Abstract
In excitable cells, membrane depolarization and activation of voltage-gated Ca2+ (CaV) channels trigger numerous cellular responses, including muscle contraction, secretion, and gene expression. Yet, while the mechanisms underlying excitation-contraction and excitation-secretion coupling have been extensively characterized, how neuronal activity is coupled to gene expression has remained more elusive. In this article, we will discuss recent progress toward understanding the relationship between patterns of channel activity driven by membrane depolarization and activation of the nuclear transcription factor CREB. We show that signaling strength is steeply dependent on membrane depolarization and is more sensitive to the open probability of CaV channels than the Ca2+ entry itself. Furthermore, our data indicate that by decoding CaV channel activity, CaMKII (a Ca2+/calmodulin-dependent protein kinase) links membrane excitation to activation of CREB in the nucleus. Together, these results revealed some interesting and unexpected similarities between excitation-transcription coupling and other forms of excitation-response coupling.
Keywords: CaM/CaMKII, Excitation-transcription coupling, Phosphorylation of cAMP response element-binding protein (pCREB), Calcium channels
1. Introduction
Excitable cells, such as neurons, convert external stimuli into signals that produce a variety of biological responses. For example, calcium flux through voltage-gated Ca2+ channels triggers communication to the nucleus to regulate gene expression. This process, known as excitation-transcription (E-T) coupling, is less well understood than other relationships between channel activation and biological output. Indeed, excitation-contraction (E-C) coupling (Armstrong et al., 1972; Chapman and Tunstall, 1981; Hodgkin and Horowicz, 1960; Schneider, 1994) and excitation-secretion (E-S) coupling (Augustine, 2001; Augustine et al., 1985; Katz and Miledi, 1967; Llinas et al., 1981; Schneggenburger and Neher, 2005) have been intensely investigated for more than half a century. E-T coupling involves many modes of signal propagation (voltage, Ca2+, proteins) over widely varying spatial and time scales. The most scrutinized example of E-T coupling is signaling to the transcription factor cAMP response element-binding protein (CREB) via phosphorylation at Ser133, which is critical for CRE-mediated gene expression and many adaptive changes in neurons (Carlezon et al., 2005; Lonze and Ginty, 2002). Compared to the other voltage-gated Ca2+ channels, CaV1 (also called L-type) channels enjoy a big advantage in such signaling over CaV2 channels (Deisseroth et al., 2003; Dolmetsch, 2003; Murphy et al., 1991; West et al., 2002), in part because of their private access to local Ca2+-dependent signaling machinery (Deisseroth et al., 1996; Dolmetsch et al., 2001; Weick et al., 2003; Zhang et al., 2005). However, the molecular mechanisms that allow CaV1 channels to decode external cues and send information to the nucleus remain incompletely understood.
In this article, we summarize progress in understanding E-T coupling by addressing the following important questions: (1) What is the stimulus-response (input-output) relationship? (2) How steeply does E-T coupling depend on membrane depolarization, channel gating, and Ca2+ influx? Is it comparable to EC and E-S coupling? (3) How do CaV1 channels decode information from external stimuli? To address these fundamental issues, we have combined molecular and cellular approaches and electrophysiology with a multidisciplinary systems analysis of cultured superior cervical ganglion (SCG) neurons. Our results indicate that E-T coupling depends on Ca2+ channel activation in a steeply cooperative manner. This steepness arises from changes in CaV1 channel open probability (Po), but not simply total Ca2+ influx. Furthermore, our data suggest that by deciphering Ca2+ channel activity, Ca2+/calmodulin (CaM)-dependent protein kinase II (CaMKII) provides a molecular link between membrane depolarization and activation of CREB.
