Abstract
OBJECTIVES
Common atrioventricular valve (CAVV) regurgitation is widely known as a risk factor for mortality and Fontan completion in patients with functional single ventricle. Hence, we reviewed our surgical experience with CAVV plasty in Fontan candidates.
METHODS
Staged Fontan strategy and extracardiac total cavopulmonary connection as Fontan modification were our principal approaches in 1995. Since then, 38 consecutive Fontan candidates (21 males, median weight at operation was 7.0 kg and median age was 17 months old) underwent CAVV plasty. Right atrial isomerism was associated with 24 patients. The initial CAVV plasty was performed before inter-stage bidirectional Glenn (BDG) in 3 patients, at BDG in 23, before Fontan in 4 and during Fontan in 8. Since 1995, the modified Alfieri technique with a tailed, expanded, polytetrafluoroethylene tube as a bridging strip was the procedure for repair and 27 patients underwent the procedure. The mean follow-up period was 7.1 years (range 0–17 years).
RESULTS
Actuarial survival and freedom from CAVV replacement rates at 1, 5 and 10 years were 81, 70 and 67% and 89, 85 and 75%, respectively. Seven patients ultimately underwent CAVV replacement with one death. Twenty-three of the 38 patients completed Fontan operation (61%). Association with total anomalous pulmonary venous connection (P= 0.01) and CAVV plasty before BDG (P= 0.05) were risk factors for mortality.
CONCLUSIONS
CAVV plasty for patients with functional single ventricle is still challenging; however, the aggressive and repeated surgical intervention may contribute to provide better life-prognosis. The ventricular volume unloading effect of BDG without additional pulmonary blood flow or Fontan operation did not contribute to maintain CAVV function. Therefore, there would not be any hesitation for CAVV replacement to control CAVVR in the setting of systemic ventricular failure. Although the statistically significant therapeutic superiority of the modified Alfieri technique was not shown so far, further follow-up may reveal the advantage of this easy and simple technique.
Keywords: Single ventricle, Fontan, Common atrioventricular valve, Atrioventricular valve plasty
Introduction
The role of the systemic atrioventricular valve is more important in Fontan or prestage bidirectional Glenn (BDG) physiology than in normal biventricular physiology. Clinically significant systemic atrioventricular valve regurgitation reduces cardiac output directly. In single-ventricle circulation, however, the elevated ventricular end-diastolic pressure by systemic atrioventricular valve regurgitation subsequently increases pulmonary vascular resistance, which also reduces systemic ventricular volume preload and causes systemic venous congestion throughout the cardiac cycle. Now, systemic atrioventricular valve regurgitation in patients with functional single ventricle is not only known as a risk factor for mortality and Fontan completion, but also for late survival after establishment of Fontan circulation [1–6].
In several types of systemic atrioventricular valves in single-ventricle physiology, common atrioventricular valve (CAVV) is the most difficult to regulate its regurgitation (CAVVR) because of the anatomical abnormality [7, 8]. Different from other valves, CAVV is an immature and undivided atrioventricular valve. Moreover, CAVV in patients with functional single ventricle is quite unbalanced, which makes repair more complicated. As a result, previously reported procedures for CAVV plasty were usually case sensitive, and the reported outcomes were not satisfied [9–15].
In this study, we reviewed our long-term surgical experiences of CAVV plasty in patients with functional single ventricle.
PATIENTS AND METHODS
Patients
The National Cerebral and Cardiovascular Center Institutional Review Board approved this retrospective study and waived patient consent. From 1995 to 2012, a total of 38 consecutive Fontan candidates with common atrioventricular valve regurgitation (CAVVR) (21 males, median age at initial CAVV plasty was 17 months old) underwent a CAVV plasty (Table 1). During the study period, 3 patients underwent CAVV replacement as the first intervention to CAVVR. The major cardiac diagnoses were unbalanced atrioventricular septal defect in 25 patients, common inlet right ventricle in 11 and variant of hypoplastic left heart syndrome in 2. Thirty-five of 38 patients (92.1%) had predominant or single right ventricle. Right atrial isomerism and total anomalous pulmonary venous connection were associated with 24 patients (63.2%) and 15 (39.5%) patients, respectively. Of note, common inlet right ventricle is defined if CAVV connects to anterior-inferiorly located predominant right ventricle and rudimental left ventricle is presented as outlet chamber [16].
