Abstract
OBJECTIVES
Fibrinogen concentrate is increasingly used in cardiac surgery when bleeding is anticipated or ongoing. Since randomized clinical studies to support this are lacking, it is relevant to know whether lower fibrinogen levels are associated with excessive bleeding. We performed a systematic review and meta-analysis to define the association between fibrinogen levels and blood loss after cardiac surgery.
METHODS
A database search (January 2013) was performed on publications assessing the association between pre- and postoperative fibrinogen levels and postoperative blood loss in adult patients undergoing cardiac surgery. Cohort studies and case–control studies were eligible for inclusion. The main outcome was the pooled correlation coefficient, calculated via Fisher's Z transformation scale, in a random-effects meta-analysis model stratified for the time point at which fibrinogen was measured.
RESULTS
A total of 20 studies were included. The pooled correlation coefficient of studies (n = 9) concerning preoperative fibrinogen levels and postoperative blood loss was −0.40 (95% confidence interval: −0.58, −0.18), pointing towards more blood loss in patients with lower preoperative fibrinogen levels. Among papers (n = 16) reporting on postoperative fibrinogen levels and postoperative blood loss, the pooled correlation coefficient was −0.23 (95% confidence interval: −0.29, −0.16).
CONCLUSIONS
Our meta-analysis indicated a significant but weak-to-moderate correlation between pre- and postoperative fibrinogen levels and postoperative blood loss in cardiac surgery. This moderate association calls for appropriate clinical studies on whether fibrinogen supplementation will decrease postoperative blood loss.
Keywords: Blood loss, Cardiac surgery, Fibrinogen
INTRODUCTION
Excessive blood loss is a frequent complication after cardiac surgery [1, 2] requiring re-operation in about 5–10% of patients. Postoperative bleeding obligating transfusions and surgical re-exploration is associated with mortality, morbidity (e.g. increased sternal wound infection, risk of transfusion-related complications) and higher costs [3–5].
Multiple factors are associated with excessive bleeding after cardiac surgery, but causal pathways have not been elucidated in full detail [5]. A surgical cause is only found in approximately half of the patients undergoing re-operation for bleeding. In the remainder of patients an acquired coagulopathy contributes to bleeding. The latter can either be caused by a pre-existing coagulation factor deficiency, a drug-induced platelet inhibition or by an acquired, operation-related haemostatic defect [3, 6]. Suggested mechanisms for the acquired defects include the use of high doses of insufficiently neutralized heparin, haemodilution and activation of the haemostatic system, resulting in disseminated intravascular coagulation, tissue trauma, platelet dysfunction and excessive fibrinolysis [7, 8]. Identification of patients at risk for excessive blood loss would offer possibilities to initiate preventive measures. However, diagnostic efficiency of the haemostasis screening tests to identify such indicators is often low.
A suggested predictor of excessive blood loss is fibrinogen, a protein that plays an essential role in coagulation. It has a dual role as it enhances platelet aggregation via binding to the GPIIb/IIIa receptors on platelets and it is converted into fibrin to form an insoluble clot. Cardiac surgery, however, can result in a significant reduction of fibrinogen concentration and function, due to blood loss, haemodilution, platelet activation by cardiopulmonary bypass (CPB), a large wound area for clot formation, hypothermia and acidosis [9, 10]. The threshold of fibrinogen at which bleeding complications are provoked is difficult to define, as it depends on the status of haematocrit, thrombin formation, platelet number and function, clotting enzyme activities [9], underlying clinical conditions (e.g. haematological malignancy and liver insufficiency [10]) and age. Several reports suggest that a fibrinogen level of >2 g/l would be sufficient to ensure adequate coagulation even in the presence of moderate thrombocytopenia [10–12].
An inverse association between pre- and postoperative fibrinogen levels, and bleeding risk, even for levels within the normal reference range (1.5–4.0 g/l) was often reported [11]. It is suggested that fibrinogen supplementation might decrease blood loss after cardiac surgery [10]. Although clinical trials are lacking [1, 13], fibrinogen supplementation is increasingly used, also in cardiac surgery. To support such a policy, the role of pre- and postoperative fibrinogen levels in the development of (excessive) blood loss after cardiac surgery should be unequivocal. We performed a systematic review and meta-analysis to define the association between fibrinogen levels and blood loss after cardiac surgery.
