Summary
The Survivor’s Health and Reaction (SHARE) study used a quality of life model adapted for cancer survivors by Dow, Ferrell and colleagues to identify factors related to global health-related quality of life (HRQL) and the prevalence of problems and healthy behaviors in breast cancer patients who participated in CALGB 8541. A total of 245 survivors (78% of those invited) who were 9.4 to 16.5 years post-diagnosis completed surveys that inquired about current HRQL, economic, spiritual, physical and psychosocial concerns, and health behaviors, (e.g. smoking, exercise, and supplement use). A regression model developed to characterize global self-assessment of HRQL from all domains showed a negative effect on global HRQL from lower social support (OR=1.03, 95% CI: 1.02, 1.05), having heart disease (OR=5.01, 95% CI: 1.39, 18.1), decreased energy (OR=1.05, 95% CI: 1.03, 1.07), and having 2 or more co-morbidities (OR=2.39, 95%CI: 1.10,5.19). Some women reported engaging in healthy behaviors since their cancer diagnosis, such as increasing exercise (31%), and reducing/quitting smoking (20%). The most prevalent problem reported was menopausal symptoms (59%). Although breast cancer survivors reported making positive lifestyle changes, physical and social factors, such as heart disease, decreased social support and having 2 or more comorbidities were found to be significantly related to HRQL. Factors related to psychological, spiritual and economic domains were not predictive of global HRQL. Suggestions are provided for areas in which to target interventions in order to improve HRQL in long-term breast cancer survivors.
Introduction
Due to greater utilization of screening mammography and improvements in diagnosis and treatment, the number of breast cancer survivors is increasing. (1–3) Survivors face ongoing concerns due to their treatment that greatly affect their lives, including fertility problems, menopausal symptoms, cardiac toxicity and second malignancies. (4, 5) Examining these topics is important as breast cancer patients progress from the crisis period of diagnosis and treatment to lifelong follow-up care, where psychological effects, changes in relationships and lingering physical symptoms become important.
Breast cancer survivors face a variety of physical, economic, spiritual, and psychosocial concerns as a result of their disease. Long-term cancer survivors have reported that while many physical concerns related to illness and treatment were resolved, problems remained in the areas of social/emotional support, health habits, spiritual/philosophical view of life, and body image concerns. (6–13) Although many studies have reported good or adequate overall quality of life among long-term survivors, issues such as sexual concerns, psychosocial problems and physical symptoms, such as pain and lymphedema, still persist. (8–13) Especially notable were the adverse effects of systemic adjuvant therapy (chemotherapy) that continued and worsened 5–10 years after diagnosis. (10, 12)
The primary goal of this study was to use a conceptual model adapted to cancer survivors (9, 14) to predict the effects of specific factors in the domains of physical, psychosocial, economic and spiritual health on global HRQL of breast cancer survivors 9–16 years post diagnosis. To our knowledge, this has not previously been done in studies of long-term HRQL in breast cancer survivors. The significance of this goal is that in identifying factors that negatively impact quality of life, interventions can be developed and targeted to those areas in order to improve the life quality of long-term breast cancer survivors.
The secondary goals of this study were to describe the prevalence of health problems among long-term breast cancer survivors, as well as to describe healthy behaviors engaged in by these survivors. This information can also be useful in developing and targeting interventions for survivors.
METHODS
1. Setting
The current study, Cancer and Leukemia Group B (CALGB) 79804, examined HRQL, health status, and lifestyle behaviors in breast cancer survivors who participated in CALGB 8541 from 1985–1991. The goal of CALGB 8541 was to determine whether a relationship existed between disease-free survival, and dose or dose-intensity for stage II breast cancer patients randomly assigned to one of three cylophosphamide/doxorubicin/ fluorouracil (CAF) regimens as adjuvant therapy for surgically resected breast cancer. (15, 16) Participants were predominantly Caucasian (86%), 48% were premenopausal and average age at diagnosis was 49 years. Sixty percent had 1–3 positive lymph nodes, and 25% had tumors less than 2 centimeters. (15, 16) The results of the trial showed that after follow-up (median 3.4 years), women treated with high or moderate CAF dose/intensity had significantly longer disease-free survival (p<0.001) and overall survival (p=0.004) than those treated with the low CAF dose/intensity in three-way log rank comparisons. The difference in overall survival between the groups treated with moderate or high dose/intensity was not significant. (15, 16)
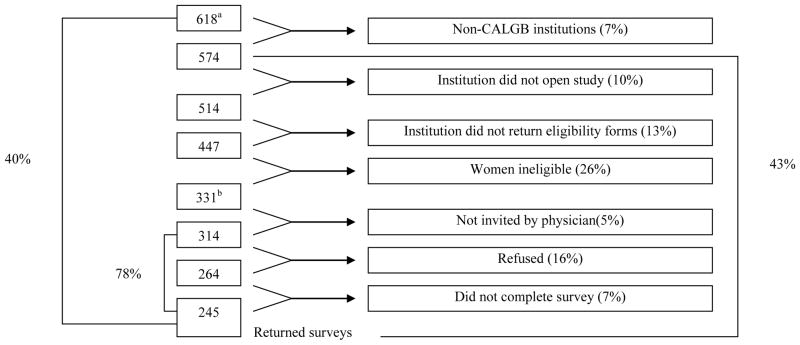
Of the 1,572 women randomized in CALGB 8541, 618 were alive and disease-free when CALGB 79804 began in 1999. Since accrual for CALGB 8541 occurred from 1985–1991, these women were 9.4 to 16.5 years post-diagnosis (median 12.5 years). CALGB 79804 was approved by the Institutional Review Board of each participating institution.
2. Procedures
Clinical Research Associates (CRAs) at CALGB treating institutions were notified of patient eligibility and selection for potential participation by the CALGB Statistical Center. The CRA confirmed the patient’s address, phone number, and disease status (alive and disease-free), which were sent to the study interviewer. CRAs also informed treating physicians of the study, and requested permission for patients to be contacted. Depending on the physician, CRAs either contacted the patient about the study or an introductory letter was sent from the principal investigator (EP) noting the physician’s permission to contact the patient. A consent form and questionnaire were also sent.
