Abstract
22q11.2 deletion syndrome (22q11DS) is a recurrent genetic mutation that is highly penetrant for psychosis. Behavioral research suggests that 22q11DS patients exhibit a characteristic neurocognitive phenotype that includes differential impairment in spatial working memory (WM). Notably, spatial WM has also been proposed as an endophenotype for idiopathic psychotic disorder, yet little is known about the neurobiological substrates of WM in 22q11DS. In order to investigate the neural systems engaged during spatial WM in 22q11DS patients, we collected functional magnetic resonance imaging (fMRI) data while 41 participants (16 22q11DS patients, 25 demographically matched controls) performed a spatial capacity WM task that included manipulations of delay length and load level. Relative to controls, 22q11DS patients showed reduced neural activation during task performance in the intraparietal sulcus (IPS) and superior frontal sulcus (SFS). In addition, the typical increases in neural activity within spatial WM-relevant regions with greater memory load were not observed in 22q11DS. We further investigated whether neural dysfunction during WM was associated with behavioral WM performance, assessed via the University of Maryland letter–number sequencing (LNS) task, and positive psychotic symptoms, assessed via the Structured Interview for Prodromal Syndromes (SIPS), in 22q11DS patients. WM load activity within IPS and SFS was positively correlated with LNS task performance; moreover, WM load activity within IPS was inversely correlated with the severity of unusual thought content and delusional ideas, indicating that decreased recruitment of working memory-associated neural circuitry is associated with more severe positive symptoms. These results suggest that 22q11DS patients show reduced neural recruitment of brain regions critical for spatial WM function, which may be related to characteristic behavioral manifestations of the disorder.
Keywords: Psychosis, Executive function, Velocardiofacial syndrome, Copy number variation, Endophenotype
Highlights
-
•
22q11DS patients show reduced activation during spatial working memory.
-
•
Typical increases in neural activity with greater memory load not seen in 22q11DS.
-
•
Neural activity is positively correlated with letter–number sequencing in 22q11DS.
-
•
Neural activity is inversely correlated with psychotic symptoms in 22q11DS.
1. Introduction
The 22q11.2 deletion syndrome (22q11DS), also referred to as velocardiofacial syndrome, is a genetic disorder caused by a hemizygous microdeletion of 1.5–3 Mb at chromosome 22q11, and is associated with variable physical phenotypic features, including cardiac defects, mild facial dysmorphology, learning impairments, hypocalcemia, and immune deficiency (Kook et al., 2010, Walter et al., 2009). In adolescence and young adulthood, up to one-third of 22q11DS patients develop schizophrenia-like psychotic disorders (Green et al., 2009, Murphy et al., 1999, Pulver et al., 1994). This genetic mutation thus represents one of the greatest known risk factors for psychosis identified to date (Jonas et al., 2013, Murphy, 2002).
A compelling aspect of the 22q11DS neurocognitive phenotype is that working memory (WM) appears to be compromised relative to other cognitive functions. In particular, children and adolescents with 22q11DS exhibit differential impairment in spatial WM (Bearden et al., 2001, Bearden et al., 2004, Wang et al., 2000), with relative strengths in performance on measures of auditory attentional capacity and verbal learning (Bearden et al., 2001, Lajiness-O'Neill, 2005). These findings support the notion that individuals with the deletion show a characteristic neurocognitive phenotype, which may be associated with disruption of neural systems involved in spatial WM function.
Nevertheless, little is currently known about the neural circuitry underlying spatial WM dysfunction in 22q11DS, nor how it relates to phenotypic expression of the disorder. Early detection of neural vulnerability markers of psychotic symptoms could potentially reduce the clinical severity and functional impairment caused by these symptoms. To our knowledge only one previous study of spatial WM has been conducted in a small sample of children and adolescents with 22q11DS, which reported hypoactivation in parietal and occipital regions during performance of a spatial WM task (Azuma et al., 2009); however, in this cross-sectional study 22q11DS youth also exhibited a different pattern of age-associated changes relative to healthy controls, involving greater age-related increases in neural activity in predominantly posterior regions in controls, while 22q11DS patients showed the opposite pattern. One other study in children with 22q11DS investigated non-spatial WM, and found hypoactivation in frontal regions in 22q11DS participants relative to unaffected controls, even when matched for performance, suggesting WM-related neural dysfunction in these patients (Kates et al., 2007). Previous behavioral work in healthy children and adolescents supports the notion that WM function develops rapidly throughout this period, suggesting that both neural and cognitive differences captured during this age range may be accounted for by developmental delays, or different trajectories (Klingberg et al., 2002, Luciana et al., 2005, Siegel and Ryan, 1989). Thus, it remains unclear whether the differential patterns of neural activity observed in the study by Azuma and colleagues are present in adult 22q11DS patients. In our study, we further hypothesized that WM dysfunction may serve as an effective marker for psychosis risk in 22q11DS, as it is considered a fundamental aspect of schizophrenia in the general population, which may underlie both cognitive and clinical disturbances associated with the illness (Glahn et al., 2003, Goldman-Rakic, 1994). Spatial WM performance is also sensitive to genetic loading for schizophrenia (Glahn et al., 2003), and in clinical high risk youth WM deficits may predict conversion to overt psychosis (Pukrop and Bechdolf, 2007).
Using functional magnetic resonance imaging (fMRI) we investigated underlying neural activity in adults with 22q11DS and healthy controls during performance on a spatial capacity WM task that included manipulations of load levels and delay length. This task has previously been shown to be sensitive to genetic liability for schizophrenia in the context of a twin study (Glahn et al., 2003), and has thus been proposed as an informative endophenotypic marker for the illness. Research on the neural circuitry underlying WM capacity in healthy adults has shown that activity within the intraparietal sulcus (IPS) is sensitive to manipulations of load levels (Todd and Marois, 2004, Todd and Marois, 2005, Xu and Chun, 2006). For example, during performance of a visual WM task, Todd and Marois (Todd and Marois, 2004) used fMRI to show that activity within posterior parietal cortex was tightly correlated with the amount of visual information maintained. Based on functional neuroimaging findings in idiopathic schizophrenia (Karlsgodt et al., 2007, Karlsgodt et al., 2009, Manoach et al., 2000) and in children with 22q11DS (Azuma et al., 2009, Kates et al., 2007), we predicted that adult 22q11DS patients would show hypoactivation within WM circuitry (i.e., superior frontal sulcus (SFS) and lateral parietal cortices) compared to healthy controls during task performance, and would not show increased activation of these regions as a function of increased WM load. Furthermore, we hypothesized that increased WM load-related neural activity would be associated with better cognitive performance on WM tasks, and reduced psychotic symptom severity in 22q11DS patients.
2. Materials and methods
2.1. Participants
The total sample consisted of 41 participants (18–43 years old, 16 22q11DS and 25 healthy controls). 22q11DS participants all had a molecularly confirmed diagnosis of 22q11.2 deletion syndrome, and were recruited from an ongoing longitudinal study at the University of California, Los Angeles (UCLA) (see (Jalbrzikowski et al., 2012) for details regarding study recruitment procedures). Healthy controls were recruited from this study and from another large-scale study of healthy adults at UCLA, the Consortium for Neuropsychiatric Phenomics. Exclusion criteria for all study participants included the following: other neurological or medical condition that might affect performance, insufficient fluency in English, substance or alcohol abuse and/or dependence with the past six months, and/or any contraindications to scanning. Healthy controls additionally did not meet criteria for any major mental disorder, with the exception of attention-deficit hyperactivity disorder (ADHD) or a past episode of depression, based on information gathered during the Structured Clinical Interview for DSM-IV Axis I Disorders (First et al., 1997). Demographic information for 22q11DS patients and matched controls are presented in Table 1.
Table 1.
Demographic and clinical information for 22q11DS and control participants.
| 22q11DS participants (n = 16) |
Control participants (n = 25) |
p-Value | |
|---|---|---|---|
| Age (years, ± SD) | 23.88 (7.28) | 24.36 (6.14) | 0.83 |
| Participant education (years, ± SD) | 12.38 (0.89) | 14.36 (1.75) | < 0.001 |
| Parental education levela | 0/2/2/6/1/5 | 2/4/6/5/1/7 | 0.658 |
| Gender (N, % female) | 10 (63%) | 15 (60%) | 0.873 |
| Handedness (left/right, % right)b | 1/14 (93%) | 0/25 (100%) | 0.390 |
| Ethnicity (N, % Latino) | 2 (12.5%) | 9 (36%) | 0.098 |
| Psychotic disorder diagnosis (N, %) | 2 (12.5%) | None | < 0.001 |
| SIPS positive symptoms (mean, ± SD)c | 8.79 (7.54) | 0.75 (2.12) | < 0.001 |
| SIPS negative symptomsc | 11.43 (9.06) | 0.25 (0.46) | < 0.001 |
| SIPS disorganized symptomsc | 5.86 (4.74) | 1.25 (1.16) | < 0.001 |
| SIPS general symptomsc | 7 (5.14) | 1.5 (1.5) | < 0.001 |
| Psychotropic medication (N, no medication/anti-depressants/antipsychotics/antianxiety/psychostimulants)d | 7/8/2/3/5 | 24/1/0/0/0 | < 0.001 |
No high school/completed high school/some college or technical schools/completed college or technical school/some graduate school or professional school/completed graduate school or professional school (based on educational attainment of most educated parent).