Table 1:
Patient characteristics (n= 38)
| Male:female (n) | 21:17 |
| Age at first CAVVP [years, median (range)] | 1.4 (0–38) |
| Body weight at first CAVVP [kg, median (range)] | 7 (3.3–43) |
| Main diagnosis (n) | |
| Unbalanced atrioventricular septal defect | 25 |
| With hypoplastic LV | 23 |
| With hypoplastic RV | 2 |
| Common inlet right ventricle | 11 |
| Hypoplastic left heart syndrome | 2 |
| Associated lesions [n (%)] | |
| Right atrial isomerism | 24 (63.2%) |
| TAPVC | 15 (39.5%) |
| Procedures of CAVVP [47 operations for 38 patients, n (%)] | |
| Bivalvation | 27 (57.4%) |
| Annuloplasty | 28 (59.6%) |
| Cleft suture | 19 (39.6%) |
CAVVP: common atrioventricular valve plasty; LV: left ventricle; RV: right ventricle; TAPVC: total anomalous of pulmonary venous connection; CAVV: common atrioventricular valve.
Timing and indication for CAVV plasty
Timing of the first CAVV plasty is summarized in Fig. 1. CAVV plasty was mainly scheduled at the timing of BDG. If moderate or greater CAVVR had already existed in the foetal period, they were treated surgically in the neonatal or early infantile period prior to BDG (n= 3). Of 23 patients undergoing CAVV plasty at BDG, 10 patients showed mild-to-moderate CAVVR. All of them had a marginal single-ventricular function and/or pulmonary arterial pressure for establishment of Fontan circulation, so CAVV plasty was prophylactically performed to improve the haemodynamic status for Fontan completion.
Figure 1:
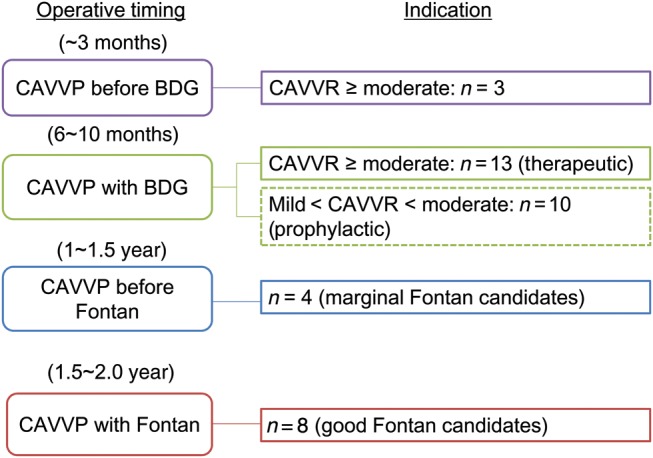
Operative timing and indication for CAVV plasty. Months after birth; years after birth; CAVVR: common atrioventricular valve regurgitation.
Developed CAVVR after BDG was treated surgically concomitantly with or without Fontan operation, which depended on the ventricular function and/or pulmonary condition. Eight patients with a good single-ventricular function and low pulmonary arterial pressure underwent CAVV plasty at the Fontan operation, and 4 patients with a mildly depressed single-ventricular function and/or relatively high pulmonary arterial pressure underwent CAVV plasty prior to Fontan completion.
Procedures of CAVV plasty and evolution of bivalvation technique
The modified Alfieri technique, also named bivalvation, as a method of CAVV plasty was introduced by several institutions in the late 1990s [17, 18]. The concept of this procedure is the shortening of the anteroposterior diameter of CAVV to maintain the coaptation of both bridging leaflets by attaching a simple ‘bridging cord’ across both leaflets, so that the common AV valve orifice is divided like two AV valves.
During the early study period, the free edges of both bridging leaflets were simply sutured like the Alfieri stitch (Fig. 2A) [19]. Then, the method was changed to the simple attachment of the strip made by an expanded polytetrafluoroethylene (ePTFE) graft to reduce the anteroposterior width of bridging leaflets (Fig. 2B). The connecting point of the remnant of the interatrial septum to the CAVV annulus was targeted to suture the strip, and the strip was not fixed to the leaflets. In some cases, the remnant of interatrial septum itself was sutured to shorten like a ‘native strip’ (Fig. 2C).
