Abstract
OBJECTIVES
Complex congenital heart defects that present earlier in life are sometimes channelled towards single-ventricle repair, because of anatomical or logistic challenges involved in two-ventricle correction. Given the long-term functional and survival advantage, we have been consciously exploring the feasibility of a biventricular repair in these patients when they present later for Fontan completion.
METHODS
Since June 2009, 71 patients were referred for staged completion of the Fontan procedure. Following detailed evaluation that included three-dimensional echocardiography and magnetic resonance imaging, 10 patients (Group 1—median age 6 years) were identified and later underwent complex biventricular repair with takedown of Glenn shunt, while completion of extracardiac Fontan repair was done in 61 patients (Group 2—median age 7 years).
RESULTS
Two-ventricle repair was accomplished in all the 10 Group 1 patients. One patient developed complete heart block requiring permanent pacemaker insertion. Late patch dehiscence occurred in another (awaiting repair). At a median follow-up of 15 months, there was no mortality among the Group 1 patients and all except for 1 patient were symptom free. There were 2 early deaths (3.3%) in the Group 2 patients.
CONCLUSIONS
Two-ventricular repair, although surgically challenging, should be considered in all patients with two functional ventricles who come for Fontan completion. Comprehensive preoperative imaging and meticulous planning helps in identifying suitable candidates.
Keywords: Univentricular heart, Bidirectional Glenn shunt, Ventricle, Congenital heart disease
INTRODUCTION
Complex congenital heart defects presenting early in life are sometimes channelled towards single-ventricle palliation, dictated by numerous factors that include relative size of ventricles, straddling of atrio-ventricular valves, complexity of intracardiac anatomy, feasibility of conduit placement, age of the patient and surgical skills [1, 2]. Additionally, in the limited resource environment, there are social and economic considerations that dictate the decision to offer single-ventricle palliation [3]. These patients typically undergo bidirectional Glenn shunt (BDGS) followed by the Fontan operation. Fontan surgery has inherent disadvantages compared with biventricular repair that are well recognized [4]. A biventricular repair in these circumstances has the theoretical advantage of maintaining a normal physiology, presumably leading to better exercise tolerance, fewer arrhythmias and potentially a much better long-term outcome.
Since June 2009, at our institution, a conscious attempt was made to explore the possibility of biventricular repair in all patients presenting for the staged Fontan procedure through careful and comprehensive reassessment of anatomy using available imaging tools that included two-dimensional (2D) and three-dimensional (3D) echocardiography, cardiac catheterization and cardiac magnetic resonance imaging (MRI). This case series will examine the specific factors that allowed consideration of two-ventricle repair and analyse their immediate and short-term follow-up.
METHODS
The records of all patients referred for staged completion of the Fontan procedure over a period of 36 months, from June 2009 to May 2012, were reviewed. The possibility of biventricular conversion was actively explored in all patients with two good-sized ventricles and functional aortic valve (AV) valves with Grade 1 or less straddling.
Suitability for biventricular repair was decided preoperatively through comprehensive cardiovascular reassessment by a team of paediatric cardiologists and paediatric cardiac surgeons. Anatomic or other concerns that had previously precluded biventricular repair were identified for each case and specifically reviewed. While 2D transthoracic echocardiography formed the cornerstone of cardiac imaging, the more recently acquired advanced imaging modalities of 3D echocardiography and cardiac MRI were put to optimal use in resolving complex anatomical questions wherever indicated. These included concerns regarding routability of the ventricular septal defect (VSD) to the aorta, estimation of ventricular volumes and functions, need for/feasibility of conduit placement and baffling of venous pathways.
Cardiac MRI was used for cardiovascular imaging in all cases where echocardiography was insufficient for decision-making. 1.5 T GE SIGNA HDxt scanner was used for imaging, with sequences including steady state free precession (SSFP), phase contrast and Gadolinium-enhanced 3D angiography, customized to the imaging needs of each patient. Functional analysis was done using GE Reportcard™. Cardiac computerized tomography (CT) was used to define coronary anatomy in the event an arterial switch operation was considered.
