Abstract
Investigations employing animal models have demonstrated that ocular growth and refractive development are regulated by visual feedback. In particular, lens compensation experiments in which treatment lenses are used to manipulate the eye's effective refractive state have shown that emmetropization is actively regulated by signals produced by optical defocus. These observations in animals are significant because they indicate that it should be possible to use optical treatment strategies to influence refractive development in children, specifically to slow the rate of myopia progression. This review highlights some of the optical performance properties of the vision-dependent mechanisms that regulate refractive error development, especially those that are likely to influence the efficacy of optical treatment strategies for myopia. In this respect, the results from animal studies have been very consistent across species; however, to facilitate extrapolation to clinical settings, results are presented primarily for nonhuman primates. In agreement with preliminary clinical trials, the experimental data show that imposed myopic defocus can slow ocular growth and that treatment strategies that influence visual signals over a large area of the retina are likely to be most effective.
Keywords: myopia, hyperopia, defocus, emmetropization
Curtin1 credits Kepler with being one of the first vision scientists to recognize the association between near work and myopia and for hypothesizing that myopia was an adaptation to near work. Although these seminal observations (circa 1610) have been replicated numerous times and much has been learnt about potential risk factors for the development of common forms of myopia, centuries of research on myopia in humans have failed to provide a clear indication of what it is about near work that promotes the development of myopia or to identify the physiological mechanisms that promote myopia in children as a result of chronic near work. However, beginning in the 1960s, research employing animal models was greatly expanded and began to provide new insights into the effects of visual experience on ocular growth and refractive development.
The nature of the visual manipulations that have been employed to characterize refractive development in animals fall into three broad categories. The first category involved either natural or imposed restrictions in viewing distance and was largely motivated by the near work hypothesis. For example, comparisons of refractive errors between feral and domesticated or laboratory-reared animals supported the idea that environments that restrict viewing distance are myopiagenic.2, 3 However, these studies suffered from many of the same confounding issues associated with human studies.4 A more controlled line of research was begun by Levinsohn5 and continued most notably by Young,6, 7, 8, 9 who demonstrated that monkeys reared in environments with a maximum viewing distance of about 20 inches developed myopia. These experiments avoided many of the confounding factors in human studies, in particular self-selection, and demonstrated that restricted viewing conditions promoted the development of myopia, but, like many human studies, failed to provide significant insights into the mechanisms responsible for the resulting myopia.
The second major category of manipulations involves alterations in ambient lighting. The three primary alterations that have been investigated include variations in the diurnal light-dark cycle and the intensity and spectral composition of ambient lighting. These studies have shown that disturbances in the circadian rhythms of ocular mechanisms can interfere with refractive development,10, 11, 12 and that both luminance13, 14, 15 and chromatic mechanisms16, 17 are involved in the visual regulation of ocular growth. In addition, it has been shown that in comparison with ordinary indoor lighting levels, elevated lighting promotes low degrees of hyperopia, whereas dim lighting levels that are sufficient to maintain normal circadian rhythms promote ocular enlargement and myopia.18 Many recent investigations of the effects of elevated ambient lighting19, 20, 21, 22, 23 were motivated by epidemiologic observations in children that indicate that time spent outdoors is protective against the onset of myopia in children24, 25, 26 and suggest that the antimyopia effects of outdoor environments are in part due to the fact that outdoor lighting levels are typically about 100 times higher than ordinary indoor lighting. This line of investigations has also strongly implicated retinal dopaminergic mechanisms in the visual regulation of ocular growth.20
The third category of experimental manipulations involves induced alterations in retinal image contrast (eg, form deprivation) and/or focus (eg, lens compensation experiments). These experiments are probably most relevant for understanding the role of vision in common forms of myopia. Moreover, the functional operating characteristics of the vision-dependent mechanisms that regulate refractive development, which are described below, provide the foundation for optical treatment strategies for myopia.
Refractive development is regulated by optical Defocus
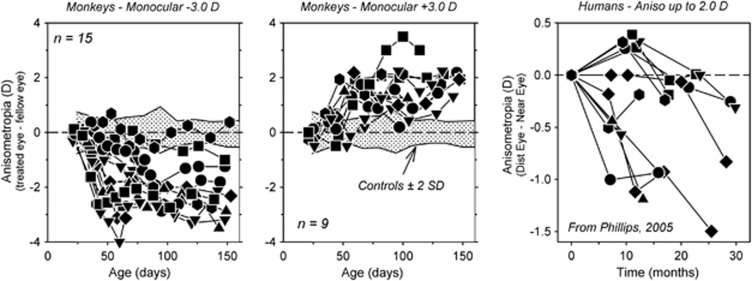
As illustrated in Figure 1, which shows the interocular differences in refractive error plotted as a function of age for monkeys reared with either −3D (left panel) or +3D lenses in front of one eye (middle panel), experimentally imposed changes in the eye's effective refractive state predictably alter refractive development in a manner that reduces the optically imposed error.27, 28, 29, 30, 31, 32, 33 In particular, the negative-powered lenses imposed relative hyperopia on the treated eyes, which in response became more myopic than their fellow control eyes. In contrast, the positive-powered lenses imposed relative myopia, which initiated relative hyperopic shifts in the treated eye refractive errors. These refractive changes were axial in nature. Specifically, the myopia was caused by an increase in the vitreous chamber elongation rate.29 The hyperopic changes were due to a reduction in vitreous chamber elongation rate coupled with the normal reduction in corneal and crystalline lens power.29 Monocular experiments like those illustrated in Figure 1 provide in-animal controls for many factors that could influence ocular growth (eg, genetic factors) and demonstrate that refractive development is largely independent in the two eyes.
