Abstract
OBJECTIVES
The extracellular matrix (XCM Biologic Tissue Matrix) is a non-cross-linked 3D patch derived from porcine dermis. Once implanted, it is infiltrated by recipient's cells and becomes incorporated in the repair. Here, we report the first series of using this device for chest wall reconstruction.
METHODS
The XCM Biologic Tissue Matrix was utilized to provide the restoration of chest wall defects. It was used either alone or in conjunction with the Synthes titanium system to provide additional support. The decision was made intraoperatively.
RESULTS
Since April 2010, 21 (12 females) patients received the device. Average age at operation was 47 ± 17 years. Eleven (52%) patients had the patch inserted alone, while the remaining 10 received it in combination with another implantable medical device. The biological tissue matrix was used to reconstruct chest wall defects in cancer involving chest wall (n = 9), chest wall deformity (n = 6), chest wall hernia (n = 5) and chest wall repair following empyema drainage (n = 1). Complications were witnessed in 3 patients receiving the combined XCM and Synthes bar mechanisms; infection (n = 2) and bar displacement and infection (n = 1).
CONCLUSIONS
The XCM patch can be safely used to provide the strength required for chest wall reconstruction and to replace previously infected reconstructions.
Keywords: XCM, Synthes, Chest wall, Biological tissue matrix
INTRODUCTION
Chest wall reconstruction remains an under-performed procedure by thoracic surgeons, despite sufficient evidence to demonstrate that reconstruction improves postoperative ventilation, shortens overall hospital stay and improves postoperative pulmonary physiology and mechanics [1–5].
Fifty years ago, autogenous reconstruction (such as fascia lata, rib, muscle or omentum) was the standard procedure in chest wall reconstruction. The main advantage was to avoid alloplastic implantable materials. This, however, was on the expense of donor site morbidity. Other limitations included the limited amount of tissue available for repairing larger defects and the technical expertise needed.
A major advancement in chest wall reconstruction was the introduction of prosthetic implantable materials. Following the trend as in other surgical specialties, the most commonly used chest wall implants are synthetic polytetrafluoroethylene (PTFE) and polypropylene with or without methylmethacrylate. However, the complications associated with these materials, such as secondary wound infections, seromas, fracture and insufficient tensile strength, to protect intrathoracic organs made their utilization admonitory [4, 6].
Although their use remains in its infancy, the introduction of the biocompatible prosthetic materials is expected to revolutionize the industry and to expand on the rate of chest wall reconstruction. This is mainly due to the near-physiological properties these materials possess.
Here, we report the first series of using the XCM Biologic Tissue Matrix (Depuy Synthes, Oberdorf, Switzerland) for chest wall reconstruction either alone or in combination with the Synthes Titanium System (Depuy Synthes, Oberdorf, Switzerland).
METHODS
Since April 2010, the XCM Biologic Tissue Matrix system was used as a reconstructive or a supportive mesh in 21 patients at the Department of Thoracic Surgery, St James's University Hospital, Leeds (Table 1).
