this SLC6 subfamily is PROT (SLC6A7), a L-proline transporter with a KM value of about 10 µM that also transports a number of other amino acids and compatible osmolytes at physiologically relevant concentrations. It has been shown to be electrogenic, inhibited by enkephalins and expressed essentially only in brain. There it is localized mainly to synaptic vesicles of some glutamatergic nerve terminals where it is suggested to play a modulatory role. The fourth member of this subfamily has a surprisingly different function, as it appears to be the concentrative amino acid transporter with the broadest selectivity range and to lack expression in the brain. It indeed transports all neutral and cationic proteinogenic amino acids with a relatively high apparent affinity (KM for essential amino acids in the 10–100-µM range) and is expressed in oocytes, large intestine and lung epithelia [74]. In view of its ability to transport all essential and most non-essential amino acids, it is not surprising that it is expressed in a large number of different cancers and corresponding cell lines [35].
The second of these two SLC6 subfamilies has been called amino acid transporters (II) or nutrient amino acid transporters. Looking at its phylogenetic tree one may actually divide this subfamily into two groups, one including B0AT2 (SLC6A15), NTT4 (SLC6A17), and the orphan transporter NTT5 (SLC6A16) and a second including SIT1 (SLC6A20), B0AT3 (SLC6A18), and B0AT1 (SLC6A19). Two transporters of the first group, B0AT2 (SLC6A15) and NTT4 (SLC6A17), appear to be expressed mostly in neurons of the brain and eye and additionally also in some other organs. They display similar amino acid selectivity, B0AT2 transporting for instance branched chain amino acids, L-methionine and L-proline with a KM in the range of 40–200 µM and some other neutral amino acids with a KM in the mM range. Both of these transporters were also proposed to co-transport amino acids and Na+ with a 1:1 stoichiometry [10]. In contrast, the quite closely related SLC6A16 transporter is still an “orphan”. Unlike B AT2 and NTT4, it is expressed in some peripheral tissues, particularly in testis, pancreas, and in the prostate. Transient transfection experiments suggested it was localized intracellularly.
The second group within the nutrient amino acid transporter subfamily is composed of three transporters which are mainly expressed at the luminal membrane of epithelia that specialize in amino acid uptake and thus mainly subserve a crucial role for the systemic intake of amino acids and thus metabolic homeostasis. The uptake of amino acids from the environment is indeed a highly conserved function of the larger NSS family of transporters that includes the metazoan SLC6 transporters and also procaryotic transporters such as for instance LeuT Unlike in bacteria, amino acids taken up from the environment by metazoa need to traverse at least three membranes to reach their intracellular site of use: (1) uptake into an epithelial cell at the surface of the organism (i.e., luminal side of gut mucosa), (2) basolateral efflux from the epithelial cell into the extracellular space (milieu intérieur) and then uptake into another (somatic) cell. SLC6 transporters are indeed potentially mediating the uptake steps (1) and (3) using the transmembrane electrochemical gradients of substrates and co-transported ions as a driving force. In contrast, the efflux step (2) is mediated by uniporters and antiporters belonging to other SLC families.
From the three mammalian SLC6 transporters expressed at the surface of epithelial cells, only two have retained an active function in humans: the main low apparent affinity (KM in millimolar range) broad selectivity neutral amino acid transporter B0AT1 and the higher affinity (KM about 100 µM) L-proline transporter SIT1. In contrast, the B0AT1-related transporter B0AT3 (SLC6A18), which, in rodents, transports neutral amino acids with a higher apparent affinity in the later segments of kidney proximal tubule, appears to have no relevant functional role anymore in human, as a frequent single-nucleotide polymorphism encodes a stop codon [73]. The broad selectivity transporter B0AT1 (SLC6A19) is the main luminal neutral amino acid transporter in the small intestine and in the kidney proximal tubule. Interestingly, this transporter needs association with the membrane-anchored peptidase ACE2 for its expression at the brush border surface membrane of intestinal enterocytes, where it was shown to be included in a digestive complex [12, 21]. In the absence of ACE2, B0AT1 protein appears to be absent from the intestinal mucosa, presumably due to its rapid ER-associated degradation [12]. Why B0AT1 in kidney proximal tubule does not associate with ACE2 expressed in the same cells is not yet known. Instead, in these cells, B0AT1 requires association with collectrin (TMEM27) to reach the plasma membrane. In the absence of this transmembrane protein, which is structurally related to ACE2 but lacks the peptidase domain, kidney proximal tubule B0AT1 is rapidly degraded [19]. Interestingly, it appears that the related transporter SIT1 requires the same partners for its expression at the surface of small intestine and kidney proximal tubule.
Uptake of essential amino acids
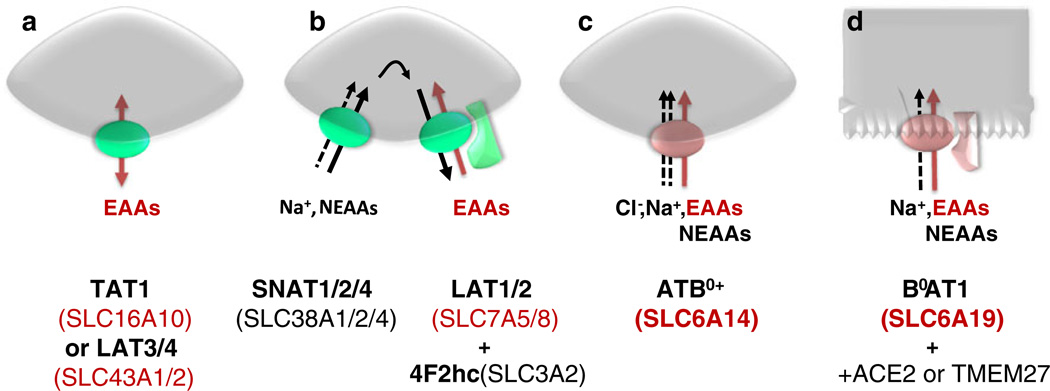
The cellular uptake of essential amino acids, in particular of branched chain and aromatic amino acids, is important for supporting cell growth and specific metabolic tasks. This requires the expression at the cell surface of transporters that import these amino acids. Interestingly, the small number of transporters known to fulfill this task belongs to three different mechanistic categories, uniporters, antiporters and symporters. It appears, however, that the uniporters, TAT1 (SLC16A10) or LAT3 (SLC43A1) and LAT4 (SLC43A2) (Fig. 3a), are not able to support rapid growth and/or high metabolic demand, because they can only equilibrate the concentration of essential amino acids across the membrane and do not actively concentrate them inside the cell. In contrast, the heterodimeric amino acid antiporter (exchanger) LAT1–4F2hc (SLC7A5-SLC3A2) is well known for its important role in the context of growth. This transporter and also LAT2–4F2hc (SLC7A8-SLC3A2) can indeed transport all essential neutral amino acids in exchange for other amino acids. Thus they can accumulate essential amino acids in the cells by taking advantage of the high intracellular concentration of free amino acids (tertiary-active transport, see Fig. 3b), providing the cell contains high amounts of non-essential efflux substrates for driving their uptake. It is probably because of this capability of LAT1–4F2hc to accumulate essential amino acids that a very large number of cancers express it [57]. From the third mechanistic category, symporters that cotransport amino acids with ions, as yet only two are known to actively transport branched and aromatic amino acids into cells and interestingly both are members of the SLC6 family. Other secondary-active amino acid transporters that also play a major role for cell growth and metabolism, in particular members of the families SLC36 (PATs) and SLC38 (system A and N), do not transport branched and aromatic amino acids but only small neutral ones. Thus cells expressing these secondary-active transporters, for instance system A, depend on the co-expression of one of the exchangers mentioned above for the active uptake of essential large neutral amino acids [82] (Fig. 3b). The two SLC6 amino acid transporters actively transporting large neutral amino acids are ATB0+ (SLC6A14) and B0AT1(SLC6A19). B0AT1 displays a low apparent affinity for its substrates (KM in millimolar range) and is expressed at the luminal surface of epithelial cells (Fig. 3d). Interestingly, this transporter has not yet been found to be associated with cancer, in contrast to the high apparent affinity (KM in micromolar range) essential amino acid transporters LAT1–4F2hc and ATB0+ possibly due to its low apparent affinity for amino acids. The broad selectivity amino acid transporter ATB0+ (SLC6A14) (Fig. 3c), which accommodates both neutral and cationic amino acids, has a much higher apparent affinity for amino acids, particularly for essential ones (KM in µM range), than B0AT1 (KM in mM range) [74]. ATB0+ had originally been characterized as transport activity in blastocytes and the transporter has been shown to be expressed also in oocytes, colon, lungs and other organs. As mentioned above, like LAT1–4F2hc, it is also highly expressed in a number of cancers, in particular, cancers of colorectal and breast origin [35].
Fig. 3.