Handedness data missing for 1 22q11DS participant.
SIPS data missing for 2 22q11DS participants; SIPS interview was not administered to 17 control participants, thus these data reflect scores for 8 control participants.
p-Value reflects a chi-square test between 22q11DS patients and control participants for medication vs. no medication.
All participants underwent a verbal and written informed consent process. The UCLA Institutional Review Board (IRB) approved all study procedures and informed consent documents.
2.2. Procedure
Participants took part in a behavioral training session immediately prior to the one-hour scan, in which they received training on the spatial capacity WM (SCAP) task in the form of one initial demonstration and a trial run before completing the experimental run inside of the scanner.
2.3. Measures
2.3.1. Spatial capacity working memory (SCAP) task
Subjects were shown a target array of 1, 3, 5, or 7 yellow circles positioned pseudo-randomly around a central fixation (Glahn et al., 2002). After a variable delay, subjects were shown a single green circle and were required to indicate whether that circle was in the same position as one of the target circles. Trial events included a 2-s target array, 1.5, 3, and 4.5-second (s) delay, 3-s probe array, and a jittered (average of 2 s) inter-trial interval (ITI) with a fixation. The rationale for the variable delay interval was twofold: (i) the jittered delay length allowed for more efficient estimates of the BOLD response (Dale, 1999), and (ii) participants were unable to anticipate the precise onset of the probe screen, which could confound the interpretation of activity during the delay period. Additionally, the number of memoranda (locations) is parametrically varied, thus allowing the investigation of differential effects of memory load on neural activation across groups (Cannon et al., 2005).
2.3.2. Neuropsychological assessments
Supervised clinical psychology doctoral students or Ph.D. staff administered a neurocognitive battery assessing multiple domains of cognitive functioning. IQ data were acquired for all 22q11DS patients and controls using the Wechsler Abbreviated Scale of Intelligence (WASI; (Wechsler, 1999)) or the Wechsler Adult Intelligence Scale (Wechsler, 2008).
WM was assessed behaviorally with the University of Maryland letter–number sequencing (LNS) task (Gold et al., 1997). In this task, the examiner presents combinations of numbers and letters of varying sequence length, and the participant must repeat the numbers in ascending order and the letters in alphabetical order. Because this task requires maintenance of spatial order information in WM (Crowe, 2000), we predicted that LNS task performance would be closely related to the cognitive demands of our SCAP fMRI task.
2.3.3. Structured Interview for Prodromal Syndromes
Master's- or Ph.D-level trained clinicians assessed study participants using the Structured Interview for Prodromal Syndromes (SIPS; (McGlashan, 2001)), which includes ratings of positive, negative, disorganized, and general symptoms. Symptoms on these scales are rated from 0 to 6, with zero representing an absence of symptoms and six referring to an extremely severe or psychotic level of symptoms. All clinical interviewers demonstrated excellent inter-reliability for symptom ratings (see (Jalbrzikowski et al., 2012) for details). These dimensional measures encompass a range of symptom severity, including sub-threshold (i.e., prodromal) and fully psychotic symptoms. We focused on the SIPS P1 subscale (unusual thought content/delusional ideas) given that the presence of unusual thought content and delusional ideas may be related most closely to WM dysfunction, as the contents of thoughts are closely related to top-down, executive control influence (or lack thereof) (Menon et al., 2001). The relationship between neural activity in IPS and SFS and total positive symptoms (unusual thought content/delusions, suspiciousness/persecutory ideas, grandiose ideas, perceptual abnormalities/hallucinations, and disorganized communication) was also assessed.
2.4. fMRI Acquisition
Data were collected at two scanning facilities at UCLA, each with a 3 T Siemens Trio MRI scanner. While participants completed the SCAP task, 291 functional T2*-weighted echoplanar images (EPIs) were collected with the following parameters: slice thickness = 4 mm, 34 slices, TR = 2 s, TE = 30 ms, flip angle = 90°, matrix 192 × 192, FOV = 192 mm. Additionally, a T2-weighted matched-bandwidth high-resolution anatomical scan (same slice prescription as EPI) and MPRAGE were collected. The parameters for the MPRAGE were the following: TR = 2.3 s, TE = 2.91 ms, FOV = 256 mm, matrix = 240 × 256, flip angle = 9°, slice thickness = 1.20 mm, 160 slices.
2.5. Statistical analysis
Analyses of neuropsychological and clinical data were performed using SPSS software v. 21 (IBM). We compared demographic characteristics between groups using independent sample t-tests for continuous variables and chi-square tests for categorical variables.
fMRI data analyses were performed using tools from the FMRIB software library (www.fmrib.ox.ac.uk/fsl), version 5.0 (Smith et al., 2004). The first 2 volumes from each scan were discarded by the scanner to allow for T1 equilibrium effects. For each scan, images for each participant were realigned to compensate for small head movements (Jenkinson and Smith, 2001). Subjects with average translational motion greater than 4 mm were excluded (n = 1). Data were spatially smoothed using a 5-mm, full-width-half-maximum Gaussian kernel. The data were filtered in the temporal domain using a nonlinear high-pass filter with a 66 second cutoff. A three-step registration process was used in which EPI images were first registered to the matched-bandwidth high-resolution scan, then to the MPRAGE structural image, and finally into standard (Montreal Neurological Institute (MNI)) space, using nonlinear transformations (Andersson et al., 2007).
Standard model fitting was conducted for all subjects. The following events were modeled after convolution with a canonical gamma hemodynamic response function: all loads, Load 1, Load 3, Load 5, Load 7, Delay 1.5 s, Delay 3 s, Delay 4.5 s. Events were modeled with the onset at the target presentation and a duration of 6.5, 8, and 9.5 s to include the variable delay and probe periods. The six motion parameters and temporal derivatives of all regressors were included as covariates of no interest to improve statistical sensitivity. For each subject, All loads, Load 1, Load 3, Load 5, Load 7, Load3 > Load1, Load5 > Load3, Load5 > Load1, Load7 > Load1, Load7 > Load5, Load7 > Load3, Delay 1.5 s, Delay 3 s, Delay 4.5 s, Delay 4.5 s > Delay 3 s, Delay 3 s > Delay 1.5 s, Delay 4.5 s > Delay 1.5 s contrasts were computed. Our primary analyses focused on the Load5 > Load1 contrast to maximize the WM load effect, as Load7 is considered to be above the 3–4 item capacity limit reported in previous studies (e.g., Todd and Marois, 2004).
Due to an administrative error, only a subset of the behavioral data was available; thus, we modeled all trials in our fMRI analyses. However, for 8 control participants with available behavioral data we directly compared results for the analysis modeling all trials vs. correct trials only. Importantly, this analysis revealed no significant differences in activation between the all trials vs. correct trials only for any of the primary analyses, i.e. the load contrasts (Load3 > Load1, Load5 > Load1, Load7 > Load3). Minor differences were detected for the comparison of all trials vs. correct trials only for analyses including all load and delay conditions: specifically, there was greater activity in IPS for all trials vs. correct trials only, whereas there was greater activity in posterior cingulate cortex for correct trials vs. all trials (see Supplementary Table 2 for details). For four 22q11DS subjects with behavioral data we performed the same analysis, comparing neural activity for all trials vs. correct trials only, and also found similar results (see Supplementary materials).
The output from the subject-specific analyses was analyzed using a mixed-effects model with FMRIB's Local Analysis of Mixed Effects (FLAME). Higher-level analyses included the following conditions and contrasts at the group-level: all loads, Load 1, Load 3, Load 5, Load 7, Load3 > Load 1, Load5 > Load3, Load5 > Load1, Load7 > Load1, Load7 > Load5, Load7 > Load3. Between-group comparisons were conducted for each of these. In order to first rule out potential scanner related differences, we checked for differences between scanners in each group. Once it was established that there were no differences in activation between scanners (p > 0.05 for all comparisons), all subsequent group-level analyses were conducted with scanner added as a covariate.