Clear surgical decisions were taken in the preoperative assessment, and surgical steps were accordingly planned. In cases where a back-up plan for Fontan was felt to be necessary, suitability for Fontan was predetermined through catheterization or cardiac MRI plus jugular venous pressure assessment.
Patients were classified into two groups: Group 1, where biventricular repair was considered feasible and takedown of Glenn shunt with conversion to two-ventricle repair was performed; and Group 2, where Fontan surgery was performed. The operative details, postoperative course and follow-up records were noted for both these groups. The results were expressed as mean and standard deviation
RESULTS
From June 2009 to March 2012, 71 patients were referred for staged completion of the Fontan procedure. Following detailed evaluation, 10 (Group 1) were found suitable for complex biventricular repair with takedown of Glenn shunt, while completion of extracardiac Fontan repair was advised in 61 patients (Group 2). There were 4 male and 6 female patients in Group 1, with a median age of 6 years (range 3–10 years) and a median weight of 16 kg (range 11.2–29.8 kg). The mean saturation of these patients was 81% (±3.4) at presentation. The median age of the Group 2 patients was 7 years (range 2–23 years) and median weight 18 kg (range 9.8–48.5 kg).
All patients in Group 1 had previously undergone BDGS (Table 1). Non-routable VSD, straddling tricuspid valve, requirement of complex heart surgery using conduit at a young age, ventricle being too small to support the systemic or pulmonary circulation were the various reasons to defer biventricular repair initially in these patients. In 2 of these patients (Patients 1 and 10), the decision for palliation with Glenn shunt had been taken on the surgical table during previous failed attempts at biventricular correction. Patient 1 with situs solitus, D-loop, D-transposed great arteries (SSD), double outlet right ventricle (DORV) and VSD following pulmonary artery banding at 2 months of age had a failed attempt at biventricular repair at 3 years of age due to prominent subaortic conus and straddling tricuspid valve tissue precluding intraventricular tunnelling of the VSD to the aorta. Patient 10, a case of situs inversus, L-loop, L-transposed great arteries (ILD) cardiac type of total anomalous pulmonary venous return (TAPVR) was found to have very small morphological left atrium and left ventricle (LV) during an attempted TAPVR repair at 2 months of age, and underwent a Damus–Kaye–Stansel (DKS) procedure with BDGS instead. All the other patients in Group 1 had undergone BDGS as per their preoperative assessment and plan.
Table 1:
Details of the Group 1 patients
| S. No. | Diagnosis | Cause for Glenn | Age at Glenn | Initial palliation | Age of final correction | Final conversion |
|---|---|---|---|---|---|---|
| 1 | DORV, VSD | Non-routable VSD with tricuspid valve straddling | 2 months, 3 years | PA band, BDGS | 9 years | Intraventricular tunnelling, TV repair, pulmonary debanding, Glenn takedown |
| 2 | DORV, VSD, PS | Non-routable VSD | 3 months | BDGS, MPA ligation and LPA plasty | 6 years | Intraventricular tunnelling, RV-PA conduit, Glenn takedown |
| 3 | DORV, VSD, PS | Non-routable VSD with tricuspid valve straddling | 6 months | BDGS | 6 years | Intraventricular tunnelling, RV-PA conduit, RPA plasty, Glenn takedown |
| 4 | DORV, VSD, PS | Non-routable VSD | 2 months, 11 months | RMBTS, BDGS | 5 years | Intraventricular tunnelling, RV-PA conduit, Glenn takedown |
| 5 | D-TGA, VSD, PS | Non-routable VSD | 4 years | BDGS with MPA interruption | 10 years | Intraventricular tunnelling, RV-PA conduit, RPA plasty, Glenn takedown |
| 6 | D-TGA, VSD, PS | Conduit requirement | 1 year | BDGS with PA banding | 7 years | Intraventricular tunnelling, RV-PA conduit, RPA plasty, Glenn takedown, TV valve repair |
| 7 | D-TGA, VSD | Complex coronary anatomy | 1 year | BDGS with PA banding | 5 years | ASO, VSD closure, Glenn takedown |
| 8 | TOF | Tricuspid valve straddling, LAD crossing RVOT | 5 months | BDGS | 3 years | VSD closure, RV-PA conduit, RPA plasty, Glenn takedown, tricuspid valve repair |
| 9 | TASVC | Hypoplastic right ventricle | 4 months | Bilateral BDGS | 4 years | Rerouting of systemic veins. Bilateral Glenn takedown |
| 10 | TAPVC | Hypoplastic LV | 10 months | Bilateral BDGS with DKS | 4 years | Rerouting of pulmonary veins, undoing of DKS with great vessel reconstruction, bilateral Glenn takedown |
ASO: arterial switch operation; BDGS: bidirectional Glenn shunt; DORV: double outlet right ventricle; DKS: Damus–Kaye–Stansel; LAD: left anterior descending artery; LPA: left pulmonary artery; PA: pulmonary artery; PS: pulmonic stenosis; RMBTS: right modified Blalock–Taussig shunt; RPA: right pulmonary artery; RV: right ventricle; TAPVC: total anomalous pulmonary venous connection; TASVC: total anomalous systemic venous connections; TGA: transposition of great arteries; TOF: tetralogy of Fallot; TV: tricuspid valve; VSD: ventricular septal defect.