Cellular uptake of essential amino acids (EAAs). Only a small number of transporters mediate the uptake of essential amino acids. Uniporters, as shown in panel a, equilibrate the concentration of aromatic or branched chain EAAs across the plasma membrane. Panel b shows a heterodimeric antiporter (obligatory exchanger) that can exchange intracellular amino acids (e.g., non-essential amino acids (NEAAs) taken up by other transporters) against extracellular EAAs (tertiary-active transport). Panels c and d (epithelial cell) show the only two symporters known to actively import EAAs into cells using the driving force of ion gradients (secondary-active transport)
Open questions
This short section highlights the fact that the SLC6 amino acid transporters are secondary active and thus concentrative uptake transporters and briefly describes their differential functions in the context of neurotransmission, cell growth/ metabolism or epithelial transport. Many different questions regarding these transporters need to be addressed, in particular because of the strong link between nutrient transport and cellular metabolism and their important physiological and pathophysiological roles. Very little is known about the cell-specific expression and regulation of SLC6 amino acid transporters at the level of expression and function. Because of their overlapping amino acid selectivity, their differential kinetic properties and their interdependence for maintaining cellular and systemic amino acid homeostasis, more systematic work also involving modeling will be required to bring our understanding to a higher level.
Modulation on the move: a perspective on the regulation of monoamine neurotransmitter transporters (Randy D. Blakely)
Even before their cloning, neuroscientists speculated that the regulation of neurotransmitter transporter expression and function could contribute to the plasticity characteristic of neuronal synapses, contributing ultimately to changes in behavior [52, 84, 85]. The availability of molecular tools in the early 1990s provided opportunities to put these speculations to the test [2]. Over the past 20 years, members of our field have shown that transporters undergo reversible phosphorylation, that activation of intracellular signaling pathways can influence transporter trafficking, that transporters reside in membrane microdomains where mobility, function and drug responses can be modulated, and that transporters associate with many kinds of proteins, from other membrane proteins, to cytoskeletal adaptors, to enzymes, that regulate their availability and actions [6]. As emphasized below, even a brief commentary on the field reveals both remarkable progress, yet so much to be done. This is particularly the case in the effort to transfer the findings of the past decades from in vitro model systems and, for the most part, non-physiological stimuli, to studies of transporter regulation by behaviorally relevant triggers in vivo. As space does not permit the depth of citation characteristic of a comprehensive treatment, the reader seeking to pursue these topics in depth should consider examination of a number of more comprehensive reviews [6, 43, 67, 75]. This review will focus on the regulation of biogenic amine neurotransmitter transporters, proteins that are responsible for dopamine (DA), norepinephrine (NE), serotonin (5-HT), and choline (Ch) transport [dopamine transporter (DAT), norepinephrine transporter (NET), serotonin transporter (SERT), and CHT, respectively], with which this author is most familiar. It is likely that the same general conclusions would apply, for example, to glutamate, γ-aminobutyric acid (GABA), or glycine transporters.
Transporter phosphorylation
A major advance with respect to biogenic amine transporter regulation came with the demonstration that all neurotransmitter transporters examined to date can be phosphorylated in response to activation of intracellular signaling pathways, with the most detailed findings arising from studies of pathways subserved by Ser/Thr protein kinases, including protein kinase A (PKA), protein kinase C (PKC), protein kinase G (PKG), mitogen-activated protein kinases such as ERK and p38 MAPK, Ca2+/calmodulin-linked protein kinase II (CamKII) [29, 67]. These studies have now been advanced to the state where all of the biogenic amine transporters have been documented to be, through one manipulation or another, phosphorylated in natively expressing tissue preparations or cultured cells. It was clear at the outset of the cloning era that biogenic amine transporters possesses multiple potential phosphorylation sites, but, up to this point, only a few of these sites have been convincingly identified and linked to a specific function. Indeed, studies from the Gether lab [36] revealed that a DAT truncation that drastically reduced PKC-dependent phosphorylation of DAT failed to alter transporter endocytosis following PKC activation, a behavior considered by many up to that point (including this author) to likely derive from direct transporter phosphorylation. That’s not to say that progress has not been achieved. Vaughan’s group has fingered a number of N-terminal residues in the phosphorylation of DAT that can influence transport function and drug responses (e.g., [30]) with evidence provided, interestingly enough, for phosphorylation associated with trafficking-independent actions of PKC activation [28]. Ramamoorthy’s team convincingly demonstrated that activation of PKG-linked SERT trafficking to the cell surface derives, at least in transfected cells, from phosphorylation of Thr276 [66], whereas Annamalai and colleagues implicated Thr258 and Ser 259 in NET in PKC-dependent endocytosis of NET [3]. Also, Khoshbouei et al. have shown that N-terminal Ser residues are critical for amphetamine (AMPH)-induced DA efflux, sites now believed to be targeted by CamKII [25]. A major caveat to all of these studies, and a key step for the field in the future, is the demonstration that these sites are modified in vivo by physiologically relevant stimuli and are responsible for behaviorally meaningful regulation of transporters at synapses. It would be exciting to see the same phosphopeptide antibody-mass spectrometry approaches that have been used successfully to implicate specific sites of phosphorylation of DAT in synaptosomes [30, 53] applied to tissue from animals receiving in vivo drug or behavioral challenges. Recently, Pizzo and colleagues [63] used the expression of human DAT mutants in Drosophila, followed by activity monitoring, to gather evidence that the N-terminal Ser residues of DAT proposed to be CamKII targets are required for the hyperlocomotory actions of AMPH in vivo. Important next steps in this story are parallel demonstrations of changes in DA efflux to validate the proposed impact of DAT mutations in vivo and a demonstration that targeting of these sites and DA efflux (versus competition for DA uptake) is critical for the behavioral actions of AMPH on DAT [18]. Additionally, our current specification of kinases and phosphatases that support transporter phosphorylation do not distinguish between direct and indirect actions (i.e., one kinase modifying another, which ultimately phosphorylates the transporter). Methods such as that recently introduced by Rudnick’s group in demonstrating that, at least in vitro, PKG does not appear to phosphorylate SERT directly, need to be more extensively incorporated into our efforts [88]. We also need to move to the use of models featuring reversible modifications of specific phosphorylation sites that could allow for timed generation or elimination of phosphorylation, since constitutively engineered modification of coding sites in transgenic animals can introduce significant compensatory issues. We and others are presently exploring the use of conditional knock-in strategies [71], but approaches where the use of drugs or peptides that could reversibly mask a phosphorylation site after systemic or local injection in vivo also seem worth considering.
More broadly, we lack an understanding of how intracellular signaling pathways, particularly those activated by endogenous receptors, converge to achieve proper temporal and spatial control of neurotransmitter action. We recently demonstrated that p38 MAPK-dependent hyperphosphorylation arises in an autism-associated mutation of SERT (Ala56) assessed in a mouse knock-in model, leading to constitutively elevated 5-HT clearance, and behavioral perturbations characteristic of the disorder [81]. But how this pathway is naturally triggered to regulate SERT, possibly through activation of inflammatory cytokine signaling, is only now coming into focus [5, 94]. Since biogenic amine transporter coding variation is rare, the definition of signaling pathways that lead to disease-associated transporter phosphorylation, as well as other regulatory, post-translational modifications (e.g., ubiquitylation, palmitoylation), will likely be key to establishing the impact of these changes on disease processes. Growing evidence supports the existence of physical and/or functional interactions of presynaptic receptors with neurotransmitter transporters [46, 93], providing opportunities for both autoreceptor and heteroreceptor regulation of neurotransmitter uptake. The possibility that disrupted receptor–transporter interactions underlie risk for neuropsychiatric disorders seems plausible and should be given further consideration [13, 45].
Transporter trafficking to sites of functional expression
Biogenic amine transporters, like all membrane proteins, must reach plasma membrane after passage through the endoplasmic reticulum (ER) and Golgi. Neurons feature highly complex processes within which membrane microdomains are well known to support specialized functions (e.g., node of Ranvier, synaptic junctions, dendritic spines). Very little is known as to how neurotransmitter transporters reach these specialized sites of expression. Three general pathways are likely to support transporter export—the budding frim the ER and intracellular movement of transporters to distal sites, the targeting or lateral entry of transporters into the microdomains of the plasma membrane, or the latter process coupled to endocytosis and relocation of internalized transporters to other sites. With respect to export from the cell soma, several studies have implicated distinct isoforms of COPII Sec24 proteins in the export of multiple neurotransmitter transporters from sites of synthesis [22, 76]. These proteins appear to help bud and/or traffic transporter-containing vesicles to sites where they can engage cytoskeletal transport machinery (e.g., microtubules) and thereby reach the cell surface. Studies to date, however, derive from heterologously expressed transporters and we may expect many nuances in this export mechanism as studies move to a native context.