Group-level statistics images were thresholded with a cluster-forming threshold of z > 2.3 and a cluster probability of p < 0.05, corrected for whole-brain multiple comparisons using Gaussian random field theory. The search region included 139,264 voxels. Brain regions were identified using the Harvard–Oxford cortical and subcortical probabilistic atlases, and all activations are reported in MNI coordinates. For reporting of clusters, we used the cluster command in FSL. Anatomical localization within each cluster was obtained by searching within maximum likelihood regions from the FSL Harvard–Oxford probabilistic atlas to obtain the maximum z-statistic and MNI coordinates within each anatomical region contained within a cluster.
Secondary analyses were conducted in order to assess the relationship of neural activity within a priori regions of interest (ROIs) with clinical and cognitive phenotypes within 22q11DS patients. These analyses focused on the Load5 > Load1 contrast to maximize the WM load effect. In particular, to determine whether performance on a WM task, performed outside of the scanner, is associated with neural activity within WM-load related regions for 22q11DS patients and controls, we examined the relationships between percent signal change in left and right IPS, and left and right SFS, and accuracy on the letter–number sequencing (LNS) task using a partial correlation analysis, controlling for age and gender.
Secondly, to determine whether neural activity within WM load-related regions was associated with psychotic symptoms in 22q11DS patients, the P1 subscale measuring unusual thought content and delusional ideas from the Structured Interview for Prodromal Syndromes (SIPS) and percent signal change in left and right IPS, and left and right SFS, was assessed using a partial correlation analysis, controlling for age and gender. We conducted this analysis within the 22q11DS patient group only, as the range of scores was (by definition) restricted among control subjects.
For the analyses of percent signal change in relation to the behavioral variables described above, mean percent signal change was extracted from four pre-defined ROIs, selected based on visual inspection of the group contrast and the previous literature, which included the right and left intraparietal sulcus, and left and right SFS. ROIs were defined using the FSL Harvard–Oxford probabilistic atlas (thresholded at 25%); we then intersected these anatomically-defined masks with our group-level Load5 > Load1 contrast in order to isolate voxels within anatomically-defined regions that were significantly active for WM load. These anatomically-defined ROIs were then used to extract average percent signal change values corresponding to 8-s stimulus convolved with a gamma HRF from the Load5 > Load1 contrast in 22q11DS patients alone (Mumford and Poldrack, 2007). As the relationship between psychosis symptoms and WM-related activation in this population has not been previously investigated, we did not correct for multiple comparisons in these exploratory analyses.
3. Results
3.1. Behavioral results
3.1.1. Demographic and clinical characteristics
As shown in Table 1, control and 22q11DS groups were matched on all demographic factors except for participant education. Patients with 22q11DS scored significantly lower than controls on measures of Full Scale IQ, Matrix Reasoning, and letter–number sequencing (Table 2). There was a non-significant trend toward lower verbal knowledge (vocabulary) in 22q11DS patients compared to controls, consistent with prior literature indicating relative sparing of verbal abilities (Bearden et al., 2001, Bearden et al., 2004, Lajiness-O'Neill, 2005, Wang et al., 2000).
Table 2.
Neuropsychological measures.
| 22q11.2 participants (n = 15)a |
Control participants (n = 25) |
t-Test | p-Value | |
|---|---|---|---|---|
| Full scale IQ (± SD)a | 76.40 (14.18) | 116.32 (17.01) | 7.54 | < 0.001 |
| Verbal knowledge | ||||
| Vocabulary (± SD)a | 38.00 (9.46) | 60.16 (10.53) | 1.91 | 0.063 |
| Visuospatial skills | ||||
| Matrix reasoning (± SD)a | 30.93 (11.28) | 56.72 (9.13) | 3.98 | < 0.01 |
| Working memory | ||||
| Letter–number sequencing (mean % correct, ± SD) | 46.44 (13.17) | 70.80 (12.39) | 5.79 | < 0.001 |
Neurocognitive data missing for 1 22q11DS participant; based on 2-subtest Wechsler Abbreviated Scale of Intelligence (WASI; vocabulary and matrix reasoning); means and standard deviations presented are T-scores; statistical analyses are based on raw score values.
3.2. fMRI results
3.2.1. Working memory activity in controls and 22q11DS patients
We first investigated the set of neural regions active for all WM conditions combined, in order to verify that the set of regions active for global WM function (e.g., not necessarily load-sensitive) are consistent with previous studies of WM encoding and maintenance in healthy adults. Healthy controls (all WM conditions, control group mean) activated a broad set of neural regions, as shown in Table 3 and Fig. 1a. Regions active included bilateral IPS, bilateral occipital cortex, middle temporal gyrus, inferior, middle, and superior frontal gyrus, cerebellum, and anterior cingulate cortex (Fig. 1a, Table 3). Patients activated a similar set of regions for all WM conditions combined (all WM conditions, patient group mean).
Table 3.
Regions of activation, maximum z-score, and MNI coordinates for direct group contrasts of combined WM conditions and load effects.
| Contrast | Regions | Voxel # | Max z-score | Max X (mm) |
Max Y (mm) |
Max Z (mm) |
|---|---|---|---|---|---|---|
| All WM conditions | ||||||
| All conditions, controls > 22q11DS | ||||||
| Left superior parietal lobule, intraparietal sulcus, supramarginal gyrus, angular gyrus | 984 | 4.28 | − 26 | − 64 | 56 | |
| All Conditions, 22q11DS > controls | ||||||
| None | ||||||
| Load effects | ||||||
| aLoad 5 > Load 1, controls > 22q11DS | ||||||
| Right supramarginal gyrus, postcentral gyrus, intraparietal sulcus | 2282 | 4.78 | 26 | − 70 | 52 | |
| Left intraparietal sulcus, superior parietal lobule | 587 | 3.53 | − 22 | − 78 | 34 | |
| Right superior frontal sulcus | 521 | 4.20 | 32 | − 10 | 52 | |
| Left superior frontal sulcus | 458 | 4.56 | − 22 | − 10 | 48 | |
| Load 5 > Load 1, 22q11DS > controls | ||||||
| None | ||||||
| Load 5 > Load 3, controls > 22q11DS | ||||||
| None | ||||||
| Load 5 > Load 3, 22q11DS > controls | ||||||
| None | ||||||
| Load 3 > Load 1, controls > 22q11DS | ||||||
| Right intraparietal sulcus, superior parietal lobule, postcentral gyrus, supramarginal gyrus, angular gyrus | 2316 | 4.42 | 28 | − 66 | 48 | |
| Bilateral occipital fusiform gyrus, lingual gyrus | 1595 | 3.74 | 6 | − 90 | − 14 | |
| Left intraparietal sulcus, superior parietal lobule, supramarginal gyrus | 1369 | 4.06 | − 24 | − 78 | 32 | |
| Left superior frontal sulcus | 682 | 5.03 | − 24 | − 8 | 52 | |
| Right superior frontal sulcus, middle frontal gyrus | 548 | 4.7 | 30 | − 4 | 52 | |
| Left occipital cortex | 494 | 3.98 | − 50 | − 58 | 0 | |
| Right occipital cortex | 459 | 3.65 | 56 | − 66 | 0 | |
| Load 3 > Load 1, 22q11DS > controls | ||||||
| None | ||||||
| Load 7 > Load 1, controls > 22q11DS | ||||||
| Right intraparietal sulcus, supramarginal gyrus, postcentral gyrus | 2237 | 4.59 | 50 | − 36 | 56 | |
| Left intraparietal sulcus, superior parietal lobule | 1120 | 4.08 | − 34 | − 40 | 32 | |
| Left superior frontal sulcus, precentral gyrus, middle frontal gyrus | 805 | 4.22 | − 24 | − 8 | 50 | |
| Right superior frontal sulcus, middle frontal gyrus, precentral gyrus | 725 | 4.44 | 22 | − 6 | 52 | |
| Load 7 > Load 1, 22q11DS > controls | ||||||
| None | ||||||
| Load 7 > Load 3, controls > 22q11DS | ||||||
| None | ||||||
| Load 7 > Load 3, 22q11DS > controls | ||||||
| Bilateral occipital cortex | 5822 | 4.33 | 26 | − 62 | − 16 | |
| Load 7 > Load 5, controls > 22q11DS | ||||||
| None | ||||||
| Load 7 > Load 5, 22q11DS > controls | ||||||
| None | ||||||
| Delay effects | ||||||
| Delay 4.5 s, controls > 22q11DS | ||||||
| Left intraparietal sulcus | 1409 | 4.33 | − 26 | − 62 | 56 | |
| Delay 4.5 s, 22q11DS > controls | ||||||
| None | ||||||
| Delay 3 s, controls > 22q11DS | ||||||
| Left intraparietal sulcus | 1058 | 5.16 | − 32 | − 44 | 40 | |
| Delay 3 s, 22q11DS > controls | ||||||
| None | ||||||
| Delay 1.5 s, controls > 22q11DS | ||||||
| Left intraparietal sulcus | 560 | 4 | − 26 | − 64 | 56 | |
| Delay 1.5 s, 22q11DS > controls | ||||||
| None | ||||||
Primary load contrast.
