Abstract
OBJECTIVES
Therapy refractory cardiogenic shock is associated with dismal outcome. Percutaneous implantation of an extracorporeal life support (ECLS) system achieves immediate cardiopulmonary stabilization, sufficient end-organ perfusion and reduction of subsequent multiorgan failure (MOF).
METHODS
Forty-one patients undergoing percutaneous ECLS implantation for cardiogenic shock from February 2012 until August 2013 were retrospectively analysed. Mean age was 52 ± 13 years, 6 (15%) were female. Mean pH values obtained before ECLS implantation were 7.15 ± 0.24, mean lactate concentration was 11.7 ± 6.4 mmol/l. Levels obtained 6 h after ECLS implantation were 7.30 ± 0.14 and 8.7 ± 5.0 mmol/l, respectively. In 23 patients (56%) cardiogenic shock resulted from an acute coronary syndrome in 13 (32%) from cardiomyopathy, in 5 (12%) from other causes. Twenty-seven (66%) had been resuscitated, in 14 (34%) implantation was performed under ongoing cardiopulmonary resuscitation (CPR). Of note, 97% of the acute coronary syndrome patients underwent percutaneous coronary intervention (PCI) either before ECLS implantation or under ECLS support. Extracorporeal life support implantation was performed on scene (Emergency Department, Cath Lab, Intensive Care Unit) by a senior cardiac surgeon and a trained perfusionist, in 8 cases (20%) in the referring hospital.
RESULTS
Thirty-day mortality was 51% [21 patients, due to MOF (n = 14), cerebral complications (n = 6) and heart failure (n = 1)]. Logistic regression analysis identified 6-h pH values as an independent risk factor of 30-day mortality (P < 0.001, OR = 0.000, 95% CI 0.000–0.042). Neither CPR nor implantation under ongoing CPR resulted in significant differences. In 26 cases (63%), the ECLS system could be explanted, after mean support of 169 ± 67 h. Seven of these patients received cardiac surgery [ventricular assist device implantation (n = 4), heart transplantation (n = 1), other procedures (n = 2)].
CONCLUSIONS
Due to the evolution of transportable ECLS systems and percutaneous techniques implantation on scene is feasible. Extracorporeal life support may serve as a bridge-to-decision and bridge-to-treatment device. Neurological evaluation before ventricular assist device implantation and PCI under stable conditions are possible. Despite substantial mortality, ECLS implantation in selected patients by an experienced team offers additional support to conventional therapy as well as CPR and allows survival in patients that otherwise most likely would have died. This concept has to be implemented in cardiac survival networks in the future.
Keywords: Extracorporeal life support, Cardiogenic shock, Myocardial infarction, Cardiomyopathy
INTRODUCTION
Therapy refractory cardiogenic shock is associated with dismal outcome, success rates of cardiopulmonary resuscitation (CPR) are variable and especially out-of-hospital cardiac arrest is associated with an unfavourable prognosis. Despite ongoing progress, therapeutic options are still limited [1]. In recent randomized studies, neither intra-aortic balloon pump (IABP) therapy nor other percutaneous ventricular assist systems were able to reduce mortality in shock patients [2, 3].
Extracorporeal life support (ECLS) is a therapeutic option for refractory cardiogenic shock due to various underlying pathologies if other therapeutic options have failed [4, 5].
Due to progress in implantation techniques as well as evolution of transport systems, ECLS implantation must not mandatorily take place in the operating theatre [6, 7]. Transportation using standard air- and ground-emergency service vehicles became possible [8].
Even though mortality rates are still high, ECLS may provide a substantial benefit in survival of patients with cardiogenic shock due to acute coronary syndrome (ACS) and other underlying pathologies [9, 10].
Here, we report on the initial experience of our interdisciplinary ECLS implantation-programme for patients in therapy refractory cardiogenic shock.
PATIENTS AND METHODS
We retrospectively analysed data from 43 patients who underwent percutaneous ECLS implantation due to refractory cardiogenic shock or cardiac arrest with ongoing resuscitation from February 2012 until August 2013. Therapy refractory cardiogenic shock was defined as requirement of increasing doses of inotropic drugs to maintain an adequate systolic and mean arterial blood pressure along with evidence of end-organ hypoperfusion. Extracorporeal life support implantation was successful in 41 patients which were further analysed. The study was approved by the institutional ethics committee, individual patient's consent was not necessary.
The ECLS team consisted of a senior cardiac surgeon and a perfusionist, both trained in ECLS implantation and management. The team was available at all times (24 h/7 days). If not on-site, team members were available within 20–30 min. Details of ECLS implantation and management have been described earlier [9, 11, 12]. Briefly, after disinfection of the implantation site and covering with sterile sheets, percutaneous cannulation of the femoral artery and vein was performed using the Seldinger technique with size 15 or 17-Fr arterial cannulas (Medtronic Bio-Medicus®, Medtronic, Meerbusch, Germany) and size 20 or 24-Fr venous cannulas (Edwards Lifesciences FemTrek®, Edwards Lifesciences, Unterschleissheim, Germany). In case palpation was not sufficient for adequate localization of the vessels, sonography was used additionally. If available, cannula position was verified immediately using transoesophageal echocardiography or radiography. In order to prevent lower limb ischaemia, a distal limb perfusion catheter (Termumo Radiofocus® Introducer, 6-Fr, Eschborn, Germany) was inserted. Tubing was coated with phosphorylcholine. After wire-positioning, 5000–10 000 IU of unfractionated heparin were administered; afterwards it was infused continuously to avoid coagulation of the ECLS system. Anticoagulant therapy control was performed using bedside activated clotting time (ACT) devices to maintain an ACT of 160–180 s. In highly suspected or confirmed cases of heparin-induced thrombocytopenia type II, anticoagulation was performed using argatroban.
If the patient's condition was too critical for transport, the ECLS team implanted the device in the referring hospital using an ECLS transport system (Sorin LifeBox®, Sorin Group, Munich, Germany). After stabilization on-site under ECLS support, the patient was transported to our clinic using standard air- and ground-emergency service vehicles, accompanied by the implanting team. The stationary ECLS systems used were Stöckert SCP systems (Sorin Group, Munich, Germany).
For data collection, the patient's past medical history and current records were reviewed. Routine laboratory parameters (including creatine kinase (CK), creatine kinase-MB isoenzyme levels (CK-MB) and troponin) were obtained before ECLS implantation, 6 h after ECLS implantation and daily within the further course by our department's central laboratory using standardized methods. pH and lactate levels were obtained from arterial blood gas samples using point of care blood gas analysis machines. In all patients, baseline left ventricular ejection fraction (LVEF) was determined either immediately before or shortly after ECLS implantation using transthoracic or transoesophageal echocardiography. Likewise, LVEF was determined before discharge. If haemodynamic stability with low levels of inotropes and vasopressors persisted, stepwise weaning by reduction of ECLS pump flow was pursued. Duration of survival was determined from ECLS implantation time to death or at a 30-day follow-up.
Explantation of the system was performed bedside in most of the cases. The arterial punction site was temporarily compressed using a Femo-Stop™ (St. Jude Medical, Eschborn, Germany) according to the manufacturer's suggestions.
Statistics
Categorical variables are given as numbers and percentages. Data concerning continuous variables are expressed as mean ± standard deviation (SD). Statistical analysis for group comparison or 30-day mortality was performed using the t-test, the χ2 or Fisher's exact test. For continuous variables, forward stepwise logistic regression analysis (likelihood ratio) was used to determine risk factors of 30-day mortality. Overall survival rates were analysed using the Kaplan–Meier estimator. Statistical differences were determined using the log-rank test. Univariant analyses for group comparison were performed using analysis of variance. IBM SPSS Statistics software, Version 20 was used for statistical analysis. A P-value of <0.05 was considered statistically significant.