One model where such mechanisms may be readily evaluated is C. elegans, which express orthologs of DAT, SERT, and CHT. Matthies and colleagues [50] demonstrated that the CHT ortholog CHO-1 is exported from the cell soma on vesicles that rely on the kinesin motor protein UNC-104. Interestingly, CHO-1 and the vesicular acetylcholine (ACh) transporter (VAChT, UNC-17) are retained in the cell soma of cholinergic neurons in unc-104 mutants. Although it is not known if these two proteins move on the same vesicles, evidence from mammalian studies indicates that they ultimately can reside on the same vesicle where VAChT imports ACh for release and where CHT resides to move to the plasma membrane upon vesicle fusion [24]. Interestingly, studies by McDonald and coworkers [51] indicate that the C. elegans DAT ortholog (DAT-1) and the vesicular monoamine transporter 2 (VMAT2) ortholog (CAT-1), do not traffic through the same pathway to DA terminals as CAT-1 trafficking out of the cell soma is blocked by the unc-104 mutation, whereas DAT-1 is exported to DA synapses. The C. elegans model is particularly attractive for future studies of native neurotransmitter transporter export owing to the transparency of the animal and the ease of transgenic expression of wild type and mutant, fluorescently tagged transporters. In another facet of the McDonald et al. study noted above, these authors demonstrated a role for C-terminal sequences in the export of DAT-1 to DA terminals. Possibly these sequences relate to the aforementioned SEC-24 protein interactions. Interestingly, DAT export to the synapse in the worm model is not dependent on the type II PDZ domain interaction sequences on the distal DAT C-terminus previously shown to be targeted by PICK-1 [80]. Finally, SERT, NET and DAT proteins also exist on dendritic membranes of 5-HT, NE and DA neurons, respectively, where they appear to serve to regulate neurotransmitter actions on firing-regulating autoreceptors (e.g., see [56]). The long dendrites associated with CEP DA neurons in the nematode model suggests that the worm model may also be very useful in understanding mechanisms that support the dendritic localization of neurotransmitter transporter proteins.
Transporter membrane trafficking
With the development of biogenic amine transporter-directed antibodies and fluorescent transporter fusions, investigators began to pursue the regulated movement of these proteins to and from the cell surface, as well as their localization to membrane microdomains. As noted above, the first evidence that biogenic amine transporter trafficking in or out of the plasma membrane could be regulated came from studies monitoring the rapid relocation of surface transporters to intracellular compartments following PKC activation. Over the years, studies have shown that such regulation rests on constitutive patterns of internalization and externalization that can be monitored separately by biotinylation or by the use of surface-epitope targeted antibodies. Melikian’s group [49] has presented evidence that distinct C-terminal sequences of DAT subserve constitutive vs PKC-modulated DAT trafficking. Galli’s group [14] has shown that plasma membrane levels of DAT proteins are also controlled by PI-3 kinase (PI3K)-linked pathways, though whether endocytic or exocytic (or both) facets of transporter recycling are modulated is unknown. PKG-linked pathways support SERT insertion into the plasma membrane [5], with evidence now available in both native and transfected cell populations. CHT proteins contain powerful endocytic sequences that are likely at work to drive the bulk of CHT proteins being resident on cholinergic synaptic vesicles [24]. As with the challenges for the future, and as noted for phosphorylation mechanisms, scant information is yet available that demonstrates the engagement of these trafficking pathways or its physiological relevance in vivo. In this regard, the Chavkin lab established that that raphe neuron p38 MAPK can modulate SERT availability and, in so doing, support the aversive actions of delta opiates [11].
Transporters in membrane microdomains
Over the past decade, multiple studies have established that biogenic amine transporters reside at the cell surface within membrane microdomains that are rich in cholesterol and the ganglioside GM1 and that, for many, are designated somewhat inappropriately as “lipid rafts”. Localization to these domains appears to restrict transporter mobility [1, 15], likely due to anchoring to cortical actin networks via a multitude of associated proteins. Using a quantum-dot conjugated antidepressant, we recently visualized natively expressed SERT in RN46A cells in vitro, associated with such membrane micro-domains, demonstrating at a single molecule level that lateral mobility of the transporter is significantly lower than that of transporters outside of these domains (Fig. 4) [15]. Although we were able to increase SERT protein mobility by cholesterol extraction, as was previously demonstrated for DAT proteins, it remains unclear whether biogenic amine transporters can move in and out of membrane microdomains as a feature of their regulation. One could envision departure from rafts occurring via the departure and free diffusion of transporters or via endocytosis and re-insertion into different compartments of the plasma membrane. Presumably, either of these models depends on reversible associations with transporter-associated proteins. To date, a host of such proteins have been identified [6], and many of them likely play a role in the organization of transporter containing microdomains. We can anticipate these studies to provide an ongoing dialogue between in vitro and in vivo approaches, as the assessment of protein interaction interfaces in reduced preparations will likely provide the tools to manipulate these interactions in the brain. This exercise is far from an ivory tower academic pursuit. Knowing how transporters achieve an appropriate membrane microdomain localization will likely identify new opportunities to influence synaptic transmission to improve human health, and also to understand how alterations in other proteins can result in changes in neurotransmitter dynamics.
Fig. 4.
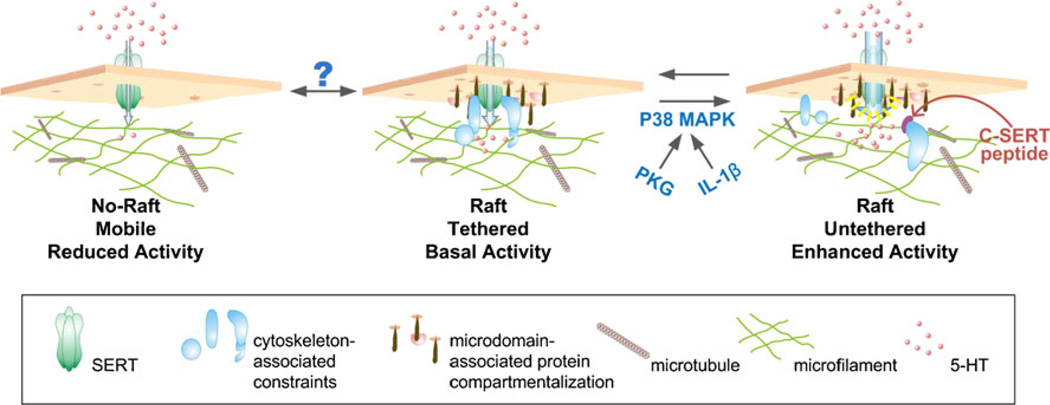
Model for SERT-cytoskeletal interactions dictating cell surface transporter regulation. In the resting state, SERT is present in two compartments, one that permits free diffusion in the membrane (left), and a second compartment that represents confinement to membrane microdomains (center) where transporters are immobilized by cytoskeleton-associated proteins (middle). When cytoskeleton- associated constraints are relaxed in response to PKG/IL-1β/p38 MAPK activation (or through actin destabilizers or C-SERT peptide treatments), SERT remains confined to membrane microdomains (right), though now transporters can adopt conformations that favor increased transport activity. Question mark overlying transitions into and out of membrane microdomains denotes the possibility that such movements could also play a role in SERT regulation, though they are not features of the PKG and p38 MAPK-dependent SERT regulation detected in the current study. Adapted from Chang et al. [15]
Two examples illustrate the point. Cremona and colleagues recently demonstrated that the interactions of DAT proteins with the membrane microdomain-associated protein flotillin 1 is required for AMPH-induced DA efflux [17], suggesting that we are likely to learn much about the actions of psychostimulants through a better understanding of these domains and the proteins they contain. Finally, we recently demonstrated that DAT proteins expressing an ADHD-associated DAT mutation (615C) fail to target properly to GM1-rich membrane microdomains and as a result display abnormal trafficking kinetics and modulation by PKC and AMPH. These examples, interestingly enough, connected by the actions of AMPH, underscore the importance of biogenic amine transporters being at the right place, at the right time.
Transporter activity states
Neurotransmitter enzymes and receptors display regulated shifts between conformational states that in turn functional capabilities, be it an alteration in neurotransmitter synthesis or probability of neurotransmitter binding triggering a downstream signal. For neurotransmitter transporters, conformational changes are often only considered as the rearrangements of the “open-out, open-in” states that describe, at a gross level, the transport cycle. However, the occurrence of stable changes in transporter conformation that lead to altered substrate affinity or the probability of transport are also beginning to be appreciated. We became interested in the possibility of regulatory transporter “activation” when we discovered that insulin, via a p38 MAPK-linked pathway, could rapidly enhance NET activity in SK-N-SH cells without elevating total transporter protein levels or transporter surface expression [4]. Interestingly, p38 MAPK-linked pathways can also rapidly enhance transport activity of SERT proteins in a trafficking-independent manner [92], a pathway that we now know can be induced in vitro by inflammatory cytokines (e.g., IL-1β, TNFα) in vitro and by peripheral immune system activation in vivo [94]. Kinetic studies indicate that these changes in activity derive from a stable shift of transporters between low and high-affinity conformations. What parts of transporter structure reorient under these conditions and how such conformations are normally constrained and mobilized are of critical importance as we move forward. In the SERT quantum dot studies noted above [15], we demonstrated that elevated SERT activity triggered by IL-1β is associated with an increased lateral mobility of transporters, though these molecules still remain lodged within GM1-enriched membrane microdomains (Fig. 4). As we found that changes in both SERT activity and mobility could be induced by cytochalasin-induced disruption of the actin cytoskeleton or by membrane permeant peptides that mimic the SERT C-terminus, we proposed that constraints on transporter lateral mobility and constraints on transporter structural conformations are different features of the same process, the tethering of SERT to the cytoskeleton by C-terminal-associated proteins.
