Abstract
OBJECTIVES
Adenocarcinoma in situ (AIS), which is considered to be pathologically non-invasive in the new International Association for the Study of Lung Cancer/the American Thoracic Society/the European Respiratory Society classification, might be present in patients who show a part-solid nodule on thin-section computed tomography (CT) scan.
METHODS
Between 2008 and 2011, 556 clinical Stage IA (c-Stage IA) lung cancer patients underwent pulmonary resection. For all the patients, the findings obtained by preoperative thin-section CT were reviewed and categorized as pure ground-glass nodule (GGN), part-solid nodule or pure-solid nodule based on the findings on thin-section CT, i.e. based on the consolidation/tumour ratio (CTR). A part-solid nodule was defined as a tumour with 0 < CTR < 1.0, which indicated focal nodular opacity that contained both solid and GGN components. All the patients were evaluated by positron emission tomography (PET), and the maximum standardized uptake value (SUVmax) was recorded. Several clinicopathological features were investigated to identify predictors of AIS in clinical Stage IA lung cancer patients with a part-solid nodule radiologically, using multivariate analyses.
RESULTS
One-hundred and twelve c-Stage IA lung cancer patients showed a part-solid appearance on thin-section CT. Among them, AIS was found in 10 (32%) of the tumours with 0 < CTR ≤ 0.5, in contrast to 3 (5%) with 0.5 < CTR < 1.0. According to multivariate analyses, SUVmax and CTR significantly predicted AIS in patients with a part-solid nodule (P = 0.04, 0.02). The mean SUVmax of the patients with AIS was 0.57 (0–1.6). Moreover, in the subgroup of part-solid nodule with a SUVmax of ≤1.0 and a CTR of ≤0.40, which were calculated as cut-off values for AIS based on the results for a receiver operating characteristic curve, 6 (40%) patients with these criteria showed a pathological non-invasive nature, even patients with a part-solid nodule.
CONCLUSIONS
Among c-Stage IA adenocarcinoma with a part-solid nodule on thin-section CT scan, an extremely low level of SUVmax could reflect a pure GGN equivalent radiologically and AIS pathologically. The preoperative tumour SUVmax on PET could yield important information for predicting non-invasiveness in patients with a part-solid nodule.
Keywords: Lung cancer, Part-solid nodule, Adenocarcinoma in situ, Positron emission tomography
INTRODUCTION
Lung cancer is the most common cause of major cancer and mortality worldwide [1]. Adenocarcinoma is the most common histological subtype of lung cancer in most countries and accounts for approximately half of all lung cancers. Moreover, recent developments in imaging technology and the widespread use of thin-section computed tomography (CT) for screening have made it possible to detect small-sized lung cancers [2, 3]. Most of these are peripherally located adenocarcinoma of the lung, and several authors have reported that lung cancers with a wide area of ground-glass nodule (GGN) on thin-section CT scan have a good prognosis, and in most cases, their pathological features are minimally invasive [4–7]. Thus, these tumours are considered to be feasible candidates for limited surgical resection, as revealed by the prospective JCOG 0201 study in Japan [8]. Nonetheless, there are still some discrepancies between CTR and the degree of pathological behaviour in patients with early stage lung cancer.
On the other hand, the maximum standardized uptake value (SUVmax) on positron emission tomography (PET) with F-18 fluorodeoxyglucose (18F-FDG) is a promising modality for predicting the prognosis and invasiveness of lung adenocarcinoma [9, 10]. Lung adenocarcinoma with a radiologically part-solid appearance exhibits a wide range of grades of malignancy (Fig. 1). To predict the biological behaviour of a part-solid nodule, preoperative findings with PET and thin-section CT must be correlated with the pathological features of these lesions, since these observations could provide more precise clues regarding proper treatment strategies for small adenocarcinoma with a part-solid appearance. In the current retrospective study, we focused on the relationships between SUVmax on PET, CTR on thin-section CT scan in clinical Stage IA (c-Stage IA) lung cancer with a part-solid nodule and its pathological invasiveness, especially regarding adenocarcinoma in situ (AIS), to develop criteria for identifying candidates for limited surgical resection by predicting pathological non-invasiveness in patients with a part-solid nodule.
