Abstract
Purpose
The combination of a vascular endothelial growth factor (VEGF) -neutralizing antibody, bevaci-zumab, and irinotecan is associated with high radiographic response rates and improved survival outcomes in patients with recurrent malignant gliomas. The aim of these retrospective studies was to evaluate tumor vascularity and expression of components of the VEGF pathway and hypoxic responses as predictive markers for radiographic response and survival benefit from the bevacizumab and irinotecan therapy.
Patients and Methods
In a phase II trial, 60 patients with recurrent malignant astrocytomas were treated with bevacizumab and irinotecan. Tumor specimens collected at the time of diagnosis were available for further pathologic studies in 45 patients (75%). VEGF, VEGF receptor-2, CD31, hypoxia-nducible carbonic anhydrase 9 (CA9), and hypoxia-inducible factor-2α were semiquantitatively assessed by immunohistochemistry. Radiographic response and survival outcomes were correlated with these angiogenic and hypoxic markers
Results
Of 45 patients, 27 patients had glioblastoma multiforme, and 18 patients had anaplastic astrocytoma. Twenty-six patients (58%) had at least partial radiographic response. High VEGF expression was associated with increased likelihood of radiographic response (P = .024) but not survival benefit. Survival analysis revealed that high CA9 expression was associated with poor survival outcome (P = .016)
Conclusion
In this patient cohort, tumor expression levels of VEGF, the moleculartarget of bevacizumab, were associated with radiographic response, and the upstream promoter of angiogenesis, hypoxia, determined survival outcome, as measured from treatment initiation. Validation in a larger clinical trial is warranted
Introduction
Glioblastomas are highly lethal cancers characterized by florid angiogenesis.1 Although several molecular mechanisms contribute to tumor angiogenesis, the vascular endothelial growth factor (VEGF) pathway seems particularly important and has been a prominent therapeutic target in cancer treatment. Recently, we have demonstrated encouraging benefit in malignant glioma patients treated with a VEGF-neutralizing antibody, bevacizumab (Avastin; Genentech, South San Francisco, CA), in combination with a topoisomerase-I inhibitor, irinotecan (Camptosar; Pfizer, New York, NY) in a phase II clinical trial.2,3 This combination demonstrated a remarkable radiographic response rate of 63%, with a 6-month progression-free survival rate of 32% for glioblastoma multiforme (GBM) and 61% for recurrent WHO grade 3 gliomas. The encouraging radiographic response rates detected in this initial phase prompted an expansion to include a total of 68 patients with recurrent malignant gliomas.3 The 6-month progression-free survival rate for all 68 patients was 43% for recurrent GBM and 61% for recurrent anaplastic gliomas.3 Despite this encouraging result of anti-VEGF therapy in malignant glioma, there are several challenges to be overcome to achieve optimal clinical benefit. Only a subset of patients who received bevacizumab experienced radiographic response or prolongation of survival. To date, there is no predictive biomarker of response or survival benefit for bevacizumab in most solid malignancies.4 Thus, there is an unmet need for predictive biomarkers to enrich for patients who are likely to have either a response to or resistance to bevacizumab. Recently, two studies using immunohistochemical (IHC) analysis of archival tumor specimens have elucidated the molecular determinants for response to epidermal growth factor receptor (EGFR) -targeted therapeutics in malignant gliomas.5,6 These studies indicate technical feasibility of tumor immunohistochemistry for biomarker identification in malignant gliomas, which may serve as a paradigm of biomarker-guided targeted therapy if independently validated in larger prospective trials.
Because VEGF is a molecular target for bevacizumab, we hypothesized that VEGF, its receptor (VEGF receptor-2 [VEGFR-2]; also known as kinase insert domain-containing receptor [KDR]), or tumor vascularity as identified by CD31 may represent a surrogate marker for therapeutic response. Hypoxia is one of the key pathophysiologic features in glioblastomas driving angiogenesis, invasion, and therapeutic resistance.7,8 Carbonic anhydrase 9 (CA9) is a hypoxiainducible transmembrane enzyme that has recently been shown to be an independent prognostic factor for malignant astrocytoma patients.9,10 Its membrane location and stability present a reliable and attractive marker for hypoxia.11 Hypoxia-inducible factor (HIF) -2α is a hypoxia-inducible transcription factor, regulating angiogenesis and other malignant phenotypes of cancer.12,13 Recently, HIF-2α has been shown to be complementary to CA9 as hypoxia determinants to predict locoregional control and survival outcome after radiotherapy in head and neck cancer patients.14 In the current study, we used semiquantitative IHC analysis of these angiogenic and hypoxic markers on tumor specimens from malignant astrocytoma patients treated with bevacizumab plus irinotecan to identify predictive biomarkers of response and survival.