RESULTS
All patients
Demographics
In 2 cases ECLS implantation under ongoing CPR was not successful. Establishing alternative access was rejected because of prolonged resuscitation. Forty-one patients were implanted successfully. Baseline characteristics of these patients are given in Table 1. Six patients (15%) were female, mean age was 52 ± 13 years (range 17–81 years). According to the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) scale, 36 patients (88%) had to be grouped into Level 1, 5 patients (12%) into Level 2. One patient had been implanted an Impella® pump (Abiomed Europe, Aachen, Germany), one a Lifebridge® system (Lifebridge Medizintechnik, Ampfing, Germany) in the referring hospital. Both systems were explanted upon arrival in our department and changed to an ECLS system because adequate cardiac output was not achievable or the system was only licensed for 6 h. Three patients (7%) were conscious during ECLS implantation and support. In 8 cases (20%) ECLS implantation was performed in the referring hospital by a team consisting of a cardiac surgeon and a trained perfusionist of our department because the patient's condition was too critical for transport. All patients were transported to our department after stabilization on-site without any adverse events or technical incidents occurring during transport. Mortality rates between in- and out-of-centre implantation did not show statistical relevant differences (P = 0.454). Twenty-seven patients (66%) had been resuscitated, in 14 (34%) implantation was performed under ongoing CPR. There was no statistical significant difference in mortality for both factors. To improve neurological outcome, patients that had been resuscitated were cooled down to 32–34°C for 24 h using an ECLS-bound heat exchanger. The underlying cause of cardiogenic shock was an ACS in 23 patients (56%) and a cardiomyopathy (CM) in 13 patients (32%). In 5 patients (12%) cardiogenic shock was due to other reasons [pulmonary embolism (n = 1), dysfunction of a mechanical mitral valve prosthesis (n = 1), unknown pathology (n = 3)]. Initial pump flow was 4.5 ± 0.7 l/min (data missing for 4 patients) and was further adapted to haemodynamics. Initial flow did not differ between survivors and patients that died (P = 0.301).
Table 1:
Baseline characteristics of all patients
| Variable | All patients | Alive within 30 days | Death within 30 days | P-value |
|---|---|---|---|---|
| n (%) | 41 | 20 (49%) | 21 (51%) | |
| ECLS duration (h), mean ± SD | 125 ± 88 | 169 ± 67 | 84 ± 87 | 0.001 |
| Initial pump flow (l/min), mean ± SD | 4.5 ± 0.7a | 4.4 ± 0.8 | 4.6 ± 0.6 | 0.3 |
| Female sex, n (%) | 6 (15%) | 5 | 1 | 0.09 |
| Age (years), mean ± SD | 52 ± 13 | 49 ± 11 | 55 ± 14 | 0.09 |
| Reason for cardiogenic shock, n (%) | ||||
| ACS | 23 (56%) | 12 | 11 | 0.4 |
| CM | 13 (32%) | 7 | 6 | |
| Other | 5 (12%) | 1 | 4 | |
| CPR, n (%) | 27 (66%) | 12 | 15 | 0.52 |
| Implantation during CPR, n (%) | 14 (34%) | 4 | 10 | 0.1 |
| Out-of-centre implantation, n (%) | 8 (20%) | 5 | 3 | 0.45 |
| Initial pH, mean ± SD | 7.15 ± 0.24 | 7.23 ± 0.21 | 7.08 ± 0.25 | 0.04 |
| 6 h pH, mean ± SD | 7.30 ± 0.14 | 7.37 ± 0.13 | 7.23 ± 0.11 | 0.001 |
| Initial lactate (mmol/l), mean ± SD | 11.7 ± 6.4 | 11.0 ± 6.4 | 12.5 ± 6.5 | 0.45 |
| 6 h lactate (mmol/l), mean ± SD | 8.7 ± 5.0 | 6.8 ± 4.3 | 10.5 ± 5.0 | 0.02 |
| Haemofiltration/-dialysis, n (%) | 19 (46%) | 8 | 11 | 0.35 |
| PCI + stenting, n (%) | 22 (54%) | 12 | 10 | |
| CABG, n (%) | 1 (2%) | n/a | 1 | |
| Heart valve surgery, n (%) | 1 (2%) | 1 | n/a | |
| LVAD, n (%) | 2 (5%) | 2 | n/a | |
| BVAD, n (%) | 2 (5%) | 1 | 1 | |
| Heart transplantation, n (%) | 1 (2%) | 1 | n/a | |
ECLS: extracorporal life support; SD: standard deviation; ACS: acute coronary syndrome; CPR: cardiopulmonary resuscitation; PCI: percutaneous coronary intervention; CABG: coronary artery bypass grafting; LVAD: left ventricular assist device; BVAD: biventricular assist device.
a4 missing (1 alive, 3 deaths).
Laboratory parameters
Comparison of preimplantation pH showed significantly lower levels in patients that died within 30 days (7.08 ± 0.25 vs 7.23 ± 0.21, P = 0.04). Analysis of preimplantation lactate levels did not reveal any statistical significance (12.5 ± 6.5 mmol/l in patients who died within 30 days vs 11.0 ± 6.4 mmol/l in patients who survived, P = 0.45). Comparison of pH and lactate levels obtained 6 h after ECLS implantation showed statistical significance for both factors: pH levels were significantly more acidic in patients who died within 30 days (7.23 ± 0.11 vs 7.37 ± 0.13, P = 0.001) and lactate levels were significantly higher (10.5 ± 5.0 vs 6.8 ± 4.3, P = 0.02). Accordingly, logistic regression analysis of initial and 6-h pH values and lactate levels identified 6-h pH values as an independent risk factor of 30-day mortality (P < 0.001, odds ratio (OR) = 0.000, 95% confidence interval (CI) 0.000–0.042).
Outcome
Fifteen patients (37%) died during ECLS therapy, 9 due to multiorgan failure (MOF), 6 because of neurological complications. All of the latter had been resuscitated. In 26 cases (63%) the ECLS system could be explanted, 7 of them received cardiac surgery. One of these patients (2%) underwent surgical coronary revascularization and died due to MOF. Two patients (5%) underwent biventricular assist device (BVAD) implantation (Berlin Heart EXCOR®). In both cases, the arterial cannula of the ECLS was explanted and an additional venous cannula was inserted into the jugular vein for further pulmonary support [extracorporeal membrane oxygenation system (ECMO)]. One died due to MOF, 1 survived. Two patients (5%) underwent left ventricular assist device (LVAD) implantation (HeartWare®). In both cases, the ECLS system initially remained in place for further right ventricular support and was removed within the following days. One patient (2%) underwent balloon atrial septostomy for left ventricular unloading and received heart transplantation in the further course. One patient (2%) received re-mitral valve replacement. In 5 (12%) of the remaining patients in which the ECLS system could be explanted, an IABP was implanted for further cardiac support. Fourteen patients (34%) did not require cardiac surgery or further support.
In 4 cases, cannulation-related complications affecting the femoral vessels and requiring surgical revision occurred. Two cases of bleeding at the cannula site requiring surgical intervention and 2 cases of wound healing disorders requiring surgical revision were observed. In most cases of bleeding complications, the patients received dual antiplatelet therapy because of recent coronary artery stenting. Five cases of lower limb ischaemia occurred, in 2 cases despite insertion of a distal limb perfusion catheter. In 2 cases, implantation of a perfusion catheter was unsuccessful due to severe peripheral artery occlusive disease. In 1 patient the distal limb perfusion catheter was inserted several hours after ECLS implantation. Altogether, 13 patients (32%) experienced ECLS implantation-related complications. In 1 case sudden thrombosis of the pump occurred, most likely due to a heparin-induced thrombocytopenia.
