Abstract
The renin-angiotensin aldosterone system is a critical mechanism for controlling blood pressure, and exerts most of its physiological effects through the action of angiotensin II. In addition to increasing blood pressure by increasing vascular resistance, angiotensin II also stimulates aldosterone secretion from the adrenal gland. Aldosterone acts to cause an increase in sodium and water reabsorption, thus elevating blood pressure. Although treatment with angiotensin converting enzyme inhibitors initially lowers circulating aldosterone, with chronic treatment aldosterone levels increase back to baseline, a phenomenon termed aldosterone escape; aldosterone blockade may therefore give added value in the treatment of hypertension. The first mineralocorticoid receptor antagonist developed was spironolactone, but its use has been severely hampered by adverse (notably oestrogenic) effects. The more recently developed mineralocorticoid receptor antagonist eplerenone exhibits a better adverse effect profile, although it is not devoid of effects similar to spironolactone. In addition, aldosterone activates non-genomic receptors that are not inhibited by either eplerenone or spironolactone. It is believed that deleterious organ remodelling is mediated by aldosterone via such non-genomic pathways. A new class of drugs, the aldosterone synthase inhibitors, is currently under development. These may offer a novel therapeutic approach for both lowering blood pressure and preventing the non-genomic effects of aldosterone. Here, we will review the cardiovascular effects of aldosterone and review the drugs available that target this hormone, with a particular focus on the aldosterone synthase inhibitors.
Keywords: Clinical studies, hypertension, cardiology, remodeling
Introduction
Hypertension is one of the most important preventable causes of premature morbidity and mortality in the developed world. It is a major risk factor for ischaemic and haemorrhagic stroke, myocardial infarction, heart failure, chronic kidney disease and cognitive decline.1 Most cases of hypertension have no known cause and are termed essential hypertension.2 Secondary causes of hypertension account for approximately 10% of cases, and treatment of secondary hypertension generally involves treating the underlying cause. Whatever the aetiology, it is clear that controlling high arterial pressure improves prognosis and is an important component of primary prevention of cardiovascular disease. Persistent hypertension can lead to left ventricular hypertrophy and remodelling of resistance arteries,3 both of which are associated with adverse cardiovascular outcomes.
First line treatment of hypertension involves lifestyle and dietary changes.4 If these measures are not effective, drug therapy is commenced. There are a wide variety of antihypertensive drugs available including angiotensin converting enzyme inhibitors (ACEi), angiotensin II receptor blockers (ARB), calcium channel blockers (CCB), beta blockers, alpha blockers and diuretics. A number of national and international guidelines exist for the treatment of hypertension, with some differences between them. The seventh Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure currently recommends thiazide diuretics should be used as an initial therapy in most patients (although new guidelines are due to be released soon); if the blood pressure remains uncontrolled (>160/100 mmHg), then a two-drug combination with a thiazide diuretic and either a CCB, ACEi or ARB is advocated.5 Guidance from the World Health Organisation dictates that the choice of antihypertensive should be based on cost effectiveness and any specific indications such as the particular benefits of ACEi or ARBs in the context of renal disease.6 In England and Wales, the latest National Institute for Health and Care Excellence (NICE) guidelines recommend starting drug treatment in those with stage 1 hypertension (>140/90 mmHg) with one or more additional risk factors (such as diabetes, end-organ damage and renal disease) and treating anyone with stage 2 hypertension (>160/100 mmHg); in Caucasian patients under 55 years of age, an ACEi or ARB is advocated, whilst in people of African/Caribbean origin and all those over 55, a CCB is recommended as these patients generally have lower plasma renin. If blood pressure is still not controlled, NICE recommends a combination of a CCB and ACEi or ARB,7 with the further addition of a diuretic if control is still not achieved.
In approximately 10% of patients with hypertension, a combination of three antihypertensives (typically diuretic, CCB plus ACEi or ARB) is not effective at controlling blood pressure.8 In these cases of ‘resistant hypertension,’ the aldosterone antagonist spironolactone is often added to the treatment regimen.9 Aldosterone antagonism with spironolactone, or indeed with the newer drug eplerenone, has been shown to be effective at lowering blood pressure in such patients and has a range of beneficial cardiovascular effects,10–12 thus highlighting the potential benefits of targeting aldosterone in the management of resistant hypertension, especially where other cardiovascular risk factors may coexist.