MATERIALS AND METHODS
Information sources and search
We searched PubMed, Embase, Web of Science, COCHRANE, CINAHL, Academic Search Premier and ScienceDirect using predefined search terms (Supplementary data, Appendix A). The search was performed in January 2013. We used both MeSH terms and free text words. Language restrictions for Dutch, English, French and German articles were set in advance. Full-text articles and meeting abstracts published since 1957 (the year in which the Clauss method to measure fibrinogen was first described) were eligible for evaluation [14].
Study selection and endpoint definitions
To be included in the analysis, studies had to assess the association between pre- and postoperative fibrinogen levels and postoperative blood loss or transfusions in adult patients undergoing cardiac surgery. The study should report either the amount of blood loss or transfusions stratified by levels of fibrinogen or the risk for blood loss (risk ratio or odds ratio [OR]) when comparing fibrinogen levels. Because most articles presented information about the association between fibrinogen level and blood loss, we focused on the correlation with bleeding. Only two studies included in the meta-analysis presented a correlation with blood transfusions. Cohort and case–control studies were considered. Intervention studies with fibrinogen supplementation were excluded.
The definition of (excessive) postoperative blood loss varied between the included articles. Excessive bleeding was defined by the authors of the included articles as a total postoperative drain volume of >400 ml in 1 h, >200 ml for 2 consecutive hours or >100 ml for 4 consecutive hours; of >200 ml per h, >150 ml for 2 consecutive hours or >100 ml for 3 consecutive hours postoperatively; or of >1500 ml within the first 24 h after surgery. Patients with excessive blood loss (PEBL) were characterized as patients with postoperative blood loss of >1000 ml in the first 24 h after operation, mean blood loss of 1650 ml ± 280 ml, median blood loss of 949 ml, blood loss >1000 ml in the first 16 h after operation, bleeding >600 ml in the first 8 h postoperatively, postoperative blood loss >200 ml per h within the first 4 h after operation with a median of 787 ml, requiring re-exploration for bleeding ≥200 ml per h for over 4 h or experiencing a sudden increase in bleeding after 2 h postoperatively, postoperative blood loss >500 ml in the first 24 h after operation or patients in the higher tertile of blood loss with a median of 1050 ml (700–2550 ml). All definitions for blood loss were accepted and PEBL stated in the article were handled as PEBL for analysis (Table 1).
Table 1:
Details of included studies
| Investigators (year of publication) | Design | n | Time point of fibrinogen determination (method) | Clinical characteristics | Outcome measurements |
|---|---|---|---|---|---|
| Blome et al. (2005) | Case–control | 98 | Preoperative Postoperative (Clauss) |
Divided into three groups of BL; lower, mid and high tertile Low (I): median 400 ml (150–475) High (III): median 1050 ml (700–2550) |
BL <12 and 24 h FGN median (10th and 90th percentiles) EE: P-value |
| Bolliger et al. (2008) | Case– control | 197 | Preoperative Postoperative (not reported) |
CABG surgery PEBL: >1000 ml/24 h Non-PEBL: <1000 ml/24 h |
BL <12 and 24 h FGN range EE: correlation |
| Davidson et al. (2008) | Case–control | 58 | Postoperative (Clauss-like) | Primary CABG surgery PEBL >200 ml/h <4 h (n = 8): median BL 787 ml vs non-PEBL (n = 50): median BL 150 ml |
BL <4 h FGN median (range) EE: P-value |
| Essell et al. (1993) | Case–control | 36 | Postoperative (not reported) | All cardiac surgical patients | BL and BT <24 h EE: P-value |
| Faraday et al. (2002) | Cohort | 57 | Postoperative (Clauss-like) | Elective CABG, (multiple) valve replacement, ascending aortic aneurysm repair and combined valve–CABG proceduresa | BL <24 and BT <24 h FGN OR EE: correlation |
| Fassin et al. (1991) | Case–control | 107 | Postoperative (not reported) | CABG surgery Group I (n = 70): <1000 ml/24 h vs Group II (n = 33): >1000 ml/24 h |
BL <24 h and BT FGN mean (SD) EE: mean differences |
