Abstract
This review aims at elucidating the interaction between genetic and environmental factors in the aetiology of primarily low myopia. Genetics greatly influence the growth of the eye, but the fine correlation between the components of refraction for the eye to become emmetrope is affected by environmental factors such as education, metabolism, physical activity, and outdoor activity.
Keywords: epidemiology, myopia, genetics, environment, aetiology, high myopia
Introduction
Since it was first realised that highly educated people are more likely to be myopic than less educated people, there has been a continuing debate over whether myopia is inherited or is environmentally determined. This debate is encapsulated in two conflicting ideas: that those born to be myopic naturally gravitate to academic studies and near-work occupations, or that engaging in these activities, particularly during development, causes myopia.
In the following study, a brief review will be presented elucidating the interaction between genetic and environmental factors in the aetiology of myopia, primarily low myopia.
Genetics
The initial approach in genetic research is family studies. This is usually followed by the search for chromosomal localisation and ultimately molecular characterisation of the gene or genes involved.
Genome-wide association studies
Several recent genetic studies on myopia that have used the genome-wide association study approach have investigated and identified genetic variants in different chromosomes associated with axial length and myopia/refractive error.1, 2, 3 Also, a recent study found that education levels influence the association between three recently discovered genetic loci and refractive error. The genetic effect on myopia was significantly larger in subjects with a higher level of education.4
Epidemiology
Recent studies have confirmed the old observation that myopia most frequently occurs and develops during school-going age. The prevalence of myopia is particularly high in college and university students, whereas myopia rarely occurs in less educated populations. On the basis of a common Refractive Error Study in Children protocol,5 it has been shown that 5-year-old children from various countries and cultures have very few refractive errors and that depending on schooling and learning systems the same children develop myopia, differing from a low percentage in Nepal to 70% in China (Table 1).6, 7, 8, 9
Table 1. Prevalence of myopia in school children.
|
Multi-Country Survey 1998–2004 | |||
|---|---|---|---|
| Age 5 |
Age 5 |
||
| M | F | ||
| Chile (n=5303) | 3.4% | 19.4% | 14.7% |
| Nepal (n=5067) | <3% | No change | |
| China Shunyi (n=5884) | 0% | 36.7% | 55.0% |
| China Guangzhou (n=4359) | 3.3% | 69.3% | 77.5% |
To illustrate the impact of learning, it has been observed that the prevalence of myopia among students is 10 times higher than among unskilled workers, based on Danish studies on conscripts from 188210 to 1964.11 The studies also showed little change in the prevalence of myopia between 1882, 1964, and 2007,12 although the prevalence of higher degrees of myopia was significantly reduced during the time between the studies.
Proband and family studies
Although family patterns of inheritance are well established in familial high myopia, there are also significant family correlations in refractive error in school myopia. In a large number of studies, children with myopic parents have been shown to be more likely myopic compared with those with non-myopic parents. Having two myopic parents generally poses a greater risk than having only one. These correlations are well established in populations of both East Asian and Caucasian origin.13
Although these correlations are consistent with a genetic basis for myopia, they do not establish it. Correlations in refractive error between parents and their children, and the heritability values calculated from these correlations, can reflect shared environments and shared genes. Where commitment to education is part of family and community culture, this could result in high correlations between parents and children, without, at the limit, any role for shared genes.
Conversely, where there are major differences in the environments in which parents and their children grow up, as was the case with the Inuit during the process of acculturation, parent–children correlations and the heritability values calculated from them can become quite low.14, 15, 16, 17 Results from family studies are in general inconclusive, but heritability studies have shown a higher inheritance of ocular dimensions than of refraction (Table 2).
Table 2. Family resemblance of ocular dimensions and refractive error.
| Husband-wife regression coefficient | Child-parent regr. coefficient | Sib-sib intrapair correlation | |
|---|---|---|---|
| Corneal radius | 0.02 | 0.32 | 0.20 |
| Axial length | 0.05 | 0.38 | 0.25 |
| Ant. chamber depth | −0.09 | 0.28 | 0.43 |
| Refractive error | 0.03 | 0.07 | 0.25 |
| SE of estimates | 0.10 | 0.08 | 0.07 |
| Maximal genetic expectation | 0.00 | 0.50 | 0.50 |
| No. of pairs examined | 108 | 159 | 160 |
All analyses based on age- and sex-independent deviation scores.