The importance of aldosterone within the renin angiotensin aldosterone pathway
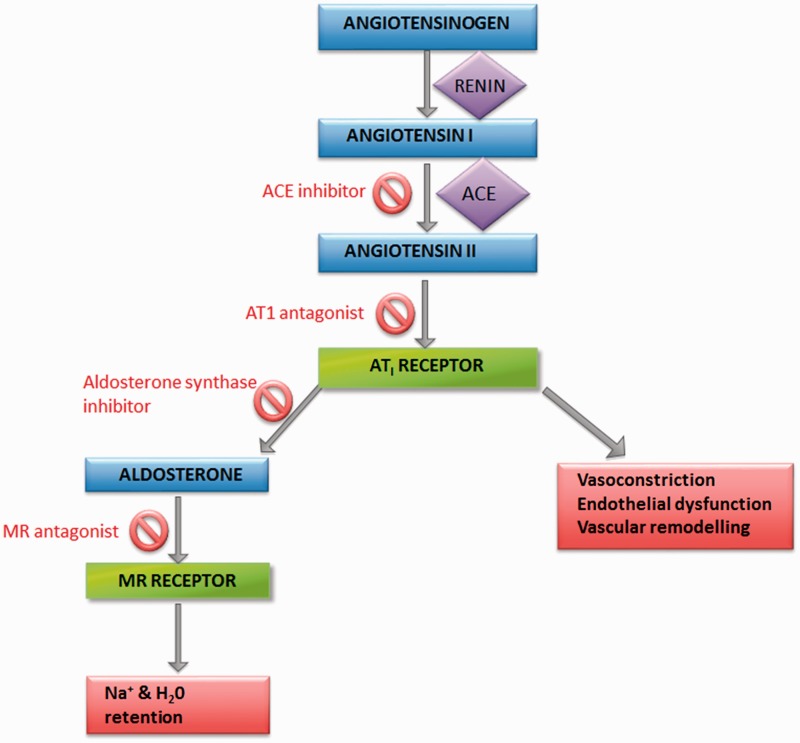
The renin angiotensin aldosterone system (RAAS; Figure 1) plays an essential role in blood pressure control through regulating fluid and electrolyte balance. Angiotensin II is the main effector of the RAAS, acting on the type I angiotensin II receptor resulting in increased blood pressure by a combination of vasoconstriction, increased cardiac output, vascular remodelling and increased aldosterone secretion.13
Figure 1.
The renin angiotensin aldosterone system. Also shown are the sites of action of ACEi, ARB, aldosterone synthase inhibitor and MR antagonist drugs.
Angiotensin II increases the synthesis of aldosterone by upregulating the CYP11B2 gene, which encodes the enzyme aldosterone synthase, in the zona glomerulosa of the adrenal cortex. Aldosterone synthase expression is also stimulated by potassium and adrenocorticotrophic hormone (ACTH).14,15
Aldosterone plays an important role in electrolyte homeostasis, through acting on mineralocorticoid receptors (MR) in the cortical collecting ducts of the kidney to increase expression of sodium channels, leading to sodium and water reabsorption which is countered by potassium loss. The resultant increase in plasma volume leads to a rise in blood pressure. The MR is a nuclear transcription factor that binds hormone response elements and increases transcription of sodium channels.16
The role of aldosterone in hypertension had first been recognised in 1954 by Conn and Louis, when they cured a case of hypertension by removal of an adrenal adenoma which secreted excess aldosterone. The largest study to date has found that primary aldosteronism accounts for 11% of patients with hypertension, although this figure may vary according to ethnicity and comorbidities.17
Aldosterone also plays an important role in hypertension in the absence of primary aldosteronism. It has been demonstrated that up to 15% of hypertensive patients have an abnormally high aldosterone / renin ratio; and in patients with resistant hypertension, this proportion rises to around 25%. Thus, higher 24-hour urinary aldosterone levels have been found in patients with resistant hypertension as compared to patients with controlled hypertension.18
The effects of aldosterone are more widespread than simply regulating sodium and potassium levels. Mineralocorticoid receptors are found in a large range of tissues including adipose, cardiac myocytes, brain and vascular endothelium, cardiac fibroblasts and vascular smooth muscle cells.15 Aldosterone activation of mineralocorticoid receptors leads to upregulation of NADPH oxidase and thereby increased production of reactive oxygen species (ROS). The generation of ROS leads to reduced bioavailability of nitric oxide, which is associated with impaired relaxation of vascular smooth muscle cells, thereby leading to increased peripheral vascular resistance and higher blood pressure. Furthermore, lower nitric oxide levels are associated with endothelial dysfunction and increased cell adhesion molecule expression, which increase the risk of atherosclerosis.19
In addition to its genomic effects, aldosterone is thought to exert non-genomic actions, which are mediated via effects on protein kinase C, intracellular calcium and cyclic adenosine monophosphate. It has been proposed that non-genomic effects could contribute to vascular smooth muscle cell hypertrophy, vascular fibrosis and interstitial fibrosis of the kidney alongside genomic effects of aldosterone, which also mediate cardiovascular fibrosis. This provides a possible additional target for therapeutic intervention.20,21
Aldosterone antagonists
Although prevention of angiotensin II generation or action would be expected to prevent the secretion of aldosterone, this is not universally the case. For example, the randomised evaluation of strategies for left ventricular dysfunction (RESOLVD) trial showed that suppression of the RAAS system with an ACEi and an ARB did not suppress aldosterone secretion in the long term.22 This phenomenon has been described as ‘aldosterone escape.’ In consequence of this, inhibiting aldosterone exerts an additive effect to inhibiting angiotensin II as regards controlling blood pressure. It is believed that use of conventional agents that target the RAAS leads to an upstream accumulation of renin, which contributes to the rise in aldosterone levels both through overcoming RAAS inhibition by the law of mass action and by generating aldosterone through alternative pathways that bypass the classical RAAS.
