Abstract
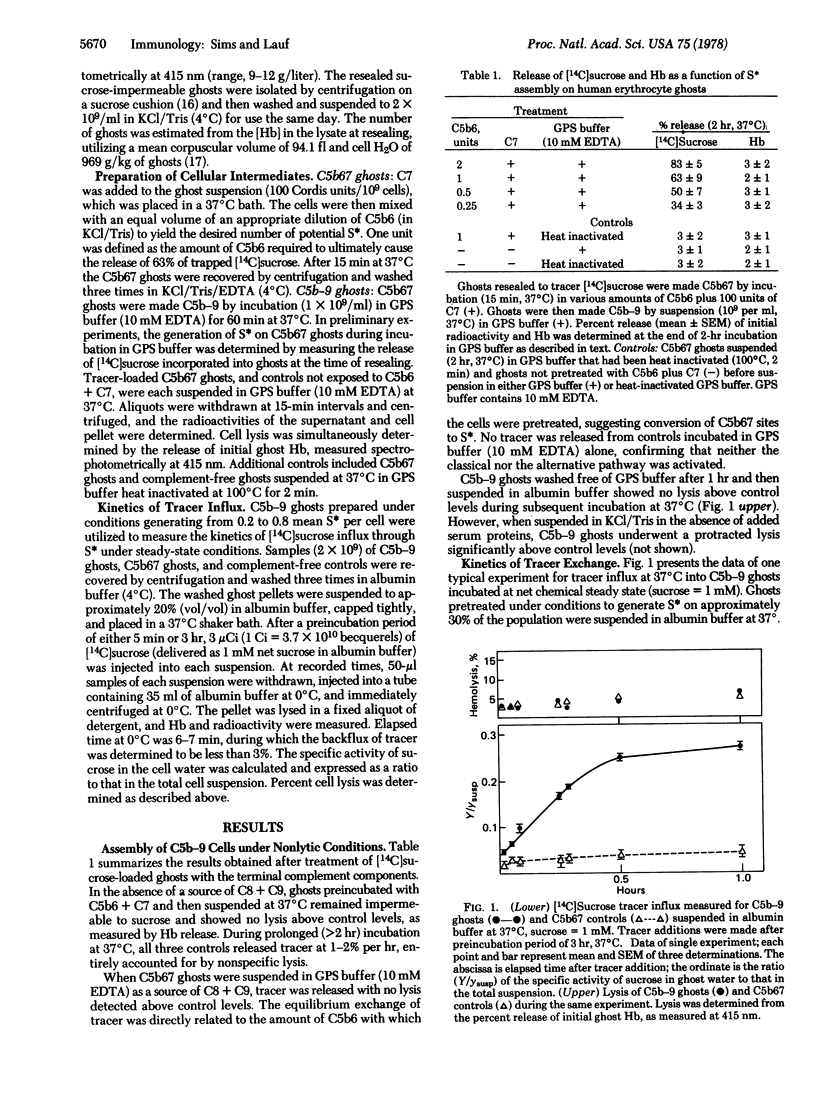
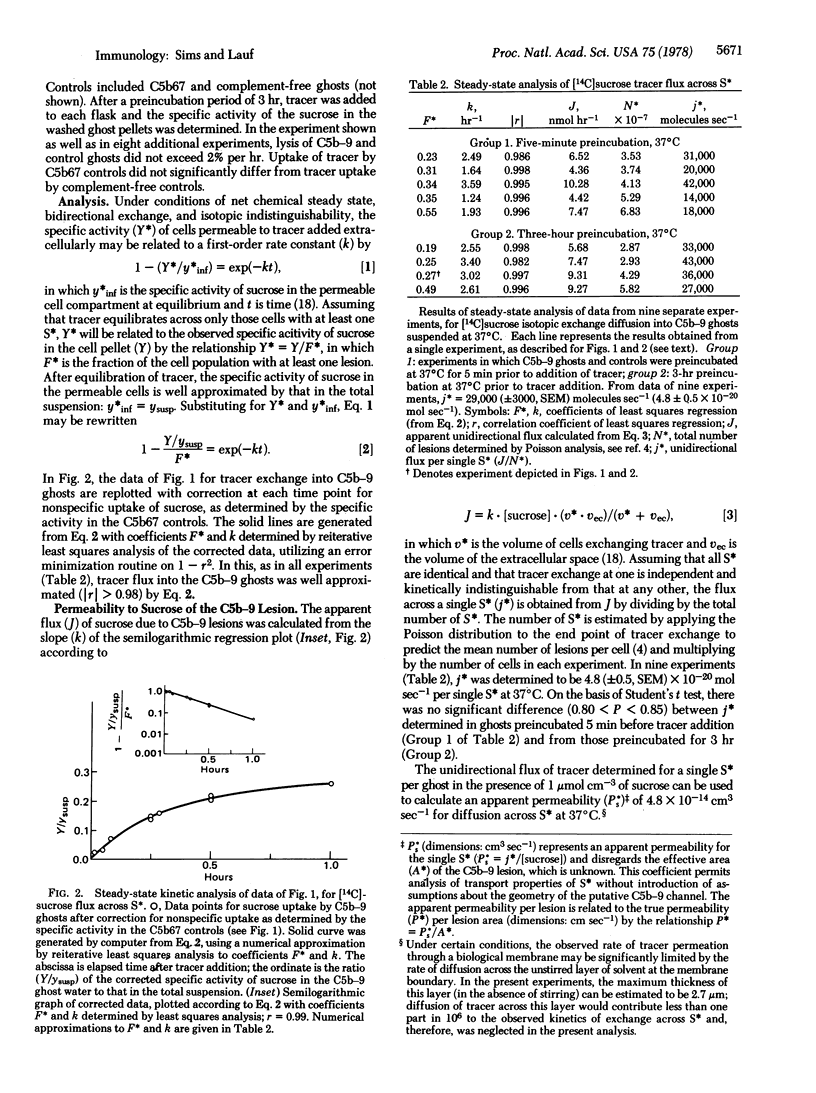
Resealed erythrocyte ghosts have been used to define the kinetics of tracer exchange across the membrane-bound terminal complex of the complement cascade (C5b-9). Under steady-state conditions and at net chemical equilibrium, C5b-9 ghosts showed no significant lysis above control levels as measured by hemoglobin efflux. In 1 mM sucrose at 37 degrees C, [14C]sucrose isotopic exchange diffusion into C5b-9 ghosts occurred at 4.8 (+/- 0.5, SEM) X 10(-20) mol sec-1 per functional lesion, equivalent to an apparent permeability coefficient of 4.8 X 10(-14) cm3 sec-1 for the single C5b-9 lesion. No significant uptake of [14C]sucrose above control levels was observed in C5b67 ghosts. The apparent rate of tracer permeation through the complement lesion is one to two orders of magnitude slower than predicted by a model of a transmembrane channel of dimensions permitting free diffusion of sucrose. The data support earlier assertions from this laboratory that diffusion of small molecules across the complement lesion in biological membranes is significantly restricted.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. J., Rubin L. G., Lint T. F., McLeod B. C., Gewurz H. Binding of the complement intermediate C56 to zymosan in acute phase human sera. Clin Exp Immunol. 1975 Apr;20(1):113–124. [PMC free article] [PubMed] [Google Scholar]
- Boyle M. D., Langone J. J., Borsos T. Studies on the terminal stages of immune hemolysis. III. Distinction between the insertion of C9 and the formation of a transmembrane channel. J Immunol. 1978 May;120(5):1721–1725. [PubMed] [Google Scholar]
- Ehrenstein G., Lecar H., Nossal R. The nature of the negative resistance in bimolecular lipid membranes containing excitability-inducing material. J Gen Physiol. 1970 Jan;55(1):119–133. doi: 10.1085/jgp.55.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg M., Hall J. E., Mead C. A. The nature of the voltage-dependent conductance induced by alamethicin in black lipid membranes. J Membr Biol. 1973 Dec 31;14(2):143–176. doi: 10.1007/BF01868075. [DOI] [PubMed] [Google Scholar]
