Abstract
Ascidians are ecologically important components of marine ecosystems yet the ascidian microbiota remains largely unexplored beyond a few model species. We used 16S rRNA gene tag pyrosequencing to provide a comprehensive characterization of microbial symbionts in the tunic of 42 Great Barrier Reef ascidian samples representing 25 species. Results revealed high bacterial biodiversity (3 217 unique operational taxonomic units (OTU0.03) from 19 described and 14 candidate phyla) and the widespread occurrence of ammonia-oxidizing Thaumarchaeota in coral reef ascidians (24 of 25 host species). The ascidian microbiota was clearly differentiated from seawater microbial communities and included symbiont lineages shared with other invertebrate hosts as well as unique, ascidian-specific phylotypes. Several rare seawater microbes were markedly enriched (200–700 fold) in the ascidian tunic, suggesting that the rare biosphere of seawater may act as a conduit for horizontal symbiont transfer. However, most OTUs (71%) were rare and specific to single hosts and a significant correlation between host relatedness and symbiont community similarity was detected, indicating a high degree of host-specificity and potential role of vertical transmission in structuring these communities. We hypothesize that the complex ascidian microbiota revealed herein is maintained by the dynamic microenvironments within the ascidian tunic, offering optimal conditions for different metabolic pathways such as ample chemical substrate (ammonia-rich host waste) and physical habitat (high oxygen, low irradiance) for nitrification. Thus, ascidian hosts provide unique and fertile niches for diverse microorganisms and may represent an important and previously unrecognized habitat for nitrite/nitrate regeneration in coral reef ecosystems.
Keywords: Sea-squirt, pyrosequencing, holobiont, thaumarchaeota, coral reef, microbiota
Introduction
Symbiotic microbial communities are a common feature of marine invertebrates and include diverse lineages of bacteria, archaea, fungi, microalgae and viruses (Rowan, 1998; Taylor et al., 2007). Prokaryotic symbionts are a particularly rich component of invertebrate microbiota and encompass nearly all major branches of bacterial and archaeal life. Many of these symbiont lineages are primarily host-associated (i.e., obligate symbionts) and represent novel microbial taxa from species level (e.g., Synechococcus spongiarum in sponges, Usher et al., 2004) to phylum level, (e.g., Poribacteria, Fieseler et al., 2004) while others exist in both free-living and host-associated states, (i.e., facultative symbionts) though generally enriched in the invertebrate microhabitat and rare in seawater communities (Sunagawa et al., 2010). The phylogenetic diversity of symbiotic microbes is associated with a diversity of metabolic pathways in the carbon, (Wilkinson, 1983) nitrogen (Hoffmann et al., 2009) and sulfur cycles (Hoffmann et al., 2005), spurred by the utilization of host waste products (e.g., ammonia), the presence of dimethylsulfoniopropionate (DMSP, Raina et al., 2010) and physico-chemical conditions of the host microenvironment (e.g., oxygen gradients; Hoffmann et al., 2008; Kühl et al., 2012). The structural and functional diversity of symbiotic microbial communities indicate that invertebrate hosts provide fertile microbial niches that contribute to prokaryotic biodiversity and nutrient cycling in coastal marine ecosystems.
Invertebrate-microbe symbioses also play critical roles in host ecological success through the provision of supplemental nutrition and production of defensive secondary metabolites. For example, sponges, corals and ascidians are able to supplement their heterotrophic filter-feeding activities with fixed carbon sourced from photosynthetic symbionts (Muscatine and Porter, 1977; Pardy and Lewin, 1981; Freeman and Thacker, 2011), utilizing autotrophic symbiont metabolism to enhance their growth rates in nutrient-limited environments. Sponge symbionts are also responsible for the synthesis of vitamin B1, which animals need to obtain from their diet (Fan et al., 2012), while the cyanobacteria in the genus Prochloron appear to provide UV-absorbing molecules to their ascidian hosts (Hirose et al., 2004). Further, symbiont biosynthesis of secondary metabolites contributes to the chemical defenses of marine invertebrates (Schmidt et al., 2005; Freeman et al., 2012), a key strategy for sessile organisms to deter predation, avoid surface fouling and compete for substrate (Armstrong et al., 2001; Pawlik, 2011). In addition to their roles in host biology and ecology, many of these unique and structurally diverse secondary metabolites have pharmaceutical applications and substantial importance for biotechnology and drug discovery (Paul and Ritson-Williams, 2008; Erwin et al., 2010).
Ascidians (Class Ascidiacea) are sessile, filter-feeding invertebrates that inhabit diverse benthic ecosystems in tropical, temperate and polar marine environments. As a basal lineage in the phylum Chordata, ascidians occupy a key stage in deuterostome evolution (Delsuc et al., 2006). Ascidians are also a prolific source of novel marine natural products (Erwin et al., 2010) and the involvement of microbial symbionts in bioactive compound production (Schmidt and Donia, 2010) has prompted recent studies of the ascidian microbiota (Donia et al., 2011; Kwan et al., 2012). Historically, most studies of microbial symbionts in ascidians have focused on cyanobacteria, in particular the genera Prochloron and Synechocystis. These symbionts associate with colonial ascidians on the colony surface, inside the common cloacal cavities or as endosymbionts in the tunic, a polysaccharide envelope surrounding the zooids (Cox et al., 1985; Cox, 1986; Hernández-Mariné et al., 1990; Hirose et al., 1996, 2006a, 2006b, 2012; Turon et al., 2005; Martínez-García et al., 2007). Even when inhabiting the colonial tunic, the symbionts are mostly extracellular, with only a few instances of intracellular associations (Hirose et al., 1996; Moss et al., 2003; Kojima and Hirose, 2010). However, few studies to date have employed the molecular approaches required to accurately assess microbial biodiversity in ascidians (Martínez-García et al., 2007, 2008, 2011; Münchhoff et al., 2007; Tait et al., 2007; López-Legentil et al., 2011; Behrendt et al., 2012; Erwin et al., 2013). For example, DNA sequence analysis and fluorescence in situ hybridization techniques only recently revealed the first archaeal symbionts in the ascidian tunic, indicating that Thaumarchaeota may be involved in nitrification inside host tissues (Martínez-García et al., 2008).
A growing body of literature suggests that ascidian-associated microbes may play a critical role in the metabolic needs of their host, (Hirose and Maruyama, 2004; Martínez-García et al., 2008; Kühl et al., 2012), yet the microbial communities inhabiting most ascidian species remain unknown. The advent of high-throughput, next-generation DNA sequencing platforms offers new opportunities for in-depth microbial diversity evaluation across large sample sets. Deep sequencing of microbial communities from soils, seawater and sponges has revealed diversity estimates over an order of magnitude higher than that recovered by traditional sequencing techniques (Huber et al., 2007; Roesch et al., 2007; Webster et al., 2010), including the detection of bacterial phyla not represented in first-generation sequencing datasets (e.g., Webster and Taylor, 2012). Similarly, the recent application of next generation sequencing to the ascidian microbiota has revealed a high diversity of symbiotic microbes and uncovered new ascidian-associated microbial lineages in the colonial host Lissoclinum patella (Behrendt et al., 2012) and solitary host Styela plicata (Erwin et al., 2013), highlighting the depth of microbial biodiversity and unknown facultative and obligate symbiotic microbes awaiting discovery within ascidian hosts.
In this study, we used 16S rRNA gene tag pyrosequencing to investigate the diversity, structure and specificity of microbial communities inhabiting the tunic of 42 samples of Great Barrier Reef (GBR) ascidians (representing 25 species, 7 families and 3 orders) in order to provide the most comprehensive characterization of the ascidian microbiome to date. The diversity and composition of ascidian-associated microbial communities were compared to free-living communities in ambient seawater and among ascidian host species, including intraspecific variability among replicates for 10 ascidian species. In addition, the spatial localization of symbionts within the ascidian tunic was visualized by electron microscopy, and the genetic identity of ascidian hosts was established by analysis of mitochondrial (cytochrome oxidase subunit I) and ribosomal (18S rRNA) gene sequences. This comprehensive assessment of microbial diversity in GBR ascidians will provide the basis for future research within the fields of symbiosis, drug discovery and ascidian holobiont resilience to environmental change or anthropogenic disturbance. Exploration of ascidian microbiomes may also highlight a hidden reservoir for primary productivity and nitrogen metabolism and enable more reliable predictions of biogeochemical cycling in coral reef environments.
Material and methods
Sample collection
Ascidian (n=42) and seawater (n=3) samples were collected by SCUBA between 2–14 m depth from several localities within the Great Barrier Reef, North Queensland, Australia (Supplementary Table S1). Ascidian samples were processed for: (1) taxonomic analyses, by preservation in 4% formaldehyde, (2) molecular analyses, by immediate submersion in liquid nitrogen and storage at −80 °C and (3) electron microscopy analyses, by preservation in 2.5% glutaraldehyde using filtered seawater as buffer. Seawater samples (2 l) were transported to the laboratory, concentrated on 0.2 μm sterivex filters (Durapore; Millipore, North Ryde, New South Wales, Australia) with a peristaltic pump and aseptically frozen at −80 °C.
DNA extraction
Frozen ascidian tissues (approximately 0.5 g per sample) were thawed, dissected under a stereomicroscope into inner tunic and zooid fractions and aseptically transferred to 1.5 ml tubes using sterile scalpels and tweezers. Inner tunic (i.e., beneath the surface layer) was chosen to avoid epibionts and ambient seawater microbes. These tunic samples were processed for microbial analysis, while zooids were processed for barcoding each ascidian specimen. DNA extraction was conducted separately for inner tunic and zooid tissue fractions with the Power Plant DNA Isolation Kit (MoBio Laboratories, Carlsbad, CA, USA) following the manufacturer's protocol. DNA extraction from concentrated seawater samples (filters) was performed by the addition of 1.8 ml lysis buffer (40 mM EDTA, 50 mM Tris and 0.75 M sucrose) and 200 μl of Lysozyme (10 mg/ml), incubation at 37 °C for 45 min, the addition of 40 μl of Proteinase K (10 μg of Proteinase K in 1 ml of 10% SDS) and incubation at 55 °C for 1 h. Lysates were transferred to sterile tubes and DNA was extracted using standard phenol:chloroform procedures and resuspended in 20 μl of distilled water. All PCR products were visualized on 1% agarose gels to assess amplification specificity and initial product quantity.
