Abstract
The development of language, social interaction and communicative skills is remarkably different in the child with autism spectrum disorder (ASD). Atypical brain connectivity has frequently been reported in this patient population. However, the neural correlates underlying their disrupted language development and functioning are still poorly understood. Using resting state fMRI, we investigated the functional connectivity properties of the language network in a group of ASD patients with clear comorbid language impairment (ASD-LI; N = 19) and compared them to the language related connectivity properties of 23 age-matched typically developing children. A verb generation task was used to determine language components commonly active in both groups. Eight joint language components were identified and subsequently used as seeds in a resting state analysis. Interestingly, both the interregional and the seed-based whole brain connectivity analysis showed preserved connectivity between the classical intrahemispheric language centers, Wernicke's and Broca's areas. In contrast however, a marked loss of functional connectivity was found between the right cerebellar region and the supratentorial regulatory language areas. Also, the connectivity between the interhemispheric Broca regions and modulatory control dorsolateral prefrontal region was found to be decreased. This disruption of normal modulatory control and automation function by the cerebellum may underlie the abnormal language function in children with ASD-LI.
Keywords: Autism spectrum disorders, Cerebellum, Language function, Resting state fMRI
Highlights
-
•
The development of language is remarkably different in the child with ASD.
-
•
Preserved intrahemispheric connectivity between Broca’s and Wernicke’s areas was found.
-
•
Decreased connectivity was found between the Broca regions and dorsolateral prefrontal region.
-
•
A loss of connectivity was found between the right cerebellar region and supratentorial language areas.
1. Introduction
Autism spectrum disorders (ASD) belong to the pervasive neurodevelopmental disorders. The prevalence of ASD has increased significantly throughout recent decades, bringing the overall estimated prevalence to 11.3 per 1000 children (Wingate et al., 2012). ASD is characterized by severe difficulties in reciprocal social interaction, stereotyped patterns of behavior and profound impairments in verbal and nonverbal communication. The ability to communicate verbally is considered to be a positive prognostic indicator for children with ASD (Groen et al., 2008; Wan et al., 2012; Williams et al., 2008). Although pragmatic language impairment is the most prominent in ASD, detailed linguistic studies have shown that for some children with ASD the linguistic abilities can be altered in pragmatic, semantic, syntactic and phonological domains extending to both receptive and expressive aspects (Groen et al., 2008; Verhoeven et al., 2010; Williams et al., 2008). One of those subgroups of ASD patients with comorbid all-encompassing language impairments, referred to as ASD-LI, has extensively been defined by Bishop (2010) and Kjelgaard and Tager-Flusberg (2001).
In contrast to the variability for most of the observations made in ASD research, the theory of a disorganized brain seems to be very consistent. This theory links the core symptoms observed in ASD to a deficient integration and synchronization of brain regions preferentially affecting long-range connections (Courchesne et al., 2007). Findings to date indicate that children with autism activate alternative and possibly less flexible networks during phonetic, semantic, syntactic and pragmatic language processing (Groen et al., 2008). Functional and structural neuroimaging are promising methods for investigating the neural correlates underlying the linguistic deficits in autism (Verhoeven et al., 2010; Courchesne et al., 2007). Several functional neuroimaging studies reported aberrant functioning between cortical areas on a range of language tasks (Harris et al., 2006; Just et al., 2004; Kana et al., 2006; Knaus et al., 2010), suggesting that alterations in cortical connectivity and the deficient communication among cortical regions may be part of the language difficulties seen in ASD (Courchesne, 2004; Courchesne et al., 2003). Further evidence for atypical brain connectivity of the language network in ASD has been corroborated by structural imaging studies using diffusion tensor imaging (DTI) (Barnea-Goraly et al., 2004; Fletcher et al., 2010; Keller et al., 2007; Nagae et al., 2012). Those studies pointed towards abnormal DTI parameters which may underlie the behavioral pattern observed in autism. Although a diagnostic imaging marker is at present still lacking, recent neuroimaging techniques seem to reveal subtle, but important, functional and/or microstructural changes in the brains of autistic children and adults. This highlights the importance of the investigation of interregional connectivity necessary for the efficient completion of higher cognitive functions such as language.
Moreover, several studies highlight a possible key role of cerebellar abnormalities in ASD such as repeated postmortem studies of ASD patients (Bailey et al., 1998; Kemper and Bauman, 2002; Ritvo et al., 1986; Williams et al., 1980). The presence of a reduced number of Purkinje cells is the most reproducible pathological observation in the autopsied autistic brain (Kemper and Bauman, 1998, 2002; Ritvo et al., 1986; Williams et al., 1980). In addition, magnetic resonance imaging (MRI) studies have described volumetric cerebellar abnormalities (Courchesne et al., 1994; Saitoh and Courchesne, 1998) and asymmetry changes (Hodge et al., 2010). During the past decade, a direct association between behavioral functioning in ASD and MRI findings was demonstrated both for cerebellar structure (Akshoomoff et al., 2004; Kates et al., 2004; Pierce and Courchesne, 2001; Webb et al., 2009) and function (Allen et al., 2004; Mostofsky et al., 2009). The involvement of the cerebellum in language, cognition and affective modulation has been overlooked for a very long time, due to its prominent role in motor functioning (Beaton and Marien, 2010). Currently, it is thought that, in addition to its contribution to the regulation and coordination of motor function, the right lateral cerebellum is actively involved in the modulation of a broad spectrum of linguistic functions (Murdoch, 2010). Much of the credit for this development goes to Leiner and colleagues. In 1989, they observed an expansion of the lateral portions of the cerebellar hemispheres and dentate nuclei in humans. They hypothesized that these regions project to pre-frontal and other association cortices in humans, forming different cortico-cerebellar loops involved in the regulation of voluntary movements and cognitive/linguistic functions (Leiner et al., 1993). Other neuroanatomical studies have further unraveled the complex reciprocal connections linking the cerebellum with higher order cortical association areas, including areas crucially involved in high-level cognitive and linguistic functions (Engelborghs et al., 1998; Middleton and Strick, 1997, 2000; Schmahmann, 1996). A number of positron emission tomography activation studies on healthy subjects demonstrated that, in addition to Broca's area, the contralateral cerebellar hemisphere was actively involved in the production of semantically related verbs in response to visually presented nouns (Papathanassiou et al., 2000; Petersen et al., 1988; Raichle et al., 1984). First, Petersen et al. observed an increased blood flow in the right lateral cerebellum, which projects to the left prefrontal language areas, supporting the involvement of the cerebellum in non-motor language (Petersen et al., 1988). Furthermore, emerging evidence for the role of the cerebellum in linguistic functions was also provided by functional MRI studies showing a consistent pattern of cerebellar activation which was not due to motor verbal responses but to non-motor cognitive processes subserving semantic word association (Frings et al., 2006; Xiang et al., 2003). Subsequent studies used variations on the original verb generation task design and reported activation of the right lateral cerebellum during performance of a language task in healthy subjects (Desmond and Fiez, 1998). Finally, cerebellar lesion studies have further confirmed this link by the presence of high level linguistic impairments in association with cerebellar pathology, including problems in verbal fluency, word retrieval, syntax, reading, writing and metalinguistic abilities (Gasparini et al., 1999; Marien et al., 2001; Riva, 1998; Schmahmann and Sherman, 1998; Silveri et al., 1994).
Motivated by a possible link between functional connectivity (FC) and language performance in ASD, the present study investigated the FC pattern of the language network, including the cerebellum. To our knowledge, the functional connections with the cerebellum were never subject of previous research. A subgroup of ASD patients with apparent structural language impairment was compared to the FC pattern of a group of age-matched typically developing (TD) children. As no specific cooperation is required and the results are not dependent on task performance, rfMRI is particularly useful for investigating pediatric or non-cooperative patient samples. The resting human brain shows low frequency (~ < 0.1 Hz) fluctuations in the blood-oxygenation-level dependent (BOLD) signals that are not random, but represent neuronal activity organized into structured spatiotemporal profiles that reflect the functional architecture of the brain (Deco and Corbetta, 2011). Resting state studies examine the level of co-activation between the functional time series of anatomically separated brain regions during rest, believed to reflect functional communication between them (Biswal et al., 1995; Damoiseaux et al., 2006; Greicius et al., 2003).