Figure 1:
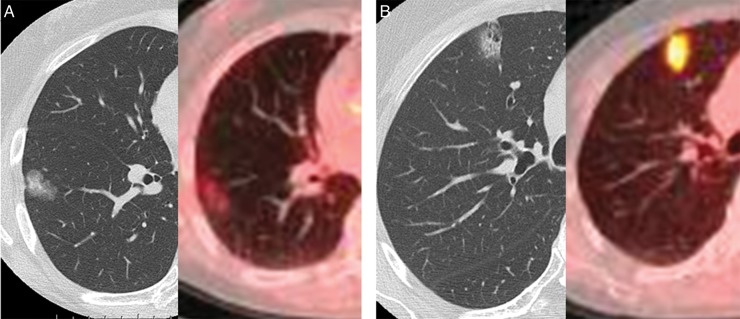
Thin-section CT scan and PET–CT reveal lung adenocarcinoma with a radiologically part-solid appearance and a wide range of grades of malignancy. (A) shows low-grade malignancy based on SUVmax on PET–CT. In contrast, (B) shows high-grade malignancy based on PET–CT despite the findings of thin-section CT.
MATERIALS AND METHODS
This protocol was approved by the ethics committee at our institute. All the patients provided their written informed consent before trial enrolment.
Between January 2008 and December 2011, 556 c-Stage IA lung cancer patients underwent pulmonary resection at our institute. For all the patients, the findings of preoperative CT were reviewed by the authors (A.H., T.M. and K.S.). A contrast-enhanced CT scan was performed to evaluate the entire lung for preoperative staging. The size of the tumours was determined preoperatively based on the findings of thin-section CT scan. In addition, all tumours were subsequently evaluated to estimate the extent of GGN with a thin-section CT scan with 2 mm collimation. The lung was photographed with a window level of −500 to −700 H and a window depth of 1000–2000 H as a ‘lung window’. The solid component was defined as an area of increased opacification that completely obscured the underlying vascular markings. Ground-glass nodule was defined as an area of a slight, homogeneous increase in density that did not obscure the underlying vascular markings. According to the radiological findings on thin-section CT, tumours were divided into three groups: pure GGN, part-solid nodule and pure-solid nodule based on the ratio of the maximum diameter of consolidation to the maximum tumour diameter (consolidation/tumour ratio, CTR). According to thin-section CT findings, pure GGN was defined as a tumour of CTR = 0, pure-solid nodule was defined as a tumour of CTR = 1.0 and part-solid nodule was defined as a tumour of 0 < CTR < 1.0, which indicated focal nodular opacity that included both solid and GGN components. The pure GGN and pure-solid nodule groups were excluded from this study.
With regard to PET–CT scanning, 112 patients underwent a PET–CT scan at the Yotsuya Medical Cube (Tokyo, Japan). The technique used for 18F-FDG-PET/CT scanning at the Yotsuya Medical Cube was as follows. All patients were asked to fast for at least 6 h before 18F-FDG injection to minimize their blood insulin level and normal tissue glucose uptake. The subjects were injected intravenously with 3.5 MBq/kg of 18F-FDG, and static emission images were obtained 60 min after injection. Image acquisition was performed using a Discovery ST PET/CT scanner (GE Medical Systems, Waukesha, WI, USA). After CT image acquisition, emission scanning was performed from the head to the mid-thighs in six bed positions. The acquired PET data were reconstructed to volumetric images with a 2D-OSEM algorithm (2 iterations/15 subsets) incorporating a CT-based attenuation correction.
All PET–CT images were interpreted by one or two experienced nuclear medicine radiologists. A workstation (Xeleris; Elegems, Haifa, Israel) was used for image display and analysis, and the SUVmax of the primary tumour was obtained.
With regard to pathological evaluations, the histological subtype of lung adenocarcinoma was determined according to a new international multidisciplinary classification published by Travis et al. [1] under the sponsorship of the International Association for the Study of Lung Cancer (IASLC), the American Thoracic Society (ATS) and the European Respiratory Society (ERS). Adenocarcinoma in situ (one of the lesions formerly known as bronchioloalveolar carcinoma (BAC)) was defined as a localized small (≤3 cm) lung adenocarcinoma with growth restricted to neoplastic cells along pre-existing alveolar structures (i.e. lepidic growth), which lacked stromal, vascular or pleural invasion.
Ultimately, 112 patients showed c-Stage IA lung cancer with a ‘part-solid’ appearance on thin-section CT scan. All the patients were evaluated by PET, and the SUVmax was recorded. The medical record of each patient was reviewed with regard to gender, sex, pack-year smoking, clinical T-status (c-T1a vs c-T1b), radiological pleural involvement, presence of air bronchogram in the tumour, serum carcinoembryonic antigen level (ng/ml, the cut-off value in our institute is 3.0) and SUVmax on PET. The relationships between these factors and pathological status were investigated to identify significant predictors of AIS in c-Stage IA patients with a part-solid nodule on thin-section CT scan. Fisher's exact test or χ2 test was used to compare two factors. Univariate and multivariate analyses were used to identify the clinical factors that predicted AIS in c-Stage IA part-solid lung cancer. Furthermore, a receiver operating characteristic (ROC) curve for predicting AIS was generated using SPSS Statistics 20 (SPSS, Inc.) by plotting sensitivity vs 1-specificity for various thresholds of several clinical factors. Regarding the way to select the optimal cut-off value from the ROC curve, we calculated the distance between the point (0, 1) and each observed cut-off point on the ROC curve. The optimal cut-off value was obtained from the point at which the distance is minimum. A multivariate analysis was performed by logistic regression analysis using SPSS Statistics 20 (SPSS, Inc.). Forward and backward stepwise procedures were used to determine the combination of factors that were essential for predicting the prognosis. Statistical analysis was considered to be significant when the probability value was <0.05.