The study interviewer called each woman to verify that study materials were received and to answer any questions. Women who wished to participate were asked to complete the questionnaire packet, sign the consent form, and return both in the postage-paid envelope provided. Non-respondents were contacted by phone to remind them to complete and return the survey. If requested, the survey was conducted by phone. Upon questionnaire completion, the research interviewer registered the patient with the CALGB Statistical Center.
A total of 618 women were identified by the CALGB Statistical Center as alive and disease-free for potential participation in this study. Reasons for exclusions, as shown in Figure 1, include institutional factors, (e.g. non-CALGB institution, not opening the study and not returning eligibility forms) and non-institutional factors (e.g. patient’s death, moving to another city or disease recurrence). Women were not invited to participate in this study if their physician did not approve. Thus, 314 women were invited to participate and 245 (78%) returned the surveys. CALGB 8541 survivors who participated (N=245) did not differ significantly from those who did not participate (N=373) in the follow-up study by age, treatment arm, number of nodes, age or year of entry in CALGB 8541 (data not shown). However, whites were more likely to participate than non-whites (93% versus 81%, respectively, p<0.0001).
Figure 1.
Accrual/Eligibility to SHARE
aThere were 618 potentially eligible patients
bThere were 331 eligible patients
3. Conceptual Framework
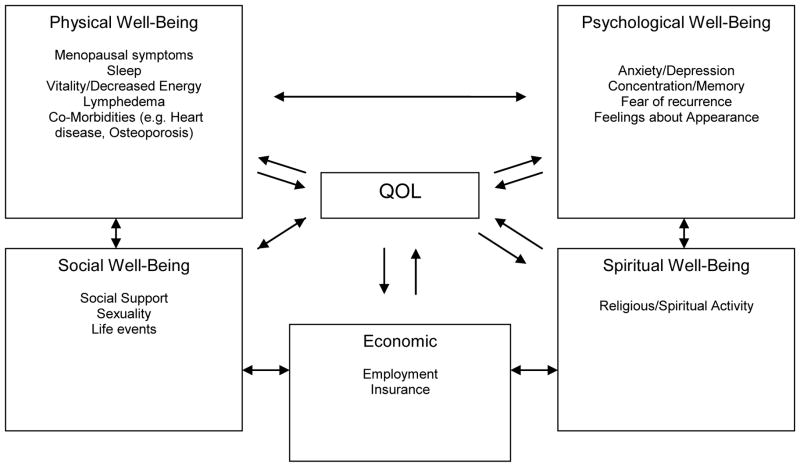
The conceptual framework for this study was derived from the Quality of Life model adapted for cancer survivors by Dow, Ferrell and colleagues (Figure 2).(9, 14) This model was chosen as there were few others developed at the time, it addressed the research questions of the investigative team, and could be readily operationalized by questionnaire assessment. The model identifies four major areas relating to global HRQL of cancer patients: physical, psychological, social and spiritual well-being. Specific issues have been identified within each domain by Dow, Ferrell and colleagues. (17, 18) For the present study, the social well-being area was subdivided into social and economic well-being. These domains, global HRQL, and specific items related to breast cancer survivors (e.g. lymphedema), were assessed in the questionnaire battery. Demographics, such as age, race and treatment variables were assessed as covariates.
Figure 2.
Quality of Life Model Adapted for Breast Cancer Survivors*
4. Measures
In keeping with the model developed by Dow, Ferrel et al., (9, 14) the following measures were used to assess the domains portrayed in Figure 2. Questionnaire items by domain are displayed in Table 1:
Table 1.
Domains and Questionnaire Items Examined in Surveys
| Domain | Questionnaire | Item |
|---|---|---|
| Physical Well-being | Menopause and Reproductive Health | Total Symptom Subscale |
| Lymphedema & Pain | Since your treatment for breast cancer, have you had any swelling in your arm or hand on the same side that you had surgery? (yes/no) | |
| Do you continue to have swelling now? (yes/no) | ||
| How would you describe this swelling? (mild, moderate, severe) | ||
| Have you had any pain in your arm or hand on the same side that you had surgery? (yes/no) | ||
| Do you have pain in your underarm or chest on the same side that you had your surgery? (yes/no) | ||
| On a scale from 1 to 10 how would you rate this pain? | ||
| Your Health-Short Form (comorbidities) | Heart Disease (yes/no) | |
| Osteoporosis (yes/no) | ||
| Interference Subscale Score: 1 interfering symptom | ||
| Interference Subscale Score: ≥2 interfering symptoms | ||
| CES-D (short form) | My sleep was restless (yes/no) | |
| SF-36 | Vitality Subscale | |
| Social Well- Being | MOS Social Support | MOS Total Score |
| MOS Affection | ||
| MOS Tangible Support | ||
| MOS Positive Interaction | ||
| MOS Emotional Support | ||
| Watt’s Sexual Functioning | Sexual Feelings | |
| Sexual Arousal | ||
| Sexual Satisfaction | ||
| Life Events Scale | Events Subscale | |
| Frequency Subscale | ||
| Psychological Well Being | CES-D (short form) | CES-D Total ≥16 vs < 16 (i.e. Depression-yes/no) |
| Breast Cancer Anxiety and Screening Scale | Anxiety/Intrusive Thoughts | |
| Avoidant Thoughts | ||
| Total Cognitive Distress | ||
| Appearance Assessment | I view myself as a… (overweight, normal, thin) person | |
| Right now are you satisfied with your breasts?* | ||
| Right now are you satisfied with overall body?* | ||
| The appearance of my breast area is important to me.** | ||
| General Appearance Feelings | ||
| Menopause and Reproductive Health | Difficulty Concentrating (yes/no) | |
| Spiritual Well-being | Systems of Belief Inventory | Subscale: Total Score |
| Subscale: Social Support | ||
| Subscale: Religious Spiritual Belief | ||
| Economic Well-Being | Effect of Cancer on Employment | Employment Problems |
| Insurance Problems | ||
| Demographic | Medical Chart | Race: White, Black, Other |
| Treatment arm: Intensive, Standard, Low Dose |
Responses were based on a 5-point Likert Scale (Strongly disagree…strongly agree)
Responses were based on a 5-point Likert Scale (Very dissatisfied…very satisfied)
Physical Well-Being
-
a)
Fatigue was measured with the vitality scale of the SF-36, which measures generic quality of life and includes eight dimensions: physical functioning, role limitations, bodily pain, general health perceptions, vitality, social functioning, emotional well-being, and general perceptions of one’s health status. Subscales are scored from 0–100, with higher scores indicating better HRQL. This scale has undergone extensive reliability and validity testing (19, 20).
