Abstract
Purpose
We examined the association between abnormal fundus autofluorescence (FAF) features on images obtained by a modified fundus camera (mFC) and geographic atrophy (GA) progression in patients with age-related macular degeneration (AMD).
Methods
Serial FAF images of 131 eyes from 131 patients with GA were included in the study. All FAF images were obtained with an mFC (excitation, ∼500–610 nm; emission, ∼675–715 nm). The GA area was quantified at baseline and 1 year later using a customized segmentation program. The yearly GA enlargement rate was then calculated. Abnormal FAF patterns in the junctional zone of GA were classified as None or Minimal change, Focal, Patchy, Banded, or Diffuse according to previously published classification based on confocal scanning laser ophthalmoscopy (cSLO). The relationship between GA enlargement and abnormal FAF was evaluated.
Results
The mean rate of GA enlargement was the fastest in eyes with Diffuse pattern (1.74 mm2 per year), followed by eyes with the Banded pattern (1.69 mm2 per year). Binary logistic regression analysis revealed that eyes with the Banded and Diffuse pattern had significantly higher risk for GA enlargement compared with eyes with the other patterns.
Conclusions
FAF image obtained by mFC appears to be acceptable for evaluating GA in accordance with an established cSLO-based classification. Eyes with the Banded or the Diffuse patterns of abnormal FAF at baseline indicate a high risk for GA progression. Identifying patients at high risk for GA progression using an mFC is broadly available method that can provide additional information to help predict disease course.
Keywords: fundus autofluorescence, fundus camera, geographic atrophy, progression, age-related macular degeneration
Introduction
Lipofuscin (LF) is known to have a significant effect on retinal pigment epithelium (RPE) function and have a key role in retinal disease. It is thought that progressive LF accumulation is mainly a byproduct of the phagocytosis of the membranous discs that are shed from the outer segment of retinal photoreceptors.1, 2, 3 LF granules accumulate over time because RPE cells have no mechanism for degrading or transporting LF material granules into the extracellular space.4 Previous studies have suggested that LF and its constituents have toxic effects on normal RPE cell function.5, 6, 7 Abnormal LF accumulation is observed in age-related macular degeneration (AMD), the leading cause of severe vision loss in developed countries.8
Although choroidal neovascularization and RPE detachment are the principle causes of vision loss in patients with AMD, geographic atrophy (GA) of the RPE also accounts for severe vision loss, comprising ∼35% of all cases with late AMD.9, 10 GA is characterized by the development of atrophic patches that enlarge slowly over time. Because no definite treatment has been proven to halt or slow GA progression, the initiation of clinical trials to evaluate therapies for GA has increased the need for an effective method of monitoring and predicting GA progression.
Fundus autofluorescence (FAF) generally refers to the autofluorescent emission obtained from LF in the RPE.11, 12 FAF imaging enables detection of LF distribution in the RPE, therefore providing information on the health and functionality of the RPE. FAF signal can be detected using specific instruments including Delori's fundus spectrophotometer, the modified fundus camera (mFC), and confocal scanning laser ophthalmoscopy (cSLO).12
Schmitz-Valckenberg et al13 described two important findings with FAF imaging in GA patients: the ability to evaluate the atrophic area quantitatively and the visualization of abnormal FAF distribution in the junctional zone surrounding the atrophic patches. Recent analyses with FAF for the junctional border of GA lesions in AMD have led to the identification of several unique phenotypes.14 The Fundus Autofluorescence in Age-related Macular Degeneration (FAM) study group developed a step-wise approach for classifying GA lesions and identified five primary phenotypes based on the presence of increased autofluorescence at the junctional zone of the atrophic patch.15 Several longitudinal studies based on these phenotypes have demonstrated that distinct FAF patterns are associated with atrophy progression. Bearelly et al16 categorized the extent of increased autofluorescence, which was termed rim area focal hyper-autofluorescence, in the 500 μm perimeter bordering the GA into three categories. However, all of these studies were performed based on the cSLO for FAF imaging.
In this study, we performed FAF imaging using an mFC in patients with AMD who developed GA, and we evaluated abnormal FAF according to the classification system described in previous report of the FAM-study group. This study aimed to determine whether abnormal FAF features on the images obtained from mFC are associated with GA progression. This determination can be made by digitally quantifying changes in junctional zone atrophy. To our knowledge, this is the first study that analyzed the impact of abnormal FAF pattern obtained by the conventional fundus camera on GA progression.