RESULTS
There were 112 c-Stage IA lung cancer patients who showed a part-solid appearance on thin-section CT scan. Forty-one patients were male and 71 were female. The patients ranged in age from 35 to 86 years, with an average of 66 years. Pathologically, all of them were adenocarcinoma. Among them, AIS was found in 13 (12%) patients with a part-solid nodule on thin-section CT scan. Conversely, postoperative nodal involvement was found in 3 (3%) of these patients (2 in N1 station and 1 in N2 station). The overall characteristics of c-Stage IA lung adenocarcinoma patients with a part-solid nodule are summarized in Table 1. AIS was found in 10 (32%) tumours with 0 < CTR ≤ 0.5, in contrast to 3 (5%) with 0.5 < CTR < 1.0 (P < 0.01). Regarding the relationship between AIS and the SUVmax value on PET, all patients with AIS showed a SUVmax of ≤2.0 (P = 0.02).
Table 1:
Results of univariate analysis for predictors of AIS in clinical Stage IA lung cancer patients with a part-solid nodule
| Clinical factors | No. of patients | No. of patients with AIS (%) | P-value* |
|---|---|---|---|
| Total | 112 | 13 (12) | |
| Gender | |||
| Male | 41 | 3 (7) | 0.28 |
| Female | 71 | 10 (14) | |
| Age (years) | |||
| ≥70 | 33 | 2 (6) | 0.24 |
| <70 | 79 | 11 (14) | |
| Pack-year smoking | |||
| ≥20 | 26 | 2 (8) | 0.48 |
| <20 | 86 | 11 (13) | |
| Clinical T-status | |||
| c-T1a | 66 | 12 (18) | <0.01 |
| c-T1b | 46 | 1 (2) | |
| Pleural involvement | |||
| Absent | 59 | 9 (15) | 0.20 |
| Present | 53 | 4 (8) | |
| Air bronchogram | |||
| Absent | 30 | 6 (20) | 0.09 |
| Present | 82 | 7 (9) | |
| Preceding malignancies | |||
| Absent | 101 | 10 (10) | 0.09 |
| Present | 11 | 3 (27) | |
| CEA | |||
| ≤3 | 77 | 9 (12) | 0.97 |
| >3 | 35 | 4 (11) | |
| CTR | |||
| ≤0.35 | 13 | 3 (23) | 0.16 |
| >0.35 | 99 | 10 (10) | |
| ≤0.40 | 26 | 7 (27) | <0.01 |
| >0.40 | 86 | 6 (7) | |
| ≤0.45 | 33 | 9 (27) | <0.01 |
| >0.45 | 79 | 4 (5) | |
| SUVmax | |||
| ≤1.0 | 43 | 9 (21) | 0.02 |
| >1.0 | 69 | 4 (6) | |
| ≤1.5 | 63 | 11 (17) | 0.03 |
| >1.5 | 49 | 2 (4) | |
| ≤2.0 | 81 | 13 (16) | 0.02 |
| >2.0 | 31 | 0 (0) | |
AIS: adenocarcinoma in situ; CEA: carcinoembryonic antigen; CTR: consolidation/tumour ratio; SUV: standardized uptake value.
*P-value in χ2 test or Fisher's exact test.
According to a multivariate analysis in c-Stage IA lung cancer patients with a part-solid nodule on thin-section CT scan, the following factors significantly predicted AIS pathologically: preceding malignancies, CTR and SUVmax level (P = 0.04, 0.02 and 0.04; Table 2). The mean SUVmax and mean CTR of the patients with AIS were 0.57 (0–1.6) and 0.47 (0.30–0.85), respectively.
Table 2:
Results of multivariate analysis for predictors of AIS in clinical Stage IA lung cancer patients with a part-solid nodule
| Variable | Odds ratio | 95% Confidence interval | P-value* |
|---|---|---|---|
| Preceding malignancies | 0.16 | 0.03–0.96 | 0.04 |
| CTR | 4.97 | 1.26–19.62 | 0.02 |
| SUVmax | 4.32 | 1.05–17.68 | 0.04 |
AIS: adenocarcinoma in situ; CTR: consolidation/tumour ratio; SUV: standardized uptake value.
