Abstract
For neonates requiring intensive care, the optimal sound environment is uncertain. Minimal disruptions from medical staff create quieter environments for sleep, but limit language exposure necessary for proper language development. There are two models of neonatal intensive care units (NICUs): open-bay, in which 6-to-10 infants are cared for in a single large room; and single-room, in which neonates are housed in private, individual hospital rooms. We compared the acoustic environments in the two NICU models. We extracted the audio tracks from video-electroencephalography (EEG) monitoring studies from neonates in an open-bay NICU and compared the acoustic environment to that recorded from neonates in a new single-room NICU. From each NICU, 18 term infants were studied (total N=36; mean gestational age 39.3±1.9 weeks). Neither z-scores of the sound level variance (0.088±0.03 vs. 0.083±0.03, p=0.7), nor percent time with peak sound variance (above 2 standard deviations; 3.6% vs. 3.8%, p=0.6) were different. However, time below 0.05 standard deviations was higher in the single-room NICU (76% vs. 70%, p=0.02). We provide objective evidence that single-room NICUs have equal sound peaks and overall noise level variability compared with open-bay units, but the former may offer significantly more time at lower noise levels.
Keywords: neonatal intensive care unit, acoustic environment, sound, open-bay, single-room
INTRODUCTION
There is increasing attention to hospitals’ acoustic environments since noise can disrupt patients’ sleep(Buxton OM, Ellenbogen JM et al. 2012). For years, neonatology care has been focused on minimizing handling and providing a quiet environment for at-risk infants. Preterm infants exhibit potentially adverse physiologic responses to hospital sounds(Kuhn P, Zores C et al. 2012), and older patients exhibit arousal responses to many hospital noises(2012; Buxton OM, Ellenbogen JM et al. 2012). For neonates, the optimal sound environment is uncertain. Quiet environments could allow for better sleep, but may not provide sufficient language exposure necessary for proper language development(Caskey M, Stephens B et al. 2011).
There are two models for neonatal intensive care units (NICUs): open-bay, in which 6-to-10 infants are cared for in a single large room; and single-room, in which each neonate is housed in a private, individual hospital room. Many newly built NICUs are designed with single rooms(Shahheidari M and Homer C 2012), but there are minimal available data regarding the acoustic environment in this setting. We hypothesized that sound in the open-bay NICU would be relatively constant, (e.g. “background noise”), without significant peaks and troughs, and therefore might be minimally disruptive. We also hypothesized that the very quiet baseline sound level in the single room unit would be abruptly disturbed by noise from medical staff, visitors, or equipment. Therefore, we designed this study to compare the variance of the acoustic environment in an open-bay to a single room NICU.
METHODS
The institutional review board at the University of Michigan approved this study and the parents of all subjects provided written informed consent. Infants studied prior to December 2011 were cared for in an open-bay NICU. In December 2011, our hospital opened a new NICU, with a single-room unit design. Infants recruited after December 2011 were therefore cared for in the single-room NICU. All subjects had conventional video electroencephalograms (EEG) recorded as part of an observational research study of neonates at risk for seizures. Inclusion criteria were: ≥36 weeks estimated gestational age and requiring care in a NICU. Exclusion criteria were: prematurity and known or suspected genetic syndromes that would be anticipated to affect neurodevelopmental outcomes. Infants’ medical records were reviewed for pertinent data, and each infant had a standardized neurological examination.
In both NICUs, the video-EEG equipment, including a microphone, was positioned at the foot of the infants’ isolette, and the recordings lasted at minimum 16 hours. For longer recordings, the first 24 hours of data were analyzed, in order to encompass both day and night shifts during the period of highest clinical acuity, when infants were first being evaluated for seizures.
