Abstract
Connective tissue disorders (CTD), which are often also termed collagen vascular diseases, include a number of related inflammatory conditions. Some of these diseases include rheumatoid arthritis, systemic lupus erythematosus, systemic sclerosis (scleroderma), localized scleroderma (morphea variants localized to the skin), Sjogren’s syndrome, dermatomyositis, polymyositis, and mixed connective tissue disease. In addition to the systemic manifestations of these diseases, there are a number of cutaneous features that make these conditions recognizable on physical exam. Lower extremity ulcers and digital ulcers are an infrequent but disabling complication of long-standing connective tissue disease. The exact frequency with which these ulcers occur is not known, and the cause of the ulcerations is often multifactorial. Moreover, a challenging component of CTD ulcerations is that there are still no established guidelines for their diagnosis and treatment. The morbidity associated with these ulcerations and their underlying conditions is very substantial. Indeed, these less common but intractable ulcers represent a major medical and economic problem for patients, physicians and nurses, and even well organized multidisciplinary wound healing centers.
Keywords: Ulcers, Connective tissue disease, vasculitis, connective tissue ulcers
1. Introduction
Connective tissue disorders (CTD), which are often also termed collagen vascular diseases, (will go forward referring to them as connective tissue disorders (CTD)), include a number of related inflammatory conditions. Some of these diseases include rheumatoid arthritis, systemic lupus erythematosus, systemic sclerosis (scleroderma), localized scleroderma (morphea variants localized to the skin), Sjogren’s syndrome, dermatomyositis, polymyositis, and mixed connective tissue disease. The various CTD are distinct entities but they have features that are common, notably they share autoantibodies, but each disease also has their own specific autoantibody (Table 1). Despite the hallmark physical exam findings that lead clinicians to a diagnosis or narrow differential diagnosis (Table 2), one cannot make a diagnosis of a CTD based on the presence of ulceration alone. The ulcerations that occur in CTD are late-onset and due to a variety of factors, most notably inflammatory vasculitis and thrombotic nonvasculitic causes.1 Vasculitides are not only associated with autoimmune forms of CTD but they themselves can be classified as a CTD.
Table 1.
Serum markers associated with Connective Tissue Disorders
| Serum markers | |
|---|---|
|
| |
| Rheumatoid Arthritis | Rheumatoid factor, anti-CCP (cyclic citrullinated peptide) |
|
| |
| Scleroderma (localized) | Anti-ssDNA, peripheral blood eosinophilia, anti-histone |
|
| |
| Scleroderma (generalized) | Anti-Scl-70, anti-centromere |
|
| |
| Systemic lupus erythematosus | ANA, Anti-dsDNA, anti-Smith, Anti-histone (drug induced SLE) |
|
| |
| Sjogren’s Syndrome | Anti-SSB(anti-La), Anti-SS-A (anti-Ro) |
|
| |
| Dermatomyositis | CPK, aldolase, Anti-Jo1 |
|
| |
| Mixed Connective tissue | Anti-nRNP |
Table 2.
Distinctive clues observed away from the ulcer location
| Clinical clues | Conditions |
|---|---|
|
| |
| Hard fingers, face | Scleroderma |
|
| |
| Facial butterfly rash | Lupus erythematosus |
|
| |
| Muscle weakness, eyelid rash | Dermatomyositis |
|
| |
| Ear redness with sparing of ear lobe | Relapsing polychondritis |
|
| |
| Livedo reticularis of microlivedo | Occlusion of small blood vessels, from vasculitis, coagulopathy, cryoglobulin, cryofibrinogen |
|
| |
| Rheumatoid hands | Rheumatoid ulcers |
|
| |
| Swelling of face, posterior neck | Cushingoid appearance (steroids) |
When patients present with an ulceration, it becomes imperative to establish the primary cause of the ulceration. Cutaneous ulcers of CTD often have unusual shapes and can be mistakenly thought of as factitial (Fig 1a).2 This is why a complete history and physical exam including extensive review of systems, family history, and current and relevant past medications is imperative for diagnosis and management.3 Having a CTD does not mean that a patient cannot have other concomitant vascular/neuropathic insufficiency like venous or pressure ulcers. This causes confusion because there is overlap. Here we review the main autoimmune connective tissue ulcers, their presentation, pathology, pathogenesis, and treatment and management.
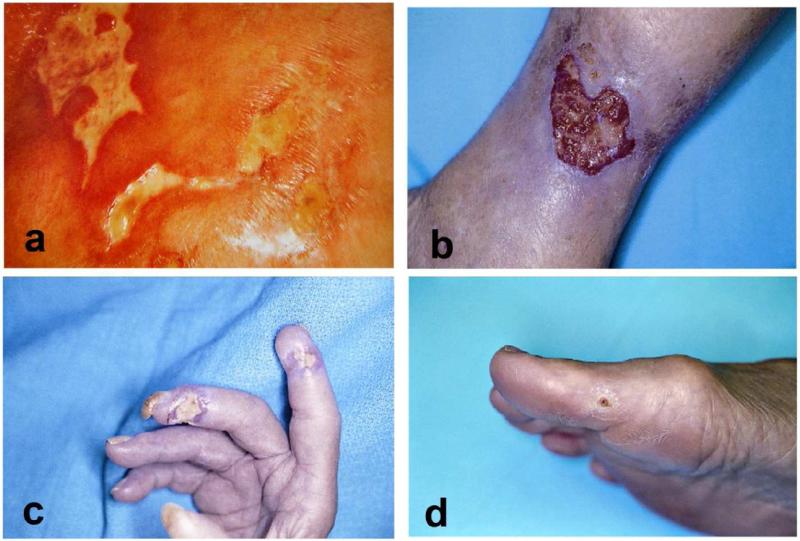
Figure 1. Examples of rheumatoid and systemic sclerosis ulcers.
A: “angular” ulcers-the unusual irregular and “angular” appearance may at first suggest factitial disease, however imaged shows a 56-year-old woman with CREST syndrome. B: rheumatoid ulcer with an angulating configuration or undulating border. C: Digital ulceration in a patient with systemic sclerosis. Ulcers located distally like the one shown here, are more likely to be the result of significant ischemia. Autoamputation is often the outcome. D: Calcinosis and a painful nonhealing ulcer on the big toe in a patient with systemic sclerosis. Patients with systemic sclerosis commonly develop calcinosis, especially in CREST syndrome.