-
b)
Menopausal symptoms were assessed using the Menopause and Reproductive Health Questionnaire, which contains a checklist asking participants to indicate if they had a particular physical symptom. A total score that assessed both frequency and severity of symptoms was calculated. (21)
-
c)
Sleep pattern was assessed with the question on the validated CES-D (short form), “My sleep was restless” (1–4). (22, 23)
-
d)
Cardiotoxicity/heart disease was assessed with the question on Your Health-Short Form inquiring if the patient had heart disease (No/Yes). The Your Health-Short Form is a modified version of the validated OARS Co-Morbidity list. (24)
-
e)
The presence of osteoporosis was assessed with the Your Health-Short Form. Additional analyses on osteoporosis were based on data from the Osteoporosis Questionnaire, which asked if women had ever experienced a spinal compression fracture or fracture of the wrist, shoulder or hip, and if a physician had ever told them that they had osteoporosis.
-
f)
Interference of co-morbidities with daily activities was assessed with an “Interference Score”, which was calculated from the Your Health-Short Form; it collected information on other disabilities and co-morbid conditions. (24) The score was assessed using a 3-point scale ranging from ‘not at all’ to ‘a great deal’.
-
g)
Lymphedema, arm pain and chest/underarm pain was assessed with the Pain and Lymphedema Questionnaire. This module documented the occurrence, duration and circumstances of any treatment-related swelling and pain in the arms and hands. (25)
Social Well-being
-
h)
Social Support was measured with the MOS Social Support Survey, a validated survey which assessed 4 areas of perceived social support of study participants: emotional/informational, tangible, affectionate, and positive social interaction of study participants. (26, 27) All subscales scores and the total score were used.
-
i)
Sexuality was assessed with the validated Watts Sexual Functioning Questionnaire. (28) The subscales of sexual satisfaction, sexual feelings and arousability were assessed.
-
j)
Stress was assessed with the Life Events Scale, which has been used in past WHI studies.22 Both the events and frequency subscales were examined.
Psychological Well-being
-
k)
Depression was measured with the CES-D (short form). A total score is calculated from this measure and was dichotomized with a score >16 reflecting the presence of depression. (22)
-
l)
Fear of recurrence was assessed with the Breast Cancer Anxiety and Screening Behavior Scale (32), a modified 21-item validated scale that assessed the emotional and cognitive aspects of breast cancer. (32–36) All subscales were examined, including intrusive thoughts, avoidance thoughts, and total cognitive distress.
-
m)
Body image/appearance was assessed with questions from the Appearance Assessment Questionnaire, (37) which used a 5-point Likert scale to assess satisfaction with the breasts and overall body, and feelings about general appearance.
-
n)
Concentration/memory was measured with an item on the Menopause and Reproductive Questionnaire symptom checklist (difficulty concentrating). (21)
Spiritual Well-being
-
o)
Religious/spiritual beliefs were measured with the System of Beliefs Inventory, a 15-item scale that measured spiritual beliefs. (38) The 2 subscales, spiritual beliefs practices and community social support, as well as total score, were assessed.
Economic Well-being
Medical Information/Demographics
-
q)
The medical file in the CALGB 8541 database provided demographics and the following information: date of study entry, treatment arm, menopausal status, number of positive nodes at diagnosis, tumor size, histological grade, estrogen receptor status, and performance status.
Health Behaviors
-
r)
The Breast Cancer Survivor Health Questionnaire obtained information on health habits (smoking, alcohol use, eating habits). This survey was designed for this study, based on literature on breast cancer survival indicating the importance of assessing these items.
Outcomes
-
s)
Overall or global QOL, the primary outcome in this study, was measured with Cantril’s quality of life ladder, which states, “Overall, how would you rate your overall quality of life?” using a scale of 0 to 10 (0=worst possible, 10=best possible). (39) The 8 SF-36 subscales, as previously described, were also assessed for descriptive purposes. (19, 20)
5. Analysis
Statistical analyses were performed by statisticians at the CALGB Statistical Center (JEH and JMD). Descriptive statistics were used to characterize the physical, psychosocial, spiritual and economic problems of survivors, as well as the SF-36 subscales. Frequency distributions were calculated to describe the prevalence of problems such as menopause, self-reported osteoporosis, lymphedema, pain, cosmesis, lack of insurance, depression, lack of social support, fear of recurrence, and problems with employment and income.
A two-step procedure was used to create a model that examined the effects of specific factors on low global HRQL, defined as a score of less than 8 on a 10-point Likert scale for the ladder, “Overall, how would you rate your quality of life?” A score of 8 was chosen due to non-normality of the item and because it appeared to be a natural cutpoint upon examining the distribution of the item. The first step involved the examination of predictors, which consisted of all items collected within each of 6 domains, as defined by the conceptual framework (see Table 1). Univariate logistic regression analyses were used to screen candidate variables within each domain. Those predictors having a univariate p-value ≤ 0.25 and lacking multicollinearity with other possible predictors (and not having large amounts of missing data) were incorporated into multivariate logistic regression analyses using backwards elimination. The following items were considered in domain-specific multivariate analyses: physical well-being (total symptom subscale, underarm/chest pain (yes/no), underarm/chest pain rating (1–10), heart disease-yes/no, health interference score—1 or 2 or more interfering symptoms, vitality subscale), social well-being (MOS total score, sexual feeling, sexual satisfaction, life events subscale), psychological well-being (depression (defined as CES-D≥16)-yes/no, total cognitive distress, general appearance feelings, difficulty concentrating-yes/no), economic well-being (employment problems) and demographic factors (race-white vs. other). No variables qualified for the model in the spiritual domain. For the second step of the modeling process, predictors that were statistically significant (p<0.10) in the domain-specific reduced models were included in an overall model incorporating predictors from all domains. Exact 95% confidence intervals were generated for odds ratios. Analyses were performed using SAS version 8.2 (Cary, NC, 2002).