Fig. 1.
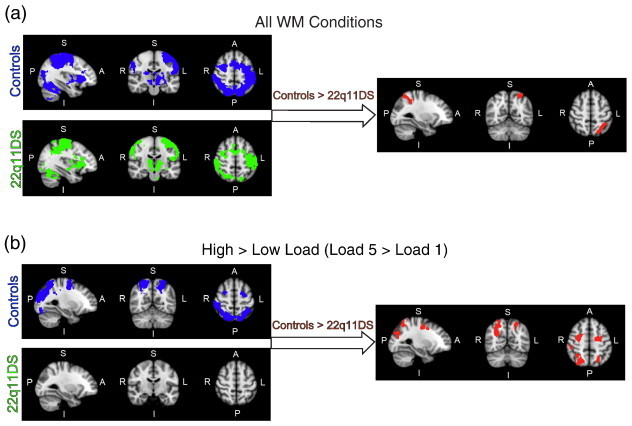
fMRI activity maps during performance on the spatial capacity working memory (SCAP) task. Blue maps represent neural activity in controls, green maps represent neural activity in 22q11DS patients, and red maps represent the between-group contrast of controls > 22q11DS patients. Brain orientations are labeled such that S = superior, I = inferior, P = posterior, and A = anterior; R = right and L = left. (a) Activation maps represent the contrast of all WM conditions combined (all loads and all delay length WM trials > Baseline), to investigate neural activity related to overall spatial WM performance. (b) Activation maps represent the contrast of high versus low WM load (Load 5 > Load 1), to investigate neural activity related to WM load. No regions showed significantly greater neural activity in 22q11DS vs. controls for these contrasts. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
A direct group contrast between controls and 22q11DS patients for all WM conditions revealed that 22q11DS patients had significantly reduced activation in the left IPS relative to control subjects. Across all WM conditions there were no regions showing greater activation for 22q11DS patients compared to controls.
3.2.2. Working memory load effects in controls and 22q11DS patients
In healthy controls, high versus low WM load (Load5 > Load1 contrast, control group mean; hereafter referred to as the WM load contrast), activated a set of regions previously identified as sensitive to parametric increases in WM load (Fukuda et al., 2010, Todd and Marois, 2004, Todd and Marois, 2005, Xu and Chun, 2006). Working memory load-related regions within controls included bilateral IPS and bilateral occipital cortex (Fig. 1b, Table 3). In 22q11DS patients, no regions were active for the WM load contrast (Load5 > Load1 contrast, patient group mean), indicating that 22q11DS patients did not show increases in neural activity as a function of load.
Our primary contrast of interest, the direct contrast between controls and 22q11DS patients for WM load (Load5 > Load1 contrast), revealed significantly greater activation in controls in bilateral IPS and SFS as a function of increased load (Fig. 1b and Table 3). There were no regions showing greater activation for 22q11DS patients compared to controls for the WM load contrast. Similarly, direct contrast between groups for Load7 > Load1 showed greater activity for controls than 22q11DS patients in bilateral IPS and SFS. Between-groups contrast for Load3 > Load1 showed a similar pattern of greater activity in controls relative to 22q11DS patients in bilateral IPS and SFS, as well as occipital cortex, and no regions showing greater activity for 22q11DS patients than controls, similar to the WM load effect seen for the primary WM load analysis (Load5 > Load1). For the Load7 > Load3 contrast, patients with 22q11DS showed greater activity than controls in occipital cortices. There were no significant differences in neural activity between controls and 22q11DS patients for the Load5 > Load3 or Load7 > Load5 contrasts.
3.2.3. Working memory delay effects
We then investigated differences in neural activity between 22q11DS patients and controls as a function of varying delay lengths, 1.5, 3, and 4.5 s (Supplementary Fig. 1 and Table 3). In both groups we found a set of fronto-parietal regions active for each delay length. Group comparison revealed that controls showed significantly greater activation than 22q11DS patients in the left IPS at each delay length, and there were no regions showing greater activity for 22q11DS patients than controls at any delay length, consistent with the findings observed for the WM load contrasts.
3.2.4. Relationship between working memory activation and behavioral performance
Partial correlations revealed that percent signal change in left IPS was significantly correlated with LNS performance for 22q11DS patients (r(11) = 0.637, p = 0.010), but not for controls (r(21) = 0.180, p = 0.20; Fig. 2b). The partial correlation between percent signal change in right IPS and LNS was marginally statistically significant for 22q11DS patients (r(11) = 0.420, p = 0.076), but not for controls (r(21) = 0.194, p = 0.187).
Fig. 2.
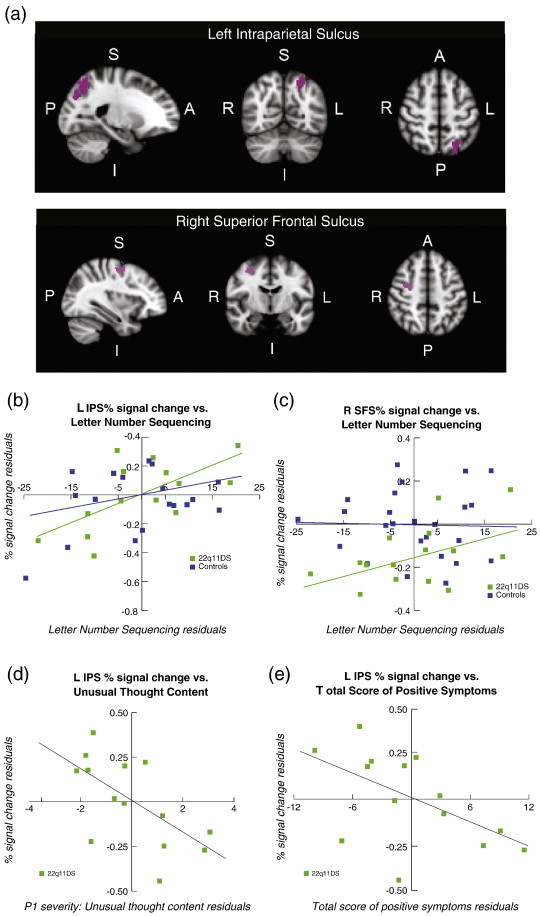
Relationship of WM load-related neural activity with behavioral measures (letter–number sequencing task performance and positive psychotic symptoms) in 22q11DS. (a) Represents the anatomically-defined left IPS and right SFS ROI (after adjusting for age and years of education) from which percent signal change was extracted for correlations with X-axis variables in b, c, and d. Brain orientations are labeled such that S = superior, I = inferior, P = posterior, and A = anterior; R = right and L = left. For b, c, and d the X-axis values represent the residuals of: (b) the letter–number sequencing task for 22q11DS patients and controls, with IPS percent signal change on the Y-axis (R2 = 0.41 for 22q11DS patients; R2 = 0.03 for controls), (c) the letter–number sequencing task for 22q11DS patients and controls, with SFS percent signal change on the Y-axis (R2 = 0.26 for 22q11DS patients; R2 = 0.052 for controls), (d) SIPS P1 (unusual thought content/delusional ideas) subscale for 22q11DS patients, with IPS percent signal change on the Y-axis, R2 = 0.38, and (e) SIPS total positive symptoms for 22q11DS patients, with IPS percent signal change on the Y-axis, R2 = 0.29. Residuals were calculated by regressing percent signal change and cognitive/clinical scores on age and gender, and are plotted for visualization purposes.
Similarly, in 22q11DS patients percent signal change in the right SFS was significantly positively correlated with LNS performance, r(11) = 0.511, p = 0.037, but this relationship was not observed in controls, r(21) = − 0.026, p = 0.453 (Fig. 2c). Partial correlations between percent signal change in left SFS, bilateral IPS and LNS for 22q11DS patients or controls were not statistically significant.
3.2.5. Relationship between working memory-related neural activation and unusual thought content
To determine whether neural activity within WM load-related regions was associated with psychotic symptoms in 22q11DS patients, the P1 subscale and the total positive psychotic symptoms scores were assessed (Fig. 2d and e).
The partial correlation between percent signal change during SCAP task performance and left IPS and unusual thought content (P1) was statistically significant (r(10) = − 0.639, p < 0.05), indicating that greater left IPS activation during task performance was associated with reduced symptom severity (Fig. 2d). The strength of this relationship, as indexed by eta2, was 0.38. The association between left IPS percent signal change and the total positive symptom score from the SIPS was also statistically significant, r(10) = − 0.538, p < 0.05 (eta2 = 0.29), indicating that greater IPS activation was also associated with lower overall psychotic symptom severity (Fig. 2e). There was also a trending negative relationship between percent signal change in right IPS versus unusual thought content, r(10) = − 0.452, p = 0.07 (eta2 = 0.20).
