Abstract
It is well established that host-associated microbial communities can interfere with the colonization and establishment of microbes of foreign origins, a phenomenon often referred to as bacterial interference or colonization resistance. However, due to the complexity of the indigenous microbiota, it has been extremely difficult to elucidate the community colonization resistance mechanisms and identify the bacterial species involved. In a recent study, we have established an in vitro mice oral microbial community (O-mix) and demonstrated its colonization resistance against an Escherichia coli strain of mice gut origin. In this study, we further analyzed the community structure of the O-mix by using a dilution/regrowth approach and identified the bacterial species involved in colonization resistance against E. coli. Our results revealed that, within the O-mix there were three different types of bacterial species forming unique social structure. They act as ‘Sensor', ‘Mediator' and ‘Killer', respectively, and have coordinated roles in initiating the antagonistic action and preventing the integration of E. coli. The functional role of each identified bacterial species was further confirmed by E. coli-specific responsiveness of the synthetic communities composed of different combination of the identified players. The study reveals for the first time the sophisticated structural and functional organization of a colonization resistance pathway within a microbial community. Furthermore, our results emphasize the importance of ‘Facilitation' or positive interactions in the development of community-level functions, such as colonization resistance.
Keywords: colonization resistance, microbial community, social structure, host-associated microbiota, facilitation
Introduction
The association with commensal microbiota is a general feature for virtually all animals, including humans (Ley et al., 2008a, 2008b; Robinson et al., 2010a, 2010b). These indigenous microbial communities co-evolve with their hosts and have crucial roles in host development and health, from nutrient acquisition in termites to the establishment of the mucosal immune system in mice and energy metabolism in humans (Hongoh et al., 2003; Backhed et al., 2004; Leslie, 2012).
Among the proposed beneficial effects, the ability of the indigenous community to resist the invasion and colonization by exogenous organisms, termed colonization resistance, has been regarded as one of the major functions of the host-associated microbiota (Brook, 1999; Reid et al., 2001; Falagas et al., 2008). It has been extensively documented in invertebrate- as well as vertebrate-associated commensal microbial communities (Veivers et al., 1982; Guarner and Malagelada, 2003; Dillon et al., 2005). The notion that colonization resistance could be a crucial component of host defense against pathogens and may be manipulated to the host's advantage makes it the subject of constant investigation for the past few decades (Freter, 1956; Lloyd et al., 1977; Van der Waaij and Van der Waaij, 1990; Rolfe, 1997).
Several mechanisms have been proposed to explain the colonization resistance observed in host-associated microbiota, including stimulating the host-immune response against invaders (Corthesy et al., 2007), competition for substrates and host-binding sites (Chan et al., 1985), generating a microenvironment that is inhibitory to potential competitors (Bernet-Camard et al., 1997), and the production of antibiotic substances (Vandenbergh, 1993). Recent work further suggested that community structure of indigenous microbiota likely has a significant role in its colonization resistance (Robinson et al., 2010b). However, due to the complexity of the indigenous microbiota, these hypotheses have scarcely been proven, and it has been extremely difficult to elucidate the community invasion resistance mechanisms by identifying the bacteria species involved and revealing the associated complex processes at the molecular level.
Recently, we established an in vitro oral microbial community (O-mix) derived from mice oral cavity (He et al., 2010a). Although it was not a defined community, the O-mix contained consistent microbial diversity of more than 10 bacteria species, as revealed by PCR-denaturing gradient gel electrophoresis (DGGE), when being recovered from frozen stock. The same microbial profile could be maintained up to 72 h in suspension culture when fresh medium was provided every 24 h (He et al., 2010a). The O-mix displayed a striking ability to restrict the integration of an E. coli strain of mice gut origin as well as E. coli reference strain MG1655, a phenomenon indicative of the colonization resistance within oral microbial community (He et al., 2010a, 2010b). Further investigation revealed that the O-mix was able to detect the presence of E. coli by recognizing its surface lipopolysaccharides (LPS), and respond by producing H2O2, a bactericidal agent that is more effective in killing E. coli cells than various oral isolates tested (He et al., 2010b).
Based on these results, we hypothesized that the presence of E. coli could trigger the activation of a colonization resistance pathway within the O-mix. The pathway might involve multiple bacterial species that have coordinated roles in preventing the colonization of E. coli. In this study, we employed PCR-DGGE analysis to track and compare the microbial profiles of the communities derived from differentially diluted O-mix. By correlating major shift in microbial profiles with the changes in communities' responsivity to E. coli, we sought to investigate the underlying pathway and identify the key players involved in the colonization resistance of the O-mix against the integration of bacteria of foreign origin.
Materials and methods
Bacterial strains and growth conditions
Mice oral isolates, including Staphylococcus saprophyticus MO-100, Streptococcus sanguinis MO-101 and Streptococcus infantis MO-102 (He et al., 2010a), as well as Escherichia coli reference strain (MG1655) (He et al., 2010b) were cultivated in brain heart infusion (BHI) broth supplemented with hemin (5 μg ml−1), vitamin K (0.5 μg ml−1), sucrose (0.1%), mannose (0.1%) and glucose (0.1%). Cultures were incubated at 37 °C under anaerobic conditions (nitrogen 85%, carbon dioxide 5% and hydrogen 10%). When needed, kanamycin (50 μg ml−1) was added to the medium for selecting E. coli MG1655.
The cultivated mice oral microbiota (O-mix) were recovered from a labarotory stock that was described in a previous study (He et al., 2010a). Briefly, 50 μl of BHI-cultivated O-mix stock was inoculated into 5 ml of supplemented BHI broth. The cultures were incubated at 37 °C under anaerobic conditions for ∼16 h until the exponential growth phase was reached, which was determined by the community growth curve measured by optical density reading.
Community dilution assay
The community dilution assay was performed as previously described (Franklin and Mills, 2006) with modifications. Briefly, an overnight culture of the O-mix (∼109 colony-forming units (c.f.u.) per ml) was subjected to serial dilution (10-fold) from 100 to 10−8, using fresh supplemented BHI as the diluent. The corresponding O-mix subcultures for each dilution were incubated at 37 °C under anaerobic condition. Each subculture was harvested and stocks were made when an OD600 of 1 (∼109 c.f.u. per ml) was reached. The resulting sub-communities were referred to as Community 100, Community 10−1, etc. up to Community 10−8. Fifty microliters of each O-mix sub-community stock was inoculated into 2 ml fresh BHI and incubated at 37 °C under anaerobic conditions until the exponential growth phase was reached. 1.5 ml of each regrown community was collected by centrifugation at 14 000 × g for 1 min and the cell pellets were used for DNA extraction and PCR-DGGE analysis as described below. The remainders of the subcultures were harvested, resuspended in fresh BHI to a final OD600 of 0.1 (∼108 c.f.u. ml−1) and were used for testing their hydrogen peroxide (H2O2) production.