3D echocardiography (Phillips IE33) provided useful insights into intracardiac anatomical details in 6 patients, especially with regard to routability of VSD to the great arteries, and abnormal atrio-ventricular valve attachments. Live 3D echocardiography and post-processing of volume data sets using QLAB™ were used to derive this information. The modality proved useful in Patients 5–8 in deciphering the relationship of the great arteries to each other, to the VSD and to the atrio-ventricular valves. This allowed planning of intraventricular tunnelling of the LV to aorta with right ventricle to pulmonary artery (RV-PA) conduit in Patient 4 (DORV, VSD and PS), Patients 5 and 6 (D-transposition of great arteries (TGA)) and Patient 8 (tetralogy of Fallot (TOF) with straddling tricuspid valve). Standard 2D echocardiography provided sufficient imaging information to plan arterial switch operation in Patient 7 (D-TGA) and systemic venous rerouting to the right atrium in Patient 9 (total anomalous systemic venous connections (TASVC)).
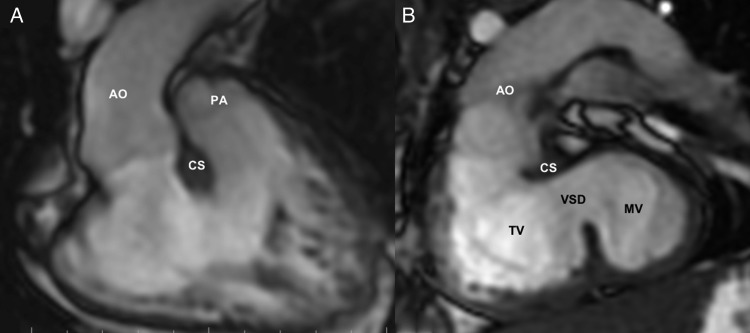
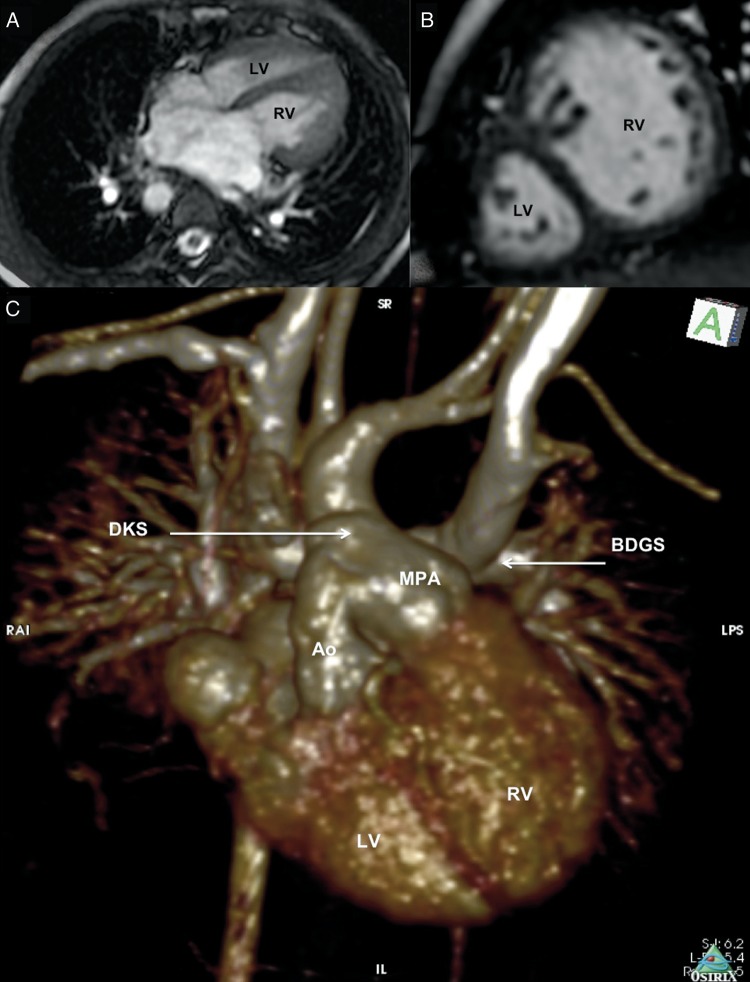
Cardiac MRI was specifically used in 4 patients in whom echocardiography provided insufficient information due to complexity of intracardiac anatomy, poor transthoracic ultrasound signals and/or inability to accurately assess the adequacy of ventricular volumes and functions. Patients 1–3 were cases of complex DORVs, previously assessed to have ‘difficult to route’ or ‘non-routable’ VSDs. Cases 1 and 3, additionally, were echocardiographically reported to have straddling tricuspid valves likely to complicate surgical routing of the LV to the aorta. The primary study question for cardiac MRI in these cases was the feasibility of biventricular correction by LV to aorta routing. In all the 3 cases, Electrocardiography-gated 2D SSFP with breath-hold sequences in multiple planes (orthogonal, four-chamber, two-chamber, ventricular short axis and oblique outflow planes) provided excellent dynamic visualization of the ventricular septal anatomy and the LV-to-aorta pathway. The ventricular