Patients and Methods
Clinical Trial and Tissue Acquisition
From April 2005 to February 2006, 68 patients with recurrent malignant gliomas (35 patients with GBM and 33 patients with WHO grade 3 gliomas) were treated in a phase II trial of bevacizumab and irinotecan at Duke University Medical Center (clinicaltrial.gov identifier: NCT00268359).2,3 Of these patients, those with anaplastic oligodendroglioma (a total of eight patients), who have different biology, treatment response, and survival, were excluded from the current analysis, leaving 60 patients for further analysis. Because concerns for intracerebral hemorrhage precluded administration of bevacizumab within 4 weeks of craniotomy, we retrospectively collected paraffin-embedded tumor material for IHC analysis from the initial diagnostic biopsy of patients who entered this trial. Before enrollment, patients underwent informed consent approved by the Duke University Institutional Review Board. This correlative study was conducted without industry support. Initial diagnosis was determined by neuropathologists at Duke University Medical Center or local facilities, where surgeries were performed. Independent confirmation of diagnosis and presence of tumor in each specimen was endorsed by a neuropathologist (R.E.M.), who was unaware of the results of IHC analysis. Histologic material was assigned by serial number and blinded for investigators (S.S. and Y.C.) who performed IHC staining blinded to patient information. Clinical data were maintained in a separate database by Duke Cancer Center biostatisticians (J.E.M. and J.E.H.), who performed statistical analyses.
IHC
Paraffin-embedded tumor sections underwent IHC staining for five protein markers with the following antibodies: VEGF-A (Santa Cruz Biotechnology, Santa Cruz, CA), VEGFR-2/KDR (LabVision, Fremont, CA), CD31 antibody (Dako North America, Carpinteria, CA), HIF-2α antibody (Chemicon, Temecula, CA), and CA9 antibody (Novus Biologicals, Littleton, CO). A modified diaminobenzidine-streptavidin technique was used for IHC, as previously described.15 Briefly, microwaving for antigen retrieval was used (Appendix Table A1, online only). All primary antibodies were applied overnight at 4°C After washing with phosphate-buffered saline (PBS), sections were incubated with appropriate secondary antibodies, antirabbit or antimouse at 1:2,000 (Jackson ImmunoResearch Laboratories, West Grove, PA), for 30 minutes and washed in PBS. Vectastain Elite ABC reagent (Vector Laboratories, Burlingame, CA) was applied for 30 minutes, and sections were washed in PBS. The color was developed by incubation with diaminobenzidine solution (Vector Laboratories), and sections were counterstained with hematoxylin.
Semiquantitative IHC Analysis
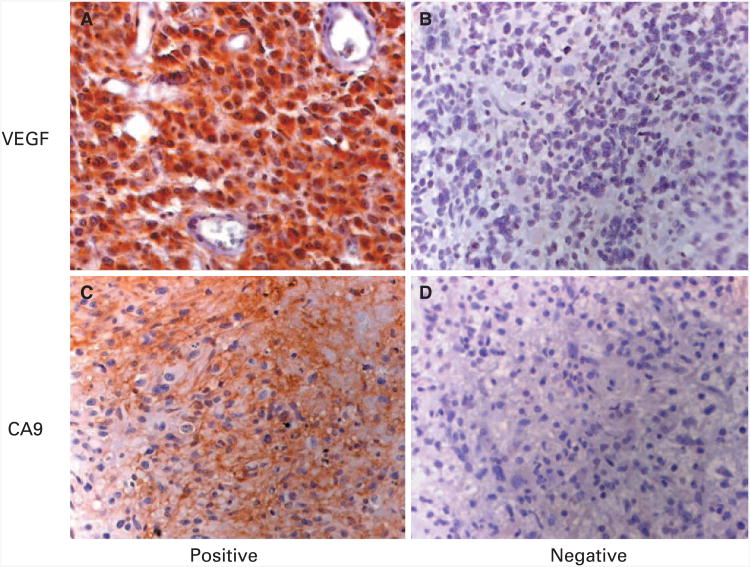
All optical fields on each sample were evaluated, and three to 10 fields of tumor area were randomly selected and imaged at ×200 magnification for CD31 staining and ×400 magnification for all other markers. Necrotic areas, normal brain tissue, and inadequate samples were excluded. Positive staining was determined qualitatively by two independent investigators (S.S. and Y.C; Figs 1A to 1D). Ten representative images of positive staining were used to establish a positive threshold for each marker on a color range program in the Adobe Photoshop 7 program (Adobe Systems, San Jose, CA), as previously described.15 Positive threshold was initially assessed on 20 randomly selected images and optimized for sensitivity and specificity. This optimal positive threshold for each marker was subsequently applied to all image samples. Positive areas (pixels/×200 to ×400 field) were semiautomatically obtained, and mean area for each sample (three to eight fields) was further used in statistical correlation with clinical parameters. Of note, one image field contains 1.339 × 106 pixels. A dichotomous scoring system (positive, high reactivity v negative, low/absent reactivity) was generated from continuous data (pixels of positive area) for each marker by using a qualitatively guided generation of cut points. In addition, all stained slides for VEGF and CA9 were independently scored as previously described16,17 by a neuropathologist (R.E.M.), who was unaware of clinical and image analysis. A semiquantitative score was derived from an intensity score of the reactivity product (absent, 0; mild, 1; moderate, 2; and strong, 3) related to endogenous positive controls multipliedby distribution score (percentage of reactive cells in tumor). Immunoreactivity scores of greater than 20 for VEGF and ≥ 20 for CA9 were considered high (positive).
