Abstract
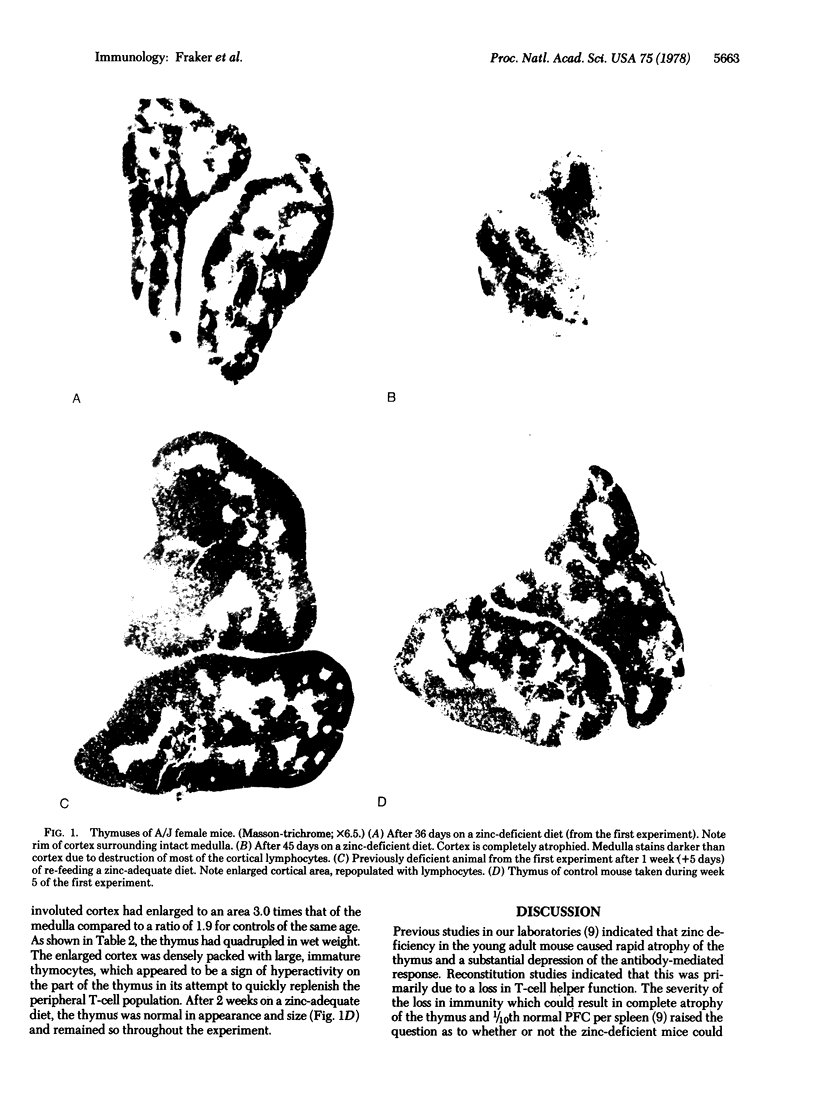
Diets deficient in zinc cause rapid atrophy of the thymus and loss of T-cell helper function in the young adult A/J mouse. Because zinc deficiency, as well as other nutritional deficiences, causes extensive damage to the immune system, the question arose as to whether zinc-deficient mice could repair the thymus and fully regenerate T-cell helper function if returned to diets containing adequate amounts of zinc. Five-week-old A/J female mice were fed either a zinc-deficient (<1 μg of Zn per g) or a zinc-adequate (50 μg of Zn per g) diet for 31 days. Histological examination of thymuses from the zinc-deficient mice revealed that the cortex was preferentially involuted and the thymus was about one-third of normal size. The direct plaque-forming cells produced per mouse spleen in response to immunization with sheep erythrocytes was 34% of normal; indirect plaque-forming cells were 18% of normal (Jerne plaque assay). After the deficient mice had been fed a zinc-adequate diet for 1 week, their response was nearly normal, except that the indirect response was 68% of controls; in this same period, the thymuses of these mice had quadrupled in size and exhibited a greatly enlarged cortex repopulated with immature thymocytes. By 2 weeks, the thymuses of the previously zinc-deficient mice were normal in size and appearance; however, there was a slight increases in numbers of indirect plaque-forming cells. By 4 weeks, the thymus weights, direct and indirect plaque-forming cell counts, and secondary response of the previously deficient mice were normal. Mice that were nearly athymic after 45 days of dietary zinc deficiency were also able to fully reconstruct the thymus and regenerate T-cell helper function. The data show that the zinc-deficient young adult mouse has the capacity to fully restore the T-cell-dependent antibody-mediated responses upon nutritional repletion.