RESULTS
Demographics/Treatment Characteristics
Table 2 displays the demographic characteristics of participants. At the time of interview, 59% of women were at least 60 years old and 94% were white. Seventy-seven percent of women had received a mastectomy and just under one-fourth had received radiation therapy.
Table 2.
Demographics of Study Participants**
| Demographic Total # of Patients |
Participants n = 245 | |
|---|---|---|
| n | % | |
| Age (years)+ | ||
| 30–39 | 3 | 1 |
| 40–49 | 20 | 8 |
| 50–59 | 78 | 32 |
| 60–69 | 92 | 38 |
| 70+ | 52 | 21 |
| Mean (SD) | 62.0(9.8) | |
|
| ||
| Race | ||
| White | 229 | 94 |
| Other | 16 | 7 |
|
| ||
| Education | ||
| 0–12 years | 122 | 55 |
| 13–16 years | 84 | 38 |
| 17–20 years | 25 | 11 |
|
| ||
| Income | ||
| Under $10,000 | 12 | 6 |
| $10,000–$19,999 | 30 | 14 |
| $20,000–$29,999 | 30 | 14 |
| $30,000–$44,999 | 38 | 18 |
| $45,000–$59,999 | 26 | 13 |
| $60,000–$79,000 | 27 | 13 |
| $80,000+ | 45 | 22 |
|
| ||
| Type of Treatment | ||
| Mastectomy | 192 | 78 |
| Breast Conservation | 53 | 22 |
|
| ||
| Estrogen Receptor Status | ||
| Negative | 76 | 31.0 |
| Positive | 159 | 64.9 |
| Borderline | 6 | 2.5 |
| Radiation Therapy | ||
| No | 187 | 77.0 |
| Yes | 56 | 23.1 |
Note: Frequencies within education, income and estrogen receptor status columns may not sum to column total due to missing data
Age at the time of interview
Domain-specific impact on HRQL
Responses to the global HRQL question ranged from 3 to 10. Twenty-nine percent of responses were below 8, which was the cutoff for lower HRQL. The overall HRQL mean scores for the SF-36 subscales are shown in Table 3. The mean scores ranged from 60.68 for vitality to 85.35 for social functioning. Physical functioning, mental health and vitality were below the corresponding population norms while role functioning (physical and emotional), social functioning and bodily pain were above the population norms.
Table 3.
SF-36 Subscale Descriptive Statistics
| MOS SF-36 Subscale | Study Participants n=245 | General U.S. Population* n=2472 |
|---|---|---|
| Mean(SD) | Mean(SD) | |
| Physical Functioning | 76.61 (28.62) | 84.15 (23.28) |
| Role Functioning-Physical | 82.31 (22.91) | 80.96 (34.00) |
| Role Functioning-Emotional | 83.74 (32.21) | 75.15 (23.69) |
| Social Functioning | 85.35 (24.33) | 71.95 (20.34) |
| Bodily Pain | 74.48 (23.96) | 60.86 (20.96) |
| Mental Health | 76.75 (16.59) | 83.28 (22.69) |
| Vitality | 60.68 (21.04) | 81.26 (33.04) |
| General Health Perceptions | 72.65 (20.21) | 74.74 (18.05) |
Normative data from the general U.S. population (19)
Using the variables listed in Table 1, multivariate analyses within each domain first identified variables related to global HRQL for that domain (Table 4). For the social well-being domain, lower social support was associated with lower reported global HRQL (OR=1.04; 95%CI=1.03,1.06). For physical well-being, reduced energy (OR=1.05; 95%CI=1.03,1.08), a history of heart disease (OR=4.11; 95%CI=1.14,14.86) and presence of co-morbidities were related to poorer global HRQL. Survivors with 1 comorbidity were 2 times more likely (OR=2.12;95%CI=0.86,5.24) to have poorer global HRQL than patients without a comorbidity and, likewise, patients with 2 or more comorbidities were 4 times more likely (OR=4.02; 95%CI=1.69,9.55) to suffer from poorer global HRQL. Within the psychological well-being domain, depression (OR=5.93; 95%CI=2.93,12.0) and negative thoughts of general appearance (OR 1.07; 95%CI=1.03,1.12) were related to poorer global HRQL. No variables in the spiritual or economic domains were significantly related to overall HRQL.
Table 4.
Variables Related to Health Quality Of Life in Multivariate Regression Models, within and across domains
| WITHIN EACH DOMAIN | Odds Ratio | 95% Confidence Limits |
|---|---|---|
|
| ||
| SOCIAL WELL-BEING | ||
| Decreased Social Support | 1.04 | (1.03,1.06) |
|
| ||
| PHYSICAL WELL-BEING | ||
| SF-36 Vitality (Decreased Energy) | 1.05 | (1.03,1.08) |
| Heart Disease | 4.11 | (1.14,14.86) |
| 1 Comorbidity* | 2.12 | (0.86,5.24) |
| 2+ Comorbidities* | 4.02 | (1.69,9.55) |
|
| ||
| PSYCHOLOGICAL WELL-BEING | ||
| Depression | 5.93 | (2.93,12.0) |
| Negative Thoughts of General Appearance | 1.07 | (1.03,1.12) |
|
| ||
| ACROSS ALL DOMAINS | ||
|
| ||
| Decreased Social Support | 1.03 | (1.02,1.05) |
|
| ||
| Heart Disease | 5.01 | (1.39,18.1) |
|
| ||
| SF-36 Vitality (Decreased Energy) | 1.05 | (1.03,1.07) |
|
| ||
| 2+ Comorbidities* | 2.39 | (1.10,5.19) |
Comorbidities included (interfered with daily life): Other cancers or leukemia, arthritis or rheumatism, or other connective tissue disorder, glaucoma, emphysema or chronic bronchitis, high blood pressure, heart disease, circulation problems in legs/arms, diabetes, stomach or intestinal disorders, osteoporosis, chronic liver or kidney disease, stroke, and depression
Across Domain Impact On HRQL
In the second step of the modeling process, predictors that were statistically significant (p<0.10) in the domain-specific reduced models (underarm/chest pain rating (1–10), heart disease (yes/no), health interference scores (1 or 2 or more interfering systems), vitality subscale, MOS total score, CES-D≥16 (yes/no), general appearance feelings, race) were included in an overall model incorporating predictors from all domains. This analysis identified four variables that significantly impacted HRQL (Table 4). Decreased social support (OR=1.03; 95%CI=1.02,1.05), heart disease (OR=5.01; 95%CI=1.39,18.1), decreased energy (OR=1.05; 95%CI=1.03,1.07) and having 2 or more co-morbidities (besides heart disease) that interfered with activities (OR=2.39; 95%CI=1.10,5.19) were all associated with poorer global HRQL.
