Abstract
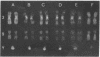
Intermolecular energy transfer between appropriately chosen pairs of dyes can be used to induce or enhance banding patterns in human metaphase chromosomes. Energy transfer, calibrated by fluorometric studies on soluble dye·DNA complexes, can also be detected by photometric measurements on cytological preparations of metaphase chromosomes stained with pairs of fluorochromes. If a fluorescent dye with one type of binding or quantum yield specificity (e.g., quinacrine, 33258 Hoechst, or chromomycin A3) is employed together with a counterstain (e.g., actinomycin D, 7-aminoactinomycin D, or methyl green) exhibiting a complementary base pair binding specificity and satisfying spectral overlap criteria for energy transfer, contrast in fluorescence from the first dye is enhanced in specific subsets of standard chromosome bands. Extensive energy transfer presumably suppress donor fluorescence except in chromosomal region containing clusters of at least 20 base pairs predominantly of one type, within which the donor but not the acceptor can bind and fluoresce. Quinacrine-bright polymorphic regions are especially resistant to fluorescence quenching by counterstains with G·C binding specificity, strengthening the evidence that these latter regions are highly enriched for A·T base pair clusters. The ability to highlight selectively many such polymorphic regions may prove of further, practical, utility in a number of cytogenetic problems.
Keywords: Förster equation, A·T or G·C base pair clustering, sensitized fluorescence, microfluorometry, chromosome polymorphisms
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badaev N. S., Borisov Iu M., Zelenin A. V., Iordanskii A. B. Mnogoobrazie strukturnogo geterokhromatina u Chironomus thummi. Dokl Akad Nauk SSSR. 1973 Dec 1;213(4):949–951. [PubMed] [Google Scholar]
- Beardsley K., Cantor C. R. Studies of transfer RNA tertiary structure by singlet-singlet energy transfer. Proc Natl Acad Sci U S A. 1970 Jan;65(1):39–46. doi: 10.1073/pnas.65.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie S., Giron J., Latt S. A. Estimation of accessibility of DNA in chromatin from fluorescence measurements of electronic excitation energy transfer. Nature. 1975 Feb 6;253(5491):470–471. doi: 10.1038/253470a0. [DOI] [PubMed] [Google Scholar]
- Caspersson T., Lindsten J., Lomakka G., Moller A., Zech L. The use of fluorescence techniques for the recognition of mammalian chromosomes and chromosome regions. Int Rev Exp Pathol. 1972;11:1–72. [PubMed] [Google Scholar]
- Chen R. F. Practical aspects of the calibration and use of the Aminco-Bowman spectrophotofluorometer. Anal Biochem. 1967 Aug;20(2):339–357. doi: 10.1016/0003-2697(67)90040-1. [DOI] [PubMed] [Google Scholar]
- Comings D. E., Drets M. E. Mechanisms of chromosome banding. IX. Are variations in DNA base composition adequate to account for quinacrine, Hoechst 33258 and daunomycin banding? Chromosoma. 1976 Jul 8;56(3):199–211. doi: 10.1007/BF00293185. [DOI] [PubMed] [Google Scholar]
- Dutrillaux B., Lejeune J. Sur une nouvelle technique d'analyse du caryotype humain. C R Acad Sci Hebd Seances Acad Sci D. 1971 May 17;272(20):2638–2640. [PubMed] [Google Scholar]