Thirty-day follow-up
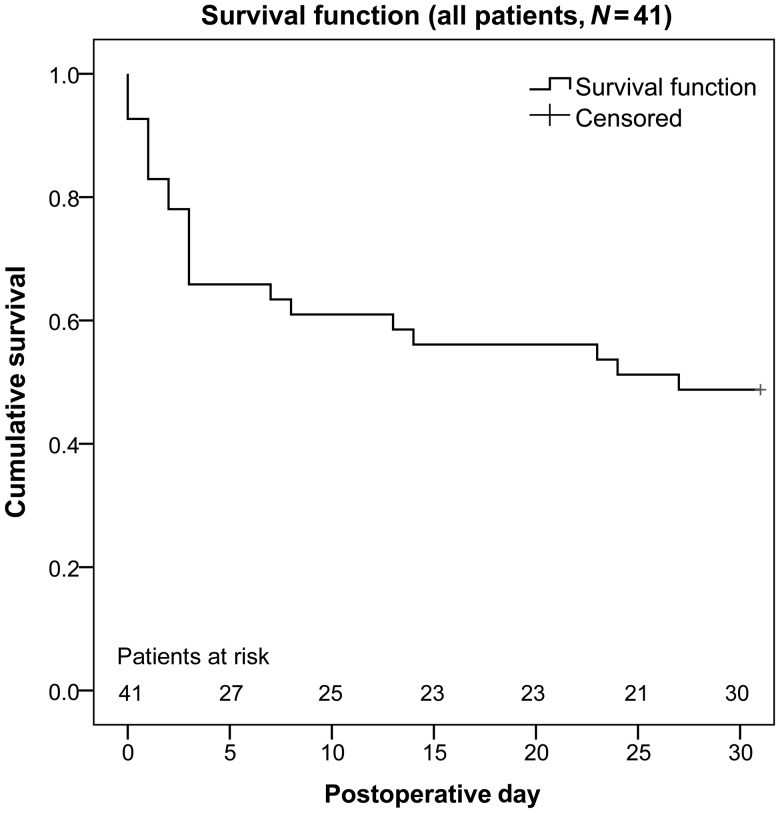
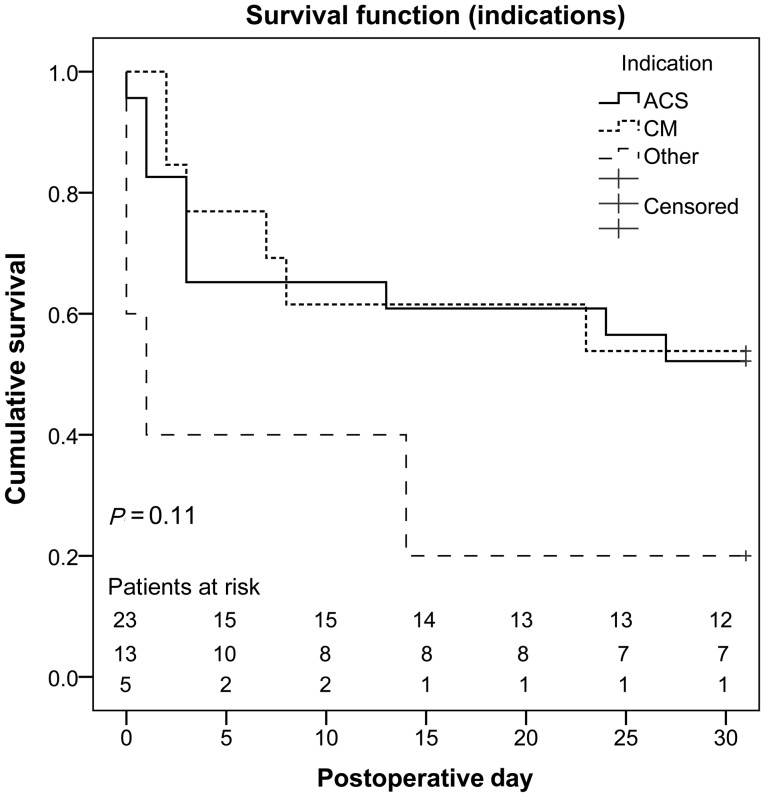
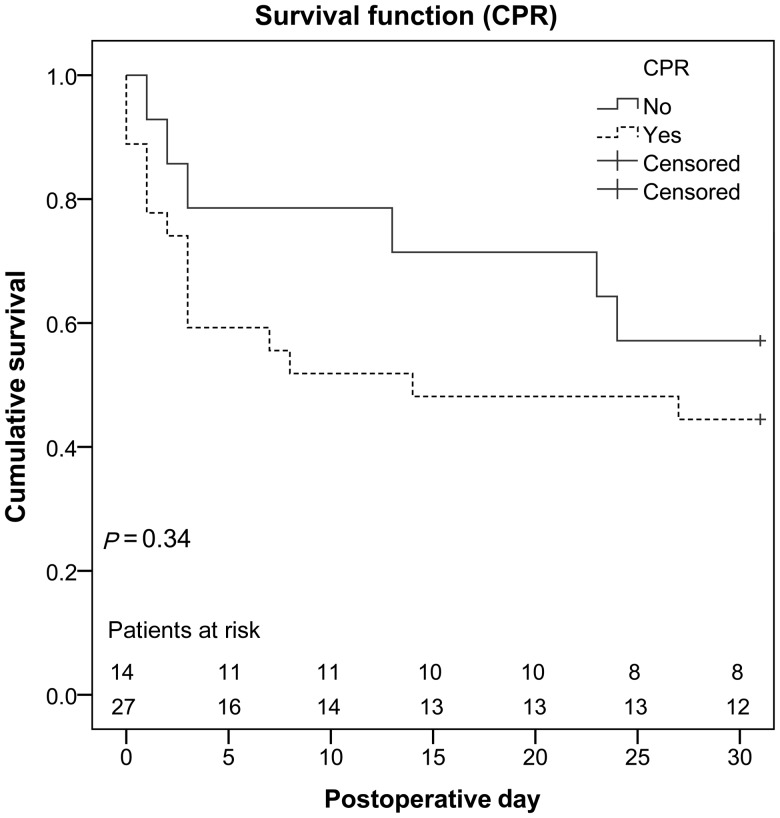
Four additional patients who initially had been weaned successfully died within the 30-day period, all of them due to MOF. Altogether, 21 patients (51%) died within 30 days. Overall survival is shown in Fig. 1, overall survival attributed to the underlying cause of cardiogenic shock in Fig. 2. Figure 3 compares non-resuscitated and resuscitated patients.
Figure 1:
Overall survival of all patients.
Figure 2:
Overall survival of all patients attributed to indication. ACS: acute coronary syndrome; CM: cardiomyopathy.
Figure 3:
Comparison of non-resuscitated and resuscitated patients. CPR: cardiopulmonary resuscitation.
Mean ICU stay of the surviving 20 patients was 24 ± 13 days, mean artificial ventilation time 16.5 ± 11.6 days. Eleven patients (55%) received surgical tracheotomy in order to facilitate weaning. Six patients (30%) still required intermittent, 1 patient (5%) full respirator support. Thirteen patients (65%) had been totally weaned. Two patients (10%) had received LVAD, 1 patient (5%) BVAD implantation, 1 patient (5%) underwent heart transplantation. Mean LVEF of the other patients was 39 ± 11%. Seven patients (35%) were compromised due to critical illness polyneuropathy. Three patients (15%) experienced neurological complications. The BVAD-patient suffered an apoplexia, 1 patient suffered cerebral ischaemia and another diffuse encephalopathy with prolonged awakening. Ten patients (50%) did not have any neurological impairment. One patient (5%) was in need of intermittent haemodialysis without having suffered from renal dysfunction preoperatively. Detailed information is given in Table 4.