septal defects in all 3 cases were large and complex. Great artery relationships were side-by-side (rightwards aorta) in Patient 1, and d-malposed great arteries in Patients 2 and 3. Patient 1 had prominent subaortic conal septum and with accessory tricuspid valve attachments in the potential LV-aorta pathway (Fig. 1). Ventricular volumes and functions were qantified using the GE ReportCARD™ software by manual tracing of the endocardial borders of the ventricles on successive short-axis 2D SSFP slices at end-diastole and end-systole. Ventricular volumes were indexed to body surface area and were found to be adequate for accomplishing biventricular repair. Patient 10 had previously undergone BDGS with the DKS operation for small left-heart structures (morphological left atrium and ventricle) with unobstructed cardiac TAPVC to morphological right atrium. Cardiac MRI helped define the anatomy well (Fig. 2). Left ventricular volume were low (LV end-diastolic indexed volume: 36 ml/m2; LV end-systolic indexed volume: 12 ml/m2); however, biventricular morphology and systolic functions were normal (ejection fraction 66% for both ventricles). There was no late gadolinium enhancement of the myocardium. In view of the morphological specifics of the case, we felt that despite the low absolute size, the left heart may be able to tolerate the increased volumes in case of total surgical correction. Distance between the BDGS anastomosis and the right atrium was recorded in all cases from the 3D MR angiographic sequences. Phase contrast sequences allowed assessment of flows and physiology in all cases subjected to cardiac MR, which were as expected for post-BDGS status with Qp:Qs ranging between 0.4 and 0.7. Internal jugular vein pressure (as surrogate for pulmonary artery pressure) was 10–13 mmHg in all 4 patients. Thus, cardiac MRI provided clearer understanding of intracardiac and extracardiac anatomy, allowed accurate assessment of ventricular volumes and provided excellent non-invasive haemodynamic assessment of flows and functions in these cases, aiding planning towards biventricular repair.