Table 1:
21 patients received the XCM Biologic Tissue Matrix for chest wall reconstruction
| No. | Sex | Age | Underlying diagnosis | Procedure | Reconstructive material | Outcome |
|---|---|---|---|---|---|---|
| 1 | M | 29 | Metastatic liposarcoma with previous chest wall resection | Redo thoracotomy and right middle lobectomy and three ribs resection with parts of costal arch | XCM patch and three Synthes bars | Well |
| 2 | M | 57 | Metastatic thyroid cancer | Partial sternectomy and claviculectomy | XCM patch and one Synthes bar | Infection |
| 3 | M | 51 | RTA with multiple rib fractures and malunion. Initial reconstruction with three Synthes bar. Complicated by herniation and single bar fracture | Replacement of one fractured Synthes bar and hernia closure | XCM patch and one Synthes bar | Well |
| 4 | M | 71 | Left upper lobe tumour (pT3N1) | Left pneumonectomy and resection of ribs 6–8 | XCM patch | Well |
| 5 | F | 35 | Pectus deformity—previous four repairs with unstable anterior chest wall | Correction of persistent chest wall deformity | XCM and two Synthes bars | Well |
| 6 | F | 18 | Ewing sarcoma of the right ninth rib | Chest wall resection and reconstruction | XCM patch and two Synthes bars | Well |
| 7 | F | 16 | Poland syndrome | Chest wall reconstruction | XCM patch and four Synthes bars | Infection-treated conservatively |
| 8 | F | 63 | Metastatic parathyroid cancer | Right VATS (wedge excision) and endoscopic chest wall reconstruction | XCM patch | Well |
| 9 | F | 65 | Left upper lobe tumour | VATS lobectomy and ribs 3–5 resection | XCM patch | Well |
| 10 | F | 58 | Chest wall deformity with previous repair and persistent deformity | Left costal cartilage resection | XCM patch | Well |
| 11 | F | 20 | Pectus deformity | Pectus repair with XCM patch to hold sternum | XCM patch | Well |
| 12 | M | 59 | Neurofibrosarcoma right upper lobe resected 25 years earlier—chest wall recurrence and tracheal stenosis | Clavicular, sternectomy, anterior chest wall resection and tracheal stenting | XCM patch and two Synthes bars | Infection, bar displacement and died (9 months) |
| 13 | F | 52 | Persistent postoperative chest wall deformity | Redo chest wall deformity repair | XCM patch and two Synthes bars | Well |
| 14 | F | 61 | Metastatic breast cancer to ribs 5–6 | Left VATS rib resection and reconstruction | XCM patch | Well |
| 15 | M | 51 | RTA with multiple rib fractures—initial repair complicated by an enlarging left subcostal hernia | Chest wall hernia repair | XCM patch | Well |
| 16 | F | 27 | Prominent costal cartilage—initial repair complicated by pain and significant abnormal movement | Redo chest wall reconstruction, removal of bone cement and Marlex Mesh | XCM patch and two Synthes bars | Well |
| 17 | F | 55 | Large cell carcinoma metastasized to the left transverse process of T11 | Chest wall resection, vertebral body resection and reconstruction | XCM patch and acrylic cement fashioned as ribs | Well |
| 18 | F | 34 | Carcinoid left upper lobe | Empyema drainage and chest wall reconstruction | XCM patch | Well |
| 19 | M | 55 | Chest wall hernia | Redo reconstruction after removal of infected Gortex patch | XCM patch | Well |
| 20 | M | 45 | Post-traumatic chest wall hernia | Reconstruction involving subcostal area | XCM patch | Well |
| 21 | M | 52 | Post-traumatic chest wall hernia | Redo reconstruction | XCM patch | Well |
RTA: road traffic accident; VATS: video assisted thoracic surgery.
The use of the biological tissue matrix was approved by our clinical directorate. When its potential use was considered in the preoperative planning, patients were advised and informed consents were obtained. For oncological patients, the inclusion criteria included: the diagnosis of primary or metastatic chest wall neoplasm different from lung cancer affecting at least three ribs, sternum, clavicle or thoracic spine, and the thoracic surrounding soft tissue. To plan the therapeutic approach in oncological patients, a multidisciplinary approach involving respiratory physicians, oncologists, radiologists and thoracic surgeons was employed. All patients underwent computed tomography scans of the chest, abdomen and brain before chest wall resection. For posterior or pancoast tumours, magnetic resonance imaging was performed. The resection involved at least two ribs, parietal muscles and when required one or part of the following: sternum, clavicle, diaphragm, soft tissue and pericardium.
The use of the biological tissue matrix for non-oncological diseases was made intraoperatively, taking into consideration the size and the location of the defects and the necessity for reinforcement when repairing chest wall deformities.