Figure 2:
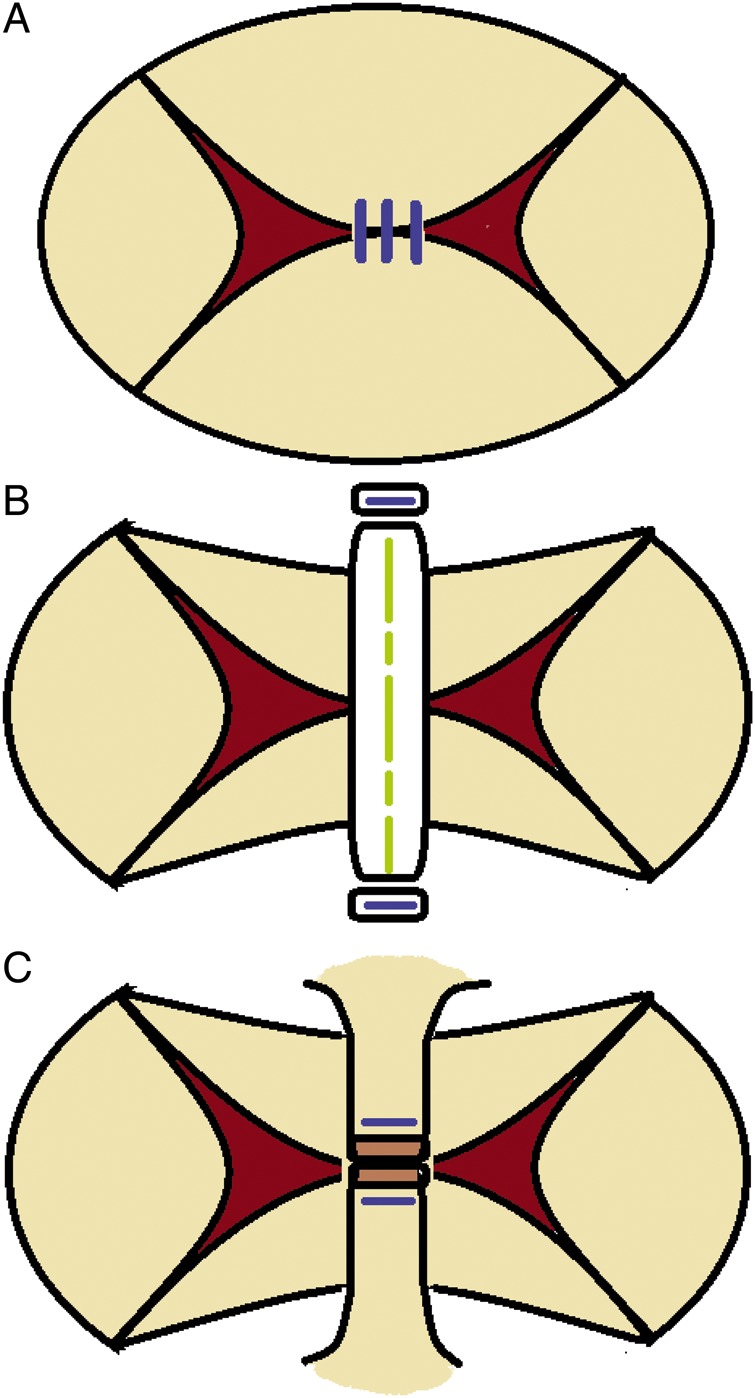
Changes of the bivalvation procedure. (A) Suturing free edge of ABL and PBL (Alfieri stitch). (B) ‘Bridging’ using ePTFE tube (reduction of diameter between ABL and PBL). (C) ‘Bridging’ by suturing IAS remnant (reduction of diameter between ABL and PBL). ABL: anterior bridging leaflet; PBL: posterior bridging leaflet; RML: right mural leaflet; LML: left mural leaflet; IAS: interatrial septum; IVS: interventricular septum.
At CAVV plasty, the length of the strip was decided in such a way that the sum of the dimensions of the divided CAVV orifices measured with bougie was equal to the targeted size. The targeted size was set to ∼100% of the normal mitral valve diameter when CAVV plasty was performed before or at Fontan operation, and to ∼150% when at BDG. Normal mitral valve dimension was calculated from normal mitral valve diameter, assuming that the valves are complete circles.
Study methods and statistical analysis
From the patients' medical records obtained from our outpatient clinic, including the echocardiography and catheter examination reports, the following variables were evaluated: (i) overall outcomes: actuarial survival rate, freedom rates from reoperation for CAVV and CAVV replacement and probability of Fontan completion, (ii) risk factor analysis for mortality, (iii) outcomes of the modified Alfieri technique, (iv) outcomes in patients undergoing the Fontan operation and (v) individual post-surgical course.
The severity of CAVVR was estimated by Doppler colour flow mapping and was graded as none to mild, moderate and severe. A grade of mild implied that the regurgitant jet crossed less than two-thirds of the systemic atrium; a grade of moderate implied that it reached beyond two-thirds of the systemic atrium; and a grade of severe implied that the jet reached the posterior wall of the atrium at a significant width. Cox proportional hazard models are used to analyse risk factors for mortality. The status of variables as being independent of the overall and late mortality was estimated with the Kaplan–Meier method, and the differences in the study groups' event-free status were assessed with a log-rank test. A value of P < 0.05 was considered statistically significant.