| Gravlee et al. (1994) | Case–control | 897 | Postoperative (Clauss-like) | Elective and emergent cardiac surgery PEBL: BL >1000 ml/16 h vs non-PEBL: BL <1000 ml/16 h |
BL <16 h and BT FGN mean (SD) EE: correlation |
| Hall et al. (2002) | Case–control | 82 | Postoperative (Clauss-like) | Elective and emergent cardiac surgery The cases with BL requiring re-exploration (n = 82, = 200 ml/h 4 h or sudden rise BL <2 h) and controls selected randomly (n = 478) | BL <18–24 h FGN EE: P-value |
| Josefy et al. (2011) | Cohort | 35 | Preoperative (Clauss-like) | Patients undergoing CABG surgery not taking clopidogrela | BT; RBCs, platelets, FFPs and cryoprecipitated AHF FGN EE: correlation |
| Karkouti et al. (2010) | Cohort | 101 | Postoperative (not reported) | Complex cardiac surgery (other than isolated CABG, single valve surgery or repair of atrial septal defect) Median BL 952 ml/24 h | BL <24 h FGN mean (SD) EE: correlation |
| Karlsson et al. (2008) | Cohort | 170 | Preoperative (Clauss) | CABG surgery. Median BL 360 ml/12 h | BL <12 h and BT during admission FGN mean (SD) EE: correlation |
| Liu et al. (2000) | Cohort | 46 | Preoperative Postoperative (not reported) |
Primary CABG surgery. Median BL 825 ml/24 h | BL <24 h FGN (correlation) EE: correlation |
| Marengo-Rowe et al. (1979) | Case–control | 774 | Postoperative (not reported) | CABG surgery PEBL >600 ml/8 h (n = 164) vs non-PEBL <600 ml/8 h (n = 610) |
BL <8 h EE: P-value |
| Nuttall et al. (1997) | Case–control | 82 | Postoperative (Clauss) | CABG, valve replacement or repair, or congenital heart surgery PEBL (n = 30): median BL 949 ml vs non-PEBL (n = 52): median BL 547 ml |
BL and BT <24 h FGN mean (SD) EE: correlation |
| Ozolina et al. (2011) | Case–control | 124 | Preoperative Postoperative (not reported) |
Cardiac surgerya Group I: <500 ml/24 h Group II: >500 ml/24 h |
BL <24 h EE: correlation |
| Prohaska et al. (2008) | Cohort | 2831 | Preoperative (Clauss-like) | CABG surgery, aortic valve replacement, combined CABG and aortic valve replacement, and other single or combined cardiac proceduresa | BT; RBCs, FFPs and PCs perioperative and <2 days FGN mean (SD) EE: OR |
| Ternström et al. (2010) | Cohort | 59 | Preoperative Postoperative (Clauss) |
Elective CABG. Median postoperative BL 380 ml/12 h | BL <12 h EE: correlation |
| Ucar et al. (2007) | Cohort | 97 | Preoperative (Clauss) | All cardiac surgical patients | BL <48 h FGN mean (SD) EE: correlation |
| Wahba et al. (1997) | Case–control | 89 | Postoperative (Clauss) | Primary CABG surgery PEBL (n = 14):1.650 ± 280 ml vs non-PEBL (n = 75): 780 ± 250 ml |
BL until removal of drains (usually Day 1) FGN mean (SD) EE: correlation |
| Welsby et al. (2006) | Cohort | 32 | Postoperative (Clauss-like) | CABG or valve operations | BL <4 h FGN mean (SD) EE: correlation |
BL: blood loss; FGN: fibrinogen; EE: effect estimate; PEBL: patients with excessive blood loss; Clauss-like: automated, probably Clauss-like, coagulation method; BT: blood transfusions; OR: odds ratio.
aCPB usage was not mandatory for study inclusion.
Data extraction and risk of bias assessment
Selection, data extraction and risk of bias assessment was done by two reviewers independently (J.E. and C.L.I.G) using a predefined extraction sheet. Concerning our research questions, we collected the following variables: study population, time point at which fibrinogen was measured, level of fibrinogen, amount of perioperative blood loss and transfusions. To assess the risk of bias, we evaluated the study design, measurement of exposure, blinding for fibrinogen level, definition and measurement of outcomes, completeness of follow-up and adjustment for confounders. These variables were used to explore sources of heterogeneity. No summary score for the risk of bias assessment was used for analytical purposes.
Summary measures and synthesis of results
For all studies the main effect-measure was extracted (OR, mean difference, correlation coefficient, P-value) with the accompanying measure of uncertainty (95% confidence interval [CI], standard error or P-value). We stratified studies based on the time point at which fibrinogen was measured (pre- versus postoperatively).