Fig 1.
Semiquantitative image analysis. Representative images used to establish the optimal positive threshold for each marker: (A) vascular endothelial growth factor (VEGF) positive; (B) VEGF negative; (C) carbonic anhydrase 9 (CA9) positive; and (D) CA9 negative.
Statistical Analysis
For each biomarker (VEGF, KDR, CD31, CA9, and HIF-2α), the agreement between measures of immunoreactivity obtained by semiquantitative image analysis and traditional semiquantitative assessment were evaluated by κ statistics. Given concerns about the validity of the normality assumption for uncategorized measures of the reactivity of each biomarker, Spearman rank correlation, instead of Pearson's correlation coefficient, was used to assess the association between biomarkers.
Survival was determined from the time of treatment initiation, unless otherwise stated, until the time of death or last follow-up. Patients alive at follow-up were censored. The Kaplan-Meier curve was used to graphically describe the survival of patients within subgroups defined by age, histologic grade, and dichotomized measures of biomarker reactivity. Log-rank tests were used to compare the survival of patients within these subgroups. Cox proportional hazards model was used to examine the effect of individual factors on survival, as well as the joint effect of several predictors. For each biomarker, Fisher's exact test was used to assess the relationship of a dichotomized measure of the biomarker's reactivity with radiographic response or the proportion of patients alive after 1 year of follow-up.
Statistical analysis was performed and graphs were generated with an SAS program (SAS Institute, Cary, NC). P < .05 was considered statistically significant.
Results
Patients
Of 60 patients with WHO grade 3 to 4 astrocytomas included in the analysis for our phase II clinical trial, we obtained paraffin-embedded tumor specimens from 45 patients (75%). The patient subset in the current IHC study is representative of clinical trial patients with similar characteristics, radiographic response rates, and survival outcomes (Table 1). Of 45 patients in the current study, 26 (58%) had at least partial response as measured by Macdonald criteria (≥ 50% reduction in size of enhancing tumor on consecutive magnetic resonance images at least 1 month apart, corticosteroids stable or reduced, and neurologically stable or improved).18 Forty-six percent of patients (12 or 26 patients) who experienced radiographic response achieved survival for more than 1 year after treatment initiation. With a median follow-up time of 70.6 weeks (95% CI, 66.6 to 85.4 weeks), 16 patients (36%) were alive at analysis, with seven of these patients completing 1 year of treatment. Ten patients (37%) with GBM and nine patients (50%) with anaplastic astrocytoma (AA) survived for more than 1 year after treatment initiation. Of note, radiographic response was not predictive of 1-year survival (Appendix Table A2, online only).
Table 1. Patient Characteristics.
| Characteristic | Patients on Clinica Trial (n = 60) | Patients on IHC Study (n = 45) |
|---|---|---|
| Sex | ||
| Male | ||
| No. of patients | 40 | 30 |
| % | 67 | 67 |
| Female | ||
| No. of patients | 20 | 15 |
| % | 33 | 33 |
|
| ||
| Age, years | ||
| Median | 47.5 | 49 |
| Range | 18-66 | 18-66 |
|
| ||
| WHO grade | ||
| Grade 4 | ||
| No. of patients | 35 | 27 |
| % | 58 | 60 |
| Grade 3 | ||
| No. of patients | 25 | 18 |
| % | 42 | 40 |
|
| ||
| KPS | ||
| Median | 80 | 80 |
| Range | 60-100 | 60-100 |
|
| ||
| Prior radiotherapy | ||
| No. of patients | 60 | 45 |
| % | 100 | 100 |
|
| ||
| No. of prior progressions | ||
| Median | 2 | 2 |
| Range | 1-5 | 1-5 |
|
| ||
| Radiographic response rate, % | 55 | 58 |
|
| ||
| Overall survival, weeks | ||
| Median | 47 | 49 |
| Range | 36.7-73.7 | 37.1-73.7 |
|
| ||
| PFS, weeks | ||
| Median | 24.8 | 27.7 |
| Range | 18.3-34 | 23-34 |
|
| ||
| PFS at 6 months, % | 48.3 | 51.1 |
Abbreviations: IHC, immunohistochemistry; KPS, Karnofsky performance score; PFS, progression-free survival.
Biomarkers
Representative images of each marker are illustrated in Figure 2. Using semiquantitative image analysis of each biomarker, we obtained means of reactive areas (pixels/high-powered field) from each sample and dichotomized them into high (positive) or absent/low (negative) using a qualitatively guided method to generate cut points (VEGF, 5,000 pixels; KDR, 5,000 pixels; CD31, 10,000 pixels; CA9, 10,000 pixels; and HIF-2α, 5,000 pixels). With these cut points, the frequencies of high reactivity of each marker were as follows: VEGF, 24%; KDR, 31%; CD31, 34%; CA9, 20%; and HIF-2α, 28%. Confirmatory traditional semiquantitative analysis by a neuropathologist (R.E.M.) was independently performed for VEGF and CA9. Immunoreactivity scores for VEGF ranged from 0 to 120, with 17% of patients having scores of more than 20 (positive), whereas immunoreactivity scores for CA9 ranged from 0 to 160, with 23% of patients having scores of 20 or greater (positive). κ statistics were used to show that the agreement between image analysis and traditional semiquantitative assessment was high (Appendix Table A3, online only).