2. Importance of a time gap in studies of coupling between depolarization and CREB phosphorylation
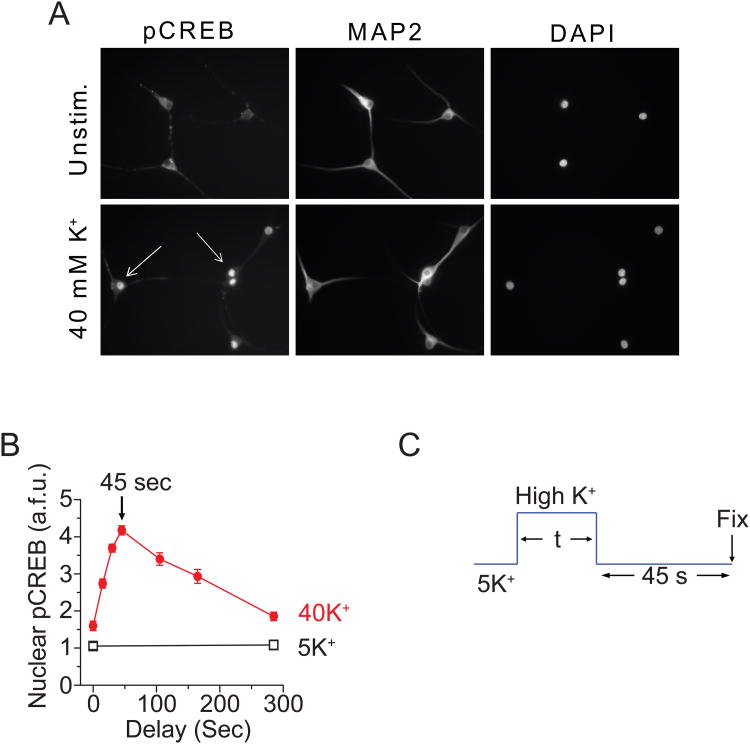
Previous studies of excitation-transcription coupling have mostly used long exposures of K+-rich solution, typically producing depolarizations lasting 30 min or more, to allow time for production of transcripts (Greenberg et al., 1986; Sheng et al., 1990). Our focus was on the initial events that trigger CREB phosphorylation (pCREB) and less on the later steps in gene activation. We found that stimulation with 40 mM K+ for only a few seconds is sufficient to generate a clearly detectable pCREB signal in individual neurons, if the depolarization is followed by an appropriate (and surprisingly brief) delay (Wheeler et al., 2008). Such a pulse-delay protocol has two obvious advantages. First, it provides a stimulus duration comparable to that achieved by a series of transmitter-induced depolarizations or a burst of action potential firing (neurons seldom undergo sustained depolarizations lasting tens of minutes under physiological circumstances). Second, the protocol allows the exploration of the signaling under biophysical conditions, in which the signaling is most input-sensitive, an advantage for gaining mechanistic insights. This is comparable to studying ion channels operating at the foot of their activation curves (Almers, 1978). Figure 1 provides an illustration of the depolarization-induced increase of pCREB and the way that we optimized the delay. pCREB immuno-intensity in the nucleus was strongly increased by 40 mM K+ stimulation for 180 s (Fig. 1A). With briefer stimulation, the pCREB response was very small if the cells were fixed immediately after the stimulation (Fig. 1B). However, if a delay was introduced between stimulation and fixation, the same stimulus could trigger a large pCREB response. By systematically varying the delay, we found that the largest pCREB response occurred with a delay of 45 s (Fig. 1B). Presumably, longer delays allow the depolarization-initiated signaling to gradually decay away while briefer delays leave less time for the signal to arrive at the nucleus (Deisseroth et al., 1998; Mermelstein et al., 2001). By keeping a 45 s gap (Fig. 1C), we were able to discern measurable pCREB responses as we varied the duration of stimulation over a range of seconds to tens of seconds.
Fig. 1. Quantification of pCREB signaling with a time delay.
(A) SCG neurons (cultured 5 days) were either mock-stimulated in 5 mM K+ Tyrode solution (containing 2 mM Ca2+) or stimulated with solutions containing 40 mM K+ for 180 s. After stimulation, cells were immediately fixed and stained for pCREB and MAP2; nuclei were counterstained with DAPI. Arrows indicate strongly activated neurons. (B) Neurons were stimulated with 5 or 40 mM K+ for 10 s followed by a variable time delay before fixation. Peak pCREB levels occurred 45 s after stimulation and decayed thereafter, becoming nearly indistinguishable from baseline within 5 min. (C) To ensure that the pCREB level measured after a given stimulation was a maximal response, we stimulated the cells for different time with a fixed 45 s delay in 5 mM K+ Tyrode solution. (Modified from Wheeler et al., 2008 or Wheeler et al. unpublished data.)
