Abstract
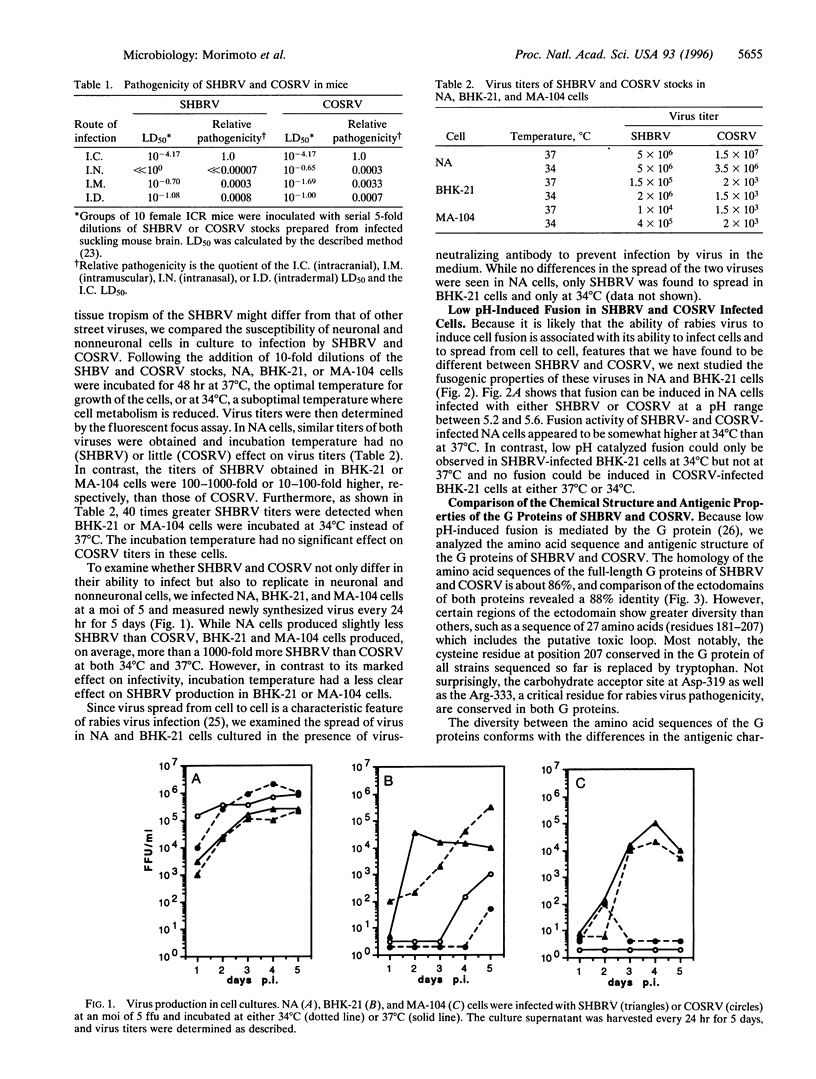
The silver-haired bat variant of rabies virus (SHBRV) has been identified as the etiological agent of a number of recent human rabies cases in the United States that are unusual in not having been associated with any known history of conventional exposure. Comparison of the different biological and biochemical properties of isolates of this virus with those of a coyote street rabies virus (COSRV) revealed that there are unique features associated with SHBRV. In vitro studies showed that, while the susceptibility of neuroblastoma cells to infection by both viruses was similar, the infectivity of SHBRV was much higher than that of COSRV in fibroblasts (BHK-21) and epithelial cells (MA-104), particularly when these cells were kept at 34 degrees C. At this temperature, low pH-dependent fusion and cell-to-cell spread of virus is seen in BHK-21 cells infected with SHBRV but not with COSRV. It appears that SHBRV may possess an unique cellular tropism and the ability to replicate at lower temperature, allowing a more effective local replication in the dermis. This hypothesis is supported by in vivo results which showed that while SHBRV is less neurovirulent than COSRV when administered via the intramuscular or intranasal routes, both viruses are equally neuroinvasive if injected intracranially or intradermally. Consistent with the above findings, the amino acid sequences of the glycoproteins of SHBRV and COSRV were found to have substantial differences, particularly in the region that contains the putative toxic loop, which are reflected in marked differences in their antigenic composition. Nevertheless, an experimental rabies vaccine based on the Pittman Moore vaccine strain protected mice equally well from lethal doses of SHBRV and COSRV, suggesting that currently used vaccines should be effective in the postexposure prophylaxis of rabies due to SHBRV.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bai X., Warner C. K., Fekadu M. Comparisons of nucleotide and deduced amino acid sequences of the glycoprotein genes of a Chinese street strain (CGX89-1) and a Chinese vaccine strain (3aG) of rabies virus. Virus Res. 1993 Feb;27(2):101–112. doi: 10.1016/0168-1702(93)90074-w. [DOI] [PubMed] [Google Scholar]
- Benmansour A., Brahimi M., Tuffereau C., Coulon P., Lafay F., Flamand A. Rapid sequence evolution of street rabies glycoprotein is related to the highly heterogeneous nature of the viral population. Virology. 1992 Mar;187(1):33–45. doi: 10.1016/0042-6822(92)90292-w. [DOI] [PubMed] [Google Scholar]
- Charlton K. M., Casey G. A. Experimental rabies in skunks: immunofluorescence light and electron microscopic studies. Lab Invest. 1979 Jul;41(1):36–44. [PubMed] [Google Scholar]