Audio tracks from the 16–24 hour video recordings from the video-EEG monitoring studies were extracted (Wondershare Video Editor; Wondershare, Shenzhen, China) and converted into MP3 files. The MP3 files were then converted into WAV files (Pazera Free Audio Extractor 1.4; Pazera Jacek, Sosnowiec, Poland). Thereafter, using statistical software, the variance of the audio signal was extracted for every 10-second recording epoch (MatLab; MathWorks, Natick, MA). Z-scores for the variances of each subject’s recording were then calculated. The average Z-score for the open-bay and single-room NICUs were calculated using the individual patients’ Z-scores from each NICU environment. To evaluate deviations from the background noise, the percent time above 2 standard deviations (sound peaks) was calculated for each subject. To evaluate the epochs with relatively little background variation, the percent of recording below 0.25, 0.01, and 0.05 standard deviations was calculated.
Statistical analysis
T-tests and chi-square tests were employed to compare demographic and acoustic environment data for subjects in the two NICUs (SAS Version 9.2; SAS Institute, Inc, Cary NC).
RESULTS
Recordings from 18 infants in the open-bay NICU and 18 infants in the single-room NICU were analyzed. Patient characteristics are summarized in Table 1. Infants in the two NICUs were of similar gestational age and birth weight, and there was no difference in their neurological examination scores. More infants in the open-bay NICU had hypoxic ischemic encephalopathy and these infants had lower Apgar scores than the more heterogeneous group of subjects from the single-room NICU. However, linear regression analyses showed that the acoustic profile was not predicted by Apgar scores (p=0.09).
Table 1.
Clinical characteristics of the 36 study subjects
| Variable | Open-Bay NICU Mean (SD) |
Single-Room NICU Mean (SD) |
p-value1 |
|---|---|---|---|
| Sex | Male=10; Female=8 | Male=10; Female=8 | >0.9 |
| Weight | 3229g ± 475 | 3174g ± 603 | 0.76 |
| Head circumference | 35 cm ± 2 | 34 cm ± 2 | 0.30 |
| Gestational Age | 39.6 weeks ± 1.6 | 39.1 weeks ± 1.2 | 0.32 |
| 5-Minute Apgar Score | Median 2 (range 0–10) | Median 9 (range 2–10) | 0.0007 |
| Neurologic Exam Score | 8.8 ± 6.1 | 5.9 ± 5.6 | 0.15 |
| Primary Neurologic Diagnosis2 | HIE3 (N = 15) Seizures, not due to HIE (N = 3) | HIE (N = 3) Seizures, not due to HIE (N = 10) Infection (N = 3) Meconium aspiration (N = 1) ICH4, no seizure (N = 1) |
p-values are derived from t-tests (weight, head circumference, and examination scores), chi squared test (sex), and Wilcoxon Rank-Sum test (Apgar scores).
All infants had suspected seizures.
HIE = hypoxic ischemic encephalopathy
ICH = intracranial hemorrhage
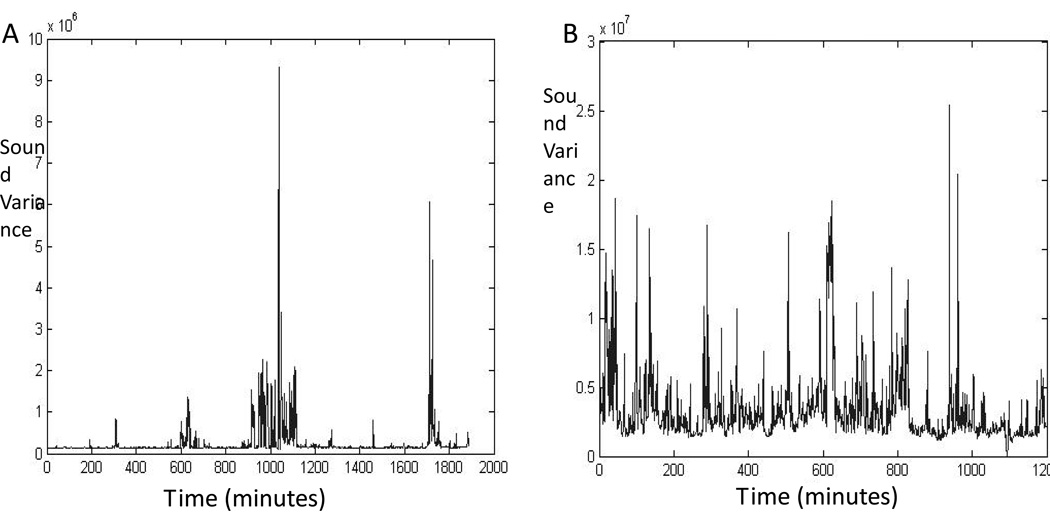
The Z-scores for the acoustic environment are presented in Table 2. The mean Z-scores for the two acoustic environments were not different (0.088±0.03 vs. 0.083±0.03, p=0.7). Similarly, there was no difference in the proportion of recording time with noise above a threshold of 2 standard deviations of the auditory signal (3.6%±1% vs. 3.8%±1%, p=0.6). There was, however significantly more time below 0.05 standard deviations in the single-room unit, compared with the open-bay NICU, compared with (76%±6% vs.70%±9%; p=0.02). This implies that infants in the single-room units experienced more time with minimal acoustic variability than those in the open-bay unit. Visual analyses of the graphs of acoustic variance corroborated this finding, with more prolonged periods of quiet in the single-room NICU (Figure 1A), compared with the open-bay NICU (Figure 1B).