3. Steep voltage-dependence of E-T coupling
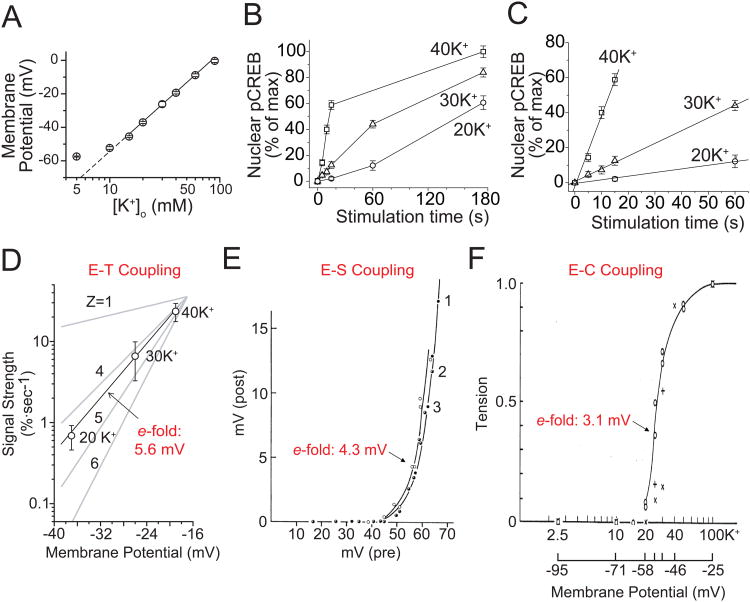
Activation of muscle contraction and neurosecretion were analyzed by Alan Hodgkin, Bernard Katz and colleagues in pioneering studies dating back to the 1960s (Hodgkin and Horowicz, 1960; Katz and Miledi, 1967). A common feature of both E-C and E-S coupling are the steep relationships between changes in membrane voltage and biological output. This inspired us to ask whether there is also a similar relationship between excitation and transcription by utilizing pCREB immunocytochemistry as the readout of responses to K+ depolarization in individual rat sympathetic neurons. Consistent with previous studies (O'Lague et al., 1974; Wheeler et al., 2006), the resting potential of SCG neurons in 5 mM K+ was approximately −60 mV (Fig. 2A). Exposure to 20, 30, and 40 mM K+ depolarized the cells to −37, −26, and −19 mV, respectively, following the Nernst relationship (Fig. 2A). Importantly, we found that although relatively weaker stimuli (e.g. 20-30 mM K+) drove much shallower signaling to pCREB, the levels eventually approached those attained with 40 mM K+ (Fig. 2B). To estimate the strength of signaling, we determined the per-unit-time effect of a given stimulus by measuring the initial slope of the time-dependent increase in pCREB (Fig. 2C). A very dramatic change in output was found with small gradations in input, demonstrating the inherent sensitivity of the signaling system. Furthermore, plotting pCREB signal strength against membrane voltage revealed a steep relationship of 5.6 mV/e-fold change (Fig. 2D), which is comparable to that found in E-C or E-S coupling (Fig. 2E, F).
Fig. 2. Strength of signaling to CREB in SCG neurons.
(A) Membrane potential plotted against [K+]o. The slope of the linear fit is 58.1 mV per 10-fold change in [K+]o, consistent with the Nernst equation. (B) Mean pCREB levels plotted against stimulation time (with a 45 s delay as determined in Figure 1); [K+]o as indicated. (C) Linear fits of initial data points, using the slope as an index of CREB signal strength, pCREB. (D) CREB signal strength plotted against membrane voltage. 100% is the full change in pCREB from baseline to maximal levels. A linear fit shows that signal strength is steeply voltage dependent, as if governed by a gating particle with a valence (z) between 4 and 5 (corresponding to 5.6 mV/e-fold change in signal strength). (E) E-S coupling is also sharply dependent on voltage, with an e-fold change per 4.3 mV. Curves 1 (full circles), curves 2 (half-filled circles) and 3 (open circles) were obtained with pre-spikes, local pulses of 1 msec and 2 msec duration, respectively. (Katz et al., 1967). (F) The e-fold change for E-C coupling is per 3.1 mV (Hodgkin et al., 1960). (A-D Modified from Wheeler et al., 2008; E modified from Katz et al., 1967; F modified from Hodgkin et al., 1960.)
4. Po, not ICa, is the critical factor for E-T coupling strength
E-C and E-S coupling have been shown to share the generic feature of a steeply cooperative relationship between Ca2+ channel activation and biological response, but vary tremendously in their dependence on Ca2+ flux (Schneggenburger and Neher, 2005; Schneider, 1994). Indeed, whereas skeletal E-C coupling operates independently of Ca2+ influx through the plasma membrane (Armstrong et al., 1972; Beam and Franzini-Armstrong, 1997; Franzini-Armstrong and Protasi, 1997; Rios and Brum, 1987; Schneider and Chandler, 1973), E-S coupling is extremely sensitive to the magnitude of Ca2+ entry (Augustine et al., 1985; Bollmann et al., 2000; Dodge and Rahamimoff, 1967; Llinas et al., 1981; Schneggenburger and Neher, 2000; Sudhof, 2004). Having found that E-T coupling also relies on a steep relationship between Ca2+ channel activation and biological response, we asked what role Ca2+ flux played in this process.
