Abstract
For many individuals, the developmental trend of lessening hyperopia from birth continues past emmetropia towards myopia during childhood. The global pattern for prevalence of refractive errors indicates that the prevalence of hyperopia is low; in contrast, the burden of myopia is on the rise because of rising prevalence and magnitude of myopia. This review highlights the need to lessen the global burden of myopia by intervening with the development and/or slowing the progression of myopia. Further, outcomes from human clinical trials of pharmaceutical, optical, and environmental approaches to control myopia will be summarised. Pharmaceutical treatments are effective in controlling eye growth but are associated with deleterious side effects. Optical strategies that induce myopic defocus at the retina such as peripheral defocus reducing lenses, simultaneous defocus lenses, bifocals, and orthokeratology as well as environmental influences such as increased outdoor activity show promise and provide a substantially risk-free environment in which to control eye growth.
Keywords: ametropia, hyperopia, optical intervention, myopia, myopic defocus
Background
The prevalence of ametropia (that is, mostly the refractive errors of myopia, hyperopia, and astigmatism) varies based on age and ethnicity, and therefore it is difficult to accurately calculate the number of those affected worldwide. However, proceeding with the understanding that those with significant ametropias are likely to have poor vision, it was estimated that in the year 2003, there were more than 2.3 billion people worldwide (37% of world's population) that were suffering from poor vision due to refractive error.1 Of the ametropias, hyperopia is the routinely observed refractive error in neonates with a trend towards lessening hyperopia in the first few years of life. Table 1 details the prevalence of hyperopia over 2.0 D across various populations from 5 years of age. The global pattern indicates that by age 12–15, the prevalence of the condition has fallen off substantially to low levels.2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12 Although moderate-to-high hyperopia may coexist with strabismus5 and associated with the risk of developing amblyopia5 or angle closure glaucoma in later life, it appears that, given the relatively low prevalence of the condition, there is a paucity of information relating to the condition and its risk factors, and thus strategies to modify and control hyperopia have been limited. There exist claims in the patent literature for modifying or preventing hyperopia with, for example, use of filters, dual focus or diffractive lenses, use of TGF-β activation inhibitor, administration of GABA receptor agonists or antagonists, and by limiting the amount of spherical aberration of the eye to less than +0.50 D; however, there have been no reports or data from human clinical studies that have considered these strategies.13, 14, 15, 16, 17
Table 1. Prevalence of hyperopia (≥+2.0 D).
| Authors | Region | Age group (years) | Prevalence of hyperopia |
|---|---|---|---|
| He et al2 | Urban China | 5 | 16.7% |
| 15 | <1% | ||
| He et al10 | Rural China | 15 | 1.5% |
| Murthy et al3 | Urban India | 5 | 15.6% |
| 15 | 10.8% | ||
| Dandona et al11 | Rural India | 7 | 0.7% |
| 15 | 1.2% | ||
| Fan et al4 | Hong Kong | 5–16 | 4.0% |
| Ip et al5 | Australia | 6 | 13.2% |
| 12 | 5.0% | ||
| Goh et al6 | Malaysia | 7 | 3.8% |
| 15 | 1% | ||
| Naidoo et al7 | South Africa | 6 | 2.4% |
| 12 | 3.2% | ||
| 15 | 0.7% | ||
| Maul et al8 | Chile | 5–7 | 21.6% |
| 14–15 | 7.5% | ||
| Zadnik et al9 (≥+1.25 D) | USA | 10±2.3 | 8.6% |
| Sapkota et al12 | Urban Nepal | 10 | 1.0% |
| 15 | 0.6% |
Astigmatism is generally seen to coexist with other refractive errors but the prevalence appears to vary widely in children aged 5–15 years. (Table 2).2, 3, 4, 6, 7, 8, 10, 11, 12, 18, 19 Although there are suggestions that astigmatism especially ‘against-the rule', might have a role in development of myopia,20 studies on this relationship have been equivocal.21, 22
Table 2. Prevalence of astigmatism (≥0.75 D, auto refraction values where provided).
| Authors | Region | Age group (years) | Prevalence of astigmatism |
|---|---|---|---|
| He et al2 | Urban China | 5–15 | 42.7% (either eye) |
| He et al10 | Rural China | 13 | 21.6% |
| 14 | 24.8% | ||
| 15 | 25.8% | ||
| 16 | 24.8% | ||
| 17 | 33.3% | ||
| Murthy et al3 | Urban India | 5 | 14.6% (either eye) |
| 15 | |||
| Dandona et al11 | Rural India | 7–15 | 9.7% (either eye) |
| Fan et al4 | Hong Kong | 5–16 | |
| Huynh et al18 | Australia | 6 | 4.8% (right eye) |
| 12 | 6.7% (right eye) | ||
| Goh et al6 | Malaysia | 7–15 | 21.3% (either eye) |
| Naidoo et al7 | South Africa | 6–15 | 14.6% (either eye) |
| Maul et al8 | Chile | 5–15 | 27% (either eye) |
| Salomão et al19 | Brazil | 11–14 | 4.6% (right eye) |
| Sapkota et al12 | Nepal | 10–15 | 7.4% (either eye) |
Significantly, the evolutionary trend with age towards lessening hyperopia or increasing myopia continues for many during childhood with an increasing number of children becoming myopic with each passing year. As discussed in later sections of the article, there is high prevalence of myopia in many parts of the world and for those affected, myopia generally progresses or increases in magnitude each year during childhood before possibly stabilising in late adolescent years. This increasing magnitude of myopia increases the risk of developing further disorders, some of which are sight threatening.
As it appears that the burden of ametropia is mostly due to myopia, this article explores the need for controlling or modifying the development and progression of myopia and the attempts that have been made to date in controlling childhood.