Figure 1:
Preoperative cardiac MRI [Patient 1] (SDD, DORV and VSD): (A) 2D-SSFP (FIESTA) image (coronal view) showing the origin of both great arteries from the right ventricle, with aorta (Ao) to the right of the pulmonary artery (PA). (B) 2D-SSFP (FIESTA) image from an oblique stack demonstrating the left ventricle (LV)-to-aorta route across the ventricular septal defect (VSD). The conoventricular VSD is seen to extend significantly into the inlet region. Tricuspid valve (TV) and the subaortic conal septum (CS) pose potential barriers to the intraventricular tunnelling. Resection of the conal septum and multiple patch tunnelling allowed an unobstructed LV-to-aorta routing for biventricular correction. Ao: aorta; CS: subaortic conal septum; LV: left ventricle; MV: mitral valve; TV: tricuspid valve.
Figure 2:
Preoperative cardiac MRI [Patient 10]: (A) 2D-SSFP (FIESTA) image four-chamber view showing a right-sided LV, which is smaller than the left-sided right ventricle (RV). (B) 2D-SSFP (FIESTA) image showing the ventricles in short axis. The anteriorly placed LV is much smaller in volume than the posterior right ventricle. (C) Gadolinium-enhanced 3D MR angiography showing extracardiac anatomical details. The outflows of the smaller, right-sided morphological LV and the larger left-sided right ventricle are joined together in a DKS anastomosis.
Based on comprehensive assessment, two-ventricle repair was considered feasible and accomplished as planned in all the Group 1 patients (Table 1). All operations were performed under hypothermic cardiopulmonary bypass. Seven patients required complex intraventricular tunnelling to connect the systemic ventricle to the aorta using single or multiple Goretex patches. Resection of conal septum with enlargement of the VSD was done in those patients when the VSD was smaller than the aortic annulus. Right ventricle to pulmonary artery conduit was used in 6 patients; while in others pulmonary debanding and tanned pericardial patch plasty preserving the pulmonary valve was done. Glenn shunt was taken down in all patients and Superior vena cava (SVC) was attached to the right atrial appendage with 5 patients requiring a Goretex tube interposition. In 1 patient arterial switch operation with VSD closure and taken down of Glenn shunt was accomplished (Patient 7). Cardiac CT helped in dissection and clearly delineating the abnormal coronary anatomy in this patient (single coronary artery with left main coronary artery traversing in between the pulmonary and aortic root). In 1 patient (Patient 10), repair involved baffling of pulmonary veins to the relatively smaller morphological LV, undoing of DKS anastomosis, undoing of Glenn shunt and reattachment of the pulmonary arteries to the main pulmonary artery. All the patients in Group 2 underwent extracardiac Fontan using polytetrafluroethylene conduit in the classical fashion. There were no instances where Fontan was performed after biventricular repair was considered feasible.
All the 10 patients survived biventricular repair, while there were two early mortality in the Group 2 patients. The duration of mechanical ventilation, inotrope use and recovery times were longer in the Group 1 patients (Table 2). Of the Group 1 patients, 1 patient (Patient 3) developed complete heart block requiring permanent pacemaker insertion. Patient 10 had a prolonged ICU and hospital stay with the initial postoperative period complicated by low cardiac output state and need for high-dose inotropic supports. Subsequently, she showed excellent recovery with normalization of left ventricular volume and functions.
Table 2:
Features of patients undergoing two-ventricle and single-ventricle repair
| Group 1, mean (SD) | Group 2, mean (SD) | |
|---|---|---|
| CPB time | 362.2 (±92.6) min | 155.9 (±95.7) min |
| Cross-clamp time | 179.9 (±83.9) min | 79 (±25.9) min |
| Ventilation | 2.1 (±1.1) days | 1.4 (±1.6) days |
| ICU stay | 7 (±3.6) days | 5.4 (±3.0) days |
| Inotrope use | 4.3 (±2.3) days | 3.3 (±2.0) days |
| Hospital stay | 18.9 (±6.3) days | 19.9 (±15.4) days |
| Mortality | 0 | 2 (3.3%) |
CPB: cardiopulmonary bypass; ICU: intensive care unit; SD: standard deviation.