Materials and Methods
Subjects
Patients with unilateral or bilateral GA associated with AMD were recruited at the Department of Ophthalmology at Kangdong Sacred Heart Hospital, Seoul, Korea, from June 2011 to August 2012. Each eye had an initial and a final (at the 1-year follow-up) FAF image. Only patients >50 years with drusen and uni- or multifocal GA were included. A single eye was selected randomly if both eyes met the inclusion criteria. Patients with a history of retinal surgery, laser photocoagulation, choroidal neovascularization, and other macular or retinal diseases (eg, diabetic retinopathy, hereditary retinal dystrophies, and myopic macular degeneration) were excluded from this study. In addition, eyes were excluded if the quality of the images were too poor for interpretation, if the GA extended beyond the margins of the image or if there was a current or previous high myopia or hyperopia (myopia<−6 diopters; hyperopia>+4 diopters). Demographic data, such as age, sex, and the presence of diabetes mellitus and hypertension, if applicable, were recorded.
Image acquisition
After pupillary dilation, color fundus photographs and FAF images were recorded from each eye using the Topcon TRC-50DX (Topcon, Paramus, NJ, USA) in an identical camera setting. For FAF imaging, custom filters were used to modify the excitation bandwidth (500–610 nm, max at 580 nm) and emission bandwidth (675–715 nm, max at 695 nm), based on the modification of Spaide.13, 17 All images were taken with the small pupil setting on the camera to reduce the possibility of insufficient pupil dilation. When taking images, optic focus was on the optic disc and surrounding major retinal vasculature. Using the setting of maximal gain for the digital camera, three individual images were obtained and the best quality images were selected and exported. The brightness and contrast of these images were adjusted manually so that the visualization of FAF abnormalities around the atrophic patch was maximally enhanced subjectively using digital imaging software (IMAGEnet Professional R-3.11; Topcon).
The initial and final AF images were registered in commercial software (MatLab R2006; The MathWorks, Inc., Natick, MA, USA). All images had a scale of ∼15 μm per pixel. For processing and analysis, images were imported into image-analysis software (MatLab, The MathWorks) as bitmap files consisting of 256 gray levels for each pixel. The image was then cropped to a 6000 μm square, which included the fovea. All subsequent analyses were performed on these images.
Measuring and grading geographic atrophy
This study used a classification system previously published by Bindewald et al15 as a part of the FAM-study group. They developed a step-wise approach to classifying GA lesions in abnormal FAF. They identified the five following primary phenotypes based on increased hyper-autofluorescence at the junctional zone of atrophy: None, Focal, Banded, Patchy, and Diffuse patterns. Eyes with no increase in FAF intensity were graded as ‘None'. When images showed very limited irregular increase of FAF in the junctional zone of atrophy without an obvious topographic pattern (Minimal change), they were included in category ‘None' because they were regarded as minimal variations from the appearance of ‘None' pattern. ‘Focal' pattern was defined by the presence of at least one spot (<200 μm) of markedly increased FAF intensity. If the spot was larger than 200 μm, it was graded as ‘Patchy'. ‘Banded pattern' was characterized by the presence of continuous ring-shaped increased FAF around GA. Eyes showing areas with increased FAF directly adjacent to the margin of the atrophic patch and elsewhere were called ‘Diffuse'. Subsequently, all subjects were classified into one of these five distinct patterns: None or Minimal change, Focal, Banded, Patchy, and Diffuse (see Figure 1). All FAF images in the present study were classified by two graders (JKC and HKK) masked to the corresponding (baseline or follow-up) image for each eye. In case of the interobserver disagreement on the classification of abnormal FAF, a third grader (PSP) participated in classifying process.