RESULTS
Overall outcomes
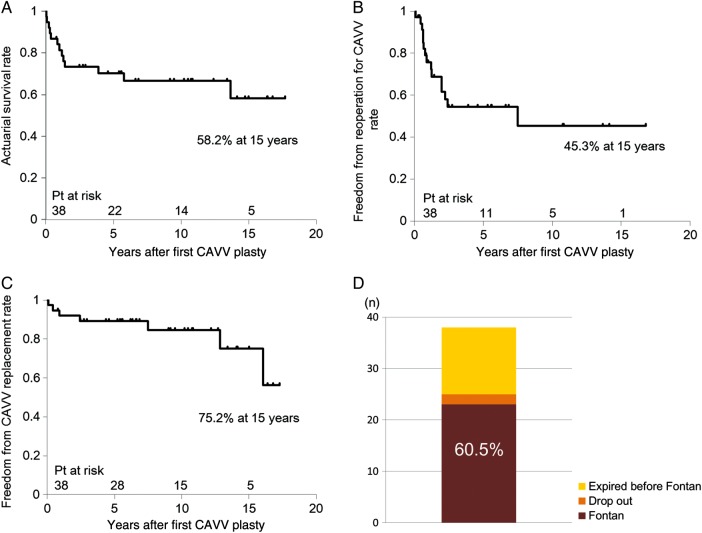
The follow-up was complete for all patients and the mean follow-up period was 7.2 ± 5.9 years (range 0–17.7). The actuarial survival rate after the first CAVV plasty was 58.2% at 15 years (Fig. 3A). There were 5 hospital deaths and 8 late deaths. Freedom from reoperation for CAVV was 45.3% at 15 years (Fig. 3B). Freedom from CAVV replacement was 75.2% at 15 years (Fig. 3C). CAVV replacement was carried out in 3 patients before Fontan completion and 4 patients after Fontan completion. As a result, 23 of the 38 patients (60.5%) completed the Fontan operation (Fig. 3D).
Figure 3:
Actuarial survival rate (A), freedom from reoperation for CAVV rate (B) and CAVV replacement rate (C) and the probability of Fontan completion (D) after first CAVV plasty in all patients.
Risk factor analysis for mortality
The Cox proportional hazard model identified associated total anomalous pulmonary venous connection (hazard ratio, 7.11; 95% confidence interval (CI), 1.51–44.8; P= 0.01) and CAVV plasty before BDG (hazard ratio, 4.64; 95% CI, 1.03–15.5, P= 0.05) as independent risk factors of overall survival. In our study cohort, all 3 patients who underwent first CAVV plasty before BDG died without undergoing Fontan operation. The cause of death was pulmonary venous obstruction in 1 and uncontrollable CAVVR and unimproved heart failure in 2. The grade of preoperative CAVVR (hazard ratio, 1.43; 95% CI, 0.38–4.53; P= 0.57), right atrial isomerism (hazard ratio, 1.53; 95% CI, 0.27–7.24; P= 0.61) and plasty method (hazard ratio, 1.03; 95% CI, 0.31–3.89; P= 0.97) were not detected as risk factors.
Outcomes of modified Alfieri technique
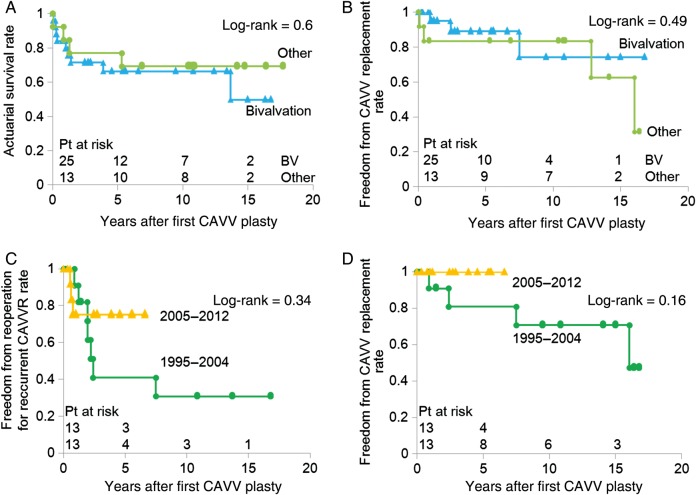
Patients who underwent the modified Alfieri technique at the first CAVV plasty showed survival and freedom from CAVV replacement rates equivalent to those in patients who underwent other plasty procedures (survival rate: P= 0.60, freedom from CAVV replacement rate: P= 0.49) (Fig. 4A and B). Patients who underwent the first CAVV plasty after 2005, when the modified Alfieri technique was simplified, showed satisfactory freedom from reoperation and CAVV replacement rate, as opposed to those before 2004. No patients required a CAVV replacement during the study period; however, the difference has not been statistically significant so far (freedom from reoperation rate: P= 0.34, freedom from replacement rate: P= 0.16) (Fig. 4C and D).\
Figure 4:
Actuarial survival rate (A), freedom from reoperation for CAVV rate (B) and CAVV replacement rate (C) after first CAVV plasty by the procedures of CAVV plasty and (D) freedom from CAVV replacement rate in patients undergoing bivalvation by surgical era.