Table 4:
Thirty-day outcome of all patients, acute coronary syndrome and cardiomyopathy patients
| Variable | All patients alive within 30 days | ACS patients alive within 30 days | CM patients alive within 30 days |
|---|---|---|---|
| n | 20 | 12 | 7 |
| ICU stay (days), mean ± SD | 24 ± 13 | 25 ± 11 | 24 ± 16 |
| Artificial ventilation time (days), mean ± SD | 16.5 ± 11.6 | 21.7 ± 11.1 | 9.0 ± 7.8 |
| Tracheotomy, n (%) | 11 (55%) | 11 (92%) | n/a |
| Cardiac function | |||
| LVEF (%), mean ± SD | 39 ± 11 | 41 ± 11 | 34 ± 9 |
| Surgical therapy | |||
| LVAD, n (%) | 2 (10%) | 2 (17%) | n/a |
| BVAD, n (%) | 1 (5%) | n/a | 1 (14%) |
| Heart transplantation, n (%) | 1 (5%) | n/a | 1 (14%) |
| Pulmonary status | |||
| Spontaneous breathing, n (%) | 13 (65%) | 6 (50%) | 6 (86%) |
| Intermittent artificial ventilation, n (%) | 6 (30%) | 5 (42%) | 1 (14%) |
| Full artificial ventilation, n (%) | 1 (5%) | 1 (8%) | n/a |
| Neurological outcome | |||
| No impairment, n (%) | 10 (50%) | 5 (42%) | 4 (57%) |
| CIP, n (%) | 7 (35%) | 6 (50%) | 1 (14%) |
| Neurological complications, n (%) | 3 (15%) | 1 (8%) | 2 (29%) |
| Renal complications | |||
| Haemodialysis, n (%) | 1 (5%) | 1 (8%) | n/a |
ACS: acute coronary syndrome; CM: cardiomyopathy; ICU: intensive care unit; LVEF: left-ventricular ejection fraction; SD: standard deviation; LVAD: left ventricular assist device; BVAD: biventricular assist device; CIP: critical illness polyneuropathy.
Acute coronary syndrome patients
Demographics
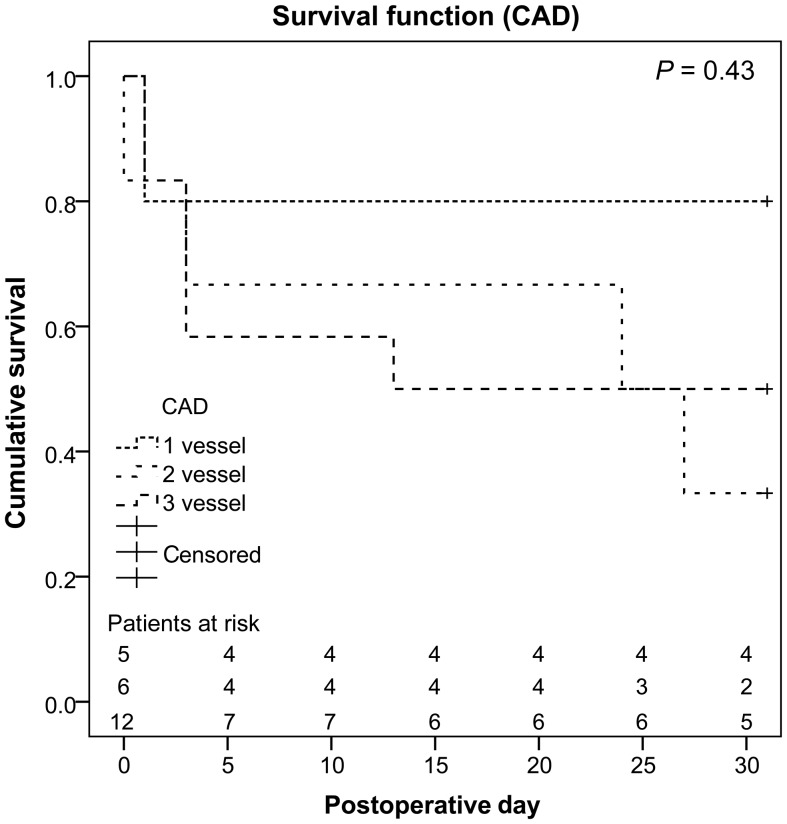
In 23 patients (56%, three females, mean age 55 ± 14 years, range 17–81 years), the underlying cause of cardiogenic shock was an ACS. Detailed patient characteristics are depicted in Table 2. All except for 1 patient (n = 22, 96%) underwent percutaneous coronary intervention (PCI) and coronary artery stenting either before, during or after ECLS support. Eighteen (78%) had been resuscitated, in 8 (35%) implantation was performed under ongoing resuscitation. Again, neither CPR (P = 0.52) nor ECLS implantation under ongoing CPR (P = 0.4) resulted in statistical significant differences between the patients that survived and those who died within 30 days. Four patients (17%) presented with a non-ST-segment-elevation myocardial infarction (STEMI), 19 (83%) with a STEMI. In 5 patients (22%) only one vessel was affected, 18 patients (78%) had a two- or three-vessel disease. No statistical relevant differences for mortality were seen for NSTEMI compared with STEMI patients (P = 0.32). Comparison of the number of coronary vessels affected did not show statistically significant differences either (Fig. 4).
Table 2:
Acute coronary syndrome patients: baseline characteristics and outcome
| Variable | All ACS patients | Alive within 30 days | Death within 30 days | P-value |
|---|---|---|---|---|
| n (%) | 23 | 12 (52%) | 11 (48%) | |
| ECLS duration (h), mean ± SD | 120 ± 81 | 162 ± 68 | 74 ± 71 | 0.006 |
| Female sex, n (%) | 3 (13%) | 2 | 1 | 1.0 |
| Age (years), mean ± SD | 55 ± 14 | 52 ± 10 | 58 ± 17 | 0.38 |
| CPR, n (%) | 18 (78%) | 11 | 7 | 0.16 |
| Implantation during CPR, n (%) | 8 (35%) | 3 | 5 | 0.4 |
| Initial pH, mean ± SD | 7.09 ± 0.23 | 7.16 ± 0.24 | 7.01 ± 0.21 | 0.11 |
| 6 h pH, mean ± SD | 7.26 ± 0.13 | 7.31 ± 0.11 | 7.20 ± 0.12 | 0.02 |
| Initial lactate (mmol/l), mean ± SD | 13.6 ± 6.4 | 11.5 ± 6.2 | 16.0 ± 6.1 | 0.1 |
| 6 h lactate (mmol/l), mean ± SD | 9.5 ± 4.8 | 7.2 ± 3.8 | 12.0 ± 4.7 | 0.01 |
| Initial CK (U/l), mean ± SD | 2692 ± 3672 | 2405 ± 2660 | 3005 ± 4656 | 0.71 |
| Maximum CK (U/l), mean ± SD | 16 601 ± 29 357 | 19 320 ± 40 354 | 13 636 ± 9285 | 0.65 |
| Initial CK-MB (U/l), mean ± SD | 267 ± 287 | 213 ± 219 | 327 ± 348 | 0.35 |
| Maximum CK-MB (U/l), mean ± SD | 506 ± 374 | 436 ± 339 | 581 ± 411 | 0.37 |
| Initial troponin (ng/ml), mean ± SD | 25.6 ± 33.8 | 34.5 ± 41.3 | 17.0 ± 22.2 | 0.25 |
| Maximum troponin (ng/ml), mean ± SD | 67.6 ± 96.0 | 52.0 ± 30.6 | 84.5 ± 136.5 | 0.43 |
| Haemofiltration/-dialysis, n (%) | 11 (48%) | 5 | 6 | 0.39 |
| NSTEMI, n (%) | 4 (17%) | 1 | 3 | 0.32 |
| STEMI, n (%) | 19 (83%) | 11 | 8 | |
| 1-vessel disease, n (%) | 5 (22%) | 4 | 1 | 0.33 |
| 2-vessel disease, n (%) | 6 (26%) | 2 | 4 | |
| 3-vessel disease, n (%) | 12 (52%) | 6 | 6 | |
| PCI + stenting, n (%) | 22 (96%) | 12 | 10 | |
| CABG, n (%) | 1 (40%) | n/a | 1 | |
| LVAD, n (%) | 2 (9%) | 2 | n/a | |
| BVAD, n (%) | n/a | n/a | n/a | |
| Heart transplantation, n (%) | n/a | n/a | n/a |
ACS: acute coronary syndrome; ECLS: extracorporeal life support; SD: standard deviation; CPR: cardiopulmonary resuscitation; CK: creatin kinase; CK-MB: creatine kinase-MB isoenzyme; NSTEMI: non-ST-segment elevation myocardial infarction, STEMI: ST-segment elevation myocardial infarction; PCI: percutaneous coronary intervention; CABG: coronary artery bypass grafting; LVAD: left ventricular assist device; BVAD: biventricular assist device.