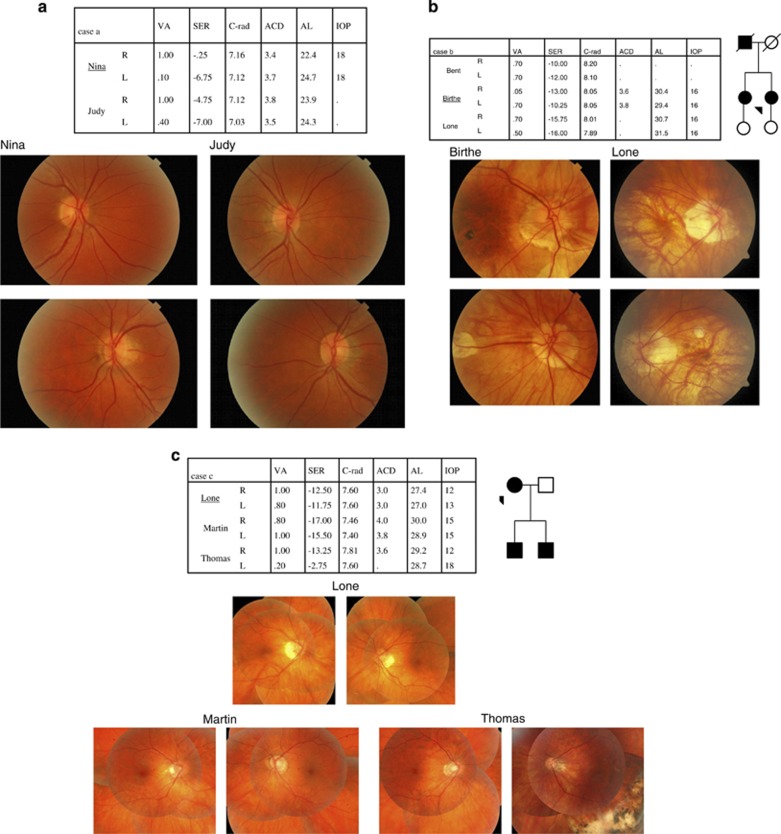
From a cohort study of unselected high myopia cases18 the following three families show not only the genetic background of high myopia cases but also the inter- and intrafamily variation in high myopia phenotype. A high degree of concordance within the three families presented (see Figure 1) indicates a marked genetic component, but in the total material there is considerable variation in the clinical course of high myopia with regard to onset, progression, eye shape, visual outcome, and prognosis. There seems to be no genetic linkage between low and high myopia.11
Figure 1.
(a) Two sisters with high myopia, one bilateral and the other unilateral. All three high myopic eyes show very steep corneas. (b) A father and two daughters with high myopia, mainly caused by axial elongation. Moderately reduced visual acuity and retinal changes in the posterior pole in all cases. (c) A mother and two sons with high myopia, mainly caused by axial elongation. The eldest son had a retinal detachment in his left eye, which is now pseudophacic.
Twin studies
In twin studies, refraction is compared in monozygotic and dizygotic twins of the same sex, and in all studies a high degree of heritability has been shown. This result has caused considerable confusion, because it has generally been interpreted as showing a predominant role for genetic factors. For example, in commenting on the evidence for rapid changes in the prevalence of myopia in Eskimo communities during the settlement process, Sorsby19 stated that:
‘The concordance shown in the substantial series of studies now available on uniovular twins have all without exception established as cumulative, direct, and incontrovertible evidence that refraction is genetically determined.'
Heritability estimates for myopia from twin studies

However, in the calculation it is assumed that mono- and dizygotic twins share the same environment, which is not the case. Already prenatal differences in environment are frequent. In a Danish study,22 dizygotic twins were more discordant than monozygotic twins in years of education, and, given the strong effects of education on the prevalence of myopia, examination of correlation in length and outcomes of education could be usefully included in future twin studies.