Spironolactone was the first MR antagonist to be developed,23 and is used for the treatment of hypertension, primary hyperaldosteronism and peripheral oedema associated with cardiac failure and other pathologies associated with secondary aldosteronism. Unfortunately, spironolactone is not well tolerated, mainly due to its lack of specificity for the MR: it also binds to progesterone and androgen receptors, leading to menstrual irregularities in women and sexual dysfunction with painful gynaecomastia in men. This adverse event profile prompted the search for better tolerated MR antagonists.24 This led to the development of eplerenone, a compound that exhibits reduced affinity for sex steroid receptors and so is less prone to cause the sex hormone-related adverse effects of spironolactone. However, as with spironolactone, electrolyte imbalances in particular hyperkalemia can occur in up to 5% of patients,25 especially in the presence of renal dysfunction and in combination with other potassium-retaining drugs such as ACEi or ARB. Eplerenone also exhibits reduced affinity for the MR compared to spironolactone.
Non-steroidal MR antagonists are currently being developed, and these may offer advantages over spironolactone and eplerenone. BAY94-8662 has been shown to be more selective for the mineralocorticoid receptor than spironolactone and has greater affinity than eplerenone. In a recent phase 2 study, BAY 94-8862 was shown to be safe and tolerable in patients with chronic heart failure and mild chronic kidney disease. Furthermore, BAY 94-8862 was shown to be as effective as spironolactone at reducing biomarkers of heart failure and CKD with a better adverse effect profile.26
Aldosterone inhibition and end-organ damage
As mentioned earlier, inhibition of aldosterone gives rise to beneficial effects on end-organ function. This was indeed proved to be the case in the 4E (eplerenone, enalapril, eplerenone and enalapril) trial, a double blind randomised 9-month-long trial in patients with hypertension and left ventricular hypertrophy. In this trial, eplerenone was equivalent to enalapril at reducing blood pressure, and the combination of enalapril and eplerenone caused a greater reduction in blood pressure than either monotherapy. Furthermore, left ventricular mass was reduced to the greatest extent by the combination therapy, followed by treatment with eplerenone alone. Treatment with enalapril alone caused the least reduction in left ventricular mass. This study demonstrated that eplerenone is highly effective at reducing target end-organ damage associated with hypertension, and more complete blockade of the RAAS using an ACEi and eplerenone combined provided the greatest degree of protection. It is important to note, however, that the short duration of this study means that extrapolation of long-term patient outcomes was not possible.11
Aldosterone is also linked to the pathogenesis of renal damage. Observational studies have shown that there is a correlation between aldosterone levels and proteinuria in patients with diabetic nephropathy; and clinical studies have shown improvements in proteinuria with eplerenone treatment.27
The role of aldosterone in heart failure is well established. The randomized aldactone evaluation study (RALES) showed a 30% reduction in mortality in patients with severe heart failure when treated with spironolactone in addition to usual therapy.28 The Eplerenone post acute MI heart efficacy and survival study (EPHESUS) trial showed that addition of eplerenone to standard treatment reduced hospital readmission by 23% in patients who had experienced an acute myocardial infarction complicated by left ventricular dysfunction. In this trial, eplerenone doses of 25 mg daily, which had only a minor effect on blood pressure, were associated with a significant reduction in mortality.29 It is possible that the beneficial effects of mineralocorticoid blockade seen in this study were due to changes in cardiac structure rather than blood pressure. From such evidence it can be inferred that aldosterone is linked to organ damage, and that inhibiting its action provides added benefit over and above lowering of blood pressure.