The studies note above suggest that biogenic amine transporter-associated proteins not only chaperone transporters from biosynthetic compartments to the cell surface and dictate their membrane trafficking, but also establish the permissible range of conformations that dictate substrate affinity and transport rates. Since some protein associations likely play roles in trafficking, localization and activity, methods must be implemented that can separate these processes, physically and temporally, if we are to capture the full extent of transporter regulation. Detached patch recording methods have provided single molecule regulation of conformational states of ion channels and the demonstration of electrical signatures of biogenic amine transporter-associated channel states offers a similar opportunity [33, 34]. One example of the power of this approach comes from the demonstration that the SNARE protein syntaxin 1A can alter NET function in a trafficking-independent manner via the elimination of NE-gated NET channel activity [77]. As with other aspects of transporter regulation, observations of biased alteration of transporter conformation from in vitro observations in reduced preparations need to be demonstrated to support the capacity for neurotransmitter uptake in vivo. This author may have his biases, but unabashedly admits that they derive from the many fruitful associations that sometimes constrain but more often enhance his activity.
The SLC6 transporter family: gene variants and mouse genetic models relevant to neuropsychiatric disorders and their treatment (Dennis L. Murphy)
By far, the greatest number of studies examining transporters in the SLC6 family related to neuropsychiatric disorders have focused on polymorphisms or rare variants in the serotonin transporter gene (SLC6A4) and its protein, SERT, the dopamine transporter gene (SLC6A3) and its protein, DAT, plus a few studies of the norepinephrine transporter gene (SLC6A2) and the GABA and glycine transporter genes. Thus, this brief review covers only major examples of genetic variants, pharmacological targets and a few associated mouse genetic models that provide useful insights into behavioral and physiological consequences of alterations in SLC6 transporters. New reviews and major papers are cited; a more complete list of references in an annotated version of this review is available upon request. Some overall perspectives and ideas for future studies are also noted in the “Conclusion”.
SLC6A4 (Chromosome 17.11–12) encodes the single SERT with characteristic 12 transmembrane-spanning segments. A promoter region length variant, 5HTTLPR and two closely associated SNPs (rs25531, rs25532), alter the expression, trafficking and function of SERT. These variants have been most clearly associated with anxiety-related traits, anxiety disorders (especially obsessive-compulsive disorder [OCD]) and perhaps depression, bipolar disorder, ADHD, and Tourette’s disorder (TD). An intron 2 VNTR shows evidence of functional consequences in model systems; only scattered clinical studies suggest an association with depressive disorders. Several rare SERT variants (e.g., I425V, which is most closely associated with OCD and TD) and a cluster of rare SNPs associated with autism are all gain-of-function variants affecting serotonin uptake and SERT regulation in cell culture systems (reviews: [39, 54]).
Selective serotonin reuptake inhibitors (SSRIs, which are inhibitors at the SERT transport site) are FDA approved as antidepressants and anti-anxiety disorder agents. Studies of the crystal structure of LeuT a bacterial homolog of SERT, DAT, and NET [60, 83, 91] indicate that antidepressants occupy the substrate site of the transporters, preventing conformational changes and thus preventing substrate transport. SRIs (including clomipramine) are the only approved class of drugs for OCD, a disorder non-responsive to other conventional anxiolytic agents that act via gabaergic and related systems (e.g., diazepam and its congeners). Some mixed evidence suggests that therapeutic responses—but more prominently side effects from SSRIs such as diarrhea—are related to the SERT promoter region polymorphisms [40].
SERT knockout mice (SERT-/- mice) and SERT deficient (+/- mice) have 5- to 9-fold increases in microdialysis-measured brain ECF serotonin, and a variable reduction according to brain region in serotonin content, associated with increased anxiety-related behaviors, exaggerated stress responsiveness, reduced locomotor activity and approximately fifty-plus related brain and peripheral phenotypic abnormalities. SERT knockout rats exhibit highly parallel findings to those found in SERT knockout mice. Transgenic SERT over-expressing mice show reduced anxiety and related behaviors and other reversed phenomena including serotonin receptor changes compared to SERT-deficient mice (reviews: [41, 55]).
SLC6A3 (Chromosome 5p15.33) encodes the single DAT. Genetic studies have indicated that common variants in DAT may be associated with ADHD, bipolar disorder as well as substance abuse, including cigarette smoking [OMIN 126455]. A rare functional DAT variant, A559V, was found in two ADHD-affected siblings and also in a patient with bipolar disorder; efflux rates of dopamine induced by amphetamine comprise the most prominent functional alteration associated with the DAT A559V mutation. Two other loss-of-function mutations, DAT P395L and L368Q, were found associated with a Mendelian recessive disorder, infantile parkinsonian dystonia, characterized by rigidity, in two independent families [44]. The partially selective DAT inhibitor, methylphenidate, is the most widely prescribed anti-ADHD agent, although d-amphetamine (which is an inhibitor of DAT, NET, and SERT) is also used in ADHD treatment. DAT knock-out mice exhibit very prominent hyperactivity, reduced body size and weight, with 5-fold increases in striatal ECF dopamine, accompanied by markedly reduced brain tissue dopamine content. Other behavioral features of these mice that resemble both some ADHD-like as well as OCD-and autism-like phenomena include stereotypic behavior, reduced habituation to novelty and some cognitive deficits [31]. The hyperactivity of the DAT knockout mice can be suppressed by d-amphetamine (which is a locomotor stimulant in wild-type mice). Other genetic mouse models of partial DAT deficiency and of DAT over-expression have been less well-characterized but suggest behavioral and biochemical alterations in the same domains as those found in DAT knockout mice.
SLC6A2 (Chromosome 16q12.2) encodes the norepinephrine transporter, NET. No common variants in SLC6A2 associated with neuropsychiatric disorders have been identified. The most prominent rare variant occurs in exon 9 yielding NET A457P, which was reported in a family constellation consisting of five siblings and their mother, all of whom suffered from orthostatic intolerance (an abnormal increase in heart rate and plasma norepinephrine upon standing, along with clinical symptoms of faintness and dizziness). The mutation leads to an almost complete loss of NET function, with greatly reduced NET surface expression. NET knockout mice have reduced locomotor activity in a novel environment together with elevated heart rates and increased blood pressure in response to activating stimuli. Like SERT and DAT knockout mice, the NET knockouts have increased ECF transmitter concentrations (here, NE) as measured by microdialysis, accompanied by markedly reduced tissue NE [32].
SLC6A5 Encodes the glycine transporter, GLYT2. Rare variants in this gene (frame shift or other mutations) have been identified in several different pedigrees with excessive startle responses (most commonly an autosomal recessive disorder, termed hyperekplexia). GLYT2 is the presynaptic neuronal glycine transporter and GLYT1 is the glial glycine transporter. Glycine is a major inhibitory neurotransmitter in the brainstem and spinal cord, regulating strychnine-sensitive glycine receptors. Glycine also acts as a co-agonist with glutamate at NMDA receptors. GLYT1 and GLYT2 knockout mice both die shortly after birth.
SLC6A1 Encodes the GABA transporter, GAT1. Only indirect human gene and post-mortem studies have raised the question of SLC6A1’s involvement in anxiety disorders, schizophrenia and seizure disorders. GAT1 knockout mice exhibited a seizure disorder, tremor and abnormal locomotor functions, although another study reported reduced anxiety-like, depression-like and aggression-related behaviors, associated with elevated brain GABA concentrations.
Conclusion
Perspectives and ideas for future studies
Perhaps most striking in this review is the consistency between genetic knockout mice models (and a few transgenic, over-expressing mouse lines) with expected consequences from transporter alterations found in biochemical and behavioral evaluations. Biochemically, transporter deficiencies lead to excess ECF neurotransmitter concentrations associated with reduced brain and other tissue concentrations of the relevant transmitter. Behavioral consequences are consistent with what had previously been observed with pharmacological manipulations of tissue homeostasis and receptors for each of these transmitters. Human genetics findings to date, particularly those associated with rare coding change variants, also seem to follow anticipated phenotypes. However, the field has to be cautious of “looking under the lamppost for lost keys” phenomena, as in many cases clinical evaluations are limited in scope. Nonetheless, the general findings, assuming support continues from studies of genetic mouse, other rodent and non-human primate models, bodes well for understanding human disorders. Current studies reviewed here certainly emphasize the importance of SLC6 transporters in multiple neuropsychiatric disorders as well as their treatment. Many study opportunities remain. The field is still searching for genetic and other biomarkers that might prove more useful than clinical features alone for the future goal of personalized medical diagnoses and treatment of neuropsychiatric disorders.