Anisometropia
Anisometropia studies are useful for showing variations in refraction in individuals in whom both genetics and environment are supposed to be equal in the two eyes. Studies show a more or less normal distribution around isometropia, but with a tendency towards a contribution of unilateral cases, particularly cases of high myopia in one eye and emmetropia or low myopia in the other eye.11 There seems to be a tendency towards more spherical myopia in right eyes (laterality) and more spherical anisometropia in myopia. Cylindrical anisometropia appeared to be independent of spherical ametropia.23
Environment
Near work and education
Numerous studies on schoolchildren and students over the last 150 years have documented a strong correlation between the development of myopia and education, and a critical analysis of data indicates that near work (accommodation) is not solely responsible for the development of myopia but only when combined with a learning process, including memorising. This was already stated beautifully by Randall24 in 1885:
Hypermetropia is the prevailing condition of the refraction of most animals, children, uncivilised peoples and eyes uninjured by the educational process.
Diet and diabetes
Another environmental risk factor that has been proposed is diet. The increasing prevalence of myopia in countries that have adopted western dietary patterns has led to the hypothesis that hyperglycaemia and hyperinsulinaemia induce myopia.25 The western lifestyle implies a larger intake of food with a high glycaemic load26 and less developed societies adopting the western dietary pattern experience increasing incidences of hyperglycaemia, insulin resistance, hyperinsulinaemia, and type 2 diabetes.27, 28 A high glycaemic load imposes acute and chronic hyperinsulinaemia,25 and a large intake of sucrose lowers the insulin sensitivity29 and blocks the binding of insulin to the receptor.30 Cordain et al25 argue that high glycaemic load and the resulting hyperinsulinaemia affect different growth factors resulting in scleral growth.
Thus, in a retrospective cohort study in type 1 diabetic patients, the influence of metabolism on the development and progression of myopia was investigated.31 The results indicated an association between hyperglycaemia (HbA1c≥8.8%) and myopia, whereas the insulin dosage was not associated with refractive error. Furthermore, the study confirmed that myopia is more prevalent in diabetic patients than in non-diabetic individuals. The underlying mechanism of the refractive changes remains unclear, but the study did support a relation between impaired metabolic control and myopia.
Having found out that myopia in diabetic patients seems to be related to metabolism, it was interesting to find out whether the refractive changes were caused by alterations of the lens or, as suggested by Cordain et al,25 by scleral growth (axial elongation). Hence, a small study was initiated at the eye clinic at Steno Diabetes Center, Denmark (unpublished data). Patients were excluded if they had onset of diabetes after 30 years of age, had undergone refractive surgery, had diabetic retinopathy causing a visual acuity of <0.2, or had cataract of a degree possibly affecting the refractive error. A total of 33 type 1 diabetic patients were included in the analyses (Table 3).
Table 3. Comparison of optical components in diabetic patients and medical students.
| Diabetic patients (n=33) | Medical students (n=143) | P-value | |
|---|---|---|---|
| Age (years) | 44.9 (14.7) | 23.2 (3.5) | |
| SEa (D) | −0.81 (2.05) | −0.79 (0.98) | 0.958 |
| Axial length (mm) | 23.49 (0.86) | 23.99 (1.12) | 0.018 |
| Corneal radius (mm) | 7.83 (0.23) | 7.79 (0.26) | 0.436 |
| Lens power (D) | 20.33 (1.92) | 18.79 (1.20) | 0.000 |
| Anterior chamber depth (mm) | 3.08 (0.41) | 3.63 (0.29) | 0.000 |
Mean, spherical equivalent in cycloplegia, right eye.