There was a trending negative relationship between percent signal change in left SFS and unusual thought content (r(10) = − 0.448, p = 0.07; eta2 = 0.29). The partial correlations between percent signal change in left and right SFS and total positive symptom severity, and between the right SFS and unusual thought content, were not statistically significant.
4. Discussion
To our knowledge, this is the first study to investigate the neural substrates of spatial WM in adults with 22q11DS, a relatively understudied population that exhibits a high rate of psychotic illness (Green et al., 2009, Pulver et al., 1994). Our fMRI findings indicate that, during performance on a spatial capacity WM task previously shown to be sensitive to genetic liability for schizophrenia (Glahn et al., 2003), patients with 22q11DS showed reduced neural activity relative to controls within the IPS and SFS, regions typically associated with WM load in previous studies of healthy individuals (Fukuda et al., 2010, Todd and Marois, 2004, Xu and Chun, 2006). While controls additionally showed the predicted increases in neural activity as a function of memory load, this pattern was not present in 22q11DS patients. The relative hypo-activation we observed within the IPS in 22q11DS patients was consistent across all delay lengths. Moreover, in 22q11DS patients (but not controls) neural activity in both the IPS and SFS was positively correlated with behavioral performance on letter–number sequencing, a task that requires manipulation of alpha-numeric information within working memory. Finally, we found that reduced neural activity within IPS was associated with more severe clinical symptoms of unusual thought content and delusional ideas in 22q11DS patients, as measured with the Structured Interview for Prodromal Syndromes. Taken together, these findings suggest a pattern of reduced neural engagement during WM in 22q11DS, and that this dysfunction is associated with both variability in working memory performance and with psychotic symptom severity. These findings thus provide initial evidence supporting neural activity during spatial WM as a potential endophenotype relevant to psychosis risk in 22q11DS.
4.1. fMRI findings
4.1.1. Group differences in neural activity during spatial WM
Consistent with prior literature on the neural underpinnings of WM in healthy adults (Awh and Jonides, 2001, Courtney et al., 1996, Curtis, 2006, Montojo and Courtney, 2008), we found that healthy controls in our study activated a distributed network of brain regions including bilateral IPS, occipital cortex, middle temporal gyrus, inferior, middle, and superior frontal gyrus, cerebellum, and anterior cingulate cortex. 22q11DS patients activated a similar set of regions across WM conditions, but showed a pattern of hypo-activation that was localized to the left IPS. These findings suggest that the neural systems globally relevant to spatial WM function, not specifically related to load level, are recruited abnormally in 22q11DS. This pattern of results supports those of the only existing prior fMRI study of spatial WM in children with 22q11DS, which found significantly reduced activation of parietal, but not prefrontal regions in 22q11DS patients (Azuma et al., 2009). A similar pattern was also observed in a high-risk cohort including children of parents with schizophrenia (Keshavan et al., 2002).
In contrast, in a study investigating the neural correlates of non-spatial WM in 22q11DS youth compared to unaffected siblings and community controls, Kates et al. (Kates et al., 2007) found that all three groups showed similar activation in parietal regions, but relative to performance-matched youth with 22q11DS both community and sibling control groups showed significantly more recruitment of frontal cortical regions during task performance, including the cingulate and precentral gyrus (Kates et al., 2007). These findings suggest that 22q11DS patients exhibit hypoactivation within WM circuitry that is not attributable to performance differences. Our study findings are in general agreement with the notion of hypoactivation within 22q11DS patients during spatial WM performance, and underscore the importance of parietal activity for intact spatial WM performance. These findings extend current understanding of WM function in 22q11DS by specifically examining the neural correlates of spatial WM in adults with this syndrome, and its behavioral consequences, which to our knowledge have not been previously investigated.
4.1.2. Working memory load effects
Research on the neural circuitry underlying WM capacity in healthy individuals has shown that activity within IPS is sensitive to manipulations of load levels. During performance of a visual WM task during fMRI, Todd and Marois (Todd and Marois, 2004) found that activity within posterior parietal cortex was tightly correlated with the limited amount of visual information maintained. The finding that neural activity in IPS increases with set size has been replicated using both simple and complex items maintained in WM, and neural activity reaches an asymptote at approximately four items (Fukuda et al., 2010, Todd and Marois, 2004, Todd and Marois, 2005, Xu and Chun, 2006). The superior frontal sulcus also appears to be particularly critical for spatial WM, as suggested by several neuroimaging studies (Awh and Jonides, 2001, Courtney et al., 1996, Curtis, 2006), and lesions of the superior frontal gyrus impair spatial WM functions to a greater extent than verbal or face WM (du Boisgueheneuc et al., 2006).
Our primary contrast of interest investigated differential effects of memory load increases between controls and 22q11DS patients. This analysis revealed a differential increase in neural activity in bilateral IPS and SFS in healthy adults compared to 22q11DS patients as a function of load level, and we found that this WM load effect was consistent regardless of delay length. These findings are in general agreement with previous research in patients with idiopathic schizophrenia, indicating hypoactivation relative to healthy controls within the dorsolateral prefrontal cortex at higher loads of a spatial capacity WM task (Cannon et al., 2005). We additionally observed greater activity in occipital cortices for 22q11DS patients compared to healthy controls at the highest load level (Load7 > Load3 contrast). However, these early sensory regions were not within the hypothesized regions associated with WM load effects or spatial WM processing/representation. Previous findings suggest that activation within the ventral-occipital cortex is driven more by the perceptual load of a scene, as opposed to the amount of information held in WM (Todd and Marois, 2004). The greater activity observed for 22q11DS patients than controls in our study may reflect differences related to the perceptual or iconic representation of the number of objects presented during the target screen. In addition, secondary analyses showed that our group differences in fMRI activity for spatial WM load effects within the IPS and SFS were not accounted for by antipsychotic or antidepressant medication usage within our 22q11DS patients.
4.1.3. Working memory delay effects in 22q11DS
While both controls and 22q11DS patients showed activity within a distributed set of frontoparietal regions for each delay length (1.5, 3, and 4.5 s), group contrasts revealed that controls exhibited greater activity within left IPS than 22q11DS patients at each delay length. These findings support the interpretation drawn from our WM load effect results, suggesting that 22q11DS patients show consistent under-recruitment of neural circuitry critical for spatial WM during task performance, suggesting disruption of a fundamental cognitive process that is relatively invariant with respect to delay length and memory set size. The results are also in general agreement with prior findings from studies investigating spatial WM in idiopathic schizophrenia and clinical high-risk groups, which show disrupted neural circuitry within WM-related regions (Brahmbhatt et al., 2006, Cannon et al., 2005, Seidman et al., 2006). Additionally, in patients with idiopathic schizophrenia and their clinically unaffected co-twins, hypo-activation was present regardless of memory load (Glahn et al., 2003). Our findings and those from previous literature on the neuropathological basis of idiopathic schizophrenia suggest a similar mechanism may be involved in 22q11DS patients, such that genes relevant to schizophrenia risk disrupted by the 22q11.2 deletion may contribute to physiological disturbances in WM neural circuitry and function.
4.1.4. Working memory-related activity and behavioral performance
Based on previous literature, we hypothesized that activity within WM-relevant neural circuitry (IPS and SFS) would be positively associated with WM performance outside the scanner. In healthy adults, strength of fMRI signal during a WM delay interval predicted task performance while inside the scanner, with the authors suggesting that increased BOLD activity corresponds to increased neural processing related to increased attention and improved task performance (Pessoa et al., 2002). We found that neural activity in the IPS and SFS correlated with WM performance in out of scanner tasks in 22q11DS patients, suggesting this locus as a critical indicator of WM dysfunction within these patients. In line with our findings, previous studies have shown an association between neural activity and behavioral performance in healthy adults using other WM task paradigms (Pessoa et al., 2002). However, this relationship was not observed in healthy controls in our study; this may be due to the reduced variability in letter–number sequencing task performance among healthy participants, who showed significantly greater overall accuracy on this task relative to 22q11DS patients.
4.1.5. Summary of fMRI findings
Collectively, our findings indicate an overall reduction in the recruitment of WM-associated neural circuitry within patients. The left IPS and SFS appear to be regions within the WM network that are particularly affected in 22q11DS patients. Morphological abnormalities have also been previously reported within these brain regions in prior structural studies investigating 22q11DS patients, showing decreased gray matter in frontal and temporal regions compared to controls (Chow et al., 2002, Van Amelsvoort et al., 2001). A more recent diffusion tensor imaging study offered evidence for reduced white matter integrity within frontal regions in 22q11DS patients compared to controls, suggesting disrupted structural connectivity in WM-related regions (da Silva Alves et al., 2011). These findings suggest that structural abnormalities may underlie the functional differences we observed between 22q11DS patients and controls, a hypothesis which we will directly test in future studies.