Hydrogen peroxide (H2O2) production assay on agar plates
An overnight culture of E. coli reference strain (MG1655) was harvested and resuspended in fresh BHI medium to a final OD600 of 0.01 (∼107 c.f.u. ml−1). 10 μl of resuspended E. coli culture was mixed with equal volumes (10 μl) of resuspended O-mix subcultures (OD600 of 0.1), resulting in a 1:10 E. coli-to-O-mix ratio and used for the H2O2 production assay.
The H2O2 production assay was performed as previously described (He et al., 2012). Briefly, spots of 5 μl of horseradish peroxide (1 ml mg−1; Thermo Scientific, Rockford, IL, USA) were added onto BHI agar plate containing 1 mg ml−1 leuco crystal violet (Acros Organics, Fair Lawn, NJ, USA). After the liquid was absorbed into the agar, 10 μl of the different mixtures was inoculated on top of the spots containing peroxidase. Plates were incubated at 37 °C under anaerobic conditions for 18 h, and the development of purple color on/around the colonies as indication of H2O2 production was monitored. Three independent replicates were performed and a representative result was shown.
PCR-DGGE analysis
PCR-DGGE was employed to track and compare the profiles of microbial communities derived from community dilution assay. Total genomic DNA of bacterial samples from the communities regrown from differentially diluted original O-mix was isolated using the MasterPure DNA Purification Kit (EPICENTRE), DNA quality and quantity were measured by a spectrophotometer at 260 nm and 280 nm (Spectronic Genesys, Spectronic Instruments, Inc., Rochester, NY, USA).
Amplification of bacterial 16S ribosomal RNA genes by PCR was carried out as described previously (Wang et al., 2012). Briefly, the universal primer set, Bac1 (5′-CGCCCGCCGCGCCCCGCGCCCGTCCCGCCGCCCCCGCCCGACTACGTGCCAGCAGCC—3′) (Sheffield et al., 1989) and Bac2 (5′-GGACTACCAGGGTATCTAATCC-3′) was used to amplify an ∼300-bp internal fragment of the 16S ribosomal RNA gene. Each 50 μl PCR contains 100 ng purified genomic DNA, 40 pmol of each primer, 200 μM of each dNTP, 4.0 mM MgCl2, 5 μl 10 × PCR buffer, and 2.5 U Taq DNA polymerase (Invitrogen). Cycling conditions were 94 °C for 3 min, followed by 30 cycles of 94 °C for 1 min, 56 °C for 1 min and 72 °C for 1 min, with a final extension period of 5 min at 72 °C. The resulting PCR products were evaluated by 1% agarose gel electrophoresis.
Polyacrylamide gels at an 8% concentration were prepared with a denaturing urea/formamide gradient between 40% [containing 2.8 M urea and 16% (vol/vol) formamide] and 70% [containing 4.9 M urea and 28% (vol/vol) formamide]. Approximately 300 ng of the PCR product were applied per well. The gels were submerged in 1 × TAE buffer (40 mM Tris base, 40 mM glacial acetic acid, 1 mM ethylenediaminetetraacetic acid) and the PCR products were separated by electrophoresis for 17 h at 58 °C using a fixed voltage of 60 V in the Bio-Rad DCode System (Bio-Rad Laboratories, Inc., Hercules, CA, USA). After electrophoresis, gels were rinsed and stained for 15 min in 1 × TAE buffer containing 0.5 μg ml−1 ethidium bromide, followed by 10 min of de-staining in 1 × TAE buffer. DGGE profile images were digitally recorded using the Molecular Imager Gel Documentation system (Bio-Rad Laboratories). Three independent biological replicates were performed and a representative gel image was shown.
Identification of candidate bacterial species involved in colonization resistance
To identify major bacterial species whose absence as a result of community dilution could correlate with the changes in communities' indigenous H2O2 production profiles as well as their responsiveness to the presence of E. coli, DGGE gel images derived from each sub-community were compared. PCR bands present in communities with lower dilution factors, while missing from cultures with higher dilution factors were excised from the DGGE gels, eluted into 20 μl sterile dH2O as preciously described (He et al., 2010a) and re-amplified with the Bac1/Bac2 universal primers. The resulting PCR products were purified and sequenced at the UCLA Sequencing and Genotyping Core Facility. The obtained partial 16S rRNA gene sequences (about 300 bp) were used to BLAST search against the HOMD (http://www.homd.org) and NCBI (http://www.ncbi.nlm.nih.gov) databases. Sequences with 98%–100% identity to those deposited in the public domain databases were considered to be positive identification of taxa.
Isolation and identification of bacterial species producing hydrogen peroxide
Hydrogen peroxide production assay revealed that the regrowth of the most diluted O-mix was still able to produce H2O2 (Figure 1), suggesting that hydrogen peroxide producing species was one of the most abundant bacteria within the O-mix. To isolate H2O2 producer, the 10−7 community derived from original O-mix via dilution was subjected to serial dilution and the diluted samples were plated onto BHI agar plates. Plates were incubated at 37 °C under anaerobic condition until distinct colonies appeared. Multiple colonies were picked and cultivated in BHI medium until turbid. Hydrogen peroxide production assay on BHI plate was performed as previously described to determine bacterial H2O2-producing capability. For isolates that were able to produce H2O2, their identities were determined by 16S sequencing as previous described (He et al., 2010a).
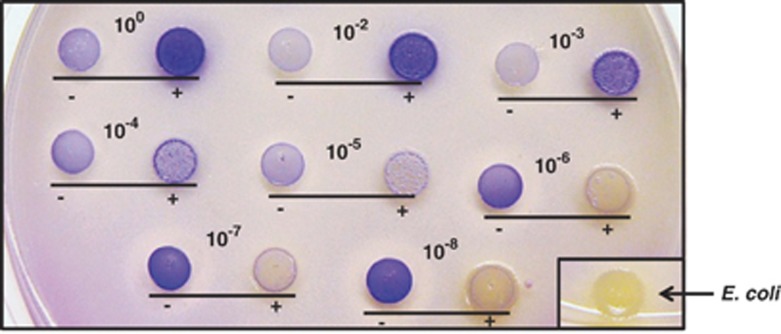
Figure 1.