- Funder J., Wieth J. O. Chloride transport in human erythrocytes and ghosts: a quantitative comparison. J Physiol. 1976 Nov;262(3):679–698. doi: 10.1113/jphysiol.1976.sp011615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN H., BARROW P., GOLDBERG B. Effect of antibody and complement on permeability control in ascites tumor cells and erythrocytes. J Exp Med. 1959 Nov 1;110:699–713. doi: 10.1084/jem.110.5.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingson D. J., Massengill R. K., Mayer M. M. The kinetics of release of 86rubidium and hemoglobin from erythrocytes damaged by antibody and complement. Immunochemistry. 1969 Mar;6(2):295–307. doi: 10.1016/0019-2791(69)90166-9. [DOI] [PubMed] [Google Scholar]
- Hoffmann L. G. Statistical evaluation of reaction mechanisms in immune hemolysis. II. The kinetics of release of 86rubidium and hemoglobin from erythrocytes damaged by antibody and complement. Immunochemistry. 1969 Mar;6(2):309–325. doi: 10.1016/0019-2791(69)90167-0. [DOI] [PubMed] [Google Scholar]
- Humphrey J. H., Dourmashkin R. R. The lesions in cell membranes caused by complement. Adv Immunol. 1969;11:75–115. doi: 10.1016/s0065-2776(08)60478-2. [DOI] [PubMed] [Google Scholar]
- Lachmann P. J., Thompson R. A. Reactive lysis: the complement-mediated lysis of unsensitized cells. II. The characterization of activated reactor as C56 and the participation of C8 and C9. J Exp Med. 1970 Apr 1;131(4):643–657. doi: 10.1084/jem.131.4.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauf P. K. Immunological and physiological characteristics of the rapid immune hemolysis of neuraminidase-treated sheep red cells produced by fresh guinea pig serum. J Exp Med. 1975 Oct 1;142(4):974–988. doi: 10.1084/jem.142.4.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C. K., Levine R. P. Rate processes in the final stage of complement hemolysis. Immunochemistry. 1977 Jun;14(6):421–428. doi: 10.1016/0019-2791(77)90167-7. [DOI] [PubMed] [Google Scholar]
- Mayer M. M. Mechanism of cytolysis by complement. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2954–2958. doi: 10.1073/pnas.69.10.2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod B., Baker P., Gewurz H. Studies on the inhibition of C56 initiated lysis (reactive lysis). I. Description of the phenomenon and methods of assay. Immunology. 1974 Jun;26(6):1145–1157. [PMC free article] [PubMed] [Google Scholar]