For analytical purposes, we transformed all effect-measures to correlation coefficients (r). P-values were transformed with Fisher's Z transformation scale and via the inverse Fisher's Z transformation to correlation coefficients. Odds ratios were transformed to a correlation scale using the tetrachoric correlation. When the association between fibrinogen and blood loss was characterized by a regression coefficient, the correlation was calculated from the regression coefficient and the standard deviation of the independent variable in the regression. These transformed correlation coefficients were pooled in a random-effects model as, for instance, described by Borenstein et al. [15]. Finally, results were transformed back to the correlation scale by the inverse Fisher's Z-transformation. All analyses were performed with STATA release 10 (StataCorp, College Station, TX, USA).
RESULTS
Study selection and characteristics
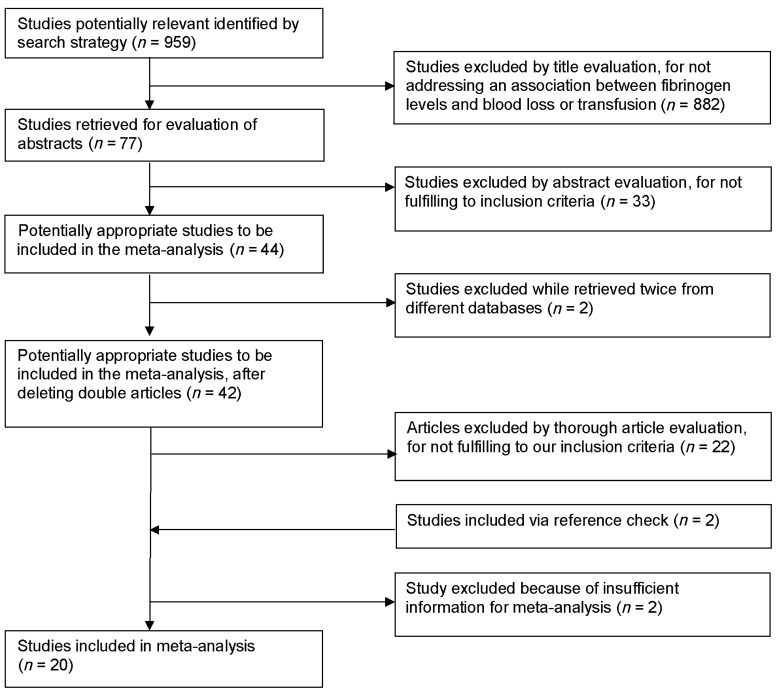
Our search strategy provided 959 articles of which 77 were retrieved for more detailed assessment. The search strategy's flow chart is shown in Fig. 1. A total of 20 articles were included, which incorporated 5972 patients. In Table 1, details of included studies are provided. Nine articles were classified as cohort studies in which fibrinogen was measured before or after operation and the presence of a correlation with blood loss was evaluated. The other 11 studies were nested case–control studies, where cases were defined as PEBL and controls as patients without excessive blood loss (non-PEBL). The definition to classify blood loss (and therefore also case–control status) differed between the articles. Fibrinogen was measured by the Clauss method in six papers, seven papers used an automated, Clauss-like, coagulation method and seven articles did not report the method used. Effect estimates were reported as correlations in 13 studies and as P-values in 5 studies; Prohaska et al. reported ORs and Fassin et al. reported mean differences.
Figure 1:
Flow chart of study selection.
Risk of bias
All studies included assessed the association between pre- and postoperative fibrinogen levels and postoperative blood loss or transfusions in adult patients undergoing cardiac surgery. Four papers reported having performed fibrinogen measurement before patients received any coagulation factors or FFPs. In the majority of articles this form of bias was not reported; two stated that they could not exclude it. Furthermore, factors that also might have influenced postoperative blood loss are drug-induced platelet dysfunction, postoperative administration of low molecular weight heparins or impairment of von Willebrand factor and factor VIII by hydroxyethyl starch (HES) solutions. Most articles do not report how these potential confounding factors were dealt with. None of the articles reported included patients lost in follow-up.