4.1.6. Working memory-related neural activity and psychotic symptoms
Of great interest was whether activity within brain regions relevant to WM performance was associated with unusual thought content in 22q11DS patients, as measured by the SIPS. We found initial evidence for a significant negative relationship between activity within left IPS and the severity of unusual thought content, such that as activity within this region decreased, the severity of unusual thought content increased. We also found a negative relationship between activity in this region and overall positive symptom severity. Our results complement those of previous studies in patients with idiopathic schizophrenia. For example, Menon et al. (2001) previously found that greater severity of unusual thought content in patients with schizophrenia was associated with reduced right dorsolateral prefrontal cortex (DLPFC) activation during auditory n-back WM task performance. Importantly, this finding was specific to symptoms of unusual thought context, as similar relationships were not observed for symptoms of hallucinatory behavior and conceptual disorganization. Our findings are the first to show that variability in WM-associated neural activity in 22q11DS patients, a population that is highly penetrant for psychosis, is associated with psychotic symptom severity. Only two of our 22q11DS patients were diagnosed with an overt psychotic disorder, suggesting that even in the absence of a clinical diagnosis, neural activity within WM circuitry is related to dimensionally measured symptoms of psychosis.
4.2. Genes relevant to WM and psychosis in 22q11DS
The greatly elevated risk for psychosis in 22q11DS offers the possibility of delineating a relatively homogenous developmental pathway to the illness. Although a larger study sample is required to investigate genetic variants within the 22q11.2 locus that may be relevant to both WM and psychosis, we offer speculative suggestions warranting further study. Notably, among the genes encoded in the deleted region, catechol-O-methyltransferase (COMT) encodes for an enzyme that is essential for the breakdown of dopamine in the prefrontal cortex (Boot et al., 2008, Lachman et al., 1996, Weinberger et al., 2001). Prefrontal dopamine dysregulation adversely affects executive neurocognitive processes, including working memory (Kellendonk et al., 2006, Murphy et al., 1996, Zahrt et al., 1997). For example, an early study found evidence for impaired spatial WM performance associated with elevated levels of dopamine turnover in the prefrontal cortex in both rat and primate models (Murphy et al., 1996). In healthy adults, a functional polymorphism within the COMT gene was related to performance on a test of prefrontal cortical function, the Wisconsin Card Sorting Test (Egan et al., 2001). The association between COMT, dopamine, and cognitive function suggests that that COMT haploinsufficiency may be relevant to altered neural activity within WM circuitry, as well as clinical symptoms, in 22q11DS patients. The proline dehydrogenase (PRODH) gene, which catalyzes the first step in proline catabolism and is involved in glutamatergic neurotransmission, also resides within the 22q11.2 locus; genetic variation in PRODH has also been shown to play a role in WM function and other neurophysiological endophenotypes relevant to schizophrenia, in both human (Kempf et al., 2008, Vorstman et al., 2009) and animal models (Paterlini et al., 2005). Our results highlight the value of 22q11DS as a model for studying the contribution of neurodevelopmental risk genes to brain function and neuropsychiatric illness.
4.3. Implications
This study sought to take advantage of an intermediate brain phenotype to help bridge the gap between a well-characterized genetic deletion and symptoms of psychosis. Our findings offer evidence for WM as a potential neural endophenotype for psychosis risk in 22q11DS, supporting its relevance for understanding gene–brain–behavior relationships in idiopathic schizophrenia. We utilized a dimensional approach to assess psychotic symptoms, which allows us to capitalize on the full range of variability rather than restricting our sample to a subset of impaired patients. The RDoC initiative encourages this approach, with the goal of promoting innovative methods for characterizing psychopathology based on dimensions of observable behaviors or neurobiological measures rather than traditional diagnostic systems (Insel et al., 2010). Previous research suggests that dimensional ratings of psychosis explain more variance in dysfunctional behavior, social adaptation, and global occupation and function than a categorical diagnosis (Rosenman et al., 2003). Translational studies in genetic mouse models of 22q11DS can be further used to directly investigate the involvement of specific neurotransmitters and/or defects in dendritic and synaptic morphology to the pathophysiology of WM dysfunction and psychosis (Karayiorgou and Gogos, 2004).
4.4. Limitations
The primary limitation in our study is the small sample size. Although 22q11DS represents the most common copy number variant associated with psychosis risk, estimated to occur in 1 out of every 4000 to 6000 live births, subject ascertainment at a single site is a challenge. Our sample size is large relative to previous studies on neural function in 22q11DS; however, our results should be considered preliminary until replicated. Additionally, our analyses modeled neural activity across all trials, rather than correct trials only; however, secondary analyses in a subset of the sample showed no substantial differences in the results for all trials vs. correct trials only, suggesting that this did not unduly influence the findings. Finally, to our knowledge the relationship between psychosis symptoms and WM-related activation in this population has not been investigated, so in these exploratory analyses we did not correct for multiple comparisons. With a FDR multiple comparison correction at p = 0.05 on the 8 comparisons made between psychosis symptoms and WM-related activation [4 predefined ROIs (left and right intraparietal sulcus and superior frontal sulcus) × 2 SIPS measures (unusual thought content and overall positive symptom score)], none of our 8 partial correlation values survived this corrected threshold. While we found a consistent pattern of relationships, indicating that higher activation in the same region critical for WM function (left IPS) was associated with both better cognitive performance and less severe psychotic symptoms, these results require validation in larger samples.
5. Conclusions
Currently, the risk and protective factors that account for variability in clinical outcome in 22q11DS are not well understood. In this study we found that 22q11DS patients exhibited reduced neural engagement of regions critical for WM relative to controls, and that this dysfunctional neural activity was associated with specific psychotic symptoms. This ‘deep phenotyping’ approach can provide a more complete and translational model to aid in the early detection and prevention of psychosis.
Acknowledgments
Disclosures: None of the authors have relevant financial disclosures or conflicts of interest.
We would like to thank the participants and their families for being a part of our research. We would also like to thank Ms. Chelsea Gilbert, Dr. Sarah Marvin, and Dr. Laurie Brenner, who assisted in conducting clinical assessments and administering neuropsychological measures to our participants; Wen-Ching Tran, who assisted in data management and scoring; and Angelica Bato who assisted with fMRI data collection.
Funding sources: This manuscript was supported by grants from the National Institute of Mental Health: RO1 MH085953 (CEB), NIH/NICHD grant P50-HD-055784 (Pilot Project Grant to CEB), NIH/NIMH 5T32MH082719-04 (Postdoctoral Training Program Fellowship given to CAM), and UL1-DE019580 and PL1MH083271 (NIH Roadmap grants to RMB). These funding sources had no further role in study design, in the collection, analysis, and interpretation of data, in the writing of the report, and in the decision to submit the paper for publication.
Footnotes
This work was performed at the Semel Institute for Neuroscience and Human Behavior (Department of Psychiatry & Biobehavioral Sciences, UCLA) and will be presented in part at the Annual Meeting of the Society for Neuroscience, November 9th–13th 2013, San Diego, CA.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nicl.2014.01.010.
Contributor Information
C.A. Montojo, Email: cmontojo@ucla.edu.
A. Ibrahim, Email: ibrahiam@umich.edu.
K.H. Karlsgodt, Email: Kkarlsgodt@nshs.edu.
C. Chow, Email: CChow@mednet.ucla.edu.
A.E. Hilton, Email: ahilton@ucla.edu.
R.K. Jonas, Email: racheljonas@ucla.edu.
T.K. Vesagas, Email: tkvesagas@ucla.edu.
C.E. Bearden, Email: cbearden@mednet.ucla.edu.
Appendix A. Supplementary data
Supplementary material.