Preparation and implantation steps
The XCM Biologic Tissue Matrix is ready to use out-of-the box. It comes in various sizes, and in every case, it was fashioned to restore the continuity of the chest wall where necessary. It was shaped to match the defect and was sutured, using vicryl/ethibond/absorbable polyclonal interrupted sutures, under adequate tension, to either the bony framework of the chest wall or the free muscle edges (Fig. 1). Where rigidity was required, Synthes plates were used on top of the biological tissue matrix apart from 1 patient where acrylic cement was used (Fig. 2).
Figure 1:
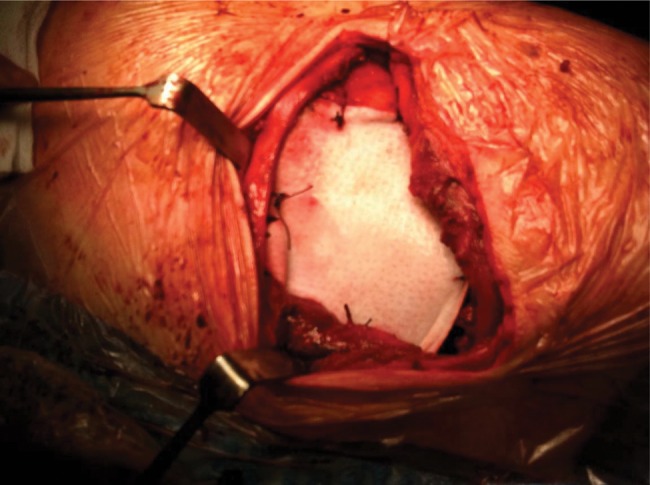
Chest wall reconstruction with the XCM Biologic Tissue Matrix following chest wall resection for invasive left upper lobe adenocarcinoma.
Figure 2:
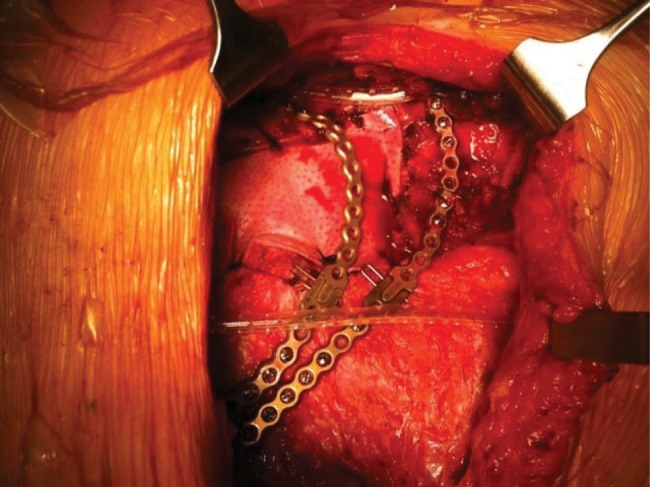
Chest wall reconstruction with the XCM Biologic Tissue Matrix enforced with two Synthes bars.
RESULTS
Between April 2010 and March 2013, 21 (12 females) patients with a mean age of 46 ± 17 years [median age 52 years, range (16–71)] had the XCM Biologic Tissue Matrix implanted in our institute. The indications for the XCM Biologic Tissue Matrix in chest wall repair/reconstruction were: cancer (n = 9), deformity (n = 6), hernia (n = 5) and following empyema drainage (n = 1). Eleven (52%) patients had the device alone, whereas the remaining 10 received it in conjunction with a reinforcing material: Synthes bars (n = 9) and acrylic cement fashioned as ribs (n = 1).
In total, 3 patients had post-implantation complications; Patients 2 and 7 with the combined XCM and Synthes mechanisms had infection and Patient 12 had infection and bar displacement. Overall, 1 patient (Patient 12) died from the progression of his underlying malignant disease.