Figure 4:
Overall survival of acute coronary syndrome patients attributed to the number of coronary vessels affected. CAD: coronary artery disease.
Laboratory parameters
Again, 6-h pH values were statistically significant lower (7.20 ± 0.12 vs 7.31 ± 0.11, P = 0.02) and 6-h lactate levels significantly higher (12.0 ± 4.7 vs 7.2 ± 3.8, P = 0.01) in patients who died. No significant differences were found for initial pH and lactate levels (7.01 ± 0.21 vs 7.16 ± 0.24, P = 0.11 and 16.0 ± 6.1 vs 11.5 ± 6.2, P = 0.1, respectively). Neither initial nor maximum levels of CK, CK-MB and troponin showed significant differences (Table 2).
Outcome
Eight patients (35%) died during ECLS support, 6 due to MOF, 2 because of neurological complications (both had been resuscitated). Fifteen patients (65%) could be weaned successfully, 14 of them had received PCI and coronary artery stenting, 2 patients (9%) additionally underwent successful LVAD implantation (HeartWare®) in the further course, in 2 other patients (9%) an IABP was implanted for further cardiac support and 1 patient (4%) received coronary artery bypass grafting (CABG).
Thirty-day follow-up
Three additional patients (13%) who initially had been weaned successfully died within the first 30 days (all because of MOF, among them the CABG patient) resulting in an overall 30-day mortality of 11 patients (48%). Mean ICU stay of the surviving 12 patients was 25 ± 11 days. Mean artificial ventilation time was 21.7 ± 11.1 days. In many cases, this was due to muscle weakness because of CIP which occurred in 6 patients (50%). In order to facilitate weaning from the respirator, surgical tracheotomy was performed in 11 patients (92%). However, after 30 days only 1 patient (8%) still required permanent respirator support, 5 (42%) received intermittent support via continuous positive airway pressure therapy, 6 (50%) were breathing spontaneously. Taken into consideration all patients except for those on LVAD support, mean LVEF was 41 ± 1%. One patient (8%) was suffering from a diffuse encephalopathy which resulted in prolonged awakening. One patient (8%) was in need of intermittent haemodialysis with no relevant kidney impairment in his past medical history (see Table 4).
Cardiomyopathy patients
Demographics
In 13 patients (32%, two females, mean age 47 ± 12 years, range 28–74 years) the underlying cause of the cardiogenic shock was a CM, in 6 of these a dilated cardiomyopathy, in 1 an arrhythmogenic right ventricular dysplasia, in 1 a valvular and in 2 cases a combined CM. In three cases, the underlying pathology remained unclear. Detailed patient characteristics are given in Table 3. Patients who survived were significantly younger than patients who died (54 ± 11 years vs 41 ± 8 years, P = 0.03). Five patients (39%) had been resuscitated, in 2 (15%) implantation was performed under ongoing resuscitation. Comparison of resuscitation rates between patients who died and those who survived showed a trend of higher resuscitation rates in patients who died (P = 0.05). There was no statistical significant difference of the initial LVEF between the two groups.
Table 3:
Cardiomyopathy patients: baseline characteristics and outcome
| Variable | All CM patients | Alive within 30 days | Death within 30 days | P-value |
|---|---|---|---|---|
| n (%) | 13 | 7 (54%) | 6 (46%) | |
| ECLS duration (h), mean ± SD | 149 ± 79 | 196 ± 55 | 95 ± 70 | 0.01 |
| Female sex, n (%) | 2 (15%) | 2 | n/a | 0.46 |
| Age (years), mean ± SD | 47 ± 12 | 41 ± 8 | 54 ± 11 | 0.03 |
| CPR, n (%) | 5 (39%) | 1 | 4 | 0.05 |
| Implantation during CPR, n (%) | 2 (15%) | 1 | 1 | 1 |
| Initial pH, mean ± SD | 7.28 ± 0.13 | 7.32 ± 0.13 | 7.24 ± 0.12 | 0.24 |
| 6 h pH, mean ± SD | 7.36 ± 0.15 | 7.44 ± 0.12 | 7.26 ± 0.12 | 0.02 |
| Initial lactate (mmol/l), mean ± SD | 8.2 ± 5.0 | 8.7 ± 6.3 | 7.5 ± 3.5 | 0.68 |
| 6 h lactate (mmol/l), mean ± SD | 6.2 ± 4.2 | 4.8 ± 3.9 | 7.9 ± 4.1 | 0.19 |
| Haemofiltration/-dialysis, n (%) | 7 (54%) | 3 | 4 | 0.59 |
| Initial LVEF (%), mean ± SD | 14 ± 6 | 13 ± 6 | 14 ± 5 | 0.69 |
| LVAD, n (%) | n/a | n/a | n/a | |
| BVAD, n (%) | 2 (15%) | 1 | 1 | |
| Heart transplantation, n (%) | 1 (8%) | 1 | n/a |
CM: cardiomyopathy; ECLS: extracorporeal life support; SD: standard deviation; CPR: cardiopulmonary resuscitation; LVEF: left-ventricular ejection fraction; LVAD: left ventricular assist device; BVAD: biventricular assist device.
Laboratory parameters
Considering only CM patients, solely 6-h pH values were significantly different in patients who died compared with those who survived (7.26 ± 0.12 vs 7.44 ± 0.12, P = 0.02). Initial pH, initial lactate and 6-h lactate levels did not show statistical relevant differences between the groups.
Outcome
Three patients (23%) died during ECLS support, 2 because of MOF, 1 because of intracranial bleeding (prolonged CPR before ECLS implantation and systemic lysis). In 10 cases (77%), the ECLS system could be explanted. Two of these patients (15%) received a BVAD (Berlin Heart EXCOR®) of whom 1 died due to MOF. One patient (8%) received heart transplantation. In this patient, balloon atrioseptostomy had to be performed during ECLS support for left ventricular unloading. In 3 patients (23%) an IABP was implanted during ECLS explantation.