At a median follow-up of 15 months, there was no mortality among the Group 1 patients and all except 1 were in New York Heart Association Functional Class I. Late VSD patch dehiscence was noticed in 1 patient (Patient 2) and awaits reoperation. The remaining patients showed good biventricular function. A gradient of 25 mmHg was found across the conduit in 1 patient at the 2-year follow-up. Laminar flows were noticed in the SVC in all the patients. The patient who underwent correction of anomalous systemic venous connections (Patient 9) was found to have unexplained pulmonary hypertension on follow-up. She was symptom-free on pulmonary vasodilators. There were no late reoperations.
DISCUSSION
It is believed that, in the presence of two adequately sized ventricles, biventricular repair is preferable to single-ventricle pathway. Several recent changes in the application of Fontan principle, like creation of a limited right-to-left shunt by a fenestration in the extracardiac tube or an adjustable atrial septal defect, appear to improve the short-term outcome [5]. But the long-term effect of venous congestion and its complications shows that even in the best of circumstances, single-ventricle pathway is clearly a palliative option [6]. The advantage of maintaining the normal physiology has prompted the reconsideration of biventricular repair even in complex congenital heart defects that have been channelled to univentricular repair.
Classifying certain complex congenital heart diseases into single or biventricular repair groups can be challenging, particularly in some cases of DORV [7–9]. For DORV with non-committed VSD, the major challenges include the presence of the tensor apparatus of the atrio-ventricular valves in the pathway from the LV to the aorta and the prospect of significant subaortic stenosis developing postoperatively [10]. The optimal surgical management of many patients presenting early with transposition of the great arteries with a VSD and pulmonary stenosis also remains controversial. Anatomic constraints such as adequacy and position of the VSD, abnormalities of atrio-ventricular valve attachment and ventricular imbalance may preclude a biventricular repair in these patients. This is especially true when surgical intervention is required early in infancy. Single-ventricle palliation has been advocated in these instances [1, 2].
Younger age at presentation, low weight and inadequacy of left-sided heart structures were shown to increase the early reintervention risk and late post-repair mortality in these patients after biventricular repair [11, 12]. Late morbidity with Rastelli operation is significant due to recurrent left ventricular outflow obstruction (LVOTO), conduit obstruction and arrhythmias [10]. In developing countries, the need for conduit replacements can impose significant financial burden on the family. Notwithstanding these limitations, in selected circumstances, the short- and intermediate-term risks of a biventricular repair may outweigh the potential long-term disadvantages of a Fontan procedure.
Aggressive resection of the infundibular septum to enlarge the VSD has mitigated the risk of LVOTO recurrence. The length between the top of the interventricular septum and the aortic valve (IVS-AV length) has been proposed as a useful predictor for performing an original Rastelli-type operation [13]. We feel that the use of preoperative cardiac MR in these doubtful cases helps to plan the tunnelling pathway and also guides in being aggressive during conal resection. In addition to the leverage of the surgery being done in a bigger child, the usage of multiple patches to refashion the pathway helps to reduce the incidence of future LVOTO.
Another factor influencing the reversal of a decision of single-ventricle palliation to biventricular repair includes the changing dynamics and alteration in cardiac morphology after previous palliative surgery [14]. In our series, a child with total anomalous systemic venous drainage (Patient 9) was thought to have a small RV at the time of initial surgery and therefore underwent Glenn procedure. On the follow-up, however, the RV was found to be adequate to support the entire systemic venous return and a biventricular correction was successfully carried out. Right ventricle growth may have been aided by leaving an antegrade PA flows at first surgery in this patient. Repair of TAPVR also may rarely be complicated due to an unusually small LV. At initial repair, the LV was found to be too small to support the whole cardiac output in one such patient (Patient 10) and hence the DKS procedure with bilateral BDGS was done. Later on follow-up, cardiac MR suggested that LV volume was adequate and a biventricular correction with takedown of DKS and rerouting of pulmonary veins was done. Even though the child required prolonged inotrope and ventilator support, she has shown excellent recovery and is currently asymptomatic.