Clinical heterogeneity, evident in major neuropsychiatric disorders such as OCD, bipolar disorder, and others, represents in clinical features multiple kinds of problems. Likewise, genetic heterogeneity, for example evident in variants within genes (e.g., SLC6A4), gene×gene, gene×environment and epigenetic variability, compounds assessment of “genetically complex” disorders. Taken together, this leaves us with much to unravel in the SLC6 scientific world.
While some current state-of-the-art GWAS (genome-wide association studies) plus GWAS metanalyses have led to “hits” within likely genes relevant to neuropsychiatric disorders, other major genome-wide single “hits” have been in unlikely genes not resolvable by pathway or network analyses (e.g.,, [70]). While the latter two approaches are predicted to prove highly valuable, they do not help with the not uncommon result of a “hit” in a large intergenic area. While this author is an avid participant in biomarker, GWAS and the network pathway study of genes, this complexity also suggests seizing upon the opportunities presented by rare genes found in maybe only a few pedigrees or 1–2 % of individuals in case–control studies of neuropsychiatric disorders. Intensive investigations of new functional rare variants using all molecular, neurochemical, electrophysiological techniques and transgenic mouse models that can mimic the variant’s consequences in vivo may point towards otherwise unforeseen areas for investigating related patient groups or new pathways. Indeed, studies this year have suggested new ideas for SLC6 investigators, for example, genes previously identified as “oncogenes” have now been preferentially found in autism patient samples vs. controls [78]. Likewise, a network of 50 embryonic developmental genes has been found expressed preferentially in the prefrontal cortex of adult schizophrenic patients versus control brains [38]. Additionally, a recent paper described a surprising overlap of associated genes across five neuropsychiatric disorders, way beyond traditional diagnostic boundaries [47]. Such new conundrums around every corner will continue to challenge transporter genetics and biology.
Acknowledgments
The laboratory of FV is supported by Swiss NSF grant 31-130471/1 and the National Centre of Competence in Research (NCCR) Kidney.CH, GR by NIH grants DA007259 and DA008213, RDB by NIH awards MH095044, MH07802, MH073159, and MH094527. DLM is funded by the NIMH Intramural Research Program, NIH, Bethesda, MD, USA.
Contributor Information
Gary Rudnick, Department of Pharmacology, Yale University School of Medicine, New Haven, CT, USA.
Reinhard Krämer, Institute of Biochemistry, University of Cologne, Cologne, Germany.
Randy D. Blakely, Department of Pharmacology and Psychiatry, Vanderbilt University School of Medicine, Nashville, TN, USA
Dennis L. Murphy, Laboratory of Clinical Science, NIMH-IRP, Bethesda, MD, USA
Francois Verrey, Institute of Physiology and Zurich Center for Integrative Human Physiology (ZIHP), University of Zurich, Winterthurerstrasse 190, 8057 Zurich, Switzerland verrey@access.uzh.ch.
References
- 1.Adkins EM, Samuvel DJ, Fog JU, Eriksen J, Jayanthi LD, Vaegter CB, Ramamoorthy S, Gether U. Membrane mobility and microdomain association of the dopamine transporter studied with fluorescence correlation spectroscopy and fluorescence recovery after photobleaching. Biochemistry. 2007;46:10484–10497. doi: 10.1021/bi700429z. [DOI] [PubMed] [Google Scholar]
- 2.Amara SG. Neurotransmitter transporters. A tale of two families. Nature. 1992;360:420–421. doi: 10.1038/360420d0. [DOI] [PubMed] [Google Scholar]
- 3.Annamalai B, Mannangatti P, Arapulisamy O, Ramamoorthy S, Jayanthi LD. Involvement of threonine 258 and serine 259 motif in amphetamine-induced norepinephrine transporter endocytosis. J Neurochem. 2010;115:23–35. doi: 10.1111/j.1471-4159.2010.06898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Apparsundaram S, Sung U, Price RD, Blakely RD. Trafficking-dependent and -independent pathways of neurotransmitter transporter regulation differentially involving p38 mitogen-activated protein kinase revealed in studies of insulin modulation of norepinephrine transport in SK-N-SH cells. J Pharmacol Exp Ther. 2001;299:666–677. [PubMed] [Google Scholar]
- 5.Blakely RD, Defelice LJ, Galli A. Biogenic amine neurotransmitter transporters: just when you thought you knew them. Physiology (Bethesda) 2005;20:225–231. doi: 10.1152/physiol.00013.2005. [DOI] [PubMed] [Google Scholar]
- 6.Blakely RD, Edwards RH. Vesicular and plasma membrane transporters for neurotransmitters. Cold Spring Harb Perspect Biol. 2012;4(2) doi: 10.1101/cshperspect.a005595. pii: a005595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Botzenhardt J, Morbach S, Kramer R. Activity regulation of the betaine transporter BetP of Corynebacterium glutamicum in response to osmotic compensation. Biochim Biophys Acta. 2004;1667:229–240. doi: 10.1016/j.bbamem.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 8.Boudko DY. Molecular basis of essential amino acid transport from studies of insect nutrient amino acid transporters of the SLC6 family (NAT-SLC6) J Insect Physiol. 2012;58:433–449. doi: 10.1016/j.jinsphys.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Broer S, Gether U. The solute carrier 6 family of transporters. Br J Pharmacol. 2012;167:256–278. doi: 10.1111/j.1476-5381.2012.01975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Broer A, Tietze N, Kowalczuk S, Chubb S, Munzinger M, Bak LK, Broer S. The orphan transporter v7-3 (slc6a15) is a Na+-dependent neutral amino acid transporter (B0AT2) Biochem J. 2006;393:421–430. doi: 10.1042/BJ20051273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruchas MR, Schindler AG, Shankar H, Messinger DI, Miyatake M, Land BB, Lemos JC, Hagan CE, Neumaier JF, Quintana A, Palmiter RD, Chavkin C. Selective p38alpha MAPK deletion in serotonergic neurons produces stress resilience in models of depression and addiction. Neuron. 2011;71:498–511. doi: 10.1016/j.neuron.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Camargo SM, Singer D, Makrides V, Huggel K, Pos KM, Wagner CA, Kuba K, Danilczyk U, Skovby F, Kleta R, Penninger JM, Verrey F. Tissue-specific amino acid transporter partners ACE2 and collectrin differentially interact with hartnup mutations. Gastroenterology. 2009;136:872–882. doi: 10.1053/j.gastro.2008.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campbell NG, Zhu CB, Lindler KM, Yaspan BL, Kistner-Griffin E, Consortium NA, Hewlett WA, Tate CG, Blakely RD, Sutcliffe JS. Rare coding variants of the adenosine A3 receptor are increased in autism: on the trail of the serotonin transporter regulome. Mol Autism. 2013;4:28. doi: 10.1186/2040-2392-4-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carvelli L, Moron JA, Kahlig KM, Ferrer JV, Sen N, Lechleiter JD, Leeb-Lundberg LM, Merrill G, Lafer EM, Ballou LM, Shippenberg TS, Javitch JA, Lin RZ, Galli A. PI 3-kinase regulation of dopamine uptake. J Neurochem. 2002;81:859–869. doi: 10.1046/j.1471-4159.2002.00892.x. [DOI] [PubMed] [Google Scholar]