Figure 1.
Interocular differences in refractive error (treated eye correction−fellow eye correction) plotted as a function of age for individual monkeys reared with −3D (left panel) or +3D lenses over one eye (middle panel).22, 46 The fellow eyes viewed through zero-powered lenses. The first symbol represents the start of the lens-rearing period. The shaded regions indicate ±2 SD around the mean anisometropia for untreated control monkeys. The right panel illustrates the interocular difference in refractive error (distance corrected eye−near corrected eye) as a function of time for children corrected using a monovision strategy that imposed up to 2.0 D of relative hyperopia on the near corrected eye.43
Optical rearing experiments like those shown in Figure 1 are most frequently conducted using very young animals because the resulting changes in refractive error are large and occur quickly. Similar experiments conducted in mature animals demonstrate that the vision-dependent mechanisms that regulate refractive development are operational well into adult life.34, 35, 36, 37 However, the type and degree of refractive changes that can be induced in adult animals is reduced in comparison with those observed in neonates. For example, form deprivation and hyperopic defocus can produce axial myopia in adolescent monkeys, but the induced myopic errors are smaller than those observed in neonates and take longer to develop.34, 35 In contrast, given the manner in which positive lens defocus produces hyperopia in neonates, it is unlikely that myopic defocus would produce significant hyperopic shifts in mature animals. The hyperopic shifts come about in young animals because reductions in axial growth occur in concert with the normal maturational decreases in corneal and lens power.29 However, corneal power reaches adult levels very early in life and lens power decreases very slowly in adolescents, normally reaching adult values relatively early in life.38, 39 As a consequence, even viewing conditions that reduce or stop axial elongation rates will not produce hyperopic shifts because the total refracting power of the eye is stable and other mechanisms such as changes in choroidal thickness are small in primates.40, 41
It is significant that the results from ‘lens compensation' experiments, which were pioneered by Schaeffel et al,28 have been qualitatively similar in every species that has been studied in a systematic manner.42 It has been consistently found that for a moderate range of treatment lens powers the eye can detect the presence of the imposed refractive error and alter its growth rate, specifically its vitreous chamber elongation rate, to eliminate the optical error. The similarities observed in different animals indicate that the mechanisms that regulate eye growth have been conserved across species. In this respect, it is notable that when humans encounter comparable viewing situations, their eyes exhibit qualitatively similar changes in refractive error. For example, the right panel in Figure 1 shows longitudinal interocular differences in refractive error for 10- to 13-year-old children who were treated using a monovision correction strategy in which one eye was corrected for distance vision and the fellow eye's correction was up to 2D more positive in power and corrected for near vision.43 In essence the treatment strategy imposed an anisometropia. In response the children developed an anisometropia that was axial in nature and in the appropriate direction to compensate for the imbalance imposed by the monovision correction strategy. The pattern of results in both laboratory animals and humans show that emmetropization is actively regulated by visual feedback associated with the eye's effective refractive state. These results are clinically significant because they indicate that optically imposed defocus could be used to effectively manage refractive development and, specifically, that imposed myopic defocus could be used to reduce the progression of myopia in children.
Refractive development is regulated in a locus-specific manner
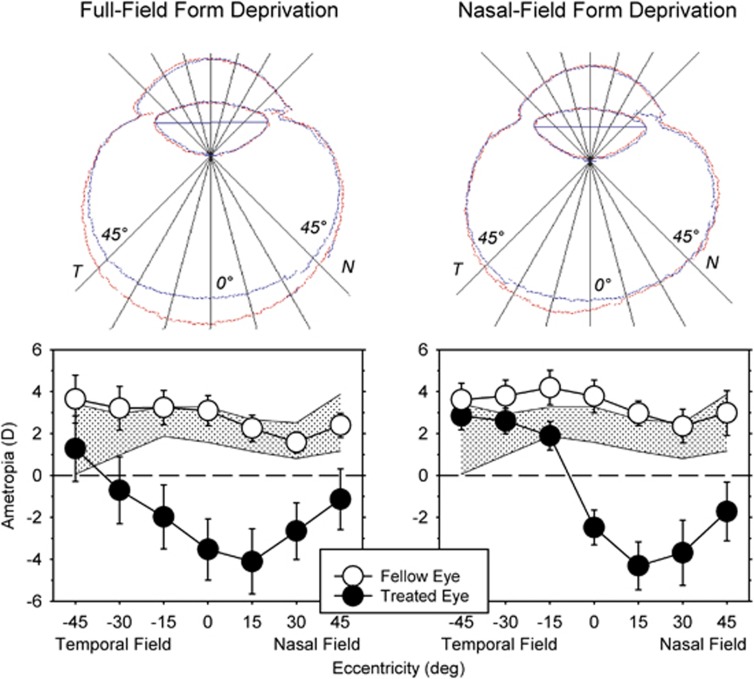
As shown first in chickens by Wallman et al,44 when visual manipulations that influence eye growth (eg, form deprivation,44, 45 myopic defocus46, 47 or hyperopic defocus47, 48) are imposed across only half the visual field, the resulting alterations in refractive error and vitreous chamber growth are restricted to the treated hemi retina. For example, when form deprivation, a strong stimulus for axial growth, is imposed across the entire visual field, the eye becomes more prolate in shape with the greatest increase in vitreous chamber depth found in the central retina with smaller symmetrical increases in the nasal and temporal retina (Figure 2 left).49 The resulting myopic changes in refractive error are also relatively symmetric across the horizontal meridian. However, when form deprivation is restricted to the nasal visual field, the greatest increases in vitreous chamber depth are found in the temporal retina and the concomitant myopic refractive errors are restricted to the nasal visual field (Figure 2 right).45
Figure 2.