Synthesis of results
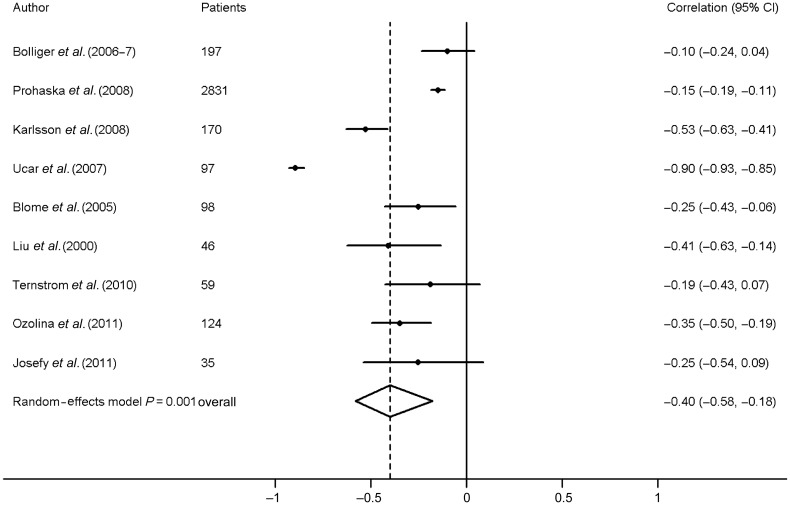
Nine studies contributed to the meta-analysis on the association between preoperative fibrinogen level and blood loss. Calculated correlations were all below −0.10 (Fig. 2). In a random-effects model, the pooled correlation for the association between preoperative fibrinogen levels and postoperative blood loss was −0.40 (95% CI, −0.58, −0.18). Clear heterogeneity was shown: I2 = 95.7%, χ2 = 187.58, P < 0.0001.
Figure 2:
Meta-analysis of the correlation between preoperative fibrinogen and postoperative blood loss.
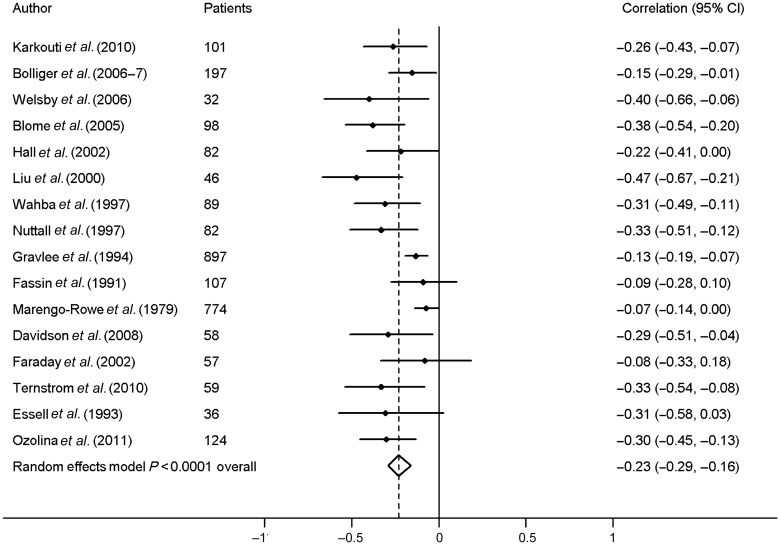
Sixteen studies contributed to the meta-analysis on the association between postoperative fibrinogen level and blood loss. Calculated correlations were all below −0.07 (Fig. 3). In a random-effects model, the pooled correlation for the association between postoperative fibrinogen levels and blood loss was −0.23 (95% CI, −0.29, −0.16). Tests for heterogeneity showed the following results: I2 = 54.5%, χ2 = 32.97, P = 0.005.
Figure 3:
Meta-analysis of the correlation between postoperative fibrinogen and postoperative blood loss.
DISCUSSION
The present meta-analysis revealed a significant pooled correlation between preoperative (r = −0.40; 95% CI, −0.58, −0.18) or postoperative (r = −0.23; 95% CI, −0.29, −0.16) fibrinogen levels on the one hand and blood loss after cardiac surgery on the other hand. This confirms the important role of fibrinogen in the coagulation process. However, since the correlation is not very strong, it is likely that other factors like haematocrit, thrombin formation, platelet number and function and clotting enzyme activities are at least as important in the origination of excessive blood loss.
Despite the presence of statistical heterogeneity among studies, likely reflecting methodological differences between studies, all included studies showed a negative correlation between fibrinogen level and postoperative blood loss. Because of this absence of qualitative heterogeneity, we decided to pool correlation coefficients in a random-effects model, which partly accounts for heterogeneity.