- Clark K. A., Neill S. U., Smith J. S., Wilson P. J., Whadford V. W., McKirahan G. W. Epizootic canine rabies transmitted by coyotes in south Texas. J Am Vet Med Assoc. 1994 Feb 15;204(4):536–540. [PubMed] [Google Scholar]
- Coulon P., Rollin P. E., Flamand A. Molecular basis of rabies virus virulence. II. Identification of a site on the CVS glycoprotein associated with virulence. J Gen Virol. 1983 Mar;64(Pt 3):693–696. doi: 10.1099/0022-1317-64-3-693. [DOI] [PubMed] [Google Scholar]
- Dietzschold B., Ertl H. C. New developments in the pre- and post-exposure treatment of rabies. Crit Rev Immunol. 1991;10(5):427–439. [PubMed] [Google Scholar]
- Dietzschold B., Wiktor T. J., Trojanowski J. Q., Macfarlan R. I., Wunner W. H., Torres-Anjel M. J., Koprowski H. Differences in cell-to-cell spread of pathogenic and apathogenic rabies virus in vivo and in vitro. J Virol. 1985 Oct;56(1):12–18. doi: 10.1128/jvi.56.1.12-18.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzschold B., Wunner W. H., Wiktor T. J., Lopes A. D., Lafon M., Smith C. L., Koprowski H. Characterization of an antigenic determinant of the glycoprotein that correlates with pathogenicity of rabies virus. Proc Natl Acad Sci U S A. 1983 Jan;80(1):70–74. doi: 10.1073/pnas.80.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudin Y., Tuffereau C., Segretain D., Knossow M., Flamand A. Reversible conformational changes and fusion activity of rabies virus glycoprotein. J Virol. 1991 Sep;65(9):4853–4859. doi: 10.1128/jvi.65.9.4853-4859.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habel K. Laboratory techniques in rabies. Habel test for potency. Monogr Ser World Health Organ. 1966;23:140–143. [PubMed] [Google Scholar]
- Lentz T. L., Burrage T. G., Smith A. L., Crick J., Tignor G. H. Is the acetylcholine receptor a rabies virus receptor? Science. 1982 Jan 8;215(4529):182–184. doi: 10.1126/science.7053569. [DOI] [PubMed] [Google Scholar]
- Lentz T. L., Wilson P. T., Hawrot E., Speicher D. W. Amino acid sequence similarity between rabies virus glycoprotein and snake venom curaremimetic neurotoxins. Science. 1984 Nov 16;226(4676):847–848. doi: 10.1126/science.6494916. [DOI] [PubMed] [Google Scholar]
- Lorenz R. J., Bögel K. Laboratory techniques in rabies: methods of calculation. Monogr Ser World Health Organ. 1973;(23):321–335. [PubMed] [Google Scholar]
- Mifune K., Ohuchi M., Mannen K. Hemolysis and cell fusion by rhabdoviruses. FEBS Lett. 1982 Jan 25;137(2):293–297. doi: 10.1016/0014-5793(82)80370-0. [DOI] [PubMed] [Google Scholar]