The data were compared with data from Danish medical students. The power of the lens was calculated by the IOL-Master with knowledge of the refractive error in cycloplegia, the radius of the corneal curvature, the depth of the anterior chamber, and the axial length. The A-constant was set at 116.9. The results of the study indicated that the axial length is shorter in diabetic patients than in non-diabetic individuals with the same refractive error and that the primary difference in the optical components is the power of the lens. The anterior chamber depth was significantly narrower in diabetic patients, which is probably explained partly by the shorter axial length and primarily by a thicker lens. Although this small study has many limitations and is not conclusive, the observations support the assumption that the refractive change in diabetic patients is due to alterations in the lens.32 Moreover, the results are supported by a twin study evaluating the influence of the duration of diabetes on refraction.33 Although the authors observed diverging results of the relations between refraction and duration of diabetes, they did observe a tendency of a decreased axial length with increased duration of diabetes, and increased lens thickness and decreased anterior chamber depth with increased duration of diabetes. The generally accepted view is that short-term fluctuations in blood glucose level alter the refraction of the lens, primarily by alteration in the osmotic pressure caused by changes in the blood glucose level and accumulation of sorbitol and fructose in the lens by the sorbitol pathway.33, 34
Recent studies have confirmed that myopia is more prevalent than hyperopia in the diabetic population.35 Other studies have evaluated the effect of acute hyperglycaemia on refractive error, but there is a need for prospective studies focusing on changes occurring over time in the ocular components (that is, cornea, lens, and axial length) in diabetic patients.
Physical activities and outdoor activities
Studies in children have indicated an association between physical activity/outdoor activity and refractive error,36, 37, 38 and it has been observed that myopes spend significantly less time engaged in sports, which is associated with myopia.38
To study the effect of physical activity on the development and progression of myopia, a 2-year longitudinal cohort study was carried out on 156 Caucasian medical students from the University of Copenhagen, Denmark, from 2005 to 2007.39
The results of the study showed an association between physical activity and myopia, suggesting a protective effect of physical activity on the development and progression of myopia. The results confirmed that intensive studying is a risk factor of myopia, and that students in the early twenties are more prone to developing myopia than are older students.
A multiple regression analysis showed that time spent reading scientific literature and younger age were both associated with a refractive change towards myopia (Table 4). Time spent being physically active was inversely associated with a refractive change towards myopia (estimated 0.175 D per hours of physical activity per day). This study did not distinguish between outdoor and indoor physical activity.
Table 4. Multiple linear regression analysis evaluating the association between personal characteristics and the refractive change from 2005 to 2007 in 143 students.
| Covariates | Estimate | 95% CI | P-value |
|---|---|---|---|
| Studying (hours per day) | −0.063 | −0.117;−0.008 | 0.024 |
| Physical activity (hours per day) | 0.175 | 0.035;0.315 | 0.015 |
| Age (years) | 0.023 | 0.003;0.043 | 0.022 |
| Gender | −0.087 | −0.278;0.105 | 0.373 |
| PC (hours per day) | 0.018 | −0.074;0.110 | 0.703 |
| Reading newspaper and so on (hours per day) | 0.017 | −0.147;0.181 | 0.835 |
| Weight (kg) | 0.003 | −0.003;0.009 | 0.268 |
| Height (cm) | 0.001 | −0.012;0.013 | 0.921 |
Dependent variable: refractive change from 2005 to 2007. N=143, R=0.298, R2=0.089, adjusted R2=0.069, SE=0.380. Bold values are statistically significant (P<0.05).
Since then, further studies have been carried out—some confirming the protective effect of physical activity and outdoor activities on the development and progression of myopia. However, the Sydney Myopia Study40 separately analysed sports performed outdoors, as well as outdoor leisure activities, and sports performed indoors. The study found that the important factor was total time spent outdoors, and that indoor sports were not protective against the development and progression of myopia.
In a recent study,41 the authors discussed possible mechanisms of this protective effect, and suggested that increased light intensity outdoors results in light-stimulated release of the retinal transmitter dopamine, which is known to be able to reduce axial elongation.
A prospective cohort study by Guggenheim et al42 reported a negative association between time spent in sports and outdoor activities and incident myopia, with the time spent outdoors having the greatest impact.
In a review and meta-analysis by Sherwin et al,43 the authors concluded that increasing the time spent outdoors may be a simple strategy by which to reduce the risk of developing myopia and its progression in adolescents and children.