Individual postoperative outcomes in patients who underwent first CAVV plasty at inter-stage bidirectional Glenn
Of 23 patients who underwent first CAVV plasty at BDG, 13 patients had moderate or greater CAVVR (Fig. 5A) and received therapeutic CAVV plasty. Excluding 1 drop-out patient and 1 awaiting Fontan operation, 9 of 11 patients underwent surgical intervention again for recurrent or remaining CAVVR, and 5 patients eventually required CAVV replacement. Only 1 patient died of thromboembolic obstruction in BDG pathway after CAVV replacement (7.7%) because of persistent high pulmonary arterial pressure. All the 9 patients who underwent Fontan operation survived.
Figure 5:
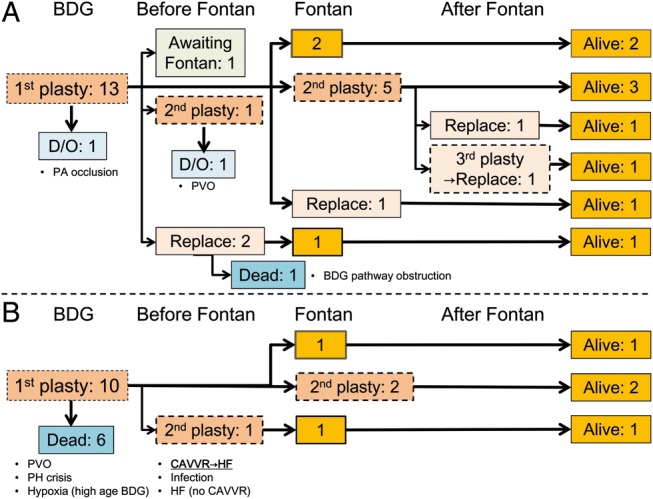
Individual clinical courses of patients undergoing first CAVV plasty at BDG with moderate or greater CAVVR (A) or less than moderate CAVVR (B). BDG: bidirectional Glenn; PA: pulmonary artery; PVO: pulmonary venous obstruction, PH: pulmonary hypertension; CAVVR: common atrioventricular valve regurgitation; HF: heart failure.
Of 10 patients who underwent prophylactic CAVV plasty at BDG (Fig. 5B), 6 patients (60%) died without undergoing Fontan operation. Although the causes of death were multifactorial, unimproved heart failure derived from uncontrollable CAVVR was the cause of death only in 1 patient. The remaining 4 patients underwent Fontan operation and survived without requiring subsequent CAVV replacement.
Outcomes in patients undergoing CAVV plasty after BDG
Twelve of the 38 patients (31.6%) underwent CAVV plasty for newly developed CAVVR after BDG. Four of the 12 patients underwent CAVV plasty before Fontan completion due to their marginal cardiopulmonary function for Fontan completion. Among them, 2 patients survived after Fontan completion and the other 2 patients died before due to pulmonary venous obstruction. Eight of the 12 patients with good cardiopulmonary function underwent concomitant CAVV plasty with Fontan operation, one of whom deceased in hospital due to unexpectedly developed pulmonary venous obstruction after the operation. Of the remaining 7 patients, 2 patients subsequently required CAVV replacement.
Outcomes after Fontan operation
In all, 23 of the 38 patients (60.5%) completed the Fontan operation. Actuarial survival rate after the Fontan operation was 95.5% at 15 years. In patients who completed the Fontan operation with their own CAVV (n= 21), freedom from CAVV replacement after Fontan operation rates were 100.0, 94.1 and 84.7% at 1, 5 and 10 years, respectively. Four patients underwent CAVV replacement after Fontan operation during the follow-up period. Latest echo-cardiography performed at 5.1 (0.78–14) years after the Fontan showed that the CAVV grade was trivial in 11 patients, mild in 3, moderate in 2 and severe in 0.
DISCUSSION
This study demonstrated that CAVV plasty for patients with functional single ventricle was challenging. Pulmonary venous obstruction, mainly derived from coexisting extracardiac TAPVC, was a risk factor for mortality. Moderate or greater CAVVR at birth was another risk factor. Whereas the outcomes of the modified Alfieri technique were not superior to those of other plasty procedures, technical modification may improve the outcomes. For the patients who underwent CAVV plasty at BDG, the mortality rate was significantly higher in patients who underwent prophylactic CAVV plasty than in those who underwent therapeutic CAVV plasty. CAVV replacement was occasionally required even after establishment of Fontan circulation; however, CAVV replacement itself was a considerable alternative to repeated CAVV plasty.