*P-value in logistic regression analysis.
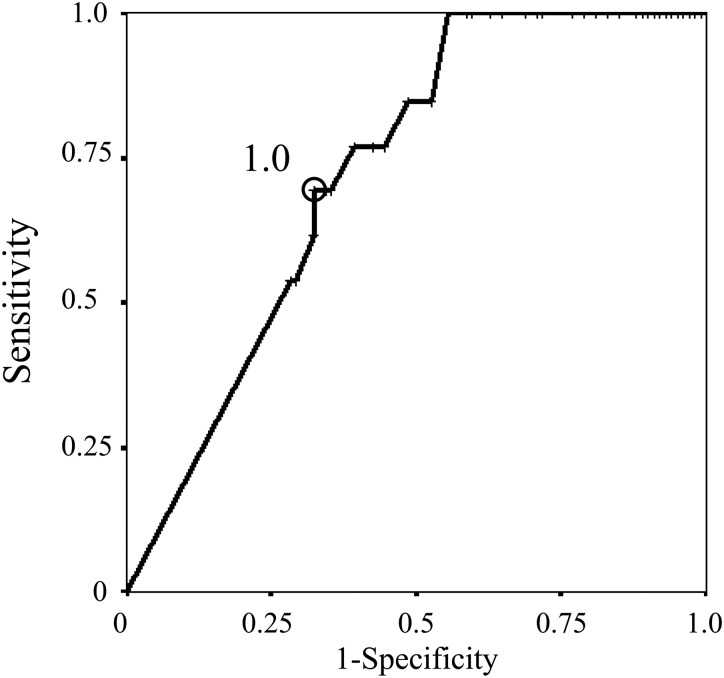
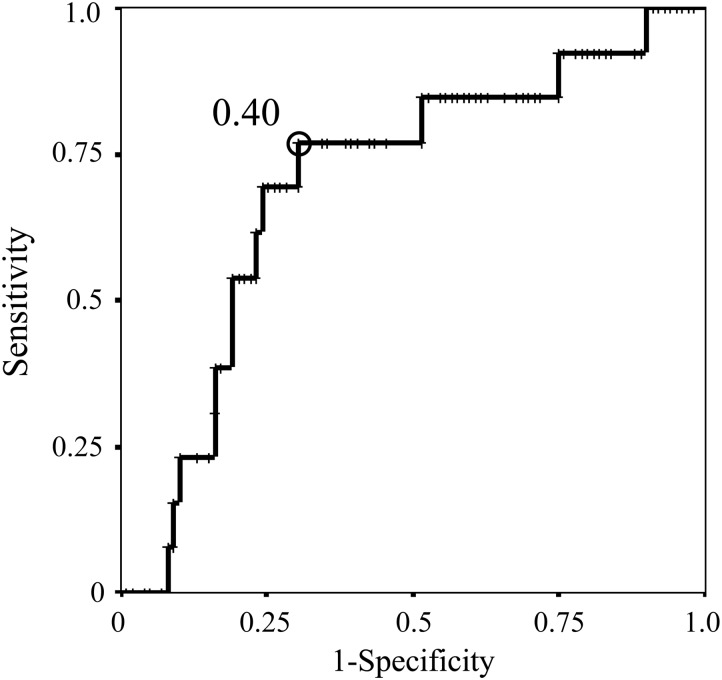
Regarding SUVmax on PET and CTR on thin-section CT scan, the ROC curve revealed that the optimal cut-off value for predicting AIS in patients with a part-solid nodule was a SUVmax of 1.0 (area under the curve 0.728; standard error 0.057) (Fig. 2) and a CTR of 0.40 (area under the curve 0.702; standard error 0.075) (Fig. 3), respectively. These cut-off values yielded a sensitivity of 0.692 and a specificity of 0.677 for SUVmax, and a sensitivity of 0.769 and a specificity of 0.697 for CTR. Based on these results, in the subgroup with a part-solid nodule with both a SUVmax of ≤1.0 and a CTR of ≤0.40, 6 (40.0%) of the 15 patients with these criteria showed AIS, i.e. a pathological non-invasive nature, even patients with a part-solid nodule. Moreover, by combining these predictors, we identified subgroups with different frequencies of pathological lymphatic vessel invasion (LVI) among c-Stage IA lung cancer with a part-solid appearance on thin-section CT scan (Table 3). The results showed that none of the patients with a part-solid nodule who showed both a SUVmax of ≤1.0 and a CTR of ≤0.40 had LVI. On the other hand, ∼26% of patients with c-Stage IA lung cancer with a part-solid nodule who did not have either of these predictors had LVI.