By applying advanced neuroimaging techniques, the current study aims to find a neurobiological substrate underlying the language difficulties seen in a subgroup of children with ASD. For this study, we applied a two-stage approach. First, we selected regions of interest (ROIs) in the language network by determining commonly activated areas using a conventional fMRI block-design with a verb generation task. Second, these ROIs were used as seed regions to evaluate the presence of altered functional connectivity in the language network during the resting state. Group differences in functional connectivity were evaluated and functional connectivity indices were correlated with PIQ, language performance and autism severity measures. We hypothesized that abnormal language function in ASD is related to a neural functional connectivity deficit of the cortical language network; particularly we hypothesized that the cortico-cerebellar connectivity might play a crucial role.
2. Materials and methods
2.1. Participants
Nineteen individuals with ASD-LI (mean age 14.0 ± 1.5 y; 15 males, 4 females) and 23 TD adolescents (mean age 14.3 ± 1.3 y; 16 males, 7 females) matched for age and PIQ were included. The majority of these individuals also participated in a previous DTI study assessing microstructural alterations in ASD-LI (Verhoeven et al., 2012). All participants were right-handed, native Dutch speakers with normal hearing with a performance or full scale IQ above 80, confirmed by an abbreviated version of the Dutch Wechsler Intelligence Scale for Children, Third Edition (WISC-III-NL) (Kort and Schittekatte, 2005) at the time of study intake (Table 1). ASD participants were selected from a clinical sample, diagnosed based on DSM-IV-TR criteria by a multidisciplinary team including a pediatric neurologist/psychiatrist. The Social Communication Questionnaire (SCQ) (Rutter and Couteur, 2003) and Social Responsiveness Scale (SRS) (Constantino and Davis, 2003) were additionally administered to ensure the current presence of substantial ASD symptoms.
Table 1.
Subject characteristics per group.
| TD | ASD-LI | p value | |
|---|---|---|---|
| Age | 14.3 (1.3) | 14.0 (1.5) | 0.460 |
| Verbal IQ | 112.5 (13.6) | 89.3 (18.7) | < 0.001 |
| Performance IQ | 105.8 (8.2) | 100.3 (13.2) | 0.098 |
| Social Responsiveness Scale | 17.3 (14.4) | 93.5 (33.9) | < 0.001 |
| Social Communication Questionnaire | 3.5 (3.8) | 20.7 (8.0) | < 0.001 |
| Sentence Formulation | 36.3 (2.2) | 31.4(4.8) | 0.001 |
| Sentence Assembly | 12.4 (0.8) | 10.4 (2.2) | 0.002 |
| Word Definition | 39.0 (6.3) | 26.6 (7.3) | < 0.001 |
| Text Comprehension | 14.0 (1.0) | 11.3 (3.9) | 0.012 |
| Semantic Relations | 18.6 (1.3) | 14.3 (4.4) | < 0.001 |
| Word Classes Receptive | 17.7 (1.8) | 13.0 (3.1) | < 0.001 |
| Word Classes Expressive | 15.5 (2.8) | 10.1 (4.0) | < 0.001 |
Overview of the participant characteristics in the patient group (ASD-LI n = 19) and their age-matched TD group (CO-ASD n = 23). Mean and standard deviation are presented for each parameter. p values are provided for between group comparisons.
Inclusion criteria for the ASD-LI group were: 1) a diagnosis of autistic disorder or pervasive developmental disorder — not otherwise specified (PDD-NOS) according to DSM-IV-TR criteria (APA, 2000), 2) SCQ score ≥ 15 and 3) SRS score ≥ 60. ASD participants with a significant history of language delay and/or impairment, defined by the absence of two-word combinations at the age of three, need for intensive speech therapy during pre-school years and the presence of language problems at the time of diagnostic assessment, were specifically selected, aiming for an ASD-LI subgroup. The identification of the ASD patients as ASD-LI patients was confirmed by extensive language testing using different subtests of the Dutch version of the Clinical Evaluation of Language Fundamentals (CELF-4NL) (Kort et al., 2008). To assess language performance of all language domains (e.g. semantics, phonology, morphology, syntax, pragmatics) in an expressive and receptive way, the following subtests of the CELF-4NL were used: Concepts and following directions (CFD), Sentence Formulation (SF), Sentence Assembly (SA), Word Definitions (WD), Word Classes Expressive (WC-E), Word Classes Receptive (WC-R), Text Comprehension (TC), Semantic Relations (SR) and Word Associations (WA). Two individuals with ASD were receiving methylphenidate therapy and one was on risperidone at the time of the acquisition. Participants were excluded if there was a chronic medical illness, metabolic disorder or an abnormal neurological examination, if ASD-LI was associated with a genetic syndrome or if conventional MRI was found to be abnormal.
None of the healthy volunteers reported a history of neurological or psychiatric conditions, nor a current medical, developmental, or psychiatric diagnosis. They did not report any (history of) language problems, which were confirmed by their adequate scores on the CELF-4NL (Table 1). The SCQ and SRS questionnaires were administered to the TD group as well, to exclude the presence of substantial ASD symptoms in this group.
The study was approved by the local Ethical Board of the University Hospitals Leuven, Belgium and informed consent was obtained from all parents/guardians according to the Declaration of Helsinki, with additional assent from all participating children.
2.2. General image acquisition parameters
Neuroimaging was performed using a Philips (Best, The Netherlands) 3T MR scanner with an 8-channel phased-array head coil. Anatomical imaging consisted of a high resolution structural volume acquired using a coronal three-dimensional turbo field echo T1-weighted images sequence with the following parameters: 182 contiguous coronal slices covering the whole brain and brainstem, slice thickness = 1.2 mm; repetition time (TR) = 9.7 ms; echo time (TE) = 4.6 ms; matrix size = 256 × 256; field-of-view (FOV) = 250 × 250 mm2; in-plane pixel size = 0.98 × 0.98 mm2; and acquisition time = 6 min 38 s.
2.3. Blocked design language related fMRI
2.3.1. Task
As the choice of the fMRI task is guided by the participants' ability to cooperate with the procedure, we chose a relatively easy-to-perform and well-known fMRI language task, i.e. a verb generation task. This task was originally used in positron emission tomography studies (Benson et al., 1999; Petersen et al., 1988) and is very robust in localizing cortical regions involved in language function. During the task a noun is visually displayed on a screen inside the magnet bore and the participant is instructed to covertly generate one or more verbs associated with it (e.g. ‘chair’ → ‘sit’). Stimuli were presented in a block design. Four task epochs (30 s each, 3 s for a single noun) are alternated with four periods of rest (30 s each). During rest epochs participants passively view a series of unpronounceable scrambled visual symbols at the center of the screen (e.g. #/°*-). Before the scan session, task performance was assessed outside the scanner. Visual stimuli were presented using Presentation software; Version 14.1 (Neurobehavioral Systems, California, USA) projected via an LCD projector on a translucent screen and viewed with a mirror placed above the head coil. Task performance assessment before the scan session showed that all participating individuals were able to generate correct verbs in response to the presented nouns. Response times outside the scanner were 1.5 s, suggesting that they had enough time in the scanner to complete each trial within the time given (TR = 3 s).
2.3.2. Acquisition parameters
For the task-related fMRI session a T2* weighted gradient echo-echo planar imaging (GE-EPI) sequence was used with the following parameters: TR = 3000 ms; TE = 33 ms; matrix size = 80 × 80; FOV = 230 mm; flip angle 90°; slice thickness 4 mm, no gap; and axial slices = 35. A total of 80 functional volumes per subject were collected.