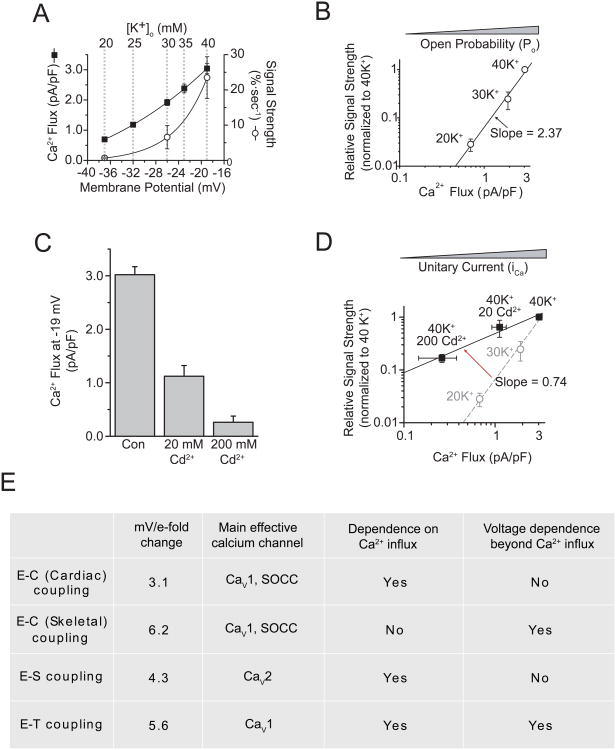
To address this question, using voltage-clamp recordings we measured Ca2+ flux at voltages corresponding to given K+ challenges and found that it displayed an exponential dependence on depolarization over the range we examined (from -37 to -19 mV) (Fig. 3A). Accordingly, we plotted pCREB signal strength against total Ca2+ flux for stimulations with 20, 30, and 40 mM K+ (Fig. 3B). On a log-log scale, the data were fit by a straight line with a slope of 2.37, indicating that CREB signal strength was steeply dependent on total Ca2+ flux, which is the sum of the integrated Ca2+ flux through each individual channel, represented by the product of channel open probability (Po) and unitary Ca2+ flux (ICa). Thus, to clarify the relationship between CREB signal strength and channel activity, it was important to determine the relative contribution of Po and ICa to the pCREB signal. Voltage changes from -37 to -19 mV have little effect on Ca2+ driving force, and thus ICa; however, Po changes steeply at this voltage range, suggesting that changes in Po may underlie the steeply increasing signal strength. However, whether ICa also contributes to pCREB signal strength was still unknown. To address this question, we used Cd2+, which has been shown to change the permeability of the channel pore to Ca2+ on a microsecond time scale, thereby decreasing the integrated Ca2+ flux through the open channel (Chow, 1991; Lansman et al., 1986), to effect changes in ICa while keeping Po fixed. As Figure 3D shows, even though Cd2+ potently blocked Ca2+ flux at -19 mV (equivalent to 40 mM K+ stimulation) (Fig. 3C), it only had a mild effect on CREB signal strength: the regression line of ICa is much shallower than that for the gradations of Po (slopes of 0.74 versus 2.37; Fig. 3D). This suggests that ICa is not the critical factor underlying the steeply increasing signal strength. Thus, for E-T coupling, the relationship between depolarization and signal strength is not a simple function of total Ca2+ flux, but is rather closely tied to voltage-dependent changes in Po (Fig. 3D, E).
Fig. 3. CREB signal strength is steeply dependent on channel Po but is largely independent of unitary Ca2+flux.
(A) Whole-cell Ca2+ flux (measured with 2 mM Ca2+ and normalized to cell capacitance) and CREB signal strength plotted versus membrane potential. Solid lines are single exponential fits. Dashed lines indicate the corresponding [K+]o yielding the given membrane potentials, derived from Figure 2A. (B) Log-log plot of signal strength versus Ca2+ flux for the [K+]o indicated. ]The solid line is a linear fit of the data. (Almers) Whole-cell Ca2+ flux, in the presence of 0, 20, or 200 μM Cd2+ (Almers)CREB signal strength for 40 K+ with 0, 20, or 200 μM Cd2+. The solid line is a linear fit of the Cd2+ data. For comparison, the dashed line and gray data points are reproduced from B. (E) Some salient features of the three major forms of excitation-response coupling. SOCC, store-operated calcium channel. (Modified from Wheeler et al., 2008.)
5. CaMKII is essential for E-T coupling
We previously found that depolarizing SCG neurons results in CaV1 channel-dependent phosphorylation of CREB and CRE-mediated gene expression (Wheeler et al., 2006; Wheeler and Cooper, 2001). Because the Po of CaV1 channels is highly voltage dependent (Reuter et al., 1982), if E-T coupling is primarily determined by channel Po then the next question is which molecule(s) are used to decode the pattern of Ca2+ transients near the CaV1 channel. An important Ca2+ sensor, CaM activates CaMKII in an activity-dependent manner; thus, CaMKII is extremely sensitive to Ca2+ spike frequency and selectively responds to high-frequency brief stimuli, such as Ca2+ fluxes at the mouth of ion channels (Kubota and Bower, 2001). It has been reported that activated CaMKII is important for signaling to CREB (Takeda et al., 2007), suggesting that CaMKII could be a logical candidate because it is tethered to CaV1 channels (Hudmon et al., 2005) and to CaV1 interacting proteins such as densin (Jenkins et al., 2010). Furthermore, autophosphorylation and persistent activation of CaMKII increase steeply with the frequency of Ca2+ pulses (De Koninck and Schulman, 1998).