Fig 2.
Representative immunostaining detection of angiogenic/hypoxic markers. (A and B) Vascular endothelial growth factor (VEGF) in tumor cytoplasm and stroma. (C and D) Kinase insert domain-containing receptor (KDR) on endothelial and tumor cell membrane and cytoplasm. (E and F) CD31 on vascular endothelial cells (arrows). (G and H) Carbonic anhydrase 9 (CA9) on tumor cell membrane and cytoplasm, adjacent to necrotic area (*) and relatively distant from blood vessels (arrows). (I and J) Hypoxia-inducible factor-2α (HIF-2α) in tumor cell nuclei and cytoplasm in a geographic distribution similar to that of CA9 reactivity.
Correlations Between Biomarkers
Spearman rank correlation was used to assess the association of uncategorized reactivitybetween markers (Appendix Table A4, online only). Higher correlations were noted among angiogenic markers (VEGF, KDR, and CD31) and among hypoxic markers (CA9 and HIF-2α), with most correlation coefficients being more than 0.5 and with highly significant P values. Significant correlations were also noted between angiogenic markers and CA9 but with lower correlation coefficients (r < 0.5). These findings suggest that all five biomarkers share regulatory mechanisms.
VEGF As a Biomarker for Radiographic Response
Fisher's exact test was used to assess the association of radiographic response and a dichotomized measure of biomarker reactivity. We found that high VEGF expression (mean positive area > 5,000 pixels/×400 field) was associated with increased likelihood of achieving radiographic response (P = .024; Table 2). This test is associated with a sensitivity of 36% (95% CI, 18% to 57.5%), specificity of 94% (95% CI, 74% to 99.9%), and positive predictive value of 90% (95% CI, 55.5% to 99.8%). Other markers, including KDR, CD31, CA9, and HIF-2α, were not significantly associated with radiographic response (all P > .1). Of note, VEGF did not predict radiographic response in patients with AAs but trended towards significance in the glioblastoma cohort (Fisher's exact test, P = .1; Appendix Table A5, online only).
Table 2. Univariate Analysis Predicts Response and Survival: Association Between VEGF Expression and Radiographic Response*.
| Radiographic Response (No. of patients) | |||
|---|---|---|---|
|
|
|||
| VEGF | CR+PR | SD+PD | Total No. of Patients |
| Positive | 9 | 1 | 10 |
|
| |||
| Negative | 16 | 16 | 32 |
|
| |||
| Total | 25 | 17 | 42 |
Abbreviations: VEGF, vascular endothelial growth factor; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease
Two-tailed P = .024 (Fisher's exact test).
CA9 As a Biomarker for Survival Outcome
High CA9 expression (mean positive area > 10,000 pixels/high-powered field) was associated with poor 1-year survival in a univariate analysis (Fisher's exact test, P = .0023; Table 3). All nine patients with high CA9 expression did not survive more than 1 year after initiation of bevacizumab treatment. Interestingly, two thirds of these patients (six of nine patients) initially experienced radiographic response. The median survival time of patients with high CA9 expression was 37 weeks (95% CI, 35.0 to 41.6 weeks), whereas the median survival time of patients with low/absent CA9 expression was 74 weeks (95% CI, 36.3 weeks to not reached). A proportional hazards model with only uncategorized CA9 expression in the model showed that high CA9 expression was associated with poor survival, with a hazard ratio (HR) of 2.72 (95% CI, 1.17 to 6.36; P = .02; Fig3A).
Table 3. Univariate Analysis Predicts Response and Survival: Association Between CA9 Expression and 1-Year Survival*.
| Overall Survival pativival (No. of patients) | |||
|---|---|---|---|
|
|
|||
| CA9 | < 1 Year | > 1 Year | Total No. of Patients |
| Positive | 9 | 0 | 9 |
|
| |||
| Negative | 15 | 19 | 34 |
|
| |||
| Total | 24 | 19 | 43 |
Abbreviation: CA9, carbonic anhydrase 9
Two-tailed P = .0023 (Fisher's exact test)
Fig 3.
Kaplan-Meier survival curves stratified by biomarker status. (A) Survival stratified by carbonic anhydrase 9 (CA9) status. (B) Survival stratified by CA9 and hypoxia-inducible factor-2α (HIF-2) status.
Additional analyses were conducted to determine whether survival analyses examining the effect of biomarkers on survival needed adjustment for traditional prognostic factors such as age and WHO grade. Proportional hazards analyses revealed that age and WHO grade were not independent prognostic factors for survival, as calculated from the time of recurrence/treatment initiation (Table 4; Appendix Table A6, online only). Thus, these factors were excluded from subsequent Cox models. Of note, advanced age (≥ 50 years) and WHO grade (GBM v AA) were independent prognostic factors for survival calculated from the time of original diagnosis when tumor samples were collected (advanced age: HR = 2.6, P = .034; WHO grade: GBM, HR = 3.8, P = .007; Appendix Table A7, online only).