Top: outlines of horizontal magnetic resonance images for the treated (red) and fellow eyes (blue) of representative monkeys reared with monocular diffuser lenses that produced form deprivation over the entire visual field (left) or only over the nasal visual field (right). T, temporal; N, nasal. Bottom: mean (±SE) spherical-equivalent refractive corrections plotted as a function of eccentricity for the treated (filled circles) and fellow eyes (open circles) of monkeys reared monocular full-field (left) and nasal-field (right) form deprivation. The shade area represents ±2 SD from the mean for normal monkeys. Replotted from Smith et al.45
These results indicate that the vision-dependent mechanisms that regulate refractive development operate in a local, regionally selective manner. In essence, visual signals from a given retinal location are integrated in a spatially restricted manner and exert their influence selectively on the subjacent sclera. Thus, during emmetropization, these mechanisms probably regulate the shape of the eye to optimize refractive error across the retina. The predominance of local acting mechanisms in regulating refractive development has a number of implications. First, it reduces the likelihood that more global mechanisms have a major role in refractive development. For example, it is difficult to imagine how the act of accommodation or increases in intraocular pressure, both of which have often been hypothesized to have a role in the genesis of myopia, could produce regionally selective changes in refractive error.42 The presence of local mechanisms also has important implications for optical treatment strategies for myopia. For instance, because the refractive state at the fovea is dependent on ocular changes at the posterior pole and in the periphery (ie, an expansion of the sclera in the periphery would displace the central retina in a posterior direction along the visual axis),50 peripheral visual signals can influence central refractive development in a manner that is independent of the nature of central vision. Moreover, optical manipulations of peripheral vision, which do not alter central vision, may be effective in controlling central refractive development.51, 52, 53
Foveal visual signals are not essential for the regulation of eye growth.
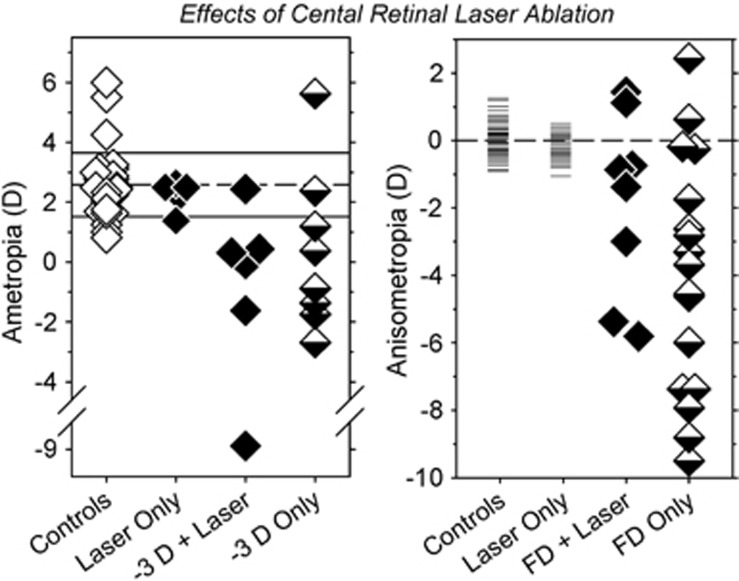
Because (1) visual acuity is highest at the fovea and decreases rapidly with eccentricity, (2) foveal vision is very sensitive to optical defocus, (3) visual signals from the fovea control accommodation, and (4) foveal vision dominates our perception of the world, it has frequently been assumed that signals from the fovea dominate refractive development.54 Although this is a very logical assumption, several observations contradict this widely held belief. For instance, if this assumption is correct then eliminating visual signals from the fovea should alter vision-dependent refractive development. However, as shown in Figure 3, foveal signals are not required for normal emmetropization.55 Laser foveal ablation does not alter the end point for emmetropization or the normal interocular balance in refractive errors. The time course and efficiency of emmetropization is also not altered by foveal ablation. In addition, laser ablation of the fovea does not prevent the eye from becoming myopic in response to either optically induced hyperopic defocus (left)53 or form deprivation (right).55 Nor does foveal laser ablation interfere with the ability of the eye to recover from either induced myopia or hyperopia.56 Overall, these results indicate that signals from the fovea are not required to detect the presence of a refractive error or to alter axial growth to eliminate an existing or an imposed optical error. In contrast, the peripheral retina (loosely meaning the area outside the central 12 degrees of the retina) in isolation can detect a refractive error and regulate emmetropization to eliminate natural or imposed errors.
Figure 3.