Identification and barcoding of host ascidians
Ascidian samples were assigned to the lowest taxonomic group possible based on morphological examination (Supplementary Text S1). Genetic identification was also performed using the mitochondrial gene cytochrome oxidase subunit I (COI) and 18S rRNA gene sequences. Both gene regions are commonly used to determine species boundaries and diversity among ascidian taxa (Tarjuelo et al., 2004; López-Legentil and Turon, 2005; Yokobori et al., 2006; Pérez-Portela et al., 2009) and COI is the metazoan standard for the Barcode of Life Project (www.barcodeoflife.org).
DNA extractions from zooid tissue were used as templates for PCR amplification of a 519 – 621 bp fragment of the COI gene. Total PCR reaction volume was 50 μl, including 10 μl of 5 × Buffer, 0.4 μl of bovine serum albumin (BSA; 10 mg/ml), 0.25 μl of My Taq DNA Polymerase (Bioline, London, United Kingdom), 2 μl of each primer (10 μM) and 1 μl of template DNA. Two sets of primer pairs were used for COI amplification, the ‘universal' primers LCO1490 and HCO2198 (Folmer et al., 1994) and the ascidian-specific primers Tun_forward and Tun_Reverse2 (Stefaniak et al., 2009). PCR conditions for amplification with universal primers were: an initial denaturing step of 94 °C for 2 min; 30 cycles of 94 °C for 45 s, 50 °C for 45 s and 72 °C for 50 s; and a final elongation step at 72 °C for 5 min. PCR conditions for amplification with ascidian-specific primers were: an initial denaturing step of 94 °C for 1 min; 60 cycles of 94 °C for 10 s, 50 °C for 30 s and 72 °C for 50 s; and a final elongation step at 72 °C for 10 min. PCR products were purified and bi-directionally sequenced at Macrogen, Inc. (Seoul, South Korea). Quality-checked sequences are archived in GenBank under accession numbers KC017426 to KC017444. Additional genetic identification and phylogenetic analyses of host ascidians were performed with 18S rRNA gene sequences recovered from the non-target, eukaryotic data component of the pyrosequencing run (Supplementary Text S2, Figure S4).
16S rRNA gene tag pyrosequencing
DNA extractions from inner tunic tissue and seawater samples were used as templates for PCR amplification of a ca. 466 bp fragment of the 16S rRNA gene encompassing the V6 – V8 regions using the primer set pyro926F (5′-AAACTYAAAKGAATTGRCGG-3′) and pyro1392R (5′-ACGGGCGGTGTGTRC-3′) complemented with adaptors B and A, respectively (Roche, Basel, Switzerland), as detailed previously (Erwin et al., 2013). Multiplex identifier (MID) barcodes unique to each sample were attached to reverse primers (Supplementary Table S2). PCR products were sent to Macrogen, Inc. for purification and further processing. Amplicon library was constructed using 5 μg of DNA from each sample (ascidian and sweater), resulting in a final concentration of 700 513 297 molecules/μl. Massively parallel 16S rRNA gene tag pyrosequencing was performed using the Roche 454 GS-FLX Titanium system, and the resulting data were deposited as flowgrams (sff file) in the Sequence Read Archive (SRA) of the National Center for Biotechnology Information under the accession number SRA056317.
Sequence data were processed with stringent filtering and screening criteria to minimize the occurrence of spurious sequences and overestimation of microbial diversity (Huse et al., 2010; Schloss et al., 2011), using the mothur software package (Schloss et al., 2009), as detailed previously (Erwin et al., 2013). Briefly, adaptor, MID and primer sequences were removed from raw sequences and the dataset de-noised (removal of reads with ambiguous base calls, long homopolymers and barcode or primer mismatches) and quality filtered (removal of short sequences and low quality reads). Non-target sequences (e.g. eukaryotic 18S rRNA, mitochondria, chloroplast) were removed using Metaxa v1.1, (Bengtsson et al., 2011) resulting in a dataset consisting solely of archaeal and bacterial 16S rRNA gene sequences. These sequences were aligned to the Greengenes database, trimmed to an overlapping alignment space (449 bp) and putatively chimeric sequences were removed (UChime; Edgar et al., 2011).
Data analysis
High quality sequences (n=94 637) were assigned to taxonomic groups based on the improved Greengenes taxonomy template (McDonald et al., 2012) with Thaumarchaeota elevated to the rank of phylum (Brochier-Armanet et al., 2008, Spang et al., 2010), grouped into OTU0.03 based on 97% sequence similarity and the average neighbor clustering algorithm, and the taxonomic assignment of each OTU0.03 was constructed by majority consensus (Schloss and Westcott, 2011).
Sampling coverage and expected total OTU diversity were calculated using rarefaction analysis and the bootstrap estimator (Smith and Van Belle, 1984) at six different OTU definitions corresponding approximately to the species (OTU0.03), genus (OTU0.05), family (OTU0.10), order (OTU0.15), class (OTU0.20) and phylum (OTU0.25) levels (97%, 95%, 90%, 85%, 80% and 75% similarity, respectively). All subsequent analyses were based on OTUs at 97% sequence identity (OTU0.03). Sub-sampling of sequence pools from samples with greater than 2000 reads were performed in the mothur software package to standardize sampling effort and determine its effect on diversity estimates. Host-specificity of the ascidian microbiota was assessed by partitioning OTUs into core (present in >70% of host species), variable (present in at least two host species) and specific (present in a single host species) groups (sensu Schmitt et al., 2012). To broaden the analysis of the specificity of the ascidian microbiota, abundant ascidian-associated OTUs (i.e., those represented by >100 total sequence reads) were compared to sequences in the GenBank database using a nucleotide-nucleotide BLAST search (Altschul et al., 1990). To compare microbial community similarity across hosts, Bray-Curtis similarity matrices were constructed using square root transformations of relative OTU abundance per host and visualized in cluster plots using Primer v6 (Plymouth Marine Laboratory, United Kingdom). Finally, Mantel tests were conducted to test for correlations between host relatedness (18S rRNA sequence similarity) and symbiont similarity (Bray-Curtis similarity) using the ade4 package for R (Dray and Dufour, 2007).
Transmission electron microscopy
Bacterial cells in the tunic of the representative ascidian species Phallusia julinea, Polycarpa aurata, Pycnoclavella sp., Clavelina meridionalis, Lissoclinum badium and Synoicum castellatum were visualized by transmission electron microscopy. Resin blocks and semi-thin and ultra-thin sections were prepared at the Microscopy Unit of the Scientific and Technical Services of the University of Barcelona as described in López-Legentil et al. (2011). Transmission electron microscopy observations were conducted on a JEOL JEM-1010 (Tokyo, Japan) electron microscope coupled with an Orius CDD camera (Gatan, Germany).
Results
Diversity and phylogeny of ascidian hosts
The 42 host ascidians examined for microbial symbionts were classified in 25 species from 7 families and all 3 recognized orders in the class Ascidiacea, with 18 species belonging to the Aplousobranchia, the largest ascidian order in terms of species and family richness (Shenkar and Swalla, 2011). Analyses of 18S rRNA gene sequences (23 of the 25 host species) and COI sequences (19 of 25 host species) confirmed morphological identifications and provide molecular datasets to facilitate additional research on the ascidian microbiota. All reference works used to identify each specimen and pertinent taxonomic remarks are provided (Supplementary Text S1), including underwater images (Supplementary Figures S1, S2 and S3) and a phylogenetic analysis using 18S rRNA sequences (Supplementary Text S2, Figure S4).
Richness and diversity of the ascidian microbiota
Collective analysis of 16S rRNA sequence reads derived from ascidian hosts (n=67 826) revealed a remarkable richness and diversity of microbial communities associated with GBR ascidians. A total of 3321 unique microbial OTU0.03 represented the combined GBR ascidian microbiome and corresponded to 19 described bacterial phyla, 14 candidate bacterial phyla and 3 described archaeal phyla (Figure 1). This increases the taxonomic diversity known to inhabit ascidians by 14 microbial phyla. Coverage estimates of total diversity sampled were high across all taxonomic levels, ranging from 82 (OTU0.03) to 85% (OTU0.25). Rarefaction analysis revealed that observed OTU diversity was approaching expected OTU diversity at higher level taxonomic rankings (e.g., phylum and class; Supplementary Figure S5) while additional sampling would continue to uncover new microbial OTUs at lower taxonomic levels (e.g., genus and species; Supplementary Figure S6) due to a rich rare component of the microbiota (1817 singletons).
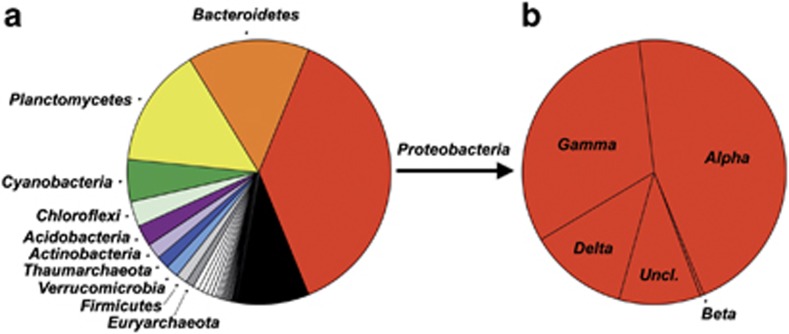
Figure 1.
Taxonomic diversity of the ascidian microbiota. (a) Phylum level distribution of the 3321 microbial OTU0.03 recovered from 42 GBR ascidian hosts, depicting common phyla (in color, >1% OTU0.03 diversity), rare phyla (in gray, <1% SBR1093, Lentisphaerae, Chlamydiae, Tenericutes, TM7, WS3, Spirochaetes, Nitrospirae, OP3, TM6, Crenarchaeota, Chlorobi, OP11, Thermi, Armatimonadetes, Fusobacteria, NKB19, Caldithrix, OP8, PAUC34f, BRC1, Elusimicrobia, GN04, KSB1 and SM2F11) and bacterial OTUs unclassified at the phylum level (in black). (b) Class level distribution of proteobacterial OTUs.