Table 4. Cox Regression Models Predicting Overall Survival.
| Biomarker | P(x2) | HR | 95% CI |
|---|---|---|---|
| VEGF | .703 | 1.18 | 0.50 to 2.83 |
| KDR | .128 | 0.52 | 0.22 to 1.21 |
| CD31 | .652 | 0.81 | 0.33 to 2.02 |
| CA9 | .020 | 2.72 | 1.17 to 6.36 |
| HIF-2α | .133 | 1.91 | 0.82 to 4.47 |
Abbreviations: HR, hazard ratio; VEGF, vascular endothelial growth factor; KDR, kinase-insert domain containing receptor (also known as VEGF receptor-2); CA9, carbonic anhydrase 9; HIF-2α, hypoxia-inducible factor-2α.
The relationship between HIF-2α expression and 1-year survival has a trend towards significance (P = .07), suggesting that HIF-2α may be associated with poor survival (Appendix Fig A1, online only). Analysis of the relationship of biomarkers by tumor grade demonstrated that CA9 was significantly associated with survival (P = .012), with a trend for HIF-2α (P = .079) in glioblastoma patients but not in the AA cohort (Appendix Table A8, online only). However, HIF-2α expression failed to show significant correlation with survival in a Cox model (Table 4).
When both CA9 and HIF-2α were simultaneously included in a Cox model as two separate factors, CA9 remained a statistically significant predictor of overall survival (HR = 2.58; 95% CI, 1.0 to 6.64; P = .049), whereas HIF-2α was not predictive of overall survival (HR = 1.40; 95% CI, 0.56 to 3.53; P = .5). The patient subgroup with negative CA9 and negative HIF-2α displayed the best prognosis, whereas the subgroup with positive CA9 and positive HIF-2α was associated with the worst prognosis (significant by the log-rank test, with P = .036, when comparing CA9 negative/HIF-2α negative with CA9 positive/HIF-2α positive; borderline significant by the log-rank test, with P = .067, when comparing all four subgroups; Fig 3B). There are no significant differences in survival for the three angiogenic markers VEGF, VEGFR-2, or CD31 (all P > .1).
Discussion
The rational development of targeted molecular therapies for cancer patients has driven the development of biomarkers to identify patient populations likely to experience benefit from an agent. Although the response of glioma patients may be enriched through the use of validated biomarkers (eg, 1p19q deletion19 and O6-methylguanine–DNA methyltransferase methylation20), biomarker discovery for targeted therapies has been more limited in neuro-oncology21 as a result of several challenges. First, brain tumors are relatively uncommon, limiting the number of patients available for analysis. Second, tissue acquisition of brain tumors poses challenges because the risk of neurosurgical complications precludes repeated tumor sampling. Because bevacizumab has a long half-life and increases the risk of postoperative hemorrhage, the collection of tumor biopsies at the time of recurrence is currently contraindicated for this protocol. Additionally, examples of targeted therapies that demonstrate striking efficacy in subgroups of patients have been rare. For example, characterization of the EGFR and PI3K-PTEN-Akt pathways informs EGFR inhibitor use,5,6 but EGFR inhibitors have not displayed sustained clinical benefit.22 The initial results from antiangiogenic therapies suggest that these agents may have clinical benefit for which biomarkers may be useful.1-3,23
Bevacizumab and irinotecan treatment induce a significant radiographic response rate and survival benefit,2,3 whereas irinotecan monotherapy is largely ineffective.24-26 Thus, the efficacy of the current regimen may be a result of bevacizumab alone or the combination with irinotecan. In the current study, we attempted to identify predictive biomarkers for radiographic response and/or survival benefit in patients with malignant astrocytomas treated with this combination regimen.
In our analysis, high tumor VEGF expression was associated with an increased radiographic response to bevacizumab but not increased survival. Current standard radiographic response assessment in neuro-oncology (Macdonald criteria) uses contrast-enhanced magnetic resonance imaging, which depicts the area of blood-brain barrier disruption.18,27 Because VEGF (also known as vascular permeability factor) regulates vascular permeability, targeting VEGF with bevacizumab may decrease contrast leakage into the tumor to produce a radiographic response. Our IHC findings, albeit limited by small sample size, support the proof-of-concept that malignant glioma patients, who have enriched tumor target (VEGF) expression, are more likely to achieve radiographic response than patients who have no or low target expression. These studies are in line with other studies of solid cancers in which VEGF expression did not correlate with survival but in which radiographic response was not assessed.28 Although bevacizumab and irinotecan are associated with a high radiographic response rate, only approximately one half of these patients achieved survival for more than 1 year, which suggests that radiographic response does not always translate into survival benefit (Appendix Table A2). Likewise, patients can achieve long-term survival even though they do not experience radiographic response during the course of bevacizumab treatment.