Left: spherical-equivalent refractive corrections obtained at ages corresponding to the end of the lens-rearing period for the right or treated eyes of individual animals reared with unrestricted vision (controls), laser ablation of the treated eye fovea (laser only),55 foveal ablation and −3D lenses, or intact retinas and −3D treatment lenses.53 Right: interocular differences in refractive error (right or treated eye−left or fellow eye) for individual controls and animals reared with laser ablation of the fovea and unrestricted vision, foveal ablation and monocular form deprivation, or only monocular form deprivation.55
From an evolutionary perspective, these results are not surprising because the vision-dependent mechanisms that regulate ocular growth evolved in species without foveas and the emmetropization process operates very efficiently in animals with comparatively low visual acuities.42, 54 With respect to optical treatment strategies for myopia, these findings are important because historically efforts to identify risk factors for myopia have concentrated on central vision, potentially missing critical factors associated with peripheral vision, and optical treatment strategies have been specifically designed to manipulate foveal vision while ignoring the potential impact of peripheral vision.
Peripheral visual signals can dominate central refractive development
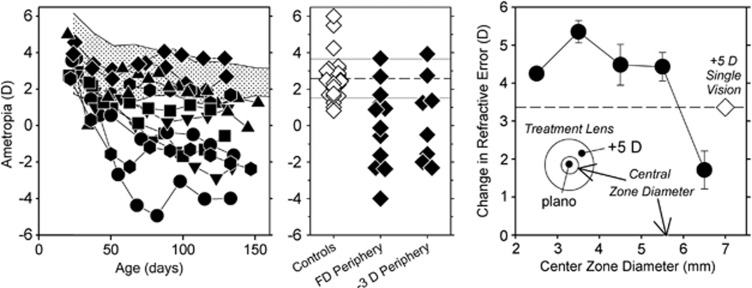
Dioptric demands can change significantly with position in the field, particularly for indoor scenes. As a consequence, the effective focus of the retinal image can vary substantially across the retina.57, 58 The impact of visual growth signals from a given retinal location on central refractive development probably depends on several factors, in particular, the sensitivity of local neurons to defocus, their absolute number and density, and the manner in which growth signals are integrated across the posterior globe. Although there is still much to learn about these factors, when there are conflicting visual signals between the central retina and the periphery, the peripheral signals can dominate central refractive development. For example, as illustrated in the left and middle panels of Figure 4, infant monkeys, which are reared with treatment lenses with central apertures that provide unrestricted central vision and produce either form deprivation53 or hyperopic defocus51, 53 in the periphery, develop central myopia, although the central retina received visual signals that should have supported normal emmetropization. Similarly, the right panel in Figure 4 shows that myopic defocus imposed in the periphery is very effective in slowing axial growth and in producing central hyperopia in chickens,51, 52 which specifically provides support for peripheral optical treatment strategies for myopia.
Figure 4.
Left: spherical-equivalent refractive corrections plotted as a function of age for monkeys reared with diffusers that had either 4 or 8 mm apertures centered over the pupils of both eyes.56 The treatment lenses provided unrestricted vision for the central 24 or 37 degrees of the retina; the rest of the retina experienced severe form deprivation. The shaded area represents the 10th to 90th percentile range of ametropias for control monkeys. Middle: ametropias measured at ages corresponding to the end of the lens-rearing period for the right eye of individual control monkeys (open symbols) and monkeys that were rearing with either peripheral form deprivation or peripheral hyperopic defocus.53 Right: changes in refractive error produced in chickens by treatment lenses with zero-powered central zones and +5D peripheral zones (filled circles) plotted as a function of the diameter of the central zone.51 The open diamond represents the changes in refraction produced by a +5D single-vision lens.
With respect to designing the optimal optical treatment strategy, it will be important to understand how peripheral signals impact central refractive development. By influencing the ability of the sclera to expand in a tangential manner, peripheral mechanisms acting locally could affect the axial position of the posterior retina and central refractive error.50 For example, tangential stretching of the sclera near the eye's equator would increase central axial length without necessarily changing the shape of the posterior globe. It is also likely that the dominate role of the periphery reflects spatial summation of growth signals across the posterior globe. Although the density of many retinal neurons is the highest in the fovea (eg, cone photoreceptors), the fovea is a very small part of the total retina. Areas outside the fovea may dominate central refractive development because the absolute numbers of neurons, particularly neurons that are part of the signal cascade that regulates emmetropization (eg, dopaminergic amacrine cells), is higher in the periphery as a result of areal summation.54 Regardless, the pattern of results indicate that optical treatment strategies that take into account the optical state of the periphery are likely to be more successful than those that do not.59