Analyses of individual hosts and ascidian species revealed up to 486 microbial OTU0.03 per individual and 697 unique OTU0.03 per species (Tables 1 and 2), with many ascidians hosting more diverse microbial communities than those recovered from ambient seawater in terms of observed and expected (Chao1) OTU richness and common diversity indices (Shannon, Simpson Inverse; Supplementary Table S3). 16S rRNA sequence reads derived from seawater (n=26 811) grouped into 385 unique OTU0.03 (129 – 284 per replicate). While high variability in sampling effort (sequence reads per sample) can obscure direct comparisons among host species and between ascidians and seawater, over 25% (n=11) of the sampled ascidians exhibited higher microbial OTU0.03 diversity than the most well-sampled seawater replicate, despite lower sampling effort (7 500–13 500 fewer sequence reads; Table 1). Further, this trend was maintained after sub-sampling of sequence pools to standardize sampling efforts across ascidian and seawater sources (Supplementary Figure S6).
Table 1. Taxonomic classification of ascidian hosts and sequence data summary for ascidian and seawater samples. Total values in bold refer to summed reads and unique OTUs.
| Species | Order | Family |
Total |
Archaea |
Bacteria |
|||
|---|---|---|---|---|---|---|---|---|
| Reads | OTU0.03 | Reads | OTU0.03 | Reads | OTU0.03 | |||
| Clavelina arafurensis | Aplousobranchia | Clavelinidae | 490 | 190 | 57 | 4 | 433 | 186 |
| Clavelina meridionalis | 249 | 103 | 15 | 8 | 234 | 95 | ||
| Clavelina meridionalis | 1207 | 333 | 44 | 10 | 1163 | 323 | ||
| Clavelina meridionalis | 1023 | 411 | 38 | 11 | 985 | 400 | ||
| Pycnoclavella sp. | 1449 | 313 | 93 | 11 | 1356 | 302 | ||
| Pycnoclavella sp. | 116 | 47 | 3 | 3 | 113 | 44 | ||
| Pycnoclavella diminuta | 2040 | 384 | 294 | 9 | 1746 | 375 | ||
| Pycnoclavella diminuta | 1188 | 301 | 434 | 9 | 754 | 292 | ||
| Pycnoclavella diminuta | 347 | 167 | 66 | 6 | 281 | 161 | ||
| Didemnum cf. albopunctatum | Didemnidae | 3654 | 154 | 906 | 12 | 2748 | 142 | |
| Didemnum cf. granulatum | 386 | 22 | 11 | 4 | 375 | 18 | ||
| Didemnum multispirale | 3035 | 102 | 10 | 2 | 3025 | 100 | ||
| Didemnum multispirale | 2799 | 142 | 21 | 3 | 2778 | 139 | ||
| Didemnum multispirale | 2979 | 209 | 25 | 6 | 2954 | 203 | ||
| Didemnum sp.1 | 6905 | 486 | 255 | 6 | 6650 | 480 | ||
| Didemnum sp.2 | 2684 | 448 | 762 | 12 | 1922 | 436 | ||
| Leptoclinides madara | 979 | 74 | 165 | 2 | 814 | 72 | ||
| Leptoclinides madara | 281 | 18 | 79 | 2 | 202 | 16 | ||
| Lissoclinum badium | 3224 | 27 | 3055 | 4 | 169 | 23 | ||
| Lissoclinum badium | 4670 | 29 | 4464 | 4 | 206 | 25 | ||
| Lissoclinum cf. capsulatum | 598 | 36 | 2 | 1 | 596 | 35 | ||
| Lissoclinum patella | 2489 | 86 | 1 | 1 | 2488 | 85 | ||
| Eudistoma amplum | Polycitoridae | 517 | 164 | 177 | 16 | 340 | 148 | |
| Eudistoma amplum | 444 | 175 | 89 | 13 | 355 | 162 | ||
| Eudistoma amplum | 825 | 286 | 112 | 11 | 713 | 275 | ||
| Polycitor giganteus | 1602 | 95 | 6 | 3 | 1596 | 92 | ||
| Aplidium protectans | Polyclinidae | 4272 | 129 | 30 | 3 | 4242 | 126 | |
| Aplidium sp. | 1968 | 176 | 64 | 7 | 1904 | 169 | ||
| Synoicum castellatum | 3846 | 382 | 4 | 2 | 3842 | 380 | ||
| Synoicum castellatum | 6447 | 344 | 60 | 3 | 6387 | 341 | ||
| Synoicum castellatum | 120 | 46 | 27 | 4 | 93 | 42 | ||
| Phallusia arabica | Phlebobranchia | Ascidiidae | 105 | 23 | 2 | 1 | 103 | 22 |
| Phallusia arabica | 338 | 53 | 39 | 8 | 299 | 45 | ||
| Phallusia arabica | 54 | 17 | 2 | 2 | 52 | 15 | ||
| Phallusia julinea | 562 | 97 | 55 | 4 | 507 | 93 | ||
| Phallusia philippinensis | 28 | 8 | 12 | 1 | 16 | 7 | ||
| Ecteinascidia diaphanis | Perophoridae | 1168 | 344 | 17 | 4 | 1151 | 340 | |
| Perophora aff. modificata | 1541 | 189 | 184 | 9 | 1357 | 180 | ||
| Polycarpa argentata | Stolidobranchia | Styelidae | 561 | 68 | 446 | 7 | 115 | 61 |
| Polycarpa aurata | 449 | 18 | 0 | 0 | 449 | 18 | ||
| Polycarpa aurata | 159 | 23 | 2 | 2 | 157 | 21 | ||
| Polycarpa aurata | 28 | 8 | 0 | 0 | 28 | 8 | ||
| Ascidian Microbiota Total = | 67 826 | 3321 | 12 128 | 104 | 55 698 | 3217 | ||
| Filtered Seawater | n.a. | n.a. | 9 573 | 221 | 289 | 24 | 9 284 | 197 |
| Filtered Seawater | n.a. | n.a. | 14 441 | 248 | 134 | 21 | 14 307 | 227 |
| Filtered Seawater | n.a. | n.a. | 2 797 | 129 | 3 | 3 | 2 794 | 126 |
| Ambient Seawater Total = | 26 811 | 385 | 426 | 26 | 26 385 | 359 | ||
| Grand Total = | 94 637 | 3604 | 12 554 | 124 | 82 083 | 3480 | ||
Table 2. Intra-specific variation in the ascidian microbiota highlighting the shared components (i.e., present in all host individuals) of each species' microbiota.
| Species | Species Cluster | No. Samples | Total Sequences | Total OTU0.03 | Shared Sequences (%) | Shared OTU0.03 (%) |
|---|---|---|---|---|---|---|
| Clavelina meridionalis | Y | 3 | 2479 | 697 | 1338 (54) | 26 (4) |
| Pycnoclavella sp. | N | 2 | 1565 | 341 | 1077 (69) | 19 (6) |
| Pycnoclavella diminuta | N | 3 | 3575 | 673 | 1731 (48) | 35 (5) |
| Didemnum multispirale | Y | 3 | 8813 | 367 | 6192 (70) | 24 (6) |
| Leptoclinides madara | Y | 2 | 1260 | 81 | 1116 (89) | 11 (14) |
| Lissoclinum badium | Y | 2 | 7894 | 41 | 7848 (99) | 15 (37) |
| Eudistoma amplum | Y | 3 | 1786 | 491 | 809 (45) | 31 (6) |
| Synoicum castellatum | N | 3 | 10 413 | 620 | 5237 (50) | 17 (3) |
| Phallusia arabica | N | 3 | 497 | 82 | 104 (21) | 2 (2) |
| Polycarpa aurata | N | 3 | 636 | 39 | 514 (81) | 3 (8) |
Species cluster refers to Figure 2.
Composition of the ascidian microbiota
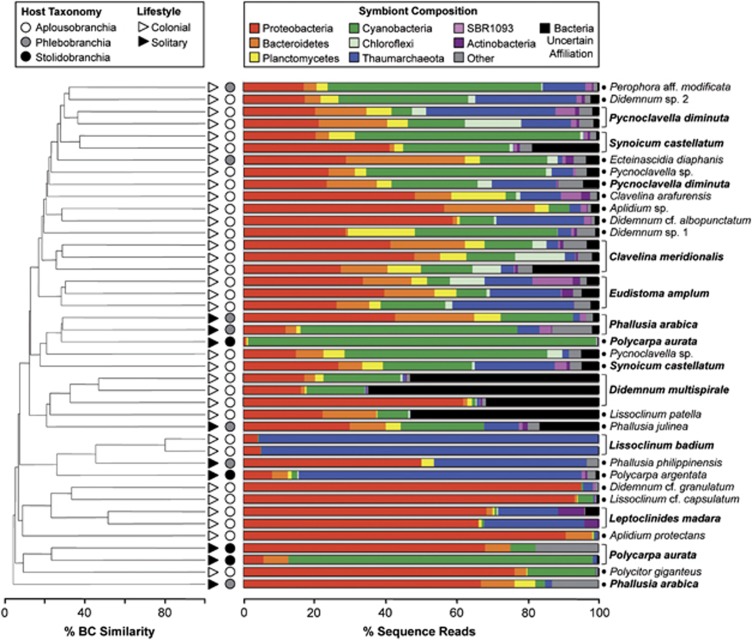
Microbial communities in GBR ascidians were composed of diverse bacterial phyla and archaeal lineages (Figure 1, Supplementary Table S4). Bacterial OTUs dominated the ascidian microbiota, accounting for 97% (n=3217) of OTU0.03 diversity and 82% of all sequence reads (n=55 698). The most dominant bacterial phylum was Proteobacteria, representing over one-third (38%) of OTU0.03 diversity (n=1251) and the only phylum detected in all examined ascidians. Proteobacteria accounted for over half of all sequence reads in 12 ascidian individuals and over 90% of sequences from Aplidium protectans, Lissoclinum cf. capsulatum and Didemnum granulatum (Figure 2). Within the Proteobacteria, the classes Alphaproteobacteria and Gammaproteobacteria were most prevalent (517 OTUs and 397 OTUs, respectively), followed by Deltaproteobacteria and Betaproteobacteria (125 OTUs and 6 OTUs, respectively). Representatives from the phyla Bacteroidetes and Planctomycetes were also common, each accounting for over 15% of OTU0.03 diversity (n=496 and 486, respectively, Figure 1) and detected in the majority (>88%) of ascidian hosts (Figure 2, Supplementary Table S4).