- 15.Chang JC, Tomlinson ID, Warnement MR, Ustione A, Carneiro AM, Piston DW, Blakely RD, Rosenthal SJ. Single molecule analysis of serotonin transporter regulation using antagonist-conjugated quantum dots reveals restricted, p38 MAPK-dependent mobilization underlying uptake activation. J Neurosci. 2012;32:8919–8929. doi: 10.1523/JNEUROSCI.0048-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Claxton DP, Quick M, Shi L, de Carvalho FD, Weinstein H, Javitch JA, McHaourab HS. Ion/substrate-dependent conformational dynamics of a bacterial homolog of neurotransmitter:sodium symporters. Nat Struct Mol Biol. 2010;17:822–829. doi: 10.1038/nsmb.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cremona ML, Matthies HJ, Pau K, Bowton E, Speed N, Lute BJ, Anderson M, Sen N, Robertson SD, Vaughan RA, Rothman JE, Galli A, Javitch JA, Yamamoto A. Flotillin-1 is essential for PKC-triggered endocytosis and membrane microdomain localization of DAT. Nat Neurosci. 2011;14:469–477. doi: 10.1038/nn.2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daberkow DP, Brown HD, Bunner KD, Kraniotis SA, Doellman MA, Ragozzino ME, Garris PA, Roitman MF. Amphetamine paradoxically augments exocytotic dopamine release and phasic dopamine signals. J Neurosci. 2013;33:452–463. doi: 10.1523/JNEUROSCI.2136-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Danilczyk U, Sarao R, Remy C, Benabbas C, Stange G, Richter A, Arya S, Pospisilik JA, Singer D, Camargo SM, Makrides V, Ramadan T, Verrey F, Wagner CA, Penninger JM. Essential role for collectrin in renal amino acid transport. Nature. 2006;444:1088–1091. doi: 10.1038/nature05475. [DOI] [PubMed] [Google Scholar]
- 20.Dierking K, Polanowska J, Omi S, Engelmann I, Gut M, Lembo F, Ewbank JJ, Pujol N. Unusual regulation of a STAT protein by an SLC6 family transporter in C. elegans epidermal innate immunity. Cell Host Microbe. 2011;9:425–435. doi: 10.1016/j.chom.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 21.Fairweather SJ, Broer A, O’Mara ML, Broer S. Intestinal peptidases form functional complexes with the neutral amino acid transporter B(0)AT1. Biochem J. 2012;446:135–148. doi: 10.1042/BJ20120307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farhan H, Reiterer V, Korkhov VM, Schmid JA, Freissmuth M, Sitte HH. Concentrative export from the endoplasmic reticulum of the gamma-aminobutyric acid transporter 1 requires binding to SEC24D. J Biol Chem. 2007;282:7679–7689. doi: 10.1074/jbc.M609720200. [DOI] [PubMed] [Google Scholar]
- 23.Farwick M, Siewe RM, Kramer R. Glycine betaine uptake after hyperosmotic shift in Corynebacterium glutamicum . J Bacteriol. 1995;177:4690–4695. doi: 10.1128/jb.177.16.4690-4695.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferguson SM, Blakely RD. The choline transporter resurfaces: new roles for synaptic vesicles? Mol Interv. 2004;4:22–37. doi: 10.1124/mi.4.1.22. [DOI] [PubMed] [Google Scholar]
- 25.Fog JU, Khoshbouei H, Holy M, Owens WA, Vaegter CB, Sen N, Nikandrova Y, Bowton E, McMahon DG, Colbran RJ, Daws LC, Sitte HH, Javitch JA, Galli A, Gether U. Calmodulin kinase II interacts with the dopamine transporter C terminus to regulate amphetamine-induced reverse transport. Neuron. 2006;51:417–429. doi: 10.1016/j.neuron.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 26.Forrest LR, Rudnick G. The rocking bundle: a mechanism for ion-coupled solute flux by symmetrical transporters. Physiology (Bethesda) 2009;24:377–386. doi: 10.1152/physiol.00030.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Forrest LR, Zhang YW, Jacobs MT, Gesmonde J, Xie L, Honig BH, Rudnick G. Mechanism for alternating access in neurotransmitter transporters. Proc Natl Acad Sci U S A. 2008;105:10338–10343. doi: 10.1073/pnas.0804659105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foster JD, Adkins SD, Lever JR, Vaughan RA. Phorbol ester induced trafficking-independent regulation and enhanced phosphorylation of the dopamine transporter associated with membrane rafts and cholesterol. J Neurochem. 2008;105:1683–1699. doi: 10.1111/j.1471-4159.2008.05262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Foster JD, Cervinski MA, Gorentla BK, Vaughan RA. Regulation of the dopamine transporter by phosphorylation. Handb Exp Pharmacol. 2006:197–214. doi: 10.1007/3-540-29784-7_10. [DOI] [PubMed] [Google Scholar]
- 30.Foster JD, Yang JW, Moritz AE, Challasivakanaka S, Smith MA, Holy M, Wilebski K, Sitte HH, Vaughan RA. Dopamine transporter phosphorylation site threonine 53 regulates substrate reuptake and amphetamine-stimulated efflux. J Biol Chem. 2012;287:29702–29712. doi: 10.1074/jbc.M112.367706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gainetdinov RR. Dopamine transporter mutant mice in experimental neuropharmacology. Naunyn Schmiedebergs Arch Pharmacol. 2008;377:301–313. doi: 10.1007/s00210-007-0216-0. [DOI] [PubMed] [Google Scholar]
- 32.Gainetdinov RR, Caron MG. Monoamine transporters: from genes to behavior. Annu Rev Pharmacol Toxicol. 2003;43:261–284. doi: 10.1146/annurev.pharmtox.43.050802.112309. [DOI] [PubMed] [Google Scholar]
- 33.Galli A, Blakely RD, DeFelice LJ. Norepinephrine transporters have channel modes of conduction. Proc Natl Acad Sci U S A. 1996;93:8671–8676. doi: 10.1073/pnas.93.16.8671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galli A, Blakely RD, DeFelice LJ. Patch-clamp and amperometric recordings from norepinephrine transporters: channel activity and voltage-dependent uptake. Proc Natl Acad Sci U S A. 1998;95:13260–13265. doi: 10.1073/pnas.95.22.13260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ganapathy V, Thangaraju M, Prasad PD. Nutrient transporters in cancer: relevance to Warburg hypothesis and beyond. Pharmacol Ther. 2009;121:29–40. doi: 10.1016/j.pharmthera.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 36.Granas C, Ferrer J, Loland CJ, Javitch JA, Gether U. N-terminal truncation of the dopamine transporter abolishes phorbol ester- and substance P receptor-stimulated phosphorylation without impairing transporter internalization. J Biol Chem. 2003;278:4990–5000. doi: 10.1074/jbc.M205058200. [DOI] [PubMed] [Google Scholar]
- 37.Gu HH, Wall S, Rudnick G. Ion coupling stoichiometry for the norepinephrine transporter in membrane vesicles from stably transfected cells. J Biol Chem. 1996;271:6911–6916. doi: 10.1074/jbc.271.12.6911. [DOI] [PubMed] [Google Scholar]
- 38.Gulsuner S, Walsh T, Watts AC, Lee MK, Thornton AM, Casadei S, Rippey C, Shahin H, Nimgaonkar VL, Go RC, Savage RM, Swerdlow NR, Gur RE, Braff DL, King MC, McClellan JM. Spatial and temporal mapping of de novo mutations in schizophrenia to a fetal prefrontal cortical network. Cell. 2013;154:518–529. doi: 10.1016/j.cell.2013.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hahn MK, Blakely RD. The functional impact of SLC6 transporter genetic variation. Annu Rev Pharmacol Toxicol. 2007;47:401–441. doi: 10.1146/annurev.pharmtox.47.120505.105242. [DOI] [PubMed] [Google Scholar]
- 40.Hu XZ, Rush AJ, Charney D, Wilson AF, Sorant AJ, Papanicolaou GJ, Fava M, Trivedi MH, Wisniewski SR, Laje G, Paddock S, McMahon FJ, Manji H, Lipsky RH. Association between a functional serotonin transporter promoter polymorphism and citalopram treatment in adult outpatients with major depression. Arch Gen Psychiatry. 2007;64:783–792. doi: 10.1001/archpsyc.64.7.783. [DOI] [PubMed] [Google Scholar]
- 41.Jennings KA, Licht CL, Bruce A, Lesch KP, Knudsen GM, Sharp T. Genetic variation in 5-hydroxytryptamine transporter expression causes adaptive changes in 5-HT(4) receptor levels. Int J Neuropsychopharmacol. 2012;15:1099–1107. doi: 10.1017/S1461145711001258. [DOI] [PubMed] [Google Scholar]
- 42.Krishnamurthy H, Gouaux E. X-ray structures of LeuT in substrate-free outward-open and apo inward-open states. Nature. 2012;481:469–474. doi: 10.1038/nature10737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kristensen AS, Andersen J, Jorgensen TN, Sorensen L, Eriksen J, Loland CJ, Stromgaard K, Gether U. SLC6 neurotransmitter transporters: structure, function, and regulation. Pharmacol Rev. 2011;63:585–640. doi: 10.1124/pr.108.000869. [DOI] [PubMed] [Google Scholar]
- 44.Kurian MA, Zhen J, Cheng SY, Li Y, Mordekar SR, Jardine P, Morgan NV, Meyer E, Tee L, Pasha S, Wassmer E, Heales SJ, Gissen P, Reith ME, Maher ER. Homozygous loss-of-function mutations in the gene encoding the dopamine transporter are associated with infantile parkinsonism-dystonia. J Clin Invest. 2009;119:1595–1603. doi: 10.1172/JCI39060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee FJ, Pei L, Liu F. Disruption of the dopamine transporter-dopamine D2 receptor interaction in schizophrenia. Synapse. 2009;63:710–712. doi: 10.1002/syn.20648. [DOI] [PubMed] [Google Scholar]