H2O2 production of oral communities when challenged with E. coli. Oral communities derived from the dilution (100 to 10−8)/regrowth of original O-mix were challenged with (+) or incubated without (−) E. coli (in a 10:1 oral cell-to-E. coli ratio) and the mixtures were spotted onto BHI agar plates containing leuco crystal violet and horseradish peroxidase; blue color development reflecting the H2O2 production was monitored after overnight incubation at 37 °C. Three independent replicates were performed and a representative result is shown.
Construction of synthetic oral microbial communities
Isolated mice oral bacterial species from previous studies, including S. saprophyticus MO-100, S. sanguinis MO-101 and S. infantis MO-102 (He et al., 2010a, 2010b) were used to construct synthetic oral microbial communities. Overnight cultures of individual oral isolates were harvested and resuspended in fresh BHI to an OD600 of 0.01. Synthetic communities with distinct microbial compositions were constructed by combining equal volumes of the selected resuspended bacterial cultures. The cultures were incubated at 37 °C under anaerobic condition for 16 h to allow the community to reach equilibrium indicated by the stable abundance of each bacterial species as determined by PCR-DGGE analysis. The cells in the synthetic community were harvested, and resuspended in fresh BHI medium to a final OD600 of 0.1 before being used for the following Community Invasion Resistance assay.
Community invasion resistance assay
Synthetic communities with distinct microbial composition (OD600 of 0.01, with ∼107 c.f.u. per ml bacterial cells) were mixed with an equal volume of E. coli MG1655 (OD600 of 0.001, with ∼106 c.f.u. per ml), resulting in a 1:10 E. coli-to-synthetic community ratio. Co-cultures were incubated at 37°C under anaerobic condition, and the viability of E. coli as well as the whole community was monitored periodically by plating the cells onto selective (with Kan 50 μg ml−1) and non-selective BHI plates. Plates were incubated at 37 °C for 2 days before colonies were counted. Two independent experiments were performed in triplicates and the average value±standard deviation (s.d.) was shown.
Quantitative measurement of H2O2 concentration in bacterial culture
The H2O2 concentration in bacterial co-cultures was measured as previously described (He et al., 2012). Briefly, 100 μl of bacterial co-culture was subjected to centrifugation at 14 000 × g for 3 min. 20 μl of supernatant or a 1:2 serial dilution of supernatant with PBS was mixed with 20 μl of horseradish peroxidase (0.5 mg ml−1) and 50 μl buffer solution (0.1 mg ml−1 of 3,3′,5,5′-tetramethylbenzidine, 0.05 M phosphate citrate buffer at pH 5.0). The mixture was incubated at room temperature for 1 min, and 100 μl of 2 M H2SO4 was added to stop the reaction. Color intensity was measured at 420 nm using a microplate reader. Three independent experiments were performed and the average value±s.d. is shown.
Two-chamber assay
The two-chamber assay was performed as previously described (He et al., 2010a) with minor modification. Briefly, 2 ml of BHI containing ∼2 × 107 total bacterial cells of different combination of oral bacterial isolates with equal number cells of each species, was inoculated into the bottom chamber of a 12-well plate containing a 0.4 μm PET membrane insert (Millipore, Billerica, MA, USA); while 0.7 ml of bacterial mixture containing E. coli MG1655 (with ∼106 c.f.u. per ml) alone, or E. coli with different oral isolates (with ∼107 c.f.u. per ml for each oral isolate) was added to the top chamber. The viability of E. coli as well as the total bacterial count within the upper and lower chamber was monitored as previously described (He et al., 2010b). Briefly, culture samples were taken periodically, vortexed for 1 min, subjected to serial dilution and plated onto selective plates. Plates were incubated for 3 days at 37 °C under anaerobic condition before colonies were counted. Two independent experiments were performed in duplicates, and average value±s.d. was presented.
Statistical analysis
Significance of differences between average values was analyzed by Student's t-tests.
Results
Communities derived from dilution/regrowth of the original O-mix displayed distinct response patterns when challenged with E. coli
Recently, we demonstrated in vitro that the cultivable mice oral microbial community (O-mix) displayed colonization resistance and responded to the presence of the Gram-negative gut isolate E. coli by elevating its H2O2 production (He et al., 2010b). To further investigate whether the observed colonization resistance required a specific community structure and the involvement of particular bacterial species within the O-mix, we applied a dilution/regrowth approach to test the effect of changes in community structure on its response to the presence of E. coli. The method was based on the premise that dilution of a relatively diverse community would remove less abundant species, resulting in communities with different species richness, and has been employed in studying community composition and structure (Franklin et al., 2001). The result of H2O2 production on agar plates showed that, the original O-mix community produced very low amounts of H2O2 when grown on BHI plates, while co-incubation with E. coli triggered a dramatic increase in H2O2 production as indicated by the development of dark blue color (Figure 1). This spike in H2O2 production was accompanied by a drastic (>90%) reduction in viability of E. coli within the mix (data not shown). Interestingly, compared with the original O-mix, the communities derived from dilution/regrowth displayed altered H2O2-production profile in the absence, as well as in the presence of E. coli.
Specifically, when the original O-mix was titrated below 10−4, the regrown communities failed to respond to the presence of E. coli with elevated H2O2 level. Meanwhile, the regrown communities derived from 10−6 (and lower) dilution of the O-mix displayed drastically increased H2O2 level even in the absence of E. coli, as reflected by the increased intensity of purple color (Figure 1). However, these regrown communities failed to achieve a similar inhibition effect against E. coli as demonstrated by the diminished hydrogen peroxide level when co-incubated with E. coli (Figure 1), as well as increased survival rate (>90%) of E. coli, compared with 10% when E. coli was co-incubated with original O-mix for 24 h (data not shown).
Identification of candidate players involved in community colonization resistance against E. coli
The H2O2 production assay revealed the differential response of the serially diluted/regrown communities to the presence of E. coli (Figure 1). The apparent transition to an ‘inversion' of the response to E. coli was associated with a reduction in species diversity in the oral communities, suggesting the involvement of different bacterial species with distinct roles in colonization resistance via regulated H2O2 production. In an effort to identify these players, microbial profiles of dilution-defined oral communities were obtained by PCR-DGGE analysis (Figure 2), the ‘banding patterns' representing the bacterial community diversity were compared and the unique 16S DNA fragments were sequenced to identify the corresponding bacterial species. All the identified species had been isolated from mice oral cavity in our previous studies (He et al., 2010a, 2010b).