Relative myopic defocus can dominate refractive development
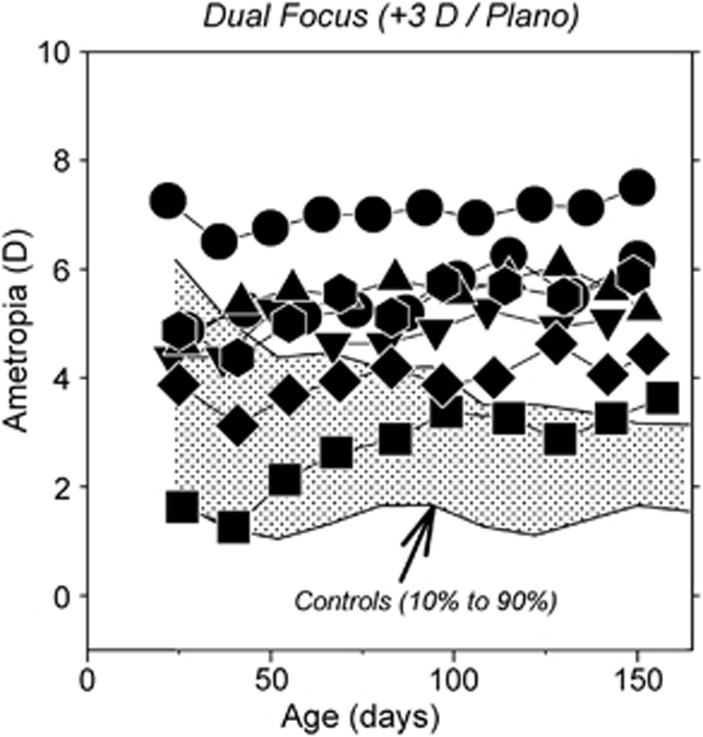
When viewing a three dimensional world, particularly indoors, the eye often experiences myopic defocus, hyperopic defocus, and in-focus retinal images interleaved over time. Moreover, in the presence of astigmatism or when wearing multifocal contact lenses, the eye can experience different types of defocus simultaneously. The manner in which these competing defocus signals are integrated by the vision-dependent mechanisms that regulate refractive development determines the overall direction of ocular growth. Figure 5 shows longitudinal refractive errors for individual monkeys reared with dual-focus Fresnel lenses that had refracting powers of +3D and plano, which established two distinct image planes across the entire visual field (Arumugam et al, IOVS 2013, E abstract 5172). All of the treated monkeys exhibited hyperopic shifts in refractive error. Similarly, the majority of infant monkeys reared with cylinder lenses that had refracting powers of +1.5 D and −1.5 D exhibited relative hyperopic refractive errors.60 In contrast, most monkeys that were treated with dual-focus lenses with powers of −3D and plano exhibited near normal emmetropization (i.e., emmetropization was controlled by the zero-powered portion of the treatment lenses). Overall, these results, which are a similar in nature to those obtained when myopic and hyperopic defocus are interleaved sequentially,61, 62 demonstrate that, when competing defocus signals occur simultaneously, refractive development is dominated by the least hyperopic/more myopic focal plane. These observations support the idea that imposing relative myopic defocus would be an effective means for slowing ocular growth and myopia progression.
Figure 5.
Spherical-equivalent refractive corrections plotted as a function of age for individual monkeys reared with dual-focus Fresnel lenses that had alternating refracting powers of 0D and +3D. The shaded area represents the 10th to 90th percentile range of ametropias for control monkeys.
The effects of imposed defocus depend on the extent of the visual field affected
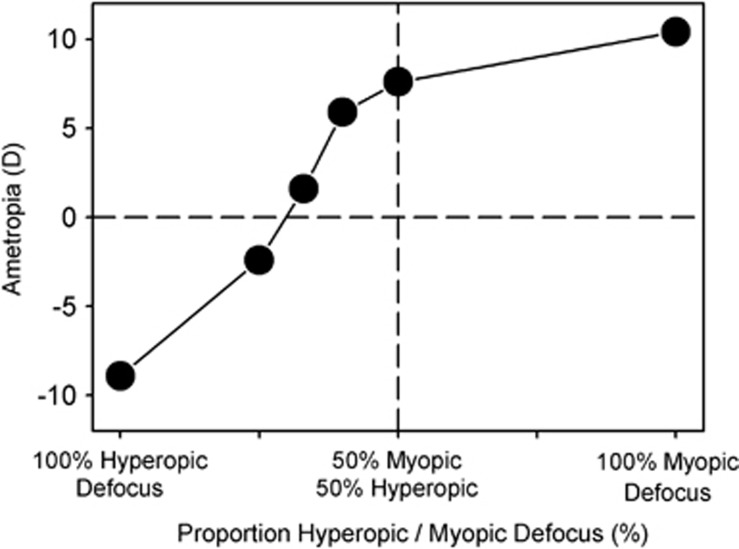
Although local mechanisms regulate refractive development, summation factors are likely to impact the overall effects of imposed defocus on refractive development. To investigate how the extend of the retinal treatment zone influences the efficacy of imposed defocus, Tse and To63 reared chickens wearing cone-like goggles that established two distinct image planes (±10 D) and that could vary the portion of the visual field dedicated to each image plane in a controlled manner. Figure 6 summarizes their results. When 100% of the field of view was devoted to either hyperopic or myopic defocus, the animals developed myopia and hyperopic errors, respectively, which completely compensated for the imposed errors. When equal portions of the field of view were devoted to hyperopic and myopic defocus, the animals developed significant degrees of hyperopia, confirming that myopic defocus has a stronger impact on refractive development than hyperopic defocus. The key point is that there was a systematic reduction in hyperopia/increase in myopia as the proportion of the field that received myopic defocus was reduced from 50 to 0%. In other words, the overall impact of a given type of defocus was dependent on the amount of the field of view that was affected.
Figure 6.
Mean interocular differences in spherical-equivalent refractive corrections for lens-reared chickens plotted as a function of the proportion of the visual field that experienced hyperopic versus myopic defocus.63
Conclusions
With respect to optical treatment strategies for common forms of myopia, animal studies indicate that optically imposed myopic defocus should be effective in slowing myopia progression. In this respect, the optical treatment lenses should ensure that relative myopic defocus is imposed on the retina and that the relative positive-powered portions of the treatment lenses are not simply used by the patient to reduce accommodative demands. To increase the likelihood that a strategy will be successful, the imposed myopic defocus should affect a large proportion of the peripheral retina. In fact, it is not necessary to include the fovea in the effective treatment zone. The emerging data from recent clinical trials show that optical strategies that take into account the peripheral retina and influence imagery over large part of the retina appear to produce larger reductions in myopia progression than those that do not.59 More importantly these novel lens designs are producing clinically significant reductions in the progression of myopia in children.