- 46.Lee FJ, Pei L, Moszczynska A, Vukusic B, Fletcher PJ, Liu F. Dopamine transporter cell surface localization facilitated by a direct interaction with the dopamine D2 receptor. Embo J. 2007;26:2127–2136. doi: 10.1038/sj.emboj.7601656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee SH, Ripke S, Neale BM, Faraone SV, Purcell SM, Perlis RH, Mowry BJ, Thapar A, Goddard ME, Witte JS, Absher D, Agartz I, Akil H, Amin F, Andreassen OA, Anjorin A, Anney R, Anttila V, Arking DE, Asherson P, Azevedo MH, Backlund L, Badner JA, Bailey AJ, Banaschewski T, Barchas JD, Barnes MR, Barrett TB, Bass N, Battaglia A, Bauer M, Bayes M, Bellivier F, Bergen SE, Berrettini W, Betancur C, Bettecken T, Biederman J, Binder EB, Black DW, Blackwood DH, Bloss CS, Boehnke M, Boomsma DI, Breen G, Breuer R, Bruggeman R, Cormican P, Buccola NG, Buitelaar JK, Bunney WE, Buxbaum JD, Byerley WF, Byrne EM, Caesar S, Cahn W, Cantor RM, Casas M, Chakravarti A, Chambert K, Choudhury K, Cichon S, Cloninger CR, Collier DA, Cook EH, Coon H, Cormand B, Corvin A, Coryell WH, Craig DW, Craig IW, Crosbie J, Cuccaro ML, Curtis D, Czamara D, Datta S, Dawson G, Day R, De Geus EJ, Degenhardt F, Djurovic S, Donohoe GJ, Doyle AE, Duan J, Dudbridge F, Duketis E, Ebstein RP, Edenberg HJ, Elia J, Ennis S, Etain B, Fanous A, Farmer AE, Ferrier IN, Flickinger M, Fombonne E, Foroud T, Frank J, Franke B, Fraser C, et al. Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat Genet. 2013;45:984–994. doi: 10.1038/ng.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu QR, Lopez-Corcuera B, Mandiyan S, Nelson H, Nelson N. Cloning and expression of a spinal cord- and brain-specific glycine transporter with novel structural features. J Biol Chem. 1993;268:22802–22808. [PubMed] [Google Scholar]
- 49.Loder MK, Melikian HE. The dopamine transporter constitutively internalizes and recycles in a protein kinase C-regulated manner in stably transfected PC12 cell lines. J Biol Chem. 2003;278:22168–22174. doi: 10.1074/jbc.M301845200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matthies DS, Fleming PA, Wilkes DM, Blakely RD. The Caenorhabditis elegans choline transporter CHO-1 sustains acetyl-choline synthesis and motor function in an activity-dependent manner. J Neurosci. 2006;26:6200–6212. doi: 10.1523/JNEUROSCI.5036-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McDonald PW, Hardie SL, Jessen TN, Carvelli L, Matthies DS, Blakely RD. Vigorous motor activity in Caenorhabditis elegans requires efficient clearance of dopamine mediated by synaptic localization of the dopamine transporter DAT-1. J Neurosci. 2007;27:14216–14227. doi: 10.1523/JNEUROSCI.2992-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meyer DC, Quay WB. Hypothalamic and suprachiasmatic uptake of serotonin in vitro: twenty-four-hour changes in male and proestrous female rats. Endocrinology. 1976;98:1160–1165. doi: 10.1210/endo-98-5-1160. [DOI] [PubMed] [Google Scholar]
- 53.Moritz AE, Foster JD, Gorentla BK, Mazei-Robison MS, Yang JW, Sitte HH, Blakely RD, Vaughan RA. Phosphorylation of dopamine transporter serine 7 modulates cocaine analog binding. J Biol Chem. 2013;288:20–32. doi: 10.1074/jbc.M112.407874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moya PR, Wendland JR, Rubenstein LM, Timpano KR, Heiman GA, Tischfield JA, King RA, Andrews AM, Ramamoorthy S, McMahon FJ, Murphy DL. Common and rare alleles of the serotonin transporter gene, SLC6A4, associated with Tourette’s disorder. Mov Disord. 2013 doi: 10.1002/mds.25460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Murphy DL, Lesch KP. Targeting the murine serotonin transporter: insights into human neurobiology. Nat Rev Neurosci. 2008;9:85–96. doi: 10.1038/nrn2284. [DOI] [PubMed] [Google Scholar]
- 56.Nirenberg MJ, Vaughan RA, Uhl GR, Kuhar MJ, Pickel VM. The dopamine transporter is localized to dendritic and axonal plasma membranes of nigrostriatal dopaminergic neurons. J Neurosci. 1996;16:436–447. doi: 10.1523/JNEUROSCI.16-02-00436.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oda K, Hosoda N, Endo H, Saito K, Tsujihara K, Yamamura M, Sakata T, Anzai N, Wempe MF, Kanai Y, Endou H. L-type amino acid transporter 1 inhibitors inhibit tumor cell growth. Cancer Sci. 2010;101:173–179. doi: 10.1111/j.1349-7006.2009.01386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ott V, Koch J, Spate K, Morbach S, Kramer R. Regulatory properties and interaction of the C- and N-terminal domains of BetP, an osmoregulated betaine transporter from Corynebacterium glutamicum . Biochemistry. 2008;47:12208–12218. doi: 10.1021/bi801325r. [DOI] [PubMed] [Google Scholar]
- 59.Patlak CS. Contributions to the theory of active transport: II. The gate type non-carrier mechanism and generalizations concerning tracer flow, efficiency, and measurement of energy expenditure. Bull Math Biophys. 1957;19:209–235. [Google Scholar]
- 60.Penmatsa A, Wang KH, Gouaux E. X-ray structure of dopamine transporter elucidates antidepressant mechanism. Nature. 2013;503:85–90. doi: 10.1038/nature12533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Perez C, Koshy C, Yildiz O, Ziegler C. Alternating-access mechanism in conformationally asymmetric trimers of the betaine transporter BetP. Nature. 2012;490:126–130. doi: 10.1038/nature11403. [DOI] [PubMed] [Google Scholar]
- 62.Peter H, Burkovski A, Kramer R. Osmo-sensing by N- and C-terminal extensions of the glycine betaine uptake system BetP of Corynebacterium glutamicum . J Biol Chem. 1998;273:2567–2574. doi: 10.1074/jbc.273.5.2567. [DOI] [PubMed] [Google Scholar]
- 63.Pizzo AB, Karam CS, Zhang Y, Ma CL, McCabe BD, Javitch JA. Amphetamine-induced behavior requires CaMKII-dependent dopamine transporter phosphorylation. Mol Psychiatry. 2013 doi: 10.1038/mp.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pramod AB, Foster J, Carvelli L, Henry LK. SLC6 transporters: structure, function, regulation, disease association and therapeutics. Mol Aspects Med. 2013;34:197–219. doi: 10.1016/j.mam.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Quick M, Yano H, Goldberg NR, Duan L, Beuming T, Shi L, Weinstein H, Javitch JA. State-dependent conformations of the translocation pathway in the tyrosine transporter Tyt1, a novel neurotransmitter:sodium symporter from Fusobacterium nucleatum . J Biol Chem. 2006;281:26444–26454. doi: 10.1074/jbc.M602438200. [DOI] [PubMed] [Google Scholar]
- 66.Ramamoorthy S, Samuvel DJ, Buck ER, Rudnick G, Jayanthi LD. Phosphorylation of threonine residue 276 is required for acute regulation of serotonin transporter by cyclic GMP. J Biol Chem. 2007;282:11639–11647. doi: 10.1074/jbc.M611353200. [DOI] [PubMed] [Google Scholar]
- 67.Ramamoorthy S, Shippenberg TS, Jayanthi LD. Regulation of monoamine transporters: role of transporter phosphorylation. Pharmacol Ther. 2011;129:220–238. doi: 10.1016/j.pharmthera.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ressl S, Terwisscha van Scheltinga AC, Vonrhein C, Ott V, Ziegler C. Molecular basis of transport and regulation in the Na(+)/ betaine symporter BetP. Nature. 2009;458:47–52. doi: 10.1038/nature07819. [DOI] [PubMed] [Google Scholar]