Our analysis demonstrated that CA9, an endogenous marker for hypoxia and acidosis, was associated with poor survival outcome in both univariate and multivariate analyses. CA9 has been shown to be an independent prognostic marker in patients with various cancers including malignant gliomas.9-11,29,30 Although bevacizumab and irinotecan have a more prominent efficacy in patients with low CA9-expressing tumors, this therapy still offers survival benefit, albeit modest, in patients with high CA9 expression (median survival time, 37 weeks) when compared with the survival of recurrent or progressive glioma patients treated with various salvage therapeutic regimens (median survival time, 28 to 30 weeks) from two independent metaanalyses of several phase II trials.31,32 HIF-2α is a transcription factor whose roles in hypoxia-induced angiogenesis differ from those of HIF-1α, an upstream transcription factor of CA9. In head and neck cancer, both CA9 and HIF-2α serve as independent and additive prognostic factors for survival and response to radiotherapy.14 In our study, we have demonstrated significant correlation between CA9 and HIF-2α status (Appendix Table A4). However, high HIF-2α expression was not significantly associated with poor survival outcome in our analyses. When combining these two hypoxic markers (ie, CA9 and HIF-2α), patients with high CA9 and high HIF-2α expression seemed to have the worst survival outcome, whereas patients with negative CA9 and negative HIF-2α had the best outcome (Fig 3).
Future clinical trials of bevacizumab may incorporate imaging techniques not dependent on neurovascular integrity to permit a dissection of radiographic response separate from direct effects on the VEGF axis. Furthermore, noninvasive modalities are under development to measure tumor hypoxia.33 For the immediate future, we advocate independent validation of these angiogenic/hypoxicbiomarkers in larger prospective studies to support the ultimate development of anti-VEGF therapy in malignant gliomas.
Acknowledgments
We thank Darell Bigner for helpful discussions and Alyssa Mathe and Kaitlyn A. Vredenburgh for their assistance in tumor specimen acquisition and coding.
Supported by the Childhood Brain Tumor Foundation, the Pediatric Brain Tumor Foundation of the United States, Accelerate Brain Cancer Cure, Duke Comprehensive Cancer Center Stem Cell Initiative Grant (J.N.R.), and National Institutes of Health Grants No. NS047409, NS054276, and CA116659 (J.N.R.). J.N.R. is a Damon Runyon-Lilly Clinical Investigator supported by the Damon Runyon Cancer Research Foundation and a Sidney Kimmel Foundation for Cancer Research Scholar.
Glossary
- Angiogenesis
The process involved in the generation of new blood vessels. While this is a normal process that naturally occurs and is controlled by “on” and “of” switches, blocking tumor angiogenesis (antiangiogenesis) disrupts the blood supply to tumors, thereby preventing tumor growth
- CA (carbonic anhydrase)
Carbonic anhydrases (CAs) are involved in several physiological processes, including pH regulation, CO2 and HCO3 transport, and water and electrolyte balance. Eight distinct CA isozymes and additional CA-related proteins have been identified
- HIF (hypoxia-inducible factor)
HIF is a transcriptional factor that regulates the adaptive responses of mammalian cells to low oxygen (hypoxia). It is composed of HIF-1α, which is upregulated in conditions of hypoxia, and HIF-1β (or, aryl hydrocarbon receptor nuclear translocators), which is expressed constitutively. Dimerization of HIF-1α with HIF-1β leads to transcription of genes such as VEGF and PDGF
- Hypoxia
Oxygen concentration below normal physiological limits in a specific tissue
- Humanized antibody
Also commonly known as a CDRgrafted antibody, it is a monoclonal antibody that has been molecularly engineered to splice the antigen-binding domains of a murine antibody (ie, the CDRs [complementarity-determining regions]) onto human immunoglobulin framework
- Immunohistochemistry
The application of antigen-antibody interactions to histochemical techniques. Typically, a tissue section is mounted on a slide and is incubated with antibodies (polyclonal or monoclonal) specific to the antigen (primary reaction). The antigen-antibody signal is then amplified using a second antibody conjugated to a complex of peroxidase-antiperoxidase (PAP), avidin-biotin-peroxidase (ABC) or avidinbiotin alkaline phosphatase. In the presence of substrate and chromogen, the enzyme forms a colored deposit at the sites of antibody-antigen binding. Immunofluorescence is an alternate approach to visualize antigens. In this technique, the primary antigen-antibody signal is am-plified using a second antibody conjugated to a fluorochrome. On UV light absorption, the fluorochrome emits its own light at a longer wavelength (fluorescence), thus allowing localization of antibody-antigen complexes
- Immunohistochemical analyses
Techniques used to evaluate the levels and patterns of expression of protein on cells or tissue specimens located on flat slides
- VEGF (vascular endothelial growth factor)
VEGF is a cytokine that mediates numerous functions of endothelial cells including proliferation, migration, invasion, survival, and permeability. VEGF is also known as vascular permeability factor. VEGF naturally occurs as a glycoprotein and is critical for angiogenesis. Many tumors overexpress VEGF, which correlates to poor prognosis. VEGF-A, -B, -C, -D, and -E are members of the larger family of VEGF-related proteins
- VEGFR (vascular endothelial growth factor receptor)
VEGFRs are transmembrane tyrosine kinase receptors to which the VEGF ligand binds. VEGFR-1 (also called Flt-1) and VEGFR-2 (also called KDR/Flk-1[murine homologue]) are expressed on endothelial cells, while VEGFR-3 (also called Flt-4) is expressed on cells of the lymphatic and vascular endothelium. VEGFR-2 is thought to be principally responsible for angiogenesis and for the proliferation of endothelial cells. Typically, most VEGFRs have seven extracellular immunoglobulin-like domains, responsible for VEGF binding, and an intracellular tyrosine kinase domain
- VEGF inhibitor
Inhibits VEGF-induced signaling by blocking ligand binding to its cognate cell surface receptor or by blocking the kinase activity of the receptor
Appendix.