Occupational myopia
A possible interaction between myopia development and certain visual demands has been studied intensively for a long time. The near-work theory was mainly based on the observations of a high prevalence of myopia among students or among workers with a short working distance, such as compositors.11
Occasionally, myopia in specific textile workers has been mentioned, with the first publication by Cramer in 1906.44 He described the working process in which young girls starting at the age of 14–15 years look for weaving faults in moving textiles, mark them, and later on repair the faults. Among 100 cloth garners, 69 were myopic with a degree of myopia between 0.75 and 9.0 D. Progression was seen until the age of 35. Only three subjects reported the onset of myopia during school years.
Cramer44 explained the myopia development with the ever-changing retinal image as the workers change fixation.
In a small study of similar working processes in a textile factory in Lillehammer, Norway, late-onset myopia was observed with a prevalence significantly higher than in a control group. The myopia was mainly caused by increase in the axial length45 (Tables 5 and 6 and Figure 2). During a later follow-up in which videos were taken of the process, it was demonstrated that the persons involved were moving their heads and eyes all the time to cover the 140–180 cm wide cloth.
Table 5. Myopisation and duration of activity in textile workers.
| Duration years | N | Emm % | Hpm % | Myopia % | Astigm % |
|---|---|---|---|---|---|
| 0–4 | 56 | 57.1 | 16.0 | 17.9 | 8.9 |
| 5–9 | 127 | 44.0 | 12.0 | 37.6 | 6.4 |
| 10–20 | 139 | 17.2 | 7.4 | 68.1 | 7.3 |
Joffe.48
Table 6. Occupational myopia.
| N | Axial length in mm mean | Refraction in D mean | P-value | |
|---|---|---|---|---|
| Textile workers | 11 | 24.4 | −2.56 | <0.01 |
| Matched controls | 11 | 22.7 | +1.19 | <0.01 |
Figure 2.
Late-onset myopia in textile workers controlling the texture for weaving faults and repairing the faults. The cause for myopia development could be a combination of eye and head movements with retinal image shift and variations in accommodation rate.
It seems that adult or late-onset myopia can develop and progress in association with certain special procedures in textile production. The cause for this development could be a combination of eye and head movements with changing retinal images and changing rate of accommodation.
A similar mechanism has been mentioned in association with Israeli Talmud students developing myopia (Table 7).46 Boys in Orthodox Schools differed from the other groups in terms of the following:
Sustained near vision (16 h a day)
Frequent changes in accommodation owing to the swaying habit during study
The variations in print size
The need for accurate accommodation when reading tiny print
Table 7. Prevalence and degree of myopia in Jewish teenagers.
| School | Sex | Number | % | Mean(SD) |
|---|---|---|---|---|
| General | F | 224 | 31.7 | −2.82 (0.22) |
| M | 175 | 27.4 | −2.10 (0.26) | |
| Orthodox | F | 278 | 36.3 | −2.70 (0.15) |
| M | 193 | 81.3 | −3.78 (0.18) |
Zylbermann et al.46
The myopia could be caused by the characteristic rocking back and forth of the upper torso creating a varying accommodative and convergent demand.
Conclusion
Genetics greatly influence the growth of the eye, but the fine correlation between the components of refraction, which is necessary for an eye to end in emmetropia, appears to be affected by environmental factors, such as education. This explains the ‘epidemic of myopia' in Far East Asia with a strong myopic shift from one generation to the next.
In individuals exposed to severe intoxication prenatally, the growth of the eye can be disturbed and can cause microphthalmus in addition to high myopia. Myopia of prematurity is an example of acquired myopia.47
The authors declare no conflict of interest.
Footnotes
Presented at 43rd Cambridge Ophthalmological Symposium 2013. Refractive Error.