- 69.Rubenhagen R, Morbach S, Kramer R. The osmoreactive betaine carrier BetP from Corynebacterium glutamicum is a sensor for cytoplasmic K+ Embo J. 2001;20:5412–5420. doi: 10.1093/emboj/20.19.5412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Scharf JM, Yu D, Mathews CA, Neale BM, Stewart SE, Fagerness JA, Evans P, Gamazon E, Edlund CK, Service SK, Tikhomirov A, Osiecki L, Illmann C, Pluzhnikov A, Konkashbaev A, Davis LK, Han B, Crane J, Moorjani P, Crenshaw AT, Parkin MA, Reus VI, Lowe TL, Rangel-Lugo M, Chouinard S, Dion Y, Girard S, Cath DC, Smit JH, King RA, Fernandez TV, Leckman JF, Kidd KK, Kidd JR, Pakstis AJ, State MW, Herrera LD, Romero R, Fournier E, Sandor P, Barr CL, Phan N, Gross-Tsur V, Benarroch F, Pollak Y, Budman CL, Bruun RD, Erenberg G, Naarden AL, Lee PC, Weiss N, Kremeyer B, Berrio GB, Campbell DD, Cardona Silgado JC, Ochoa WC, Mesa Restrepo SC, Muller H, Valencia Duarte AV, Lyon GJ, Leppert M, Morgan J, Weiss R, Grados MA, Anderson K, Davarya S, Singer H, Walkup J, Jankovic J, Tischfield JA, Heiman GA, Gilbert DL, Hoekstra PJ, Robertson MM, Kurlan R, Liu C, Gibbs JR, Singleton A, Hardy J, Strengman E, Ophoff RA, Wagner M, Moessner R, Mirel DB, Posthuma D, Sabatti C, Eskin E, Conti DV, Knowles JA, Ruiz-Linares A, Rouleau GA, Purcell S, Heutink P, Oostra BA, McMahon WM, Freimer NB, Cox NJ, Pauls DL. Genome-wide association study of Tourette’s syndrome. Mol Psychiatry. 2013;18:721–728. doi: 10.1038/mp.2012.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schnutgen F, Ghyselinck NB. Adopting the good reFLEXes when generating conditional alterations in the mouse genome. Transgenic Res. 2007;16:405–413. doi: 10.1007/s11248-007-9089-8. [DOI] [PubMed] [Google Scholar]
- 72.Shi L, Quick M, Zhao Y, Weinstein H, Javitch JA. The mechanism of a neurotransmitter:sodium symporter-inward release of Na+ and substrate is triggered by substrate in a second binding site. Mol Cell. 2008;30:667–677. doi: 10.1016/j.molcel.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Singer D, Camargo SM, Huggel K, Romeo E, Danilczyk U, Kuba K, Chesnov S, Caron MG, Penninger JM, Verrey F. Orphan transporter SLC6A18 is renal neutral amino acid transporter B0AT3. J Biol Chem. 2009;284:19953–19960. doi: 10.1074/jbc.M109.011171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sloan JL, Mager S. Cloning and functional expression of a human Na(+) and Cl(-)-dependent neutral and cationic amino acid transporter B(0+) J Biol Chem. 1999;274:23740–23745. doi: 10.1074/jbc.274.34.23740. [DOI] [PubMed] [Google Scholar]
- 75.Steiner JA, Carneiro AM, Blakely RD. Going with the flow: trafficking-dependent and -independent regulation of serotonin transport. Traffic. 2008;9:1393–1402. doi: 10.1111/j.1600-0854.2008.00757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sucic S, El-Kasaby A, Kudlacek O, Sarker S, Sitte HH, Marin P, Freissmuth M. The serotonin transporter is an exclusive client of the coat protein complex II (COPII) component SEC24C. J Biol Chem. 2011;286:16482–16490. doi: 10.1074/jbc.M111.230037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sung U, Apparsundaram S, Galli A, Kahlig KM, Savchenko V, Schroeter S, Quick MW, Blakely RD. A regulated interaction of syntaxin 1A with the antidepressant-sensitive norepinephrine transporter establishes catecholamine clearance capacity. J Neurosci. 2003;23:1697–1709. doi: 10.1523/JNEUROSCI.23-05-01697.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tabares-Seisdedos R, Rubenstein JL. Chromosome 8p as a potential hub for developmental neuropsychiatric disorders: implications for schizophrenia, autism and cancer. Mol Psychiatry. 2009;14:563–589. doi: 10.1038/mp.2009.2. [DOI] [PubMed] [Google Scholar]
- 79.Talvenheimo J, Fishkes H, Nelson PJ, Rudnick G. The serotonin transporter-imipramine “receptor”. J Biol Chem. 1983;258:6115–6119. [PubMed] [Google Scholar]
- 80.Torres GE, Yao WD, Mohn AR, Quan H, Kim KM, Levey AI, Staudinger J, Caron MG. Functional interaction between monoamine plasma membrane transporters and the synaptic PDZ domain-containing protein PICK1. Neuron. 2001;30:121–134. doi: 10.1016/s0896-6273(01)00267-7. [DOI] [PubMed] [Google Scholar]
- 81.Veenstra-VanderWeele J, Muller CL, Iwamoto H, Sauer JE, Owens WA, Shah CR, Cohen J, Mannangatti P, Jessen T, Thompson BJ, Ye R, Kerr TM, Carneiro AM, Crawley JN, Sanders-Bush E, McMahon DG, Ramamoorthy S, Daws LC, Sutcliffe JS, Blakely RD. Autism gene variant causes hyperserotonemia, serotonin receptor hypersensitivity, social impairment and repetitive behavior. Proc Natl Acad Sci USA. 2012;109:5469–5474. doi: 10.1073/pnas.1112345109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Verrey F. System L: heteromeric exchangers of large, neutral amino acids involved in directional transport. Pflugers Arch. 2003;445:529–533. doi: 10.1007/s00424-002-0973-z. [DOI] [PubMed] [Google Scholar]
- 83.Wang H, Goehring A, Wang KH, Penmatsa A, Ressler R, Gouaux E. Structural basis for action by diverse antidepressants on biogenic amine transporters. Nature. 2013;503:141–145. doi: 10.1038/nature12648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Welch BL, Hendley ED, Turek I. Norepinephrine uptake into cerebral cortical synaptosomes after one fight or electroconvulsive shock. Science. 1974;183:220–221. doi: 10.1126/science.183.4121.220. [DOI] [PubMed] [Google Scholar]
- 85.Wenk G, Hepler D, Olton D. Behavior alters the uptake of [3H]choline into acetylcholinergic neurons of the nucleus basalis magnocellularis and medial septal area. Behav Brain Res. 1984;13:129–138. doi: 10.1016/0166-4328(84)90143-8. [DOI] [PubMed] [Google Scholar]
- 86.Weyand S, Shimamura T, Yajima S, Suzuki S, Mirza O, Krusong K, Carpenter EP, Rutherford NG, Hadden JM, O’Reilly J, Ma P, Saidijam M, Patching SG, Hope RJ, Norbertczak HT, Roach PC, Iwata S, Henderson PJ, Cameron AD. Structure and molecular mechanism of a nucleobase-cation-symport-1 family transporter. Science. 2008;322:709–713. doi: 10.1126/science.1164440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wong FH, Chen JS, Reddy V, Day JL, Shlykov MA, Wakabayashi ST, Saier MH., Jr The amino acid-polyamine-organocation superfamily. J Mol Microbiol Biotechnol. 2012;22:105–113. doi: 10.1159/000338542. [DOI] [PubMed] [Google Scholar]
- 88.Wong A, Zhang YW, Jeschke GR, Turk BE, Rudnick G. Cyclic GMP-dependent stimulation of serotonin transport does not involve direct transporter phosphorylation by cGMP-dependent protein kinase. J Biol Chem. 2012;287:36051–36058. doi: 10.1074/jbc.M112.394726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yamashita A, Singh SK, Kawate T, Jin Y, Gouaux E. Crystal structure of a bacterial homologue of Na+/Cl-dependent neurotransmitter transporters. Nature. 2005;437:215–223. doi: 10.1038/nature03978. [DOI] [PubMed] [Google Scholar]
- 90.Zhao Y, Terry DS, Shi L, Quick M, Weinstein H, Blanchard SC, Javitch JA. Substrate-modulated gating dynamics in a Na+-coupled neurotransmitter transporter homologue. Nature. 2011;474:109–113. doi: 10.1038/nature09971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhou Z, Zhen J, Karpowich NK, Law CJ, Reith ME, Wang DN. Antidepressant specificity of serotonin transporter suggested by three LeuT-SSRI structures. Nat Struct Mol Biol. 2009;16:652–657. doi: 10.1038/nsmb.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhu CB, Carneiro AM, Dostmann WR, Hewlett WA, Blakely RD. p38 MAPK activation elevates serotonin transport activity via a trafficking-independent, protein phosphatase 2A–dependent process. J Biol Chem. 2005;280:15649–15658. doi: 10.1074/jbc.M410858200. [DOI] [PubMed] [Google Scholar]
- 93.Zhu CB, Lindler KM, Campbell NG, Sutcliffe JS, Hewlett WA, Blakely RD. Colocalization and regulated physical association of presynaptic serotonin transporters with A(3) adenosine receptors. Mol Pharmacol. 2011;80:458–465. doi: 10.1124/mol.111.071399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhu CB, Lindler KM, Owens AW, Daws LC, Blakely RD, Hewlett WA. Interleukin-1 receptor activation by systemic lipopolysaccharide induces behavioral despair linked to MAPK regulation of CNS serotonin transporters. Neuropsychopharmacology. 2010;35:2510–2520. doi: 10.1038/npp.2010.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ziegler C, Bremer E, Kramer R. The BCCT family of carriers: from physiology to crystal structure. Mol Microbiol. 2010;78:13–34. doi: 10.1111/j.1365-2958.2010.07332.x. [DOI] [PubMed] [Google Scholar]