References
- Andersson J.L.R., Jenkinson M., Smith S. 2007. Non-linear registration aka spatial normalization FMRIB technical report TR07JA2, FMIRB Analysis Group of the University of Oxford. [Google Scholar]
- Awh E., Jonides J. Overlapping mechanisms of attention and spatial working memory. Trends Cogn. Sci. 2001;5:119–126. doi: 10.1016/s1364-6613(00)01593-x. [DOI] [PubMed] [Google Scholar]
- Azuma R.R., Daly E.M.E., Campbell L.E.L., Stevens A.F.A., Deeley Q.Q., Giampietro V.V. Visuospatial working memory in children and adolescents with 22q11.2 deletion syndrome; an fMRI study. J. Neurodev. Disord. 2009;1:46–60. doi: 10.1007/s11689-009-9008-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearden C.E., Woodin M.F., Wang P.P., Moss E., Mcdonald-Mcginn D., Zackai E. The neurocognitive phenotype of the 22Q11.2 deletion syndrome: selective deficit in visual–spatial memory. J. Clin. Exp. Neuropsychol. 2001;23:447–464. doi: 10.1076/jcen.23.4.447.1228. [DOI] [PubMed] [Google Scholar]
- Bearden C.E., Jawad A.F., Lynch D.R., Sokol S., Kanes S.J., Mcdonald-Mcginn D.M. Effects of a functional COMT polymorphism on prefrontal cognitive function in patients with 22q11.2 deletion syndrome. Am. J. Psychiatr. 2004;161:1700–1702. doi: 10.1176/appi.ajp.161.9.1700. [DOI] [PubMed] [Google Scholar]
- Boot E., Booij J., Zinkstok J., Abeling N., De Haan L., Baas F. Disrupted dopaminergic neurotransmission in 22q11 deletion syndrome. Neuropsychopharmacology. 2008;33:1252–1258. doi: 10.1038/sj.npp.1301508. [DOI] [PubMed] [Google Scholar]
- Brahmbhatt S.B.S., Haut K.K., Csernansky J.G.J., Barch D.M.D. Neural correlates of verbal and nonverbal working memory deficits in individuals with schizophrenia and their high-risk siblings. Schizophr. Res. 2006;87:191–204. doi: 10.1016/j.schres.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Cannon T.D.T., Glahn D.C.D., Kim J.J., van Erp T.G.M.T., Karlsgodt K.K., Cohen M.S.M. Dorsolateral prefrontal cortex activity during maintenance and manipulation of information in working memory in patients with schizophrenia. Arch. Gen. Psychiatry. 2005;62:1071–1080. doi: 10.1001/archpsyc.62.10.1071. [DOI] [PubMed] [Google Scholar]
- Chow E.W.C.E., Zipursky R.B.R., Mikulis D.J.D., Bassett A.S.A. Structural brain abnormalities in patients with schizophrenia and 22q11 deletion syndrome. Biol. Psychiatry. 2002;51:208–215. doi: 10.1016/s0006-3223(01)01246-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney S.M., Ungerleider L.G., Keil K., Haxby J.V. Object and spatial visual working memory activate separate neural systems in human cortex. Cereb. Cortex. 1996;6:39–49. doi: 10.1093/cercor/6.1.39. [DOI] [PubMed] [Google Scholar]
- Crowe S.F. Does the letter number sequencing task measure anything more than digit span? Assessment. 2000;7:113–117. doi: 10.1177/107319110000700202. [DOI] [PubMed] [Google Scholar]
- Curtis C.E. Prefrontal and parietal contributions to spatial working memory. Neuroscience. 2006;139(1):173–180. doi: 10.1016/j.neuroscience.2005.04.070. [DOI] [PubMed] [Google Scholar]
- da Silva Alves F., Schmitz N., Bloemen O. White matter abnormalities in adults with 22q11 deletion syndrome with and without schizophrenia. Schizophrenia. 2011;132(1):75–83. doi: 10.1016/j.schres.2011.07.017. [DOI] [PubMed] [Google Scholar]
- Dale A.M.A. Optimal experimental design for event-related fMRI. Hum. Brain Mapp. 1999;8:109–114. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- du Boisgueheneuc F., Levy R., Volle E., Seassau M., Duffau H., Kinkingnehun S. Functions of the left superior frontal gyrus in humans: a lesion study. Brain. 2006;129:3315–3328. doi: 10.1093/brain/awl244. [DOI] [PubMed] [Google Scholar]
- Egan M.F., Goldberg T.E., Kolachana B.S., Callicott J.H., Mazzanti C.M., Straub R.E. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc. Natl. Acad. Sci. U. S. A. 2001;98:6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M.B., Spitzer R.L., Williams J.B.W. American Psychiatric Publishing, Incorporated; 1997. Structured Clinical Interview for DSM-IV Axis I Disorders. [Google Scholar]
- Fukuda K., Awh E., Vogel E.K. Discrete capacity limits in visual working memory. Curr. Opin. Neurobiol. 2010;20:177–182. doi: 10.1016/j.conb.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glahn D.C., Kim J., Cohen M.S., Poutanen V.P., Therman S., Bava S. Maintenance and manipulation in spatial working memory: dissociations in the prefrontal cortex. NeuroImage. 2002;17:201–213. doi: 10.1006/nimg.2002.1161. [DOI] [PubMed] [Google Scholar]
- Glahn D., Therman S., Manninen M., Huttunen M., Kaprio J., Lönnqvist J. Spatial working memory as an endophenotype for schizophrenia. Biol. Psychiatry. 2003;53:624–626. doi: 10.1016/s0006-3223(02)01641-4. [DOI] [PubMed] [Google Scholar]
- Gold J.M., Carpenter C., Randolph C. Auditory working memory and Wisconsin Card Sorting Test performance in schizophrenia. Arch. Gen. Psychiatry. 1997;54(2):159–165. doi: 10.1001/archpsyc.1997.01830140071013. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic P.S.P. Working memory dysfunction in schizophrenia. J. Neuropsychiatry Clin. Neurosci. 1994;6:348–357. doi: 10.1176/jnp.6.4.348. [DOI] [PubMed] [Google Scholar]
- Green T., Gothelf D., Glaser B., Debbane M., Frisch A., Kotler M. Psychiatric disorders and intellectual functioning throughout development in velocardiofacial (22q11.2 deletion) syndrome. J. Am. Acad. Child Adolesc. Psychiatry. 2009;48:1060–1068. doi: 10.1097/CHI.0b013e3181b76683. [DOI] [PubMed] [Google Scholar]
- Insel T.T., Cuthbert B.B., Garvey M.M., Heinssen R.R., Pine D.S.D., Quinn K.K. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am. J. Psychiatr. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Jalbrzikowski M.M., Carter C.C., Senturk D.D., Chow C.C., Hopkins J.M.J., Green M.F.M. Social cognition in 22q11.2 microdeletion syndrome: relevance to psychosis? Schizophr. Res. 2012;142:99–107. doi: 10.1016/j.schres.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M.M., Smith S.S. A global optimisation method for robust affine registration of brain images. Med. Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jonas R.K., Montojo C.A., Bearden C.E. The 22q11.2 deletion syndrome as a window into complex neuropsychiatric disorders over the lifespan. Biol. Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karayiorgou M.M., Gogos J.A.J. The molecular genetics of the 22q11-associated schizophrenia. Mol. Brain Res. 2004;132(2):95–104. doi: 10.1016/j.molbrainres.2004.09.029. [DOI] [PubMed] [Google Scholar]
- Karlsgodt K.H.K., Glahn D.C.D., van Erp T.G.M.T., Therman S.S., Huttunen M.M., Manninen M.M. The relationship between performance and fMRI signal during working memory in patients with schizophrenia, unaffected co-twins, and control subjects. Schizophr. Res. 2007;89(1-3):191–197. doi: 10.1016/j.schres.2006.08.016. [DOI] [PubMed] [Google Scholar]
- Karlsgodt K.H., Sanz J., Erp T.G.M.V., Bearden C.E., Nuechterlein K.H., Cannon T.D. Re-evaluating dorsolateral prefrontal cortex activation during working memory in schizophrenia. Schizophr. Res. 2009;108:143–150. doi: 10.1016/j.schres.2008.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates W.R., Krauss B.R., AbdulSabur N., Colgan D., Antshel K.M., Higgins A.M. The neural correlates of non-spatial working memory in velocardiofacial syndrome (22q11.2 deletion syndrome) Neuropsychologia. 2007;45:2863–2873. doi: 10.1016/j.neuropsychologia.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellendonk C.C., Simpson E.H.E., Polan H.J.H., Malleret G.G., Vronskaya S.S., Winiger V.V. Transient and selective overexpression of dopamine D2 receptors in the striatum causes persistent abnormalities in prefrontal cortex functioning. Neuron. 2006;49:603–615. doi: 10.1016/j.neuron.2006.01.023. [DOI] [PubMed] [Google Scholar]