2.3.3. Data analysis
SPM8 was used for image pre-processing and statistical analysis (Ashburner and Friston, 2011). Single subject functional image time-series were realigned to each other using a two-pass procedure, registering the images to the mean image after a first realignment. Next, the structural image was coregistered with the mean of the functional time series. Finally, the images were normalized to standard MNI EPI space, resliced to a voxel size of 2 × 2 × 2 mm and smoothed with a full width at half-maximum (FWHM) Gaussian Smoothing kernel of 8 mm.
After pre-processing, the following analyses were carried out. First, for each subject a general linear model was used to regress the time-course of verb generation versus rest as predictors and with the realignment parameters as regressors of no interest. A high-pass filter with a cutoff of 128 s was used to remove slow signal drifts. Voxel-wise T-maps were constructed for each subject. Second, the contrast maps were carried to a second level analysis to test for significant group effects using a two level, one-way ANOVA (Analysis of Variance). Third, a conjunction analysis was performed starting from the single-group fMRI statistical activation maps in order to select those voxels active in both subject groups during the execution of the language task. The conjunction maps were thresholded at p = 0.001 uncorrected and 10 voxel cluster size. At each peak of activation a seed was selected and the volume of the seed was extended to a maximum of approximately 200 voxels, which corresponds to a sphere of 11 mm. The selected seeds are assumed to encompass the language network (see below).
2.4. Seed definitions for the resting state functional connectivity analysis
2.4.1. Functional connectivity analysis of the language network
The conjunction map of the brain responses during the active language task revealed the following eight activation peaks which were used as seeds for the functional connectivity analysis of the language network: 1–2) The left and right inferior frontal gyrus (IFG-L, IFG-R) including parts of Brodmann areas (BA) 44, 45, and 47, constituting the ‘expressive’ language area or Broca's area; 3) the left dorsolateral prefrontal cortex (DLPF-L) including parts of BA 46 and BA 10, implicated in semantic working memory (Gabrieli et al., 1998); 4–5) the left and right middle and superior temporal gyrus (STG-L, STG-R) including BA 21 and BA 22, constituting the receptive language region or Wernicke's area; 6) the left medial frontal gyrus including the medial side of BA 6 or supplementary motor area (SMA), crucial in the programming and fluent execution of extended action sequences (Alario et al., 2006), and BA 32 which is the dorsal part of the anterior cingulate cortex linked to error detection and online monitoring of performance (Carter et al., 1998); 7) the left premotor cortex (Premotor) at BA 6 implicated in planning of articulation and naming (Duffau et al., 2003); and 8) the right cerebellar lobule VI, crus I (cerebellar), similar to the activations found in a meta-analysis of neuroimaging studies investigating the functional topography of the human cerebellum (Stoodley and Schmahmann, 2009). MNI coordinates of the peak activation (center of the seed), the T-value of the peak and the cluster size are given in Table 2.
Table 2.
Seed definitions: language related areas.
| Region of interest (ROI) | MNI coordinates of peak activation x y z | Cluster size | T peak value |
|---|---|---|---|
| IFG left | − 52 14 − 2 | 165 | 6.06 |
| IFG right | 48 14 − 2 | 132 | 3.93 |
| MTG left | − 52 − 44 2 | 113 | 3.88 |
| MTG right | 54 − 34 − 6 | 108 | 3.80 |
| DLPF left | − 36 50 14 | 144 | 4.63 |
| Premotor cortex left | − 44 2 54 | 127 | 4.08 |
| SMA | − 4 16 48 | 210 | 6.24 |
| Cerebellar cortex right | 36 − 62 − 32 | 132 | 5.36 |
Characteristics of the seeds defining the language network are presented with MNI coordinates of the peak activations, T-value of the peak and cluster size.
2.5. Resting state fMRI data acquisition and analysis
2.5.1. Participant instruction
All participants were instructed to relax (but not sleep), keep their eyes closed and think of nothing in particular during the rfMRI scanning.
2.5.2. Acquisition parameters
For the non-task related resting state fMRI (rfMRI), data was acquired using a T2* weighted GE-EPI sequence with the following parameters: TR = 1700 ms; TE = 33 ms; matrix size = 64 × 64, FOV = 230 mm; flip angle 90°; slice thickness = 4 mm, no gap; and axial slices = 32. Two hundred and fifty functional volumes were obtained in 7 min.
2.5.3. Functional connectivity analysis
Functional connectivity analysis was performed using in-house developed software (Ebisch et al., 2011). To check for potential differences in head movement between participants of the TD group and the ASD-LI group, we compared both groups with respect to motion parameters, using the root mean squared variance (rmsvariance) (Church et al., 2009). Pre-processing of the rfMRI data was very similar to the pre-processing of the task-related fMRI time series, comprising realignment to the mean, followed by a coregistration to the structural image and normalization into standard MNI EPI space. Images were resliced to a 3 × 3 × 3 mm voxel size and smoothed with a FWHM Gaussian Smoothing kernel of 8 mm. Further pre-processing included 1) band-pass filtering between 0.009 and 0.08 Hz; 2) regression of the white matter and cerebrospinal fluid signals based on the MNI white matter and the ventricular segmentation mask and 3) regression of the 3D motion parameters. To avoid the introduction of false negative correlations, the global signal was not regressed out (Weissenbacher et al., 2009). For each ROI, a representative BOLD time-course was obtained by averaging the signal of all the voxels within the ROI.
To assess functional connectivity, we first calculated the Pearson correlation coefficient between the mean signal intensity time courses of each ROI pair. A Fisher's r-to-z transformation was applied to each correlation map, to obtain an approximately normal distribution of the functional connectivity values and accordingly apply parametric statistics. Next, a random-effect analysis was performed independently for each of the two groups of participants in order to reveal coherent functional connectivity patterns between ROIs that were consistent across participants. Statistical significance was assessed using a statistical threshold p < 0.001 corrected by the False Discovery Rate (FDR). Using the results from the random-effect analysis, an independent-sample t-test was used to calculate direct contrasts between ASD-LI patients and TD with respect to all the different ROIs. Here, a statistical threshold of p < 0.05 FDR corrected was applied.
Secondly, a whole brain connectivity analysis was performed. Here, the Pearson correlation coefficient between the signal intensity time courses of each ROI and the time courses of all the residual brain voxels (p < 0.001 FDR corrected) was calculated. Again independent-samples t-tests were used to calculate direct contrasts between ASD-LI patients and TD, according to a random-effect analysis between the whole-brain connectivity maps for the different seed ROIs of the TD and ASD-LI patients. Statistical significance was assessed using a statistical threshold p < 0.01 FDR corrected.
2.6. Functional connectivity linked to behavioral parameters
In a final step we evaluated the relation between functional connectivity and behavioral measures in the ASD-LI group, focusing on PIQ, language performance [verbal IQ (VIQ), Sentence Formulation (SF), Sentence Assembly (SA), Word Definitions (WD), Text Comprehension (TC), Semantic Relations (SR), Word Classes Receptive (WCR), Word Classes Expressive (WCE)] and autism severity scores [SRS, SCQ]. Although the age range is not too large within the ASD-LI sample, age is a crucial component while evaluating language skills. Therefore, we calculated Pearson correlations corrected for age when testing the association between behavioral scores and neural connectivity. To account for errors due to multiple comparisons we constrained the correlation analysis to those ROI-pairs that showed significant functional connectivity difference in the direct contrasts between ASD-LI patients and TD. To reduce the number of behavioral components related to language performance and autism severity, we performed a principal component factor analysis with orthogonal varimax rotation on all behavioral measures and extracted the main factors.
3. Results
3.1. Demographics
Nineteen right-handed children with ASD and 23 age-matched right-handed TD control children were included in the study. An overview of the group characteristics is shown in Table 1. ASD-LI patients and TD controls were well matched for age and performance IQ (PIQ). VIQ was significantly lower in the ASD-LI patients compared to the TD group (p < 0.001), reflecting the inherent language problems. These language problems were confirmed by a significantly weaker performance of the ASD-LI patients on all assessed language subtests of the CELF-4NL. The ASD-LI subjects scored on average − 2.82 SD below the mean of their controls across all language tests.