Keywords: thymic atrophy, re-feeding of zinc-adequate diet, repair and repopulation of thymus, young adult A/J mice
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chandra R. K. Immunocompetence in undernutrition. J Pediatr. 1972 Dec;81(6):1194–1200. doi: 10.1016/s0022-3476(72)80262-2. [DOI] [PubMed] [Google Scholar]
- Claman H. N. Corticosteroids and lymphoid cells. N Engl J Med. 1972 Aug 24;287(8):388–397. doi: 10.1056/NEJM197208242870806. [DOI] [PubMed] [Google Scholar]
- Davis S. D., Nelson T., Shepard T. H. Teratogenicity of vitamin B6 deficiency: omphalocele, skeletal and neural defects, and splenic hypoplasia. Science. 1970 Sep 25;169(3952):1329–1330. doi: 10.1126/science.169.3952.1329. [DOI] [PubMed] [Google Scholar]
- Faulk W. P., Demaeyer E. M., Davies A. J. Some effects of malnutrition on the immune response in man. Am J Clin Nutr. 1974 Jun;27(6):638–646. doi: 10.1093/ajcn/27.6.638. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., Haas S. M., Luecke R. W. Effect of zinc deficiency on the immune response of the young adult A/J mouse. J Nutr. 1977 Oct;107(10):1889–1895. doi: 10.1093/jn/107.10.1889. [DOI] [PubMed] [Google Scholar]
- GORDON J. E., JANSEN A. A., ASCOLI W. MEASLES IN RURAL GUATEMALA. J Pediatr. 1965 Apr;66:779–786. doi: 10.1016/s0022-3476(65)80016-6. [DOI] [PubMed] [Google Scholar]
- Gershoff S. N., Gill T. J., 3rd, Simonian S. J., Steinberg A. I. Some effects of amino acid deficiencies on antibody formation in the rat. J Nutr. 1968 Jun;95(2):184–190. doi: 10.1093/jn/95.2.184. [DOI] [PubMed] [Google Scholar]
- Hirokawa K., Makinodan T. Thymic involution: effect on T cell differentiation. J Immunol. 1975 Jun;114(6):1659–1664. [PubMed] [Google Scholar]
- Hurley L. S. Approaches to the study of nutrition in mammalian development. Fed Proc. 1968 Jan-Feb;27(1):193–198. [PubMed] [Google Scholar]
- Luecke R. W., Simonel C. E., Fraker P. J. The effect of restricted dietary intake on the antibody mediated response of the zinc deficient A/J mouse. J Nutr. 1978 May;108(5):881–887. doi: 10.1093/jn/108.5.881. [DOI] [PubMed] [Google Scholar]
- McFarlane H. Malnutrition and impaired immune response to infection. Proc Nutr Soc. 1976 Dec;35(3):263–272. doi: 10.1079/pns19760045. [DOI] [PubMed] [Google Scholar]
- Smythe P. M., Brereton-Stiles G. G., Grace H. J., Mafoyane A., Schonland M., Coovadia H. M., Loening W. E., Parent M. A., Vos G. H. Thymolymphatic deficiency and depression of cell-mediated immunity in protein-calorie malnutrition. Lancet. 1971 Oct 30;2(7731):939–943. doi: 10.1016/s0140-6736(71)90267-4. [DOI] [PubMed] [Google Scholar]
- Watts T. Thymus weights in malnourished children. J Trop Pediatr (1967) 1969 Dec;15(4):155–158. doi: 10.1093/tropej/15.4.155. [DOI] [PubMed] [Google Scholar]