Prevalence of Problems/Behaviors
Table 5 lists the reported prevalence of problems and healthy behaviors. Many women reported engaging in health-promoting behaviors since their cancer diagnosis, such as changing eating habits (53%) and increasing exercise (31%). Fifty-two out of 245 women (22%) reported making changes in smoking habits, with 36 of the 52 women (69%) reporting that they stopped smoking, 13 women (25%) reduced the number of cigarettes smoked, and 3 women increased their smoking.
Table 5.
Prevalence of Healthy Behaviors and Problems Among Study Participants
| Healthy Behaviors | Participants n = 245 | |
|---|---|---|
| n | % | |
|
| ||
| Taking Prescription Meds | 130 | 53 |
|
| ||
| Taking Vitamins | 177 | 72 |
|
| ||
| Taking Herbs | 55 | 22 |
|
| ||
| Taking Supplements | 32 | 13 |
|
| ||
| Exercise Increased | 77 | 31 |
|
| ||
| Changed Eating Habits | 131 | 53 |
|
| ||
| Stopped or Reduced Smoking | 49 | 20 |
|
| ||
| Problems | ||
|
| ||
| Lymphedema | 57 | 23 |
|
| ||
| Fatigue | 52 | 21 |
|
| ||
| Pain in Arm/Hand | 54 | 22 |
|
| ||
| Pain in Underarm/Chest* | 52 | 22 |
|
| ||
| Osteoporosis | 61 | 25 |
|
| ||
| Menopausal Symptoms | 145 | 59 |
|
| ||
| Depression | 51 | 21 |
Note: Missing data was trivial (would not change %) except where noted (n=237)
Over half of participants reported taking prescription medications. The most prevalent problems reported were menopausal symptoms (59%), which included hot flashes and vaginal dryness, followed by self-reported osteoporosis (24%), lymphedema (23%), arm/hand pain (22%), underarm/chest pain (22%), fatigue (21%), and depression (21%), defined as ≥16 on the CES-D.
Additional osteoporosis analyses revealed that forty-one women (17%) reported ever having a fracture of the shoulder (n=6), wrist (n=26), hip (n=5) or spine (n=9). Sixty-one patients (25%) reported being told by their physicians that they had osteoporosis. Of these, 26 (43%) women reported taking some form of prescription medication, with 9 (35%) receiving only hormonal therapy (such as Raloxifene, Tamoxifen, or Estrogen pills/patch or vaginal cream), 14 (54%) taking only bisphosphonates and 3 (12%) receiving both.
DISCUSSION
One of the primary goals of this study was to develop a predictive model to identify factors related to global HRQL, based on a conceptual model by Dow, Ferrell and colleagues.(9, 14) While factors in the physical, psychological and social domains were significant in the within-domain analyses, only physical and social factors were significantly related to global HRQL. Spiritual and economic factors were not significant in any of the models. Physical factors that were significantly related to global HRQL included having heart disease, fatigue and one or 2 or more comobidities. In the social well-being domain, higher social support was significantly related to better global HRQL.
Several studies support our findings on the influence of social support on HRQL. Social support has been shown to influence HRQL (40–42) by independently influencing adjustment to life events such as cancer rather than buffering against stressful life events. (42) Other studies of breast cancer patients observed that affective social support influenced optimism and distress, (40) and that older breast cancer survivors (over age 55) had more positive interpersonal relationships than younger survivors. (41)
Although heart disease was prevalent only among a small number of participants (N=17), it was significantly related to poorer HRQL in this study. Heart disease among survivors may be due to cardiotoxicity as a result of breast cancer treatment, (i.e., chemotherapy and ionizing radiation or other types of heart disease) or some other treatment-related factor. (43) Since patients in CALGB 8541 received different doses of doxorubicin and other chemotherapeutic agents and a subset of patients (15%) received radiotherapy, treatment-related heart disease may have occurred.
The presence of symptoms and co-morbidities have long been associated with poorer HRQL. Fatigue, which was significantly related to poorer HRQL in this study, has often been reported in women with breast cancer, (44–47) but is only now being reported as a significant problem among long-term survivors. (48) The prevalence of lymphedema, a treatment-related side effect that often becomes a common comorbidity among survivors, was reported by 23% of women in this study and has also been associated with lower HRQL. Similar results were found by Kornblith et al., (13) suggesting that lymphedema is a distressing complication associated with poorer HRQL for many years following cancer treatment.
Osteoporosis was another common comorbidity reported by 24% of study participants. Tamoxifen, used to decrease the risk of breast cancer recurrence, particularly in women with estrogen receptor (ER) positive tumors, appears to have some protective effect on bone mineral density in post-menopausal patients,(49, 50) but may cause loss of bone density in premenopausal women.(51) A direct correlation has been reported between risk of postmenopausal breast cancer and bone mineral density.(52) Nevertheless, a high risk of osteoporosis in breast cancer survivors is likely related to an increased incidence of chemotherapy-induced menopause with higher chemotherapy doses.(53) Since breast cancer survivors are usually seen regularly by physicians, the opportunity exists for osteoporosis screening and treatment to help prevent fractures.
Psychological factors were not significantly related to global HRQL in this study. Others have found, however, that while survivors are well-adjusted overall, 30% experienced depression and anxiety four years post-treatment, as well as problems such as distress regarding body image, fear of recurrence and sexual problems. (54, 55)
Secondary goals of this study were to assess health problems and healthy behaviors of long-term breast cancer survivors. Overall, study participants had few serious health problems and made healthy changes regarding diet, exercise and smoking. These results are similar to those of Stewart et al., who found that women who had survived cancer for 5 years or longer held the strongest beliefs that a healthy lifestyle helped prevent cancer recurrence.(56) These women were more likely to make positive changes in their lifestyle, such as eating healthier and exercising regularly, which may have enhanced their personal sense of control and overall sense of well-being.