Thirty-day follow-up
One patient refusing assist device implantation was initially weaned successfully but died on Day 23 due to heart failure. Another patient who had been weaned died due to MOF in the further course. Overall 30-day mortality therefore was 46% (6 patients), 7 patients (54%) were alive after 30 days. Mean ICU stay of the surviving patients was 24 ± 16 days, mean time on respirator was 9 ± 7.8 days. Tracheotomy was necessary in none of the patients. Only 1 patient (14%) was still in need of intermittent respirator support. One patient (14%) had received heart transplantation, another (14%) underwent BVAD implantation. Mean LVEF of the remaining 5 patients was 34 ± 9%. Two patients (29%) suffered apoplexia, 1 of them during BVAD therapy. Detailed information is given in Table 4.
DISCUSSION
Possible indications for ECLS therapy include acute exacerbation of chronic heart failure, acute heart failure due to ACSs, myocarditis, drug intoxication, intractable arrhythmias, pulmonary embolism and cardiac arrest requiring CPR due to several reasons [6]. As far as applicable in emergency situations, contraindications for ECLS institution are terminal malignancies, severe coagulation disorders, suspected or confirmed acute stroke, irreversible brain or end-organ damage, patients in an acute exacerbation of chronic severe heart failure not eligible for assist device implantation or heart transplantation or a ‘do not resuscitate’ order [4, 6]. However, ideal patient selection and preimplant factors being indicative of in-hospital and long-term survival are still debated. In our institution, main indications for non-postcardiotomy ECLS implantation are severe therapy refractory cardiogenic shock due to ACS, CM, myocarditis, valvular heart diseases, pulmonary embolism or ongoing CPR without return of spontaneous circulation. Almost no ultimate exclusion criteria exist and decision is taken in each individual case based on interdisciplinary consent of treating cardiac surgeons, cardiologists and anaesthesiologists. In case the underlying pathology is unclear and no obvious contraindications exist, ECLS implantation may be performed as a bridge-to-decision therapy. Even in cases of unobserved collapse and unknown duration of CPR ECLS implantation is possible.
We observed a general 30-day survival rate of 49%. Acute coronary syndrome patients survived in 52%, CM patients in 54%. Other groups report around 28–53% survival [9, 10, 13, 14]; however, a lot of the studies available in current literature only included patients with cardiac arrest resulting in reduced comparability and accounting for the wide range of survival rates.
Patients of our study population who died within 30 days did not only show significantly lower pH levels preimplantation, but were also significantly more acidic and had higher blood lactate levels at 6 h after ELCS implantation. Similar results were found by other authors [8, 10, 15]. Consecutively, we could identify 6-h pH values as an independent risk factor contributing to 30-day mortality. Considering lactate and acidosis as parameters of systemic malperfusion and impaired tissue oxygenation, these results show that controlling cardiac shock as soon as possible and thus not only normalizing acid–base metabolism but also achieving normal perfusion of vital organs is crucial. A cardiac index of 2.2–2.8, in critically ill patients up to 3 l min−1 m−2 should be attained [6]. Since other studies including a smaller one with 28 patients showed no impact of preimplant pH and lactate as well as 6-h lactate [13], further studies are warranted. A more integrating approach considering additional parameters of shock and MOF might have an even better predictive value. However, it must be taken into consideration that it is not feasible to determine numerous parameters if the patient's condition is critical. Possible parameters might be those used in the sequential organ failure Assessment Score or the peripheral venous oxygen saturation which is easily obtainable even in emergency situations [16, 17]. Given that all patients had been critically ill, classical scales and scores such as the INTERMACS scale do not help to triage the patients because all of them had to be assigned to low levels.
Since neither resuscitation nor ECLS implantation under ongoing resuscitation did show statistical significant differences when comparing all patients or the subgroups and since several studies showed that early ECLS implantation in patients undergoing CPR significantly improves survival rates [13], this explicitly is not a contraindication for ECLS implantation. In contrast, ELCS implantation and support is a feasible way of stabilizing patients presenting with cardiac arrest.
For ACS patients no relevant differences were seen for the underlying pathology (NSTEMI vs STEMI, one-vessel disease vs two-vessel disease or three-vessel disease). However, taking into consideration the corresponding Kaplan–Meier survival estimate this might be due to the little number of patients presenting with a one-vessel disease. As we demonstrated earlier, mortality in patients presenting with STEMI and cardiogenic shock who undergo immediate surgical coronary revascularization is substantially high [18]. Especially in these patients ECLS therapy allowing for stabilization and bridging to surgical revascularization should be taken into consideration. Further studies addressing this question in detail are warranted.
In general, stepwise weaning from ECLS is pursued (bridge-to-recovery), however if causal therapy is not available or recovery of the myocardium not achievable, ECLS may serve as a bridging therapy to either assist device implantation or heart transplantation [5, 6]. One CM patient was successfully bridged to heart transplantation and—as 2 other patients—was conscious during ECLS therapy [19]. Extracorporeal life support therapy as a bridge-to-transplant option has also been reported by other groups in selected cases [20]; however, the feasibility of this approach highly depends on the availability of donor organs. Another approach is the assist device implantation as destination therapy (bridge-to-destination) or to span waiting time to heart transplantation (bridge-to-bridge) after initial ECLS therapy. The benefits of primary ECLS therapy might be end-organ and right ventricle recovery allowing assist device implantation to happen under more stable conditions and possibly allowing the use of a univentricular respectively intracorporeal ventricular assist device. Additionally, other cardiopulmonary support than ECLS implantation is barely feasible under ongoing CPR [20, 21]. Especially in high-risk patients ventricular assist device implantation might even be performed on ECLS without switching to a conventional cardiopulmonary bypass system in order to reduce its side-effects [22]. In our study, 2 patients were implanted a Berlin Heart EXCOR®, 2 patients a HeartWare®; one of the BVAD-patients died due to MOF. If necessary, the ECLS system may remain in situ for right ventricular support within the first days or might be switched to a veno-pulmonary artery right heart bypass. For pulmonary support, the ECLS system might be changed into a veno-venous ECMO system.
Beurtheret et al. showed that in-hospital mortality rates were equal in patients who received ECLS implantation in-house or in the referring hospital even though the distribution of diagnoses was significantly different. They were able to stabilize and transport 86% of the patients who underwent ECLS implantation in the referring hospital of whom 36.8% survived. The remaining 14% could not be transported due to haemodynamic instability and ultimately died after a median of 2 days [5]. Arlt et al. implanted hand-held Mini-ECMO systems out-of-centre in 21 patients with cardiogenic shock due to myocardial infarction (20 patients) or pulmonary embolism (1 patient). All patients could be transported to the corresponding tertiary-care centre; 62% survived [8]. In our cohort, 20% of the patients were implanted in other hospitals and were transported to our department without complications. Thirty-day mortality rates between in-house and out-of-centre implantations did not differ either.
Other studies showed that duration of CPR [10], the time interval between collapse and starting ECLS [23] and the door-to-ECLS-implantation time in patients with out-of-hospital cardiac arrest [13] are crucial. This emphasizes the necessity of well-organized ECLS-programmes in order to make ECLS implantation feasible as soon as possible.
Commonly observed ECLS access site-related complications are vessel injury, bleeding, lower limb ischaemia and infection [6]. We saw 23 cases (32%) of implantation-related complications. Other groups observed with 30–40% comparable or even higher rates of complications [5, 7, 9, 10] even though varying circumstances of ECLS implantation and varying definitions of ECLS complications need to be taken into account. Peripheral artery disease is a major risk factor of ischaemic complications [24]. In order to avoid lower limb ischaemia, insertion of a distal limb perfusion catheter is essential [25]. Isolated cases of unsuccessful percutaneous ECLS implantation occur, in such cases subsequent surgical cannulation might be considered [23]. In general, implantation should be performed by an experienced cardiac surgeon able to cope with complications and being well-versed in establishing alternative access (subclavian artery, femoral cut-down and aortic cannulation).