In this study group, more than half of the patients had complications derived from right atrial isomerism, and TAPVC was frequently associated with this anatomical property. Preoperative pulmonary venous obstruction and postoperative pulmonary venous stenosis are well-recognized risk factors for survival and Fontan completion [20]. Indeed, 5 of the 13 deaths were complicated with postoperative pulmonary venous stenosis. Another patient who died from a pulmonary hypertension crisis after CAVV plasty concomitant with BDG also showed a preoperative pulmonary venous obstruction. Because this study targeted a relatively small number of patients, statistical analysis was not possible after stratification to exclude the influences by coexisting TAPVC. However, the influence of this associated anatomical issue should be taken into consideration at the interpretation of the outcomes of CAVV plasty itself.
Patients with significant CAVVR at birth showed a poor life-prognosis. All the 3 patients who underwent CAVV plasty before BDG passed away without achieving a Fontan operation. In patients with hypoplastic left heart syndrome, significant tricuspid regurgitation presented at birth was a risk factor for mortality after Norwood operation [21, 22]. Procedures to apply for valvuloplasty were very limited in small babies. The primary CAVV replacement might improve the outcome without attempting CAVV plasty for them; however, we hesitate to do so because the commercially available mechanical valves are so large that restrictive ventricular motion after implantation or frequent thromboembolism formation are feared. In addition to the 9 patients who underwent therapeutic CAVV plasty at BDG and required repeated surgical intervention for recurrent CAVVR before or at Fontan, progression of CAVVR continued in 4 patients even after Fontan operation. Both BDG circulation without additional pulmonary blood flow and Fontan circulation were known to reduce systemic ventricular volume preload [23, 24]. The cause of regurgitation in CAVV was mainly derived from a congenital abnormality of the leaflet itself and subvalvular structures rather than annulus dilatation. Hence, ventricular volume reduction and changes in papillary muscle geometry may sometimes provide adverse effects for the CAVV function.
The mode of death after prophylactic CAVV plasty varied, and subsequently progressing CAVVR was the main aetiology in only 1 of the 6 patients. Whereas 3 of the 4 surviving patients underwent redo CAVV plasty, all the 4 patients are now surviving long after Fontan completion without CAVV replacement. In these 4 patients, the successful regulation of CAVVR provided their haemodynamic improvement and enabled them to reach Fontan operation. These facts indicate that the severity of the cardiopulmonary function, rather than the severity of preoperative CAVVR, for Fontan completion determined the outcomes in this group of patients [1]. The optimal timing of prophylactic CAVVR and the decision to treat or not to treat this mild-to-moderate CAVVR are still unclear. Now, we prefer to do it concomitantly with inter-stage BDG to reduce the frequency of on-pump heart surgery and also to prepare for better Fontan circulation.
Our modified Alfieri technique is simple and standardized. The point where the remnant of the interatrial septum attaches to the CAVV annulus was targeted to shorten the anteroposterior diameter. The conduction system can be theoretically avoided, but sometimes with difficulty in patients with right atrial isomerism. The bridging strip made from a ePTFE tube was used to divide the valve orifice without suturing the leaflet itself. Instead, if present, the remnant of the interatrial septum can be directly shortened. The advantage of these modifications is that the anteroposterior diameter can be shortened without suturing the leaflet itself. No patients have required CAVV replacement since 2005, when the above-described technical modifications were introduced. The other considerable advantage is its repeatability. Of 4 patients who underwent second CAVV plasty, recurrent CAVVR after the simple ‘bridging’ technique could be easily regulated by re-‘bridging’ with ePTFE strip in 1 patient. Although the aetiology of recurrent regurgitation was usually multifocal and also mainly from abnormality of the leaflet itself, further follow-up may reveal the statistically significant superiority of this technique.
Various complications such as thromboembolic events, heart block and single-ventricular basal wall dysfunction have been reported in patients with functionally single ventricle who underwent CAVV replacement [12, 25], which may be partly explained by the fact that the implanted prosthetic valve was too large for patients with single-ventricular physiology. This study included 7 patients (18.4%) who underwent subsequent CAVV replacement after several times of CAVV repairs. One of the 7 patients died of a thromboembolic obstruction in the BDG pathway early after the CAVV replacement, and another patient developed complete atrioventricular block and required permanent pacemaker implantation. The remaining 5 long-term survivors are free from any complications. Now, we set the size of the mechanical valve used with CAVV replacement at 100% or smaller of normal mitral valve diameter before Fontan operation and at 80% or smaller at or after Fontan operation [24].
Study limitations
At first, details of the repaired valve function were not revealed except grade of regurgitation. Superiority of this repair method to others in any aspect was not detected so far. Although varied aetiology of recurrent CAVVR after the modified Alfieri technique was thought to be derived from each valvular native structural abnormality, further study is mandatory to identify the therapeutic effect of this technique.