Physical symptoms, especially hot flashes and vaginal dryness were the most prevalent physical problems reported by women. Studies have shown that menopausal symptoms, such as sleep disorders, hot flashes, sweats, headaches, and vaginal dryness can have a negative effect on women’s sexuality and overall well-being.(57–60) Symptoms may more severe in breast cancer survivors if menopause occurs as a result of breast cancer treatment.(61, 62)
There are several strengths to the current study. Data presented here are from a quality of life and lifestyle follow-up study of participants in CALGB 8541. This study provides a unique perspective on the quality of life of long-term breast cancer survivors for several reasons. First, this study examines survivors who were 9–16 years post-diagnosis, which few previous studies have included. Secondly, the women were diagnosed at relatively the same stage of disease and received one of 3 known chemotherapy regimens within a clinical trial, thus reducing variability due to treatment regimen and stage of diagnosis. Many previous studies used heterogeneous populations. Thirdly, in addition to quality of life measures, this study provides information on lifestyle and healthy behaviors of long-term survivors, which have not been included in many past studies. Lastly, the study identifies modifiable factors that contribute to poor quality of life, thus suggesting areas for physicians and survivors to direct interventions to improve quality of life in this population.
Limitations include reliance on self-report of co-morbidities, such as heart disease, lymphedema and osteoporosis. Information on temporal changes in HRQL was not possible because HRQL was examined at only one time point. Thus, future studies should collect serial measures of HRQL at pre-specified intervals after treatment has concluded. Also, a response bias may have occurred in that only patients with better HRQL may have chosen to participate in the follow-up study. This emphasizes the need for more longitudinal studies of breast cancer survivors from treatment through follow-up.
In conclusion, the modeling performed in this study provides an important framework in which to view the health status of long-term breast cancer survivors. Although survivors made positive lifestyle changes as a result of their diagnosis and treatment, physical symptoms, such as heart disease and other comorbidities, fatigue, and social support, were all identified as potential areas of intervention to improve the HRQL of long-term survivors. Interventions such as education about ways to detect and manage problems, such as lymphedema and cardiotoxicity, in order to minimize their severity could promote a better overall quality of life among long-term survivors.
References
- 1.Smigel K. Breast cancer death rates decline for white women. J Natl Cancer Inst. 1995;87(3):173. doi: 10.1093/jnci/87.3.173. [DOI] [PubMed] [Google Scholar]
- 2.Nasseri K. Secular trends in the incidence of female breast cancer in the United States, 1973–1998. Breast J. 2004;10:129–35. doi: 10.1111/j.1075-122x.2004.21276.x. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, et al. Cancer statistics, 2006 CA Cancer J Clin. 2006;56(2):106–30. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 4.Partridge AH, Winer EP, Burstein HJ. Follow-up care of breast cancer survivors. Semin Oncol. 2003;30:817–25. doi: 10.1053/j.seminoncol.2003.08.026. [DOI] [PubMed] [Google Scholar]
- 5.Partridge AH, Winer EP. Long-term complications of adjuvant chemotherapy for early stage breast cancer. Breast Dis. 2004;21:55–64. doi: 10.3233/bd-2004-21108. [DOI] [PubMed] [Google Scholar]
- 6.Polinsky ML. Functional status of long-term breast cancer survivors: demonstrating chronicity. Health Soc Work. 1994;19(3):165–73. doi: 10.1093/hsw/19.3.165. [DOI] [PubMed] [Google Scholar]
- 7.Wyatt G, Kurtz ME, Liken M. Breast cancer survivors: An exploration of quality of life issues. Cancer Nurs. 1993;16:440–48. [PubMed] [Google Scholar]
- 8.Dorval M, Maunsell E, Deschenes L, Brisson J, Masse B. Long-term quality of life after breast cancer: comparison of 8-year survivors with population controls. J Clin Oncol. 1998;16:487–94. doi: 10.1200/JCO.1998.16.2.487. [DOI] [PubMed] [Google Scholar]
- 9.Dow KH, Ferrell BR, Leigh S, Ly J, Gulasekaram P. An evaluation of the quality of life among long-term survivors of breast cancer. Breast Cancer Res Treat. 1996;39(3):261–273. doi: 10.1007/BF01806154. [DOI] [PubMed] [Google Scholar]
- 10.Ganz PA, Desmond KA, Leedham B, Rowland JH, Meyerowitz BE, Belin TR. Quality of life in long-term disease-free survivors of breast cancer: a follow-up study. J Natl Cancer Inst. 2002;94:39–49. doi: 10.1093/jnci/94.1.39. [DOI] [PubMed] [Google Scholar]
- 11.Ashing-Giwa K, Ganz PA, Petersen L. Quality of life of African-American and white long term breast carcinoma survivors. Cancer. 1999;85:418–26. doi: 10.1002/(sici)1097-0142(19990115)85:2<418::aid-cncr20>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 12.Casso D, Buist DS, Taplin S. Quality of life of 5–10 year breast cancer survivors diagnosed between age 40 and 49. Health Qual Life Outcomes. 2004;2:25. doi: 10.1186/1477-7525-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kornblith AB, Herndon JE, Weiss RB, Zhang C, Zuckerman EL, Rosenberg S, et al. Long-term adjustment of survivors of early-stage breast carcinoma 20 years after adjuvant chemotherapy. Cancer. 2003;98(4):679–689. doi: 10.1002/cncr.11531. [DOI] [PubMed] [Google Scholar]
