Abstract
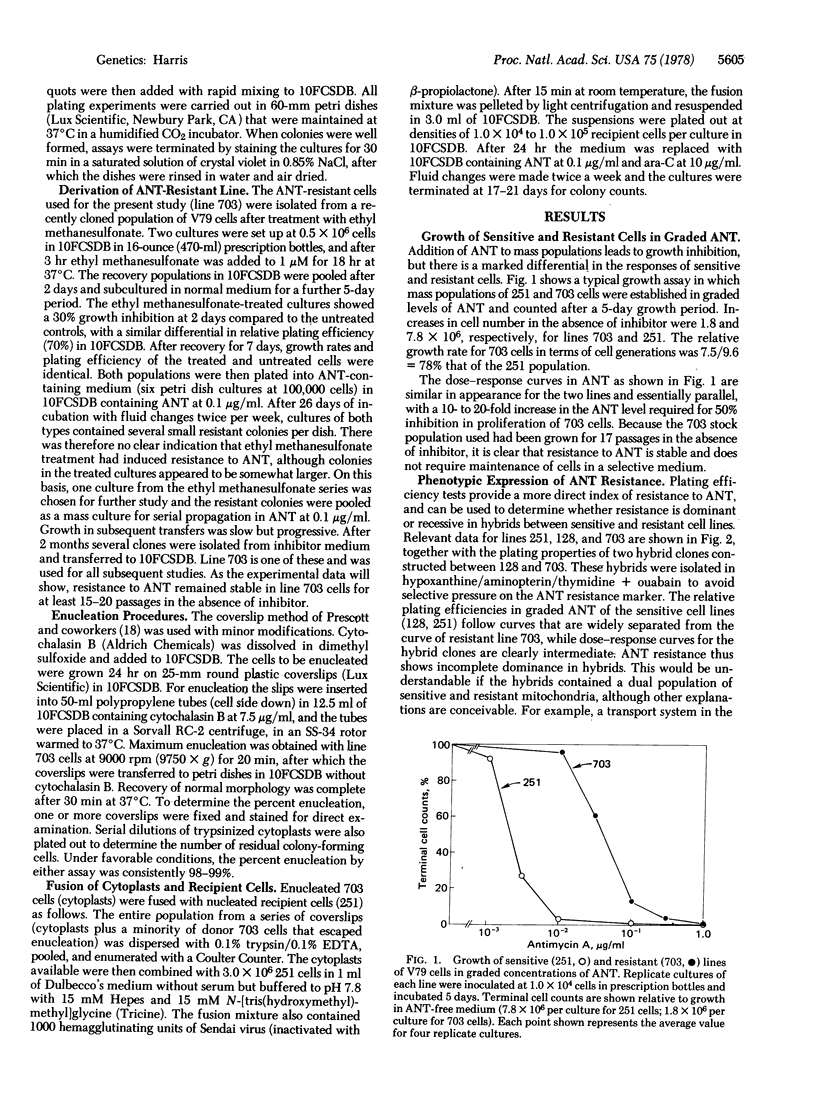
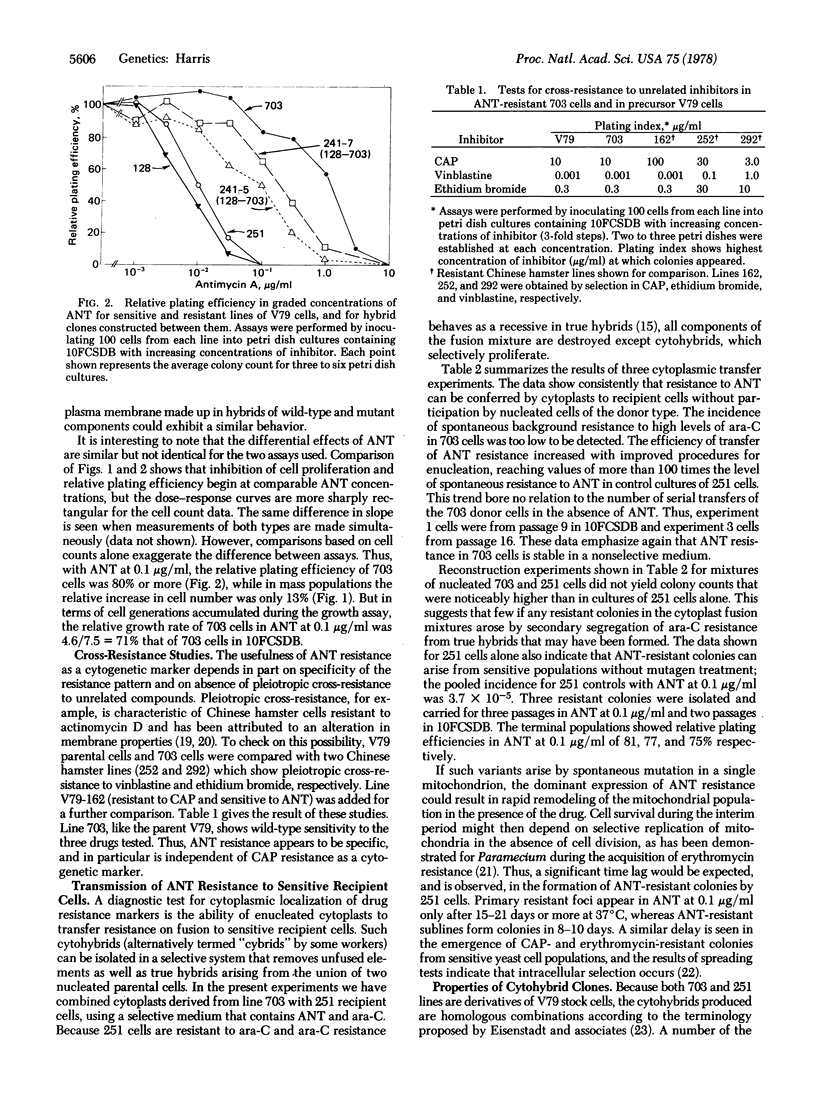
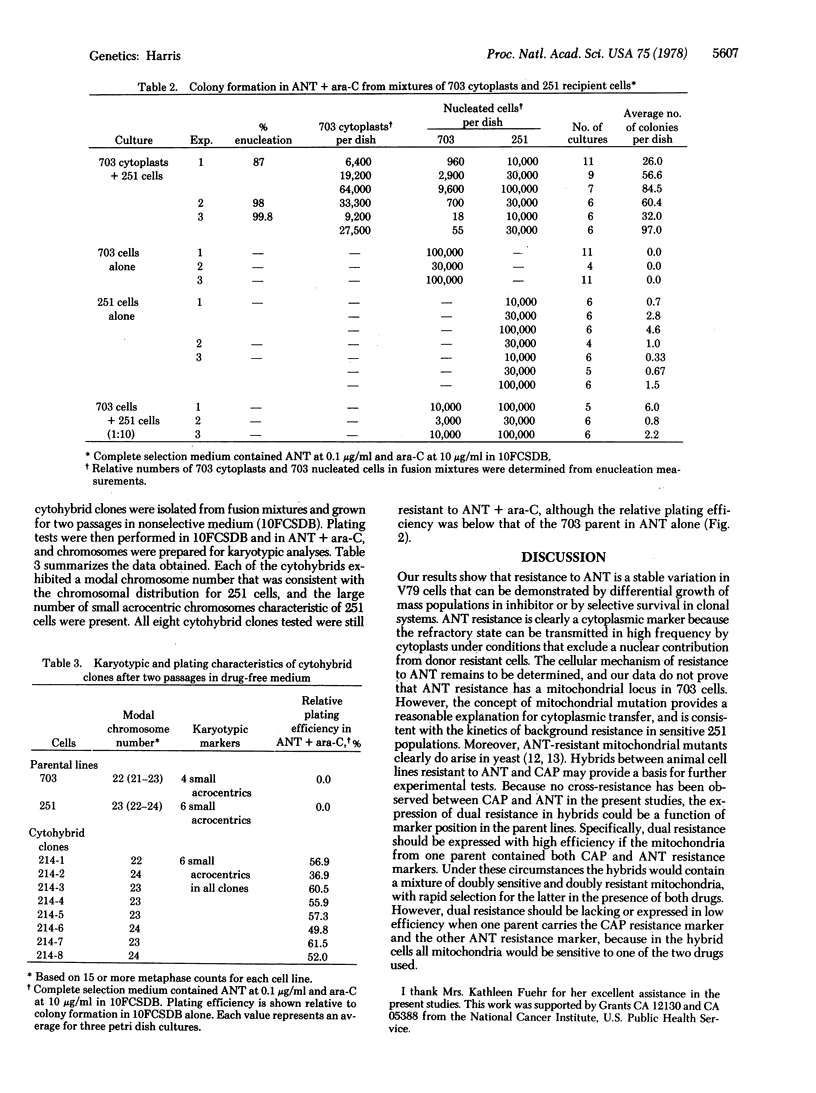
A mutant subline of V79 Chinese hamster cells resistant to antimycin A (ANT) was obtained by treatment with ethyl methanesulfonate followed by serial selection in ANT-containing medium. Clonal derivatives of the resistant line are less susceptible than parent cells to growth inhibition by ANT in mass populations and show a higher plating efficiency in graded levels of inhibitor. Resistance to ANT is stable in drug-free medium and follows a pattern of incomplete dominance in hybrids between resistant and sensitive cells. No cross-resistance to chloramphenicol or other unrelated compounds was observed. Cytoplasmic transmission of ANT resistance can be readily demonstrated by fusing enucleated cytoplasts from resistant donors to sensitive recipient cells. The resulting cytohybrids ("cybrids") grow selectively in ANT but are identical karyotypically with the recipient cell type. As a putative mitochondrial marker, ANT resistance may be usefully combined with chloramphenicol resistance for studies on segregation and recombination in the mitochondrial genome of mammalian cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biedler J. L., Riehm H. Cellular resistance to actinomycin D in Chinese hamster cells in vitro: cross-resistance, radioautographic, and cytogenetic studies. Cancer Res. 1970 Apr;30(4):1174–1184. [PubMed] [Google Scholar]