Acknowledgments
This work was supported by grants from the National Eye Institute (EY-03611 and EY-07551) and funds from the Vision Cooperative Research Centre, Sydney Australia.
Dr Smith is a co-author on patents for anti-myopia lens designs that involve manipulating peripheral optics. Dr Hung and Dr Arumugam have no potential conflicts of interest.
References
- Curtin BJ. Basic Science and Clinical Management. Harper & Row: Philadelphia; 1985. The Myopias. [Google Scholar]
- Rose L, Yinon U, Belkin M. Myopia induced in cats deprived of distance vision during development. Vision Res. 1974;14:1029–1032. doi: 10.1016/0042-6989(74)90172-2. [DOI] [PubMed] [Google Scholar]
- Young FA. The distribution of refractive errors in monkeys. Exp Eye Res. 1964;3:230–238. doi: 10.1016/s0014-4835(64)80015-4. [DOI] [PubMed] [Google Scholar]
- Smith EL., IIIEnvironmentally induced refractive errors in animalsIn: Rosenfield M, Gilmartin B (eds).Myopia and Nearwork Butterworth-Heinemann: Oxford; 199857–90. [Google Scholar]
- Levinsohn FG. Zur frage der kunstlich erzeugten kurzsichtigkeit bei affen. Klinische Monatsblatter fur Augenheilkunde. 1919;61:794–803. [Google Scholar]
- Young FA. The effect of restricted visual space on the primate eye. Am J Ophthalmol. 1961;52:799–806. doi: 10.1016/0002-9394(61)90904-7. [DOI] [PubMed] [Google Scholar]
- Young FA. The development and retention of myopia by monkeys. Am J Optom Arch Am Acad Optom. 1961;38:545–555. doi: 10.1097/00006324-196110000-00001. [DOI] [PubMed] [Google Scholar]
- Young FA. The effect of nearwork illumination level on monkey refraction. Am J Optom Arch Am Acad Optom. 1962;39:60–67. doi: 10.1097/00006324-196202000-00002. [DOI] [PubMed] [Google Scholar]
- Young FA. The effect of restricted visual space on the refractive error of the young monkey eye. Invest Ophthalmol. 1963;2:571–577. [PubMed] [Google Scholar]
- Lauber JK, Kinnear A. Eye enlargement in birds induced by dim light. Canad J Ophthal. 1979;14:265–269. [PubMed] [Google Scholar]
- Lauber JK, McGinnis J. Eye lesions in domestic fowl reared under continuous light. Vision Res. 1966;6:619–626. doi: 10.1016/0042-6989(66)90073-3. [DOI] [PubMed] [Google Scholar]
- Li T, Troilo D, Glasser A, Howland H. Constant light produces severe corneal flattening and hyperopia in chickens. Vision Res. 1995;35:1203–1209. doi: 10.1016/0042-6989(94)00231-a. [DOI] [PubMed] [Google Scholar]
- Liu R, Qian Y-F, He JC, Hu M, Zhou X-T, Dai J-H, et al. Effects of different monochromatic lights on refractive development and eye growth in guinea pigs. Exp Eye Res. 2011;92:447–453. doi: 10.1016/j.exer.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Seidemann A, Schaeffel F. Effects of longitudinal chromatic aberration on accommodation and emmetropization. Vision Res. 2002;42:2409–2417. doi: 10.1016/s0042-6989(02)00262-6. [DOI] [PubMed] [Google Scholar]
- Kroger RH, Wagner HJ. The eye of the blue acara (Aequidens pulcher, Cichlidae) grows to compensate for defocus due to chromatic aberration. J Comp Physiol A. 1996;179:837–842. doi: 10.1007/BF00207362. [DOI] [PubMed] [Google Scholar]
- Rucker RJ, Wallman J. Chick eyes compensate for chromatic simulation of hyperopic and myopic defocus: Evidence that the eye uses longitudinal chromatic aberration to guide eye-growth. Vision Res. 2009;49:1775–1783. doi: 10.1016/j.visres.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rucker FJ, Wallman J. Chicks use changes in luminance and chromatic contrast as indicators of the sign of defocus. J Vis. 2012;12:1–13. doi: 10.1167/12.6.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen Y, Peleg E, Belkin M, Polat U, Solomon AS. Ambient illuminance, retinal dopamine release and refractive development in chicks. Exp Eye Res. 2012;103:33–40. doi: 10.1016/j.exer.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Ashby R, Ohlendorf A, Schaeffel F. The effect of ambient illuminance on the development of deprivation myopia in chicks. Invest Ophthalmol Vis Sci. 2009;50 (11:5348–5354. doi: 10.1167/iovs.09-3419. [DOI] [PubMed] [Google Scholar]
- Ashby RS, Schaeffel F. The effect of bright light on lens compensation in chicks. Invest Ophthalmol Vis Sci. 2010;51 (10:5247–5253. doi: 10.1167/iovs.09-4689. [DOI] [PubMed] [Google Scholar]