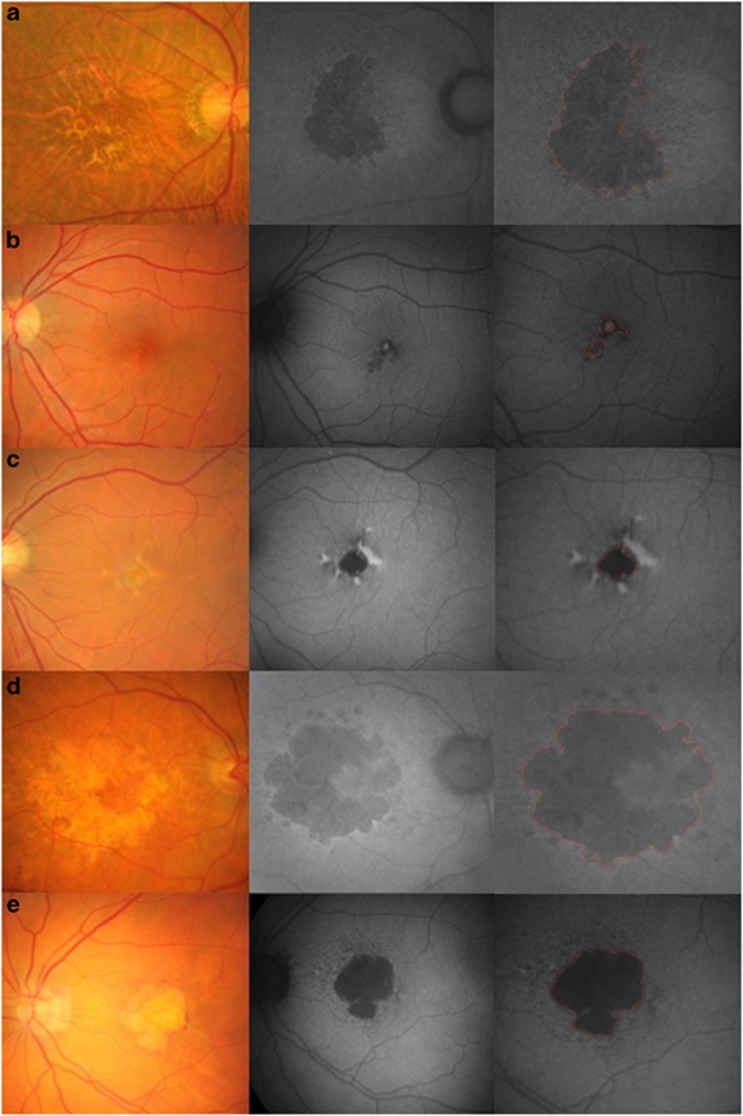
Figure 1.
Examples of fundus autofluorescence (FAF) images showing quantification of geographic atrophy (GA) in patients with age-related macular degeneration (AMD) using a modified fundus camera. Three images on the same line include the fundus image (Left), the original FAF image (middle) and the cropped image (right). Atrophic areas are highlighted in red. Images were classified in accordance with different patterns of hyper-autofluorescence in the junctional zone of GA. (a) Right eye of a 73-year-old patient showing GA with no increased autoflorescence intensity (None or Minimal change). Very small area of hyper-autofluorescence at the junctional zone of GA is minimal variation of the None pattern. (b) Left eye of a 68-year-old patient with small areas of focally increased autofluorescence at the margin of the atrophic area (Focal). (c) Right eye of a 58-year-old patient with areas of focally increased autofluorescence, which is larger than that of the Focal pattern (Patchy). (d) Left eye of a 71-year-old patient with a band of increased autofluorescence surrounding the geographic atrophy (Banded). (e) Left eye of a 79-year-old patient with many variable shapes of hyper-autofluorescent spot around GA (Diffuse).
The other two examiners (IHH and K.L.K) calculated the total GA area on the digital images using a customized segmentation program in MATLAB (Mathworks 7.0, The MathWorks). These examiners used a computer mouse to trace all areas of GA around the macula manually. Geographic atrophy was recognized as well-demarcated black areas corresponding to the dead or absent RPE. Areas of intact autofluorescence within GA were also traced, but a different color was used to signify RPE sparing and these areas were excluded from the calculation of GA. Figure 1 shows the atrophic area highlighted in red in the cropped images. The MATLAB automates conversion of pixels to square mm, based on the magnification factors of the imaging device. Areas of atrophy smaller than 0.02 mm2 were excluded. The total GA for each baseline and follow-up image was calculated by summing all of the GA areas and subtracting demarcated areas of sparing. Only the mean value of GA areas measured by two examiners was used for calculating the enlargement rate of GA.