- Birky C. W., Jr On the origin of mitochondrial mutants: evidence for intracellular selection of mitochondria in the origin of antibiotic-resistant cells in yeast. Genetics. 1973 Jul;74(3):421–432. doi: 10.1093/genetics/74.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunn C. L., Wallace D. C., Eisenstadt J. M. Cytoplasmic inheritance of chloramphenicol resistance in mouse tissue culture cells. Proc Natl Acad Sci U S A. 1974 May;71(5):1681–1685. doi: 10.1073/pnas.71.5.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunn C. L., Wallace D. C., Eisenstadt J. M. Mitotic segregation of cytoplasmic determinants for chloramphenicol resistance in mammalian cells. I: Fusion with mouse cell lines. Somatic Cell Genet. 1977 Jan;3(1):71–92. doi: 10.1007/BF01550988. [DOI] [PubMed] [Google Scholar]
- Gillham N. W. Genetic analysis of the chloroplast and mitochondrial genomes. Annu Rev Genet. 1974;8:347–391. doi: 10.1146/annurev.ge.08.120174.002023. [DOI] [PubMed] [Google Scholar]
- Harris M. Phenotypic expression of drug resistance in hybrid cells. J Natl Cancer Inst. 1973 Feb;50(2):423–429. doi: 10.1093/jnci/50.2.423. [DOI] [PubMed] [Google Scholar]
- LITTLEFIELD J. W. SELECTION OF HYBRIDS FROM MATINGS OF FIBROBLASTS IN VITRO AND THEIR PRESUMED RECOMBINANTS. Science. 1964 Aug 14;145(3633):709–710. doi: 10.1126/science.145.3633.709. [DOI] [PubMed] [Google Scholar]
- Lichtor T., Getz G. S. Cytoplasmic inheritance of rutamycin resistance in mouse fibroblasts. Proc Natl Acad Sci U S A. 1978 Jan;75(1):324–328. doi: 10.1073/pnas.75.1.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obbink D. J., Spithill T. W., Maxwell R. J., Linnane A. W. Biogenesis of mitochondria 48: mikamycin resistance in Saccharomyces cerevisiae--a mitochondrial mutation conferring resistance to an antimycin A-like contaminant in mikamycin. Mol Gen Genet. 1977 Mar 7;151(2):127–136. doi: 10.1007/BF00338687. [DOI] [PubMed] [Google Scholar]