It is important to state that the primary aim of this study was to evaluate the correlation an association between the pre- and postoperative fibrinogen levels and blood loss after cardiac surgery. This perhaps is most validly represented by the preoperative measurement. Owing to operation influences, e.g. CPB usage, haemodilution, platelet dysfunction etc. [5, 16], the postoperative fibrinogen levels are probably more biased and, therefore, less accurate for this evaluation, even when no FFP or fibrinogen administration is reported in the original paper. These surgery-related haemostatic defects may have had more impact causing bleeding than those existing prior to the operation [17]. Still, the preoperative correlation found is stronger than the postoperative one, suggesting that surgical procedures also might have a negative, downsizing effect on the correlation. Ternström et al. [18] demonstrated a correlation between pre- and postoperative fibrinogen level (r = 0.80, P < 0.001), suggesting that the time point at which fibrinogen is measured might be less important.
Haemodilution due to the use of CPB and volume supplementation reduces coagulation factor levels to ∼25–50% of baseline, parallel with the decrease of the haematocrit level [1, 18, 19]. Each coagulation factor has to be present at a minimum level adequate to support haemostasis, often well below the normal range. Simultaneous reductions in multiple coagulation factor levels that develop during CPB, therefore, generally do not result in bleeding complications [16]. However, patients with a lower baseline value are more prone to develop a fibrinogen level insufficient to ensure adequate coagulation. Moreover, if a low fibrinogen level occurs in combination with other factors impairing the strength of clot formation, the risk of bleeding may increase. In this context, Fenger-Erikson et al. [20] demonstrated that a dilutional coagulopathy due to administration of HES products resulted in less stable clotting assessed by thromboelastometry. Also, patients undergoing urgent surgery while using (dual) antiplatelet therapy may need higher fibrinogen levels to avoid excessive bleeding. Unfortunately, only a few studies in our review included urgent surgery patients and no correlation between fibrinogen levels and bleeding in this population was possible. The lowest average of fibrinogen levels reported in the assessed papers for this meta-analysis is 2.6 ± 0.79 g/l pre- and 1.6 ± 1.18 g/l postoperatively, the normal reference range being 1.5–4.0 g/l.
Other laboratory parameters, e.g. activated partial thromboplastin time, prothrombin time (PT) and platelet count have also been studied in this context [2, 5, 7, 13], but all these tests, including fibrinogen, have (as single parameter) limited utility for diagnosis of postoperative coagulopathy because their results are only weakly associated with parameters of clinical bleeding or because they are not rapidly available [7, 21]. Collective assessment of several laboratory values using thromboelastography or whole blood haemostatometry may provide more applicable diagnostic information [5, 21], but their association with blood loss remains low.
Although the correlation between fibrinogen levels and blood loss is not very strong, fibrinogen concentrate administration in patients with ongoing bleeding could be considered as a possible treatment option. Indeed, several investigators advocate that fibrinogen can act as first-line therapy to correct postoperative bleeding and reduce the use of allogeneic blood products without evidence of thrombotic complications [10]. A recent meta-analysis on the effect of fibrinogen concentrate in patients with various bleeding conditions reported no outcome differences for bleeding, mortality, length of stay at the intensive care unit or side effects. Patients treated with fibrinogen concentrate used significantly less other blood products. The authors concluded that the six included RCTs were of low quality and comprised altogether only 248 patients, not all eligible for evaluation of all outcome effects [22]. In this context, it is important to note that fibrinogen is an acute-phase protein the level of which increases gradually after surgical procedures [1, 10, 18]. Therefore, the use of fibrinogen concentrate, in combination with this physiological increase postoperative, might increase the risk of thrombosis.
Many studies concluding that there is no statistically significant correlation between fibrinogen and postoperative blood loss did not give information about the strength of the correlation or exact P-value, and could therefore not be included. This reporting bias could have been further influenced by unpublished studies failing to demonstrate significant correlations. Therefore, the association between fibrinogen and postoperative blood loss that we calculated could be overestimated. Other important limitations of this meta-analysis are the low power and quality of the included studies, the substantial heterogeneity among studies and the variability in the definition of the endpoint blood loss. Systematic reviews on the effect of intervention with fibrinogen concentrate reveal a similar lack of quality of the studies [22, 23]. However, fibrinogen supplementation is currently solely substantiated on this questionable information. The different assays used among studies should not be of influence on the analysis of the association with bleeding, while the comparison between PEBL and non-PEBL within the paper is performed by the same assay. Also, although the definition of blood loss or a bleeding patient appeared to be highly divergent among studies, overall, blood loss of >200 ml per h or >1 l in the postoperative period was considered as excessive bleeding. Furthermore, in some of the evaluated studies there is a lack of differentiation between patients who bleed from surgical causes and those who apparently have a severe coagulopathy without a specific surgical source of bleeding [24]. Moreover, it is not always clear whether fibrinogen supplementation, transfusions of FFPs or platelets, took place before sampling, influencing the results, or after the postoperative fibrinogen measurements. Finally, the usage of other methods to measure fibrinogen, like the PT-derived method, or the presence of HES products may lead to an overestimation of the fibrinogen amount [25].