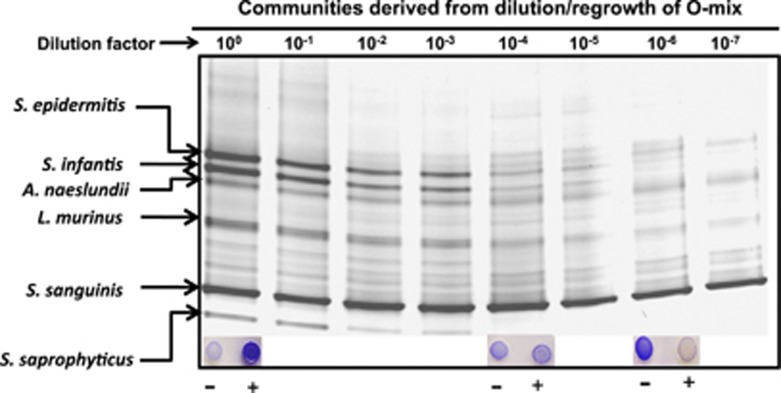
Figure 2.
PCR-DGGE analysis of microbial communities derived from dilution/regrowth of original O-mix. Original O-mix was subjected to serial dilution (10−0–10−7)/regrowth to establish new bacterial communities. The microbial profile of each derived community was assessed by PCR-DGGE. The major bacterial species within O-mix were identified as indicated by the arrows. Inlet shows the H2O2 production of the specific communities in response to the absence (−) and presence (+) of E. coli. Three independent biological replicates were performed and a representative gel image is shown.
Our data revealed that when O-mix was titrated to 10−4, the absence of certain bacterial species including Staphylococcus saprophyticus, was concurrent with the reduced community response to the presence of E. coli. Meanwhile, the absence of Streptococcus infantis from community 10−6 was coincident with the community's spontaneous high-level H2O2 production (Figure 2). The results implicated the involvement of these bacterial species in the resistance pathway. Furthermore, H2O2 has been shown to be responsible for killing invading E. coli, which makes H2O2-producing bacteria one of the important players in this pathway (He et al., 2010b). As community 10−7 comprised less bacterial species while still containing H2O2 producers, colony isolation coupled with the H2O2 production assay was used to identify the H2O2 producer within the community10−7, the result revealed that the H2O2-producing Streptococcus sanguinis was the most abundant species within the communities 10−7 and 10−8. This was corroborated by our DGGE data showing that the band with the highest density within these communities was S. sanguinis (Figure 2).
Synthetic oral community comprised of identified players was sufficient to exert colonization resistance against E. coli
Based on the concurrence of the removal of specific bacteria species from communities derived from dilution and the corresponding community functional shift, we identified three potential players that were likely to be involved in the pathway leading to the resistance against E. coli. The candidates were searched against the panel of isolated oral bacterial species from mice oral cavity. All three candidates, S. saprophyticus, S. infantis and S. sanguinis, had been isolated during our previous studies (He et al., 2010a, 2010b). In an effort to investigate if the identified bacterial species were required and sufficient to elicit the colonization resistance when challenged with E. coli, we established synthetic communities composed of different combination of three identified potential players and tested their responsivity to the presence of E. coli, whose initial inoculum was about 10% of the total cell numbers within synthetic communities.
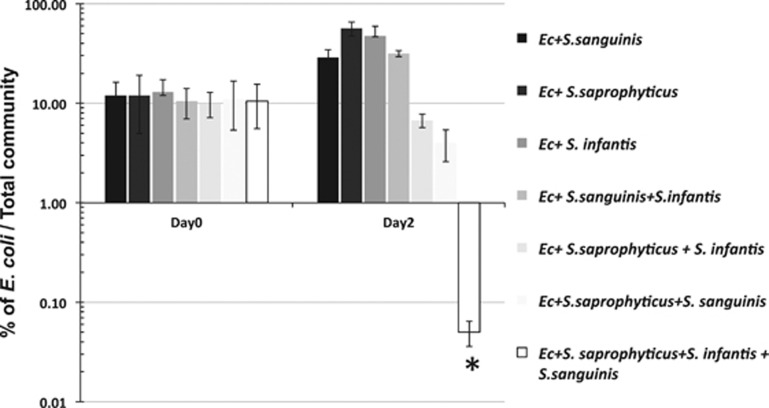
Results showed that, when E. coli was added to mono-species communities, no significant inhibition effect was observed (Figure 3). There was a two- to fivefold increase in the percentage of viable count of E. coli in respect to the total viable cells within the co-culture during the 2-day period. Similar outcome was observed when synthetic duo-species community containing S. infantis and S. sanguinis was challenged with E. coli. However, when co-cultivated with a two-species community composed of S. saprophyticus/S. infantis, or S. saprophyticus/S. sanguinis, a two- to threefold reduction in the percentage of viable E. coli was observed. Most strikingly, when E. coli encountered the synthetic community composed of all three species, it suffered drastic viability loss, its proportion relative to the total bacteria count dropped from around 10% to less than 0.05% after 48 h of co-cultivation, while no significant change in the total bacterial count was apparent (data not shown).
Figure 3.
Colonization resistance of synthetic communities against E. coli (Ec). E. coli was co-cultivated with synthetic communities comprised different combination of identified bacterial species in a 1:10 ratio, and its viability was monitored after 48 h incubation at 37 °C under anaerobic condition. Two independent experiments were performed in triplicates and the average value±s.d. is shown. The asterisk indicates that the value of that specific experimental setup is significantly lower than that of other setups (Student's t-test P-value <0.05).