Figure 2.
Microbial community similarity and composition in 42 samples of GBR ascidians. Dendrogram (left) based on Bray-Curtis (BC) similarity of microbial communities in ascidian hosts. Ordinal classifications of ascidians hosts are shown as circles, Aplousobranchia (white), Phlebobranchia (gray) and Stolidobranchia (black) and zooid organization as triangles, colonial (white) and solitary (black). Bar charts (right) show the relative abundance of microbial phyla in each host ascidian, with host species names listed on the right. Bold names indicate species with replicate samples.
Cyanobacteria was the fourth most diverse phyla associated with ascidians (172 OTUs, 5% of OTU0.03 diversity) and included the genus Procholoron, present only in Lissoclinum patella (OTU0810), and 4 OTUs that were closely related (95–98% sequence identity) to the recently described candidatus ‘Acaryochloris bahamiensis' (López-Legentil et al., 2011). Most notably, two Acaryochloris OTUs (OTU0125, 0126) were common in all 3 individuals of the host Eudistoma amplum (0.7 to 8.9% relative abundance). An additional 5 described phyla were common in ascidians, including Chloroflexi (103 OTU0.03), Acidobacteria (87), Actinobacteria (62), Verrucomicrobia (51) and Firmicutes (45), each accounting for 1 to 3% of OTU0.03 diversity and detected in at least half of the ascidian hosts examined. The remaining 24 described and candidate phyla present in the ascidian microbiota were rare overall (each <1% of total OTU0.03 diversity) and within each host ascidian (<2% of sequence reads; Figure 2, Supplementary Table S4), with the exception of Spirochaetes in Polycarpa aurata (18% relative abundance) and SBR1093 in Eudistoma amplum (11%).
Archaeal OTUs accounted for 18% (n=12 128) of sequence reads but only 3% (n=104) of the OTU0.03 richness in the ascidian microbiota. Thaumarchaeota were particularly abundant (n=11 993; 53 OTUs) and common (present in 93% of host individuals), with most archaeal sequence reads (98%) matching to the ammonia-oxidizing genera Nitrosopumilus (n=11 630; 36 OTUs) and Cenarchaeum (n=261; 5 OTUs). In fact, the most common OTU0.03 in the ascidian microbiota (OTU0001, Nitrosopumilus sp.) was present in 37 of the 42 host individuals (22 of 25 host species) at relative abundances up to 95% (Lissoclinum badium), while extremely rare in ambient seawater (0–0.04%). In addition, a common archaeal symbiont in Leptoclinides madara (OTU0025, 17–28% relative abundance) was classified to the genus Cenarchaeum and closely matched (98% sequence identity) an uncultivated archaeon reported in the marine sponge Axinella verrucosa (GenBank accession number AF420237).
Specificity of the ascidian microbiota
Comparison of the rich ascidian microbiota with ambient seawater microbes revealed low overlap between free-living and host-associated microbial communities. A total of 283 OTUs were present in the seawater communities and absent from the ascidian microbiota, while 102 OTUs were present in both ascidian and seawater samples, representing only 3% of total OTU0.03 diversity in the ascidian microbiota. Further, over one-third (n=40) of these shared microbial OTUs exhibited greater than an order of magnitude difference in relative abundance in seawater and ascidians assemblages, including 5 OTUs that were 200x to 700x more abundant in host ascidians (Figure 3). For example, OTU0001 (Nitrosopumilus sp.) accounted for 17% of sequence reads from the ascidian microbiota. The remaining 4 OTUs were specific to particular host families (e.g., OTU0301 in Didemnidae), species (e.g., OTU1798 in 3 individuals of Clavelina meridionalis) or individuals (e.g., OTU0225 in 1 of 3 Didemnum multispirale individuals) and rare or absent in most ascidian hosts (Figure 3).
Figure 3.
Relative abundance of seawater microbes in the ascidian microbiota. (a) Rank-abundance plots showing the relative abundance of 102 microbial OTUs present in both seawater (black line) and ascidian hosts (gray bars). Asterisks denote OTUs>200 times more abundant in ascidian hosts than seawater. (b) Classification and relative abundance of 5 rare seawater biosphere OTUs among ascidian hosts.
Additional analysis of abundant components of the ascidian microbiota revealed symbiont overlap between ascidians and other invertebrate hosts, as well as a unique component of the ascidian microbiota (Table 3). A total of 56 microbial OTUs accounted for 78% of sequences obtained from ascidian hosts. Over two-thirds of these OTUs (n=38) matched closely (>97% sequence identity) to previously characterized sequences (Table 3), most commonly derived from seawater (n=14), corals (n=9), sponges (n=6) and sediment (n=3). In some cases, OTUs that were widespread among ascidians hosts and in the rare biosphere of seawater matched closely to other invertebrate-associated sequences. For example, OTU0264 (Bacteroidetes, Flavobacteriaceae) was present in 24 ascidian individuals, was rare in seawater (<0.05% relative abundance) and matched identically to coral-derived sequences from Caribbean (Montastraea faveolata) and Indo-Pacific (Montipora aequituberculata) stony corals and an Indo-Pacific soft coral (Sinularia sp.). The remaining 18 OTUs exhibited greater divergence from both free-living and host-associated microbes, including 11 OTUs that exhibited <95% sequence identity to known microbial sequences (Table 3).
Table 3. Abundant OTUs in the ascidian microbiota, showing their representation in ascidian (ASC) and seawater (SW) datasets, number of host species, closest known relative and taxonomic classification.
| OTU | Reads (ASC) | Hosts (ASC) | Reads (SW) | BLAST Match Source (Identity, Acc. No.) | Phylum | Lowest Taxonomic Rank |
|---|---|---|---|---|---|---|
| 0001 | 11338 | 39 | 9 | Sponge (98.3, AF420237) | Thaumarchaeota | G. Nitrosopumilus |
| 0140 | 9981 | 42 | 11105 | Seawater (100, GU119217) | Cyanobacteria | G. Prochlorococcus |
| 0287 | 4964 | 11 | 0 | Bivalve (92.9, EU857739) | Unclassified | K. Bacteria |
| 0364 | 3669 | 13 | 13 | Sponge (100, HQ241801) | γ-proteobacteria | G. Coxiella |
| 0188 | 2836 | 30 | 33 | Seawater (100, HQ338142) | α-proteobacteria | F. Rhodobacteraceae |
| 0292 | 1836 | 34 | 30 | Seawater (100, GU119442) | Cyanobacteria | G. Prochlorococcus |
| 0189 | 1790 | 28 | 13 | Seawater (100, JF514245) | α-proteobacteria | G. Mesorhizobium |
| 0225 | 1633 | 25 | 1 | Seawater (100, JF769651) | α-proteobacteria | O. Rhizobiales |
| 0301 | 1432 | 10 | 1 | Ascidian (100, DQ860066) | α-proteobacteria | O. Rhizobiales |
| 1128 | 1346 | 3 | 0 | Seafloor Lava (93.2, EU491218) | Proteobacteria | P. Proteobacteria |
| 1129 | 985 | 2 | 0 | Sediment (88.7, GU046335) | Unclassified | K. Bacteria |
| 0851 | 858 | 5 | 0 | Sponge (94.1, EU883386) | α-proteobacteria | O. Rhodospirillales |
| 0310 | 779 | 32 | 10685 | Seawater (100, JN547429) | Cyanobacteria | G. Prochlorococcus |
| 1063 | 671 | 3 | 0 | Soil (97.9, JQ059148) | α-proteobacteria | F. Rhodospirillaceae |
| 1798 | 567 | 5 | 1 | Seawater (95.8, HQ715140) | α-proteobacteria | O. Rhizobiales |
| 1379 | 379 | 5 | 0 | Soil (90.4, GQ127925) | Unclassified | K. Bacteria |
| 3180 | 354 | 1 | 0 | Sediment (95.4, AB374687) | Bacteroidetes | F. Flammeovirgaceae |
| 1101 | 336 | 16 | 118 | Seawater (100, AB540006) | Bacteroidetes | F. Flavobacteriaceae |
| 0355 | 333 | 25 | 0 | Coral (100, FJ809316) | SBR1093 | C. VHS-B5-50 |
| 0862 | 329 | 16 | 0 | Sponge (100, EU335078) | Chloroflexi | C. Anaerolineae |
| 0931 | 327 | 13 | 0 | Algae (96.7, HM474939) | Chloroflexi | C. Anaerolineae |
| 0164 | 326 | 30 | 3 | Seawater (100, GU119490) | Planctomycetes | O. Pirellulales |
| 1032 | 326 | 3 | 0 | Sediment (96.6, JQ989595) | α-proteobacteria | O. Rhizobiales |
| 0866 | 324 | 22 | 0 | Coral (100, DQ416621) | Bacteroidetes | F. Flavobacteriaceae |
| 0293 | 261 | 11 | 0 | Sponge (100, FJ625530) | Planctomycetes | O. Pirellulales |
| 2687 | 260 | 2 | 0 | Biofilm (94.6, FJ901434) | Cyanobacteria | F. Phormidiaceae |
| 0296 | 246 | 2 | 0 | Sediment (96.7, JN977252) | γ-proteobacteria | C. γ-proteobacteria |
| 0273 | 245 | 15 | 0 | Coral (99.6, JQ347330) | Cyanobacteria | F. Pseudanabaenaceae |
| 0025 | 241 | 2 | 0 | Sponge (98.3, AF420237) | Thaumarchaeota | G. Cenarchaeum |
| 0875 | 211 | 3 | 0 | Coral (97.1, FJ425620) | Bacteroidetes | F. Flammeovirgaceae |
| 0003 | 208 | 19 | 0 | Cyanobacteria (100, JX197041) | Thaumarchaeota | G. Nitrosopumilus |
| 0335 | 206 | 26 | 59 | Seawater (100, EU592360) | α-proteobacteria | F. Rhodobacteraceae |
| 0300 | 202 | 4 | 0 | Sponge (100, JN128259) | γ-proteobacteria | G. Microbulbifer |
| 0344 | 198 | 9 | 0 | Sponge (100, DQ097259) | α-proteobacteria | G. Pseudovibrio |
| 2656 | 193 | 3 | 0 | Diatom Bloom (94.4, EU734047) | β-proteobacteria | C. β-proteobacteria |
| 0318 | 186 | 20 | 0 | Coral (100, FJ489710) | SBR1093 | C. EC214 |
| 2229 | 183 | 3 | 0 | Seawater (98.3, HM798908) | α-proteobacteria | F. Rhodospirillaceae |
| 0187 | 179 | 17 | 2 | Seawater (100, HM103531) | α-proteobacteria | F. Rhodobacteraceae |
| 0161 | 165 | 19 | 0 | Sediment (100, GQ249478) | γ-proteobacteria | F. Chromatiaceae |