Why control myopia?
It may be argued that the transition of the human society from an outdoor-oriented, hunter-gatherer-farming society to more indoor-oriented, technology, and desk-based society in the modern world obviates the need for clear distance vision and shifts the focus to more intermediate and near vision. The rural-urban divide in the prevalence of myopia, the intergenerational shift towards increasing prevalence of myopia provides support for this adaptive process.2, 3, 10, 11, 23 However, alarmingly, this myopic shift appears to be insidious in its development and progression. The prevalence of the condition has reached significant proportions in some East Asian countries, with nearly 80% of those aged 18 years and over myopic.2, 24, 25, 26 The evidence for the rise in prevalence of myopia is compelling and is also evident in the Western population27, 28 and suggests that in addition to increasing prevalence, there is a shift in recent decades to an earlier onset of myopia and increased prevalence of myopia at any given age comparable to that seen in previous generations resulting in a higher prevalence of highly myopic eyes. In a study of 395 children and their families, it was found that at the age of 11 years, the children's refractive errors were similar to those of their parents and it was estimated that at 18 years of age, the children would be more myopic by ∼2.0 D compared with their parent's refractive error.29 In a study involving randomly selected preschool children (age 4.6±0.9 years), the prevalence of myopia in 1996–1997 was 2.3% but was 6.3% in 2006–2007.30 Five nationwide myopia surveys conducted over a 20-year period in Taiwan showed a significant myopic shift in refraction from 1983 to 2000. In addition, the study also reported that in 1983, the age at which a myopic refraction was first registered was 11 years old and this shifted to 8 years old in 2000.24 This trend towards an earlier onset and a more myopic refraction appears to be associated with a concomitant rise in the prevalence of high myopia (>−6.0 D).24
Each dioptre increase in myopia increases the risk of developing structural changes at the retina and optic disc, and the risk of developing sight-threatening eye disorders and high myopia is now a leading cause of visual impairment and blindness in many countries across Asia.31, 32, 33 In addition, these highly myopic eyes are likely to experience reduced visual performance due to spectacle minification and reduced neural sampling density associated with retinal stretching.34, 35 Cone photoreceptor packing density (cells per square millimetre) was shown to be significantly lower in myopic eyes than in emmetropic eyes.36
Thus, there is an urgent need to intervene with both development and/or progression of myopia to reduce the burden and prevent the pathological consequences associated with high levels of myopia.
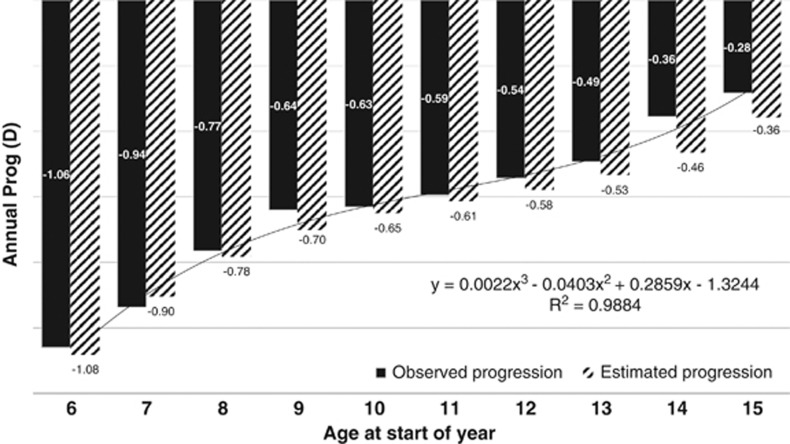
Figure 1 provides the annual and estimated progression data for 508 spectacle wearers of mostly Asian ethnicity (data from Guangzhou, China, Hong Kong, and Singapore) aged 6–16 years. These data indicate that the younger the onset of myopia, the greater the annual progression and greater the risk of having high levels of myopia. An intervention that can delay the onset of myopia by 1 or 2 years can significantly delay or prevent the eye from reaching −6.0 D or more of myopia.
Figure 1.
Annual progression data (observed and estimated) for children with myopia aged 6–12 years.
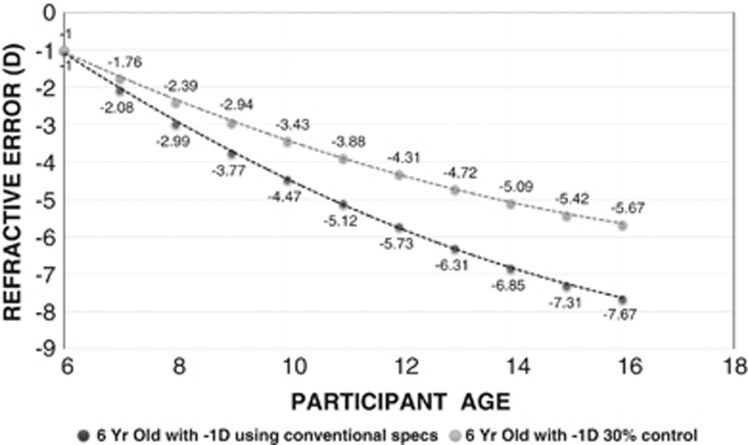
Using the estimated progression data, it can be seen that a 6-year old with −1.0 D myopia is likely to reach −6.0 D at 12.9 years of age and −7.0 D at 14.6 years of age (Figure 2). Using these estimates, an intervention that can slow the rate of progress by 30% delays the age at which the eye reaches −6.0 D to past 16 years of age. Assuming that the progression of the eye stabilises after 16 years, this eye is unlikely to become −7.0 D.
Figure 2.
Estimated refractive error progression for a 6-year old with conventional correction and a myopia control strategy with 30% slowing of progression.