- Morimoto K., Ni Y. J., Kawai A. Syncytium formation is induced in the murine neuroblastoma cell cultures which produce pathogenic type G proteins of the rabies virus. Virology. 1992 Jul;189(1):203–216. doi: 10.1016/0042-6822(92)90696-M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy F. A., Bauer S. P., Harrison A. K., Winn W. C., Jr Comparative pathogenesis of rabies and rabies-like viruses. Viral infection and transit from inoculation site to the central nervous system. Lab Invest. 1973 Mar;28(3):361–376. [PubMed] [Google Scholar]
- Murphy F. A. Rabies pathogenesis. Arch Virol. 1977;54(4):279–297. doi: 10.1007/BF01314774. [DOI] [PubMed] [Google Scholar]
- Ravkov E. V., Smith J. S., Nichol S. T. Rabies virus glycoprotein gene contains a long 3' noncoding region which lacks pseudogene properties. Virology. 1995 Jan 10;206(1):718–723. doi: 10.1016/s0042-6822(95)80095-6. [DOI] [PubMed] [Google Scholar]
- Reagan K. J., Wunner W. H. Rabies virus interaction with various cell lines is independent of the acetylcholine receptor. Arch Virol. 1985;84(3-4):277–282. doi: 10.1007/BF01378980. [DOI] [PubMed] [Google Scholar]
- Rupprecht C. E., Smith J. S., Fekadu M., Childs J. E. The ascension of wildlife rabies: a cause for public health concern or intervention? Emerg Infect Dis. 1995 Oct-Dec;1(4):107–114. doi: 10.3201/eid0104.950401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar V., Dietzschold B., Koprowski H. Direct entry of rabies virus into the central nervous system without prior local replication. J Virol. 1991 May;65(5):2736–2738. doi: 10.1128/jvi.65.5.2736-2738.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Superti F., Hauttecoeur B., Morelec M. J., Goldoni P., Bizzini B., Tsiang H. Involvement of gangliosides in rabies virus infection. J Gen Virol. 1986 Jan;67(Pt 1):47–56. doi: 10.1099/0022-1317-67-1-47. [DOI] [PubMed] [Google Scholar]
- Warrell M. J. Human deaths from cryptic bat rabies in the USA. Lancet. 1995 Jul 8;346(8967):65–66. doi: 10.1016/s0140-6736(95)92106-0. [DOI] [PubMed] [Google Scholar]
- Whitt M. A., Buonocore L., Prehaud C., Rose J. K. Membrane fusion activity, oligomerization, and assembly of the rabies virus glycoprotein. Virology. 1991 Dec;185(2):681–688. doi: 10.1016/0042-6822(91)90539-n. [DOI] [PubMed] [Google Scholar]
- Wiktor T. J. Laboratoty techniques in rabies: tissue culture methods. Monogr Ser World Health Organ. 1973;(23):101–123. [PubMed] [Google Scholar]
- Wiktor T. J., Macfarlan R. I., Foggin C. M., Koprowski H. Antigenic analysis of rabies and Mokola virus from Zimbabwe using monoclonal antibodies. Dev Biol Stand. 1984;57:199–211. [PubMed] [Google Scholar]
- Wunner W. H., Dietzschold B. Rabies virus infection: genetic mutations and the impact on viral pathogenicity and immunity. Contrib Microbiol Immunol. 1987;8:103–124. [PubMed] [Google Scholar]