Data analysis
Data were compiled with a standard spreadsheet program (Microsoft Excel) and were analyzed using commercially available statistical software (SPSS 18.0; SPSS Inc., Chicago, IL, USA). Statistical analysis included frequency and descriptive statistics. The baseline characteristics of the study subjects were analyzed using t-tests for comparison of means and χ2-tests for comparison of proportions. Interclass correlation coefficient (ICC) was calculated for the reproducibility of quantifying process performed by two examiners. To determine the risk factors for the progression of GA, it was neccessary to compare the group with a high GA enlargement rate with the group with a low GA enlargement rate. Therefore, we divided all of the samples into two groups in which the GA enlargement rate was over or under the average and regarded a GA enlargement rate greater than the average as a meaningful progression of atrophic area. All of the baseline characteristics were compared between the two groups, and binary logistic regression was performed to identify risk factors. Statistical significance was set at P<0.05.
Results
A total of 131 eyes from 131 AMD patients with GA were initially recruited for the study and had FAF images taken. Upon review of the medical history, 20 eyes were excluded because 15 eyes had diabetic retinopathy or another retinal disease and 5 eyes had undergone previous retinal surgery. One patient with unilateral GA was also excluded because he developed CNV in one eye. In addition, images from 24 eyes were of poor quality due to media opacities and GA could not be clearly demarcated. Therefore, a total of 86 eyes from 86 patients (43 male, 43 female) met all enrollment criteria and were used in study analyses. Baseline demographics are described in Table 1. The mean size of GA area was 11.12 mm2 at baseline (range, 0.42–44.65) and 12.25 mm2 (range, 0.43–46.95) at 1-year follow-up. The GA area showed a continuous enlargement over a year with a mean rate of 1.14 mm2 per year.
Table 1. Baseline demographics and descriptive data.
| Variable | Frequency (n=86) |
|---|---|
| Age, mean (range) in years | 69.78 (51–93) |
| Male (%) | 43 (50.0) |
| Diabetes (%) | 25 (29.1) |
| Hypertension (%) | 43 (50.0) |
| Pattern of abnormal FAFa | |
| None or Minimal change (%) | 18 (20.9) |
| Focal (%) | 12 (14.0) |
| Banded (%) | 16 (18.6) |
| Patchy (%) | 15 (17.4) |
| Diffuse (%) | 25 (29.1) |
Abbreviation: FAF, fundus autofluorescence.
Patterns of the abnormal FAF junctional zone were determined in accordance with the classification of the FAM-study group.15
In accordance with the classification system developed by the FAM-study group, the number of eyes phenotyped as None or Minimal change, Focal, Banded, Patchy, or a Diffuse pattern was 18, 12, 16, 15, and 25, respectively (Table 2). The GA enlargement rate was the slowest in eyes with the None or Minimal change abnormal FAF pattern (mean, 0.20 mm2 per year), followed by eyes with Focal pattern (mean, 0.53 mm2 per year), followed by eyes with the Patchy FAF pattern (mean, 1.16 mm2 per year), and eyes with the Banded and Diffuse FAF pattern (mean, 1.69 mm2 per year and 1.74 mm2 per year, respectively). Agreement between examiners quantifying the extent of GA on FAF was high (intraclass correlation: 0.95, P<0.01)
Table 2. Growth rates of GA by pattern of abnormal FAF.
| Pattern | N | Mean growth rates (mm2 per year) | Median growth rates (mm2 per year) | SD (mm2 per year) | IQR |
|---|---|---|---|---|---|
| None or Minimal change | 18 | 0.20 | 0.22 | 0.89 | 0.01–0.67 |
| Focal | 12 | 0.53 | 0.56 | 0.92 | 0.20–0.90 |
| Banded | 16 | 1.69 | 1.69 | 1.47 | 0.69–2.97 |
| Patchy | 15 | 1.16 | 1.02 | 1.18 | 0.40–2.04 |
| Diffuse | 25 | 1.74 | 1.41 | 1.60 | 0.82–2.29 |
Abbreviations: FAF, fundus autofluorescence; IQR, interquartile range; N, number of eyes; SD, standard deviation.
The comparisons of different demographic and clinical characteristics between eyes with a GA enlargement rate under average and eyes with a GA enlargement rate over average are summarized in Table 3. Age, sex, and the presence of diabetes or hypertension were not different between the two groups; however, the GA area and the patterns of abnormal FAF were significantly different between the two groups.