Myopia control strategies
Commonly used optical devices such as spectacles and contact lenses employ the use of concave lenses to correct for the refractive error and restore normal vision but do not counter the mechanism driving the axial length increase that effects the increase in myopia.
Although both genetic and environmental factors appear to have a role in the development and progression of myopia, the overwhelming evidence that ocular growth can be modulated by environmental influences, for example, form deprivation and optical defocus models, has provided avenues for strategies to modify myopia.
In addition, over the years, a number of interventions have been trialled to delay the onset or slowing the progression of myopia in humans. Table 3 lists the results from human clinical trials with interventions mainly aimed at slowing the progress of myopia. Each of the strategies are further explored in the section below.
Table 3. Myopia control strategies in human clinical studies (randomised or comparitive studies).
| Authors | Intervention | Control group | Progression(test vs control D) | % Slowing of myopia progression |
|---|---|---|---|---|
| Wu et al45 | Outdoor activity- 80 min per day | Yes | −0.25 vs −0.38 D | 34% (1 year) |
| Morgan et al46 | Outdoor activity- 60 min per day | Yes | −0.75±0.69 vs −0.86±0.77 D | 15% (2 years) |
| Chung et al47 | Undercorrection +0.75 D | Fully corrected SPL | −1.00±0.33 vs−0.77±0.33 D | Worsening myopia 29% (1 year) |
| Adler and Millidot48 | Undercorrection +0.50 D | Fully corrected SPL | −0.99±0.35 vs −0.82±0.38 D | Worsening myopia 20% (1 year) |
| Yang et al55 | PAL +1.50 D | Single-vision SPL | −1.24±0.56 vs −1.50±0.67 D | 17% (2 years) |
| COMET 258 | PAL +2.00 D | Single-vision SPL | −0.87 vs −1.15 D | 24% (3 years) |
| Gwiazda et al54 | PAL +2.00 D | Single-vision SPL | −1.28±0.06 vs −1.48±0.06 D | 14% (3 years) |
| Hasebe et al56 | PAL +1.50 D | Single-vision SPL | −0.89±0.06 vs −1.20±0.08 D | 26% (18 months) |
| Edwards et al57 | PAL +1.50 D | Single-vision SPL | −1.12±0.67 vs −1.26±0.74 D | 11% (2 years) |
| Cheng et al59 | Exec bifocal +1.50 D | Single-vision SPL | −0.96±0.09 (B/F) vs | 38% (B/F) |
| Exec bifocal +1.50 D and 3Δ D | −0.70±0.10 (B/FwithΔ) vs | 56% (B/F with Δ) 2 years | ||
| Base in | −1.55±0.12 D | |||
| Fulk et al50 | Bifocal spectacles | Single-vision SPL | −0.99±0.68 vs −1.24±0.65 D | 20% (2.5 years) |
| Chua et al72 | 1% atropine | Placebo treated | −0.28±0.92 vs | 72% (2 years) |
| −1.20±0.69 D | ||||
| Chia et al73 | 0.5, 0.1, 0.01 atropine | No | −0.30±0.60 D (0.5%) | — |
| −0.38±0.60 D (0.1%) | ||||
| −0.49±0.63 D (0.01%) | ||||
| Tan et al66 | 2% gel twice daily | Placebo treated | −0.47 D (gel/gel) | 44% (1 year) |
| 2% gel once daily, once | −0.70 D (gel/placebo) | 17% | ||
| Placebo; placebo twice daily | −0.84 D (placebo/placebo) | |||
| Siatkowski et al65 | Pirenzepine 2% | Placebo treated | −0.58 vs −0.99 D | 41% (2 years) |
| Sankaridurg et al68 | Novel designs I, II, III | Single-vision SPL | −0.81±0.43 (I) vs | 15% (1 yr, Lens III) |
| −0.81±0.46 (II) vs | ||||
| −0.66±0.41 (III) vs | ||||
| −0.78±0.50 D (control) | ||||
| Sankaridurg et al69 | Novel contact lens design | Single-vision SPL | −0.57±0.33 vs −0.84±0.47 D | 34% (1 year) |
| Anstice and Phillips71 | Dual focus lens +2.0 D | Single-vision CL | −0.44±0.33 vs −0.69±0.38 D | 36% (10 months) |
Abbreviations: CL, contact lens; SPL, spectacle.
Outdoor activity
It has been long held that near-work activity is associated with myopia, and recent epidemiological evidence indicates that outdoor activity, rather than indoor activity, appears to be protective for development of myopia.37, 38, 39 In addition, it appears that progression of myopia might be slower in summer months compared with other months40, 41 indicating a possible protective role for light, and some preliminary work with animal models has shown that bright light can prevent the development of form deprivation myopia.42, 43 Although the exact mechanism by which outdoor activity mediates development and progression of refractive errors remains unclear, elevation of retinal dopamine activity, reduced accommodative response, and spectral composition of light have been suggested as possible factors.44 Although it is not clear from the above that outdoor activity per se influences the progression of myopia, clinical trials have been undertaken to determine whether outdoor activity reduces the progression of myopia.45, 46 Table 1 summarises the results from two clinical studies. Although both report a difference in the progression of myopia with improved outdoor activity of as little as 60–80 min, the dioptric difference at the end of the treatment period was <0.25 D and not of clinical relevance. However, of interest is the finding of a decrease in the number of new myopes during the study period. After 1 year, in the study by Wu et al,45 prevalence of new myopia was 8.4% in the group randomised to outdoor activity compared with 17.7% in the control group. In the other study39 at 2 years, the prevalence of myopia was 25.2% in the group randomised to outdoor activity compared with 20.7% in the control group.