The Appendix is included in the full-text version of this article, available online at www.jco.org. It is not included in the PDF version
Footnotes
Authors' Disclosures Of Potential Conflicts Of Interest: Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: None Research Funding: Henry S. Friedman, Genentech Expert Testimony: None Other Remuneration: None
Author Contributions: Conception and design: Sith Sathornsumetee, Jeremy N. Rich Administrative support: Henry S. Friedman
Provision of study materials or patients: Sith Sathornsumetee, Annick Desjardins, Henry S. Friedman, Mark W. Dewhirst, James J. Vredenburgh, Jeremy N. Rich
Collection and assembly of data: Sith Sathornsumetee, Yiting Cao, Roger E. McLendon
Data analysis and interpretation: Sith Sathornsumetee, Yiting Cao, Jennifer E. Marcello, James E. Herndon II, Roger E. McLendon, Jeremy, N. Rich
Manuscript writing: Sith Sathornsumetee, Jeremy N. Rich
Final approval of manuscript: Sith Sathornsumetee, Yiting Cao, Jennifer, E. Marcello, James E. Herndon II, Roger E. McLendon, Annick, Desjardins, Henry S. Friedman, Mark W. Dewhirst, James J., Vredenburgh, Jeremy N. Rich
References
- 1.Jain RK, di Tomaso E, Duda DG, et al. Angiogenesis in brain tumours. Nat Rev Neurosci. 2007;8:610–622. doi: 10.1038/nrn2175. [DOI] [PubMed] [Google Scholar]
- 2.Vredenburgh JJ, Desjardins A, Herndon JE, II, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13:1253–1259. doi: 10.1158/1078-0432.CCR-06-2309. [DOI] [PubMed] [Google Scholar]
- 3.Vredenburgh JJ, Desjardins A, Herndon JE, II, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25:4722–729. doi: 10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
- 4.Jubb AM, Oates AJ, Holden S, et al. Predictng benefit from anti-angiogenic agents in malignancy. Nat Rev Cancer. 2006;6:626–635. doi: 10.1038/nrc1946. [DOI] [PubMed] [Google Scholar]
- 5.Haas-Kogan DA, Prados MD, Tihan T, et al. Epidermal growth factor receptor protein kinase B/Akt, and glioma response to erlotinib. J Natl Cancer Inst. 2005;97:880–887. doi: 10.1093/jnci/dji161. [DOI] [PubMed] [Google Scholar]
- 6.Mellinghoff IK, Wang MY, Vivanco I, et al. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N Engl J Med. 2005;353:2012–2024. doi: 10.1056/NEJMoa051918. [DOI] [PubMed] [Google Scholar]
- 7.Pouysségur J, Dayan F, Mazure NM. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature. 2006;441:437–443. doi: 10.1038/nature04871. [DOI] [PubMed] [Google Scholar]
- 8.Kaur B, Khwaja FW, Severson EA, et al. Expression of carbonic anhydrase IX in astrocytic tumors predicts poor prognosis. Clin Cancer Res. 7:153–2005. doi: 10.1158/1078-0432.CCR-05-0848. [DOI] [PubMed] [Google Scholar]
- 9.Haapasalo JA, Nordfors KM, Hilvo M, et al. Expression of carbonic anhydrase IX in astrocytictumors predicts poor prognosis. Clin Cancer Res. 2006;473:477. doi: 10.1158/1078-0432.CCR-05-0848. [DOI] [PubMed] [Google Scholar]
- 10.Korkolopoulou P, Perdiki M, Thymara I, et al. A multivariate survival study with emphasis upon carbonic anhydrase IX. Hum Pathol. 2007;38:629–638. doi: 10.1016/j.humpath.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 11.Potter C, Harris AL. Hypoxia inducible carbonic anhydrase IX, marker of tumour hypoxia, survival pathway and therapy target. Cell Cycle. 2004;3:164–167. [PubMed] [Google Scholar]
- 12.Sowter HM, Raval R, Moore J, et al. Predominant role of hypoxia inducible transcription factor Hif-1α versus Hif-2α in regulation of the transcriptional response to hypoxia. Cancer Res. 2003;63:6130–6134. [PubMed] [Google Scholar]
- 13.Hu CJ, Wang LY, Chodosh LA, et al. Differential roles of hypoxia inducible factor 1 alpha (HIF-1α) and HIF-2α in hypoxic gene regulation. Mol Cell Biol. 2003;23:9361–9374. doi: 10.1128/MCB.23.24.9361-9374.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koukourakis MI, Bentzen SM, Giatromanolaki A, et al. Endogenous markers of two separate hypoxia response pathways (hypoxia inducible factor 2 alpha and carbonic anhydrase 9) are associated with radiotherapy failure in head and neck cancer patients recruited in the CHART randomized trial. J Clin Oncol. 24:727–735. 2006. doi: 10.1200/JCO.2005.02.7474. [DOI] [PubMed] [Google Scholar]