Aldosterone synthase inhibitors
Although spironolactone and eplerenone effectively lower blood pressure, they can cause reactive increases in circulating renin and aldosterone levels, reducing the effectiveness of treatment. Furthermore, experimental data suggest that some of the deleterious effects of aldosterone may occur through non-genomic pathways, independently of stimulation of the MR. If non-genomic pathways do contribute importantly to the effects induced by aldosterone, drugs which inhibit aldosterone synthase would potentially offer an advantage over MR antagonists in preventing activation of both genomic and non-genomic pathways.30
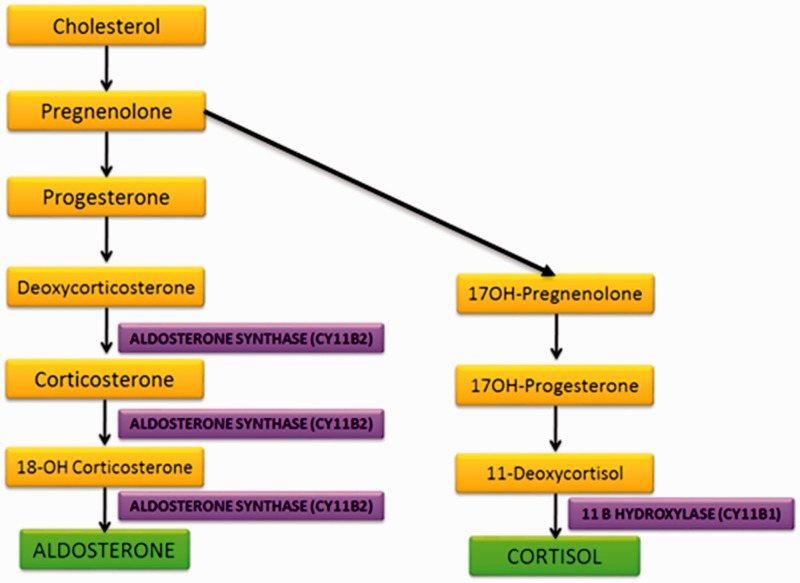
Aldosterone production is largely controlled by regulation of transcription of the CYP11B2 gene which encodes aldosterone synthase. This enzyme catalyses the final three rate-limiting steps of aldosterone synthesis (Figure 2). It is located in the mitochondria and is part of the cytochrome P450 family.13 The synthesis of aldosterone and cortisol are closely linked. Cortisol production occurs in the zona fasiculata of the adrenal cortex, the final step of cortisol synthesis involving the enzyme 11-β-hydroxylase which is encoded by the CYP11B1 gene. The CYP11B1 and the CYP11B2 gene are both located on the short arm of chromosome 8. Transcription of the CYP11B1 gene is stimulated by ACTH and that of the CYP11B2 gene by angiotensin II and potassium. The similarity of aldosterone synthase and of 11-β-hydroxylase has rendered it difficult to find a specific a specific inhibitor that selectively targets aldosterone synthase.31
Figure 2.
Schematic of adrenal steroid biosynthesis.
Preclinical studies of aldosterone synthase inhibition
Fadrozole and its dextroenantiomer FAD 286, which have inhibitory properties against aldosterone synthase, have been experimentally tested preclinically but have never been investigated in patients.32–34 In spontaneously hypertensive rats, FAD286 dose dependently reduced urine and plasma aldosterone levels; however, in combination with spironolactone it caused severe dehydration and hyperkalemia, thus highlighting the potential danger of complete aldosterone inhibition using combination therapies.32
In transgenic mice overexpressing human renin and aldosterone, FAD286 reduced mortality, prevented cardiac hypertrophy and matrix deposition in the heart and kidney. However, it was not as effective as an ARB at reducing blood pressure.33
In rat models of congestive heart failure, FAD286 showed beneficial haemodynamic effects and improved left ventricular function and remodelling to a similar extent as spironolactone.34
In spontaneously hypertensive rats, reduction in myocardial fibrosis by the two enantiomers of fadrozole were compared to a mineralocorticoid receptor antagonist. At high doses, both the S and the R enantiomers of fadrozole lowered plasma aldosterone levels to similar extents; but interestingly, only R-fadrozole reduced cardiac fibrosis (by 50%) whereas S-fadrozole had no such effect. This raises the question of whether the antifibrotic effects of this drug are truly mediated via reduction in plasma aldosterone or through other as yet unrecognised mechanisms.35
In uninephrectomised rats on a high salt diet treated with angiotensin II, both FAD286 and spironolactone reduced blood pressure. After 8 weeks, FAD286 was associated with a reduction in plasma aldosterone levels whereas spironolactone was associated with increased plasma aldosterone. Nevertheless, both FAD286 and spironolactone reduced renal and cardiac hypertrophy and interstitial fibrosis to a similar extent.36
In summary, preclinical studies of FAD286 have generally been positive, although they have raised new questions. The drug has been shown to inhibit aldosterone synthase, produce dose-dependent reductions in aldosterone levels, reduce blood pressure and prevent end-organ damage. However, aldosterone synthase inhibition prevented end-organ damage to a similar extent as mineralocorticoid receptor antagonism, suggesting that aldosterone does in fact mediate end-organ damage via mineralocorticoid receptor dependent mechanisms. On the other hand, the discordant enantioselectivity of fadrozole on cardiac fibrosis, despite equal effects on circulating aldosterone levels, argues for an effect of this drug which may be non-aldosterone-mediated. Further studies are required to dissect out any possible effects of this drug which are not exerted through modulating aldosterone synthesis. The translational potential of FAD286 is also hampered by the fact that it lacks selectivity for aldosterone synthase, since it also inhibits 11-β-hydroxylase to a large extent; this leads to inhibition of cortisol synthesis secondary to ACTH or stress. This lack of selectivity is likely to reduce the potential clinical usefulness of FAD286.