Undercorrection
Undercorrection of myopia is a widely practiced procedure that has been in use for many years. The objective of undercorrection is to achieve (a) the goal of myopic defocus, which in animal models has demonstrated a reduction in progression of myopia and (b) a reduction of stress on accommodation in near-point environments. However, data from prospective clinical trials suggest that the procedure may, in fact, result in acceleration of the progression of myopia (Table 1). Chung et al47 showed that undercorrection of myopia by ∼0.75 D compared with full correction actually worsened myopia by 0.23 D over a period of 2 years.47 Similar results were seen in a later study wherein eyes that were undercorrected by 0.50 D showed an increase in myopic progression compared with those who were fully corrected.48
Bifocal and progressive addition spectacles
Bifocal spectacles are another of the earliest procedures aimed at controlling the progression of myopia. The rationale of the approach is to reduce accommodative stress at near point. Indeed, myopic eyes are said to have greater lag of accommodation compared with non-myopic eyes with resultant hyperopic defocus at near.49 Clinical trials were conducted using bifocal spectacles50, 51, 52 with an aim to reduce the accommodative demand but the results were equivocal with some studies showing benefit and others reporting no significant difference. It was suggested that poor compliance and/or improper alignment of the spectacles might have confounded results. In 1999, progressive addition spectacles (PALs) were suggested as an alternative, as they allow clear vision at all working distances while maintaining the ability to reduce accommodative demand. Although an initial trial found PALs to slow the progress of myopia significantly in comparison with single-vision spectacles,53 a large, prospective, multicentre trial conducted in the United States (COMET study) found that the difference in terms of dioptric values between eyes wearing PALs and single-vision spectacles was too small to be of any clinical significance.54 Other studies found similar results with a small difference between PAL and conventional lenses.55, 56, 57 Similarly, a more recent trial involving high-risk groups that is, children with high accommodative lags and near-point esophoria also failed to produce any significant benefits.58 A more recent trial involving children with myopia randomised to wear single-vision spectacles or executive bifocals with +1.50 D add or +1.50 D add with 3Δ base in prism found that over a period of 24 months, myopia progression was on average −1.55 D for those wearing single-vision spectacles, −0.96 D for those with executive bifocals, and −0.70 D for those with executive bifocals with base in prisms.59 Although the spherical equivalent data showed a greater benefit with executive bifocals incorporating prisms, axial length change showed similar changes with and without prisms (0.62 mm increase with single vision vs 0.41 mm with bifocals and 0.41 mm with bifocals and prism). Even after taking into account the differences in efficacy for axial length and spherical equivalent measures, the slowing of progression is substantial and of significance. The reasons for these results remain unclear but could possibly be attributed to factors such as improved effect because of the larger segment size of the bifocal and the plus segment imposing myopic defocus on the peripheral retina. In addition, the use of bifocal contact lenses has also been investigated and found to be effective in slowing the progression of myopia.60
Pharmaceutical agents—atropine and pirenzepine
The initial premise for using atropine was its ability to reduce the accommodative effort. In clinical studies, atropine 1% was found to be quite effective in retarding progression of myopia in children61 but the side effects and inconvenience associated with its long-term use has limited its uptake, especially in Western countries. In addition, rebound of myopia was found to occur upon discontinuation of the drug.62 However, in countries such as Taiwan, where the prevalence of myopia is in epidemic proportions with a lack of alternatives to stem the tide, the use of atropine is popular and on the rise.62 More recent studies with low-dose atropine of 0.5, 0.1, and 0.01% show mean progression of myopia at 2 years to be −0.30±0.60, −0.38±0.60, and −0.49±0.63 D with the above doses compared with −0.28±0.92 with atropine 1% and −1.20±0.69 D with a placebo group.63 Yet, it remains to be seen whether rebound also occurs with low doses. Atropine prevents myopia in a chick model that incorporates a striated ciliary muscle innervated by nicotinic receptors rather than muscarinic receptors. This, therefore, suggests that the mechanism of action is likely to be non-accommodative, possibly affecting the retina and/or sclera.64
In addition to atropine, pirenzepine a selective M1 receptor antagonist was evaluated in clinical trials as it was considered less likely to induce mydriasis and cycloplegia. Pirenzepine was effective in reducing the progression (see Table 1); however, pirenzepine is not yet commercially available.65, 66
Peripheral retinal defocus
There is accumulating evidence for the role of the peripheral retina and peripheral vision in the development and progression of refractive errors. Primate studies indicate that form deprivation at the peripheral retina produced axial myopia despite of clear vision at the fovea and foveal ablation did not disrupt the emmetropization process.67 Specifically, human myopic eyes were found to have hyperopic defocus at the peripheral retina relative to the centre, and the presence of continuing hyperopic retinal defocus at the periphery with conventional corrective devices has been thought to influence progression. Human clinical trials with treatment strategies aimed at reducing the peripheral retinal hyperopic defocus show that it may be possible to slow the progression of myopia. After 12 months of spectacle lens wear, progression of myopia with a rotationally asymmetrical novel spectacle lens was not significant with all myopia. However, for children aged 6–12 years whose parents are myopic, spectacle wear was found to reduce the progression of myopia significantly when higher rates of progression were evident.68 Similarly, the rate of progression of myopia was reduced by ∼30% in eyes wearing contact lenses designed to reduce hyperopic defocus compared with single-vision spectacles.69 Also of interest, orthokeratology has also been found to be effective in reducing progression of myopia, and it has been suggested that the underlying mechanism involves reduction in peripheral hyperopic defocus. One 5 year study found an increase in axial length of 0.99±0.47 and 1.41±0.68 mm for orthokeratology groups and a control group of spectacle lens wearers and indicates a significant slowing of ocular growth with orthokeratology.70
Simultaneous defocus
The rationale for simultaneous defocus is that, in addition to having a clear image on the retina, simultaneously having another image in myopic defocus serves as a signal to confuse and stop, or delay, eye growth. In a prospective, contralateral clinical study, one eye of each participant wore a dual focus contact lens with +2.0 D of myopic defocus for 10 months, whereas the other eye wore a single-vision contact lens. After 10 months, there was a significant slowing of myopia in the eyes that wore dual focus lenses (average of −0.44 D progression in the dual focus lens-wearing eye vs −0.69 D in the control lens-wearing eye).71
Conclusions
Clearly, there is a need to control the progression of myopia to prevent or limit the morbidity associated with rising myopia. Although a number of approaches have been explored to control myopia, many of these are directed at slowing the progress of myopia rather than delaying the onset of myopia. Although the pharmaceutical agents are able to slow the progress significantly, problems associated with their use have limited their implementation. At the present time, of the optical strategies, contact lenses that induce myopic defocus (peripheral retinal defocus reducing lenses, simultaneous defocus lenses, and orthokeratology) appear to be superior in slowing the progress compared with spectacles but this might simply be related to close alignment of the contact lens with the eye, thus eliminating the possibility of aberrations with eye movements and also ensuring constant delivery of stimulus for as long as the lens is worn. Although these results need validation and also longer-term data, overall, the optical strategies appear to operate in an environment with minimal to nil risk compared with drugs and thus can be considered for incorporation into day-to-day practice. In addition, although the role of outdoor activity in the onset and progression of myopia needs further exploration, the use of environmental influences is an exciting and promising avenue in the quest to reduce the rate of progression of myopia.