In view of the long-term survival and functional advantage of biventricular repair, identification of potential candidates is critical. Failure to identify potential candidates prospectively may result in unnecessary long-term single-ventricle palliation in some patients. The main advantage of biventricular repair is the availability of a pulmonary ventricle to perform at low systemic venous pressures and to provide flexibility and adaptation to exercise and increases in pulmonary vascular resistance. If incorporation of a poorly functioning pulmonary ventricle in the circulation leads to high venous pressures and poor adaptation to exercise or increases in pulmonary vascular resistance, the whole exercise of biventricular conversion becomes wasteful. Newer advances in diagnostic tools facilitate better preoperative assessment in complex congenital heart diseases. 3D echocardiography has been found to be especially valuable in surgical planning of intraventricular tunnelling in complex TGA and DORV [15–17]. Cardiac MRI is a valuable method for evaluating complex abnormalities and obtaining detailed anatomic and functional information [18]. Cardiac MRI may be especially useful in older patients, particularly those with complex or treated malformations, where information acquired with transthoracic echocardiography may not be satisfactory. Cardiac CT is specifically useful when information on coronary arteries cannot be obtained by other means.
The threshold for opting for a technically simpler single-ventricle strategy instead of a complex biventricular repair is likely to be much lower in a limited resource environment—governed by factors as varied as costs, access to advanced imaging and surgical expertise. However, as this series shows, it is possible to overcome these barriers and provide optimal anatomical and physiological solutions to selected patients with complex heart disease, even in limited resource environments such as ours.
STUDY LIMITATIONS
This study has the limitations of a single-centre retrospective case series and serves to demonstrate the feasibility of performing biventricular repairs in selected patients with complex heart defects who were originally considered for single-ventricle palliation. Additionally, there are challenges in selected patients that include the need for future conduit replacements. The technical challenges are considerable and recovery times are longer compared with performing the Fontan operation.
CONCLUSION
Two-ventricular repair should be considered in all patients with two functional ventricles who come for Fontan completion. Comprehensive preoperative evaluation including cardiac MRI may help in identifying suitable candidates and in the planning of the surgical technique or other details like the need for conduit or pacemaker. Although technically challenging, the eventual results may be gratifying.
Conflict of interest: none declared.
REFERENCES
- 1.Kleinert S, Sano T, Weintraub RG, Mee RBB, Karl TR, Wilkinson JL. Anatomic features and surgical strategies in double-outlet right ventricle. Circulation. 1997;96:1233–9. doi: 10.1161/01.cir.96.4.1233. [DOI] [PubMed] [Google Scholar]
- 2.Puga FJ. The role of the Fontan procedure in the surgical treatment of congenital heart malformations with double-outlet right ventricle. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2000;3:57–62. doi: 10.1053/tc.2000.6504. [DOI] [PubMed] [Google Scholar]
- 3.Kumar RK, Shrivastava S. Pediatric heart care in India. Heart. 2008;94:984–90. doi: 10.1136/hrt.2007.139360. [DOI] [PubMed] [Google Scholar]
- 4.Cetta F, Boston US, Dearani JA, Hagler DJ. Double outlet right ventricle: opinions regarding management. Curr Treat Options Cardiovasc Med. 2005;7:385–90. doi: 10.1007/s11936-005-0022-2. [DOI] [PubMed] [Google Scholar]