S. saprophyticus initiates the colonization resistance pathway
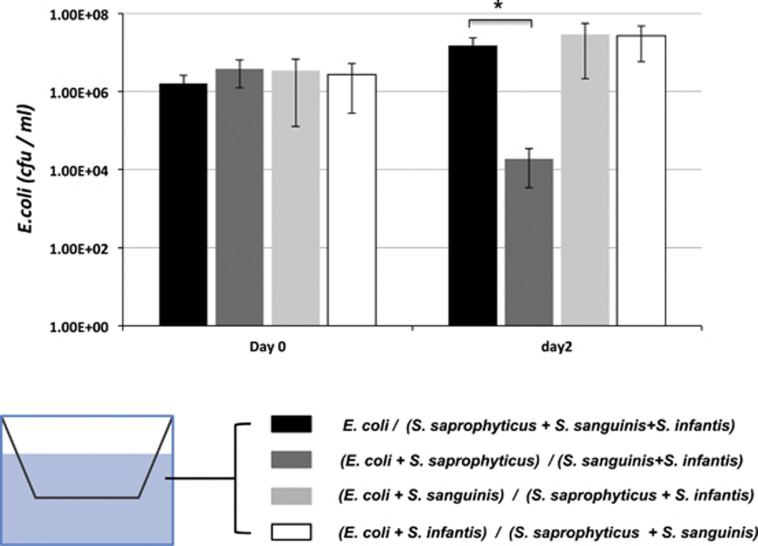
Our previous study showed that when E. coli was co-cultivated with the O-mix, it suffered drastic reduction in viability. However, when E. coli and O-mix cells were separated in a two-chamber vessel with a 0.4-μm pore size membrane, no inhibition was observed even though they shared the same culture medium (He et al., 2010b). Meanwhile, E. coli LPS mutants displayed much reduced ability in triggering the production of H2O2 by the O-mix, which was the main factor responsible for killing E. coli (He et al., 2010b). These data indicated that certain bacterial species within the O-mix could potentially detect the presence of E. coli via cell–cell contact (possibly through direct contact with E. coli LPS) and initiate the resistance response (He et al., 2010b). In an effort to determine if such a role can be carried out by one of the identified players, two-chamber assay was applied to study the possible contact-dependent activation of the resistance against E. coli by a synthetic oral community composed of S. saprophyticus, S. infantis and S. sanguinis. When E. coli (in the upper chamber) was physically separated from the rest of the synthetic community (in the low chamber) by a 0.4-μm pore size membrane while sharing the same culturing medium, no inhibition was observed, which confirmed our recent data that cell–cell contact might be required for triggering the response (He et al., 2010b). To test which oral isolate could have the role in sensing E. coli and initiating the response, each one of the three oral isolates was co-cultured with E. coli in the upper chamber, whereas other community members were grown in the lower chamber. Our result showed that a drastic reduction in E. coli viability was monitored only when E. coli was co-incubated with S. saprophyticus in the upper chamber (Figure 4). As co-cultivation with S. saprophyticus alone did not cause any growth inhibition in E. coli (Figure 3), the co-incubation of the two species in the upper chamber might induce the production of diffusible signal(s), which could relay the response to the rest of the community members in the lower chamber, and eventually result in the production of H2O2.
Figure 4.
Survival of E. coli within synthetic oral communities in two-chamber assay. E. coli alone, or mixed with different oral isolates was cultured in the upper chamber of the two-chamber system, while the rest of the members of the synthetic communities were inoculated to the lower chamber. Viability of E. coli in the upper chamber in each setup (represented by different bars) was monitored after 48 h. Two independent experiments were performed in duplicates, and average value±s.d. is presented. The asterisk indicates significant differences between two values (Student's t-test P-value <0.05).
S. infantis inhibitsS. sanguinis H2O2 production and responds to the diffusible signal(s) derived from E. coli-activated S. saprophyticus
Our result suggested that S. saprophyticus might be able to sense the presence of E. coli via cell–cell contact and activate the resistance pathway by producing diffusible signal(s) (Figure 4). To further determine the role of S. infantis in the resistance pathway, we tested the effect of spent medium of E. coli, S. saprophyticus monoculture or their dual-species culture on the H2O2 production of either S. sanguinis, or its co-culture with S. infantis. The co-cultivation with S. infantis highly suppressed the H2O2 production of S. sanguinis (reduced from 0.58±0.10 mM to 0.05±0.01 mM), while the addition of the spent medium of S. saprophyticus and E. coli co-culture not only rescued the repression but also resulted in a significantly higher H2O2 level (0.97±0.09 mM) within the community (Table 1).
Table 1. Quantitative measurement of H2O2 production of synthetic communities under different treatments.
|
Community |
Added spent medium |
|||
|---|---|---|---|---|
| E. coli | S. saprophyticus | S. saprophyticus+ E. coli | ||
| S. sanguinis | 0.58±0.10 | 0.65±0.10 | 0.72±0.12 | 0.57±0.09 |
| S. sanguinis+S. infantis | 0.05±0.01 | 0.03±0.01 | 0.02±0.01 | 0.97±0.09a |
Three replicates were performed for each assay. The values represent the H2O2 production (mM) of synthetic communities under different treatments. Average values±s.d. are shown.
Indicates significant differences between the indicated value and the value obtained from same community without adding spent medium (Student's t-test P-value <0.05).
H2O2 production of synthetic oral communities in response to the presence of E. coli
Quantitative measurement of H2O2 production revealed that H2O2 can only be detected in synthetic communities containing H2O2-producing S. sanguinis. The H2O2 level in S. sanguinis monoculture grown under community growth condition was determined to be ∼0.62 mM, a concentration below the minimum inhibitory concentration (0.8 mM) against E. coli (He et al., 2010b). The inclusion of S. saprophyticus in the community did not affect the H2O2 level. However, when these two cultures were challenged with E. coli (in a 10:1 ratio) for 24 h, there was a significant reduction in the H2O2 level detected within the community. Furthermore, when S. infantis was used to establish two-species community with S. sanguinis, or tri-species community with S. sanguinis/S. saprophyticus, the H2O2 level dropped to ∼0.02 mM (Table 2). Most strikingly, when the synthetic community contained all three bacterial species, it demonstrated the most drastic response to the presence of E. coli by increasing its H2O2 level almost 40-fold, reaching to about 1 mM (Table 2), a concentration that could exert significant inhibitory effect on E. coli (He et al., 2010b). It is worth noting that, although no H2O2 could be detected in duo-species community comprised of S. saprophyticus and S. infantis, either in the presence or absence of E. coli (Table 2), it still exerted certain inhibitory effect toward E. coli (Figure 3), suggesting the involvement of mechanism(s) other than the production of H2O2 in inhibiting E. coli.
Table 2. Quantitative measurement of H2O2 production in synthetic communities 24 h after challenged with and without E. coli.
| Synthetic communities | H2O2 production (mM) without E. coli | H2O2 production with E. coli |
|---|---|---|
| S. sanguinis | 0.62±0.08 | 0.19±0.05 |
| S. saprophyticus | ND | ND |
| S. infantis | ND | ND |
| S. sanguinis+S. infantis | 0.02±0.01 | 0.03±0.01 |
| S. saprophyticus+S. infantis | ND | ND |
| S. saprophyticus+S. sanguinis | 0.54±0.05 | 0.17±0.08 |
| S. saprophyticus+S. infantis+S. sanguinis | 0.03±0.01 | 1.17±0.14a |
Abbreviation: ND, not detected.
Three replicates were performed for each assay. Average values±s.d. are shown.
Significant differences between the indicated value and value obtained from same community without challenged with E. coli (Student's t-test P-value <0.05).
Discussion
Colonization resistance is an intrinsic property of ecological communities and has significant roles in protecting the residential niches and maintaining the stability of established communities. This phenomenon has been well documented in a variety of ecosystems, ranging from grassland, marine ecosystem to host-associated microbiota (Brook, 1999; Stachowicz et al., 1999; Dillon et al., 2005; Thomsen et al., 2006).