3.2. Control analyses
Statistical comparison of the amount of head movement between patients and TD participants was performed. The average subject rmsvariance (Church et al., 2009) of motion was 0.084 mm (SD 0.026) in the TD group and 0.091 mm (SD 0.038) in the ASD-LI group and did not significantly differ between groups (p = 0.47).
A general linear model analysis of the functional data in response to the verb generation task showed statistically significant (p < 0.001, minimal cluster size 10 voxels) activations of the main language-related regions, consistent with previous descriptions of BOLD activations related to verb generation (Allendorfer et al., 2012; Frings et al., 2006; Holland et al., 2001; Wood et al., 2004), in both TD and ASD groups (Fig. 1). A conjunction map confirms the presence of activation overlap in the language related areas: DLPF, MTG, SMA, Premotor, IFG and Cerebellum (Fig. 1).
Fig. 1.
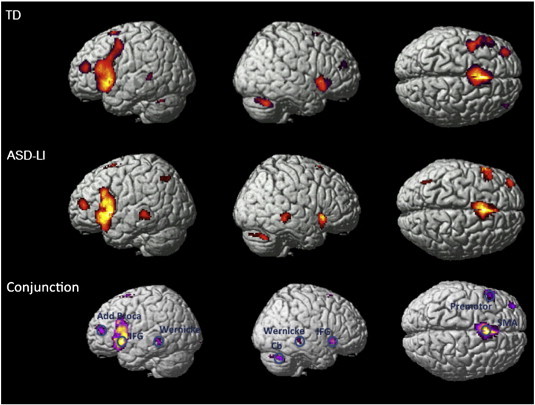
This figure shows the statistical group activation maps of language in TD and ASD-LI patients as well as the conjunction map that displays the common activated voxels in TD and ASD-LI patients (p < 0.001, minimal cluster size 10 voxels). The center of the seeds was defined by the peak activations of the conjunction map.
3.3. Results of functional connectivity analysis
Based on the resting state data, we first evaluated the functional connectivity in the language network by correlating the mean signal intensity time courses for each ROI pair combination in the eight language network components described above. The mean functional connectivity of the language network was significantly reduced in ASD individuals compared to TD subjects (p < 0.001). Fig. 2A, B shows the functional connectivity correlation map as well as a schematic representation of the significant connections between the language nodes, plotted on an axial slice. In the TD group, significant connectivity (p < 0.001 FDR corrected) was identified in 21 of 28 functional connectivity links (Fig. 2A). In the patient group, only 9 of 28 functional connectivity links were significant (p < 0.001 FDR corrected) (Fig. 2B). Note the preserved connectivity between IFG left (Broca's area) and STG left (Wernicke's area) in the ASD group and the profound loss of connectivity with the right cerebellar seed in this group.
Fig. 2.
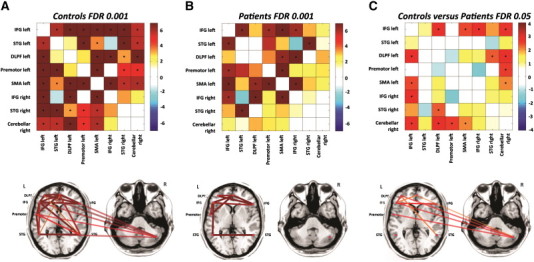
A and B show the correlation matrices representing functional connectivity links among the eight identified language network regions evaluated in this study in TD (n = 19) (A) and ASD-LI patients (n = 13) (B). C presents the correlation matrices showing differences in functional connectivity between TD and patients among the eight network regions involved in language processing. The color represents the T value of connectivity between the two connected brain regions. Significant correlations (p < 0.001 FDR for within group and p < 0.05 FDR for between group comparison) are indicated with a dot at the center of the matrix square. Below each correlation matrix, significant correlations are schematically represented as lines on an axial slice. Line thickness and color vary according to the scale above. The semi-transparent lines represent the connections to the cerebellum.
Fig. 2C contrasts the functional connectivity strength between ASD-LI patients and TD participants and shows a significant reduction (p < 0.05 FDR corrected) in the following connections: right cerebellum with left DLPF, right cerebellum with left premotor, right cerebellum with SMA, right cerebellum with left IFG, left IFG with right IFG, left IFG with SMA, left IFG with left DLPF cortex and left DLPF cortex with right STG. To examine the specificity of the seed-based findings, a whole brain connectivity analysis was performed estimating the spatial pattern of whole brain cortical connectivity seeded from a predefined language network component. For each study group, a group-level voxel wise functional connectivity map (FDR corrected p < 0.001) was created starting from the right cerebellar seed and the left IFG seed (Broca's area). These maps were statistically compared between ASD-LI patients and TD participants (FDR corrected p < 0.01). Fig. 3 illustrates these group-level voxel wise functional connectivity maps for all TD subjects (Fig. 3; TD) and all ASD-LI patients (Fig. 3; ASD-LI). Here, Fig. 3 (TD) confirms that almost all areas that co-activate with the right cerebellum are involved in language processing, even when all brain voxels were evaluated in this analysis. Furthermore, in Fig. 3 (ASD-LI) one can appreciate that connectivity starting from the right cerebellar seed and left IFG seed is very limited in ASD-LI patients. Also, Fig. 3 (TD > ASD-LI) shows the areas of significantly different connectivity seeding from the right cerebellar area and left IFG seed. Table 3 gives a more detailed description of the brain regions with significant connectivity differences between both groups concerning their connectivity with the seed ROI (right cerebellar seed and left IFG seed).
Fig. 3.
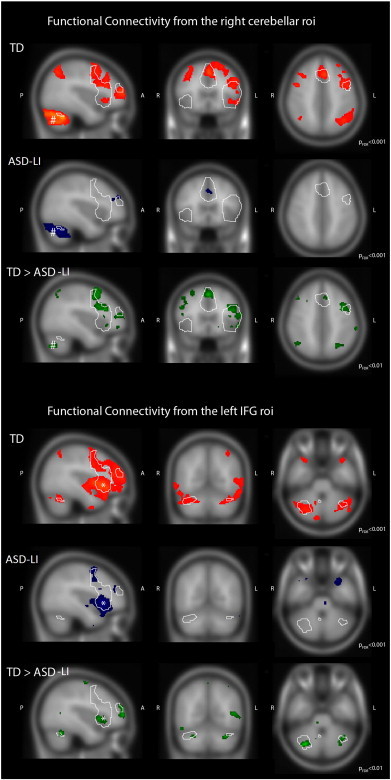
This figure represents the voxel-wise functional connectivity maps using the right cerebellar network region (#) and left Broca region (*) as a seed for whole brain connectivity analyses. The outline of the task-based language activation map of the TD is overlaid to ease the comparison. TD maps present a high degree of spatial specificity, showing correlation almost exclusively with other regions belonging to the language network (p < 0.001, FDR corrected). In the ASD-LI maps an important reduction of connectivity starting from the right cerebellar seed and left IFG (Broca's area) is shown (p < 0.001, FDR corrected). Also, significant differences for a statistical threshold of p < 0.01 FDR-corrected are presented (TD > ASD-LI).
Table 3.