Table 3. Comparison of baseline characteristics in the study groups: 1-year follow-up.
|
GA growth rate |
GA growth rate |
P-value | |
|---|---|---|---|
| <1.14 mm2 per yeara (n=52) | >1.14 mm2 per yeara (n=34) | ||
| Age (years) | 69.87±11.48 | 69.65±12.16 | 0.93b |
| Female | 27 (51.9%) | 16 (47.1%) | 0.83c |
| Diabetes | 15 (28.8%) | 10 (29.4%) | 0.98c |
| Hypertension | 26 (50.0%) | 17 (50.0%) | 0.98c |
| Baseline GA (mm2) | 7.97±7.69 | 15.93±12.00 | <0.01b |
| Pattern of abnormal FAF | 0.05c | ||
| None or Minimal change | 15 | 3 | |
| Focal | 9 | 3 | |
| Banded | 6 | 10 | |
| Patchy | 9 | 6 | |
| Diffuse Pattern | 13 | 12 |
Abbreviations: FAF, fundus autofluorescence; GA, geographic atrophy.
1.14 was drawn from the average of GA growth rate over a year of all subjects.
Independent t-test.
Chi-square test.
Binary logistic regression analysis revealed that the size of GA area was a statistically significant risk factor for GA progression (odds ratio (OR) 1.09; P<0.01) (Table 4). Age, sex, diabetes, and hypertension were not statistically significant. In the respect of the patterns of abnormal FAF, the Banded pattern (OR 8.33; P=0.01) and the Diffuse pattern (OR 4.62; P=0.04) of abnormal FAF had significantly high risk compared with the None or Minimal change pattern. When pooling the data of the Banded and the Diffuse patterns and comparing to the other pattern, the Banded and Diffuse patterns of the abnormal FAF was a highly significant risk factor (OR 3.18; P=0.01).
Table 4. Independent risk factors for progression of geographic atrophy in fundus autofluorescence in patients with age-related macular degeneration.
| Risk factor |
Progression of GA |
||
|---|---|---|---|
| OR | P-value | 95% CI | |
| Age | 0.998 | 0.932 | 0.96–1.04 |
| Sex | |||
| Male | 1 | ||
| Female | 0.82 | 0.66 | 0.35–1.96 |
| GA area | 1.09 | 0.001 | 1.03–0.14 |
| Diabetes | |||
| No | 1 | ||
| Yes | 1.03 | 0.96 | 0.40–2.67 |
| Hypertension | |||
| No | 1 | ||
| Yes | 1.00 | 0.99 | 0.42–2.37 |
| FAF pattern | |||
| None or Minimal change | 1 | ||
| Focal | 1.67 | 0.58 | 0.28–10.09 |
| Banded | 8.33 | 0.01 | 1.68–41.29 |
| Patchy | 3.33 | 0.14 | 0.66–16.74 |
| Diffuse | 4.62 | 0.04 | 1.06–20.01 |
| None+Focal+Patchy | 1 | ||
| Banded+Diffuse | 3.18 | 0.01 | 0.30–2.27 |
Abbreviations: CI, confidence interval; FAF, fundus autofluorescence; OR, Odd ratio.
Discussion
FAF imaging was originally obtained by using the cSLO. The cSLO device, which provides a wide field of retinal scanning (55°), is not influenced by the AF from structures other than the RPE because it uses a confocal optic. It has been used increasingly because of its perceived technical advantages and good image quality, and most of the studies analyzing FAF in patients with GA secondary to AMD obtained their data using cSLO. However, the relatively high purchase and maintenance costs of the cSLO limit its availability for use. On the other hand, the conventional fundus camera, which uses the excitation and emission filters that are applied for fluorescein angiography, is available in almost all ophthalmologic clinics. Although the FAF images obtained by using the conventional fundus camera are of lower quality than the images obtained by using the cSLO because of low contrast, high background noise, and the influence of media opacity, its broad availability makes it a good alternative to the cSLO. In addition, Spaide17 proposed a modification in 2003 in which the filters for FAF imaging were changed by moving the excitation and emission wavelengths more toward the red end of the spectrum, limiting the influence of the lens on image quality. Schmitz-Valckenberg et al13 classified FAF image quality in age-related GA into three different categories: insufficient, intermediate, and good. Among the 32 eyes (16 patients) examined, 22 eyes had a good image quality, 8 eyes had an intermediate quality, and only 2 eyes were insufficient in quality when using the ‘optimized' fundus camera. The cSLO was better, with the number of eyes with a good image quality increasing to 28. Therefore, the cSLO is still the preferred imaging system for clinical or laboratory trials, when systematically assessing eyes with GA. Despite the fact that mFC is inferior to cSLO for visualizing abnormal FAF patterns, GA quantification with the two instruments was similar when using good quality images. These findings highlight the clinical use of the fundus camera as a tool for evaluating FAF in patients with AMD. In our study, 24 of the 110 eyes that met the inclusion criteria were excluded from this study because of image quality issues, similar to the previous study.