Figure 2:
The ROC curve of SUVmax on PET for the prediction of AIS in clinical Stage IA lung cancer patients with a part-solid nodule, with a cut-off value of 1.0.
Figure 3:
The ROC curve of CTR on thin-section CT scan for the prediction of AIS in clinical Stage IA lung cancer patients with a part-solid nodule, with a cut-off value of 0.40.
Table 3:
Probability of the presence of lymphatic vessel invasion in patients with clinical Stage IA lung cancer patients with a part-solid nodule
| Subgroups | No. of patients | No. of patients with LVI (%) | P-value* |
|---|---|---|---|
| Total number | 112 | 19 (17) | |
| Presence of SUVmax ≤ 1.0 and CTR ≤ 0.40 | |||
| with both factors | 15 | 0 (0) | 0.02 |
| with either factors | 39 | 4 (10) | |
| with neither factors | 58 | 15 (26) | |
LVI: lymphatic vessel invasion; CTR: consolidation/tumour ratio; SUV: standardized uptake value.
*P-value in χ2 test.
DISCUSSION
Recently, limited surgical resection has been actively indicated for multiple primary lung cancers and/or very early lung cancers that are located peripherally and show a GGN appearance on thin-section CT scan. Based on rapid advances in clinical, radiological, pathological and molecular aspects of lung adenocarcinoma, a new international multidisciplinary classification was published in 2011 under the sponsorship of IASLC/ATS/ERS [1]. In this classification, AIS is a newly defined concept that refers to a localized non-invasive adenocarcinoma with lepidic growth, which lacks stromal, vascular or pleural invasion. Adenocarcinoma in situ is best demonstrated typically as a pure GGN on thin-section CT, but is sometimes seen as a part-solid or occasionally a pure-solid nodule (Fig. 4). Since several studies have demonstrated that AIS with pure lepidic growth was associated with 100% disease-free survival [11–14], the ability to predict non-invasiveness in c-Stage IA lung cancer patients with a part-solid nodule could be important for identifying appropriate surgical treatments.
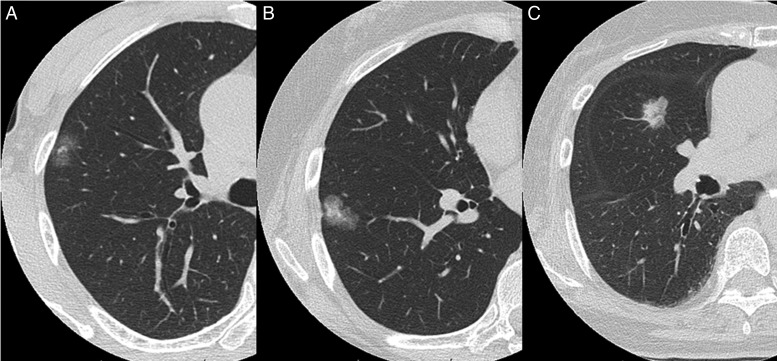
Figure 4:
Adenocarcinoma in situ is sometimes seen radiologically as a part-solid nodule or occasionally as a pure-solid nodule. The CTRs are: (A) 0.31, (B) 0. 40 and (C) 0.85.
Notably, a prospective trial of the Japan Clinical Oncology Group, JCOG 0201 [8], revealed proper candidates for limited surgery based on the results of radiological findings to predict non-invasive lung cancer, which was defined as an adenocarcinoma of 2 cm or less with a CTR of ≤0.25. This indicates a significant correlation between CTR and pathological findings. The prognostic significance of a solid part in small-sized GGN adenocarcinoma has already been addressed in the literature [15, 16]. Nonetheless, as indicated in this study, there are still some discrepancies between CTR and the degree of pathological behaviour in patients with early stage lung cancer. Lung adenocarcinoma with a part-solid appearance has a wide range of pathological characteristics, since the solid part on thin-section CT scan represents various features, such as collapse of the alveoli, the subsequent formation of an active fibrotic focus and the proliferation of cancer cells [15, 17]. Actually, delayed loco-regional recurrence after limited resection has been reported even for GGN adenocarcinoma [18].
On the other hand, a previous report suggested that a higher SUVmax may indicate aggressive malignant behaviour in small-sized adenocarcinoma, which was independent of the in situ components pathologically [19–21]. An elevated SUVmax on PET reflects cellular proliferation and the aggressiveness of the primary lung cancer; however, the sensitivity of PET for AIS is usually very low due to the lower metabolic activity of cancer cells and the likelihood that they will escape detection with the use of FDG [22]. Therefore, the efficacy of PET for predicting the biological features of small-sized adenocarcinoma, especially AIS, remains unclear. However, this disadvantage of FDG-PET for detecting very early lung cancer could provide more important information. By combining the latest radiological imaging of CTR on thin-section CT scan and SUVmax on PET, more precise clues for predicting a pathological non-invasive nature can be obtained for lung cancer patients with part-solid lesions, and this could aid in the selection of appropriate candidates for sublobar resection.