Whole brain functional connectivity from right cerebellar and left Broca's area.
| Seed ROI | Brain region functionally connected with seed ROI | MNI coordinates (x, y, z) |
T-score TD group |
T-score ASD-LI group |
T-score TD vs ASD-LI group |
||
|---|---|---|---|---|---|---|---|
| Cerebellum right | |||||||
| Left superior frontal gyrus, SEF | − 2 | 22 | 48 | 8.15 | 1.36 | 4.01 | |
| Anterior cingulate gyrus | 0 | 34 | 36 | 7.40 | 1.83 | 2.24 | |
| Left superior frontal gyrus, sup orb | − 26 | 58 | − 2 | 7.77 | 0.68 | 3.81 | |
| Left middle frontal gyrus, premotor, SMA | − 32 | 6 | 62 | 6.48 | 0.23 | 4.09 | |
| Left middle frontal gyrus | − 34 | 4 | 46 | 6.24 | 0.30 | 3.59 | |
| Left inferior frontal gyrus | − 44 | 12 | 24 | 7.82 | 0.07 | 5.13 | |
| Left parietal lobe | − 38 | − 62 | 46 | 6.42 | 0.55 | 2.49 | |
| Right middle frontal gyrus, premotor, SMA | 34 | 12 | 56 | 5.87 | 0.99 | 2.23 | |
| Right precentral gyrus | 48 | 4 | 46 | 6.68 | 1.28 | 1.52 | |
| Left thalamus | − 6 | − 8 | 6 | 6.84 | 1.88 | 3.38 | |
| Left cerebellum crus I, declive | − 24 | − 76 | − 26 | 10.79 | 7.30 | 3.00 | |
| Left cerebellum crus II, pyramis | − 34 | − 72 | − 44 | 7.71 | 1.92 | 3.66 | |
| Right cerebellum crus II, pyramis | 26 | − 70 | − 42 | 8.69 | 3.27 | 4.12 | |
| Right cerebellum crus I, tuber | 40 | − 70 | − 30 | 22.64 | 13.93 | 2.92 | |
| Left nodule, vermis | − 2 | − 80 | − 16 | 9.60 | 5.89 | 0.35 | |
| Broca left | |||||||
| Left inferior frontal gyrus | − 52 | 16 | 16 | 21.96 | 10.61 | 0.44 | |
| Left superior frontal gyrus | − 2 | 14 | 54 | 10.02 | 5.93 | 3.26 | |
| Anterior cingulate gyrus | 0 | 26 | 28 | 7.28 | 3.13 | 2.54 | |
| Left precentral gyrus | − 50 | 4 | 38 | 6.76 | 2.40 | 3.39 | |
| Left middle temporal gyrus | − 60 | − 46 | 0 | 8.01 | 3.87 | 3.10 | |
| Left inferior parietal lobule, supramarginal gyrus | − 54 | − 46 | 44 | 7.21 | 4.99 | 2.12 | |
| Right middle frontal gyrus | 42 | 38 | 30 | 6.19 | 1.33 | 2.97 | |
| Right inferior frontal gyrus | 50 | 14 | − 6 | 11.48 | 7.13 | 2.61 | |
| Right inferior frontal gyrus | 54 | 14 | 30 | 8.37 | 1.50 | 3.53 | |
| Right middle temporal gyrus | 52 | − 40 | 0 | 8.85 | 2.25 | 2.63 | |
| Right parietal lobule, supramarginal gyrus | 56 | − 32 | 50 | 7.78 | 2.28 | 2.20 | |
| Frontal operculum | 34 | 26 | 0 | 8.85 | 3.74 | 2.76 | |
| Right cerebellum crus I | 30 | − 66 | − 32 | 6.73 | 1.00 | 4.63 | |
| Left cerebellum crus I | − 34 | − 62 | − 32 | 5.53 | 0.89 | 3.18 | |
Brain regions showing seed-based functional connectivity differences at a significance level of p < 0.01 (FDR corrected) starting from the right cerebellar seed region and starting from the left Broca's area, respectively.
3.4. Links between functional connectivity and behavioral parameters
We limited our analysis to those ROI pairs for which the functional connectivity was significantly different (p < 0.05 FDR corrected) between ASD-LI and TD participants and we correlated them with PIQ and the factor scores of the language and autism severity measures.
Based on the eigenvalue > 1 criterion we extracted three factors accounting for 71% of the total variance. Language subtests were randomly assigned to two factors. The first language factor had factor loading above 0.7 on SF (0.77), DW (0.75), WC-R (0.87), and WC-E (0.85). The second language component was determined by high loadings on TC (0.88) and SR (0.71). The third component describes ASD severity and was determined by high loadings on SRS (0.88) and SCQ (0.83). Factor scores were calculated using the regression method. The results of correlating the pair-wise functional connectivity measures with the behavioral measures are shown in Table 4. The ASD severity index showed a significant negative correlation with the quality of functional connectivity between the right cerebellum and left DLPF seed (r = − 0.43; p = 0.04; two tailed probability). No individual behavioral measures correlated with the connectivity measures.
Table 4.
Correlations between FC strengths and behavioral factors.
| r | Language 1 | Language 2 | Autism severity | PIQ |
|---|---|---|---|---|
| L-Broca–L DLPF | 0.14 | 0.10 | − 0.05 | 0.19 |
| L-Broca–R Broca | 0.16 | 0.23 | − 0.16 | 0.29 |
| L Broca–SMA | 0.04 | 0.02 | − 0.26 | 0.03 |
| L Broca–R cerebellum | 0.43 | 0.34 | − 0.19 | 0.39 |
| L DLPF–R Wernicke | 0.22 | 0.17 | 0.15 | 0.16 |
| L DLPF–R cerebellum | 0.02 | − 0.01 | − 0.43⁎ | 0.20 |
| L Premotor–R cerebellum | 0.42 | 0.31 | − 0.20 | − 0.37 |
| SMA–R cerebellum | 0.31 | 0.22 | − 0.14 | 0.01 |
p < .05.
4. Discussion
Many children with ASD show a marked delay in the initial onset of speech and language development (Rapin and Dunn, 1997). The fraction of language problems that persist throughout life, however, can vary from subtle isolated pragmatic problems to extensive language deficits including syntactic, semantic and phonological domains (Rapin and Dunn, 2003; Riches et al., 2011; Roberts et al., 2004). In a previous study, we assessed the white matter microstructural properties in a subgroup of ASD patients with clear co-occurring language impairment (ASD-LI) (Verhoeven et al., 2012). We focused on the integrity of the superior longitudinal fascicle (SLF), a major association white matter tract involved in language processing connecting Broca's and Wernicke's area. DTI, however, showed no microstructural differences comparing the mean fractional anisotropy values and mean diffusivity values of the SLF in the ASD-LI patients to those of the age-matched TD controls. The present study investigated the functional connectivity properties of the language network in those ASD-LI patients using resting state fMRI. Two important findings emerge from this study. Firstly, a global decrease of functional connectivity in the language network of ASD-LI patients has been found. More specifically, major decreases in connectivity were observed in the connections with the right cerebellar region as well as in the connectivity of left Broca's area with its contra-hemispheric analog, left SMA and with the left DLPF cortex. Secondly, the loss of connectivity was related to a lower language performance in ASD-LI patients.
Firstly, in this study we focused on the functional connectivity properties of the language network in a group of ASD-LI children. We did not limit our analysis to the classical Broca–Wernicke connection, but investigated the distributed brain network for semantic retrieval. Eight common language components were identified using a well-established easy-to-perform verb-generation task, which has proven to consistently activate the regions involved in language processing (Benson et al., 1999; Petersen et al., 1988). Functional connectivity between those components was assessed using task-independent fMRI. In agreement with previous DTI findings (Verhoeven et al., 2012), the functional connectivity analysis confirmed a preserved intra-hemispheric connectivity between left IFG and left STG in the ASD-LI group. In contrast, a profound loss of functional connectivity was found between the interhemispheric Broca regions, SMA and modulatory control dorsolateral prefrontal region. Also, a prominent decrease in functional connectivity was found in the cortico-cerebellar circuits, especially in the indirect links to the main Broca's and Wernicke's area. Both the ROI-based functional connectivity maps as well as the whole-brain voxel-wise functional connectivity maps show a disruption of the cerebello-DLPF, cerebello-SMA, cerebello-IFG and cerebello-premotor loops; all suggesting a dysfunction in cerebellar control and/or modulation of language functioning. This indicates, as previously suggested by Hodge et al. (2010), that these fronto–cortico–cerebellar interconnections could facilitate most areas of cognitive function, including language, executive function, working memory, attention and emotion.