- Gill J. E., Jotz M. M., Young S. G., Modest E. J., Sengupta S. K. 7-Amino-actinomycin D as a cytochemical probe. I. Spectral properties. J Histochem Cytochem. 1975 Nov;23(11):793–799. doi: 10.1177/23.11.1194669. [DOI] [PubMed] [Google Scholar]
- Hollander D. H., Litton L. E., Liang Y. W. Ethidium bromide counterstain for differentiation of quinacrine stained interphase bodies and brilliant metaphase bands. Exp Cell Res. 1976 Apr;99(1):174–175. doi: 10.1016/0014-4827(76)90692-3. [DOI] [PubMed] [Google Scholar]
- Jensen R. H., Langlois R. G., Mayall B. H. Strategies for choosing a deoxyribonucleic acid stain for flow cytometry of metaphase chromosomes. J Histochem Cytochem. 1977 Aug;25(8):954–964. doi: 10.1177/25.8.70463. [DOI] [PubMed] [Google Scholar]
- Kornberg R. D. Structure of chromatin. Annu Rev Biochem. 1977;46:931–954. doi: 10.1146/annurev.bi.46.070177.004435. [DOI] [PubMed] [Google Scholar]
- LATT S. A., CHEUNG H. T., BLOUT E. R. ENERGY TRANSFER. A SYSTEM WITH RELATIVELY FIXED DONOR-ACCEPTOR SEPARATION. J Am Chem Soc. 1965 Mar 5;87:995–1003. doi: 10.1021/ja01083a011. [DOI] [PubMed] [Google Scholar]
- Latt S. A., Brodie S., Munroe S. H. Optical studies of complexes of quinacrine with DNA and chromatin: implications for the fluorescence of cytological chromosome preparations. Chromosoma. 1974;49(1):17–40. doi: 10.1007/BF00284985. [DOI] [PubMed] [Google Scholar]
- Latt S. A. Fluorescent probes of chromosome structure and replication. Can J Genet Cytol. 1977 Dec;19(4):603–623. doi: 10.1139/g77-065. [DOI] [PubMed] [Google Scholar]
- Latt S. A. Optical studies of metaphase chromosome organization. Annu Rev Biophys Bioeng. 1976;5:1–37. doi: 10.1146/annurev.bb.05.060176.000245. [DOI] [PubMed] [Google Scholar]
- Latt S. A., Wohlleb J. C. Optical studies of the interaction of 33258 Hoechst with DNA, chromatin, and metaphase chromosomes. Chromosoma. 1975 Nov 11;52(4):297–316. doi: 10.1007/BF00364015. [DOI] [PubMed] [Google Scholar]
- LePecq J. B., Paoletti C. A fluorescent complex between ethidium bromide and nucleic acids. Physical-chemical characterization. J Mol Biol. 1967 Jul 14;27(1):87–106. doi: 10.1016/0022-2836(67)90353-1. [DOI] [PubMed] [Google Scholar]
- Manuelidis L. Chromosomal localization of complex and simple repeated human DNAs. Chromosoma. 1978 Mar 22;66(1):23–32. doi: 10.1007/BF00285813. [DOI] [PubMed] [Google Scholar]
- Modest E. J., Sengupta S. K. 7-Substituted actinomycin D (NSC-3053) analogs as fluorescent DNA-binding and experimental antitumor agents. Cancer Chemother Rep. 1974 Jan-Feb;58(1):35–48. [PubMed] [Google Scholar]
- Müller W., Gautier F. Interactions of heteroaromatic compounds with nucleic acids. A - T-specific non-intercalating DNA ligands. Eur J Biochem. 1975 Jun;54(2):385–394. doi: 10.1111/j.1432-1033.1975.tb04149.x. [DOI] [PubMed] [Google Scholar]
- Nayak R., Sirsi M., Podder K. Mode of action of antitumour antibiotics. spectrophotometric studies on the interaction of chromomycin A3 with DNA and chromatin of normal and neoplastic tissue. Biochim Biophys Acta. 1975 Jan 20;378(2):195–204. [PubMed] [Google Scholar]
- Pachmann U., Rigler R. Quantum yield of acridines interacting with DNA of defined sequence. A basis for the explanation of acridine bands in chromosomes. Exp Cell Res. 1972 Jun;72(2):602–608. doi: 10.1016/0014-4827(72)90045-6. [DOI] [PubMed] [Google Scholar]