2. Connective tissue disorders
2.1 Rheumatoid Arthritis
Rheumatoid arthritis (RA) is a chronic, inflammatory autoimmune disorder expressed most commonly as a symmetrical, deforming arthropathy. Physical exam findings include symmetric swelling of the small joints of the hand and feet, ulnar deviation, swan neck, and boutonniere deformity.2 Although RA primarily affects the joints, extraarticular manifestations are frequent, such as rheumatoid nodules and skin ulcerations. The cause of leg ulcerations in RA is multifactorial, including, vasculitis, paraproteinemias, anticardiolipin antibodies, venous insufficiency, toxic effects of medications, superficial ulcerating rheumatoid necrobiosis, pressure ulcers, neuropathic ulcers, and pyoderma gangrenosum.1,2 The ulcers as seen on physical exam have an angular configuration or an undulating border (Fig 1b).2
Venous insufficiency can be a complication of impaired movement of the ankle joint as a result of RA because of poor muscle pump action.1,3 Toxic effects of medications include use of corticosteroids that cause skin atrophy where minor trauma leads to ulceration.2 RA patients can be debilitated from their disease and bedridden making them prone to pressure ulcers.2 Superficial ulcerating rheumatoid necrobiosis (SURN) lesions are bilateral over pretibial areas and are refractory to treatment. They are characterized by yellow-red plaques that ulcerate.4 A well-known cause of ulceration in RA is vasculitis. Vessels of different sizes may be affected. These include small to medium sized muscular arteries, arterioles, and venules. Small to medium size vessels, when involved, can mimic polyarteritis nodosa and can be a severe rheumatoid vasculitis. These patients will require systemic therapy because mortality can be high.5 Milder vasculitic disease also occur in RA patients, where postcapillary venules are affected. These patients present with palpable purpura.6
Workup for the patients should include complete history and thorough physical exam, screening laboratory studies (Table 1) and biopsies. It should be noted that the histological picture does not always lead to a diagnosis and repeated biopsies can be necessary. Treatment is a challenge, but stabilizing the autoimmune disease is imperative. When venous insufficiency is the underlying component of ulceration, compression dressings can be help, and in those cases pinch grafting has been performed and was successful in relieving pain and inducing healing.2,7 Adalimumab with methotrexate (MTX) have shown promise in RA associated ulcers (Table 3).8 Improving wound bed preparation by the application of moisture-retentive dressings has shown to be beneficial.2
Table 3.
Summary of treatments for connective tissue ulcers
| Treatment | |
|---|---|
|
| |
| Rheumatoid arthritis | Pinch grafting Compression stockings when venous insufficiency present Adalimumab 40mg twice a month with Methotrexate 20mg weekly Topical tacrolimus Granulocyte monocyte adsorption |
|
| |
| Scleroderma | Nifedipine Iloprost Warfarin Tretinoin Enzymatic debridement with occlusive dressing |
|
| |
| Systemic lupus erythematosus | With vasculitis- systemic corticosteroids with cytotoxic agents (azathioprine or cyclophosphamide) |
|
| |
| Sjogren’s Syndrome | Immunosuppressive agents |
|
| |
| Dermatomyositis | Cyclophosphamide With Calcinosis – calcium channel blockers or colchicine |
|
| |
| Mixed connective tissue disorder | Depends on etiology of ulcer |
2.2 Systemic sclerosis (scleroderma) and localized scleroderma (morphea variants localized to the skin)
Localized scleroderma (that is localized to the skin), has a number of morphological variants. Generalized morphea, linear scleroderma with a subgroup of en coup de sabre, but there are also variants such as subacute morphea that are difficult to manage. Diagnosis of systemic sclerosis is more likely in the face of Raynaud’s phenomenon and internal organ disease. In addition to induration of the skin, other physical findings in systemic sclerosis are areas of hypo or hyperpigmented skin, a small oral aperture, thinning of the lips, and flat polygonal telangiectasia affecting either or both the lips or palms. Nail fold capillaroscopy shows capillary dropout and tortuous blood vessels.2,3ENREF 9 Patients with systemic sclerosis may have serious and life threatening systemic complications affecting the lungs, GI tract, heart, and kidneys. The systemic complications highlight the need for a multidisciplinary approach.