- 14.Ferrell BR, Dow KH, Grant M. Measurement of the quality of life in cancer survivors. Qual Life Res. 1995;4:523–31. doi: 10.1007/BF00634747. [DOI] [PubMed] [Google Scholar]
- 15.Wood WC, Budman DR, Korzun AH, Cooper MR, Younger J, Hart RD, et al. Dose and dose intensity of adjuvant chemotherapy for stage II, node-positive breast carcinoma. N Engl J Med. 1994;330(18):1253–59. doi: 10.1056/NEJM199405053301801. [DOI] [PubMed] [Google Scholar]
- 16.Budman D, Berry DA, Cirrincione CT, Henderson IC, Wood WC, Weiss RB, et al. Dose and dose intensity as determinants of outcome in the adjuvant treatment of breast cancer. J Natl Cancer Inst. 1998;90(16):1205–11. doi: 10.1093/jnci/90.16.1205. [DOI] [PubMed] [Google Scholar]
- 17.Dow KH, Ferrell BR, Haberman MR, Eaton L. The meaning of quality of life in cancer survivorship. OncoI Nurs Forum. 1999;26:519–28. [PubMed] [Google Scholar]
- 18.Ersek M, Ferrell BR, Dow KH, Melancon CH. Quality of life in women with ovarian cancer. West J Nurs Res. 1997;19:334–50. doi: 10.1177/019394599701900305. [DOI] [PubMed] [Google Scholar]
- 19.Ware JE, Snow KK, Kosinski M, Gandek B. SF-36 Health Survey Manual and Interpretation Guide. Boston, MA: New England Medical Center, The Health Institute; 1993. [Google Scholar]
- 20.Stewart AL, Ware J. Measure of Functioning and Well-Being: The Medical Outcomes Study Approach. Durham, NC: Duke University Press; 1992. [Google Scholar]
- 21.Bauer SM, Hseih SF, Hseih CH, Lundquist D, Terrill I, Habin K, et al. Treatment-related menopause in female cancer survivors: Prevalence and quality of life issues [Abstract 102]. Proceedings of the 6th Annual Cancer Nursing Research Conference, and Oncology Nursing Forum; 2001. [Google Scholar]
- 22.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Applied Psychol Measurement. 1977;1:385–401. [Google Scholar]
- 23.Andersen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale) Am J Prev Med. 1994;10:77–84. [PubMed] [Google Scholar]
- 24.George LK, Fillenbaum G. OARS methodology: A decade of experience in geriatric assessment. J Am Geriatrics Soc. 1985;33:607–15. doi: 10.1111/j.1532-5415.1985.tb06317.x. [DOI] [PubMed] [Google Scholar]
- 25.Paskett E. Lymphedema: knowledge, treatment and impact among breast cancer survivors. Breast Journal. 1999;6(6):373–8. doi: 10.1046/j.1524-4741.2000.99072.x. [DOI] [PubMed] [Google Scholar]
- 26.Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32(6):705–714. doi: 10.1016/0277-9536(91)90150-b. [DOI] [PubMed] [Google Scholar]
- 27.Anderson D, Bilodeau B, Deshaies G, Gilbert M, Jobin J. French-Canadian validation of the MOS Social Support Survey. Can J Cardiol. 2005;21:867–73. [PubMed] [Google Scholar]
- 28.Watts RJ. Sexual functioning, health beliefs and compliance with high blood pressure medications. Nurs Res. 1982;31:278–83. [PubMed] [Google Scholar]
- 29.Holland JC, Herndon J, Kornblith AB, Cella DF, Cooper MR, Green M, et al. A sociodemographic data collection model for cooperative clinical trials. Proc ASCO. 1992;445:157. [Google Scholar]
- 30.Greenberg DB, Kornblith AB, Herndon JE, Zuckerman E, Schiffer CA, Weiss RB, et al. Quality of life of adult leukemia survivors treated on clinical trials of the Cancer and Leukemia Group B from 1971–1988: Predictors for later psychological distress. Cancer. 1997;80:1936–44. doi: 10.1002/(sici)1097-0142(19971115)80:10<1936::aid-cncr10>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 31.Kornblith AB, Anderson J, Cella DF, Tross S, Zuckerman E, Cherin E, et al. Hodgkin’s disease survivors at increased risk for problems in psychosocial adaptation. Cancer. 1992;70:2214–24. doi: 10.1002/1097-0142(19921015)70:8<2214::aid-cncr2820700833>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 32.Kash KM, Jacobsen PB, Holland JC, Osborne MP, Miller DG. Measuring breast cancer anxiety. Proceedings of the 42nd annual meeting of the Academy of Psychosomatic Medicine; November 1995; Palm Springs, CA. p. 13. [Google Scholar]
- 33.Taylor JA. A personality scale of manifest anxiety. J Abnorm Psychol. 1953;48:285–290. doi: 10.1037/h0056264. [DOI] [PubMed] [Google Scholar]
- 34.Spielberger CD, Gorsuch RL, Lushene RD. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1970. [Google Scholar]
- 35.Lerman C, Trock BBR. Psychological Side Effects of Breast Cancer Screening. Health Psychology. 1991;10(4):259–267. doi: 10.1037//0278-6133.10.4.259. [DOI] [PubMed] [Google Scholar]
- 36.Lerman C, Kash KM, Stefanick ML. Younger women at increased risk for breast cancer: perceived risk, psychological well-being, and surveillance behavior. J Natl Cancer Inst Monogr. 1994;16:171–6. [PubMed] [Google Scholar]
- 37.Polivy J. Psychological effects of mastectomy on a woman’s feminine self-concept. J Nerv Ment Dis. 1977;164(2):77–87. doi: 10.1097/00005053-197702000-00001. [DOI] [PubMed] [Google Scholar]
- 38.Holland JC, Kash KM, Passik S, Gronert MK, AS A brief spiritual beliefs inventory for use in quality of life research in life-threatening illnesses. Psycho-Oncology. 1998;7:460–69. doi: 10.1002/(SICI)1099-1611(199811/12)7:6<460::AID-PON328>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 39.Cantril H. The Patterns of Human Concerns. New Brunswick, New Jersey: Rutgers University Press; 1965. [Google Scholar]