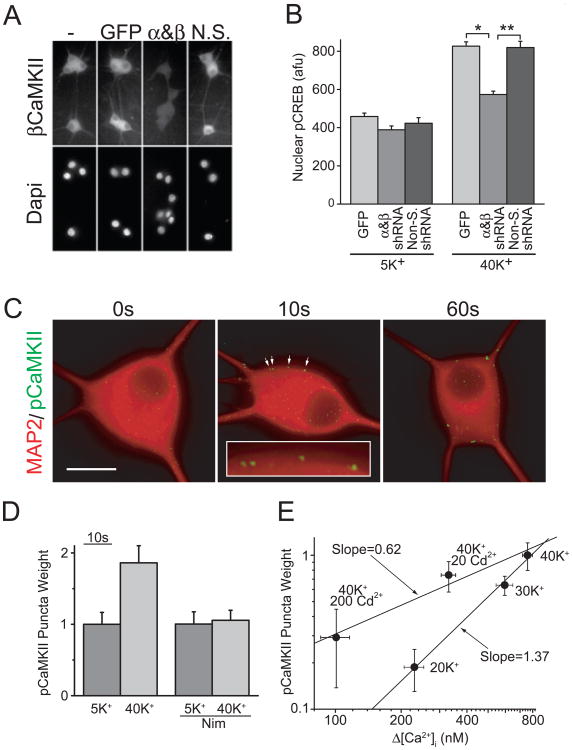
If CaMKII is important for E-T coupling, direct evidence could come from its role in pCREB signaling. To test this, we examined pCREB formation after knocking down the expression of α and βCaMKII, the main CaMKII isoforms in neurons (Fig. 4A). Interestingly but not unexpectedly, signaling to the nucleus was reduced by >65% after CaMKII was knocked down (Fig. 4A, B), which indicates CaMKII is important for E-T coupling. However, whether CaMKII could decode the external information in E-T coupling was still unknown. To approach this issue, two critical questions were addressed. First, is CaMKII activated locally, near the source of Ca2+ entry (that is, near the CaV1 channel itself)? And second, is CaMKII also highly Po-sensitive but only weakly ICa-sensitive? As Figure 4C shows, brief stimulation with 40 mM K+ recruited autophosphorylation of CaMKII (pCaMKII) to form puncta near the cell surface, which is consistent with local CaMKII activation. Furthermore, a CaV1 channel inhibitor, nimodipine prevented the formation of these pCaMKII puncta upon stimulation, indicating that CaV1 channel activity is also required for CaMKII activation (Fig. 4D). By using Cd2+ to decrease ICa, we found that grading ICa had a much milder effect on CaMKII activation than changing Po (Fig. 4E), similar to its effect on pCREB signaling (compare Figs. 4E and 3D). Taken together, these data suggest that in E-T coupling, CaM/CaMKII serves as a local Ca2+ sensor to help CaV1 channels decode the external inputs, a critical early step for signaling to the nucleus.
Fig. 4. CaMKII is critical for CREB signaling.
(A) SCG neurons infected with lentiviruses expressing GFP alone, shRNAs against α and βCaMKII, or nonsilencing control shRNAs, and stained with an antibody against βCaMKII; nuclei were counterstained with DAPI. (B) pCREB levels from neurons stimulated for 2.5 s with 5 mM K+ or 40 mM K+ and transferred to a 5 mM K+ solution for 45 s before fixation. *p<5×10-17; **p< 5×10-10. (C) SCG neurons stimulated with 40 mM K+ for 0, 10, or 60 s and immediately fixed and stained for MAP2 (red) and pCaMKII (green). Arrows point to pCaMKII puncta (magnified in the inset). Background pCaMKII staining was subtracted to highlight punctuate staining. Scale bar, 20 μm. (D) Nimodipine blocks pCaMKII puncta formation. (E) Plot of pCaMKII puncta weight versus the rise in bulk Ca2+ measured using Fura-2 ratiometric Ca2+ imaging for the stimulation conditions indicated. (Modified from Wheeler et al., 2008.)
6. Conclusion
Activity-dependent changes in neuronal morphology, excitability, and synaptic strength are all dependent on gene expression and protein synthesis. Accordingly, a thorough understanding of the mechanisms by which neurons link membrane depolarization to nuclear signaling is of critical importance. It is well known that CaV1 channels can couple membrane depolarization to nuclear transcription (Greenberg et al., 1986; Morgan and Curran, 1986; Murphy et al., 1991), acting on local signaling machinery (Deisseroth et al., 1996; Dolmetsch et al., 2001; Oliveria et al., 2007; Weick et al., 2003). However, the downstream mechanisms following Ca2+ influx have remained unclear. In this article, we used sympathetic neurons as model cells to characterize the basic properties of E-T coupling and make comparisons with E-C and E-S coupling. We found that signal strength in E-T coupling is steeply dependent on Ca2+ channel activity, similar to E-C and E-S coupling (Fig. 2D-F). Importantly, E-T coupling strength is not simply a reflection of the integrated strength of Ca2+ influx. Instead, our experiments indicate that it is highly sensitive to Po, but only weakly sensitive to ICa, even though Po and ICa are both multiplicative factors in determining Ca2+ flux overall (Fig. 3D).