- 5.Bridges ND, Mayer JE, Jr, Lock JE, Jonas RA, Hanley FL, Keane JF, et al. Effect of baffle fenestration on outcome of the modified Fontan operation. Circulation. 1992;86:1762–9. doi: 10.1161/01.cir.86.6.1762. [DOI] [PubMed] [Google Scholar]
- 6.Camposilvan S, Milanesi O, Stelling G, Pettenazzo A, Zancan L, D'Antiga L. Liver and cardiac function in the long term after Fontan operation. Ann Thorac Surg. 2008;86:177–82. doi: 10.1016/j.athoracsur.2008.03.077. [DOI] [PubMed] [Google Scholar]
- 7.Serraf A, Nakamura T, Lacour-Gayet F, Piot D, Bruniaux J, Touchot A, et al. Surgical approaches for double-outlet right ventricle or transposition of the great arteries associated with straddling atrioventricular valves. J Thorac Cardiovasc Surg. 1996;111:527–35. doi: 10.1016/s0022-5223(96)70304-5. [DOI] [PubMed] [Google Scholar]
- 8.Kanter K, Anderson R, Lincoln C, Firmin R, Rigby M. Anatomic correction of double-outlet right ventricle with sub pulmonary ventricular septal defect (the “Taussig-Bing” anomaly) Ann Thorac Surg. 1986;41:287–92. doi: 10.1016/s0003-4975(10)62771-3. [DOI] [PubMed] [Google Scholar]
- 9.Lacour-Gayet F. Intracardiac repair of double outlet right ventricle. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2008;11:39–43. doi: 10.1053/j.pcsu.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Alsoufi B, Awan A, Al-Omrani A, Al-Ahmadi M, Canver CC, Bulbul Z, et al. The Rastelli procedure for transposition of the great arteries: resection of the infundibular septum diminishes recurrent left ventricular outflow tract obstruction risk. Ann Thorac Surg. 2009;88:137–43. doi: 10.1016/j.athoracsur.2009.03.099. [DOI] [PubMed] [Google Scholar]
- 11.Bradley TJ, Karamlou T, Kulik A, Mitrovic B, Vigneswaran T, Jaffer S, et al. Determinants of repair type, reintervention, and mortality in 393 children with double-outlet right ventricle. J Thorac Cardiovasc Surg. 2007;134:967–73. doi: 10.1016/j.jtcvs.2007.05.061. [DOI] [PubMed] [Google Scholar]
- 12.Ruzmetov M, Rodefeld MD, Turrentine MW, Brown JW. Rational approach to surgical management of complex forms of double outlet right ventricle with modified Fontan operation. Congenit Heart Dis. 2008;3:397–403. doi: 10.1111/j.1747-0803.2008.00220.x. [DOI] [PubMed] [Google Scholar]
- 13.Fujii Y, Kotani Y, Takagaki M, Arai S, Kasahara S, Otsuki S, et al. The impact of the length between the top of the interventricular septum and the aortic valve on the indications for a biventricular repair in patients with a transposition of the great arteries or a double outlet right ventricle. Interact CardioVasc Thorac Surg. 2010;10:900–5. doi: 10.1510/icvts.2009.223982. [DOI] [PubMed] [Google Scholar]
- 14.Al Qethamy HO, El Oakley RM, Tageldin MM, Abdulhamed JM, Al Faraidi Y. Late complex biventricular repair after bidirectional cavopulmonary shunt. J Card Surg. 2008;23:719–21. doi: 10.1111/j.1540-8191.2008.00616.x. [DOI] [PubMed] [Google Scholar]
- 15.Roemer U, Hoch M, Kozlik-Feldmann R, Daebritz S, Netz H. Live 3D echocardiography in infants and children: visualization of ventricular septal defects (VSD) in complex congenital heart disease. Eur J Echocardiogr. 2005;6:S79. [Google Scholar]
- 16.Vogel M, Ho SY, Lincoln C, Anderson RH. Transthoracic three-dimensional echocardiography for the assessment of straddling tricuspid or mitral valves. Cardiol Young. 2000;10:603–9. doi: 10.1017/s104795110000888x. [DOI] [PubMed] [Google Scholar]
- 17.Bharucha T, Roman KS, Anderson RH, Vettukattil JJ. Impact of multiplanar review of three-dimensional echocardiographic data on management of congenital heart disease. Ann Thorac Surg. 2008;86:875–81. doi: 10.1016/j.athoracsur.2008.04.106. [DOI] [PubMed] [Google Scholar]
- 18.Boechat MI, Ratib O, Williams PL, Gomes AS, Child JS, Allada V. Cardiac MR imaging and MR angiography for assessment of complex tetralogy of Fallot and pulmonary atresia. Radiographics. 2005;25:1535–46. doi: 10.1148/rg.256045052. [DOI] [PubMed] [Google Scholar]