Table 2.
Comparisons of acoustic variability between the open-bay and single-room NICU. The proportion of quiet time (z-score less than 0.05 SD) was significantly higher in the single-room NICU, but the overall z-scores were the same in both units.
| Variable | Open-Bay NICU (Mean ± SD) |
Single-Room NICU (Mean ± SD) |
P-value* |
|---|---|---|---|
| Audio background z-score | 0.088 ± 0.03 | 0.083 ± 0.03 | 0.66 |
| % time above 2 SD | 3.6% ± 1.0 | 3.8% ± 1.0 | 0.60 |
| % time below 0.25 SD | 78% ± 7.0 | 82% ± 5.0 | 0.073 |
| % time below 0.1 SD | 72% ± 9.0 | 78% ± 6.0 | 0.034 |
| % time above 0.05 SD | 70% ± 9.0 | 76% ± 6.0 | 0.022 |
P-values are derived from Student’s t-tests
Figure 1.
These graphs depict the variance of the audio recording for two infants. Panel A demonstrates the frequent silent periods observed in the single-room NICU. Panel B displays the background noise variability from a patient in the open-bay NICU. Note that the variance for these two individuals was quite different (the y-axis sound variance units are displayed as 106 in Panel A and 107 in Panel B), but overall, there was no significant difference in variance between the two study groups.
DISCUSSION
We have developed an innovative approach to assessing auditory environments for critically ill newborn infants, with somewhat surprising results: overall acoustic variance was not different between the open-bay and single room NICU and peaks in sound occurred equally frequently in the two NICUs; however, periods of presumed silence (with minimal sound variability) were significantly more common in the single-room NICU.
Because many sick neonates spend their first weeks, and sometimes months, in a NICU, the impact of the unit’s physical design and acoustic environment may be significant. Efforts to diminish physical stimulation and minimize noise in the NICU have included housing neonates in single rooms. Contrary to our hypothesis, we found that the proportion of noise peaks (above 2 standard deviations of the baseline signal) was just as high in the single-room NICU as the open-bay unit. However, infants in private rooms had much more time in relatively silent conditions.
Silence in an ICU setting may be advantageous, as neonates exhibit potentially adverse physiologic responses to noise, particularly during active sleep(Kuhn P, Zores C et al. 2012). However, this environment is still quite different from the in utero experience, during which the fetus is exposed to sounds from the mother’s body, as well as to language as the mother speaks(Krueger C 2010). Restricting language exposure, by isolating neonates in private rooms, could have unintended negative consequences. The impact of the NICU environment on language development remains to be elucidated. If limited language exposure (or excessive exposure to a very quiet environment) is a risk factor for abnormal language development, then interventions to optimize the NICU’s acoustic environment may be required.