References
- Guggenheim JA, McMahon G, Kemp JP, Akhtar S, St Pourcain B, Northstone K, et al. A genome-wide association study for corneal curvature identifies the platelet -derived growth factor receptor α gene as a quantitative trait locus for eye size in white Europeans. Mol Vis. 2013;19:243–253. [PMC free article] [PubMed] [Google Scholar]
- Verhoeven VJ, Hysi PG, Wojciechowski R, Fan Q, Guggenheim JA, Höhn R, et al. Genome-wide meta-analyses of multiancestry cohorts identify multiple new susceptibility loci for refractive error and myopia. Nat Genet. 2013;45 (3:314–318. doi: 10.1038/ng.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guggenheim JA, Zhou X, Evans DM, Timpson NJ, McMahon G, Kemp JP, et al. Coordinated genetic scaling of the human eye: shared determination of axial eye length and corneal curvature Invest Ophthalmol Vis Sci 2013. 754(31715–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Q, Wojciechowski R, Ikram MK, Cheng CY, Chen P, Zhou X, et al. Education influences the association between genetic variants and refractive error: a meta-analysis of five Singapore studies Hum Mol Genet 2013. e-pub ahead of print 6 September 2013 doi: 10.1093/hmg/ddt431 [DOI] [PMC free article] [PubMed]
- Negrel AD, Maul E, Pokharel GP, Zhao J, Ellwein LB. Refractive Error Study in Children: sampling and measurement methods for a multi-country survey. Am J Ophthalmol. 2000;129:421–426. doi: 10.1016/s0002-9394(99)00455-9. [DOI] [PubMed] [Google Scholar]
- Maul E, Barroso S, Munoz SR, Sperduto RD, Ellwein LB. Refractive Error Study in Children: results from La Florida, Chile. Am J Ophthalmol. 2000;129:445–454. doi: 10.1016/s0002-9394(99)00454-7. [DOI] [PubMed] [Google Scholar]
- Pokharel GP, Negrel AD, Munoz SR, Ellwein LB. Refractive Error Study in Children: results from Mechi Zone, Nepal. Am J Ophthalmol. 2000;129:436–444. doi: 10.1016/s0002-9394(99)00453-5. [DOI] [PubMed] [Google Scholar]
- Zhao J, Pan X, Sui R, Munoz SR, Sperduto RD, Ellwein LB. Refractive Error Study in Children: results from Shunyi district China. Am J Ophthalmol. 2000;129:427–435. doi: 10.1016/s0002-9394(99)00452-3. [DOI] [PubMed] [Google Scholar]
- He M, Zeng J, Liu Y, Xu J, Pokharel GP, Ellwein LB. Refractive error and visual impairment in urban children in southern China. Invest Ophthalmol Vis Sci. 2004;45:793–799. doi: 10.1167/iovs.03-1051. [DOI] [PubMed] [Google Scholar]
- Tscherning M. Studier Over Myopiens Aetiologi. C Myres Boghandel: Copenhagen, Denmark; 1882. [Google Scholar]
- Goldschmidt E. On the etiology of myopia. An epidemiological study. Acta Ophthalmol (Copenh) 1968;98 (Suppl:11–137. [PubMed] [Google Scholar]
- Jacobsen N, Jensen H, Goldschmidt E. Prevalence of myopia in Danish conscripts. Acta Ophthalmol Scand. 2007;85:165–170. doi: 10.1111/j.1600-0420.2006.00789.x. [DOI] [PubMed] [Google Scholar]
- Morgan I, Rose K. How genetic is school myopia. Prog Retin Eye Res. 2005;24:1–38. doi: 10.1016/j.preteyeres.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Young FA, Leary GA, Baldwin WR, West DC, Box RA, Harris E, et al. The transmission of refractive errors within eskimo families. Am J Optom Arch Am Acad Optom. 1969;46:676–685. doi: 10.1097/00006324-196909000-00005. [DOI] [PubMed] [Google Scholar]
- Morgan RW, Speakman JS, Grimshaw SE. Inuit myopia: an environmentally induced "epidemic". Can Med Assoc J. 1975;112:575–577. [PMC free article] [PubMed] [Google Scholar]