DISCUSSION
The advance of biological prosthesis into chest surgery is a new challenge. Although synthetic tissue material provides strong tissue reinforcement, it remains a source of a foreign body reaction, which can result in serious complications. Extracellular biological meshes were introduced in the 1990s and provide the extracellular scaffold necessary for tissue healing. They are either derived from human (allograft; derived from dermis, intestinal mucosa or pericardium) or animal (xenograft; usually porcine or bovine) tissues.
Most published studies on biological meshes are case series, and their use was limited to contaminated or infected fields for abdominal wall reconstruction where a synthetic mesh would be strongly contraindicated [7]. This is the first series to report the elective use of the XCM Biologic Tissue Matrix system in chest wall reconstruction in thoracic surgery.
In 1983, LeRoux and Shama set the primary characteristics of an ideal prosthetic material: rigidity, malleability and radiolucency [8]. Secondary characteristics include inexpensiveness, incorporation by the body, durability, physically and chemically inert, resistance to infection and strain, inability to elicit inflammatory or foreign body reaction, non-carcinogenic and hypoallergenic, sterilizable.
The XCM Biologic Tissue Matrix is a sterile non-cross-linked 3D matrix derived from porcine dermis. Following the Kensey Nash's Optrix™ cleansing process, the device maintains a non-cross-linked collagen structure needed for strength and durability and preserves the natural fibrous architecture necessary for scaffold formation for cell in-growth and proliferation. Unlike cross-linked biological meshes, non-cross-linked meshes contract and degrade more rapidly following implantation resulting in an increased tensile strength of the tissue before a strong, mature wound has formed [9]. Immunohistochemical studies confirmed that biological tissue meshes retain the biological integrity of the extracellular matrix, namely: elastin, fibronectin, lamini, glycosaminoglycans, proteoglycans, growth factors and cytokines [10, 11]. In reconstructing the abdominal wall or perineum, longer-term persistence is more critical, and consequently, biological mesh durability have shown to reduce the recurrence rate [12]. Furthermore, Wiegman et al. [13] reported their chest wall repair experience using the Peri-Guard Repair Biological Patch on 3 patients with secondary incisional herniation after lung transplantation. At follow-up, they had physiological breathing mechanics and ultrasound confirmed the absence of adhesions, seroma and haematoma. They concluded the suitability of the biological mesh in the immunocompromised patients.
The main objective of an en bloc chest wall resection in malignant invasion was to achieve disease-free margins (R0). R0 can only be accomplished by radical and aggressive bone resection and depending on the extent of resection distortion of chest wall dynamics may ensue. Failure of the remaining respiratory musculoskeletal system to provide the physiological passive circumferential chest wall needed throughout the respiratory cycle will result in a restrictive disease pattern in the acute postoperative phase and thoracic insufficiency syndrome in the long term. The latter occurs in 26% of patients with large chest wall resection and failure of the non-rigid reconstruction [14, 15]. Thus, chest wall reconstruction at the same time as the resection favours early extubation and reduces the risk of mortality [4, 16].
Lin et al. [17] reported their experience with the biological mesh Permacol (Covidien, Mansfield, MA, USA) in 5 children with chest wall malignancy to determine the safety of the biological mesh in children with no postoperative complications. The Gore® DualMesh® biomaterial, an expanded PTFE prosthesis, was used in 11 cancer patients [18]. The mesh proved excellent durability and biocompatibility even after an average of 23 months of follow-up. The authors concluded that the Gore® DualMesh® biomaterial has the potential to become an ideal prosthesis for the bony chest wall as an alternative to conventional PTFE or polypropylene grafts.