Clinical studies of aldosterone synthase inhibition
The success of FAD286 in preclinical studies led to the development of LCI699, which has greater selectivity for inhibition of CY11B2 over CY11B1, thereby having less of an influence on cortisol. LC1699 is the first orally active aldosterone synthase inhibitor that has been tested in humans.
Phase 1 study
In a double blind, placebo controlled trial in 12 healthy normotensive subjects, LCI699 at doses of 0.5–3.0 mg once daily caused a dose-dependent decrease in plasma and urine aldosterone levels. It did not affect cortisol levels at lower doses and was well tolerated. However, at 3 mg daily selectivity was lost and it blunted cortisol responses to ACTH, and also caused signs of hypoaldosteronism (postural tachycardia, mild hyponatraemia and reduced body weight). These results supported further evaluation of LC1699.37
Phase 2 studies
In a single blind, proof of concept phase 2 a study, 14 hypertensive patients with primary aldosteronism received LCI699 0.5 mg daily for the first two weeks followed by 1 mg daily for a further two weeks, and finally placebo for one further week. Plasma aldosterone levels were reduced by up to 80% in a dose-dependent manner with treatment, and this was accompanied by dose-dependent increases in deoxycorticosterone (the aldosterone precursor), confirming the inhibition of aldosterone synthase. The inhibitory effects on aldosterone synthase were maintained throughout the study, with no escape phenomena and no rebound effect seen after treatment was withdrawn.38 Plasma potassium increased by a mean of 0.73 mmol/L and there was a mild increase in plasma renin concentration. Although plasma cortisol levels were unaffected, there was evidence of inhibition of 11-β-hydroxylase as both plasma ACTH and 11-dehydroxycortisol (the precursor to cortisol) were raised. Furthermore, all patients had a blunted cortisol response to ACTH. The lack of selectivity for CYP11B1 and CYP11B2 is consistent with preclinical studies using fadrozole. Additionally, ambulatory systolic blood pressures were modestly reduced by 4 mmHg over 4 weeks. It was concluded that up to 1 mg daily of LC1699 was safe and well tolerated, and effectively reduced plasma aldosterone levels as well as partially correcting hypokalaemia in patients with primary hyperaldosteronism.38
Calhoun et al. recently carried out a randomised controlled, double blind phase 2 trial, in which 534 patients with mild to moderate primary hypertension received LCI699 0.25–1 mg daily, eplerenone 50 mg twice daily or placebo. After 8 weeks, there was a dose-dependent reduction of mean systolic blood pressure with LCI699. The daily 1.0 mg dose significantly reduced systolic blood pressure by a mean of 12.6 mmHg, while eplerenone 50 mg twice daily reduced systolic blood pressure by a mean of 13.8 mmHg, compared with placebo; 24-hour mean ambulatory diastolic and systolic blood pressure were reduced to similar extents by eplerenone and LCI699 1.0 mg daily after 8 weeks. Twenty percent of patients receiving LCI699 1.0 mg daily had suppression of ACTH stimulated release of cortisol, indicating partial inhibition of 11-β-hydroxylase (CYP11B1) although it did not reduce plasma cortisol concentrations and no patients developed signs of hypocortisolism. The most frequently occurring adverse effects were headache, dizziness and nasopharyngitis, which were similar in the LCI699 and placebo groups. Hyperkalemia occurred in 3% of patients in both the LCI699 and placebo groups. The compound was safe and well tolerated.39
A study by Andersen et al set out to find the maximum tolerated dose of LCI699 with respect to cortisol suppression by ACTH. In all, 63 patients were randomised in a double-blind fashion to either placebo or various doses of LCI699. The trial found dose-dependent reductions in both systolic and diastolic blood pressures by LCI699. The maximum tolerated dose was found to be 1.3 mg once daily. LCI699 had no serious adverse effects and no patients developed signs or symptoms of adrenal insufficiency.40
In a study of 155 patients with treatment-resistant hypertension, patients were randomised in a double blind fashion to receive over 8 weeks either placebo, eplerenone 50 mg twice daily or varying doses of LCI699 (0.25 mg twice daily, 0.5 mg once daily, 1 mg once daily or 1 mg twice daily). Eplerenone gave rise to a significant reduction in systolic and diastolic blood pressure (9.9 and 2.9 mmHg, respectively). Surprisingly there was no significant reduction in either systolic or diastolic blood pressure with any dose of LCI699. However, LCI699 did suppress plasma aldosterone and increase deoxycorticosterone levels in a dose-dependent manner. The reasons for this discrepancy are not clear, and the findings are contradictory to those of the studies by Calhoun et al. and Andersen et al. This merits further evaluation in larger trials and suggests that aldosterone synthase inhibition may be of clinical use only in combination with other antihypertensive agents; or at any rate may be more useful in reducing end-organ damage by aldosterone rather than in lowering blood pressure.41