- Alsbirk P. Refraction in West Greenland eskimos. Acta Ophthalmologica. 1979;57:84–95. doi: 10.1111/j.1755-3768.1979.tb06663.x. [DOI] [PubMed] [Google Scholar]
- Guggenheim JA, Kirov G, Hodson SA. The heritability of high myopia: a reanalysis of Goldschmidt's data. J Med Genet. 2000;37:227–231. doi: 10.1136/jmg.37.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt E, Fledelius HC. Clinical features in high myopia. A Danish cohort study of high myopia cases followed from age 14 to age 60. Acta Ophthalmol. 2011;89:97–98. doi: 10.1111/j.1755-3768.2010.02104.x. [DOI] [PubMed] [Google Scholar]
- Sorsby A, Young FA. Transmission of refractive errors within Eskimo families. Am J Optom Arch Am Acad Optom. 1970;47:244–249. doi: 10.1097/00006324-197003000-00010. [DOI] [PubMed] [Google Scholar]
- Sorsby A, Sheridan M, Leary G.Refraction and its components in twinsMRC Report No 303.HMSO: London, UK; 1962 [Google Scholar]
- Hammond CJ, Snieder H, Gilbert CE, Spector TD. Genes and environment in refractive error: the twin eye study. Invest Ophthalmol Vis Sci. 2001;42:1232–1236. [PubMed] [Google Scholar]
- Lyhne N, Sjolie AK, Kyvik KO, Green A. The importance of genes and environment for ocular refraction and its determiners: a population based study among 20-45 year old twins. Br J Ophthalmol. 2001;85:1470–1476. doi: 10.1136/bjo.85.12.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt E, Lyhne N, Lam CSY. Ocular anisometropia and laterality. Acta Ophthalmol Scand. 2004;82 (2:175–178. doi: 10.1111/j.1600-0420.2004.00230.x. [DOI] [PubMed] [Google Scholar]
- Randall BA. The refraction of the human eye. Am J Med Sci. 1885;179:123–151. [Google Scholar]
- Cordain L, Eaton SB, Brand MJ, Lindeberg S, Jensen C. An evolutionary analysis of the aetiology and pathogenesis of juvenile-onset myopia. Acta Ophthalmol Scand. 2002;80:125–135. doi: 10.1034/j.1600-0420.2002.800203.x. [DOI] [PubMed] [Google Scholar]
- Cordain L. Cereal grains: humanity's double-edged sword. World Rev Nutr Diet. 1999;84:19–73. doi: 10.1159/000059677. [DOI] [PubMed] [Google Scholar]
- Ebbesson SO, Schraer CD, Risica PM, Adler AI, Ebbesson L, Mayer AM, et al. Diabetes and impaired glucose tolerance in three Alaskan Eskimo populations. The Alaska-Siberia Project. Diabetes Care. 1998;21:563–569. doi: 10.2337/diacare.21.4.563. [DOI] [PubMed] [Google Scholar]
- Daniel M, Rowley KG, McDermott R, Mylvaganam A, O'Dea K. Diabetes incidence in an Australian aboriginal population. An 8-year follow-up study. Diabetes Care. 1999;22:1993–1998. doi: 10.2337/diacare.22.12.1993. [DOI] [PubMed] [Google Scholar]
- Beck-Nielsen H, Pedersen O, Sorensen NS. Effects of diet on the cellular insulin binding and the insulin sensitivity in young healthy subjects. Diabetologia. 1978;15:289–296. doi: 10.1007/BF02573821. [DOI] [PubMed] [Google Scholar]
- Beck-Nielsen H, Pedersen O, Lindskov HO. Impaired cellular insulin binding and insulin sensitivity induced by high-fructose feeding in normal subjects. Am J Clin Nutr. 1980;33:273–278. doi: 10.1093/ajcn/33.2.273. [DOI] [PubMed] [Google Scholar]
- Jacobsen N, Jensen H, Lund-Andersen H, Goldschmidt E. Is poor glycaemic control in diabetic patients a risk factor of myopia. Acta Ophthalmol. 2008;86 (5:510–514. doi: 10.1111/j.1600-0420.2007.01104.x. [DOI] [PubMed] [Google Scholar]