The present study reveals a functional dissociation of the right cerebellum and the supratentorial cortical language network consisting of the contralateral Broca's and Wernicke's area, DLPF, SMA and premotor area. These findings are in agreement with the findings of Mostofsky et al. that describe a relative dissociation of the cerebral and cerebellar motor regions in ASD children (Mostofsky et al., 2009). Connections with other modulatory areas (DLPF, SMA, premotor area) are particularly affected. Those results strongly support the hypothesis that the cortical functioning of Broca's area is not affected per se, but that language dysfunction in ASD-LI is the result of a dysfunctional regulatory control of this region. The significantly reduced functional connectivity between Broca's area and DLPF, which is an important modulatory area for language, together with an intact Broca–Wernicke connection, further supports this hypothesis (Kovelman et al., 2012; Nardone et al., 2011; Romanski et al., 1999).
Secondly, we correlated our behavioral measures with the connectivity strengths in those language related ROI-pairs that showed significant group differences. Here, we demonstrated a significant negative correlation between the functional quality of the DLPF — right cerebellar connections and an ASD severity index. These findings suggest that underconnectivity of the DLPF-right cerebellar circuit is directly linked to the autistic phenotype. On the other hand, the quality of the functional connections between the right cerebellum and left premotor area, right cerebellum and left Broca area showed a trend (defined as p < 0.10) to be associated with general language ability.
This study has limitations, some of which relate to the included study population and others to the technical aspects of fMRI. By focusing on a specific ASD-LI subsample, we reduced the complex heterogeneity prevalent in ASD and hence increased statistical power. The use of more homogeneous patient samples may facilitate unraveling the brain–behavior association in the complex neurobiology of ASD. However, this limits the conclusions that can be drawn regarding the findings of this study. Therefore, the complicated relationship between language impairment and neuroconnectivity in ASD requires further investigation using a more general group of ASD patients with intact language ability, as well as a group of patients with specific language impairment without ASD. These additional analyses are necessary to further disentangle connectivity findings specifically linked to language deficits and to ASD features. We believe that further study will give us a clearer view to know if the brain characteristics reported in this study are related to ASD, to language impairments or to both. Thirdly, currently we do not have information on the microstructural white matter characteristics of the bundles connecting the cerebellum with the supratentorial language network. Refinement of the neurobiological properties of these bundles is wanted to correctly reconstruct these fibers using DTI tractography and to correlate our functional connectivity findings with anatomical connectivity measures derived from white matter microstructure. However, partial volume effects and complex multiple fiber orientations within a single voxel detract from the accuracy of DTI based fiber tracking (Vos et al., 2011; Wedeen et al., 2008). Such challenges could be addressed by other techniques such as, probabilistic fiber tracking, Qball and Q-space imaging, constrained spherical deconvolution which hold promise for future work (Jeurissen et al., 2011; Tournier et al., 2008). Although these recently developed techniques might provide more accurate and unambiguous results, the more complex data processing, long imaging times and strong demands on the magnetic field gradient hardware still impede practical application in a clinical setting, particularly with an autistic pediatric population. Finally, it is important to acknowledge the general limitations of rfMRI. Using a task-based fMRI in children with autism may have its limitations because of the potential influence of subject performance on fMRI activity. Interpretation becomes complicated when performance on the well-known and well-validated language task is not matched. More specifically, in such cases, the abnormal fMRI response may be either the result or the cause of the performance deficit. Therefore, drawing extended conclusions may not be preferable. We did use the task-based fMRI to identify the language system in both patients and controls. Activation peaks were used as seeds for the rfMRI analysis. In addition, resting state connectivity is vulnerable to noise and physiological artifacts. Although we ruled out differences due to head motion and regressed out physiological variations of the time series data, it might be of additional value to perform cardiac gating and synchronize the respiratory signal. Because we were working with an autistic and young population, we were not able to perform this extra monitoring.
In conclusion, understanding the neuronal basis of ASD is crucial for gaining further insight into several clinical aspects of this complex neurodevelopmental disorder: differentiation between subgroups, treatment options, correlations between behavior, underlying anatomy, refinement of the language network and genetics. Our results contain novel connectivity data describing a relative dissociation of cerebral and cerebellar language regions in children with ASD-LI. The detachment from the normal modulatory control and automation function of the cerebellum might alter normal language development and functioning in this subgroup of children with ASD. Furthermore, it might also explain in part the great diversity of language deficits found in ASD with respect to both the extent as well as the type of linguistic problems seen. Larger study groups, combining structural and functional connectivity with extensive psychometric assessments are required to further unravel the role of the cerebellum in the severity of the language deficits in ASD. However, we believe this study has put some steps forward into a better understanding of the neurobiology of language problems in ASD.
Conflict of interest
None.
Acknowledgments
The authors are grateful to our participants and healthy volunteers who made this research possible. This study was supported by the Fund for Scientific Research-Flanders (F.W.O.) (research grant G.0354.06, doctoral mandate to JSV, post-doctoral mandate to DM and BB), by the Belgian Inter University Attraction Pole (grant 6/29) and by the KU Leuven Research Council (grant IDO/08/013).
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- Akshoomoff N. Outcome classification of preschool children with autism spectrum disorders using MRI brain measures. J. Am. Acad. Child Adolesc. Psychiatry. 2004;43(3):349–357. doi: 10.1097/00004583-200403000-00018. [DOI] [PubMed] [Google Scholar]
- Alario F.X. The role of the supplementary motor area (SMA) in word production. Brain Res. 2006;1076(1):129–143. doi: 10.1016/j.brainres.2005.11.104. [DOI] [PubMed] [Google Scholar]
- Allen G., Muller R.A., Courchesne E. Cerebellar function in autism: functional magnetic resonance image activation during a simple motor task. Biol. Psychiatry. 2004;56(4):269–278. doi: 10.1016/j.biopsych.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Allendorfer J.B. Females and males are highly similar in language performance and cortical activation patterns during verb generation. Cortex. 2012;48(9):1218–1233. doi: 10.1016/j.cortex.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APA . American Psychiatric Association; Washington (DC): 2000. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- Ashburner J., Friston K. 2011. http://www.fil.ion.ucl.ac.uk/spm/software/spm8