Acknowledgments
The work is supported by grants from the Australian Federal Government through the CRC scheme and the Brien Holden Vision Institute.
The authors declare no conflict of interest.
Footnotes
The subject of this article was presented at the 2013 Cambridge Ophthalmological Symposium held at Cambridge 12–13 September 2013.
References
- Naidoo KS, Jaggernath J. Uncorrected refractive errors. Ind J Ophthalmol. 2012;60:432–437. doi: 10.4103/0301-4738.100543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M, Zeng J, Liu Y, Xu J, Pokharel GP, Ellwein LB. Refractive error and visual impairment in urban children in southern China. Invest Ophthalmol Vis Sci. 2004;45:793–799. doi: 10.1167/iovs.03-1051. [DOI] [PubMed] [Google Scholar]
- Murthy GVS, Gupta SK, Ellwein LB, Munoz SR, Pokharel GP, Sanga L, et al. Refractive error in children in an urban population in New Delhi. Invest Ophthalmol Vis Sci. 2002;43:623–631. [PubMed] [Google Scholar]
- Fan DS, Lam DSC, Lam RF, Lau JTF, Chong KS, Cheung EYY, et al. Prevalence, incidence and progression of myopia in school children in Hong Kong. Invest Ophthalmol Vis Sci. 2004;45:1071–1075. doi: 10.1167/iovs.03-1151. [DOI] [PubMed] [Google Scholar]
- IP JM, Robaei D, Kifley A, Wang JJ, Rose KA, Mitchell P. Prevalence of hyperopia and associations with eye findings in 6 and 12 year olds. Ophthalmology. 2008;115:678–685. doi: 10.1016/j.ophtha.2007.04.061. [DOI] [PubMed] [Google Scholar]
- Goh PP, Abqariyah Y, Pokharel GP, Ellwein LB. Refractive error and visual impairment in school-age children in Gombak District, Malaysia. Ophthalmology. 2005;112:678–685. doi: 10.1016/j.ophtha.2004.10.048. [DOI] [PubMed] [Google Scholar]
- Naidoo KS, Raghunandan KP, Mashige KP, Govender P, Holden BA, Pokharel GP, et al. Refractive error and visual impairment in African children in South Africa. Invest Ophthalmol Vis Sci. 2003;44:3764–3770. doi: 10.1167/iovs.03-0283. [DOI] [PubMed] [Google Scholar]
- Maul E, Barroso S, Munoz SR, Sperduto RD, Ellwein LB. Refractive error study in children. Results from La Florida, Chile. Am J Ophthalmol. 2000;129:445–454. doi: 10.1016/s0002-9394(99)00454-7. [DOI] [PubMed] [Google Scholar]
- Zadnik K, Manny RE, Yu JA, Mitchell GL, Cotter SA, Quiralte JC, et al. Ocular component data in schoolchildren as a function of age and gender. Optom Vis Sci. 2003;80:226–236. doi: 10.1097/00006324-200303000-00012. [DOI] [PubMed] [Google Scholar]
- He M, Huang W, Zheng Y, Huang L, Ellwein LB. Refractive error and visual impairment in school children in rural southern China. Ophthalmology. 2007;114:374–382. doi: 10.1016/j.ophtha.2006.08.020. [DOI] [PubMed] [Google Scholar]
- Dandona R, Dandona L, Srinivas M, Saharae P, Narsaiah S, Munoz SR, et al. Refractive error in children in a rural population in India. Invest Ophthalmol Vis Sci. 2002;43:615–622. [PubMed] [Google Scholar]
- Sapkota YD, Adhikari BN, Pokharel GP, Poudyal BK, Ellwein LB. The prevalence of visual impairment in school children of upper-middle socioeconomic status in Kathmandu. Ophthalmic Epidemiol. 2008;15:17–23. doi: 10.1080/09286580701772011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland S.US 5838419. Method and apparatus for treating refractive eye abnormalities. USPTO patent full-text and image database accessed on http://patft.uspto.gov/ .