- Fledelius HC, Miyamoto K. Diabetic myopia - is it lens-induced? An oculometric study comprising ultrasound measurements. Acta Ophthalmol (Copenh) 1987;65:469–473. doi: 10.1111/j.1755-3768.1987.tb07025.x. [DOI] [PubMed] [Google Scholar]
- Logstrup N, Sjolie AK, Kyvik KO, Green A. Long-term influence of insulin dependent diabetes mellitus on refraction and its components: a population based twin study. Br J Ophthalmol. 1997;81:343–349. doi: 10.1136/bjo.81.5.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantyjarvi M. Myopia and diabetes. A review. Acta Ophthalmol Suppl. 1988;185:82–85. doi: 10.1111/j.1755-3768.1988.tb02672.x. [DOI] [PubMed] [Google Scholar]
- Chen SJ, Tung TH, Liu JH, Lee AF, Lee FL, Hsu WM, et al. Prevalence and associated factors of refractive errors among type 2 diabetics in Kinmen, Taiwan. Ophthalmic Epidemiol. 2008;15 (1:2–9. doi: 10.1080/09286580701585736. [DOI] [PubMed] [Google Scholar]
- Parssinen O, Era P, Leskinen AL. Some physiological and psychological characteristics of myopic and non-myopic young men. Acta Ophthalmol Suppl. 1985;173:85–87. doi: 10.1111/j.1755-3768.1985.tb06852.x. [DOI] [PubMed] [Google Scholar]
- Parssinen O, Lyyra AL. Myopia and myopic progression among schoolchildren: a three-year follow-up study. Invest Ophthalmol Vis Sci. 1993;34:2794–2802. [PubMed] [Google Scholar]
- Mutti DO, Mitchell GL, Moeschberger ML, Jones LA, Zadnik K. Parental myopia, near work, school achievement, and children's refractive error. Invest Ophthalmol Vis Sci. 2002;43:3633–3640. [PubMed] [Google Scholar]
- Jacobsen N, Jensen H, Goldschmidt E. Does the level of physical activity in university students influence development and progression of myopia? a 2 year prospective cohort study. Invest Ophthalmol Vis Sci. 2008;49 (4:1322–1327. doi: 10.1167/iovs.07-1144. [DOI] [PubMed] [Google Scholar]
- Rose KA, Morgan IG, Ip J, Kifley A, Huynh S, Smith W, et al. Outdoor activity reduces the prevalence of myopia in children. Ophthalmology. 2008;115:1279–1285. doi: 10.1016/j.ophtha.2007.12.019. [DOI] [PubMed] [Google Scholar]
- French AN, Ashby RS, Morgan IG, Rose KA. Time outdoors and the prevention of myopia. Exp Eye Res. 2013;114:58–68. doi: 10.1016/j.exer.2013.04.018. [DOI] [PubMed] [Google Scholar]
- Guggenheim JA, Northstone K, McMahon G, Ness AR, Deere K, Mattocks C, et al. Time outdoors and physical activity as predictors of incident myopia in childhood: a prospective cohort study. Invest Ophthalmol Vis Sci. 14. 2012;53 (6:2856–2865. doi: 10.1167/iovs.11-9091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin JC, Reacher MH, Keogh RH, Khawaja AP, Mackey DA, Foster PJ. The association between time spent outdoors and myopia in children and adolescents. A systematic review and meta-analysis. Ophthalmology. 2012;119:2141–2151. doi: 10.1016/j.ophtha.2012.04.020. [DOI] [PubMed] [Google Scholar]
- Cramer E. Die Arbeitsmyopie der Tuchstopferinnen. Klin Monatsbl Augenheilk. 1906;44:60–67. [Google Scholar]
- Simensen B, Thorud LO. Adult onset myopia and occupation. Acta Ophthalm. 1994;72:469–471. doi: 10.1111/j.1755-3768.1994.tb02799.x. [DOI] [PubMed] [Google Scholar]
- Zylbermann R, Landau D, Berson D. The influence of study habits on myopia in Jewish teenagers. J Pediatr Ophthalmol Strabismus. 1993;30:319–322. doi: 10.3928/0191-3913-19930901-12. [DOI] [PubMed] [Google Scholar]
- Fledelius H. Prematurity and the eye. Acta Ophthalmol. 1976;128(Suppl):3–245. [PubMed] [Google Scholar]
- Joffe CM.Vestn Oftal1949281951-1994. (In Russian).