We analyzed the variance of the acoustic background, since abrupt sound changes are alerting and known to disrupt sleep(Buxton OM, Ellenbogen JM et al. 2012), and our equipment was not specifically calibrated in order to allow reliable comparisons of absolute sound levels. We used normalized values (Z-scores) in order to allow evaluation of sound variability among infants with a range of disease severity and, therefore, a spectrum of need for intensive bedside nursing care. Future studies, and individual NICUs, should also assess absolute noise levels.
Our pilot study has some other limitations. The audio recordings were extracted from clinically indicated video-EEG monitoring studies. Therefore, there was some inherent variability in the duration of recording. However, we only selected records with more than 16 hours of audio so that both day and night shifts were represented. We specifically chose the first 16–24 hours of monitoring, since these are typically the periods of highest acuity for at-risk infants.
Although there were more infants with low Apgar scores (due to hypoxic ischemic encephalopathy) in the open-bay NICU, there was no difference in the two groups’ neurological examination abnormalities. Regression analyses showed that Apgar scores were not significant predictors of the acoustic environment. Future studies, with larger cohorts, could match for diagnosis or illness severity scores. The use of transcription software could also allow for analysis of the specific sources of noise in the NICU environment.
We demonstrate here that infants in single-room NICUs are exposed to similar peaks in sound as those in open-bay NICUs. However, infants in single rooms have a larger proportion of time with very little background variability – periods of presumed minimal auditory stimulation. If, as we now hypothesize, infants in single rooms have reduced language exposure, compared to those in open-bay units, then future nursing interventions should be designed to address this issue. Finding an optimal balance of physiologic sound exposure and protection against sleep disruption could contribute to enhanced patient-centered care and optimized outcomes for vulnerable neonates.
ACKNOWLEDGEMENTS
The authors are grateful to Joseph Burns, PhD, for his assistance with data analysis.
Research Support: This research was supported by the Child Neurology Foundation’s Shields Fellowship Award and the NICHD (5K23HD068402), as well as the University of Michigan Undergraduate Research Opportunities Program.
Role of the funding sources: The funding sources had no role in the research design, execution, data analysis, or decision to submit the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: The authors have no conflicts of interest to disclose.
BIBLIOGRAPHY
- Buxton O, et al. Sleep Disruption Due to Hospital Noise A Prospective Evaluation. Annals of Internal Medicine. 2012:1–10. doi: 10.7326/0003-4819-157-3-201208070-00472. [DOI] [PubMed] [Google Scholar]
- Caskey M, et al. Importance of Parent Talk on the Development of Preterm Infant Vocalization. Pediatrics. 2011;128:910–916. doi: 10.1542/peds.2011-0609. [DOI] [PubMed] [Google Scholar]
- Edrick M, et al. A Review of the Effects of Sleep During the First Year of LIfe on Cognitive, Psychomotor, and Temperament Development. Infant Sleep and Development. 2009;11:1449–1455. doi: 10.1093/sleep/32.11.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger Charlene. Exposure to Maternal Voice in Preterm Infants. Advances in Neonatal Care. 2010;1:13–18. doi: 10.1097/ANC.0b013e3181cc3c69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn P, et al. Infants born very preterm react to variations of the acoustic environment in their incubator from a minimum signal-to-noise ration threshold of 5 to 10 dBA. Pediatric Research. 2012;71:386–392. doi: 10.1038/pr.2011.76. [DOI] [PubMed] [Google Scholar]
- Shahheidari M, Homer C. Impact of the Design of Neonatal Intensive Care Units on Neonates, Staff, and Families A Systematic Literature Review. The Journal of Perinatal & Neonatal Nursing. 2012;3:260–266. doi: 10.1097/JPN.0b013e318261ca1d. [DOI] [PubMed] [Google Scholar]