- Bailey A. A clinicopathological study of autism. Brain. 1998;121(Pt 5):889–905. doi: 10.1093/brain/121.5.889. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N. White matter structure in autism: preliminary evidence from diffusion tensor imaging. Biol. Psychiatry. 2004;55(3):323–326. doi: 10.1016/j.biopsych.2003.10.022. [DOI] [PubMed] [Google Scholar]
- Beaton A., Marien P. Language, cognition and the cerebellum: grappling with an enigma. Cortex. 2010;46(7):811–820. doi: 10.1016/j.cortex.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Benson R.R. Language dominance determined by whole brain functional MRI in patients with brain lesions. Neurology. 1999;52(4):798–809. doi: 10.1212/wnl.52.4.798. [DOI] [PubMed] [Google Scholar]
- Bishop D.V. Overlaps between autism and language impairment: phenomimicry or shared etiology? Behav. Genet. 2010;40(5):618–629. doi: 10.1007/s10519-010-9381-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 1995;34(4):537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Carter C.S. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280(5364):747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Church J.A. Control networks in paediatric Tourette syndrome show immature and anomalous patterns of functional connectivity. Brain. 2009;132(Pt 1):225–238. doi: 10.1093/brain/awn223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino J., Davis S. Validation of a brief quantitative measure of autistic traits: comparison of the Social Responsiveness Scale with the Autism Diagnostic Interview—Revised. J. Autism Dev. Disord. 2003;33(4):427–433. doi: 10.1023/a:1025014929212. [DOI] [PubMed] [Google Scholar]
- Courchesne E. Brain development in autism: early overgrowth followed by premature arrest of growth. Ment. Retard. Dev. Disabil. Res. Rev. 2004;10(2):106–111. doi: 10.1002/mrdd.20020. [DOI] [PubMed] [Google Scholar]
- Courchesne E., Townsend J., Saitoh O. The brain in infantile autism: posterior fossa structures are abnormal. Neurology. 1994;44(2):214–223. doi: 10.1212/wnl.44.2.214. [DOI] [PubMed] [Google Scholar]
- Courchesne E., Carper R., Akshoomoff N. Evidence of brain overgrowth in the first year of life in autism. JAMA. 2003;290(3):337–344. doi: 10.1001/jama.290.3.337. [DOI] [PubMed] [Google Scholar]
- Courchesne E. Mapping early brain development in autism. Neuron. 2007;56(2):399–413. doi: 10.1016/j.neuron.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Damoiseaux J.S. Consistent resting-state networks across healthy subjects. Proc. Natl. Acad. Sci. U. S. A. 2006;103(37):13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deco G., Corbetta M. The dynamical balance of the brain at rest. Neuroscientist. 2011;17(1):107–123. doi: 10.1177/1073858409354384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmond J.E., Fiez J.A. Neuroimaging studies of the cerebellum: language, learning and memory. Trends Cogn. Sci. 1998;2(9):355–362. doi: 10.1016/s1364-6613(98)01211-x. [DOI] [PubMed] [Google Scholar]
- Duffau H. The role of dominant premotor cortex in language: a study using intraoperative functional mapping in awake patients. Neuroimage. 2003;20(4):1903–1914. doi: 10.1016/s1053-8119(03)00203-9. [DOI] [PubMed] [Google Scholar]
- Ebisch S.J. Altered intrinsic functional connectivity of anterior and posterior insula regions in high-functioning participants with autism spectrum disorder. Hum. Brain Mapp. 2011;32(7):1013–1028. doi: 10.1002/hbm.21085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelborghs S. Functional anatomy, vascularisation and pathology of the human thalamus. Acta Neurol. Belg. 1998;98(3):252–265. [PubMed] [Google Scholar]
- Fletcher P.T. Microstructural connectivity of the arcuate fasciculus in adolescents with high-functioning autism. Neuroimage. 2010;51(3):1117–1125. doi: 10.1016/j.neuroimage.2010.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frings M. Cerebellar involvement in verb generation: an fMRI study. Neurosci. Lett. 2006;409(1):19–23. doi: 10.1016/j.neulet.2006.08.058. [DOI] [PubMed] [Google Scholar]
- Gabrieli J.D., Poldrack R.A., Desmond J.E. The role of left prefrontal cortex in language and memory. Proc. Natl. Acad. Sci. U. S. A. 1998;95(3):906–913. doi: 10.1073/pnas.95.3.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparini M. Linguistic impairment after right cerebellar stroke: a case report. Eur. J. Neurol. 1999;6(3):353–356. doi: 10.1046/j.1468-1331.1999.630353.x. [DOI] [PubMed] [Google Scholar]
- Greicius M.D. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc. Natl. Acad. Sci. U. S. A. 2003;100(1):253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groen W.B. The phenotype and neural correlates of language in autism: an integrative review. Neurosci. Biobehav. Rev. 2008;32(8):1416–1425. doi: 10.1016/j.neubiorev.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Harris G.J. Brain activation during semantic processing in autism spectrum disorders via functional magnetic resonance imaging. Brain Cogn. 2006;61(1):54–68. doi: 10.1016/j.bandc.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Hodge S.M. Cerebellum, language, and cognition in autism and specific language impairment. J. Autism Dev. Disord. 2010;40(3):300–316. doi: 10.1007/s10803-009-0872-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland S.K. Normal fMRI brain activation patterns in children performing a verb generation task. Neuroimage. 2001;14(4):837–843. doi: 10.1006/nimg.2001.0875. [DOI] [PubMed] [Google Scholar]
- Jeurissen B. Probabilistic fiber tracking using the residual bootstrap with constrained spherical deconvolution. Hum. Brain Mapp. 2011;32(3):461–479. doi: 10.1002/hbm.21032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just M.A. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain. 2004;127(Pt 8):1811–1821. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- Kana R.K. Sentence comprehension in autism: thinking in pictures with decreased functional connectivity. Brain. 2006;129(Pt 9):2484–2493. doi: 10.1093/brain/awl164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates W.R. Neuroanatomic variation in monozygotic twin pairs discordant for the narrow phenotype for autism. Am. J. Psychiatry. 2004;161(3):539–546. doi: 10.1176/appi.ajp.161.3.539. [DOI] [PubMed] [Google Scholar]
- Keller T.A., Kana R.K., Just M.A. A developmental study of the structural integrity of white matter in autism. Neuroreport. 2007;18(1):23–27. doi: 10.1097/01.wnr.0000239965.21685.99. [DOI] [PubMed] [Google Scholar]
- Kemper T.L., Bauman M. Neuropathology of infantile autism. J. Neuropathol. Exp. Neurol. 1998;57(7):645–652. doi: 10.1097/00005072-199807000-00001. [DOI] [PubMed] [Google Scholar]
- Kemper T.L., Bauman M.L. Neuropathology of infantile autism. Mol. Psychiatry. 2002;7(Suppl. 2):S12–S13. doi: 10.1038/sj.mp.4001165. [DOI] [PubMed] [Google Scholar]
- Kjelgaard M.M., Tager-Flusberg H. An investigation of language impairment in autism: implications for genetic subgroups. Lang. Cogn. Process. 2001;16(2–3):287–308. doi: 10.1080/01690960042000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaus T.A. Language laterality in autism spectrum disorder and typical controls: a functional, volumetric, and diffusion tensor MRI study. Brain Lang. 2010;112(2):113–120. doi: 10.1016/j.bandl.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kort W., Schittekatte M. David Wechsler. Handleiding en Verantwoording. Harcourt Test Publishers; Amsterdam: 2005. Wechsler Intelligence Scale for Children. (Amsterdam: NIP Dienstencentrum) [Google Scholar]
- Kort W., Schittekatte M., Compaan E., editors. CELF-4-NL: Clinical Evaluation of Language Fundamentals. 4th ed. Pearson Assessment and Information B.V.; Amsterdam: 2008. [Google Scholar]
- Kovelman I. Brain basis of phonological awareness for spoken language in children and its disruption in dyslexia. Cereb. Cortex. 2012;22(4):754–764. doi: 10.1093/cercor/bhr094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiner H.C., Leiner A.L., Dow R.S. Cognitive and language functions of the human cerebellum. Trends Neurosci. 1993;16(11):444–447. doi: 10.1016/0166-2236(93)90072-t. [DOI] [PubMed] [Google Scholar]