Tuffanelli and Winkelmann studied a large series of patients with ulcers secondary to systemic sclerosis. They determined that ulcers of systemic sclerosis most frequently occur on the fingertips and on the dorsa of the interphalangeal or metacarpophalangeal joints (Fig 1c). Various parts of the legs were also affected. The ulcers tended to be painful, where slower to heal, and relatively refractory to standard methods of treatment. 9 The ulcerations that occur in scleroderma are the results of very fibrotic skin, vascular compromise, abnormalities in coagulation, and tissue calcium deposition.2 There is a typical indentation (pitting scar) present on the pulp of the finger in many patients with scleroderma, probably due to ischemia and collapse of the underlying tissues.2
In calcinosis cutis (especially in CREST variant of systemic sclerosis) one sees cutaneous deposits of calcium containing material that grows in size. As the skin thins out it becomes more susceptible to breakdown and to the formation of ulcers (Fig 1d).2 Symptoms may resolve once the calcium is spontaneously extruded. Low dose warfarin (in doses not enough to affect coagulation) have been prescribed to decrease the inflammatory component and pain associated with these ulcers.2
Ulcers on the knuckles are frequently secondary to trauma, whereas ulcers on the finger tips are typically the result of ischemia.2 The majority of patients with digital ulcers have Raynaud’s phenomenon. The vascular compromise seen in ulcers due to systemic sclerosis show endarteritis obliterans with intimal proliferation, hence systemic vasodilators are not as effective in Raynaud’s disease (without a CTD) because the latter are more secondary to vascular spasms.1
In systemic sclerosis, the blood vessels are occluded and do not respond to vasodilators. Therefore, however, a trial of systemic vasodilator therapy is worthwhile, in particular calcium channel blockers and sildenafil.2 Treatment (Table 3) can include moisture retentive dressings that lead to pain control and painless debridement of these ulcers. One should always be sure that osteomyelitis or septic arthritis is not present, which may require imaging with MRI. If bone is not involved, enzymatic debridement can be initiated, with care that maceration of the surrounding skin does not occur.2 Nifedipine use has been shown to reduce the number of ulcers compared to baseline, and Iloprost has been shown to be effective in reducing recurrence of new digital ulcers.10,11 Supportive management for the patients is imperative; they should be advised to avoid exposure to cold surfaces and environment and to avoid smoking. In recalcitrant ulcers, the goal should be to keep the patient comfortable and to help facilitate their daily activities.2
2.3 Systemic lupus erythematosus
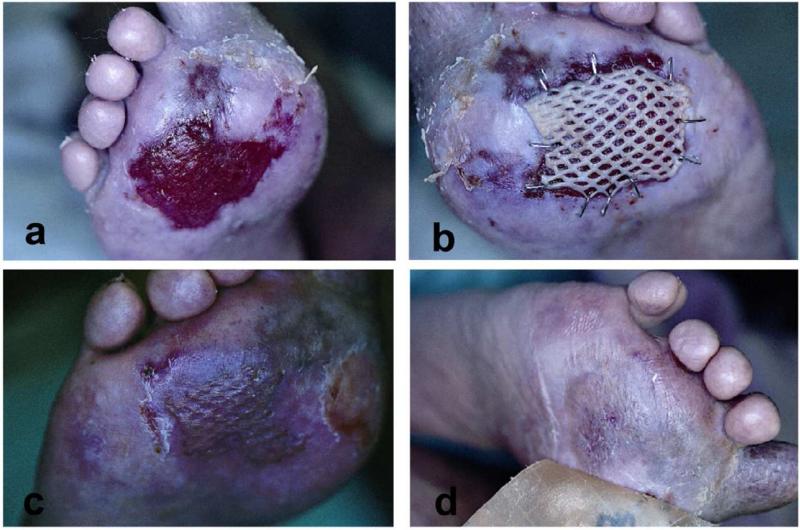
Systemic lupus erythematosus (SLE) is a systemic autoimmune connective tissue disease that can affect most organ systems. The immune system attacks the body’s cells and tissue, resulting in inflammation and tissue damage. SLE most often harms the heart, joints, skin, lungs, blood vessels, liver, kidneys, and nervous system. Ulcerations are not infrequent and like other CTD are multifactorial. Vasculitis, noninflammatory thrombosis of small or large vessels, venous insufficiency, lupus profundus, lichen planus overlap, and drug induced lupus syndrome has been associated with leg ulcerations.1 Most leg ulcers of SLE are located over the malleolar, supramalleolar, or pretibial areas.12 The ulcers are usually painful, sharply marginated, or punched out. Adjacent skin can appear erythematous, purpuric, or rolled and violaceous.1 Lupus panniculitis can mimic factitial ulcers because of their odd location and distribution.2 Lichen planus (LP) typically spares the palms and soles. When lichen planus is present with SLE, LP can present as an inflammatory condition that involves the palms and soles (Fig 2A-D). These LP cases highlight the point that ulcerations in SLE are multifactorial and a biopsy is important for diagnosis and proper management.
Figure 2. Erosive lichen planus/systemic lupus erythematosus overlap.
A: Lichen planus rarely presents on the palms and soles. However, in an overlap of lichen planus and SLE, palms and soles can be involved. B: After failure of occlusive dressings and corticosteroid injections, split-thickness autologous skin grafting was used to stimulate healing. C: 4 weeks after grafting, there is complete healing. Satellite lesions of lichen planus are still visible at the edges however. D: Several weeks after grafting, with the use of hydrocolloid dressings, the affected area is looking more like normal skin.
Histological examination of vaculitic ulcers in SLE shows a leukocytoclastic vasculitis with fibrinoid necrosis of the vessel walls and prominent polymorphonuclear cell infiltration. Thrombocclusive histologic findings can be associated with the presence of antiphopholipid antibodies (lupus anticoagulant).1 Venous insufficiency as a cause of SLE ulcerations may or may not be associated with the presence of antiphospholipid antibodies.13,14
SLE-associated leg ulcers are a therapeutic challenge, as local wound care is not always sufficient. The underlying cause of the ulceration needs to be established and treated. If vasculitis is present, systemic corticosteroids with cytotoxic agents should to be utilized (Table 3).2
2.4 Sjogren’s Syndrome
Sjogren’s syndrome is characterized by a decrease in lacrimal and salivary secretions. The cutaneous manifestations include Raynaud’s phenomenon, dryness of the skin, angular stomatitis, livedo reticularis, episodic purpura of the legs, erythema nodosum, ulcers of the legs.15 Ulcerations of Sjogren’s syndrome have been associated in the literature with cryoglobulinemic, vasculitis, anticardiolipin antibody, and Felty’s syndrome (characterized by the presence of leukopenia, splenomegaly, and arthritis).15-18 There is no absolute established proven treatment with patients with vasculitis and Sjorgren’s syndrome, but immunosuppressive agents have shown some success (Table 3).15