In conclusion, although the results of our meta-analysis support the association between lower fibrinogen levels and a higher bleeding risk after cardiac surgery, the correlation is weak to moderate, the studies evaluated contain substantial heterogeneity, and are of low power and quality. However, this cumbersome available information forms the current base to substantiate fibrinogen supplementation. Fibrinogen supplementation for patients with a lower preoperative fibrinogen level or concomitant factors impairing coagulation such as the use of antiplatelet drugs might have a beneficial effect in reducing the risk of excessive blood loss, but studies of better quality, preferentially randomized, are required before any specific recommendation can be proposed.
Supplementary material
Supplementary material is available at ICVTS online.
Conflict of interest: none declared.
REFERENCES
- 1.Ucar HI, Oc M, Tok M, Dogan OF, Oc B, Aydin A, et al. Preoperative fibrinogen levels as a predictor of postoperative bleeding after open heart surgery. Heart Surg Forum. 2007;10:284–8. doi: 10.1532/HSF98.20071065. [DOI] [PubMed] [Google Scholar]
- 2.Welsby IJ, Jiao K, Ortel TL, Brudney CS, Roche AM, Bennett-Guerrero E, et al. The kaolin-activated thrombelastograph predicts bleeding after cardiac surgery. J Cardiothorac Vasc Anesth. 2006;20:531–5. doi: 10.1053/j.jvca.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 3.Paparella D, Brister SJ, Buchanan MR. Coagulation disorders of cardiopulmonary bypass: a review. Intensive Care Med. 2004;30:1873–81. doi: 10.1007/s00134-004-2388-0. [DOI] [PubMed] [Google Scholar]
- 4.Davidson SJ, McGrowder D, Roughton M, Kelleher AA. Can ROTEM thromboelastometry predict postoperative bleeding after cardiac surgery? J Cardiothorac Vasc Anesth. 2008;22:655–61. doi: 10.1053/j.jvca.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Nuttall GA, Oliver WC, Ereth MH, Santrach PJ. Coagulation tests predict bleeding after cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 1997;11:815–23. doi: 10.1016/s1053-0770(97)90112-9. [DOI] [PubMed] [Google Scholar]
- 6.Menichetti A, Tritapepe L, Ruvolo G, Speziale G, Cogliati A, Di GC, et al. Changes in coagulation patterns, blood loss and blood use after cardiopulmonary bypass: aprotinin vs. tranexamic acid vs. epsilon aminocaproic acid. J Cardiovasc Surg (Torino) 1996;37:401–7. [PubMed] [Google Scholar]
- 7.Gravlee GP, Arora S, Lavender SW, Mills SA, Hudspeth AS, Cordell AR, et al. Predictive value of blood clotting tests in cardiac surgical patients. Ann Thorac Surg. 1994;58:216–21. doi: 10.1016/0003-4975(94)91103-7. [DOI] [PubMed] [Google Scholar]
- 8.Despotis GJ, Avidan MS, Hogue CW., Jr Mechanisms and attenuation of hemostatic activation during extracorporeal circulation. Ann Thorac Surg. 2001;72:S1821–31. doi: 10.1016/s0003-4975(01)03211-8. [DOI] [PubMed] [Google Scholar]
- 9.Martini WZ. Coagulopathy by hypothermia and acidosis: mechanisms of thrombin generation and fibrinogen availability. J Trauma. 2009;67:202–8. doi: 10.1097/TA.0b013e3181a602a7. [DOI] [PubMed] [Google Scholar]
- 10.Solomon C, Pichlmaier U, Schoechl H, Hagl C, Raymondos K, Scheinichen D, et al. Recovery of fibrinogen after administration of fibrinogen concentrate to patients with severe bleeding after cardiopulmonary bypass surgery. Br J Anaesth. 2010;104:555–62. doi: 10.1093/bja/aeq058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karlsson M, Ternstrom L, Hyllner M, Baghaei F, Nilsson S, Jeppsson A. Plasma fibrinogen level, bleeding, and transfusion after on-pump coronary artery bypass grafting surgery: a prospective observational study. Transfusion. 2008;48:2152–8. doi: 10.1111/j.1537-2995.2008.01827.x. [DOI] [PubMed] [Google Scholar]