The most recent trial compared aldosterone synthase inhibition using LCI699 with mineralocorticoid receptor blockade in patients with primary hyperaldosteronism. In addition to usual medications, patients were treated with LC1699 for 30 days followed by placebo washout for 1 week followed by another 30 days of treatment with eplerenone. Thirty days of treatment with eplerenone reduced 24-hour ambulatory blood pressure by 5 mmHg more than LCI699; however, LCI699 was associated with a 75% decrease in plasma aldosterone whilst eplerenone increased aldosterone levels by 89%.42
Conclusions
The development of LCI699 has allowed assessment of the benefits and safety of inhibiting aldosterone synthesis in hypertensive patients. Unfortunately, the lack of selectivity of LCI699 at higher doses (above 3 mg daily) giving rise to inhibition of 11-β-hydroxylase (CYP11B1) and alteration of the glucocorticoid axis limits the dose that can be used. It is unlikely that LCI699 will supplant mineralocorticoid receptor blockers clinically as the latter are more effective at lowering blood pressure. The development in due course of a second generation of more selective blockers of aldosterone synthase should make it possible to test the value of this approach, hopefully to achieve greater reductions in blood pressure without affecting the glucocorticoid axis.
It should be noted that inhibition of aldosterone synthesis is not free of risks. As with mineralocorticoid receptor blockade, aldosterone synthase inhibitors are likely to cause hyperkalemia and hyponatraemia. Furthermore, their long-term effect on kidney function is not known. In the absence of aldosterone, mineralocorticoid receptors may become activated by cortisol or deoxycorticosterone (aldosterone precursor), and the combination of mineralocorticoid receptor blockade and aldosterone synthase inhibition to prevent this may lead to severe hypoaldosteronism as already seen in early trials. Moreover, the possible long-term effects of inhibition of cortisol stimulation by ACTH with these drugs warrant further study.
Further studies are required to establish whether there is indeed differential cardiovascular benefit of suppressing aldosterone production compared with blocking activation of the mineralocorticoid receptor, independent of any antihypertensive effect. The preclinical studies have shown positive results with regard to end-organ damage in the kidneys, heart and blood vessels, however this needs to be confirmed in large-scale trials in humans.
An interesting avenue to explore would be to examine whether the use of high doses of LCI699 to inhibit 11-β-hydroxylase (CYP11B1) and prevent cortisol production may have a place in the treatment of cortisol excess. Indeed, in a recent assessment of patients with Cushing’s disease who received varying doses of LCI699 ranging from 2 to 50 mg twice daily, eight of the nine patients had normalised urinary cortisol levels after 70 days.43
The usage of aldosterone blocking agents in the treatment of hypertension is low in most countries, and their place in most hypertension treatment guidelines is generally as fourth- or fifth-line therapy in treatment-resistant cases. More studies showing end-organ protection in hypertensive patient populations may pave the way for a broader use of aldosterone blockers, and in the future possibly also of aldosterone synthase inhibitors, especially if more selective agents can be developed.
Guarantor
Albert Ferro
Contributorship
Milan Hargovan performed the literature search. Milan Hargovan and Albert Ferro wrote the paper
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
None declared.
References
- 1.Vasan RS, Larson MG, Leip EP, et al. Impact of high-normal blood pressure on the risk of cardiovascular disease. N Engl J Med 2001; 245: 1291–1297 [DOI] [PubMed] [Google Scholar]
- 2.Flynn JT. Differentiation between primary and secondary hypertension in children using ambulatory blood pressure monitoring. Pediatrics 2002; 110: 89–93 [DOI] [PubMed] [Google Scholar]
- 3.Devereux RB, Pickering TG, Harshfield GA, et al. Left ventricular hypertrophy in patients with hypertension: importance of blood pressure response to regularly recurring stress. Circulation 1983; 68: 470–476 [DOI] [PubMed] [Google Scholar]
- 4.Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary approaches to Stop Hypertension (DASH) diet. N Engl J Med 2001; 344: 3–10 [DOI] [PubMed] [Google Scholar]
- 5.Chobanian A, Bakris G, Black H, et al. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension 2003; 42: 1206–1252 [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization, International Society of Hypertension Writing Group 2003 World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. J Hypertension 2003; 21: 1983–1992 [DOI] [PubMed] [Google Scholar]
- 7. National Clinical Guideline Centre (NCGC). Hypertension: the clinical management of primary hypertension in adults. Clinical Guideline 127. Methods, evidence and recommendations. Commissioned by the National Institute for Health and Clinical Excellence. London: NCGC, 2011, http://www.nice.org.uk/nicemedia/live/13561/56007/56007.pdf.