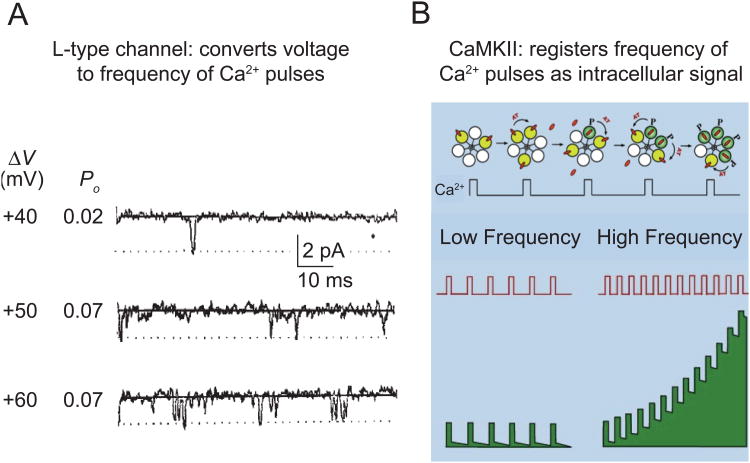
The Po of CaV1 channels increases in a steeply voltage-dependent manner (Fig. 5A). On the other hand, local saturation of Ca2+ sensors in the immediate vicinity of an open channel would reduce the importance of changes in ICa, effectively transforming analog Ca2+ signals into a digital format. To support this hypothesis, we have determined that CaM/CaMKII serves as the Ca2+ sensor for decoding this digital frequency of Ca2+ transients near the channel. Activated CaMKII is in the immediate vicinity of CaV1 channels (Grueter et al., 2006; Hudmon et al., 2005; Jenkins et al., 2010) and is steeply dependent on the frequency of Ca2+/CaM pulses (Fig. 5B). Three lines of evidence support its participation in signaling to the nucleus. First, decreasing CaMKII levels reduced the strength of CREB signaling (Fig. 4B). Second, pCaMKII puncta formed at the cell surface in a CaV1 channel activity-dependent manner (Fig. 4C, D). Third, pCaMKII formation was much more sensitive to changing Po than ICa (Fig. 4E), thus accounting for the disparate effects of Po and ICa in signaling to CREB. Taken together, our findings provide evidence that local CaMKII activation is critical in decoding Ca2+ transients generated by CaV1 channel openings, which is an initial step for E-T coupling in the sympathetic neurons.
Fig. 5. CaM/CaMKII decodes the digital frequency code generated by CaV1 channels.
(A) The frequency of CaV1 channel opening (Po) is steeply voltage-dependent (Reuter et al., 1982). (B) The activity of CaMKII increases in a steeply frequency-dependent way (top) (De Koninck and Schulman, 1998). Quantitative modeling suggests that the frequency-dependence holds for brief pulses of Ca2+ of the kind produced by the activity of CaV1 channels (Dupont et al., 2003). Thus, the combination of CaV1 and CaMKII supports a highly sensitive transduction of cell membrane potential to intracellular biochemical signaling (bottom). (A modified from Reuter et al., 1982; B was kindly provided by A. Hudmon.)