There is a consensus that there is no indication in reconstructing the defects of <5 cm regardless of topography, and that posterior defects are reconstructed only when they are >10 cm [14]. In our series, two-thirds of the patients underwent a minimum of two ribs resection for cancer invasion. Reconstruction using the XCM Biologic Tissue Matrix alone was successfully achieved in 4 patients, whereas in the remaining 5 the repair was achieved by adding the MatrixRib system (n = 4) or acrylic cement fashioned as ribs (n = 1). A key principle of chest reconstruction is the restitution of a thoracic volume sufficient to ensure an early proper lung expansion [19]. The use of a strong mesh under titanium bars/plates increases the strength of the reconstruction but not without risk of infection [14]. In our cases, infection occurred in 2 of the 5 cancer patients receiving the combined materials (40%). This is significantly higher than previously reported figures (5–10%) [2]. Traditional surgical teaching advocates the removal of all synthetic materials; however, recent evidences suggested that the resorbable feature of the biological patches does not require their removal even if infected [11, 20]. In our cases, re-exploration did not show evidence of infectious involvement in the XCM Biologic Tissue Matrix.
The XCM Biologic Tissue Matrix withstands a higher tensile and suture pull-out forces than products made from either PTFE or midweight microporous prolene/cellulose/polydioxanone materials [21]. The net result is a strong biological scaffold incorporated into the repair with the necessary properties to facilitate soft tissue healing. We therefore utilized this feature to reconstruct chest wall in non-malignant patients. The device was effectively implanted on its own in 5 cases to repair chest wall hernial defects with neither postoperative complication nor further herniation. It was also used as a reinforcing material in 4 patients with chest wall deformity. In the pectus patient, the device was fashioned like a hammock and placed retrosternally replacing pectus bars. The patient with Poland syndrome (Patient 7) developed postoperative wound-related infection and was treated conservatively. In 1 patient, the device was used to replace a previously inserted infected Marlex Mesh and in another to close the chest wall cavity following empyema drainage and rib resection.
CONCLUSION
The XCM patch can be safely used to provide the strength required for chest wall reconstruction and to replace previously infected reconstructions. Randomized controlled trials with long-term follow-up studies are lacking for all of the available scaffolds, particularly those derived from animal tissue.
Conflict of interest: Kostas Papagiannopoulos is on the Speaker's bureau panel of DePuy Synthes.
APPENDIX. CONFERENCE DISCUSSION
Dr A. Turna (Istanbul, Turkey): I have difficulty in understanding why you use synthetic material in chest wall deformities in addition to bars, and why you didn't use muscle flaps during these reconstructions. Did you use this material very selectively? Can you recommend its use in all patients?
Dr Papagiannopoulos: Maybe I wasn't very well understood. The XCM patch is not a synthetic material. It is a biological material. I did not use synthetic materials on the chest wall deformities and I did not use bars.
But the patients with deformities in whom I had to reconstruct the chest wall, they were an absolute mess. They had received pectus repairs in other institutions, where they had essentially taken not just the cartilages but the cartilaginous bed, and they came with very unstable sternums. These people needed to have stabilization, and you need metal work to do that.
Dr Turna: Is it a much better material than the muscle flap?
Dr Papagiannopoulos: In an area that has been operated on in the past that has potential ischaemia, has a lot of fibrosis and a questionable vascular supply, why would you want to embark on actually mobilizing muscle flaps and causing more damage to the blood supply? I'm afraid I don't agree with this.
Dr D. Miller (Atlanta, GA, USA): Last year at the Southern Thoracic we presented a series of biological chest wall reconstructions using bovine pericardium and absorbable BioBridge, which is PDS bars which are absorbable at two years. These were infected cases. We really enjoyed that technique. We didn't have to remove any at a later date.
The only thing with some of these patches, like bovine pericardium and maybe in this one, because of the stretchability of the patch, you may run into some issues with paradoxical motion. I know you mentioned that in the one done thoracoscopically you were okay, but did you have any other issues when you did it through an open technique?
Dr Papagiannopoulos: I haven't yet dared to put it into massive chest wall reconstructions without any support. I have used it for chest wall reconstructions thoracoscopically when I had to remove up to three ribs. So the largest defect we had was essentially 12 × 15 cm in the anterolateral part of the chest wall.