Secondly, CAVV repair in single-ventricular physiology is still challenging. As shown in this study cohort, not so few patients required repeated repair and/or replacement. Detection of haemodynamic improvement by successful regulation of CAVVR is quite difficult. Several other factors like associated pulmonary venous obstruction strongly affected overall survival. CAVVR was sometimes the result of impaired systemic ventricular function, whereas intrinsic anatomical abnormality of CAVV would conversely deteriorate single-ventricular function. Therefore, there would not be any hesitation for CAVV replacement to control CAVVR in the setting of systemic ventricular failure.
CONCLUSION
CAVV plasty for patients with functionally single ventricle is still challenging; however, the aggressive and repeated surgical intervention may contribute to a better life-prognosis. The ventricular volume unloading effect of BDG without additional pulmonary blood flow or Fontan operation did not contribute to maintain CAVV function. CAVV replacement before the impairing cardiopulmonary function should not be hesitated for uncontrollable CAVV regurgitation because of their acceptable outcomes. Although the statistically significant therapeutic superiority of the modified Alfieri technique has not been shown so far, further follow-up may reveal the advantage of this easy and simple technique.
Funding
None.
Conflict of interest: none declared.
REFERENCES
- 1.Honjo O, Atlin CR, Mertens L, Al-Radi OO, Redington AN, Caldarone CA, et al. Atrioventricular valve repair in patients with functional single-ventricle physiology: impact of ventricular and valve function and morphology on survival and reintervention. J Thorac Cardiovasc Surg. 2011;142:326–35. doi: 10.1016/j.jtcvs.2010.11.060. [DOI] [PubMed] [Google Scholar]
- 2.d'Udekem Y, Iyengar AJ, Cochrane AD, Grigg LE, Ramsay JM, Wheaton GR, et al. The Fontan procedure: contemporary techniques have improved long-term outcomes. Circulation. 2007;116(Suppl 11):I157–64. doi: 10.1161/CIRCULATIONAHA.106.676445. [DOI] [PubMed] [Google Scholar]
- 3.Knott-Craig CJ, Danielson GK, Schaff HV, Puga FJ, Weaver AL, Driscoll DD. The modified Fontan operation. An analysis of risk factors for early postoperative death or takedown in 702 consecutive patients from one institution. J Thorac Cardiovasc Surg. 1995;109:1237–43. doi: 10.1016/S0022-5223(95)70208-3. [DOI] [PubMed] [Google Scholar]
- 4.Ohuchi H, Kagisaki K, Miyazaki A, Kitano M, Yazaki S, Sakaguchi H, et al. Impact of the evolution of the Fontan pperation on early and late mortality: a single-center experience of 405 patients over 3 decades. Ann Thorac Surg. 2011;92:1457–67. doi: 10.1016/j.athoracsur.2011.05.055. [DOI] [PubMed] [Google Scholar]
- 5.Friedman KG, Salvin JW, Wypij D, Gurmu Y, Bacha EA, Brown DW, et al. Risk factors for failed staged palliation after bidirectional Glenn in infants who have undergone stage one palliation. Eur J Cardiothorac Surg. 2011;40:1000–6. doi: 10.1016/j.ejcts.2011.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Imai Y, Takanashi Y, Hoshino S, Terada M, Aoki M, Ohta J. Modified Fontan procedure in ninety-nine cases of atrioventricular valve regurgitation. J Thorac Cardiovasc Surg. 1997;113:262–8. doi: 10.1016/S0022-5223(97)70322-2. [DOI] [PubMed] [Google Scholar]
- 7.Uemura H, Ho SY, Anderson RH, Kilpatrick LL, Yagihara T, Yamashita K. The nature of the annular attachment of a common atrioventricular valve in hearts with isomeric atrial appendages. Eur J Cardiothorac Surg. 1996;10:540–5. doi: 10.1016/s1010-7940(96)80421-0. [DOI] [PubMed] [Google Scholar]
- 8.Anderson RH, Spicer D. Anatomy of common atrioventricular junction with complex associated lesions. World J Pediatr Congenit Heart Surg. 2010;1:112–8. doi: 10.1177/2150135110361362. [DOI] [PubMed] [Google Scholar]
- 9.Okita Y, Miki S, Kusuhara K, Ueda Y, Tahata T, Yamanaka K, et al. Annuloplastic reconstruction for common atrioventricular valvular regurgitation in right isomearism. Ann Thorac Surg. 1989;47:302–4. doi: 10.1016/0003-4975(89)90295-6. [DOI] [PubMed] [Google Scholar]