2.5 Dermatomyositis
Dermatomyositis (DM) ENREF 15 is characterized by muscle weakness and characteristic cutaneous findings that include a heliotrope rash, periungual telangiectasias, dystrophic cuticles, photodistributed violaceous erythema, Gottron’s papules.1,19 Ulcerations seen in dermatomyositis have been reported to involve calcinosis of the dermis, which leads to skin breakdown most notably after trauma. Vasculitis related cutaneous ulcers are rarely reported in adult-onset DM, and have been shown to be resistant to systemic corticosteroids. In juvenile dermatomyositis, the subgroup Banker type, is characterized by vasculitic ulceration which is often resistant to systemic corticosteroid therapy as well and is associated with a poor prognosis.20,21 One study reported the effectiveness of intravenous cyclophosphamide pulse therapy resulting in induction of clinical remission of DM with cutaneous vasculitis in an adult (Table 3).20 Ulcerations secondary to calcinosis do not readily heal because the ulcers are filled with calcium deposits, but colchicine and calcium channel blockers have been shown to be beneficial for treatment of the calcinosis (Table 3).2
2.6 Mixed Connective Tissue Disease
Mixed connective tissue disease (MCTD) is an overlap syndrome combining features of systemic lupus erythematosus, rheumatoid arthritis, systemic sclerosis and dermatomyositis together with the presence of antibodies to U1-RNP (Table 1).22 Skin manifestations of MCTD include Raynaud’s phenomenon associated with edema of the hands, sclerodactyly, calcinosis, telangiectasia, photosensitivity, malar rash and the rash of dermatomyositis. Chronic leg ulcers are not rare in MCTD. Not unlike the other CTD, ulcerations of MTCD have been reported to be due to subcutaneous calcification, vasculitis, nonspecific inflammation, Antiphospholipid antibodies.23-26 The prognosis in MCTD is relatively good, and systemic corticosteroids have shown some promise. However, treatment of the ulceration depends on the etiology. At times, these leg ulcers can be recalcitrant to treatment and require a multitude of treatment modalities before healing can occur.26 Treatment with pentoxifylline, calcium channel blockers, and sildenafil have been investigated but usually amputation is the end result (Table 3).2
3. Pyoderma Gangrenosum
Pyoderma gangrenosum (PG) is categorized under the neutrophilic dermatoses, with histological findings showing dense neutrophilc infiltrates in the dermis in the absence of infection and malignancy. The etiology of PG is unknown. However, many systemic diseases are associated with the development of PG, including inflammatory bowel disease, myeloproliferative disease, arthritis, or monoclonal IgA gammopathy.3 In addition, many patients with CTD have ulcerations consistent with PG. The typical PG ulcer has been reported to start as a papule, pustule, or vesicle, which rapidly ulcerates. The classic physical exam finding is an ulcer with purple, undermined border, with a necrotic wound bed. Cribiform scarring (with strands of epithelium across the wound bed) develops as the ulcer heals.2 Pathergy, which represents a worsening of the ulcer secondary to debridement and manipulation of the ulcer, is a concern when dealing with PG. However, adequate biopsy to rule out other conditions and removing necrotic tissue to improve the wound bed is imperative and should be done. Biopsy from the ulcer edge should be investigated for routine histology as well as for culture for bacteria, fungi, and mycobacteria. Laboratory investigation should include complete blood count, comprehensive metabolic profile, liver function tests, as well as other symptom-directed work-up which is dependent on the clinical circumstances. Colonoscopy should be done in patients with gastrointestinal symptoms.3
There are no guidelines for the treatment of PG. Systemic corticosteroids are administered at >60mg/day orally for prolonged periods of time. Methylprednisolone 1g I.V. for five days has been shown to be effective. We strongly advise telemetry monitoring, as electrolyte disturbances can cause fatal arrhythmias. Thus, concomitant use of diuretics is a relative contraindication. TNF-alpha inhibitors have been used with success as well, but they are associated with the development of lymphoma. Potent topical corticosteroids are not effective, except in very early ulcerations. Nonadherent dressings are preferred over adherent dressings because the latter are associated with pain upon removal. Bioengineered skin has been useful in stimulating healing once the wound bed is optimal.2,3ENREF 3
4. Vasculitis
Vasculitis is an immune-complex mediated event in which circulating immune complexes are deposited in vessel walls, this leads to activation of complement, which then leads to accumulation of inflammatory cells which cause damage to vessel wall.27 The different types of vasculitis that can cause cutaneous ulceration include small vessel vasculitis (such as leukocytoclastic vasculitis), medium sized vessel involvement such as polyarteritis nodosa (Fig 3a,b), microscopic polyangiitis, and granulomatous vasculitis, which includes Wegner’s granulomatosis (Fig 3C-E).2
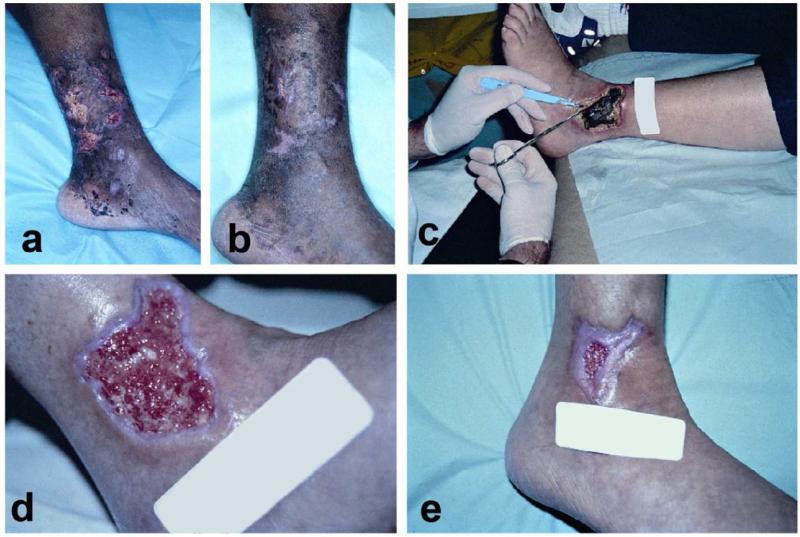
Figure 3. Examples of vasculitic ulcers.
A-B: Biopsy proven periarteritis nodosa with destruction of medium-sized vessels. Notice the slight microlivedo pattern surrounding the ulcer as well as the necrotic appearance of some of the ulcers. Treatment included methotrexate, sulfamethoxazole for pnuemocystis pneumonia (PCP) prophylaxis, and with a gel that promoted a moist wound environment and helped with autolytic debridement. C-E: This patient has an established diagnosis of Wegners granulomatosis c-ANCA positive small-medium sized vessel vasculitis. C: This large necrotic ulcer needed surgical debridement. D Three weeks after initial debridement, the wound bed is optimal with evidence of re-epithelialization. E: Six weeks after initial presentation. These cases illustrate the importance of attention to proper wound care in vasculitic ulcers in addition to systemic therapy.