I have videos of these people, and I have to apologize, but I didn't have enough time to show those. But we have taken videos of these people giving us Valsalva manoeuvres and coughing on postoperative day 2 after surgery, and I didn't see any paradoxical breathing. I would be a little bit reluctant to start using it in massive chest wall reconstructions, obviously. You need a lot of soft tissue on top of that to make sure you're not going to have paradoxical breathing.
Dr Miller: I agree with that, because in our infected chest walls, infected breast cancer or radionecrosis and so forth, it has worked out very well because we did not have to remove that. And if you have to use methylmethacrylate or some other type of synthetic mesh, there is a 20 to 30% chance you will have to remove that at a later date.
Dr Papagiannopoulos: Just to make a small comment on what you said, I know all of us hate infections, but sometimes infections can be good, because in those cases that you mentioned, the surrounding tissues have such a lot of fibrosis that might be helpful for this material without actually needing any further support, but if you just work in cancer patients and you disrupt the soft and the osseus elements of the chest wall, if you don't reconstruct it with both elements, if it is very large you might have paradoxical breathing.
Dr Miller: Yes, I agree. When we go back for infection and we have to remove it, with methylmethacrylate, as you recall, you have a nice fibrotic capsule there and you usually don't have to do anything else.
Dr Papagiannopoulos: Yes, I agree.
REFERENCES
- 1.Arnold PG, Pairolero PC. Chest-wall reconstruction: an account of 500 consecutive patients. Plast Reconstr Surg. 1996;98:804–10. doi: 10.1097/00006534-199610000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Deschamps C, Tirnaksiz BM, Darbandi R, Trastek VF, Allen MS, Miller DL, et al. Early and long-term results of prosthetic chest wall reconstruction. J Thorac Cardiovasc Surg. 1999;117:588–91. doi: 10.1016/s0022-5223(99)70339-9. [DOI] [PubMed] [Google Scholar]
- 3.Mansour KA, Thourani VH, Losken A, Reeves JG, Miller JI, Jr, Carlson GW, et al. Chest wall resections and reconstruction: a 25-year experience. Ann Thorac Surg. 2002;73:1720–5. doi: 10.1016/s0003-4975(02)03527-0. [DOI] [PubMed] [Google Scholar]
- 4.Weyant MJ, Bains MS, Venkatraman E, Downey RJ, Park BJ, Flores RM, et al. Results of chest wall resection and reconstruction with and without rigid prosthesis. Ann Thorac Surg. 2006;81:279–85. doi: 10.1016/j.athoracsur.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Mahabir RC, Butler EB. Stabilisation of the chest wall: autologous and alloplastic reconstructions. Semin Plast Surg. 2011;25:34–42. doi: 10.1055/s-0031-1275169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watanabe A, Watanabe T, Obama T, Ohsawa H, Mawatari T, Ichimiya Y, et al. New material for reconstruction of the anterior chest wall including the sternum. J Thorac Cardiovasc Surg. 2003;126:1212–4. doi: 10.1016/s0022-5223(03)00933-4. [DOI] [PubMed] [Google Scholar]
- 7.Smart NJ, Bloor S. Durability of biologic implants for use in hernia repair: a review. Surg Innov. 2012a;19:221–9. doi: 10.1177/1553350611429027. [DOI] [PubMed] [Google Scholar]
- 8.LeRoux BT, Shama DM. Resection of tumours of the chest wall. Curr Probl Surg. 1983;20:345–86. doi: 10.1016/s0011-3840(83)80007-0. [DOI] [PubMed] [Google Scholar]