- Smith EL, III, Hung L-F, Huang J. Protective effects of high ambient lighting on the development of form-deprivation myopia in rhesus monkeys. Invest Ophthalmol Vis Sci. 2012;53:421–428. doi: 10.1167/iovs.11-8652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EL, III, Hung L-F, Arumugam B, Huang J. Negative lens-induced myopia in infant monkeys: Effects of high ambient lighting. Invest Ophthalmol Vis Sci. 2013;54:2959–2969. doi: 10.1167/iovs.13-11713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton TT, Siegwart JT., Jr Light levels, refractive development, and myopia - A speculative review. Exp Eye Res. 2013;114:48–57. doi: 10.1016/j.exer.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guggenheim JA, Northstone K, McMahon G, Ness AR, Deere K, Mattocks C, et al. Time outdoors and physical activiity as predictors of incident myopia in childhood: A prospective cohort study. Invest Ophthalmol Vis Sci. 2012;53:2856–2865. doi: 10.1167/iovs.11-9091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones-Jordan LA, Sinnott LT, Cotter SA, Kleinstein RN, Manny RE, Mutti DO, et al. Time outdoors, visual acuity, and myopia progression in juvenile-onset myopes. Invest Ophthalmol Vis Sci. 2012;53:7169–7175. doi: 10.1167/iovs.11-8336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose K, Morgan I, Ip J, Kifley A, Huynh S, Smith W, et al. Outdoor activity reduces the prevalence of myopia in children. Ophthalmology. 2008;115:1279–1285. doi: 10.1016/j.ophtha.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Hung L-F, Crawford MLJ, Smith EL., III Spectacle lenses alter eye growth and the refractive status of young monkeys. Nature Med. 1995;1:761–765. doi: 10.1038/nm0895-761. [DOI] [PubMed] [Google Scholar]
- Schaeffel F, Glasser A, Howland HC. Accommodation, refractive error and eye growth in chickens. Vision Res. 1988;28:639–657. doi: 10.1016/0042-6989(88)90113-7. [DOI] [PubMed] [Google Scholar]
- Smith EL, III, Hung L-F. The role of optical defocus in regulating refractive development in infant monkeys. Vision Res. 1999;39:1415–1435. doi: 10.1016/s0042-6989(98)00229-6. [DOI] [PubMed] [Google Scholar]
- Wildsoet C, Wallman J. Choroidal and scleral mechanisms of compensation for spectacle lenses in chicks. Vision Res. 1995;35:1175–1194. doi: 10.1016/0042-6989(94)00233-c. [DOI] [PubMed] [Google Scholar]
- Howlett MH, McFadden SA. Spectacle lens compensation in the pigmented guinea pig. Vision Res. 2009;49:219–227. doi: 10.1016/j.visres.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Shaikh AW, Siegwart JT, Norton TT. Effect of interrupted lens wear on compensation for a minus lens in tree shrews. Optom Vis Sci. 1999;76:308–315. doi: 10.1097/00006324-199905000-00019. [DOI] [PubMed] [Google Scholar]
- Whatham A, Judge S. Compensatory changes in eye growth and refraction induced by daily wear of soft contact lenses in young marmosets. Vision Res. 2001;41:267–273. doi: 10.1016/s0042-6989(00)00250-9. [DOI] [PubMed] [Google Scholar]
- Smith EL, III, Bradley DV, Fernandes A, Boothe RG. Form deprivation myopia in adolescent monkeys. Optom Vis Sci. 1999;76:428–432. doi: 10.1097/00006324-199906000-00023. [DOI] [PubMed] [Google Scholar]
- Zhong X, Ge J, Nie H, Smith EL., III Compensation for experimentally induced hyperopic anisometropia in adolescent monkeys. Invest Ophthalmol Vis Sci. 2004;45:3373–3379. doi: 10.1167/iovs.04-0226. [DOI] [PubMed] [Google Scholar]
- Papastergiou GI, Schmid GF, Laties AM, Pendrak K, Lin T, Stone RA. Induction of axial elongation and myopic refractive shift in one year old chickens. Vision Res. 1998;38:1883–1888. doi: 10.1016/s0042-6989(97)00347-7. [DOI] [PubMed] [Google Scholar]
- Siegwart JT, Norton TT. The susceptible period for deprivation-induced myopia in tree shrew. Vision Res. 1998;38:3505–3515. doi: 10.1016/s0042-6989(98)00053-4. [DOI] [PubMed] [Google Scholar]
- Qiao-Grider Y, Hung L-F, Kee C-s, Ramamirtham R, Smith E., III Normal ocular development in young rhesus monkeys (Macaca mulatta) Vision Res. 2007;47:1424–1444. doi: 10.1016/j.visres.2007.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadnik K, Mutti DO.Incidence and distribution of refractive anomaliesIn: Benjamin WJ (ed).Borish's Clinical Refraction W.B. Saunders: Philadelphia; 199830–46. [Google Scholar]
- Hung L-F, Wallman J, Smith EL., III Vision dependent changes in the choroidal thickness of macaque monkeys. Invest Ophthalmol Vis Sci. 2000;41:1259–1269. [PubMed] [Google Scholar]
- Zhu X, McBrien NA, Smith EL, 3rd, Troilo D, Wallman J. Eyes in various species can shorten to compensate for myopic defocus. Invest Ophthalmol Vis Sci. 2013;54:2634–2644. doi: 10.1167/iovs.12-10514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EL, III, Charles F. Prentice Award Lecture 2010: A case for peripheral optical treatment strategies for myopia. Optom Vis Sci. 2011;88:1029–1044. doi: 10.1097/OPX.0b013e3182279cfa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips JR. Monovision slows juvenile myopia progression unilaterally. Brit J Ophthalmol. 2005;89:1196–1200. doi: 10.1136/bjo.2004.064212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallman J, Gottlieb MD, Rajaram V, Fugate-Wentzek L. Local retinal regions control local eye growth and myopia. Science. 1987;237:73–77. doi: 10.1126/science.3603011. [DOI] [PubMed] [Google Scholar]