According to the present results in 112 part-solid lung adenocarcinoma patients of c-N0 status, both CTR on thin-section CT scan and the SUVmax level on PET of primary tumours were significantly correlated with AIS, i.e. pathologically non-invasive, by a multivariate analysis. Our results showed that, if they had both a SUVmax of ≤1.0 and a CTR of ≤0.40, even patients with radiologically part-solid tumours had an extremely high incidence of AIS (40%). Furthermore, LVI was never found in patients who had both a SUVmax of ≤1.0 and a CTR of ≤0.40, even if they had a part-solid nodule. On the other hand, if patients had neither of these predictors, we found an extremely high incidence of LVI (26%) in c-Stage IA lung cancer with a part-solid nodule. Our results suggest that limited surgical resection could be an effective therapy for these patients with a SUVmax of ≤1.0 and a CTR of ≤0.40, due to the significantly non-invasive nature of these cancers. Thus, our findings regarding the combination of CTR on thin-section CT and SUVmax on PET more accurately reflect the pathological nature of lung cancer with a part-solid appearance. These findings may be applicable to a specific surgical approach, such as a sublobar resection for early stage lung cancer, the extent of lymph node dissection and the treatment of multiple small-sized lung cancers. In addition, these findings to predict AIS in patients with a part-solid nodule could be used more effectively in our daily practice when we continue the close observation for small-sized lung cancer with GGN predominance as well as selecting appropriate operative modes.
Basically, it is certain that AIS is found in patients with a pure GGN on thin-section CT scan, and the information about the relationship between pure GGN and AIS is an interesting matter. In contrast, AIS is found very occasionally in patients with a part-solid nodule [1]. Actually, we encounter AIS in resected patients with lung cancers showing part-solid nodules on thin-section CT scan. The important point is that 100% disease-free survival is expected if AIS is completely resected. Moreover, lung adenocarcinoma with a radiologically part-solid appearance exhibits a wide range of grades of malignancy. So it is worth investigating the clinical predictors of AIS in patients with part-solid nodules on thin-section CT scan. These data would be informative for deciding the appropriate treatment strategies of part-solid nodules.
The improved quality of CT images and the frequent application of CT examinations in screening programmes have enhanced the capability to detect small-sized lung cancers, which has raised surgical issues that do not yet have definitive answers. Lobectomy has been recommended as a standard surgical procedure [23], even for small-sized lung cancers, since lymph node metastasis can be found in ∼15% of lung cancers that are 2 cm or smaller [24, 25]. Nevertheless, our radiological criteria that combine thin-section CT and FDG-PET could be used more precisely to predict pathological non-invasiveness for patients with a part-solid appearance. These patients would be feasible candidates for limited surgical resection, such as wide wedge resection or segmentectomy. In the future, however, whether or not limited surgical resection is an appropriate therapy for lung cancers with a GGN appearance on thin-section CT could be strictly based on the results of three randomized trials (JCOG 0802 and JCOG 0804 in Japan and CALGB 140503 in North America).
This study was limited by a short median follow-up period. And the number of patients with AIS was relatively small, because our cohorts were composed by c-Stage IA lung adenocarcinoma with a ‘part-solid’ appearance on thin-section CT scan. Further investigations are warranted in the future. Moreover, c-Stage IA lung adenocarcinoma is a heterogeneous cohort. Basically, AIS reveals pure GGN radiologically [1]. On the other hand, like some radiological findings we indicated in this study, we encounter AIS that presents sometimes a part-solid or occasionally a solid nodule in our daily practice. Actually, lung adenocarcinoma with a radiological part-solid appearance exhibits a wide range of grade of malignancies. Generally, radiological part-solid lesions reveal excellent prognosis. Nonetheless, there are some discrepancies among CTR, SUVmax and pathological status. To predict the biological behaviour of a part-solid nodule, preoperative findings with PET and thin-section CT must be correlated with pathological features of these lesions. So in this study, we aimed to predict AIS in c-Stage IA lung cancer patients with a part-solid appearance on thin-section CT scan, since these observations could provide more precise clues regarding proper treatment strategies for small-sized adenocarcinoma with a part-solid appearance. In contrast, regarding c-Stage IA pure-solid lung cancer, the most important topic is the appropriate operative strategy for resectable NSCLC <2 cm in size [25]. From this point, a Phase III trial as to the feasibility of segmentectomy for lung cancer 2 cm or less in size is now ongoing in Japan (JCOG 0802) and the USA. But historically, lung cancers with a pure-solid appearance on thin-section CT scan are considered to be of invasive nature with a high incidence of nodal involvement of >20%, despite their small size [5, 25]. Regarding the proper indication of segmentectomy for pure-solid lung cancer, some controversies are still surrounded such as possible high local recurrence rate and insufficiency of interlobar lymph node dissection in patients who underwent segmentectomy. So the study to investigate radiological features for negative nodal involvement and to elucidate the candidate for limited surgery even in patients with radiologically pure-solid lung cancer is warranted. Therefore, in the future, we have to address the identification of the clinical and radiological factors to stratify the prognosis of c-Stage IA lung cancer patients. Further studies are needed.