The FAM-Study Group developed the classification of abnormal FAF according to distinct patterns in the junctional zone of the GA area and identified the following five primary phenotypes based on the presence of increased hyper-autofluorescence: None, Focal, Banded, Patchy, and Diffuse.15 The Diffuse phenotype was further subdivided into four additional patterns. Holz et al14 showed that the phenotypic features of FAF abnormalities had a much stronger impact on atrophy progression than any other risk factor that has been addressed in previous studies on progression of GA attributable to AMD, and introduced the ‘diffuse trickling' pattern that is associated with an extremely rapid progression of atrophy. In the current study, we also grouped samples into five primary phenotypes in accordance with the classification developed by the FAM-Study group, but we did not further subgroup the Diffuse FAF pattern for the sake of simplicity. As previously described, eyes with only the Minimal change pattern were grouped with eyes in the None category because the Minimal change pattern was thought to be similar in scope to the None pattern.
We found that the GA enlargement rate was the fastest in eyes with the Diffuse FAF pattern and the Banded pattern. These results are consistent with Holz et al,14 who demonstrated faster median rates of progression for eyes with a Diffuse pattern (median, 1.77 mm2 per year) and Banded pattern (median, 1.81 mm2 per year) compared with eyes with a Focal pattern (median, 0.81 mm2 per year) and eyes without any identified patterns (median, 0.38 mm2 per year). Although our study was based not on the cSLO but on the mFC as an imaging tool for abnormal FAF, the results of present study implied that the classification of previous cSLO-based study may be applicable to the abnormal FAF obtained from mFC. However, some detailed differences exists between two studies. Our study showed a relatively slow GA progression rate, both overall and each FAF pattern. This may have resulted from two possible factors. First, epidemiologic factors, including race and smoking status, were different between the two study cohorts. It is well known from the Age-Related Eye Disease Study that whites have a higher risk of developing advanced AMD than blacks, although the incidence of early AMD is not different between the two races.18 This indicates that race, or genetics, is associated with disease progression. The previous FAM-Study Group study examined patients who had visited European hospitals, while our patients were all Asians. Furthermore, modifiable factors associated with advanced AMD progression (eg, smoking status, body mass index) were different between the two study cohorts. The slow progression rates observed in our study might have been, in part, because of these epidemiologic differences. The use of different FAF imaging devices may have also influenced the results. Because FAF images obtained by the mFC had low contrast and high background noise, the GA area may have been over- or underestimated, compared with cSLO images. The abnormal FAF patterns identified on mFC images may also have been different if FAF had been examined with cSLO, but a direct comparison of abnormal FAF pattern appearance on both devices has not yet been performed.
As previously described, we divided all of the samples into two groups in which the GA enlargement rate was over or under 1.14, and we regarded a GA enlargement rate greater than 1.14 as a meaningful progression. By comparing the two groups using binary logistic regression analysis, the present study showed that the Diffuse and the Banded FAF patterns are risk factors for the progression of GA. The eyes with the Diffuse FAF pattern had a greater than fourfold higher probability of GA progression than eyes with None or Minimal change FAF pattern. The eyes with the Banded FAF pattern had a greater than eightfold higher probability of GA progression than eyes with None or Minimal change FAF pattern. These findings suggest that different patterns of abnormal FAF around GA have a significant predictive value for the progression of atrophy. In addition, the present study demonstrated that previously published classification systems are considerably reliable for identifying prognostic determinants.