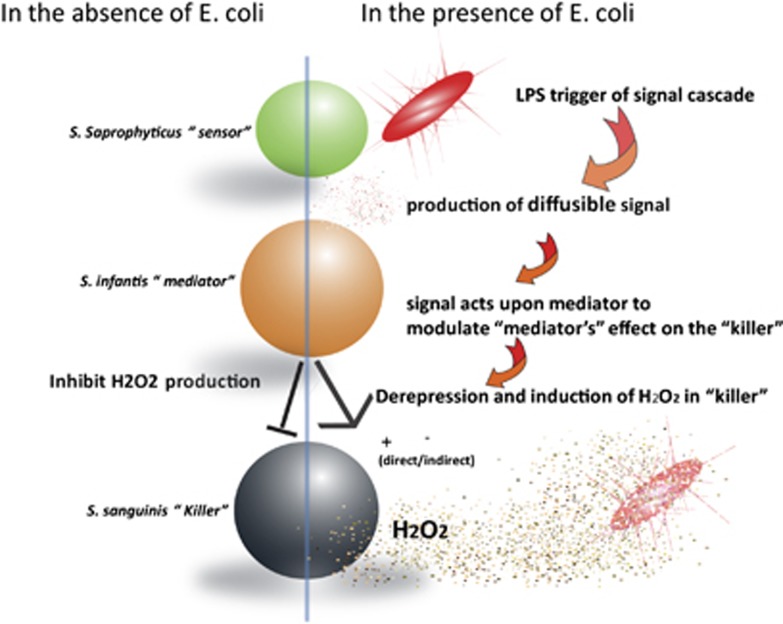
The past decade has witnessed an increasing interest in studying the ecology of host-associated microbiota. As one of the potential beneficial effects conferred by these indigenous microbial communities, colonization resistance has been implicated in protecting host against pathogens and has received great attention (Rolfe, 1997; Brook, 1999; Adlerberth et al., 2000; Fons et al., 2000; Reid et al., 2001; Guarner and Malagelada, 2003; Reid et al., 2003; Alain, 2004; Dillon et al., 2005; Falagas et al., 2008). However, due to the complexity of host-associated indigenous microbial community, it is very difficult to identify the bacterial species responsible for colonization resistance and to elucidate the mechanisms involved. In this study, using an established in vitro microbial community (O-mix) derived from the mice oral cavity, we have revealed one of its important colonization resistance mechanisms by identifying the key bacterial species in a pathway involved in regulated H2O2 production to prevent E. coli integration (Figure 5).
Figure 5.
Tentative model of colonization resistance pathway of the O-mix against integration of E. coli. Green, orange and black balls represent S. saprophyticus, S. infantis and S. sanguinis, respectively.
This particular colonization resistance pathway is comprised of three key bacterial species: S. saprophyticus (the ‘Sensor'), S. infantis (the ‘Mediator') and S. sanguinis (the ‘Killer') (Figure 5). In the absence of foreign invader (for example, E. coli), S. infantis represses S. sanguinis' capacity to produce H2O2, resulting in minimal H2O2 level within the community (Tables 1 and 2). When encountering E. coli, S. saprophyticus acts as the ‘Sensor' due to its ability to detect the presence of E. coli (Figure 4) and initiates the invasion resistance response by producing diffusible signal(s) (Figure 4; Table 1). The signal(s) could relay the information to S. infantis (Table 1), which not only alleviates its suppression on S. sanguinis' H2O2 production but also stimulates the Killer's H2O2-producing capability (Table 1). The resultant increased H2O2 level exerts inhibitory effect on the invading E. coli (Figure 5).
A few lines of evidence suggested S. saprophyticus might sense the presence of E. coli via direct cell–cell contact. First of all, two-chamber assay showed that only when co-incubating with S. saprophyticus in the upper chamber, while the rest of the two members of the synthetic community remained in the lower chamber, did E. coli suffer drastic inhibition (Figure 4). As co-cultivation of S. saprophyticus with E. coli alone did not result in the inhibition of E. coli (Figure 3), the result suggested that the interaction between E. coli and S. saprophyticus was the initial step in initiating the response, while the inhibition of E. coli was mainly due to the production of H2O2 by other players in the same pathway. Meanwhile, the membrane used for separating the two chambers has a pore size of 0.4 μm, which should be permeable to most, if not all, of the diffusible factors. If the initial triggering event were due to the released metabolites or secretary substances by E. coli, we would expect to observe similar inhibition regardless of which community member was co-incubated with E. coli in the upper chamber. These results, together with our previous studies showing E. coli LPS was required for triggering the resistance response in the O-mix (He et al., 2010b), indicated a direct physical contact between E. coli and the ‘Sensor' in triggering the response. Furthermore, the ability of the spent medium of E. coli/S. saprophyticus co-culture to induce H2O2 production of the community (Table 1), as well as the observed relay of the inhibition event even when E. coli and S. saprophyticus were physically separated from the rest of the community members in the two-chamber assay (Figure 4) were indicative of the diffusible nature of the signal(s) produced by S. saprophyticus upon physical contact with E. coli.
Our data also showed that a two-species community comprised S. saprophyticus and S. infantis, but missing H2O2-producing S. sanguinis still exerted inhibitory effect against E. coli, although at a much reduced level, suggesting other potential mechanisms might also contribute to the observed resistance against E. coli (Figure 3). All three isolates or their close relatives have been identified in mice oral cavities from a separate study using 454-pyrosequecning analysis (Chun et al., 2010), however, their physiological or biological functions have not been investigated. Our synthetic community data demonstrated that the E. coli-induced colonization resistance required the participation of these three players, and missing any one of them resulted in a greatly reduced colonization resistance against the integration of E. coli.