Small-sized blood vessel cutaneous vasculitis generally includes capillaries, postcapillary venules, and nonmuscular arterioles (< 50 μm in diameter) that are found mainly within the superficial papillary dermis. In contrast, medium-sized blood vessels (between 50 and 150 μm in diameter) have muscular walls and are found principally in the deep reticular dermis, near the junction of the dermis and subcutaneous tissue. Larger vessels are not found within the skin.28
Unlike ulcerations associated with SLE or scleroderma for instance, where the diagnosis of a CTD has been established probably years before the presentation of the ulcer, patients presenting with ulceration due to a primary vasculitis are unaware of their underlying diagnosis. When managing these patients, taking a practical approach in evaluating their signs and symptoms will lead to proper diagnosis. As stated before, a thorough history and physical exam needs to be performed with a focus on review of systems and new medications.3 The diagnosis can be obtained by observing areas of any palpable purpura or skin necrosis. The shape and size of these lesions help us determine if small or medium sized vessels are involved. A superficial vasculitis leads to wedge shaped area of necrosis and, thus, a well-defined and regular skin purpura and necrosis. Occlusion of a deep vessel leaves open the chance for anastomosing vessels to alter the effect at the skin surface, so the areas of purpura and necrosis are irregular in shape.3
The presence of livedo reticularis or microlivedo is suggestive of vasculitis. Routine blood work should include a complete blood count, comprehensive metabolic profile, erythrocyte sedimentation rate, liver function test, hepatitis profile, antineutrophil cytoplasmic antibody (ANCA), which may be present when small to medium sized vessels are suspected. HIV testing and symptom directed work up for autoimmune diseases or malignancies may be required. Urinalysis and chest x-ray should also be done. Multiple skin biopsies often need to be performed to reduce sampling error. Excisional biopsies are preferred over punch biopsies.2,3ENREF 3 Since cutaneous vasculitis is a dynamic process, biopsies should be taken from a lesion less than 24 hours old; this approach has been shown to be most effective from the diagnostic standpoint.27 Biopsies should extend into the reticular dermis and subcutaneous tissue.2ENREF 27
Livedoid vasculitis is characterized by irregularly shaped ulcers overlying areas of purpura on the lower extremities. The ulcers are chronic and painful. Pathology shows thrombosis of dermal vessels with hyalinization of the vessel wall and sparse inflammatory infiltrate, and focal thrombi. These ulcers tend to heal with a characteristic white depressed scar studded with telangiestasia which has been termed atrophie blanche. These ulcers can be idiopathic in nature, but have been associated with a variety of conditions, including cyroglobulinemia, lymphoma, polyarteritis nodosa. Therapy can include nicotinic acid, antiplatelet regimens like aspirin, nifedipine, and pentoxifylline. 1,2
Since vasculitic lesions have an average duration of 28 months, and have no standard therapy, treatment is with proper wound dressings, oral medications for control of disease, and pain control.3,29 Oral prednisone administered at a dose of 30-60mg daily for several weeks has been used for deteriorating cases. Pentoxifylline at 800mg three times a day may be effective as well. Colchicine at oral doses of 0.5mg twice daily and or dapsone 150-200mg daily have also been successfully used. Immunosuppressive agents like etanercept are reserved for more recalcitrant cases and should be used with caution because of the risk of developing lymphoma (Table 4).2,3ENREF 3 For pain control we have success with a lidocaine 2% gel or with Benzocaine 20% spray to the wound and periwound area.
Table 4.
Summary of treatments in vasculitis and microthrombotic diseases
| Treatment | |
|---|---|
| Vasculitis | Prednisone 30-60mg daily for several weeks Pentoxifylline 800mg three times a day Colchicine 0.5mg twice daily Dapsone 150-200mg daily |
| Cryoglobulinemia | Patients that are hepatitis C positive - interferon alpha with ribavirin (jaymie 36) |
| Cryofibrinogenemia | Danazol (the alternative to stanozolol which is no longer available). Prednisone Plasmapheresis Low dose warfarin Avoid cold exposure |
| Calciphylaxis | Parathyroidectomy Thiosulfate, bisphosphonates Debridement of areas with calcified blood vessels followed by skin grafting with bioengineered skin |
| Cholesterol embolization | Statins Surgical intervention for revascularization |
| Antiphospholipid syndrome | Aspirin as prophylaxis Anticoagulation in the setting of thrombosis |
5.0 Cutaneous microthrombic ulcers
These are diseases characterized by occlusion of small dermal vessels either by thrombi or an opaque material. These ulcers are caused by hypercoagulable states induced by autoimmune disease (antiphospholipid antibodies), paraproteinemia (cryoglobulins, cryofibrinogen), occlusion of vessels by cholesterol, calcium deposition (calciphylaxis), and primary hypercoagulable states.3 These ulcers are characterized by severe pain, atrophy blanche, livedo reticularis, microlivedo, and dark eschars. These findings can be observed within the ulcer bed or at the edges.3,30 The lower extremities may have palpable pulses and are warm to touch. Work up should include a complete blood count, comprehensive metabolic profile, hepatitis panel, antiphospholipid antibodies, total cholesterol, cryofibrinogen and cryoglobulin.3 When testing for paraproteins in serum or plasma, the blood samples need to be collected and kept at 37°C before a determination i s made. If the temperature of the plasma or serum fall prior to clot formation, paraproteins will precipitate out, leading to a false-negative test result. Skin biopsies are essential for the diagnosis of microthrombotic ulcers. The histological presence of needle-shaped clefts by dissolution of crystals is pathognomonic for cholesterol emboli.3,31 See Table 4 for a review of treatments.