| 0306 | 157 | 16 | 0 | Coral (100, FJ203575) | α-proteobacteria | F. Hyphomicrobiaceae |
| 0850 | 153 | 3 | 0 | Biofilm (98.7, DQ167245) | α-proteobacteria | G. Kiloniella |
| 2389 | 152 | 3 | 0 | Coral (95.8, EF206859) | γ-proteobacteria | C. γ-proteobacteria |
| 0133 | 147 | 20 | 0 | Coral (100, GU118991) | Bacteroidetes | F. Flammeovirgaceae |
| 0264 | 147 | 24 | 13 | Coral (100, FJ809398) | Bacteroidetes | F. Flavobacteriaceae |
| 0294 | 145 | 3 | 0 | Algae (99.6, GU451475) | α-proteobacteria | G. Pseudovibrio |
| 1065 | 143 | 4 | 0 | Coral (93.2, GU118840) | α-proteobacteria | O. Rhodospirillales |
| 0186 | 138 | 17 | 0 | Sediment (99.6, FJ358900) | Bacteroidetes | F. Flammeovirgaceae |
| 0307 | 137 | 13 | 0 | Algae (99.6, HM474882) | α-proteobacteria | F. Rhodospirillaceae |
| 2811 | 137 | 2 | 0 | Seawater (94.5, EF572701) | Bacteroidetes | F. Flavobacteriaceae |
| 0297 | 132 | 12 | 0 | Coral (99.6, FJ203345) | Planctomycetes | O. Pirellulales |
| 0939 | 130 | 13 | 0 | Sediment (100, DQ256661) | Cyanobacteria | G. Leptolyngbya |
| 2749 | 124 | 1 | 0 | Sediment (96.2, EU287328) | α-proteobacteria | O. Rhizobiales |
| 0686 | 121 | 7 | 0 | Bivalve (92.5, EU857738) | Unclassified | K. Bacteria |
| 2875 | 117 | 1 | 0 | Seawater (98.3, JN216763) | α-proteobacteria | C. α-proteobacteria |
| 1132 | 107 | 1 | 0 | Mammal Gut (89.2, EU459272) | Unclassified | K. Bacteria |
| 0172 | 101 | 4 | 0 | Seawater (99.2, GQ349494) | δ-proteobacteria | G. Nitrospina |
Core, variable and specific microbial OTUs
Comparison of the microbial communities among ascidian hosts revealed a high degree of host specificity in the ascidian microbiota and the presence of a small number of very abundant and widespread microbial OTUs. No universal symbiont OTUs (i.e., present in all host species) were detected and core OTUs (present in >70% of host species) were represented by 7 OTUs at high relative abundance, accounting for 40% of all sequence reads. These OTUs corresponded to 2 Prochlorococcus sp. (Cyanobacteria; OTU0140, OTU0310) that were also common in seawater communities (41 and 40% relative abundance, respectively), as well as Nitrosopumilus sp. (Thaumarchaeota; OTU0001), Prochlorococcus sp. (Cyanobacteria; OTU0292), Rhodobacteraceae sp. (Alphaproteobacteria; OTU0188), Pirellulales sp. (Planctomycetes; OTU0164) and an OTU from the candidate phylum SBR1093 (OTU0355) that were rare (0.01–0.12% relative abundance) or absent in seawater samples. Variable OTUs (present in at least 2 host species) were represented by 950 OTUs and accounted for 49% of sequence reads, while specific OTUs (present in a single host species) were represented by 2364 OTUs and accounted for 11% of sequence reads.
Community-level analysis of tunic-associated microbes among ascidian species revealed a significant correlation between host relatedness (18S rRNA sequence similarity) and symbiont community similarity (Mantel test, r=0.37, P<0.001). This relationship was maintained when replicate samples were removed (r=0.28, P<0.001) and when using sub-sampled sequence pools to standardize sampling effort (r=0.50, P<0.001), indicating that high symbiont similarity among individuals of the same species and sampling artifacts were not the sole drivers of the observed correlation. Indeed, while symbiont communities were consistent across replicate individuals for 5 colonial ascidian species, other host species exhibited high intra-specific variability among replicates, including two solitary and three colonial species (Table 2). The lowest intra-specific diversity in symbiont structure was seen in Lissoclinum badium, where shared symbionts accounted for 37% of OTU0.03 diversity and 99% of sequence reads. The highest intra-specific diversity was seen in Phallusia arabica, where shared symbionts only accounted for 2% of OTU0.03 diversity and 21% of sequence reads (Table 2). Symbiont communities did not strictly cluster by higher-level host taxonomy (order to genus-level) or lifestyle (solitary or colonial; Figure 2), likely obscured by the observed variability in symbiont specificity among hosts.
Bacterial ultrastructure in the ascidian tunic
Transmission electron microscopy examination of the solitary ascidians Phallusia julinea and Polycarpa aurata revealed randomly distributed and extremely rare bacterial cells in the inner tunic of these two species. All bacterial morphotypes observed in P. julinea were ovoid to rod-shaped cells (ca. 0.4 μm × 2 μm; Supplementary Figure S7A), while ovoid cells (ca. 0.12 μm), cyanobacteria (ca. 0.15 μm, with ca. 5 thylakoids evenly spaced along the periphery of the cell), and a spiral bacterium (Supplementary Figure S7B) were observed in P. aurata. Colonial ascidians were characterized by a higher number of bacteria in their tunic. Pycnoclavella sp. featured groups of 2 to 5 cyanobacteria encased in a network of fibers (Supplementary Figure S7C). Both clavelinids (Pycnoclavella sp. and C. meridionalis) contained ovoid-shaped bacteria often surrounded by irregular inclusions spread throughout the tunic (Supplementary Figure S7D). In Lissoclinum badium and Synoicum castellatum, all bacterial cells were ovoid or rod-shaped (ca. 0.5 μm × 2 μm, and ca. 0.3 μm × 1 μm, respectively) and observed either in isolation or forming small groups of 2–6 bacteria in close proximity to ascidian cells (Supplementary Figure S7E and S7F, respectively).
Discussion
Bacterial biodiversity hotspots in the ascidian tunic
In this study, we provide the most comprehensive characterization of the ascidian microbiota to date and reveal exceptional bacterial biodiversity inhabiting the tunic of GBR ascidians. Encompassing 3321 unique OTU0.03 from 19 described bacterial phyla, 14 candidate bacterial phyla and 3 described archaeal phyla, the ascidian microbiota exhibited comparable diversity to the rich microbiota associated with marine sponges (Schmitt et al., 2012) and corals (Sunagawa et al., 2010) and indicates that the ascidian tunic represents a previously unrecognized hotspot for marine microbial diversity. Visualization of microbial cells by transmission electron microscopy confirmed the presence of microbes in the ascidian tunic and was consistent with results from 16S rRNA gene tag pyrosequencing, for example, the prevalence of cyanobacterial OTUs (>50% of sequence reads) and cyanobacterial cells encased in a fiber network in Pycnoclavella sp. and the detection of a Spirochaetes OTU (18% relative abundance) and a bacterium with spiral morphology in Polycarpa aurata.
Phylum-level composition of the ascidian microbiota retrieved herein was similar to what has been described for other ascidian species and was comprised of mostly Proteobacteria, Bacteroidetes and Planctomycetes (Martínez-García et al., 2007; Tait et al., 2007; Behrendt et al., 2012). Moreover, as found for other tropical ascidians (e.g., Behrendt et al., 2012), Cyanobacteria were particularly abundant in most of our ascidian samples. In addition, the ascidian microbiota demonstrated some overlap with other host-associated microbial communities yet clear distinction from ambient planktonic communities in coral reef seawater, except for the widespread presence of Cyanobacteria from the Prochlorococcus genus. Consistently, previous studies have noted multiple shared symbiont lineages among microbiota of sponges and corals (Taylor et al., 2007; Simister et al., 2012), indicating microbial lineages adapted to host-associated lifestyles may disperse among disparate host organisms. However, the ascidian microbiota also maintained distinguishing characteristics in comparison to other host-associated communities. For example, the phylum Planctomycetes exhibited high diversity in ascidian hosts, whereas members of this phylum are typically rare in microbiota of sponge (Schmitt et al., 2012; Webster and Taylor, 2012) and coral hosts (Sunagawa et al., 2010; Barott et al., 2011). Further, 11 of the 56 most common OTUs in the ascidian microbiota exhibited high sequence divergence (>5%) from any previously described marine microbe. The unique niches inside invertebrate tissues are becoming recognized hotspots for microbial biodiversity and our results suggest that ascidian tunics offer a similarly fertile habitat for marine microorganisms.
Rare seawater microbes enriched in the ascidian tunic
The vast majority of OTUs in the ascidian microbiota were not present in planktonic communities. However, a cautionary note is necessary here as seawater samples were collected in only one of our sampling sites and at one given time (October 2011), while ascidian samples were collected from different locations (separated by less than 120 km) and times (May through November 2011; Supplementary Table S1). Microbes in seawater are known to vary seasonally, occur in patches or be stratified according to their microenvironmental requirements or to microscale turbulences (e.g., Giovannoni and Stingl, 2005). Accordingly, the low number of shared OTUs (3%) between the seawater and the ascidian samples may be partly due to an insufficient sampling of the surrounding seawater.