The suggested associations or the predictive value of GA progression in this study should be interpreted with caution because of several study limitations. The most important of these limitations is that the accuracy of quantifying GA area using Matlab has not been proven in the literature. Hwang et al19 used Matlab to quantify GA and to evaluate the relationship between GA progression and an increased FAF in eyes with AMD. In their study, they precisely measured atrophic areas in a semiautomated segmentation manner. However, their FAF images were obtained using cSLO. To our knowledge, there is no previous study about analyzing GA area in FAF images obtained by fundus camera with Matlab. Because the mFC uses only one image, it is inevitable to improve signal visualization by adjusting the contrast and brightness; this modification process may influence the accuracy of Matlab. Therefore, it would be necessary to verify the accuracy of Matlab to measure GA in FAF images obtained by a fundus camera, not cSLO. Some other factors including refraction of studied eyes may have influenced on the acquisition of paired images. Refraction of the studied eyes were different from each other, and the ratio of atrophic area to pixel might vary depending on refraction. We tried to minimize this error by excluding the eyes with severe refractive error from subjects as well as by focusing on the constant structure such as the optic disc and the retinal vasculature when taking images using fundus camera. Although our efforts to make identical image scale between initial and final images were not perfect, any remaining influence of different refractive errors should have been so subtle that their effect on the accuracy of quantification process should have been limited. Second, a potential limitation of the study is the lack of reproducibility in classifying abnormal FAF. Evaluating the intra- and interobserver agreement in the classification of FAF patterns in all subjects could increase the reliability of the present study. Biarnés et al20 described intra- and interobserver agreement in the evaluation of FAF patterns in AMD patients with GA and reported high intraobserver reproducibility and variable interobserver agreement; however, their study was based on images obtained by cSLO. Other limitations include the relatively small sample size and the short follow-up period. Further studies are needed to clarify the reproducibility of FAF patterns obtained by mFC in a large number of subjects with a long-term follow-up period.
In conclusion, FAF imaging using mFC is a good noninvasive tool for visualizing variable FAF patterns in vivo. FAF image acquisition by mFC appears to be adaptable for evaluation in accordance with the published classification systems assessed by using cSLO. On the basis of these classification systems, the Diffuse and Banded FAF pattern on baseline FAF images indicates a high risk of GA progression compared with other patterns in patients with AMD. The identification of high-risk characteristics with mFC is a broadly available method for providing additional information for predicting the disease course.
The authors declare no conflict of interest.
References
- Feeney-Burns L, Berman ER, Rothmann H. Lipofuscin of human retinal pigment epithelium. Am J Ophthalmol. 1980;90:783–791. doi: 10.1016/s0002-9394(14)75193-1. [DOI] [PubMed] [Google Scholar]
- Weiter JJ, Delori FC, Wing GL, Fitch KA. Retinal pigment epithelial lipofuscin and melanin and choroidal melanin in human eyes. Invest Ophthalmol Vis Sci. 1986;27:145–152. [PubMed] [Google Scholar]
- Wing GL, Blanchard GC, Weiter JJ. The topography and age relationship of lipofuscin concentration in the retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1978;17:601–607. [PubMed] [Google Scholar]
- Holz FG, Bellmann C, Margaritidis M, Schütt F, Otto TP, Völcker HE. Patterns of increased in vivo fundus autofluorescence in the junctional zone of geographic atrophy of the retinal pigment epithelium associated with age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 1999;237:145–152. doi: 10.1007/s004170050209. [DOI] [PubMed] [Google Scholar]