5.1 Antiphospholipd Syndrome
Antiphospholipid antibodies ENREF 17 (APLA) are a group of immunoglobulins that specifically react with the phospholipid portion of the prothrombin activator complex, namely factor Xa, factor V, phospholipids, and ionized calcium.1,32ENREF 32 ENREF 1 Anticardiolipin antibody and lupus anticoagulant are both antibodies with antiphospholipid specificity. Lab tests of lupus anticoagulant are confirmed with clotting tests that depend on phospholipids. Lab results for anticardiolipin antibody are positive for medium to high levels of anticardiolipin IgG and IgM antibodies.32 Skin biopsies reveal small vessels in dermis with evidence of thrombosis and sparse inflammatory cell infiltration.1 Physical exam findings include livedo reticularis, splinter hemorrhages, leg ulcers, superficial thrombophlebitis, and focal ischemia (Fig 4a).1 When APLA are present in the setting of a CTD, one refers to the presentation as secondary antiphospholipid syndrome. Treatment is with anticoagulants and aspirin for prevention (Table 4).32 Patients with primary antiphospholipid syndrome commonly have a history of a cerebrovascular accident, myocardial infarction, recurrent fetal losses, deep vein thrombosis, and pulmonary embolism.32
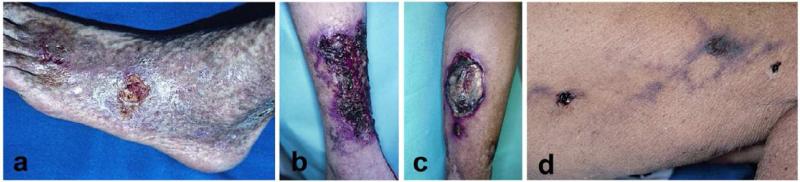
Figure 4. Examples of cutaneous microthrombotic ulcers.
Necrosis and livedo reticularis should always raise the suspicion of an obstruction of small blood vessels as illustrated in the following examples. A: This Caucasian man with antiphospholipid syndrome has extensive livedo reticularis and very painful ulcers. B: In this case, the underlying condition is cryoglobulinemia. Concomitant hepatitis C infection should always be excluded. Patients often need therapy with systemic immunosuppressive agents. C: Cyrofibrinogenemia was diagnosed in this 87-year-old man with multiple medical problems by plasma measurements and histology. His ulcer healed with stanozolol (which is no longer commercially available, danazol is the alternative). D: This women’s thigh shows livedo reticularis and necrotic ulcers. Histology would show deposition of calcium crystals in the wall of blood vessels.
5.2 Cryoglobulinemia
Cryoglobulins are immunoglobulins or complexes of immunoglobulins and complement that precipitate in serum at temperatures below 37°C.27 Three types have been characterized. Type I cryoglobulins are composed of monoclonal immunoglobulins, usually IgM and less commonly IgG or IgA, and are associated with hematologic abnormalities. Types II and III cryoglobulins are collectively labeled as “mixed” cryoglobulins, and are comprised of monoclonal or polyclonal immunoglobulins (IgM) respectively with rheumatoid factor activity that recognize polyclonal IgG.33ENREF 15 Type II is the most common type of cryoglobulinemia. Mixed cryoglobulins are associated with chronic inflammation and infection, including hepatitis B and C viral infections, systemic lupus erythematosus, rheumatoid arthritis, and Sjogren’s syndrome.27,33,34 Cryoglobulins may also be present in the sera of patients with chronic inflammatory conditions without clinical significance. However, when cryoglobulins deposit in small and medium-sized vessels in the skin, kidneys, joints, and nerves, tissue injury may occur.27 Mixed essential cryoglobulinemia is the common cause of skin ulceration. ENREF 34 These proteins precipitate at low temperatures, occlude the vessels and subsequently cause ulceration (Fig 4b).33
5.3 Cryofibrinogenemia
Cryofibrinogenemia (CF) is defined by complexes of fibrin, fibrinogen, fibronectin, and fibrin split products with albumin, immunoglobulins and plasma proteins that precipitate from the patient’s plasma in the cold. CF present in plasma and not in serum. Some patients are asymptomatic, while others have purpura, livedo reticularis, ulcerations, gangrene, and necrosis resulting from thrombosis (Fig 4c).35
Cryofibrinogenemic purpura is a skin condition that manifests as painful purpura with slow healing ulcerations and edema of both feet during winter months, due to the precipitation of CF.36
5.4 Cholesterol embolism
Cholesterol emboli are small deposits of cholesterol laden-material that becomes lodged inside the blood vessels of the skin or other internal organs. Typically these emboli are <100 μm in diameter, which explains their distribution in very small blood vessels. Cholesterol embolization occurs in the elderly population with severe atherosclerotic disease. The condition leads to a blockage of the flow of blood through small caliber vessels and causes malfunction or death of the tissue supplied by the affected blood vessels. Cholesterol crystals embolize after a vascular surgical procedure or after starting anticoagulant therapy. Skin findings include livedo reticularis, gangrene, cyanosis, ulceration, painful red nodules, and purpura (purple patches). Treatment includes removal or stenting of unstable atheromatous plaques, as well statins (Table 4).3,31
5.5 Calciphylaxis
Calciphylaxis is classically associated with renal disease and secondary parathyroidism. Calciphylaxis can occur in those with high or normal levels of serum calcium and phosphate, with or without vitamin D replacement, less often in those who have received a renal transplant.2,37ENREF 37 Skin biopsy shows calcium deposition in and around the blood vessel walls, but the histology is highly variable. Calciphylaxis may begin as microlivedo and eventually lead to tissue necrosis (Fig 4d). Treatment includes reversing the cause of the elevated calcium with parathyroidectomy or bisphosphonates. Ulcers recalcitrant to treatment have been successfully managed with debridement and bioengineered skin (Table 4).2
6.0 Discussion