- 8.August P. Initial treatment of hypertension. N Engl J Med 2003; 348: 610–617 [DOI] [PubMed] [Google Scholar]
- 9.McCormack T, Arden C, Begg A, et al. Optimising hypertension treatment: NICE/BHS guideline implementation and audit for best practice. Br J Cardiol 2013; 20: S1–S16 [Google Scholar]
- 10.Weinberger MH, Roniker B, Krause SL, et al. Eplerenone, a selective aldosterone blocker, in mild-to-moderate hypertension. Am J Hypertens 2002; 15: 709–716 [DOI] [PubMed] [Google Scholar]
- 11.Pitt B, Reichek N, Willenbrock R, et al. Effects of eplerenone, enalapril, and eplerenone/enalapril in patients with essential hypertension and left ventricular hypertrophy: the 4E-left ventricular hypertrophy study. Circulation 2003; 108: 1831–1838 [DOI] [PubMed] [Google Scholar]
- 12.Chapman N, Dobson J, Wilson S, et al. Effect of spironolactone on blood pressure in subjects with resistant hypertension. Hypertension 2007; 49: 839–845 [DOI] [PubMed] [Google Scholar]
- 13.Atlas SA. The renin-angiotensin aldosterone system: pathophysiological role and pharmacologic inhibition. J Manag Care Pharm 2007; 13: 9–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bassett MH, White PC, Rainey WE. The regulation of aldosterone synthase expression. Mol Cell Endocrinol 2004; 217: 67–74 [DOI] [PubMed] [Google Scholar]
- 15.Tomaschitz A, Pilz S, Ritz E, et al. Aldosterone and arterial hypertension. Nat Rev Endocrinol 2010; 6: 83–93 [DOI] [PubMed] [Google Scholar]
- 16.Ruilope LM. Aldosterone, hypertension, and cardiovascular disease: an endless story. Hypertension 2008; 52: 207–208 [DOI] [PubMed] [Google Scholar]
- 17.Rossi GP, et al. A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J Am Coll Cardiol 2006; 48: 2293–2300 [DOI] [PubMed] [Google Scholar]
- 18.Calhoun D, Nishizaka M, Zaman M. Hyperaldosteronism among Black and White subjects with resistant hypertension. Hypertension 2002; 40: 892–896 [DOI] [PubMed] [Google Scholar]
- 19.Brown NJ. Aldosterone and end-organ damage. Curr Opin Nephrol Hypertension 2005; 14: 235–241 [DOI] [PubMed] [Google Scholar]
- 20.Williams JS. Evolving research in nongenomic actions of aldosterone. Curr Opin Endocrinol Diabetes Obes 2013; 20: 198–203 [DOI] [PubMed] [Google Scholar]
- 21.Lin YH, Wu XM, Lee HH, et al. Adrenalectomy reverses myocardial fibrosis in patients with primary aldosteronism. J Hypertens 2012; 30: 1606–1613 [DOI] [PubMed] [Google Scholar]
- 22.McKelvie RS, Yusuf S, Pericak D, et al. Comparison of candesartan, enalapril, and their combination in congestive heart failure: randomized evaluation of strategies for left ventricular dysfunction (RESOLVD) pilot study. The RESOLVD Pilot Study Investigators. Circulation 1999; 100: 1056–1064 [DOI] [PubMed] [Google Scholar]
- 23.Wolf RL, Mendlowitz M, Roboz J, et al. Treatment of hypertension with spironolactone. Double-blind study. JAMA 1966; 198: 1143–1149 [PubMed] [Google Scholar]
- 24.Parthasarathy HK, Menard J, White WB, et al. A double-blind, randomized study comparing the antihypertensive effect of eplerenone and spironolactone in patients with hypertension and evidence of primary aldosteronism. J Hypertens 2011; 29: 980–990 [DOI] [PubMed] [Google Scholar]
- 25.Weinberger MH, Roniker B, Krause SL, et al. Eplerenone, a selective aldosterone blocker, in mild-to-moderate hypertension. Am J Hypertens 2002; 15: 709–716 [DOI] [PubMed] [Google Scholar]
- 26.Pitt B, Kober L, Ponikowski P, et al. Safety and tolerability of the novel non-steroidal mineralocorticoid receptor antagonist BAY 94-8862 in patients with chronic heart failure and mild or moderate chronic kidney disease: a randomized, double-blind trial. Eur Heart J 2013; 34: 2453–2463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garg R, Hurwitz S, Williams GH, et al. Aldosterone production and insulin resistance in healthy adults. J Clin Endocrinol Metab 2010; 95: 1986–1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pitt B, Zannad F, Remme WJ, et al. Effectiveness of spironolactone added to an angiotensin-converting enzyme inhibitor and a loop diuretic for severe chronic congestive heart failure (the Randomized Aldactone Evaluation Study [RALES]). Am J Cardiol 1996; 78: 902–907 [DOI] [PubMed] [Google Scholar]