- 40.Trunzo J, Pinto B. Social support as a mediator of optimism and distress in breast cancer survivors. J Consult Clin Psychol. 2003;71(4):805–811. doi: 10.1037/0022-006x.71.4.805. [DOI] [PubMed] [Google Scholar]
- 41.Stava CJ, Lopez A, Vassilopoulou-Sellin R. Health profiles of younger and older breast cancer survivors. Cancer. 2006;107:1752–9. doi: 10.1002/cncr.22200. [DOI] [PubMed] [Google Scholar]
- 42.Kornblith AB, Herndon JE, Zuckerman E, Viscoli CM, Horwitz RI, Cooper MR, et al. Social support as a buffer to the psychological impact of stressful life events in women with breast cancer. Cancer. 2001;91:443–54. doi: 10.1002/1097-0142(20010115)91:2<443::aid-cncr1020>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 43.Eltringham JR. Cardiac response to combined modality therapy. Front Radiat Ther Oncol. 1979;t3:161–174. [Google Scholar]
- 44.Bower JE, Ganz PA, Desmond KA, Bernaards C, Rowland JH, Meyerowitz BE, et al. Fatigue in long-term breast carcinoma survivors: a longitudinal investigation. Cancer. 2006;106:751–8. doi: 10.1002/cncr.21671. [DOI] [PubMed] [Google Scholar]
- 45.Blesch KS, Paice JA, Wickham R, Harte N, Schnoor DK, Purl S, et al. Correlates of fatigue in people with breast or lung cancer. Oncol Nurs Forum. 1991;18:81–87. [PubMed] [Google Scholar]
- 46.Mock V, Burke MB, Sheehan P, Creaton EM, Winningham ML, McKenney-Tedder S, et al. A nursing rehabilitation program for women receiving adjuvant chemotherapy for breast cancer. Oncol Nurs Forum. 1994;21:899–907. [PubMed] [Google Scholar]
- 47.Winningham M, MacVicar M, Bondoc M, Anderson JI, Minton JP. Effect of aerobic exercise on body weight and composition in patients with breast cancer on adjuvant chemotherapy. Oncol Nurs Forum. 1989;16:683–89. [PubMed] [Google Scholar]
- 48.Broeckel JA, Thors CL, Jacobsen PB, Small M, Cox CE. Sexual functioning in long-term breast cancer survivors treated with adjuvant chemotherapy. Breast Cancer Res Treat. 2002;75:241–8. doi: 10.1023/a:1019953027596. [DOI] [PubMed] [Google Scholar]
- 49.Love RR, Mazess RB, Barden HS, Epstein S, Newcomb PA, Jordan VC, et al. Effects of tamoxifen on bone mineral density in postmenopausal women with breast cancer. N Engl J Med. 1992;326:852–56. doi: 10.1056/NEJM199203263261302. [DOI] [PubMed] [Google Scholar]
- 50.Kristensen B, Ejlersten B, Dagaard P, Larsen L, Homegaard SN, Trasnbol I, et al. Tamoxifen and bone metabolism in postmenopausal low-risk breast cancer patients: a randomized study. J Clin OncoI. 1994;12:992–997. doi: 10.1200/JCO.1994.12.5.992. [DOI] [PubMed] [Google Scholar]
- 51.Powles TJ, Hickish T, Kanis JL, Tidy A, Ashley S. Effect of tamoxifen on bone mineral density measured by dual-energy x-ray absorptiometry in healthy premenopausal and postmenopausal women. J Clin OncoI. 1996;14:78–84. doi: 10.1200/JCO.1996.14.1.78. [DOI] [PubMed] [Google Scholar]
- 52.Nelson RL, Turyk M, Kim J, Persky V. Bone mineral density and the subsequent risk of cancer in the NHANES I follow-up cohort. BMC Cancer. 2002;2:22. doi: 10.1186/1471-2407-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kanis JA, McCloskey EV, Powles T, Paterson AHG, Ashley S, Spector T. A high incidence of vertebral fracture in women with breast cancer. Brit J Cancer. 1999;79:1179–81. doi: 10.1038/sj.bjc.6690188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kornblith AB, Ligibel J. Psychosocial and sexual functioning of survivors of breast cancer. Review. Semin Oncol. 2003;30:799–813. doi: 10.1053/j.seminoncol.2003.08.025. [DOI] [PubMed] [Google Scholar]
- 55.Deimling GT, Bowman KF, Sterns S, Wagner LJ, Kahana B. Cancer-related health worries and psychological distress among older adult, long-term cancer survivors. Psychooncology. 2006;15:306–20. doi: 10.1002/pon.955. [DOI] [PubMed] [Google Scholar]
- 56.Stewart DE, Cheng AM, Duff S, Wong F, McQuestion M, Cheung T, et al. Attributions of cause and recurrence in long-term breast cancer survivors. Psycho-oncology. 2001;10:179–183. doi: 10.1002/pon.497. [DOI] [PubMed] [Google Scholar]
- 57.Sutton R, Buzdar AU, Hortobagyi GN. Pregnancy and offspring after adjuvant chemotherapy in breast cancer patients. Cancer. 1990;65:847–850. doi: 10.1002/1097-0142(19900215)65:4<847::aid-cncr2820650402>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 58.Blume E. Sex after chemo: a neglected issue. J Natl Cancer Inst. 1993;85:768–70. doi: 10.1093/jnci/85.10.768. [DOI] [PubMed] [Google Scholar]
- 59.Dupont WD, Page DL. Menopausal estrogen replacement therapy and breast cancer. Arch Int Med. 1991;67–72:151. [PubMed] [Google Scholar]
- 60.Nagamani M, Kelver ME, Smith ER. Treatment of menopausal hot flashes with transdermal administration of clonidine. Am J Obstet Gynecol. 1987;156:561–65. doi: 10.1016/0002-9378(87)90050-0. [DOI] [PubMed] [Google Scholar]
- 61.Crandall C, Peterson L, Ganz PA, Greendale GA. Association of breast cancer and its therapy with menopause related symptoms. Menopause. 2004;11(5):519–30. doi: 10.1097/01.gme.0000117061.40493.ab. [DOI] [PubMed] [Google Scholar]
- 62.Carpenter JS, Elam JL, Ridner SH, Carney PH, Cherry GJ, Cucullu HL. Sleep, fatigue, and depressive symptoms in breast cancer survivors and matched healthy women experiencing hot flashes. Oncol Nurs Forum. 2004;31(3):591–598. doi: 10.1188/04.onf.591-598. [DOI] [PubMed] [Google Scholar]