Skin ulcerations present a challenge to health care providers and ulcers associated with connective tissue disorders are no exception. A multidisciplinary approach is often desirable when caring for these patients. One cannot focus on just the ulcer since the underlying cause can be a serious systemic condition. Connective tissue ulcers can be associated with rheumatoid arthritis, systemic lupus erythematosus, scleroderma, sjogren’s, dermatomyositis, and mixed connective tissue disease. The reasons for ulcer formation are multifactorial and include vasculitis and microthrombotic diseases, in addition to concomitant venous insufficiency. When examining an ulcer, one most look away from the ulcer for distinctive clues to lead you to the primary cause of the ulcer; hard fingers, butterfly rash, muscle weakness, livedo reticularis, rheumatoid hands (table 2). When examining ulcers, the presence of livedo reticularis or microlivedo, purple edges, undermined or undulating borders, unusual location should make you consider ulcers of inflammatory etiology.3 Work up should include extensive history and physical exam, laboratory exam should include a complete blood count, comprehensive metabolic profile, hepatitis panel, anticardiolipin antibodies, total cholesterol, relevant serum factors which aid in diagnosis (see table 1), cryofibrinogen and cryoglobulin, P-ANCA and C-ANCA. Skin biopsy is imperative but not always diagnostic. It is recommended to perform a narrow and long elliptical excisional biopsy when vasculitis is in the differential diagnosis. The biopsy should include reticular dermis and subcutaneous tissue.2 One of the main treatments has been the use of systemic corticosteroid, but this is somewhat counterintuitive because steroids can contribute to delayed wound healing by suppressing the inflammatory response, it decreases neovascularization, collagen synthesis and re-epithelialization.38 Therefore, even if the ulceration heals, it tends to recur because of poor microcirculation or location in a friction prone region. Newer systemic immunosuppressant medications with advances in local wound care have provided more therapeutic options for these patients, but more still needs to be done. There is emerging evidence that mesenchymal stem cell (MSC) therapy in autoimmune disorders may have therapeutic potential.39 In mice with active SLE and some patients with SLE, transplantation of MSC reduced the production autoantibodies and reversed renal dysfunction in mice.40 Recent evidence from our own work has shown that autologous MSC accelerated the healing of chronic wounds in mouse wound healing models and in humans.41 Phase I and Phase II trials of MSC therapy show promise but controlled multicenter trials are still needed. Only time will tell how effective stem cell therapy will be on healing wounds acute or chronic and regardless of etiology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goslen JB. Autoimmune Ulceration of the leg. Leg Ulcers. 1990;3:92–117. doi: 10.1016/0738-081x(90)90050-b. [DOI] [PubMed] [Google Scholar]
- 2.Falanga V, Lindholm C, Carson PA, et al. Text Atlas of Wound Managament (ed 2) Informa Healthcare; London, UK: 2012. [Google Scholar]
- 3.Panuncialman J, Falanga V. Basic approach to inflammatory ulcers. Dermatologic Therapy. 2006;19:365–376. doi: 10.1111/j.1529-8019.2006.00097.x. [DOI] [PubMed] [Google Scholar]
- 4.Jorrizo JL, O AJ, Stanely RJ. Superficial Ulcerating Necrobiosis in Rheumatoid Arthritis. A Variant of the Necrobiosis Lipoidica-Rheumatoid Nodule Spectrum? Arch Dermatol. 1982;118 [PubMed] [Google Scholar]
- 5.Danning CL, Illei GG, Boumpas DT. Vasculitis associated with primary rheumatologic diseases. Curr opin Rheumatol. 1998;10:58–65. doi: 10.1097/00002281-199801000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Jorrizo JL, Daniels JC. Dermatologic conditons reported in patients with rheumatoid arthritis. JAAD. 1983;8:439–457. doi: 10.1016/s0190-9622(83)70049-6. [DOI] [PubMed] [Google Scholar]
- 7.Oien RF, Hakansson A, Hansen BU. Leg ulcers in patients with rheumatoid arthritis- a prospective study of aeitology, wound healing, and pain reduction after pinch grafting. Rheumatoloy. 2001;40:816–820. doi: 10.1093/rheumatology/40.7.816. [DOI] [PubMed] [Google Scholar]
- 8.Hirche D, Rubbert A, Lunau L, et al. Successful treatment of refractory rheumatoid arthritis-associated leg ulcerations with adalimumab. Br J Dermatol. 2005;152:1062–4. doi: 10.1111/j.1365-2133.2005.06520.x. [DOI] [PubMed] [Google Scholar]
- 9.Tuffanelli DL, Winkelmann RK. Systemic Scleroderma: A Clinical Study of 727 Cases. Arch Dermatol. 1961;84:359–357. doi: 10.1001/archderm.1961.01580150005001. [DOI] [PubMed] [Google Scholar]
- 10.Rademaker M, Cooke ED, Almond NE, et al. Comparison of intravenous infusions of iloprost and oral nifedipine in treatment of Raynaud’s phenomenon in patients with systemic sclerosis: a double blind randomised study. British Journal of Dermatology. 1989;298:561–564. doi: 10.1136/bmj.298.6673.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wigley FM, Wise RA, Seibold JR, et al. Intravenous Iloprost Infusion in Patients with Raynaud Phenomenon Secondary to Systemic Sclerosis A Multicenter, Placebo-controlled, Double-Blind Study. Ann Intern Med. 120:199–206. doi: 10.7326/0003-4819-120-3-199402010-00004. [DOI] [PubMed] [Google Scholar]
- 12.Reddy V, Dziadzio M, Hamdulay S, et al. Lupus and leg ulcers--a diagnostic quandary. Clin Rheumatol. 2007;26:1173–5. doi: 10.1007/s10067-006-0306-2. [DOI] [PubMed] [Google Scholar]
- 13.Fink AM, Kottas-Heldenberg A, Bayer PM, et al. Lupus Anticoagulant in Patients with Chronic Venous Insuffiency. Acta Derm Venereol. 2003;83:287–289. doi: 10.1080/00015550310016553. [DOI] [PubMed] [Google Scholar]