- Paoletti J., Magee B. B., Magee P. T. The structure of chromatin: interaction of ethidium bromide with native and denatured chromatin. Biochemistry. 1977 Feb 8;16(3):351–357. doi: 10.1021/bi00622a002. [DOI] [PubMed] [Google Scholar]
- Parkman R., Mosier D., Umansky I., Cochran W., Carpenter C. B., Rosen F. S. Graft-versus-host disease after intrauterine and exchange transfusions for hemolytic disease of the newborn. N Engl J Med. 1974 Feb 14;290(7):359–363. doi: 10.1056/NEJM197402142900703. [DOI] [PubMed] [Google Scholar]
- Peakman D. C., Moreton M. F., Robinson A. Prenatal diagnosis: techniques used to help in ruling out maternal cell contamination. J Med Genet. 1977 Feb;14(1):37–39. doi: 10.1136/jmg.14.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlammadinger J., Poulsen H., Mikkelsen M. Inhibition of the development of Q-bands on human chromosomes by netropsin. Hum Genet. 1977 Dec 23;39(3):309–313. doi: 10.1007/BF00295425. [DOI] [PubMed] [Google Scholar]
- Schweizer D., Ambros P., Andrle M. Modification of DAPI banding on human chromosomes by prestaining with a DNA-binding oligopeptide antibiotic, distamycin A. Exp Cell Res. 1978 Feb;111(2):327–332. doi: 10.1016/0014-4827(78)90177-5. [DOI] [PubMed] [Google Scholar]
- Schweizer D. Reverse fluorescent chromosome banding with chromomycin and DAPI. Chromosoma. 1976 Nov 29;58(4):307–324. doi: 10.1007/BF00292840. [DOI] [PubMed] [Google Scholar]
- Stryer L. Fluorescence energy transfer as a spectroscopic ruler. Annu Rev Biochem. 1978;47:819–846. doi: 10.1146/annurev.bi.47.070178.004131. [DOI] [PubMed] [Google Scholar]
- Stryer L., Haugland R. P. Energy transfer: a spectroscopic ruler. Proc Natl Acad Sci U S A. 1967 Aug;58(2):719–726. doi: 10.1073/pnas.58.2.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. C. The degree of unwinding of the DNA helix by ethidium. I. Titration of twisted PM2 DNA molecules in alkaline cesium chloride density gradients. J Mol Biol. 1974 Nov 15;89(4):783–801. doi: 10.1016/0022-2836(74)90053-9. [DOI] [PubMed] [Google Scholar]
- Weisblum B., De Haseth P. L. Quinacrine, a chromosome stain specific for deoxyadenylate-deoxythymidylaterich regions in DNA. Proc Natl Acad Sci U S A. 1972 Mar;69(3):629–632. doi: 10.1073/pnas.69.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisblum B. Why centric regions of guinacrine-tested mouse chromosomes show diminished fluorescence. Nature. 1973 Nov 16;246(5429):150–151. doi: 10.1038/246150a0. [DOI] [PubMed] [Google Scholar]
- Wells R. D., Larson J. E. Studies on the binding of actinomycin D to DNA and DNA model polymers. J Mol Biol. 1970 Apr 28;49(2):319–342. doi: 10.1016/0022-2836(70)90248-2. [DOI] [PubMed] [Google Scholar]
- Zelenin A. V., Kirianova E. A., Kolesnikov V. A., Stepanova N. G. Use of actinomycin D for the specific quenching of fluorescence of deoxyribonucleic acid in cells stained with acridine aminoderivatives. J Histochem Cytochem. 1976 Nov;24(11):1169–1172. doi: 10.1177/24.11.63505. [DOI] [PubMed] [Google Scholar]
- van de Sande J. H., Lin C. C., Jorgenson K. F. Reverse banding on chromosomes produced by a guanosine-cytosine specific DNA binding antibiotic: olivomycin. Science. 1977 Jan 28;195(4276):400–402. doi: 10.1126/science.63994. [DOI] [PubMed] [Google Scholar]