- Marien P. The lateralized linguistic cerebellum: a review and a new hypothesis. Brain Lang. 2001;79(3):580–600. doi: 10.1006/brln.2001.2569. [DOI] [PubMed] [Google Scholar]
- Middleton F.A., Strick P.L. Dentate output channels: motor and cognitive components. Prog. Brain Res. 1997;114:553–566. doi: 10.1016/s0079-6123(08)63386-5. [DOI] [PubMed] [Google Scholar]
- Middleton F.A., Strick P.L. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res. Brain Res. Rev. 2000;31(2–3):236–250. doi: 10.1016/s0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- Mostofsky S.H. Decreased connectivity and cerebellar activity in autism during motor task performance. Brain. 2009;132(Pt 9):2413–2425. doi: 10.1093/brain/awp088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch B.E. The cerebellum and language: historical perspective and review. Cortex. 2010;46(7):858–868. doi: 10.1016/j.cortex.2009.07.018. [DOI] [PubMed] [Google Scholar]
- Nagae L.M. Elevated mean diffusivity in the left hemisphere superior longitudinal fasciculus in autism spectrum disorders increases with more profound language impairment. AJNR Am. J. Neuroradiol. 2012;33(9):1720–1725. doi: 10.3174/ajnr.A3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardone R. Theta burst stimulation of dorsolateral prefrontal cortex modulates pathological language switching: a case report. Neurosci. Lett. 2011;487(3):378–382. doi: 10.1016/j.neulet.2010.10.060. [DOI] [PubMed] [Google Scholar]
- Papathanassiou D. A common language network for comprehension and production: a contribution to the definition of language epicenters with PET. Neuroimage. 2000;11(4):347–357. doi: 10.1006/nimg.2000.0546. [DOI] [PubMed] [Google Scholar]
- Petersen S.E. Positron emission tomographic studies of the cortical anatomy of single-word processing. Nature. 1988;331(6157):585–589. doi: 10.1038/331585a0. [DOI] [PubMed] [Google Scholar]
- Pierce K., Courchesne E. Evidence for a cerebellar role in reduced exploration and stereotyped behavior in autism. Biol. Psychiatry. 2001;49(8):655–664. doi: 10.1016/s0006-3223(00)01008-8. [DOI] [PubMed] [Google Scholar]
- Raichle M.E. Dynamic measurements of local blood flow and metabolism in the study of higher cortical function in humans with positron emission tomography. Ann. Neurol. 1984;(15 Suppl.):S48–S49. doi: 10.1002/ana.410150710. [DOI] [PubMed] [Google Scholar]
- Rapin I., Dunn M. Language disorders in children with autism. Semin. Pediatr. Neurol. 1997;4(2):86–92. doi: 10.1016/s1071-9091(97)80024-1. [DOI] [PubMed] [Google Scholar]
- Rapin I., Dunn M. Update on the language disorders of individuals on the autistic spectrum. Brain Dev. 2003;25(3):166–172. doi: 10.1016/s0387-7604(02)00191-2. [DOI] [PubMed] [Google Scholar]
- Riches N.G. Non-word repetition in adolescents with specific language impairment and autism plus language impairments: a qualitative analysis. J. Commun. Disord. 2011;44(1):23–36. doi: 10.1016/j.jcomdis.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Ritvo E.R. Lower Purkinje cell counts in the cerebella of four autistic subjects: initial findings of the UCLA-NSAC Autopsy Research Report. Am. J. Psychiatry. 1986;143(7):862–866. doi: 10.1176/ajp.143.7.862. [DOI] [PubMed] [Google Scholar]
- Riva D. The cerebellar contribution to language and sequential functions: evidence from a child with cerebellitis. Cortex. 1998;34(2):279–287. doi: 10.1016/s0010-9452(08)70755-x. [DOI] [PubMed] [Google Scholar]
- Roberts J.A., Rice M.L., Tager-Flusberg H. Tense marking in children with autism. Appl. Psycholinguist. 2004;25(3):429–448. [Google Scholar]
- Romanski L.M. Dual streams of auditory afferents target multiple domains in the primate prefrontal cortex. Nat. Neurosci. 1999;2(12):1131–1136. doi: 10.1038/16056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M., Couteur A.L. Western Psychological Services; Los Angeles (CA): 2003. Social Communication Questionnaire. [Google Scholar]
- Saitoh O., Courchesne E. Magnetic resonance imaging study of the brain in autism. Psychiatry Clin. Neurosci. 1998;(52 Suppl.):S219–S222. doi: 10.1111/j.1440-1819.1998.tb03226.x. [DOI] [PubMed] [Google Scholar]
- Schmahmann J.D. From movement to thought: anatomic substrates of the cerebellar contribution to cognitive processing. Hum. Brain Mapp. 1996;4(3):174–198. doi: 10.1002/(SICI)1097-0193(1996)4:3<174::AID-HBM3>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Schmahmann J.D., Sherman J.C. The cerebellar cognitive affective syndrome. Brain. 1998;121(Pt 4):561–579. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- Silveri M.C., Leggio M.G., Molinari M. The cerebellum contributes to linguistic production: a case of agrammatic speech following a right cerebellar lesion. Neurology. 1994;44(11):2047–2050. doi: 10.1212/wnl.44.11.2047. [DOI] [PubMed] [Google Scholar]
- Stoodley C.J., Schmahmann J.D. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage. 2009;44(2):489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- Tournier J.D. Resolving crossing fibres using constrained spherical deconvolution: validation using diffusion-weighted imaging phantom data. Neuroimage. 2008;42(2):617–625. doi: 10.1016/j.neuroimage.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Verhoeven J.S. Neuroimaging of autism. Neuroradiology. 2010;52(1):3–14. doi: 10.1007/s00234-009-0583-y. [DOI] [PubMed] [Google Scholar]
- Verhoeven J.S. Is there a common neuroanatomical substrate of language deficit between autism spectrum disorder and specific language impairment? Cereb. Cortex. 2012;22(10):2263–2271. doi: 10.1093/cercor/bhr292. [DOI] [PubMed] [Google Scholar]
- Vos S.B. Partial volume effect as a hidden covariate in DTI analyses. Neuroimage. 2011;55(4):1566–1576. doi: 10.1016/j.neuroimage.2011.01.048. [DOI] [PubMed] [Google Scholar]
- Wan C.Y. Atypical hemispheric asymmetry in the arcuate fasciculus of completely nonverbal children with autism. Ann. N. Y. Acad. Sci. 2012;1252(1):332–337. doi: 10.1111/j.1749-6632.2012.06446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb S.J. Cerebellar vermal volumes and behavioral correlates in children with autism spectrum disorder. Psychiatry Res. Neuroimaging. 2009;172(1):61–67. doi: 10.1016/j.pscychresns.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedeen V.J. Diffusion spectrum magnetic resonance imaging (DSI) tractography of crossing fibers. Neuroimage. 2008;41(4):1267–1277. doi: 10.1016/j.neuroimage.2008.03.036. [DOI] [PubMed] [Google Scholar]
- Weissenbacher A. Correlations and anticorrelations in resting-state functional connectivity MRI: a quantitative comparison of preprocessing strategies. Neuroimage. 2009;47(4):1408–1416. doi: 10.1016/j.neuroimage.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Williams R.S. Autism and mental-retardation — neuropathologic studies performed in 4 retarded persons with autistic behavior. Arch. Neurol. 1980;37(12):749–753. doi: 10.1001/archneur.1980.00500610029003. [DOI] [PubMed] [Google Scholar]
- Williams D., Botting N., Boucher J. Language in autism and specific language impairment: where are the links? Psychol. Bull. 2008;134(6):944–963. doi: 10.1037/a0013743. [DOI] [PubMed] [Google Scholar]
- Wingate M., M.B., Kirby R.S., Pettygrove S., Cunniff C., Meaney F., Schulz E., Miller L., Robinson C., Quintana G., Kaiser M.Y., Lee L.C., Landa R., Newschaffer C., Constantino J., Fitzgerald R., Zahorodny W., Daniels J., Giarelli E., Pinto-Martin J., Levy S.E., Nicholas J., Charles J., Zimmerman J., Maenner M.J., Durkin M., Rice C., Baio J., Van Naarden Braun K., Phillips K., Doernberg N., Yeargin-Allsopp M. Prevalence of autism spectrum disorders—Autism and Developmental Disabilities Monitoring Network, 14 sites, United States, 2008. MMWR Surveill. Summ. 2012;61(3):1–19. [PubMed] [Google Scholar]
- Wood A.G. Language cortex activation in normal children. Neurology. 2004;63(6):1035–1044. doi: 10.1212/01.wnl.0000140707.61952.ca. [DOI] [PubMed] [Google Scholar]
- Xiang H. Involvement of the cerebellum in semantic discrimination: an fMRI study. Hum. Brain Mapp. 2003;18(3):208–214. doi: 10.1002/hbm.10095. [DOI] [PMC free article] [PubMed] [Google Scholar]