In conclusion, the radiological diagnosis of non-invasive lung cancer using the CTR on thin-section CT scan and SUVmax on PET correlated well with pathological AIS. Our results support the notion that limited surgical resection is an effective therapy even for lung cancers with a part-solid appearance, in patients who have both a SUVmax of ≤1.0 and a CTR of ≤0.40.
Funding
This work was supported in part by a Grant-in-Aid for Cancer Research from the Ministry of Health, Labour and Welfare, Japan.
Conflict of interest: none declared.
REFERENCES
- 1.Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, Yatabe Y, et al. International association for the study of lung cancer/American thoracic society/European respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6:244–85. doi: 10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henschke CI, Yankelevitz DF, Libby DM, Pasmantier MW, Smith JP, Miettinen OS. Survival of patients with stage I lung cancer detected on CT screening. N Engl J Med. 2006;355:1763–71. doi: 10.1056/NEJMoa060476. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki K, Asamura H, Kusumoto M, Kondo H, Tsuchiya R. ‘Early’ peripheral lung cancer: prognostic significance of ground glass opacity on thin-section computed tomographic scan. Ann Thorac Surg. 2002;74:1635–9. doi: 10.1016/s0003-4975(02)03895-x. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki K, Kusumoto M, Watanabe S, Tsuchiya R, Asamura H. Radiologic classification of small adenocarcinoma of the lung: radiologic-pathologic correlation and its prognostic impact. Ann Thorac Surg. 2006;81:413–9. doi: 10.1016/j.athoracsur.2005.07.058. [DOI] [PubMed] [Google Scholar]
- 6.Takamochi K, Nagai K, Yoshida J, Suzuki K, Ohde Y, Nishimura M, et al. The role of computed tomographic scanning in diagnosing mediastinal node involvement in non-small cell lung cancer. J Thorac Cardiovasc Surg. 2000;119:1135–40. doi: 10.1067/mtc.2000.105830. [DOI] [PubMed] [Google Scholar]
- 7.Kodama K, Higashiyama M, Yokouchi H, Takami K, Kuriyama K, Mano M, et al. Prognostic value of ground-glass opacity found in small lung adenocarcinoma on high-resolution CT scanning. Lung Cancer. 2001;33:17–25. doi: 10.1016/s0169-5002(01)00185-4. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki K, Koike T, Asakawa T, Kusumoto M, Asamura H, Nagai K, et al. A prospective radiological study of thin-section computed tomography to predict pathological noninvasiveness in peripheral clinical IA lung cancer (Japan Clinical Oncology Group 0201) J Thorac Oncol. 2011;6:751–6. doi: 10.1097/JTO.0b013e31821038ab. [DOI] [PubMed] [Google Scholar]
- 9.Vansteenkiste J, Fischer BM, Dooms C, Mortensen J. Positron-emission tomography in prognostic and therapeutic assessment of lung cancer: systematic review. Lancet Oncol. 2004;5:531–40. doi: 10.1016/S1470-2045(04)01564-5. [DOI] [PubMed] [Google Scholar]
- 10.Nair VS, Barnett PG, Ananth L, Gould MK. PET scan 18F-fluorodeoxyglucose uptake and prognosis in patients with resected clinical stage IA non-small cell lung cancer. Chest. 2010;137:1150–6. doi: 10.1378/chest.09-2356. [DOI] [PubMed] [Google Scholar]
- 11.Noguchi M, Morikawa A, Kawasaki M, Matsuno Y, Yamada T, Hirohashi S, et al. Small adenocarcinoma of the lung. Histologic characteristics and prognosis. Cancer. 1995;75:2844–52. doi: 10.1002/1097-0142(19950615)75:12<2844::aid-cncr2820751209>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 12.Koike T, Togashi K, Shirato T, Sato S, Hirahara H, Sugawara M, et al. Limited resection for noninvasive bronchioloalveolar carcinoma diagnosed by intraoperative pathologic examination. Ann Thorac Surg. 2009;88:1106–11. doi: 10.1016/j.athoracsur.2009.06.051. [DOI] [PubMed] [Google Scholar]