Ecological theory suggests that species-rich communities are more resistant to invasion than species-poor communities, this negative invasibility–diversity relationship have been documented in a wide range of community types, including animals, plants, marine invertebrates, protists and bacteria (Case, 1990; McGrady-Steed et al., 1997; Stachowicz et al., 1999; Hodgson et al., 2002; Cook et al., 2006; Thomsen et al., 2006). It has been proposed that, the observed invasibility–diversity relationship in established ecological communities is due to three non-mutually exclusive mechanisms, including dominance, niche complementarity and positive interactions (Bruno et al., 2003). Our synthetic community data are consistent with those from numerous community ecology studies (Case, 1990; McGrady-Steed et al., 1997; Stachowicz et al., 1999; Hodgson et al., 2002; Cook et al., 2006; Thomsen et al., 2006), further confirming the importance of species diversity or richness within the community in developing colonization resistance. However, the observed negative diversity–invasibility relationship is not simply because more diverse communities have higher probability of containing an oral bacterial species that exerts particularly high colonization resistance against E. coli (dominance), but rather it emphasized on the importance of ‘Facilitation', or positive species interactions, in the colonization resistance of mice oral community. Facilitation are encounters between organisms that benefit at least one of the participants and cause harm to neither (Bruno et al., 2003). It is an important community-level process that can be found in many ecological communities, particularly bacterial ecosystems, and could potentially increase colonization resistance against invasion and contribute to community stability (Hodgson et al., 2002; Burmolle et al., 2006). Within established microbial communities, different types of residential bacterial species are often involved in synergistic metabolism, or engaged in cooperative beneficial interactions, which have been shown to have striking influence on the survival of the communities (Kuramitsu et al., 2007). Within our synthetic community, the colonization resistance is neither dependent on one particular species nor manifested by simple additive inhibitory effect of each player. Instead, it requires a complex pathway with multiple players, including S. saprophyticus (the ‘Sensor'), S. infantis (the ‘Mediator') and S. sanguinis (the ‘Killer'), which are actively engaged in information exchange and relay, eventually resulting in the production of an antimicrobial compound to prevent the integration of foreign bacterium.
Within the in vitro mice oral community, multiple Streptococci with H2O2-producing capability have been identified (He et al., 2010a), the replacement of S. sanguinis with another H2O2 producer, S. gordonii, resulted in a similar E. coli-specific response of the synthetic community (data not shown), suggesting the functional redundancy within the O-mix. This result is consistent with the observation that even for communities with relatively low biodiversity, functional redundancy could have an important role in the stability of microbial communities (Franklin et al., 2001; Franklin and Mills, 2006). Because of the limitation of cultivation-dependent methods, the microbial profile within the O-mix in this study could not fully represent microbial diversity of the original mice oral community. Thus, we cannot rule out the possibility that multiple sensors or mediators participate in the colonization resistance pathway.
Over the last decade, we have begun to appreciate the fact that the residential bacteria are often engaged in extensive interactions, which could result in many new physiological and biological functions that cannot be observed with individual species (Kuramitsu et al., 2007). The introduction of the term ‘Sociomicrobiology' (Parsek and Greenberg, 2005) further emphasizes the significance of the community level, or group behavior observed in microbial communities. However, due to their complex nature, our knowledge of the structures within most of the studied microbial communities is often limited to their residential bacterial profiles or simplified one-on-one interactions, while lacking more detailed understanding of the community organizations. Our study revealed a sophisticated colonization resistance pathway involving multiple bacterial species that have differential roles in preventing the integration of bacterium of foreign origin. Although the molecular details of this pathway require further investigation, our result clearly demonstrates the presence of sophisticated community structures and their important roles in specific community-level functions, such as colonization resistance.
Acknowledgments
The study was supported by US National Institutes of Health (NIH) Grants (DE020102 and DE021108) and International Science and Technology Cooperation Program of China (2011DFA30940).
The authors declare no conflict of interest.
References
- Adlerberth I, Cerquetti M, Poliane I, Wold A, Collignon A. Mechanisms of colonisation and colonisation resistance of the digestive tract part 1: bacteria/host interactions. Microb Ecol Health Dis. 2000;12:223–239. [Google Scholar]
- Alain LS. Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS Microbiol Rev. 2004;28:405–440. doi: 10.1016/j.femsre.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Backhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Nati Acad Sci USA. 2004;101:15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernet-Camard MF, Lievin V, Brassart D, Neeser JR, Servin AL, Hudault S. The human Lactobacillus acidophilus strain LA1 secretes a nonbacteriocin antibacterial substance(s) active in vitro and in vivo. Appl Environ Microbiol. 1997;63:2747–2753. doi: 10.1128/aem.63.7.2747-2753.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook I. Bacterial interference. Crit Rev Microbiol. 1999;25:155–172. doi: 10.1080/10408419991299211. [DOI] [PubMed] [Google Scholar]
- Bruno JF, Stachowicz JJ, Bertness MD. Inclusion of facilitation into ecological theory. Trends Ecol Evol. 2003;18:119–125. [Google Scholar]
- Burmolle M, Webb JS, Rao D, Hansen LH, Sorensen SJ, Kjelleberg S. Enhanced biofilm formation and increased resistance to antimicrobial agents and bacterial invasion are caused by synergistic interactions in multispecies biofilms. Appl Environ Microbiol. 2006;72:3916–3923. doi: 10.1128/AEM.03022-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case TJ. Invasion resistance arises in strongly interacting species-rich model competition communities. Proc Nati Acad Sci USA. 1990;87:9610–9614. doi: 10.1073/pnas.87.24.9610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan RC, Reid G, Irvin RT, Bruce AW, Costerton JW. Competitive exclusion of uropathogens from human uroepithelial cells by Lactobacillus whole cells and cell wall fragments. Infect Immun. 1985;47:84–89. doi: 10.1128/iai.47.1.84-89.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun J, Kim K, Lee JH, Choi Y. The analysis of oral microbial communities of wild-type and toll-like receptor 2-deficient mice using a 454 GS FLX Titanium pyrosequencer. BMC Microbiol. 2010;10:101. doi: 10.1186/1471-2180-10-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook KL, Garland JL, Layton AC, Dionisi HM, Levine LH, Sayler GS. Effect of microbial species richness on community stability and community function in a model plant-based wastewater processing system. Microb Ecol. 2006;52:725–737. doi: 10.1007/s00248-006-9105-1. [DOI] [PubMed] [Google Scholar]