- 10.Ando M, Takahashi Y. Edge-to-edge repair of common atrioventricular or tricuspid valve in patients with functionally single ventricle. Ann Thorac Surg. 2007;84:1571–7. doi: 10.1016/j.athoracsur.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 11.Kotani Y, Chetan D, Atlin CR. Longevity and durability of atrioventricular valve repair in single-ventricle patients. Ann Thorac Surg. 2012;94:2061–9. doi: 10.1016/j.athoracsur.2012.04.048. [DOI] [PubMed] [Google Scholar]
- 12.Wong DJ, Iyengar AJ, Wheaton GR, Ramsay JM, Grigg LE, Horton S, et al. Long-term outcomes after atrioventricular valve operations in patients undergoing single-ventricle palliation. Ann Thorac Surg. 2012;94:606–13. doi: 10.1016/j.athoracsur.2012.03.058. [DOI] [PubMed] [Google Scholar]
- 13.Nakata T, Fujimoto Y, Hirose K, Tosaka Y, Ide Y, Tachi M, et al. Atrioventricular valve repair in patients with functional single ventricle. J Thorac Cardiovasc Surg. 2010;140:514–21. doi: 10.1016/j.jtcvs.2010.05.024. [DOI] [PubMed] [Google Scholar]
- 14.Tatsuno K, Suzuki K, Kikuchi T, Takahashi Y, Murakami Y, Mori K. Valvuloplasty for common atrioventricular valve regurgitation in cyanotic heart diseases. Ann Thorac Surg. 1994;58:154–6. doi: 10.1016/0003-4975(94)91090-1. [DOI] [PubMed] [Google Scholar]
- 15.Sallehuddin A, Bulbul Z, Otero F, Al Dhafiri K, Al-Halees Z. Repair of atrioventricular valve regurgitation in the modified Fontan operation. Eur J Cardiothorac Surg. 2004;26:54–9. doi: 10.1016/j.ejcts.2004.03.031. [DOI] [PubMed] [Google Scholar]
- 16.Keeton BR, Macartney FJ, Hunter S, Mortera C, Rees P, Shinebourne EA, et al. Univentricular heart of right ventricular type with double or common inlet. Circulation. 1979;59:403–11. doi: 10.1161/01.cir.59.2.403. [DOI] [PubMed] [Google Scholar]
- 17.Oku H, Iemura J, Kitayama H, Saga T, Shirotani H. Bivalvation with bridging for common atrioventricular valve regurgitation in right isomerism. Ann Thorac Surg. 1994;57:1324–6. doi: 10.1016/0003-4975(94)91386-2. [DOI] [PubMed] [Google Scholar]
- 18.Ito M, Kikuchi S, Hachiro Y, Abe T. Bivalvation for common atrioventricular valve regurgitation in cyanotic heart disease. Ann Thorac Surg. 1999;5:340–2. [PubMed] [Google Scholar]
- 19.Alfieri O, De Bonis M. The role of the edge-to-edge repair in the surgical treatment of mitral regurgitation. J Card Surg. 2010;25:536–41. doi: 10.1111/j.1540-8191.2010.01073.x. [DOI] [PubMed] [Google Scholar]
- 20.Hoashi T, Kagisaki K, Oda T, Kitano M, Kurosaki K, Shiraishi I. Long-term results of treatments for functional single ventricle associated with extracardiac type total anomalous pulmonary venous connection. Eur J Cardiothorac Surg. 2013;43:965–70. doi: 10.1093/ejcts/ezs594. [DOI] [PubMed] [Google Scholar]
- 21.Dinh DC, Gurney JG, Donohue JE, Bove EL, Hirsch JC, Devaney EJ, et al. Tricuspid valve repair in hypoplastic left heart syndrome. Pediatr Cardiol. 2011;32:599–606. doi: 10.1007/s00246-011-9924-9. [DOI] [PubMed] [Google Scholar]
- 22.Barber G, Helton JG, Aglira BA, Chin AJ, Murphy JD, Pigott JD, et al. The significance of tricuspid regurgitation in hypoplastic left-heart syndrome. Am Heart J. 1988;116(6 Pt 1):1563–7. doi: 10.1016/0002-8703(88)90744-2. [DOI] [PubMed] [Google Scholar]
- 23.Forbes TJ, Gajarski R, Johnson GL, Reul GJ, Ott DA, Drescher K, et al. Influence of age on the effect of bidirectional cavopulmonary anastomosis on left ventricular volume, mass and ejection fraction. J Am Coll Cardiol. 1996;28:1301–7. doi: 10.1016/S0735-1097(96)00300-2. [DOI] [PubMed] [Google Scholar]
- 24.Shimada M, Hoashi T, Kagisaki K, Shiraishi I, Yagihara T, Ichikawa H. Clinical outcomes of prophylactic Damus-Kaye-Stansel anastomosis concomitant with bidirectional Glenn procedure. J Thorac Cardiovasc Surg. 2012;143:137–43. doi: 10.1016/j.jtcvs.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 25.Mahale WT, Gaynor JW, Spray TL. Atrioventricular valve replacement in patients with a single ventricle. Ann Thorac Surg. 2001;72:182–86. doi: 10.1016/s0003-4975(01)02699-6. [DOI] [PubMed] [Google Scholar]