Acknowledgments
We would like to thank members of the Tsien lab for helpful suggestions and discussions. We are grateful to Andrew Hudmon for providing the model in Fig. 5B. This paper is based on the work of Wheeler et al. 2008 and was supported by research grants to R.W.T. from the National Institute of General Medical Sciences and the National Institute of Neurological Disorders and Stroke.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Almers W. Gating currents and charge movements in excitable membranes. Rev Physiol Biochem Pharmacol. 1978;82:96–190. doi: 10.1007/BFb0030498. [DOI] [PubMed] [Google Scholar]
- Armstrong CM, Bezanilla FM, Horowicz P. Twitches in the presence of ethylene glycol bis(-aminoethyl ether)-N,N'-tetracetic acid. Biochimica et biophysica acta. 1972;267:605–608. doi: 10.1016/0005-2728(72)90194-6. [DOI] [PubMed] [Google Scholar]
- Augustine GJ. How does calcium trigger neurotransmitter release? Current opinion in neurobiology. 2001;11:320–326. doi: 10.1016/s0959-4388(00)00214-2. [DOI] [PubMed] [Google Scholar]
- Augustine GJ, Charlton MP, Smith SJ. Calcium entry and transmitter release at voltage-clamped nerve terminals of squid. The Journal of physiology. 1985;367:163–181. doi: 10.1113/jphysiol.1985.sp015819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beam KG, Franzini-Armstrong C. Functional and structural approaches to the study of excitation-contraction coupling. Methods Cell Biol. 1997;52:283–306. doi: 10.1016/s0091-679x(08)60384-2. [DOI] [PubMed] [Google Scholar]
- Bollmann JH, Sakmann B, Borst JG. Calcium sensitivity of glutamate release in a calyx-type terminal. Science. 2000;289:953–957. doi: 10.1126/science.289.5481.953. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Duman RS, Nestler EJ. The many faces of CREB. Trends in neurosciences. 2005;28:436–445. doi: 10.1016/j.tins.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Chapman RA, Tunstall J. The tension-depolarization relationship of frog atrial trabeculae as determined by potassium contractures. The Journal of physiology. 1981;310:97–115. doi: 10.1113/jphysiol.1981.sp013539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow RH. Cadmium block of squid calcium currents. Macroscopic data and a kinetic model. J Gen Physiol. 1991;98:751–770. doi: 10.1085/jgp.98.4.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Koninck P, Schulman H. Sensitivity of CaM kinase II to the frequency of Ca2+ oscillations. Science. 1998;279:227–230. doi: 10.1126/science.279.5348.227. [DOI] [PubMed] [Google Scholar]
- Deisseroth K, Bito H, Tsien RW. Signaling from synapse to nucleus: postsynaptic CREB phosphorylation during multiple forms of hippocampal synaptic plasticity. Neuron. 1996;16:89–101. doi: 10.1016/s0896-6273(00)80026-4. [DOI] [PubMed] [Google Scholar]
- Deisseroth K, Heist EK, Tsien RW. Translocation of calmodulin to the nucleus supports CREB phosphorylation in hippocampal neurons. Nature. 1998;392:198–202. doi: 10.1038/32448. [DOI] [PubMed] [Google Scholar]
- Deisseroth K, Mermelstein PG, Xia H, Tsien RW. Signaling from synapse to nucleus: the logic behind the mechanisms. Current opinion in neurobiology. 2003;13:354–365. doi: 10.1016/s0959-4388(03)00076-x. [DOI] [PubMed] [Google Scholar]
- Dodge FA, Jr, Rahamimoff R. Co-operative action a calcium ions in transmitter release at the neuromuscular junction. The Journal of physiology. 1967;193:419–432. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolmetsch R. Excitation-transcription coupling: signaling by ion channels to the nucleus. Sci STKE. 2003;2003:PE4. doi: 10.1126/stke.2003.166.pe4. [DOI] [PubMed] [Google Scholar]
- Dolmetsch RE, Pajvani U, Fife K, Spotts JM, Greenberg ME. Signaling to the nucleus by an L-type calcium channel-calmodulin complex through the MAP kinase pathway. Science. 2001;294:333–339. doi: 10.1126/science.1063395. [DOI] [PubMed] [Google Scholar]
- Dupont G, Houart G, De Koninck P. Sensitivity of CaM kinase II to the frequency of Ca2+ oscillations: a simple model. Cell Calcium. 2003;34:485–497. doi: 10.1016/s0143-4160(03)00152-0. [DOI] [PubMed] [Google Scholar]
- Franzini-Armstrong C, Protasi F. Ryanodine receptors of striated muscles: a complex channel capable of multiple interactions. Physiol Rev. 1997;77:699–729. doi: 10.1152/physrev.1997.77.3.699. [DOI] [PubMed] [Google Scholar]
- Greenberg ME, Ziff EB, Greene LA. Stimulation of neuronal acetylcholine receptors induces rapid gene transcription. Science. 1986;234:80–83. doi: 10.1126/science.3749894. [DOI] [PubMed] [Google Scholar]