Nevertheless, we found that several microbes from the rare biosphere of seawater exhibited high relative abundance in ascidian-associated communities. Five microbial OTUs exhibited 200 to 700 times higher relative abundance in the ascidian tunic than in the plankton, suggesting the selective enrichment of rare seawater microbes in ascidian hosts as observed for the microbiota in marine sponges (Webster et al., 2010; Taylor et al., 2013) and reef-building corals (Sunagawa et al., 2010). Notably, 3 of the 5 OTUs enriched in the ascidian microbiota were classified to the order Rhizobiales, a lineage of Alphaproteobacteria well known for their nitrogen-fixation capacity and mutualistic relationships with terrestrial plants (Lodwig et al., 2003) and more recently documented as dominant nitrogen-fixing symbionts in the coral microbiome (Lema et al., 2012). In this study, a total of 176 OTUs affiliated with Rhizobiales were present in the ascidian microbiota and detected in all 25 ascidian host species prompting further study of nitrogen-fixing bacteria in the ascidian microbiota and their potential contribution to nitrogen cycles in the ascidian holobiont. These results also indicate the potential for horizontal symbiont transfer among hosts with the rare biosphere of seawater acting as a conduit among host habitats.
Host specificity of the ascidian microbiota
The vast majority of symbiont OTUs (71%) were present in a single host species and absent in seawater, indicating a high degree of host specificity in the microbiota of coral reef ascidians. Indeed, no universal symbionts (i.e., present in all ascidian hosts) occurred and only 7 core OTUs (of 3321 total OTUs) were detected. While few 16S rRNA gene sequence datasets from ascidians are available for comparative analyses, several OTUs exhibited specific associations with particular host taxa across a broad geographic range. For example, OTU3073 from Ecteinascidia diaphanis matched to the candidate genus Endoecteinascidia, a distinct lineage of Gammaproteobacteria described solely from ascidians in the genus Ecteinascidia, including E. turbinata from the Mediterranean (Moss et al., 2003) and Caribbean (Pérez-Matos et al., 2007). The detection of this candidate genus from a GBR ascidian expands the known geographic range of this symbiont taxon and further supports its specificity to the host genus Ecteinascidia. In addition, this symbiont lineage is particularly notable for its putative role in secondary metabolite synthesis within the animal cell, including the production of the anticancer agent ET-743 (Rath et al., 2011), which may constitute a key functional aspect of ascidian-bacterial symbioses (Kwan et al., 2012).
Even among replicate individuals of the same ascidian species, some intra-specific variability was observed. Consistent microbial community structure was observed in 5 of the 10 ascidian species where multiple individuals were analyzed, while the remaining half exhibited greater similarity to the microbiota of unrelated species than to conspecific hosts, suggesting a non-obligate symbiosis. These results suggest different factors structuring the symbiont communities in different ascidian species, with more homogenous communities potentially maintained in some hosts by vertical symbiont transmission or specific functional requirements and more heterogeneous communities in other hosts determined by more stochastic or dynamic factors. This observation is in agreement with mounting evidence suggesting that colonial ascidians, such as the Didemnidae, establish stable symbiotic microbial associations that are vertically transmitted (Kott, 1980, 1982, 2001; Hirose, 2000; Schuett et al., 2005; Hirose et al., 2006a, 2006b; Hirose and Hirose, 2007; Bright and Bulgheresi, 2010; López-Legentil et al., 2011; Kojima and Hirose, 2012), while others, such as solitary ascidians may selectively acquire symbionts from the surrounding seawater (Erwin et al., 2013).
Widespread ammonia-oxidizing archaea (AOA) in the ascidian microbiota
Nitrification is a key process in the global nitrogen cycle that results in the conversion of ammonia to nitrite (ammonia-oxidation) and nitrite to nitrate (nitrite-oxidation), a two-step process mediated solely by prokaryotic organisms (Ward et al., 2007). The archaeal component of the ascidian microbiota was notably comprised of lineages with known ammonia-oxidization capabilities. In particular, sequences affiliated with the genus Nitrosopumilus dominated the archaeal communities in GBR ascidians and several Nitrosopumilus OTUs exhibited a widespread distribution among hosts and high relative abundance within hosts. In coral reef waters, observations of high nitrite/nitrate concentrations compared to adjacent, open water habitats have long suggested active nitrification among reef-associated microbes (Webb et al., 1975). More recent studies have reported that host-associated microbes in sponges and corals contributed to nitrification in these reef habitats to a larger extent than reported for free-living communities in sediments and seawater (Diaz and Ward, 1997; Southwell et al., 2008). The finding herein of widespread ammonia-oxidizing archaea in coral reef ascidians suggests an additional and potentially important source of nitrification in reef habitats.
In fact, the most dominant of all OTUs in the ascidian microbiota (17% of total reads) was classified in the genus Nitrosopumilus and matched nearly identically (>99% sequence identity) to a symbiotic ammonia-oxidizing archaea (AOA) previously described in the Mediterranean ascidian Cystodytes dellechiajei, where active nitrification was detected in the tunic layer (Martínez-García et al., 2008). Another OTU recovered from two individuals of the ascidian Leptoclinides madara at high relative abundance (17–28%) was classified in the genus Cenarchaeum, a candidate taxon erected for the sponge-associated symbiont Cenarchaeum symbiosum (Preston et al., 1996) whose genome includes homologues of genes associated with chemolithotrophic ammonia oxidation (Hallam et al., 2006). Finally, some ascidians (e.g., Lissoclinum badium) hosted Nitrospina symbionts, a genus of Deltaproteobacteria whose members are capable of nitrite-oxidation, in addition to dominant AOA lineages, suggesting that the complete nitrification process may occur in the ascidian tunic of at least some species.
Ammonia is the primary form of nitrogenous waste produced by ascidians (Goodbody, 1974) and may be recycled via uptake or oxidation by resident microbes. For example, the widespread AOA reported herein may utilize the ammonia-rich waste products of their host ascidians as substrate for nitrification reactions. Indeed, nitrifying microbes require not only a reduced form of inorganic nitrogen, but also high oxygen and low irradiance levels, as marine AOA are particularly susceptible to photoinhibition at higher irradiance levels (Merbt et al., 2012). Thus, the ascidian tunic habitat not only satisfies the ammonia and oxygen requirements of AOA (Kühl et al., 2012), but may also shelter these populations from the high irradiance levels characteristic of shallow water reefs (e.g., Vermeij and Bak, 2002) and represent important habitats for nitrite/nitrate regeneration in coral reef environments. Further, the dynamic chemical landscapes in and around ascidians (Behrendt et al., 2012, Kühl et al., 2012) may offer periodic windows of optimal conditions for additional metabolic pathways and maintain the complex microbiota observed in ascidian tunics.
While the taxonomic scope of the ascidian species examined herein was broad, the geographic scope was restricted to shallow water habitats of the GBR. Yet even within this single biome, our results show a remarkably rich and diverse microbial community associated with coral reef ascidians. Given the broad distribution of ascidians in the marine environment, (Lambert, 2005) expanded efforts to document the diversity of the ascidian microbiota will continue to clarify the role of ascidians as habitats for novel microbial communities and their importance for microbial-mediated processes in marine biogeochemical cycles.
Acknowledgments
This research was funded by the Marie Curie International Reintegration Grant FP7-PEOPLE-2010-RG 277038 (within the 7th European Community Framework Program), the Spanish Government projects CTM2010-17755 and CTM2010-22218 and the Catalan Government grant 2009SGR-484 for Consolidated Research Groups. NSW was funded through an Australian Research Council Future Fellowship (FT1200100480).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on The ISME Journal website (http://www.nature.com/ismej)
Supplementary Material
References
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Armstrong E, Yan L, Boyd KG, Wright PC, Burgess JG. The symbiotic role of marine microbes on living surfaces. Hydrobiologia. 2001;461:37–40. [Google Scholar]
- Barott KL, Rodriguez-Brito B, Janouškovec J, Marhaver KL, Smith JE, Keeling P, et al. Microbial diversity associated with four functional groups of benthic reef algae and the reef-building coral Montastraea annularis. Environ Microb. 2011;13:1192–1204. doi: 10.1111/j.1462-2920.2010.02419.x. [DOI] [PubMed] [Google Scholar]
- Behrendt L, Larkum Anthony WD, Trampe E, Norman A, Sørensen SJ, Kühl M. Microbial diversity of biofilm communities in microniches associated with the didemnid ascidian Lissoclinum patella. ISME J. 2012;6:1222–1237. doi: 10.1038/ismej.2011.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtsson J, Eriksson KM, Hartmann M, Wang Z, Shenoy BD, Grelet G-A, et al. Metaxa: a software tool for automated detection and discrimination among ribosomal small subunit (12S/16S/18S) sequences of archaea, bacteria, eukaryotes, mitochondria, and chloroplasts in metagenomes and environmental sequencing datasets. Anton Leeuw. 2011;100:471–475. doi: 10.1007/s10482-011-9598-6. [DOI] [PubMed] [Google Scholar]