- Smith EL, III, Huang J, Hung L-F, Ramamirtham R, Blasdel T, Humbird T, et al. Hemiretinal form deprivation: evidence for local control of eye growth and refractive development in infant monkeys. Invest Ophthalmol Vis Sci. 2009;50:5057–5069. doi: 10.1167/iovs.08-3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EL, 3rd, Hung L-F, Huang J, Arumugam B. Effects of local myopic defocus on refractive development in monkeys. Optom Vis Sci. 2013;90:1176–1186. doi: 10.1097/OPX.0000000000000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diether S, Schaeffel F. Local changes in eye growth induced by imposed local refractive error despite active accommodation. Vision Res. 1997;37:659–668. doi: 10.1016/s0042-6989(96)00224-6. [DOI] [PubMed] [Google Scholar]
- Smith EL, III, Hung L-F, Huang J, Blasdel T, Humbird T, Bockhorst K. Effects of optical defocus on refractive development in monkeys: evidence for local, regionally selective mechanisms. Invest Ophthalmol Vis Sci. 2010;51:3864–3873. doi: 10.1167/iovs.09-4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Hung L-F, Ramamirtham R, Blasdel T, Humbird T, Bockhorst K, et al. Effects of form deprivation on peripheral refractions and ocular shape in infant rhesus monkeys (Macaca mulatta) Invest Ophthalmol Vis Sci. 2009;50:4033–4044. doi: 10.1167/iovs.08-3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidemann A, Schaeffel F, Guirao A, Lopez-Gil N, Artal P. Peripheral refractive errors in myopic, emmetropic, and hyperopic young subjects. J Opt Soc Am A. 2002;19:2363–2373. doi: 10.1364/josaa.19.002363. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wildsoet CF. The effect of 2-zone concentric bifocal spectacle lenses on refractive error development and eye growth in young chicks. Invest Ophthalmol Vis Sci. 2011;52:1078–1086. doi: 10.1167/iovs.10-5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wildsoet CF. The effective add inherent in 2-zone negative lenses inhibits eye growth in myopic young chicks. Invest Ophthalmol Vis Sci. 2012;53:5085–5093. doi: 10.1167/iovs.12-9628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EL, III, Hung L-F, Huang J. Relative peripheral hyperopic defocus alters central refractive development in monkeys. Vision Res. 2009;49:2386–2392. doi: 10.1016/j.visres.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallman J, Winawer J. Homeostasis of eye growth and the question of myopia. Neuron. 2004;43:447–468. doi: 10.1016/j.neuron.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Smith EL, III, Ramamirtham R, Qiao-Grider Y, Hung L-F, Huang J, Kee CS, et al. Effects of foveal ablation on emmetropization and form-deprivation myopia. Invest Ophthalmol Vis Sci. 2007;48:3914–3922. doi: 10.1167/iovs.06-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EL, III, Kee C-s, Ramamirtham R, Qiao-Grider Y, Hung L-F. Peripheral vision can influence eye growth and refractive development in infant monkeys. Invest Ophthalmol Vis Sci. 2005;46:3965–3972. doi: 10.1167/iovs.05-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charman WN. Myopia, posture and the visual environment. Ophthal Physiol Opt. 2011;31:494–501. doi: 10.1111/j.1475-1313.2011.00825.x. [DOI] [PubMed] [Google Scholar]
- Flitcroft DI. The complex interactions of retinal, optical and environmental factors in myopia aetiology. Prog Retin Eye Res. 2012;31:622–660. doi: 10.1016/j.preteyeres.2012.06.004. [DOI] [PubMed] [Google Scholar]
- Smith EL., III Optical treatment strategies to slow myopia progression: effects of the visual extent of the optical treatment zone. Exp Eye Res. 2013;114:77–88. doi: 10.1016/j.exer.2012.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kee C-s, Hung L-F, Qiao-Grider Y, Roorda A, Smith EL., III Effects of optically imposed astigmatism on emmetropization in infant monkeys. Invest Ophthalmol Vis Sci. 2004;45:1647–1659. doi: 10.1167/iovs.03-0841. [DOI] [PubMed] [Google Scholar]
- Kee C-s, Hung LF, Qiao-Grider Y, Ramamirtham R, Winawer J, Wallman J, et al. Temporal constraints on experimental emmetropization in infant monkeys. Invest Ophthalmol Vis Sci. 2007;48:957–962. doi: 10.1167/iovs.06-0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winawer J, Zhu X, Choi J, Wallman J. Ocular compensation for alternating myopic and hyperopic defocus. Vision Res. 2005;45:1667–1677. doi: 10.1016/j.visres.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Tse DY, To C-H. Graded competing regional myopic and hyperopic defocus produce summated emmetropization set points in chick. Invest Ophthalmol Vis Sci. 2011;52:8056–8062. doi: 10.1167/iovs.10-5207. [DOI] [PubMed] [Google Scholar]