- To CH, Lam SY, Wan SK.US7506983. Method of optical treatment. USPTO patent full-text and image database http://patft.uspto.gov/ .
- Honda S, Ogawa T, Watanabe N.US6010699. Method for controlling axial length of the eye. USPTO patent full-text and image database http://patft.uspto.gov/ .
- Collins MJ, Wildsoet C.US 6045578. Optical treatment method. USPTO patent full-text and image database http://patft.uspto.gov/ .
- Laties AM, Stone RA.US5385939. GABA-ergic modulation of eye growth. USPTO patent full-text and image database http://patft.uspto.gov/ .
- Huynh SC, Kifley A, Rose KA, Morgan IG, Mitchell P. Astigmatism in 12 year old Australian children: comparisons with a 6 year old population. Invest Ophthalmol Vis Sci. 2007;48:73–82. doi: 10.1167/iovs.06-0263. [DOI] [PubMed] [Google Scholar]
- Salomão SR, Cinoto RW, Berezovsky A, Mendieta L, Nakanami CR, Lipener C, et al. Prevalence and causes of visual impairment in low-middle income school children in Sao Paulo, Brazil. Invest Ophthalmol Vis Sci. 2008;49:4308–4313. doi: 10.1167/iovs.08-2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwiazda J, Grice K, Held R, McLellan J, Thorn F. Astigmatism and the development of myopia in children. Vision Res. 2000;40:1019–1026. doi: 10.1016/s0042-6989(99)00237-0. [DOI] [PubMed] [Google Scholar]
- Heidary G, Ying GS, Maguire MG, Young TL. The association of astigmatism and spherical refractive error in a high myopia cohort. Optom Vis Sci. 2005;82:244–247. doi: 10.1097/01.opx.0000159361.17876.96. [DOI] [PubMed] [Google Scholar]
- Leung TW, AK LAM, Deng L, Kee CS. Characteristics of astigmatism as a function of age in a Hong Kong clinical population. Optom Vis Sci. 2012;89:984–992. doi: 10.1097/OPX.0b013e31825da156. [DOI] [PubMed] [Google Scholar]
- Wu MM, Edwards MH. The effect of having myopic parents: an analysis of myopia in three generations. Optom Vis Sci. 1999;37:952–957. doi: 10.1097/00006324-199906000-00018. [DOI] [PubMed] [Google Scholar]
- Lin LL, Chen CJ, Hung PT, Ko LS. Nation-wide survey of myopia among schoolchildren in Taiwan, 1986. Acta Ophthalmol Suppl. 1988;185:29–33. doi: 10.1111/j.1755-3768.1988.tb02657.x. [DOI] [PubMed] [Google Scholar]
- Lin LL, Shih YF, Hsiao CK, Chen CJ. Prevalence of myopia in Taiwanese schoolchildren: 1983 to 2000. Ann Acad Med Singapore. 2004;33:27–33. [PubMed] [Google Scholar]
- Sun J, Zhou J, Zhao P, Lian J, Zhu H, Zhou Y, et al. High prevalence of myopia and high myopia in 5060 Chinese University students in Shanghai. Invest Ophthalmol Vis Sci. 2012;53:7504–7509. doi: 10.1167/iovs.11-8343. [DOI] [PubMed] [Google Scholar]
- Vitale S, Sperduto RD, Ferris FL.3rd. Increase prevalence of myopia in the United States between 1971–9172 and 1999–2004 Arch Ophthalmol 20091271632–1639. [DOI] [PubMed] [Google Scholar]
- Kempen JH, Mitchell P, Lee KE, Tielsch JM, Broman AT, Taylor HR, et al. The prevalence of refractive errors among adults in the United States, Western Europe and Australia. Arch Ophthalmol. 2004;122 (4:495–505. doi: 10.1001/archopht.122.4.495. [DOI] [PubMed] [Google Scholar]
- Laing YB, Lin Z, Vasudevan B, Jahanki V, Young A, Gao TY, et al. Generational difference of refractive error in the baseline study of the Beijing Myopia Progression Study. Br J Ophthalmol. 2013;97:765–769. doi: 10.1136/bjophthalmol-2012-302468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan DSP, Lai C, Lau HHW, Cheung EYY, Lam DSC. Change in vision disorders among Hong Kong pre-schoolers in 10 years. Clin Exp Ophthalmol. 2011;39:398–403. doi: 10.1111/j.1442-9071.2010.02470.x. [DOI] [PubMed] [Google Scholar]
- Hsu WM, Cheng JH, Liu JH, Tsai SY, Chou P. Prevalence and causes of visual impairment in an elderly Chinese population in Taiwan: the Shihpai Eye Study. Ophthalmology. 2004;111:62–69. doi: 10.1016/j.ophtha.2003.05.011. [DOI] [PubMed] [Google Scholar]
- Van Newkirj MR. The Hong Kong vision study: a pilot assessment of visual impairment in adults. Trans Am Ophthalmol Soc. 1997;95:715–749. [PMC free article] [PubMed] [Google Scholar]
- Iwase A, Araie M, Tomidokoro A, Yamamoto T, Shimizu H, Kitazawa Y, Tajimi Study Group Prevalence and causes of low vision and blindess in a Japanese adult population: the Tajimi study. Ophthalmology. 2006;113:1354–1362. doi: 10.1016/j.ophtha.2006.04.022. [DOI] [PubMed] [Google Scholar]
- Atchison DA, Schmid KL, Pritchard N. Neural and optical limits to visual performance in myopia. Vision Res. 2006;46:3707–3722. doi: 10.1016/j.visres.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Strang NC, Winn B, Bradley A. The role of neural and optical factors in limiting visual resolution in myopia. Vision Res. 1998;38:1712–1721. doi: 10.1016/s0042-6989(97)00303-9. [DOI] [PubMed] [Google Scholar]