- Bright M, Bulgheresi S. A complex journey: transmission of microbial symbionts. Nat Rev Microbiol. 2010;8:218–230. doi: 10.1038/nrmicro2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brochier-Armanet C, Boussau B, Gribaldo S, Forterre P. Mesophilic Crenarchaeota: proposal for a third archaeal phylum, the Thaumarchaeota. Nat Rev Microbiol. 2008;6:245–252. doi: 10.1038/nrmicro1852. [DOI] [PubMed] [Google Scholar]
- Cox G. Comparison of Prochloron from different hosts: I. Structural and ultrastructural characteristics. New Phytol. 1986;104:429–445. [Google Scholar]
- Cox GC, Hiller RG, Larkum AWD. An unusual cyanophyte, containing phycourobilin and symbiotic with ascidians and sponges. Mar Biol. 1985;89:149–163. [Google Scholar]
- Diaz MC, Ward BB. Sponge-mediated nitrification in tropical benthic communities. Mar Ecol Prog Ser. 1997;156:97–107. [Google Scholar]
- Donia MS, Fricke WF, Partensky F, Cox J, Elshahawi SI, White JR, et al. Complex microbiome underlying secondary and primary metabolism in the tunicate-Prochloron symbiosis. Proc Natl Acad Sci USA. 2011;108:E1423–E1432. doi: 10.1073/pnas.1111712108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dray S, Dufour A. The ade4 package: Implementing the duality diagram for ecologists. J. Stat Softw. 2007;22:1–20. [Google Scholar]
- Delsuc F, Brinkmann H, Chourrout D, Philippe H. Tunicates and not cephalochordates are the closest living relatives of vertebrates. Nature. 2006;439:965–968. doi: 10.1038/nature04336. [DOI] [PubMed] [Google Scholar]
- Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 2011;27:2194–2200. doi: 10.1093/bioinformatics/btr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erwin PM, López-Legentil S, Schuhmann PW. The pharmaceutical value of marine biodiversity for anti-cancer drug discovery. Ecol Econ. 2010;70:445–451. [Google Scholar]
- Erwin PM, Pineda MC, Webster N, Turon X, López-Legentil S. Small core communities and high variability in bacteria associated with the introduced ascidian Styela plicata. Symbiosis. 2013;59:35–46. [Google Scholar]
- Fan L, Reynolds D, Liu M, Stark M, Kjelleberg S, Webster NS, et al. Functional equivalence and evolutionary convergence in complex communities of microbial sponge symbionts. Proc Natl Acad Sci USA. 2012;109:E1878–E1887. doi: 10.1073/pnas.1203287109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fieseler L, Horn M, Wagner M, Hentschel U. Discovery of the novel candidate phylum “Poribacteria” in marine sponges. Appl Environ Microbiol. 2004;70:3724–3732. doi: 10.1128/AEM.70.6.3724-3732.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechy. 1994;3:294–299. [PubMed] [Google Scholar]
- Freeman CJ, Thacker RW. Complex interactions between marine sponges and their symbiotic microbial communities. Limnol Oceanogr. 2011;56:1577–1586. [Google Scholar]
- Freeman MF, Gurgui C, Helf MJ, Morinaka BI, Uria AR, Oldham NJ, et al. Metagenome mining reveals polytheonamides as posttranslationally modified ribosomal peptides. Science. 2012;338:387–390. doi: 10.1126/science.1226121. [DOI] [PubMed] [Google Scholar]
- Giovannoni SJ, Stingl U. Molecular diversity and ecology microbial plankton. Nature. 2005;437:343–348. doi: 10.1038/nature04158. [DOI] [PubMed] [Google Scholar]
- Goodbody I. The physiology of ascidians. Adv Mar Biol. 1974;12:121–149. [Google Scholar]
- Hallam SJ, Konstantinidis KT, Putnam N, Schleper C, Watanabe Y, Sugahara J, et al. Genomic analysis of the uncultivated marine crenarchaeote Cenarchaeum symbiosum. Proc Natl Acad Sci USA. 2006;103:18296–18301. doi: 10.1073/pnas.0608549103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Mariné M, Turon X, Catalan J. A marine Synechocystis (Chroococcales, Cyanophyta) epizoic on didemnid ascidians from the Mediterranean Sea. Phycologia. 1990;29:275–284. [Google Scholar]
- Hirose E. Plant rake and algal pouch of the larvae in the tropical ascidian Diplosoma similis: an adaptation for verticaltransmission of photosynthetic symbionts Prochloron sp. Zool Sci. 2000;17:233–240. [Google Scholar]
- Hirose E, Hirose M. Morhological process of vertical transmission of photosymbionts in the colonial ascidian Trididemnum miniatum Kott, 1977. Mar Biol. 2007;150:359–367. [Google Scholar]
- Hirose E, Maruyama T. What are the benefits in the ascidian-Prochloron symbiosis. Endocyt Cell Res. 2004;15:51–62. [Google Scholar]
- Hirose E, Adachi R, Kuze K. Sexual reproduction of the Prochloron-bearing ascidians, Trididemnum cyclops and Lissoclinum bistratum, in subtropical waters: seasonality and vertical transmission of photosymbionts. J Mar Biol Ass UK. 2006;86:175–179. [Google Scholar]
- Hirose E, Hirose M, Neilan BA. Localization of symbiotic cyanobacteria in the colonial ascidian Trididemnum miniatum (Didemnidae, Ascidiacea) Zool Sci. 2006;23:435–442. doi: 10.2108/zsj.23.435. [DOI] [PubMed] [Google Scholar]
- Hirose E, Maruyama T, Cheng L, Lewin RA. Intracellular symbiosis of a photosynthetic prokaryote, Prochloron sp., in a colonial ascidian. Invertebr Biol. 1996;115:343–348. [Google Scholar]
- Hirose E, Ohtsuka K, Ishikura M, Maruyama T. Untraviolet absorption in ascidian tunic and ascidian-Prochloron symbiosis. J Mar Biol Assoc UK. 2004;84:789–794. [Google Scholar]
- Hirose E, Turon X, López-Legentil S, Erwin PM, Hirose M. First records of didemnid ascidians harboring Prochloron from the Caribbean Panama: genetic relationships between Caribbean and Pacific photosymbionts and host ascidians. Sys Biodivers. 2012;10:435–445. [Google Scholar]
- Hoffmann F, Larsen O, Thiel V, Rapp HT, Pape T, Michaelis W, et al. An anaerobic world in sponges. Geomicrobiol J. 2005;22:1–10. [Google Scholar]
- Hoffmann F, Radax R, Woebken D, Holtappels M, Lavik G, Rapp HT, et al. Complex nitrogen cycling in the sponge Geodia barretti. Environ Microb. 2009;11:2228–2243. doi: 10.1111/j.1462-2920.2009.01944.x. [DOI] [PubMed] [Google Scholar]
- Hoffmann F, Røy H, Bayer K, Hentschel U, Pfannkuchen M, Brümmer F, et al. Oxygen dynamics and transport in the Mediterranean sponge Aplysina aerophoba. Mar Biol. 2008;153:1257–1264. doi: 10.1007/s00227-008-0905-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber JA, Mark Welch DB, Morrison HG, Huse SM, Neal PR, Butterfield DA, et al. Microbial population structures in the deep marine biosphere. Science. 2007;318:97–100. doi: 10.1126/science.1146689. [DOI] [PubMed] [Google Scholar]
- Huse SM, Welch DM, Morrison HG, Sogin ML. Ironing out the wrinkles in the rare biosphere through improved OTU clustering. Environ Microbiol. 2010;12:1889–1898. doi: 10.1111/j.1462-2920.2010.02193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima A, Hirose E. Transmission of cyanobacterial symbionts during embryogenesis in the coral reef ascidians Trididemnum nubilum and T. clinides (Didemnidae, Ascidiacea, Chordata) Biol Bull. 2012;222:63–73. doi: 10.1086/BBLv222n1p63. [DOI] [PubMed] [Google Scholar]
- Kojima A, Hirose E. Transfer of prokaryotic algal symbionts from a tropical ascidian (Lissoclinum punctatum) colony to its larvae. Zool Sci. 2010;27:124–127. doi: 10.2108/zsj.27.124. [DOI] [PubMed] [Google Scholar]
- Kott P. Algal-bearing didemnid ascidians in the Indo-West-Pacific. Mem Queensl Mus. 1980;20:1–47. [Google Scholar]
- Kott P. Didemnid-algal symbioses: host species in the Western Pacific with notes on the symbiosis. Micronesica. 1982;18:95–127. [Google Scholar]
- Kott P. The Australian Ascidiacea. Part 4, Aplousobranchia (3), Didemnidae. Mem Queensl Mus. 2001;47:1–407. [Google Scholar]
- Kühl M, Behrendt L, Trampe E, Qvortrup K, Schreiber U, Borisov SM, et al. Microenvironmental ecology of the chlorophyll b-containing symbiotic cyanobacterium Prochloron in the didemnid ascidian Lissoclinum patella. Front Microbiol. 2012;3:73–90. doi: 10.3389/fmicb.2012.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan JC, Donia MS, Han AW, Hirose E, Haygood MG, Schmidt EW. Genome streamlining and chemical defense in a coral reef symbiosis. Proc Natl Acad Sci USA. 2012;109:20655–20660. doi: 10.1073/pnas.1213820109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert G. Ecology and natural history of the protochordates. Can J Zool. 2005;83:34–50. [Google Scholar]
- Lema KA, Willis BL, Bourne DG. Corals form characteristic associations with symbiotic nitrogen-fixing bacteria. Appl Environ Microbiol. 2012;78:3136–3144. doi: 10.1128/AEM.07800-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodwig EM, Hosie AHF, Bourdès A, Findlay K, Allaway D, Karunakaran R, et al. Amino-acid cycling drives nitrogen fixation in the legume-Rhizobium symbiosis. Nature. 2003;422:722–726. doi: 10.1038/nature01527. [DOI] [PubMed] [Google Scholar]
- López-Legentil S, Song B, Bosch M, Pawlik JR, Turon X. Cyanobacterial diversity and a new Acaryochloris-like symbiont from Bahamian sea-squirts. PLoS One. 2011;6:e23938. doi: 10.1371/journal.pone.0023938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Legentil S, Turon X. How do morphotypes and chemotypes relate to genotypes? The colonial ascidian Cystodytes (Polycitoridae) Zool Scr. 2005;34:3–14. [Google Scholar]
- Martínez-García M, Díaz-Valdés M, Wanner G, Ramos-Esplá A, Antón J. Microbial community associated with the colonial ascidian Cystodytes dellechiajei. Environ Microbiol. 2007;9:521–534. doi: 10.1111/j.1462-2920.2006.01170.x. [DOI] [PubMed] [Google Scholar]