- Bergmann M, Schutt F, Holz FG, Kopitz J. Inhibition of the ATP-driven proton pump in RPE lysosomes by the major lipofuscin fluorophore A2-E may contribute to the pathogenesis of age-related macular degeneration. Faseb J. 2004;18:562–564. doi: 10.1096/fj.03-0289fje. [DOI] [PubMed] [Google Scholar]
- Hammer M, Richter S, Guehrs KH, Schweitzer D. Retinal pigment epithelium cell damage by A2-E and its photoderivatives. Mol Vis. 2006;12:1348–1354. [PubMed] [Google Scholar]
- Holz FG, Schütt F, Kopitz J, Eldred GE, Kruse FE, Völcker HE, et al. Inhibition of lysosomal degradative functions in RPE cells by a retinoid component of lipofuscin. Invest Ophthalmol Vis Sci. 1999;40:737–743. [PubMed] [Google Scholar]
- van Leeuwen R, Klaver CC, Vingerling JR, Hofman A, de Jong PT. Epidemiology of age-related maculopathy: a review. Eur J Epidemiol. 2003;18:845–854. doi: 10.1023/a:1025643303914. [DOI] [PubMed] [Google Scholar]
- Klein R, Klein BE, Knudtson MD, Meuer SM, Swift M, Gangnon RE. Fifteen-year cumulative incidence of age-related macular degeneration: the Beaver Dam Eye Study. Ophthalmology. 2007;114:253–262. doi: 10.1016/j.ophtha.2006.10.040. [DOI] [PubMed] [Google Scholar]
- Sunnes JS. The natural history of geographic atrophy, the advanced atrophic form of age-related macular degeneration. Mol Vis. 1999;5:25–29. [PubMed] [Google Scholar]
- Bindewald-Wittich A, Han M, Schmitz-Valckenberg S, Snyder SR, Giese G, Bille JF, et al. Two-photon-excited fluorescence imaging of human RPE cells with a femtosecond Ti:Sapphire laser. Invest Ophthalmol Vis Sci. 2006;47:4553–4557. doi: 10.1167/iovs.05-1562. [DOI] [PubMed] [Google Scholar]
- Delori FC, Dorey CK, Staurenghi G, Arend O, Goger DG, Weiter JJ. In vivo fluorescence of the ocular fundus exhibits retinal pigment epithelium lipofuscin characteristics. Invest Ophthalmol Vis Sci. 1995;36:718–729. [PubMed] [Google Scholar]
- Schmitz-Valckenberg S, Fleckenstein M, Göbel AP, Sehmi K, Fitzke FW, Holz FG, et al. Evaluation of autofluorescence imaging with the scanning laser ophthalmoscope and the fundus camera in age-related geographic atrophy. Am J Ophthalmol. 2008;146:183–192. doi: 10.1016/j.ajo.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Holz FG, Bindewald-Wittich A, Fleckenstein M, Dreyhaupt J, Scholl HP, Schmitz-Valckenberg S, FAM-Study Group Progression of geographic atrophy and impact of fundus autofluorescence patterns in age-related macular degeneration. Am J Ophthalmol. 2007;143:463–472. doi: 10.1016/j.ajo.2006.11.041. [DOI] [PubMed] [Google Scholar]
- Bindewald A, Schmitz-Valckenberg S, Jorzik JJ, Dolar-Szczasny J, Sieber H, Keilhauer C, et al. Classification of abnormal fundus autofluorescence patterns in the junctional zone of geographic atrophy in patients with age related macular degeneration. Br J Ophthalmol. 2005;89:874–878. doi: 10.1136/bjo.2004.057794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearelly S, Khanifar AA, Lederer DE, Lee JJ, Ghodasra JH, Stinnett SS, et al. Use of fundus autofluorescence images to predict geographic atrophy progression. Retina. 2011;31:81–86. doi: 10.1097/IAE.0b013e3181e0958b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaide RF. Fundus autofluorescence and age-related macular degeneration. Ophthalmology. 2003;110:392–399. doi: 10.1016/S0161-6420(02)01756-6. [DOI] [PubMed] [Google Scholar]
- Clemons TE, Milton RC, Klein R, Seddon JM, Ferris FL, 3rd, Age-Related Eye Disease Study Research Group Risk factors for the incidence of Advanced Age-Related Macular Degeneration in the Age-Related Eye Disease Study (AREDS) AREDS report no. 19. Ophthalmology. 2005;112:533–539. doi: 10.1016/j.ophtha.2004.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JC, Chan JW, Chang S, Smith RT. Predictive value of fundus autofluorescence for development of geographic atrophy in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2006;47 (6:2655–2661. doi: 10.1167/iovs.05-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biarnés M, Monés J, Trindade F, Alonso J, Arias L. Intra and interobserver agreement in the classification of fundus autofluorescence patterns in geographic atrophy secondary to age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2012;250 (4:485–490. doi: 10.1007/s00417-011-1846-y. [DOI] [PubMed] [Google Scholar]