- Michaels D. W., Abramovitz A. S., Hammer C. H., Mayer M. M. Increased ion permeability of planar lipid bilayer membranes after treatment with the C5b-9 cytolytic attack mechanism of complement. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2852–2856. doi: 10.1073/pnas.73.8.2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Eberhard H. J. Complement. Annu Rev Biochem. 1975;44:697–724. doi: 10.1146/annurev.bi.44.070175.003405. [DOI] [PubMed] [Google Scholar]
- RENKIN E. M. Filtration, diffusion, and molecular sieving through porous cellulose membranes. J Gen Physiol. 1954 Nov 20;38(2):225–243. [PMC free article] [PubMed] [Google Scholar]
- Schwoch G., Passow H. Preparation and properties of human erythrocyte ghosts. Mol Cell Biochem. 1973 Dec 15;2(2):197–218. doi: 10.1007/BF01795474. [DOI] [PubMed] [Google Scholar]
- TOSTESON D. C., HOFFMAN J. F. Regulation of cell volume by active cation transport in high and low potassium sheep red cells. J Gen Physiol. 1960 Sep;44:169–194. doi: 10.1085/jgp.44.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranum-Jensen J., Bhakdi S., Bhakdi-Lehnen B., Bjerrum O. J., Speth V. Complement lysis: the ultrastructure and orientation of the C5b-9 complex on target sheep erythrocyte membranes. Scand J Immunol. 1978;7(1):45–46. doi: 10.1111/j.1365-3083.1978.tb00425.x. [DOI] [PubMed] [Google Scholar]
- Valet G., Franz G., Lauf P. K. Different red cell populations in newborn, genetically low potassium sheep: relation to hematopoietic, immunologic and physiologic differentiation. J Cell Physiol. 1978 Feb;94(2):215–227. doi: 10.1002/jcp.1040940211. [DOI] [PubMed] [Google Scholar]
- Valet G., Opferkuch W. Mechanism of complement-induced cell lysis. Demonstration of a three-step mechanism of EAC1-8 cell lysis by C9 and of a non-osmotic swelling of erythrocytes. J Immunol. 1975 Oct;115(4):1028–1033. [PubMed] [Google Scholar]
- Wobschall D., McKeon C. Step conductance increases in bilayer membranes induced by antibody-antigen-complement action. Biochim Biophys Acta. 1975 Dec 1;413(2):317–321. doi: 10.1016/0005-2736(75)90117-0. [DOI] [PubMed] [Google Scholar]