- Chui TY, Song H, Burns SA. Individual variations in human cone photoreceptor packing density: variations with refractive error. Invest Ophthalmol Vis Sci. 2008;49:4679–4687. doi: 10.1167/iovs.08-2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose KA, Morgan IG, Ip J, Kifley A, Huynh S, Smith W, et al. Outdoor activity reduces the prevalence of myopia in children. Ophthalmology. 2008;115:1279–1285. doi: 10.1016/j.ophtha.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Dirani M, Tong L, Gazzard G, Zhang X, Chia A, Young TL, et al. Outdoor activity and myopia in Singapore teenage children. Br J Ophthalmol. 2009;93:997–1000. doi: 10.1136/bjo.2008.150979. [DOI] [PubMed] [Google Scholar]
- Guo Y, Liu LJ, Xu L, Lv YY, Tang P, Feng Y. Outdoor activity and myopia among primary students in rural and urban regions of Beijing. Ophthalmology. 2013;120:277–283. doi: 10.1016/j.ophtha.2012.07.086. [DOI] [PubMed] [Google Scholar]
- Fulk GW, Cyert LA, Parker DA. Seasonal variation in myopia progression and ocular elongation. Optom Vis Sci. 2002;79:46–51. doi: 10.1097/00006324-200201000-00012. [DOI] [PubMed] [Google Scholar]
- Donovan LA, Sankaridurg P, Ho A, Chen X, Lin Z, Thomas V, et al. Myopia progression in Chinese children is slower in summer than in winter. Optom Vis Sci. 2012;89:1196–1202. doi: 10.1097/OPX.0b013e3182640996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EL, 3rd, Hung LF, Huang J. Protective effects of high ambient lighting on the development of form-deprivation myopia in rhesus monkeys. Invest Ophthalmol Vis Sci. 2012;53:421–428. doi: 10.1167/iovs.11-8652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashby RS, Schaeffel F. The effect of bright light on lens compensation in chicks. Invest Ophthalmol Vis Sci. 2010;51:5247–5253. doi: 10.1167/iovs.09-4689. [DOI] [PubMed] [Google Scholar]
- French AN, Ashby RS, Morgan IG, Rose KA. Time outdoors and the prevention of myopia. Exp Eye Res. 2013;114:58–68. doi: 10.1016/j.exer.2013.04.018. [DOI] [PubMed] [Google Scholar]
- Wu PC, Tsai CL, Wu HL, Yang YH, Kuo HK. Outdoor activity during class recess reduces myopia onset and progression in school children. Ophthalmology. 2013;120 (5:1080–1085. doi: 10.1016/j.ophtha.2012.11.009. [DOI] [PubMed] [Google Scholar]
- Morgan IG, Xiang F, Rose KA, Chen Q, He M.Two year results from the Guangzhou Outdoor Activity Longitudinal Study (GOALS). ARVO E abstract 27352012
- Chung K, Mohidin N, O'Leary DJ. Undercorrection of myopia enhances rather than inhibits myopia progression. Vision Res. 2002;42:2555–2559. doi: 10.1016/s0042-6989(02)00258-4. [DOI] [PubMed] [Google Scholar]
- Adler D, Millidot M. The possible effect of undercorrection on myopic progression in children. Clin Exp Optom. 2006;89 (5:315–321. doi: 10.1111/j.1444-0938.2006.00055.x. [DOI] [PubMed] [Google Scholar]
- Gwiazda J, Thorn F, Bauer J, Held R. Myopic children show insufficient accommodative response to blur. Invest Ophthalmol Vis Sci. 1993;34:690–694. [PubMed] [Google Scholar]
- Fulk GW, Cyert LA, Parker DE. A randomized trial of the effect of single-vision vs bifocal lenses on myopia progression in children with esophoria. Optom Vis Sci. 2000;77 (8:395–401. doi: 10.1097/00006324-200008000-00006. [DOI] [PubMed] [Google Scholar]
- Mandell RB. Myopia control with bifocal correction. Am J Optom Arch Am Acad Optom. 1959;36:652–658. doi: 10.1097/00006324-195912000-00005. [DOI] [PubMed] [Google Scholar]
- Goss DA, Grosvenor T. Rates of childhood myopia progression with bifocals as a function of nearpoint esophoria: consistency of three studies. Optom Vis Sci. 1990;67:637–640. doi: 10.1097/00006324-199008000-00015. [DOI] [PubMed] [Google Scholar]
- Leung JT, Brown B. Progression of myopia in Hong Kong Chinese schoolchildren is slowed by wearing progressive lenses. Optom Vis Sci. 1999;76:346–354. doi: 10.1097/00006324-199906000-00013. [DOI] [PubMed] [Google Scholar]
- Gwiazda J, Hyman L, Hussein M, Everett D, Norton TT, Kurtz D, et al. randomized clinical trial of progressive addition lenses vs single vision lenses on the progression of myopia in children. Invest Ophthalmol Vis Sci. 2003;44 (4:1492–1500. doi: 10.1167/iovs.02-0816. [DOI] [PubMed] [Google Scholar]
- Yang Z, Lan W, Ge J, Liu W, Chen X, Chen L, et al. The effectiveness of progressive addition lenses on the progression of myopia in Chinese children. Ophthalmic Physiol Opt. 2009;29 (1:41–48. doi: 10.1111/j.1475-1313.2008.00608.x. [DOI] [PubMed] [Google Scholar]
- Hasebe S, Ohtsuki H, Nonaka T, Nakatsuka C, Miyata M, Hamasaki I, et al. Effect of progressive addition lenses on myopia progression in Japanese children: a prospective, randomized, double-masked, crossover trial. Invest Ophthalmol Vis Sci. 2008;49 (7:2781–2789. doi: 10.1167/iovs.07-0385. [DOI] [PubMed] [Google Scholar]