- Martínez-García M, Koblížek M, López-Legentil S, Antón J. Epibiosis of oxygenic phototrophs containing chlorophylls a, b, c, and d on the colonial ascidian Cystodytes dellechiajei. Microb Ecol. 2011;61:13–19. doi: 10.1007/s00248-010-9694-6. [DOI] [PubMed] [Google Scholar]
- Martínez-García M, Stief P, Díaz-Valdés M, Wanner G, Ramos-Esplá A, Dubilier N, et al. Ammonia-oxidizing Crenarchaeota and nitrification inside the tissue of a colonial ascidian. Environ Microbiol. 2008;10:2991–3001. doi: 10.1111/j.1462-2920.2008.01761.x. [DOI] [PubMed] [Google Scholar]
- Merbt SN, Stahl DA, Casamayor EO, Martí E, Nicol GW, Prosser JI. Differential photoinhibition of bacterial and archaeal ammonia oxidation. FEMS Microbiol Lett. 2012;327:41–46. doi: 10.1111/j.1574-6968.2011.02457.x. [DOI] [PubMed] [Google Scholar]
- McDonald D, Price MN, Goodrich J, Nawrocki EP, DeSantis TZ, Probst A, et al. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J. 2012;6:610–618. doi: 10.1038/ismej.2011.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss C, Green DH, Pérez B, Velasco A, Henríquez R, McKenzie JD. Intracellular bacteria associated with the ascidian Ecteinascidia turbinata: phylogenetic and in situ hybridisation analysis. Mar Biol. 2003;143:99–110. [Google Scholar]
- Muscatine L, Porter JW. Reef corals: mutualistic symbioses adapted to nutrient-poor environments. BioScience. 1977;27:454–460. [Google Scholar]
- Münchhoff J, Hirose E, Maruyama T, Sunairi M, Burns BP, Neilan BA. Host specificity and phylogeography of the prochlorophyte Prochloron sp., an obligate symbiont in didemnid ascidians. Environ Microbiol. 2007;9:890–899. doi: 10.1111/j.1462-2920.2006.01209.x. [DOI] [PubMed] [Google Scholar]
- Pardy RL, Lewin RA. Colonial ascidians with Prochlorophyte symbionts: evidence for translocation of metabolites from alga to host. Bull Mar Sci. 1981;31:817–823. [Google Scholar]
- Paul VJ, Ritson-Williams R. Marine chemical ecology. Nat Prod Rep. 2008;25:662–695. doi: 10.1039/b702742g. [DOI] [PubMed] [Google Scholar]
- Pawlik JR. The chemical ecology of sponges on Caribbean reefs: natural products shape natural systems. Bio Science. 2011;61:888–898. [Google Scholar]
- Preston CM, Wu KY, Molinski TF, DeLong EF. A psychrophilic crenarchaeon inhabits a marine sponge: Cenarchaeum symbiosum gen. nov., sp. nov. Proc Natl Acad Sci USA. 1996;93:6241–6246. doi: 10.1073/pnas.93.13.6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Matos AE, Rosado W, Govind NS. Bacterial diversity associated with the Caribbean tunicate Ecteinascidia turbinata. A van Leeuw J Microb. 2007;92:155–164. doi: 10.1007/s10482-007-9143-9. [DOI] [PubMed] [Google Scholar]
- Pérez-Portela R, Bishop JDD, Davis AR, Turon X. Phylogeny of the families Pyuridae and Styelidae (Stolidobranchiata, Ascidiacea) inferred from mitochondrial and nuclear DNA sequences. Mol Phylogenet Evol. 2009;50:560–570. doi: 10.1016/j.ympev.2008.11.014. [DOI] [PubMed] [Google Scholar]
- Raina JB, Dinsdale EA, Willis BL, Bourne DG. Do the organic sulfer compounds DMSP and DMS drive coral microbioal associations. Trends Microbiol. 2010;18:101–108. doi: 10.1016/j.tim.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Rath CM, Janto B, Earl J, Ahmed A, Hu FZ, Hiller L, et al. Meta-omic characterization of the marine invertebrate microbial consortium that produces the chemotherapeutic natural product ET-743. ACS Chem Biol. 2011;6:1244–1256. doi: 10.1021/cb200244t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesch LFW, Fulthorpe RR, Riva A, Casella G, Hadwin AKM, Kent AD, et al. Pyrosequencing enumerates and contrasts soil microbial diversity. ISME J. 2007;1:283–290. doi: 10.1038/ismej.2007.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowan R. Diversity and ecology of zooxanthellae on coral reefs. J Phycol. 1998;34:407–417. [Google Scholar]
- Schloss PD, Gevers D, Westcott SL. Reducing the effects of PCR amplification and sequencing artifacts on 16S rRNA-based studies. PLoS One. 2011;6:e27310. doi: 10.1371/journal.pone.0027310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloss PD, Westcott SL. Assessing and improving methods used in operational taxonomic unit-based approaches for 16S rRNA gene sequence analysis. App Environ Microbiol. 2011;77:3219–3226. doi: 10.1128/AEM.02810-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. App Environ Microbiol. 2009;75:7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt EW, Donia MS. Life in cellulose houses: symbiotic bacterial biosynthesis of ascidian drugs and drug leads. Curr Opin Biotech. 2010;21:827–833. doi: 10.1016/j.copbio.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt EW, Nelson JT, Rasko DA, Sudek S, Eisen JA, Haygood MG, et al. Patellamide A and C biosynthesis by a microcin-like pathway in Prochloron didemni, the cyanobacterial symbiont of Lissoclinum patella. Proc Natl Acad Sci USA. 2005;102:7315–7320. doi: 10.1073/pnas.0501424102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt S, Tsai P, Bell J, Fromont J, Ilan M, Lindquist N, et al. Assessing the complex sponge microbiota: core, variable and species-specific bacterial communities in marine sponges. ISME J. 2012;J6:564–576. doi: 10.1038/ismej.2011.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuett C, Doepke H, Groepler W, Wichels A. Diversity of intratunical bacteria in the tunic matrix of the colonial ascidian Diplosoma migrans. Helgoland Mar Res. 2005;59:136–140. [Google Scholar]
- Shenkar N, Swalla BJ. Global diversity of Ascidiacea. PLoS One. 2011;7:e20657. doi: 10.1371/journal.pone.0020657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simister RL, Deines P, Botté ES, Webster NS, Taylor MW. Sponge-specific clusters revisited: a comprehensive phylogeny of sponge-associated microorganisms. Environ Microbiol. 2012;14:517–524. doi: 10.1111/j.1462-2920.2011.02664.x. [DOI] [PubMed] [Google Scholar]
- Smith EP, Van Belle G. Nonparametric estimation of species richness. Biometrics. 1984;40:119–129. [Google Scholar]
- Southwell MW, Weisz JB, Martens CS, Lindquist N. In situ fluxes of dissolved inorganic nitrogen from the sponge community on Conch Reef, Key Largo, Florida. Limnol Oceanogr. 2008;53:986–996. [Google Scholar]
- Spang A, Hatzenpichler R, Brochier-Armanet C, Rattei T, Tischler P, Spieck E, et al. Distinct gene set in two different lineages of ammonia-oxidizing archaea supports the phylum Thaumarchaeota. Trends Microbiol. 2010;18:331–340. doi: 10.1016/j.tim.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Stefaniak L, Lambert G, Gittenberger A, Zhang H, Lin S, Whitlatch RB. Genetic conspecificity of the worldwide populations of Didemnum vexillum Kott, 2002. Aquat Invasions. 2009;4:29–44. [Google Scholar]
- Sunagawa S, Woodley CM, Medina M. Threatened corals provide underexplored microbial habitats. PLoS One. 2010;5:e9554. doi: 10.1371/journal.pone.0009554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait E, Carman M, Sievert SM. Phylogenetic diversity of bacteria associated with ascidians in Eel Pond (Woods Hole, Massachusetts, USA) J Exp Mar Biol Ecol. 2007;342:138–146. [Google Scholar]
- Tarjuelo I, Posada D, Crandall KA, Pascual M, Turon X. Phylogeography and speciation of colour morphs in the colonial ascidian Pseudodistoma crucigaster. Mol Ecol. 2004;13:3125–3136. doi: 10.1111/j.1365-294X.2004.02306.x. [DOI] [PubMed] [Google Scholar]
- Taylor MW, Radax R, Steger D, Wagner M. Sponge-associated microorganisms: evolution, ecology, and biotechnological potential. Microbiol Mol Biol Rev. 2007;71:295–347. doi: 10.1128/MMBR.00040-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MW, Tsai P, Simister RL, Deines P, Botte E, Ericson G, et al. “Sponge-specific” bacteria are widespread (but rare) in diverse marine environments. ISME J. 2013;7:438–443. doi: 10.1038/ismej.2012.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turon X, López-Legentil S, Banaigs B. Cell types, microsymbionts, and pyridoacridine distribution in the tunic of three color morphs of the genus Cystodytes (Ascidiacea, Polycitoridae) Invertebr Biol. 2005;124:355–369. [Google Scholar]
- Usher KM, Toze S, Fromont J, Kuo J, Sutton DC. A new species of cyanobacterial symbiont from the marine sponge Chondrilla nucula. Symbiosis. 2004;36:183–192. [Google Scholar]
- Vermeij MJA, Bak RPM. How are coral populations structured by light? Marine light regimes and the distribution of Madracis. Mar Ecol Prog Ser. 2002;233:105–116. [Google Scholar]
- Ward BB, Capone DG, Zehr JP. What's new in the nitrogen cycle. Oceanography. 2007;20:101–109. [Google Scholar]
- Webb KL, DuPaul WD, Wiebe W, Sottile W, Johannes RE. Enewetak (Eniwetok) Atoll: aspects of the nitrogen cycle on a coral reef. Limnol Oceanogr. 1975;20:198–219. [Google Scholar]
- Webster NS, Taylor MW. Marine sponges and their microbial symbionts: love and other relationships. Environ Microbiol. 2012;14:335–346. doi: 10.1111/j.1462-2920.2011.02460.x. [DOI] [PubMed] [Google Scholar]
- Webster NS, Taylor MW, Behnam F, Lücker S, Rattei T, Whalan S, et al. Deep sequencing reveals exceptional diversity and modes of transmission for bacterial sponge symbionts. Environ Microbiol. 2010;12:2070–2082. doi: 10.1111/j.1462-2920.2009.02065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson CR. Net primary productivity in coral reef sponges. Science. 1983;219:410–412. doi: 10.1126/science.219.4583.410. [DOI] [PubMed] [Google Scholar]
- Yokobori S, Kurabayashi A, Neilan BA, Maruyama T, Hirose E. Multiple origins of the ascidian-Prochloron symbiosis: molecular phylogeny of photosymbiotic and non-symbiotic colonial ascidians inferred from 18S rDNA sequences. Mol Phylogenet Evol. 2006;40:8–19. doi: 10.1016/j.ympev.2005.11.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.