Limitations
The study design was retrospective and single-centre based. The study included only a relatively small number of patients with various underlying pathologies. The number of patients presented is too low to establish an accurate risk model. Additionally, no control group existed. So far, only limited data on long-term survival and quality-of-life exist. Further studies addressing these issues are warranted.
CONCLUSION
Extracorporeal life support therapy in patients with refractory cardiogenic shock or in patients undergoing CPR is feasible and may serve as a bridge-to-decision and bridge-to-treatment device. It allows acceptable 30-day survival in about 50% of the patients that otherwise most likely would have died. In patients with severe heart failure refractory to conventional therapy ECLS implantation should therefore be considered early. Ideally, ECLS should be initiated before inadequate circulation and thus end-organ failure results. Establishment of ECLS teams available all time and establishing the necessary infrastructure for making ECLS implantation possible in remote hospitals might serve to further increase survival of patients suffering from cardiogenic shock or cardiac arrest and undergoing CPR.
APPENDIX. CONFERENCE DISCUSSION
Dr A. Moritz (Frankfurt, Germany): Thank you for sending me the manuscript so that I can go into some details you could not show here in your talk. This is in some respects a peculiar series. There were two other ECMO sessions. So we have to remember it's 41 patients, it's non-postcardiotomy, and it's all percutaneous, so that's the group.
Lactate levels and pH at the beginning were prognostic for survival, at least 30-day survival, but also the 6-hour lactate levels. In the manuscript you did not show any other parameters, such as whether or not you achieved sufficient flow for these patients. The absolute numbers are something but the flow needed by these patients may be a different thing. So I have three questions.
You also had in your series, as in many others, limb ischaemia complications, but you did not delineate the fate of these patients. Did they all die once they had ischaemia? In our series, that's a very dismal parameter. The second question refers to what I said. You achieved a flow of 2.2 to 2.8 litres per square metre. Is this sufficient? For postcardiotomy we know that it's not necessarily enough for these patients to cope with their metabolic disorder and they actually, at least to our knowledge, need higher flows to recover and to repair. And how did you check this? In the paper there is only the pH shown. There is no indication of central venous oxygenation or any other measurement of effective cardiac output. That's the second question.
And the third, you restricted your follow-up to 30 days which is a bit short for these patients. You also had neurological complications, so my question is how many of these patients, of this critically ill group, made it to a reasonable quality of life 90 days after your implantation?
Dr Khaladj: Limb ischaemia, as we heard before, is a serious problem. Three of the five patients with lower limb ischaemia died in hospital. In this retrospective study, flow rates of up to 6.5 l/min could be achieved. Flow rates were adapted to haemodynamic and metabolic parameters to control the shock. The initial flow rates of the patients that died and those who survived were not different. We started our ECLS programme in 2012. This is our early experience. We are following the patients up to 90 days, including a quality of life assessment.
Dr Moritz: I just want to point out absolute flow and blood pressure is not necessarily a means to determine the metabolic needs of these patients. So the failed recovery of pH and lactate in your patient group was a bad prognostic factor.
Dr Khaladj: Yes.
Dr Moritz: So my question is, could you achieve, not only by ECLS flow but also by other measures, sufficient flow in these patients? Why did they not recover?
Dr Khaladj: In some of the patients, ECLS implantation was performed too late or after prolonged CPR with lactate levels up to 300 mg/dl and pH values around 6.5. Subsequently controlling shock was impossible due to advanced organ failure. These patients were treated on different ICUs, so for this patient cohort no standardized protocol for measuring the central venous saturation existed and the data are therefore not presented, but this is warranted in further studies.
Dr A. Martens (Hannover, Germany): I think the most important thing is that these patients have to be on ECLS very early. So what is your experience with the cardiologists over the last months and years? Do they know that they can send patients to you? Are there centres that have a cardiac life support system on site and are there patients who arrive at your hospital with a previously implanted ECMO?
Dr Khaladj: From the patients presented today, one patient was on the Lifebridge® system and another was supported by an Impella pump; both were switched to our system upon hospital arrival. To date, two cardiology departments near Munich are able to implant Lifebridge® systems. All other departments inform us if they have patients too unstable to transport; in these cases we implant the system in the referring hospital before transfer to our centre.
Dr D. Zimpfer (Vienna, Austria): You stated in your presentation that eight patients received the ECMO system out of the hospital. Did you go to other hospitals to implant it or was it in the setting of CPR?
Dr Khaladj: This was not in the setting of CPR. Most of the patients suffered from decompensated cardiomyopathies (five of them), and three had an acute coronary syndrome after prior PCI in the referring hospital. After conservative medical therapy had failed, ECLS implantation was performed at the bedside by our team.
Dr Zimpfer: And the second question I have, the diagram on your last slide, it looks like you considered going directly to the site of CPR if you replace the initial defibrillation by ECLS. Did you consider that or is this just very provocative?
Dr Khaladj: It could be anywhere in the chain of survival. Of course, ECLS therapy cannot replace early defibrillation.
Dr H. Feier (Timisoara, Romania): I would like to know if you have some kind of temperature management. I saw that six of the deaths were due to ischaemic brain damage. Once you are on ECLS, do you lower the body temperature?
Dr Khaladj: All patients that had been resuscitated before or during ECLS implantation were cooled for 24 to 48 h to 32–34°C core body temperature.