- 29.Pitt B, Bakris G, Ruilope LM, et al. Serum potassium and clinical outcomes in the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS). Circulation 2008; 118: 1643–1650 [DOI] [PubMed] [Google Scholar]
- 30.Amar L, Azizi M, Menard J, et al. Sequential comparison of aldosterone synthase inhibition and mineralocorticoid blockade in patients with primary aldosteronism. J Hypertens 2013; 31: 624–629 [DOI] [PubMed] [Google Scholar]
- 31.Azizi M, Amar L, Menard J, et al. Aldosterone synthase inhibition in humans. Nephrol Dial Transplant 2013; 28: 36–43 [DOI] [PubMed] [Google Scholar]
- 32.Menard J, Gonzalez MF, Guyene TT, et al. Investigation of aldosterone-synthase inhibition in rats. J Hypertens 2006; 24: 1147–1155 [DOI] [PubMed] [Google Scholar]
- 33.Fiebeler A, Nussberger J, Shagdarsuren E, et al. Aldosterone synthase inhibitor ameliorates angiotensin II-induced organ damage. Circulation 2005; 111: 3087–3094 [DOI] [PubMed] [Google Scholar]
- 34.Mulder P, Mellin V, Favre J, et al. Aldosterone synthase inhibition improves cardiovascular function and structure in rats with heart failure: a comparison with spironolactone. Eur Heart J 2008; 29: 2171–2179 [DOI] [PubMed] [Google Scholar]
- 35.Minnaard-Huiban M, Emmen JM, Roumen L, et al. Fadrozole reverses cardiac fibrosis in spontaneously hypertensive heart failure rats: discordant enantioselectivity versus reduction of plasma aldosterone. Endocrinology 2008; 149: 28–31 [DOI] [PubMed] [Google Scholar]
- 36.Lea WB, Kwak ES, Luther JM, et al. Aldosterone antagonism or synthase inhibition reduces end-organ damage induced by treatment with angiotensin and high salt. Kidney Int 2009; 75: 936–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Menard J, Watson C, Rebello S, et al. Hormonal and electrolyte responses to the aldosterone synthase inhibitor LCI699 in sodium depleted healthy subjects. J Am Coll Cardiol 2010; 55: A61–E583 [Google Scholar]
- 38.Amar L, Azizi M, Joël M, et al. Aldosterone synthase inhibition with LCI699. A proof-of-concept study in patients with primary aldosteronism. Hypertension 2010; 56: 831–838 [DOI] [PubMed] [Google Scholar]
- 39.Calhoun DA, White WB, Krum H, et al. Effects of a novel aldosterone synthase inhibitor for treatment of primary hypertension: results of a randomized, double-blind, placebo and active-controlled phase 2 trial. Circulation 2011; 124: 1945–1955 [DOI] [PubMed] [Google Scholar]
- 40.Andersen K, Hartman D, Peppard T, et al. The effects of aldosterone synthase inhibition on aldosterone and cortisol in patients with hypertension: a phase II, randomized, double-blind, placebo controlled, multicenter study. J Clin Hypertens 2012; 14: 580–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karns AD, Bral JM, Hartman D, et al. Study of aldosterone synthase inhibition as an add-on therapy in resistant hypertension. J Clin Hypertens 2013; 15: 186–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Amar L, Azizi M, Menard J, et al. Sequential comparison of aldosterone synthase inhibition and mineralocorticoid blockade in patients with primary aldosteronism. J Hypertens 2013; 31: 624–629 [DOI] [PubMed] [Google Scholar]
- 43.Bertagna X, Pivonello R, Fleseriu M, et al. Patients with Cushing’s disease achieve normal urinary cortisol with LCI699, a potent 11b-hydroxylase inhibitor: preliminary results from a multicenter, proof-of-concept study. Endocrine Abstracts 2012, pp. OR012–OR012 [DOI] [PubMed] [Google Scholar]