- 15.Cao Y, Sonveaux P, Liu S, et al. Systemic overexpression of angiopoietin-2 promotes tumor microvessel regression and inhibits angiogenesis and tumor growth. Cancer Res. 2007;67:3835–3844. doi: 10.1158/0008-5472.CAN-06-4056. [DOI] [PubMed] [Google Scholar]
- 16.McLendon RE, Wikstrand CJ, Matthews MR, et al. Glioma-associated antigen expression in oligodendroglial neoplasms: Tenascin and epiderma growth factor receptor. J Histochem Cytochem. 2000;48:1103–1110. doi: 10.1177/002215540004800807. [DOI] [PubMed] [Google Scholar]
- 17.Choe G, Horvath S, Cloughesy TF, et al. Analysis of the phosphatidylinositol 3′-kinase signalingpathway in glioblastoma patients in vivo. Cancer Res. 2003;63:2742–2746. [PubMed] [Google Scholar]
- 18.Macdonald DR, Cascino TL, Schold SC, et al. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 19.Cairncross JG, Ueki K, Zlatescu MC, et al. Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J Natl Cancer Inst. 1998;90:1473–1479. doi: 10.1093/jnci/90.19.1473. [DOI] [PubMed] [Google Scholar]
- 20.Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 21.Mischel PS, Cloughesy T. Using molecular information to guide brain tumor therapy. Nat Clin Pract Neurol. 2006;2:232–233. doi: 10.1038/ncpneuro0145. [DOI] [PubMed] [Google Scholar]
- 22.Prados MD, Lamborn KR, Chang S, et al. combined with temozolomide in patients with stable or recurrent malignant glioma. Neuro Oncol. 2006;8:67–78. doi: 10.1215/S1522851705000451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Batchelor TT, Sorensen AG, di Tomaso E, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11:83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friedman HS, Petros WP, Friedman AH, et al. Irinotecan therapy in adults with recurrent or progressive malignant glioma. J Clin Oncol. 1999;17:1516–1525. doi: 10.1200/JCO.1999.17.5.1516. [DOI] [PubMed] [Google Scholar]
- 25.Batchelor TT, Gilbert MR, Supko JG, et al. Phase 2 study of weekly irinotecan in adults with recurrent malignant glioma: Final report of NABTT 97-11. Neuro Oncol. 2004;6:21–27. doi: 10.1215/S1152851703000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prados MD, Lamborn K, Yung WK, et al. A phase 2 trial of irinotecan (CPT-11) in patients with recurrent malignant glioma: A North American Brain Tumor Consortium study. Neuro Oncol. 2006;8:189–193. doi: 10.1215/15228517-2005-010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pope WB, Lai A, Nghiemphu P, et al. MRI in patients with high-grade gliomas treated with bevacizumab and chemotherapy. Neurology. 2006;66:1258–1260. doi: 10.1212/01.wnl.0000208958.29600.87. [DOI] [PubMed] [Google Scholar]
- 28.Jubb AM, Hurwitz HI, Bai W, et al. Impact of vascular endothelial growth factor-A expression, thrombospondin-2 expression, and microvessel density on the treatment effect of bevacizumab in metastatic colorectal cancer. J Clin Oncol. 2006;24:217–227. doi: 10.1200/JCO.2005.01.5388. [DOI] [PubMed] [Google Scholar]
- 29.Swietach P, Vaughan-Jones RD, Harris AL. Regulation of tumor pH and the role of carbonic anhydrase 9. Cancer Metastasis Rev. 2007;26:299–310. doi: 10.1007/s10555-007-9064-0. [DOI] [PubMed] [Google Scholar]
- 30.Atkins M, Regan M, McDermott D, et al. Carbonic anhydrase IX expression predicts outcome of interleukin 2 therapy for renal cancer. Clin Cancer Res. 2005;11:3714–3721. doi: 10.1158/1078-0432.CCR-04-2019. [DOI] [PubMed] [Google Scholar]
- 31.Wong ET, Hess KR, Gleason MJ, et al. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol. 1999;17:2572–2578. doi: 10.1200/JCO.1999.17.8.2572. [DOI] [PubMed] [Google Scholar]
- 32.Carson KA, Grossman SA, Fisher JD, et al. Prognostic factors for survival in adult patients with recurrent glioma enrolled onto the New Approaches to Brain Tumor Therapy CNS Consortium phase I and II clinical trials. J Clin Oncol. 2007;25:2601–2606. doi: 10.1200/JCO.2006.08.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Serganova I, Humm J, Ling C, et al. Tumor hypoxia imaging. Clin Cancer Res. 2006;12:5260–5264. doi: 10.1158/1078-0432.CCR-06-0517. [DOI] [PubMed] [Google Scholar]