- Edwards MH, Li RW, Lam CS, Lew JK, Yu BS. The Hong Kong progressive lens myopia control study: study design and main findings. Invest Ophthalmol Vis Sci. 2002;43:2852–2858. [PubMed] [Google Scholar]
- Correction of Myopia Evaluation Trial 2 study group Progressive addition lenses vs single vision lenses for slowing progression of myopia in children with high accommodative lag and near esophoria. Invest Ophthalmol Vis Sci. 2011;52:2749–2757. doi: 10.1167/iovs.10-6631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng D, Schmid KL, Woo GC, Drobe B. Randomized trial of effect of bifocal and prismatic bifocal spectacles on myopic progression: two year results. Arch Ophthalmol. 2010;128 (1:12–19. doi: 10.1001/archophthalmol.2009.332. [DOI] [PubMed] [Google Scholar]
- Aller TA, Wildsoet C. Bifocal soft contact lenses as a possible myopia control treatment: a case report involving identical twins. Clin Exp Optom. 2008;91:479. doi: 10.1111/j.1444-0938.2007.00230.x. [DOI] [PubMed] [Google Scholar]
- Song YY, Wang H, Wang BS, Qi H, Rong ZX, Chen HZ. Atropine in ameliorating the progression of myopia in children with mild to moderate myopia: a meta-analysis of controlled clinical trials. J Ocul Pharmacol Ther. 2011;27:361–368. doi: 10.1089/jop.2011.0017. [DOI] [PubMed] [Google Scholar]
- Tong L, Huang XL, Koh AL, Zhang X, Tan DT, Chua WH. Atropine for the treatment of childhood myopia: effect on myopia progression after cessation of atropine. Ophthalmology. 2009;116:572–579. doi: 10.1016/j.ophtha.2008.10.020. [DOI] [PubMed] [Google Scholar]
- Fang YT, Chou YJ, Pu C, Lin PJ, Liu TL, Huang N, et al. Prescription of atropine eye drops among children diagnosed with myopia in Taiwan from 2000 to 2007: a nationwide study. Eye. 2013;27:418–424. doi: 10.1038/eye.2012.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBrien NA, Moghaddam HO, Reeder AP. Atropine reduces experimental myopia and eye enlargement via a nonaccommodative mechanism. Invest Ophthalmol Vis Sci. 1993;34:205–215. [PubMed] [Google Scholar]
- Siatkowski RM, Cotter SA, Crockett RS, Miller JM, Novack GD, Zadnik K, U.S Pirenzepine Study Group Two-year multicenter, randomized, double-masked, placebo-controlled, parallel safety and efficacy study of 2% pirenzepine ophthalmic gel in children with myopia. J AAPOS. 2008;12 (4:332–339. doi: 10.1016/j.jaapos.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Tan DT, Lam DS, Chua WH, Shu-Ping DF, Crockett RS, Asian Pirenzepine study Group One year multicentre, double-masked, placebo-controlled, parallel safety and efficacy study of 2% pirenzepine ophthalmic gel in children with myopia. Ophthalmology. 2005;112:84–91. doi: 10.1016/j.ophtha.2004.06.038. [DOI] [PubMed] [Google Scholar]
- Smith EL, 3rd, Ramamirtham R, Qiao-Grider Y, Hung LF, Huang J, Kee CS, et al. Effects of foveal ablation on emmetropization and form-deprivation myopia. Invest Ophthalmol Vis Sci. 2007;48:3914–3922. doi: 10.1167/iovs.06-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaridurg P, Donoval L, Varnas S, Ho A, Chen X, Martinez A, et al. Spectacle lenses designed to reduce progression of myopia: 12 month results. Optom Vis Sci. 2010;87 (9:631–641. doi: 10.1097/OPX.0b013e3181ea19c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaridurg P, Holden B, Smith E, 3rd, Naduvilath T, Chen X, de la Jara PL, et al. Decrease in rate of myopia progression with a contact lens designed to reduce relative peripheral hyperopia: one year results. Invest Ophthalmol Vis Sci. 2011;52 (13:9362–9367. doi: 10.1167/iovs.11-7260. [DOI] [PubMed] [Google Scholar]
- Hiroaka t, Kakita T, Okamoto F, Takahashi H, Oshika T. Long term effect of overnight orthokeratology on axial length elongation in childhood myopia: a 5-year follow-up study. Invest Ophthalmol Vis Sci. 2012;53 (7:3913–3919. doi: 10.1167/iovs.11-8453. [DOI] [PubMed] [Google Scholar]
- Anstice NS, Phillips JR. Effect of dual focus soft contact lens wear on axial myopia progression in children. Ophthalmology. 2011;118 (6:1152–1161. doi: 10.1016/j.ophtha.2010.10.035. [DOI] [PubMed] [Google Scholar]
- Chua WH, Balakrishnan V, Chan YH, Tong L, Ling Y, Quah BL, et al. Atropine for the treatment of childhood myopia. Ophthalmology. 2006;113 (12:2285–2291. doi: 10.1016/j.ophtha.2006.05.062. [DOI] [PubMed] [Google Scholar]
- Chia A, Chua WH, Cheung YB, Wong WL, Lingham A, Fong A, et al. Atropine for the treatment of childhood myopia: safety and efficacy of 0.5, 0.1 and 0.01% doses (Atropine for the Treatment of Myopia 2) Ophthalmology. 2012;119 (2:347–354. doi: 10.1016/j.ophtha.2011.07.031. [DOI] [PubMed] [Google Scholar]