- Corthesy B, Gaskins HR, Mercenier A. Cross-talk between probiotic bacteria and the host immune system. J Nutr. 2007;137:781S–790S. doi: 10.1093/jn/137.3.781S. [DOI] [PubMed] [Google Scholar]
- Dillon RJ, Vennard CT, Buckling A, Charnley AK. Diversity of locust gut bacteria protects against pathogen invasion. Ecol Lett. 2005;8:1291–1298. [Google Scholar]
- Falagas ME, Rafailidis PI, Makris GC. Bacterial interference for the prevention and treatment of infections. Int J Antimicrob Agents. 2008;31:518–522. doi: 10.1016/j.ijantimicag.2008.01.024. [DOI] [PubMed] [Google Scholar]
- Fons M, Gomez A, Karjalainen T. Mechanisms of colonisation and colonisation resistance of the digestive tract part 2: bacteria/bacteria interactions. Microb Ecol Health Dis. 2000;12:240–246. [Google Scholar]
- Franklin R, Mills A. Structural and functional responses of a sewage microbial community to dilution-induced reductions in diversity. Microb Ecol. 2006;52:280–288. doi: 10.1007/s00248-006-9033-0. [DOI] [PubMed] [Google Scholar]
- Franklin RB, Garland JL, Bolster CH, Mills AL. Impact of dilution on microbial community structure and functional potential: comparison of numerical simulations and batch culture experiments. Appl Environ Microbiol. 2001;67:702–712. doi: 10.1128/AEM.67.2.702-712.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freter R. Experimental enteric Shigella and Vibrio infections in mice and guinea pigs. J Exp Med. 1956;104:411–418. doi: 10.1084/jem.104.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarner F, Malagelada JR. Gut flora in health and disease. Lancet. 2003;361:512–519. doi: 10.1016/S0140-6736(03)12489-0. [DOI] [PubMed] [Google Scholar]
- He X, Hu W, Kaplan C, Guo L, Shi W, Lux R. Adherence to streptococci facilitates fusobacterium nucleatum integration into an oral microbial community. Microb Ecol. 2012;63:532–542. doi: 10.1007/s00248-011-9989-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Tian Y, Guo L, Ano T, Lux R, Zusman D, et al. In vitro communities derived from oral and gut microbial floras inhibit the growth of bacteria of foreign origins. Microb Ecol. 2010;60:665–676. doi: 10.1007/s00248-010-9711-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Tian Y, Guo L, Ano T, Lux R, Zusman D, et al. Oral-derived bacterial flora defends its domain by recognizing and killing intruders—A molecular analysis using Escherichia coli as a model intestinal bacterium. Microb Ecol. 2010;60:655–664. doi: 10.1007/s00248-010-9708-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson DJ, Rainey PB, Buckling A. Mechanisms linking diversity, productivity and invasibility in experimental bacterial communities. Proc R Soc Lond. Series B: Biological Sciences. 2002;269:2277–2283. doi: 10.1098/rspb.2002.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hongoh Y, Ohkuma M, Kudo T. Molecular analysis of bacterial microbiota in the gut of the termite Reticulitermes speratus (Isoptera; Rhinotermitidae) FEMS Microbiol Ecol. 2003;44:231–242. doi: 10.1016/S0168-6496(03)00026-6. [DOI] [PubMed] [Google Scholar]
- Kuramitsu HK, He X, Lux R, Anderson MH, Shi W. Interspecies interactions within oral microbial communities. Microbiol Mol Biol Rev. 2007;71:653. doi: 10.1128/MMBR.00024-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie M. Gut microbes keep rare immune cells in Line. Science. 2012;335:1428. doi: 10.1126/science.335.6075.1428. [DOI] [PubMed] [Google Scholar]
- Ley RE, Hamady M, Lozupone C, Turnbaugh PJ, Ramey RR, Bircher JS, et al. Evolution of mammals and their gut microbes. Science. 2008;320:1647–1651. doi: 10.1126/science.1155725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley RE, Lozupone CA, Hamady M, Knight R, Gordon JI. Worlds within worlds: evolution of the vertebrate gut microbiota. Nat Rev Micro. 2008;6:776–788. doi: 10.1038/nrmicro1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd AB, Cumming RB, Kent RD. Prevention of Salmonella typhihurium infection in poultry by pretreatment of chickens and poults with intestinal extracts. Aust Vet J. 1977;53:82–87. doi: 10.1111/j.1751-0813.1977.tb14891.x. [DOI] [PubMed] [Google Scholar]
- McGrady-Steed J, Harris PM, Morin PJ. Biodiversity regulates ecosystem predictability. Nature. 1997;390:162–165. [Google Scholar]
- Parsek MR, Greenberg EP. Sociomicrobiology: the connections between quorum sensing and biofilms. Trends Microbiol. 2005;13:27–33. doi: 10.1016/j.tim.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Reid G, Howard J, Gan BS. Can bacterial interference prevent infection. Trends Microbiol. 2001;9:424–428. doi: 10.1016/s0966-842x(01)02132-1. [DOI] [PubMed] [Google Scholar]
- Reid G, Jass J, Sebulsky MT, McCormick JK. Potential uses of probiotics in clinical practice. Clin Microbiol Rev. 2003;16:658–672. doi: 10.1128/CMR.16.4.658-672.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson C, Schloss P, Ramos Y, Raffa K, Handelsman J. Robustness of the bacterial community in the cabbage white butterfly larval midgut. Microb Ecol. 2010;59:199–211. doi: 10.1007/s00248-009-9595-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson C, Bohannan BJM, Yong VB. From structure to function: the ecology of host-associated microbial communities. Microbiol Mole Biol Rev. 2010;74:453–476. doi: 10.1128/MMBR.00014-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfe RD.1997Colonization resistanceIn: Mackie RI, White BA, Isaacson RE (eds).Gastrointestinal Microbiology Chapman and Hall: New York, NY; 501–535. [Google Scholar]
- Sheffield VC, Cox DR, Lerman LS, Myers RM. Attachment of a 40-base-pair G+C-rich sequence (GC-clamp) to genomic DNA fragments by the polymerase chain reaction results in improved detection of single-base changes. Proc Natl Acad Sci USA. 1989;86:232–236. doi: 10.1073/pnas.86.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachowicz JJ, Whitlatch RB, Osman RW. Species diversity and invasion resistance in a marine ecosystem. Science. 1999;286:1577–1579. doi: 10.1126/science.286.5444.1577. [DOI] [PubMed] [Google Scholar]
- Thomsen MA, D'antonio CM, Suttle KB, Sousa WP. Ecological resistance, seed density and their interactions determine patterns of invasion in a California coastal grassland. Ecol Lett. 2006;9:160–170. doi: 10.1111/j.1461-0248.2005.00857.x. [DOI] [PubMed] [Google Scholar]
- Van Der Waaij D, Van Der Waaij BD. The colonization resistance of the digestive tract in different animal species and in man; a comparative study. Epidemiol Infect. 1990;105:237–243. doi: 10.1017/s0950268800047841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbergh PA. Lactic acid bacteria, their metabolic products and interference with microbial growth. FEMS Microbiol Rev. 1993;12:221–237. [Google Scholar]
- Veivers PC, O'Brien RW, Slaytor M. Role of bacteria in maintaining the redox potential in the hindgut of termites and preventing entry of foreign bacteria. J Insect Physiol. 1982;28:947–951. [Google Scholar]
- Wang R, Kaplan A, Guo L, Shi W, Lux R, He X. The influence of iron availability on human salivary microbial community composition. Microb Ecol. 2012;64:152–161. doi: 10.1007/s00248-012-0013-2. [DOI] [PMC free article] [PubMed] [Google Scholar]