REFERENCES
- 1.Nolan JP, Hazinski MF, Billi JE, Boettiger BW, Bossaert L, de Caen AR, et al. Part 1: executive summary: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Resuscitation. 2010;81(Suppl 1):e1–25. doi: 10.1016/j.resuscitation.2010.08.002. doi:10.1016/j.resuscitation.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thiele H, Zeymer U, Neumann FJ, Ferenc M, Olbrich HG, Hausleiter J, et al. Intra-aortic balloon counterpulsation in acute myocardial infarction complicated by cardiogenic shock (IABP-SHOCK II): final 12 month results of a randomised, open-label trial. Lancet. 2013 doi: 10.1016/S0140-6736(13)61783-3. [DOI] [PubMed] [Google Scholar]
- 3.Seyfarth M, Sibbing D, Bauer I, Frohlich G, Bott-Flugel L, Byrne R, et al. A randomized clinical trial to evaluate the safety and efficacy of a percutaneous left ventricular assist device versus intra-aortic balloon pumping for treatment of cardiogenic shock caused by myocardial infarction. J Am Coll Cardiol. 2008;52:1584–8. doi: 10.1016/j.jacc.2008.05.065. doi:10.1016/j.jacc.2008.05.065. [DOI] [PubMed] [Google Scholar]
- 4.Belohlavek J, Kucera K, Jarkovsky J, Franek O, Pokorna M, Danda J, et al. Hyperinvasive approach to out-of hospital cardiac arrest using mechanical chest compression device, prehospital intraarrest cooling, extracorporeal life support and early invasive assessment compared to standard of care. A randomized parallel groups comparative study proposal. ‘Prague OHCA study. J Transl Med. 2012;10:163. doi: 10.1186/1479-5876-10-163. doi:10.1186/1479-5876-10-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beurtheret S, Mordant P, Paoletti X, Marijon E, Celermajer DS, Leger P, et al. Emergency circulatory support in refractory cardiogenic shock patients in remote institutions: a pilot study (the cardiac-RESCUE program) Eur Heart J. 2013;34:112–20. doi: 10.1093/eurheartj/ehs081. doi:10.1093/eurheartj/ehs081. [DOI] [PubMed] [Google Scholar]
- 6.Beckmann A, Benk C, Beyersdorf F, Haimerl G, Merkle F, Mestres C, et al. Position article for the use of extracorporeal life support in adult patients. Eur J Cardiothorac Surg. 2011;40:676–80. doi: 10.1016/j.ejcts.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 7.Arlt M, Philipp A, Voelkel S, Schopka S, Husser O, Hengstenberg C, et al. Early experiences with miniaturized extracorporeal life-support in the catheterization laboratory. Eur J Cardiothorac Surg. 2012;42:858–63. doi: 10.1093/ejcts/ezs176. doi:10.1093/ejcts/ezs176. [DOI] [PubMed] [Google Scholar]
- 8.Arlt M, Philipp A, Voelkel S, Camboni D, Rupprecht L, Graf BM, et al. Hand-held minimised extracorporeal membrane oxygenation: a new bridge to recovery in patients with out-of-centre cardiogenic shock. Eur J Cardiothorac Surg. 2011;40:689–94. doi: 10.1016/j.ejcts.2010.12.055. [DOI] [PubMed] [Google Scholar]
- 9.Sakamoto S, Taniguchi N, Nakajima S, Takahashi A. Extracorporeal life support for cardiogenic shock or cardiac arrest due to acute coronary syndrome. Ann Thorac Surg. 2012;94:1–7. doi: 10.1016/j.athoracsur.2012.01.032. doi:10.1016/j.athoracsur.2012.01.032. [DOI] [PubMed] [Google Scholar]
- 10.Haneya A, Philipp A, Diez C, Schopka S, Bein T, Zimmermann M, et al. A 5-year experience with cardiopulmonary resuscitation using extracorporeal life support in non-postcardiotomy patients with cardiac arrest. Resuscitation. 2012;83:1331–7. doi: 10.1016/j.resuscitation.2012.07.009. doi:10.1016/j.resuscitation.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 11.Chen YS, Yu HY, Huang SC, Lin JW, Chi NH, Wang CH, et al. Extracorporeal membrane oxygenation support can extend the duration of cardiopulmonary resuscitation. Crit Care Med. 2008;36:2529–35. doi: 10.1097/CCM.0b013e318183f491. doi:10.1097/CCM.0b013e318183f491. [DOI] [PubMed] [Google Scholar]
- 12.Ganslmeier P, Philipp A, Rupprecht L, Diez C, Arlt M, Mueller T, et al. Percutaneous cannulation for extracorporeal life support. Thorac Cardiovasc Surg. 2011;59:103–7. doi: 10.1055/s-0030-1250635. doi:10.1055/s-0030-1250635. [DOI] [PubMed] [Google Scholar]
- 13.Leick J, Liebetrau C, Szardien S, Fischer-Rasokat U, Willmer M, van Linden A, et al. Door-to-implantation time of extracorporeal life support systems predicts mortality in patients with out-of-hospital cardiac arrest. Clin Res Cardiol. 2013;102:661–9. doi: 10.1007/s00392-013-0580-3. doi:10.1007/s00392-013-0580-3. [DOI] [PubMed] [Google Scholar]
- 14.Wu MY, Lee MY, Lin CC, Chang YS, Tsai FC, Lin PJ. Resuscitation of non-postcardiotomy cardiogenic shock or cardiac arrest with extracorporeal life support: the role of bridging to intervention. Resuscitation. 2012;83:976–81. doi: 10.1016/j.resuscitation.2012.01.010. doi:10.1016/j.resuscitation.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 15.Schopka S, Philipp A, Lunz D, Camboni D, Zacher R, Rupprecht L, et al. Single-center experience with extracorporeal life support in 103 nonpostcardiotomy patients. Artif Organs. 2013;37:150–6. doi: 10.1111/j.1525-1594.2012.01544.x. doi:10.1111/j.1525-1594.2012.01544.x. [DOI] [PubMed] [Google Scholar]
- 16.Megarbane B, Deye N, Aout M, Malissin I, Resiere D, Haouache H, et al. Usefulness of routine laboratory parameters in the decision to treat refractory cardiac arrest with extracorporeal life support. Resuscitation. 2011;82:1154–61. doi: 10.1016/j.resuscitation.2011.05.007. doi:10.1016/j.resuscitation.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 17.Wu MY, Lin PJ, Tsai FC, Haung YK, Liu KS. Impact of preexisting organ dysfunction on extracorporeal life support for non-postcardiotomy cardiopulmonary failure. Resuscitation. 2008;79:54–60. doi: 10.1016/j.resuscitation.2008.05.002. doi:10.1016/j.resuscitation.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Khaladj N, Bobylev D, Peterss S, Guenther S, Pichlmaier M, Bagaev E, et al. Immediate surgical coronary revascularisation in patients presenting with acute myocardial infarction. J Cardiothorac Surg. 2013;8:167. doi: 10.1186/1749-8090-8-167. doi:10.1186/1749-8090-8-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peterss S, Pfeffer C, Reichelt A, Born F, Franz W, Netz H, Kaczmarek I, Hagl C, Khaladj N. Extracorporal life support and left ventricular unloading in a non-intubated patient as bridge to heart transplantation. Int J Artif Organs. 2013;36 doi: 10.5301/ijao.5000251. [DOI] [PubMed] [Google Scholar]
- 20.Barth E, Durand M, Heylbroeck C, Rossi-Blancher M, Boignard A, Vanzetto G, et al. Extracorporeal life support as a bridge to high-urgency heart transplantation. Clin Transpl. 2012;26:484–8. doi: 10.1111/j.1399-0012.2011.01525.x. doi:10.1111/j.1399-0012.2011.01525.x. [DOI] [PubMed] [Google Scholar]
- 21.Pagani FD, Lynch W, Swaniker F, Dyke DB, Bartlett R, Koelling T, et al. Extracorporeal life support to left ventricular assist device bridge to heart transplant: a strategy to optimize survival and resource utilization. Circulation. 1999;100:II206–10. doi: 10.1161/01.cir.100.suppl_2.ii-206. doi:10.1161/01.CIR.100.suppl_2.II-206. [DOI] [PubMed] [Google Scholar]
- 22.Haneya A, Philipp A, Puehler T, Ried M, Hilker M, Zink W, et al. Ventricular assist device implantation in patients on percutaneous extracorporeal life support without switching to conventional cardiopulmonary bypass system. Eur J Cardiothorac Surg. 2012;41:1366–70. doi: 10.1093/ejcts/ezr203. doi:10.1093/ejcts/ezr203. [DOI] [PubMed] [Google Scholar]
- 23.Kagawa E, Inoue I, Kawagoe T, Ishihara M, Shimatani Y, Kurisu S, et al. Assessment of outcomes and differences between in- and out-of-hospital cardiac arrest patients treated with cardiopulmonary resuscitation using extracorporeal life support. Resuscitation. 2010;81:968–73. doi: 10.1016/j.resuscitation.2010.03.037. doi:10.1016/j.resuscitation.2010.03.037. [DOI] [PubMed] [Google Scholar]
- 24.Bisdas T, Beutel G, Warnecke G, Hoeper MM, Kuehn C, Haverich A, et al. Vascular complications in patients undergoing femoral cannulation for extracorporeal membrane oxygenation support. Ann Thorac Surg. 2011;92:626–31. doi: 10.1016/j.athoracsur.2011.02.018. doi:10.1016/j.athoracsur.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 25.Madershahian N, Nagib R, Wippermann J, Strauch J, Wahlers T. A simple technique of distal limb perfusion during prolonged femoro-femoral cannulation. J Cardiac Surg. 2006;21:168–9. doi: 10.1111/j.1540-8191.2006.00201.x. doi:10.1111/j.1540-8191.2006.00201.x. [DOI] [PubMed] [Google Scholar]