- 9.Yao C, Markowicz M, Pallua N, Noah EM, Steffens G. The effect of cross-linking of collagen matrices on their angiogenic capability. Biomaterials. 2008;29:66–74. doi: 10.1016/j.biomaterials.2007.08.049. [DOI] [PubMed] [Google Scholar]
- 10.Hoganson DM, Owens GE, O'Doherty EM, Bowley CM, Goldman SM, Harilal DO, et al. Preserved extracellular matrix components and retained biological activity in decellualrized porcine mesothelioma. Biomaterials. 2010;31:6934–40. doi: 10.1016/j.biomaterials.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 11.Hoganson DM, O'Doherty EM, Owens GE, Harilal DO, Goldman SM, Bowley CM, et al. The retention of extracellular matrix proteins and angiogenic and mitogenic cytokines in a decellularized porcine dermis. Biomaterials. 2010;31:6730–7. doi: 10.1016/j.biomaterials.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 12.Smart NJ, Bryan N, Hunt JA. A scientific evidence for the efficacy of biologic implants for soft tissue reconstruction. Colorectal Dis. 2012;14(Suppl 3):1–6. doi: 10.1111/codi.12042. [DOI] [PubMed] [Google Scholar]
- 13.Wiegmann B, Zardo P, Dickgreber N, Länger F, Fegbeutel C, Haverich A, et al. Eur J Cardiothorac Surg. 2010;37:602–5. doi: 10.1016/j.ejcts.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 14.Berthet JP, Whilm JM, Canaud L, Joyeux F, Cosmar C, Hireche K, et al. The combination of polytetrafluoroethylene mesh and titanium rib implants: an innovative process for reconstructing large full thickness chest wall defects. Eur J Cardiothorac Surg. 2012;42:444–53. doi: 10.1093/ejcts/ezs028. [DOI] [PubMed] [Google Scholar]
- 15.Berthet JP, D'Annoville T, Canaud L, Marty-Ané CH. Delayed syndrome of thoracic insufficiency: a consequence of non-rigid reconstruction of a large chest wall defect. Eur J Cardiothorac Surg. 2012;41:953–4. doi: 10.1093/ejcts/ezr120. [DOI] [PubMed] [Google Scholar]
- 16.Lardinois D, Müller M, Furrer M, Banic A, Gugger M, Krueger T, et al. Functional assessment of chest wall integrity after methylmethacrylate reconstruction. Ann Thorac Surg. 2000;69:919–23. doi: 10.1016/s0003-4975(99)01422-8. [DOI] [PubMed] [Google Scholar]
- 17.Lin SR, Katenberg ZJ, Bruzoni M, Albanese CT, Dutta S. Chest wall reconstruction using implantable cross-linked porcine dermal collagen matrix (Permacol) J Paediatr Surg. 2012;47:1472–5. doi: 10.1016/j.jpedsurg.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Nagayasu T, Yamasaki N, Tagawa T, Tsuchiya T, Miyazaki T, Nanashima A, et al. Long-term results of chest wall reconstruction with DualMesh. Interact CardioVasc Thorac Surg. 2010;11:581–4. doi: 10.1510/icvts.2010.242040. [DOI] [PubMed] [Google Scholar]
- 19.Coonar AS, Wihlm JM, Wells FC, Qureshi N. Intermediate outcome and dynamic computerised tomography after chest wall reconstruction with the STRATOS titanium rib bridge system: video demonstration of preserved bucket-handle rib motion. Interact CardioVasc Thorac Surg. 2011;12:80–1. doi: 10.1510/icvts.2010.249615. [DOI] [PubMed] [Google Scholar]
- 20.DiCocco JM, Fabian TC, Emmett KP, Magnotti LJ, Goldberg SP, Croce MA. Components separation for abdominal wall reconstruction: the Memphis modification. Surgery. 2012;151:118–25. doi: 10.1016/j.surg.2011.06.034. [DOI] [PubMed] [Google Scholar]
- 21.Hackett ES, Harilal D, Bowley C, Hawes M, Turner AS, Goldmans SM. Evaluation of porcine hydrated dermis augmented repair in a fascial defect model. J Biomed Mater Res B Appl Biomater. 2011;96:134–8. doi: 10.1002/jbm.b.31751. [DOI] [PubMed] [Google Scholar]