- Perasso R., Adoutte A. The process of selection of erythromycin-resistant mitochondria by erythromycin in Paramecium. J Cell Sci. 1974 May;14(3):475–497. doi: 10.1242/jcs.14.3.475. [DOI] [PubMed] [Google Scholar]
- Prescott D. M., Myerson D., Wallace J. Enucleation of mammalian cells with cytochalasin B. Exp Cell Res. 1972;71(2):480–485. doi: 10.1016/0014-4827(72)90322-9. [DOI] [PubMed] [Google Scholar]
- Riehm H., Biedler J. L. Potentiation of drug effect by Tween 80 in Chinese hamster cells resistant to actinomycin D and daunomycin. Cancer Res. 1972 Jun;32(6):1195–1200. [PubMed] [Google Scholar]
- Spolsky C. M., Eisenstadt J. M. Chloramphenicol-resistant mutants of human HeLa cells. FEBS Lett. 1972 Sep 15;25(2):319–324. doi: 10.1016/0014-5793(72)80514-3. [DOI] [PubMed] [Google Scholar]
- Wallace D. C., Bunn C. L., Eisenstadt J. M. Cytoplasmic transfer of chloramphenicol resistance in human tissue culture cells. J Cell Biol. 1975 Oct;67(1):174–188. doi: 10.1083/jcb.67.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. B., Freeman K. B. Selection of mammalian cells resistant to a chloramphenicol analog. J Cell Biol. 1975 May;65(2):492–498. doi: 10.1083/jcb.65.2.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YU C. K., SINCLAIR W. K. HOMOGENEITY AND STABILITY OF CHROMOSOMES OF CHINESE HAMSTER CELLS IN VITRO. Can J Genet Cytol. 1964 Mar;6:109–115. doi: 10.1139/g64-013. [DOI] [PubMed] [Google Scholar]