- 14.Fink AM, Kottas-Heldenberg A, Mayer W, et al. Lupus anticoagulant and venous leg ulceration. British Journal of Dermatology. 2002;146:308–310. doi: 10.1046/j.0007-0963.2001.04546.x. [DOI] [PubMed] [Google Scholar]
- 15.Chapnick SL, Merkel PA. Skin ulcers in a patient with Sjogren’s syndrome. Arthritis Care Res (Hoboken) 2010;62:1040–6. doi: 10.1002/acr.20181. [DOI] [PubMed] [Google Scholar]
- 16.Foley JF, Karlen M, Watson CJ, et al. Anticoagulant Therapy of Cryoglobulinemic Ulcers in a Case of Sjogrens Syndrome. JAMA. 1961;176:149–154. [Google Scholar]
- 17.Jedryka-Goral A, Jagiello P, D’Cruz DP, et al. Isotype profile and clinical relevance of anticardiolipin antibodies in Sjogren’s syndrome. ANNALS OF THE RHEUMATIC DISEASES. 1992;51:889–891. doi: 10.1136/ard.51.7.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gurling KJ. Association of Sjorgrens and Felty’s syndrome. ANNALS OF THE RHEUMATIC DISEASES. 1953;12:212–216. doi: 10.1136/ard.12.3.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clarke JT, Werth VP. Rheumatic manifestations of skin disease. Curr Opin Rheumatol. 2010;22:78–84. doi: 10.1097/BOR.0b013e328333b9e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsujimura S, Saito K, Tanaka Y. Complete Resolution of Dermatomyositis with Refractory Cutaneous Vasculitis by Intravenous Cyclophosphamide Pulse Therapy. Inter Med. 2008;47:1935–1940. doi: 10.2169/internalmedicine.47.1289. [DOI] [PubMed] [Google Scholar]
- 21.Martin N, Li CK, Wedderburn LR. Juvenile dermatomyositis: new insights and new treatment strategies. Ther Adv Musculoskel Dis. 2012;4:41–50. doi: 10.1177/1759720X11424460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mukerji B, H JB. Undifferentiated, overlapping and mixed connective tissue diseases. Am J Med Sci. 1993;305:114–119. doi: 10.1097/00000441-199302000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Yamamura K, Takahara M, Masunaga K, et al. Subcutaneous calcification of the lower legs in a patient with mixed connective tissue disease. J Dermatol. 2011;38:791–3. doi: 10.1111/j.1346-8138.2010.01177.x. [DOI] [PubMed] [Google Scholar]
- 24.Rozin AP, Egozi D, Ramon Y, et al. Large leg ulcers due to autoimmune diseases. Med Sci Monit. 2011;17:CS1–CS7. doi: 10.12659/MSM.881308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doria A, Ruffatti A, Calligaro A, et al. Antiphospholipid Antibodies in Mixed Connective Tissue Disease. Clin Rheumatol. 1992;11:48–50. doi: 10.1007/BF02207083. [DOI] [PubMed] [Google Scholar]
- 26.Rozin AP, Braun-Moscovici Y, Bergman R, et al. Recalcitrant leg ulcer due to mixed connective tissue disease. The Journal of Medicine. 2006;64:91–94. [PubMed] [Google Scholar]
- 27.Chen KR, Carlson JA. Clinical Approach to Cutaneous Vasculitis. Am J Clin Dermatol. 2008;9:71–92. doi: 10.2165/00128071-200809020-00001. [DOI] [PubMed] [Google Scholar]
- 28.Gonzalez-Gay MA, Garcia-Porrua C, Pujol RM. Clinical approach to cutaneous vasculitis. Current Opinion in Rheumatology. 2004;17:56–61. doi: 10.1097/01.bor.0000145519.68725.5a. [DOI] [PubMed] [Google Scholar]
- 29.Sais G, Vidaller A, Jucgla A, et al. Prognostic Factors in Leukocytoclastic Vasculitis. Seminars in Arthritis and Rheumatism. 1998;134:309–315. doi: 10.1001/archderm.134.3.309. [DOI] [PubMed] [Google Scholar]
- 30.Paquette D, Falanga V. Leg Ulcers. Clin Geriatr Med. 2002;18:77–88. doi: 10.1016/s0749-0690(03)00035-1. [DOI] [PubMed] [Google Scholar]
- 31.Donohue KG, Saap L, Falanga V. Cholesterol cystal embolization an artheresclerotic disease with frequent and varied cutaneous manifestations. J Eur Acad Dermatol Venereol. 2003;17:504–511. doi: 10.1046/j.1468-3083.2003.00710.x. [DOI] [PubMed] [Google Scholar]
- 32.Misita CP, Moll S. Antiphospholipid antibodies. Circulation. 2005;112:e39–44. doi: 10.1161/CIRCULATIONAHA.105.548495. [DOI] [PubMed] [Google Scholar]
- 33.Ferri C, Zignego AL, Pileri SA. Cryoglobulins. J Clin Pathol. 2002;55:4–13. doi: 10.1136/jcp.55.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramos-Casals M, Cerevera R, Yague J, et al. Cryoglobulinemia in Primary Sj6gren’s Syndrome: Prevalence and Clinical Characteristics in a Series of 115 Patients. Seminars in Arthritis and Rheumatism. 1998;28:200–205. doi: 10.1016/s0049-0172(98)80037-1. [DOI] [PubMed] [Google Scholar]
- 35.Saadoun D, Elalamy I, Ghillani-Dalbin P, et al. Cryofibrinogenemia: new insights into clinical and pathogenic features. Am J Med. 2009;122:1128–35. doi: 10.1016/j.amjmed.2009.03.040. [DOI] [PubMed] [Google Scholar]
- 36.Amdo TD, Welker JA. An approach to the diagnosis and treatment of cryofibrinogenemia. Am J Med. 2004;116:332–7. doi: 10.1016/j.amjmed.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 37.Howe SC, Murray JD, Reeves RT, et al. Calciphylaxis, a poorly understood clinical syndrome: three case reports and a review of the literature. Ann Vasc Surg. 2001;15:470–3. doi: 10.1007/s100160010122. [DOI] [PubMed] [Google Scholar]
- 38.Sagg KG, Furst DE. In: Major side effects of systemic glucocorticoids. Matteson EL, editor. 2011. UptoDate. [Google Scholar]
- 39.Ren G, Chen X, Dong F, et al. Concise review: mesenchymal stem cells and translational medicine: emerging issues. Stem Cells Transl Med. 2012;1:51–8. doi: 10.5966/sctm.2011-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun L, Akiyama K, Zhang H, et al. Mesenchymal stem cell transplantation reverses multiorgan dysfunction in systemic lupus erythematosus mice and humans. Stem Cells. 2009;27:1421–32. doi: 10.1002/stem.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Falanga V, Iwamoto S, Chartier M, et al. Autologous bone marrow-derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng. 2007;13:1299–312. doi: 10.1089/ten.2006.0278. [DOI] [PubMed] [Google Scholar]