- Kempf L.L., Nicodemus K.K.K., Kolachana B.B., Vakkalanka R.R., Verchinski B.A.B., Egan M.F.M. Functional polymorphisms in PRODH are associated with risk and protection for schizophrenia and fronto-striatal structure and function. Audio Trans. IRE Prof. Group. 2008;4 doi: 10.1371/journal.pgen.1000252. (e1000252-e1000252) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavan M.S.M., Diwadkar V.A.V., Spencer S.M.S., Harenski K.A.K., Luna B.B., Sweeney J.A.J. A preliminary functional magnetic resonance imaging study in offspring of schizophrenic parents. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2002;26:1143–1149. doi: 10.1016/s0278-5846(02)00249-x. [DOI] [PubMed] [Google Scholar]
- Klingberg T.T., Forssberg H.H., Westerberg H.H. Increased brain activity in frontal and parietal cortex underlies the development of visuospatial working memory capacity during childhood. J. Cogn. Neurosci. 2002;14:1–10. doi: 10.1162/089892902317205276. [DOI] [PubMed] [Google Scholar]
- Kook S.D., An S.K., Kim K.R., Kim W.J., Lee E., Namkoong K. Psychotic features as the first manifestation of 22q11.2 deletion syndrome. Psychiatry Investig. 2010;7:72–74. doi: 10.4306/pi.2010.7.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachman H.M., Papolos D.F., Saito T., Yu Y.M., Szumlanski C.L., Weinshilboum R.M. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6:243–250. doi: 10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- Lajiness-O'Neill R.R. 22q11.2 deletion syndrome: introduction. Child Neuropsychol. 2005;11:1–3. doi: 10.1080/09297040590911176. [DOI] [PubMed] [Google Scholar]
- Luciana M., Conklin H.M., Hooper C.J., Yarger R.S. The development of nonverbal working memory and executive control processes in adolescents. Child Dev. 2005;76:697–712. doi: 10.1111/j.1467-8624.2005.00872.x. [DOI] [PubMed] [Google Scholar]
- Manoach D.S., Gollub R.L., Benson E.S., Searl M.M., Goff D.C., Halpern E. Schizophrenic subjects show aberrant fMRI activation of dorsolateral prefrontal cortex and basal ganglia during working memory performance. Biol. Psychiatry. 2000;48:99–109. doi: 10.1016/s0006-3223(00)00227-4. [DOI] [PubMed] [Google Scholar]
- McGlashan T.H. Psychosis treatment prior to psychosis onset: ethical issues. Schizophr. Res. 2001;51:47–54. doi: 10.1016/s0920-9964(01)00238-9. [DOI] [PubMed] [Google Scholar]
- Menon V.V., Anagnoson R.T.R., Mathalon D.H.D., Glover G.H.G., Pfefferbaum A.A. Functional neuroanatomy of auditory working memory in schizophrenia: relation to positive and negative symptoms. NeuroImage. 2001;13:433–446. doi: 10.1006/nimg.2000.0699. [DOI] [PubMed] [Google Scholar]
- Montojo C.A., Courtney S.M. Differential neural activation for updating rule versus stimulus information in working memory. Neuron. 2008;59:173–182. doi: 10.1016/j.neuron.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumford J.A., Poldrack R.A. Modeling group fMRI data. Soc. Cogn. Affect. Neurosci. 2007;2:251–257. doi: 10.1093/scan/nsm019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K.C. Schizophrenia and velo-cardio-facial syndrome. Lancet. 2002;359:426–430. doi: 10.1016/S0140-6736(02)07604-3. [DOI] [PubMed] [Google Scholar]
- Murphy B.L.B., Arnsten A.F.A., Goldman-Rakic P.S.P., Roth R.H.R. Increased dopamine turnover in the prefrontal cortex impairs spatial working memory performance in rats and monkeys. Proc. Natl. Acad. Sci. U. S. A. 1996;93:1325–1329. doi: 10.1073/pnas.93.3.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K.C., Murphy K.C., Jones L.A., Owen M.J. High rates of schizophrenia in adults with velo-cardio-facial syndrome. Arch. Gen. Psychiatry. 1999;56:940–945. doi: 10.1001/archpsyc.56.10.940. [DOI] [PubMed] [Google Scholar]
- Paterlini M., Zakharenko S.S., Lai W.-S., Qin J., Zhang H., Mukai J. Transcriptional and behavioral interaction between 22q11.2 orthologs modulates schizophrenia-related phenotypes in mice. Nat. Neurosci. 2005;8:1586–1594. doi: 10.1038/nn1562. [DOI] [PubMed] [Google Scholar]
- Pessoa L., Gutierrez E., Bandettini P., Ungerleider L. Neural correlates of visual working memory — fMRI amplitude predicts task performance. Neuron. 2002;35:975–987. doi: 10.1016/s0896-6273(02)00817-6. [DOI] [PubMed] [Google Scholar]
- Pukrop Ruhrmann, Bechdolf Klosterkotter. Neurocognitive indicators for a conversion to psychosis: comparison of patients in a potentially initial prodromal state who did or did not convert to a psychosis. Schizophr. Res. 2007;92:10-10. doi: 10.1016/j.schres.2007.01.020. [DOI] [PubMed] [Google Scholar]
- Pulver A.E., Nestadt G., Goldberg R. Psychotic illness in patients diagnosed with velo-cardio-facial syndrome and their relatives. J. Nerv. Ment. Dis. 1994;182:476–477. doi: 10.1097/00005053-199408000-00010. [DOI] [PubMed] [Google Scholar]
- Rosenman S.S., Korten A.A., Medway J.J., Evans M.M. Dimensional vs. categorical diagnosis in psychosis. Acta Psychiatr. Scand. 2003;107:378–384. doi: 10.1034/j.1600-0447.2003.00059.x. [DOI] [PubMed] [Google Scholar]
- Seidman L.J.L., Thermenos H.W.H., Poldrack R.A.R., Peace N.K.N., Koch J.K.J., Faraone S.V.S. Altered brain activation in dorsolateral prefrontal cortex in adolescents and young adults at genetic risk for schizophrenia: an fMRI study of working memory. Schizophr. Res. 2006;85:58–72. doi: 10.1016/j.schres.2006.03.019. [DOI] [PubMed] [Google Scholar]
- Siegel L.S.L., Ryan E.B.E. The development of working memory in normally achieving and subtypes of learning disabled children. Child Dev. 1989;60:973–980. doi: 10.1111/j.1467-8624.1989.tb03528.x. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Woolrich M.W., Beckmann C.F., Behrens T., Johansen-Berg H. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Todd J.J., Marois R. Capacity limit of visual short-term memory in human posterior parietal cortex. Nature. 2004;5:751–754. doi: 10.1038/nature02466. [DOI] [PubMed] [Google Scholar]
- Todd J.J., Marois R. Posterior parietal cortex activity predicts individual differences in visual short-term memory capacity. Cognitive. 2005;5:144–155. doi: 10.3758/cabn.5.2.144. [DOI] [PubMed] [Google Scholar]
- Van Amelsvoort T., Daly E., Robertson D., Suckling J., Ng V., Critchley H. Structural brain abnormalities associated with deletion at chromosome 22q11: quantitative neuroimaging study of adults with velo-cardio-facial syndrome. Br. J. Psychiatry. 2001;178:412–419. doi: 10.1192/bjp.178.5.412. [DOI] [PubMed] [Google Scholar]
- Vorstman J.A.S., Turetsky B.I., Sijmens-Morcus M.E.J., de Sain M.G., Dorland B., Sprong M. Proline affects brain function in 22q11DS children with the low activity COMT 158 allele. Neuropsychopharmacology. 2009;34:739–746. doi: 10.1038/npp.2008.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter E.E., Mazaika P.K.P., Reiss A.L.A. Insights into brain development from neurogenetic syndromes: evidence from fragile X syndrome, Williams syndrome, Turner syndrome and velocardiofacial syndrome. Neuroscience. 2009;164:257–271. doi: 10.1016/j.neuroscience.2009.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P.P.P., Woodin M.F.M., Kreps-Falk R.R., Moss E.M.E. Research on behavioral phenotypes: velocardiofacial syndrome (deletion 22q11.2) Dev. Med. Child Neurol. 2000;42:422–427. doi: 10.1017/s0012162200000785. [DOI] [PubMed] [Google Scholar]
- Wechsler D. 1999. Wechsler Abbreviated Scale of Intelligence (WASI) [Google Scholar]
- Wechsler D. 2008. WAIS-IV. [Google Scholar]
- Weinberger D.R.D., Egan M.F.M., Bertolino A.A., Callicott J.H.J., Mattay V.S.V., Lipska B.K.B. Prefrontal neurons and the genetics of schizophrenia. Biol. Psychiatry. 2001;50:825–844. doi: 10.1016/s0006-3223(01)01252-5. [DOI] [PubMed] [Google Scholar]
- Xu Y.D., Chun M.M. Dissociable neural mechanisms supporting visual short-term memory for objects. Nature. 2006;440:91–95. doi: 10.1038/nature04262. [DOI] [PubMed] [Google Scholar]
- Zahrt J.J., Taylor J.R.J., Mathew R.G.R., Arnsten A.F.A. Supranormal stimulation of D1 dopamine receptors in the rodent prefrontal cortex impairs spatial working memory performance. J. Neurosci. 1997;17:8528–8535. doi: 10.1523/JNEUROSCI.17-21-08528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.