- 13.Watanabe S, Watanabe T, Arai K, Kasai T, Haratake J, Urayama H. Results of wedge resection for focal bronchioloalveolar carcinoma showing pure ground-glass attenuation on computed tomography. Ann Thorac Surg. 2002;73:1071–5. doi: 10.1016/s0003-4975(01)03623-2. [DOI] [PubMed] [Google Scholar]
- 14.Sakurai H, Dobashi Y, Mizutani E, Matsubara H, Suzuki S, Takano K, et al. Bronchioloalveolar carcinoma of the lung 3 centimeters or less in diameter: a prognostic assessment. Ann Thorac Surg. 2004;78:1728–33. doi: 10.1016/j.athoracsur.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki K, Yokose T, Yoshida J, Nishimura M, Takahashi K, Nagai K, et al. Prognostic significance of the size of central fibrosis in peripheral adenocarcinoma of the lung. Ann Thorac Surg. 2000;69:893–7. doi: 10.1016/s0003-4975(99)01331-4. [DOI] [PubMed] [Google Scholar]
- 16.Breathnach OS, Kwiatkowski DJ, Finkelstein DM, Godleski J, Sugarbaker DJ, Johnson BE, et al. Bronchioloalveolar carcinoma of the lung: recurrences and survival in patients with stage I disease. J Thorac Cardiovasc Surg. 2001;121:42–7. doi: 10.1067/mtc.2001.110190. [DOI] [PubMed] [Google Scholar]
- 17.Shimosato Y, Suzuki A, Hashimoto T, Nishiwaki Y, Kodama T, Yoneyama T, et al. Prognostic implications of fibrotic focus (scar) in small peripheral lung cancers. Am J Surg Pathol. 1980;4:365–73. doi: 10.1097/00000478-198008000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Yoshida J, Ishii G, Yokose T, Aokage K, Hishida T, Nishimura M, et al. Possible delayed cut-end recurrence after limited resection for ground-glass opacity adenocarcinoma, intraoperatively diagnosed as Noguchi type B, in three patients. J Thorac Oncol. 2010;5:546–50. doi: 10.1097/JTO.0b013e3181d0a480. [DOI] [PubMed] [Google Scholar]
- 19.Okada M, Nakayama H, Okumura S, Daisaki H, Adachi S, Yoshimura M, et al. Multicenter analysis of high-resolution computed tomography and positron emission tomography/computed tomography findings to choose therapeutic strategies for clinical stage IA lung adenocarcinoma. J Thorac Cardiovasc Surg. 2011;141:1384–91. doi: 10.1016/j.jtcvs.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 20.Nakayama H, Okumura S, Daisaki H, Kato Y, Uehara H, Adachi S, et al. Value of integrated positron emission tomography revised using a phantom study to evaluate malignancy grade of lung adenocarcinoma: a multicenter study. Cancer. 2010;116:3170–7. doi: 10.1002/cncr.25244. [DOI] [PubMed] [Google Scholar]
- 21.Miyasaka Y, Suzuki K, Takamochi K, Matsunaga T, Oh S. The maximum standardized uptake value of fluorodeoxyglucose positron emission tomography of the primary tumour is a good predictor of pathological nodal involvement in clinical N0 non-small-cell lung cancer. Eur J Cardiothorac Surg. 2013;44:83–7. doi: 10.1093/ejcts/ezs604. [DOI] [PubMed] [Google Scholar]
- 22.Gould MK, Maclean CC, Kuschner WG, Rydzak CE, Owens DK. Accuracy of positron emission tomography for diagnosis of pulmonary nodules and mass lesions: a meta-analysis. JAMA. 2001;285:914–24. doi: 10.1001/jama.285.7.914. [DOI] [PubMed] [Google Scholar]
- 23.Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg. 1995;60:615–22. doi: 10.1016/0003-4975(95)00537-u. discussion 622–23. [DOI] [PubMed] [Google Scholar]
- 24.Asamura H, Nakayama H, Kondo H, Tsuchiya R, Shimosato Y, Naruke T. Lymph node involvement, recurrence, and prognosis in resected small, peripheral, non-small-cell lung carcinomas: are these carcinomas candidates for video-assisted lobectomy? J Thorac Cardiovasc Surg. 1996;111:1125–34. doi: 10.1016/s0022-5223(96)70213-1. [DOI] [PubMed] [Google Scholar]
- 25.Hattori A, Suzuki K, Matsunaga T, Fukui M, Kitamura Y, Miyasaka Y, et al. Is limited resection appropriate for radiologically ‘solid’ tumor in small lung cancers? Ann Thorac Surg. 2012;94:212–5. doi: 10.1016/j.athoracsur.2012.03.033. [DOI] [PubMed] [Google Scholar]