- 12.Rahe-Meyer N, Solomon C, Winterhalter M, Piepenbrock S, Tanaka K, Haverich A, et al. Thromboelastometry-guided administration of fibrinogen concentrate for the treatment of excessive intraoperative bleeding in thoracoabdominal aortic aneurysm surgery. J Thorac Cardiovasc Surg. 2009;138:694–702. doi: 10.1016/j.jtcvs.2008.11.065. [DOI] [PubMed] [Google Scholar]
- 13.Ramsey G, Arvan DA, Stewart S, Blumberg N. Do preoperative laboratory tests predict blood transfusion needs in cardiac operations? J Thorac Cardiovasc Surg. 1983;85:564–9. [PubMed] [Google Scholar]
- 14.CLAUSS A. [Rapid physiological coagulation method in determination of fibrinogen] Acta Haematol. 1957;17:237–46. doi: 10.1159/000205234. [DOI] [PubMed] [Google Scholar]
- 15.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta-Analysis. 1 edn. John Wiley & Sons; 2009. [Google Scholar]
- 16.Essell JH, Martin TJ, Salinas J, Thompson JM, Smith VC. Comparison of thromboelastography to bleeding time and standard coagulation tests in patients after cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 1993;7:410–5. doi: 10.1016/1053-0770(93)90161-d. [DOI] [PubMed] [Google Scholar]
- 17.Eika C, Havig O, Godal HC. The value of preoperative haemostatic screening. Scand J Haematol. 1978;21:349–54. doi: 10.1111/j.1600-0609.1978.tb00376.x. [DOI] [PubMed] [Google Scholar]
- 18.Ternstrom L, Radulovic V, Karlsson M, Baghaei F, Hyllner M, Bylock A, et al. Plasma activity of individual coagulation factors, hemodilution and blood loss after cardiac surgery: a prospective observational study. Thromb Res. 2010;126:e128–33. doi: 10.1016/j.thromres.2010.05.028. [DOI] [PubMed] [Google Scholar]
- 19.Marengo-Rowe AJ, Lambert CJ, Leveson JE, Thiele JP, Geisler GF, Adam M, et al. The evaluation of hemorrhage in cardiac patients who have undergone extracorporeal circulation. Transfusion. 1979;19:426–33. doi: 10.1046/j.1537-2995.1979.19479250180.x. [DOI] [PubMed] [Google Scholar]
- 20.Fenger-Eriksen C, Jensen TM, Kristensen BS, Jensen KM, Tonnesen E, Ingerslev J, et al. Fibrinogen substitution improves whole blood clot firmness after dilution with hydroxyethyl starch in bleeding patients undergoing radical cystectomy: a randomized, placebo-controlled clinical trial. J Thromb Haemost. 2009;7:795–802. doi: 10.1111/j.1538-7836.2009.03331.x. [DOI] [PubMed] [Google Scholar]
- 21.Faraday N, Guallar E, Sera VA, Bolton ED, Scharpf RB, Cartarius AM, et al. Utility of whole blood hemostatometry using the clot signature analyzer (R) for assessment of hemostasis in cardiac surgery. Anesthesiology. 2002;96:1115–22. doi: 10.1097/00000542-200205000-00014. [DOI] [PubMed] [Google Scholar]
- 22.Wikkelso A, Lunde J, Johansen M, Stensballe J, Wetterslev J, Moller AM, et al. Fibrinogen concentrate in bleeding patients. Cochrane Database Syst Rev. 2013;8:CD008864. doi: 10.1002/14651858.CD008864.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kozek-Langenecker S, Sorensen B, Hess JR, Spahn DR. Clinical effectiveness of fresh frozen plasma compared with fibrinogen concentrate: a systematic review. Crit Care. 2011;15:R239. doi: 10.1186/cc10488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hall TS, Sines JC, Spotnitz AJ. Hemorrhage related reexploration following open heart surgery: the impact of pre-operative and post-operative coagulation testing. Cardiovasc Surg. 2002;10:146–53. doi: 10.1016/s0967-2109(01)00129-6. [DOI] [PubMed] [Google Scholar]
- 25.Adam S, Karger R, Kretschmer V. Influence of different hydroxyethyl starch (HES) formulations on fibrinogen measurement in HES-diluted plasma. Clin Appl Thromb Hemost. 2010;16:454–60. doi: 10.1177/1076029609336855. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.