- Grueter CE, Abiria SA, Dzhura I, Wu Y, Ham AJ, Mohler PJ, Anderson ME, Colbran RJ. L-type Ca2+ channel facilitation mediated by phosphorylation of the beta subunit by CaMKII. Mol Cell. 2006;23:641–650. doi: 10.1016/j.molcel.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Hodgkin AL, Horowicz P. Potassium contractures in single muscle fibres. The Journal of physiology. 1960;153:386–403. doi: 10.1113/jphysiol.1960.sp006541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudmon A, Schulman H, Kim J, Maltez JM, Tsien RW, Pitt GS. CaMKII tethers to L-type Ca2+ channels, establishing a local and dedicated integrator of Ca2+ signals for facilitation. J Cell Biol. 2005;171:537–547. doi: 10.1083/jcb.200505155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins MA, Christel CJ, Jiao Y, Abiria S, Kim KY, Usachev YM, Obermair GJ, Colbran RJ, Lee A. Ca2+-dependent facilitation of Cav1.3 Ca2+ channels by densin and Ca2+/calmodulin-dependent protein kinase. II J Neurosci. 30:5125–5135. doi: 10.1523/JNEUROSCI.4367-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins MA, Christel CJ, Jiao Y, Abiria S, Kim KY, Usachev YM, Obermair GJ, Colbran RJ, Lee A. Ca2+-dependent facilitation of Cav1.3 Ca2+ channels by densin and Ca2+/calmodulin-dependent protein kinase II. J Neurosci. 2010;30:5125–5135. doi: 10.1523/JNEUROSCI.4367-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B, Miledi R. A study of synaptic transmission in the absence of nerve impulses. The Journal of physiology. 1967;192:407–436. doi: 10.1113/jphysiol.1967.sp008307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota Y, Bower JM. Transient versus asymptotic dynamics of CaM kinase II: possible roles of phosphatase. J Comput Neurosci. 2001;11:263–279. doi: 10.1023/a:1013727331979. [DOI] [PubMed] [Google Scholar]
- Lansman JB, Hess P, Tsien RW. Blockade of current through single calcium channels by Cd2+, Mg2+, and Ca2+. Voltage and concentration dependence of calcium entry into the pore. J Gen Physiol. 1986;88:321–347. doi: 10.1085/jgp.88.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas R, Steinberg IZ, Walton K. Relationship between presynaptic calcium current and postsynaptic potential in squid giant synapse. Biophysical journal. 1981;33:323–351. doi: 10.1016/S0006-3495(81)84899-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonze BE, Ginty DD. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35:605–623. doi: 10.1016/s0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- Mermelstein PG, Deisseroth K, Dasgupta N, Isaksen AL, Tsien RW. Calmodulin priming: nuclear translocation of a calmodulin complex and the memory of prior neuronal activity. Proc Natl Acad Sci U S A. 2001;98:15342–15347. doi: 10.1073/pnas.211563998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JI, Curran T. Role of ion flux in the control of c-fos expression. Nature. 1986;322:552–555. doi: 10.1038/322552a0. [DOI] [PubMed] [Google Scholar]
- Murphy TH, Worley PF, Baraban JM. L-type voltage-sensitive calcium channels mediate synaptic activation of immediate early genes. Neuron. 1991;7:625–635. doi: 10.1016/0896-6273(91)90375-a. [DOI] [PubMed] [Google Scholar]
- O'Lague PH, Obata K, Claude P, Furshpan EJ, Potter DD. Evidence for cholinergic synapses between dissociated rat sympathetic neurons in cell culture. Proc Natl Acad Sci U S A. 1974;71:3602–3606. doi: 10.1073/pnas.71.9.3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveria SF, Dell'Acqua ML, Sather WA. AKAP79/150 anchoring of calcineurin controls neuronal L-type Ca2+ channel activity and nuclear signaling. Neuron. 2007;55:261–275. doi: 10.1016/j.neuron.2007.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter H, Stevens CF, Tsien RW, Yellen G. Properties of single calcium channels in cardiac cell culture. Nature. 1982;297:501–504. doi: 10.1038/297501a0. [DOI] [PubMed] [Google Scholar]
- Rios E, Brum G. Involvement of dihydropyridine receptors in excitation-contraction coupling in skeletal muscle. Nature. 1987;325:717–720. doi: 10.1038/325717a0. [DOI] [PubMed] [Google Scholar]
- Schneggenburger R, Neher E. Intracellular calcium dependence of transmitter release rates at a fast central synapse. Nature. 2000;406:889–893. doi: 10.1038/35022702. [DOI] [PubMed] [Google Scholar]
- Schneggenburger R, Neher E. Presynaptic calcium and control of vesicle fusion. Current opinion in neurobiology. 2005;15:266–274. doi: 10.1016/j.conb.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Schneider MF. Control of calcium release in functioning skeletal muscle fibers. Annual review of physiology. 1994;56:463–484. doi: 10.1146/annurev.ph.56.030194.002335. [DOI] [PubMed] [Google Scholar]
- Schneider MF, Chandler WK. Voltage dependent charge movement of skeletal muscle: a possible step in excitation-contraction coupling. Nature. 1973;242:244–246. doi: 10.1038/242244a0. [DOI] [PubMed] [Google Scholar]
- Sheng M, McFadden G, Greenberg ME. Membrane depolarization and calcium induce c-fos transcription via phosphorylation of transcription factor CREB. Neuron. 1990;4:571–582. doi: 10.1016/0896-6273(90)90115-v. [DOI] [PubMed] [Google Scholar]
- Sudhof TC. The synaptic vesicle cycle. Annu Rev Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- Takeda H, Kitaoka Y, Hayashi Y, Kumai T, Munemasa Y, Fujino H, Kobayashi S, Ueno S. Calcium/calmodulin-dependent protein kinase II regulates the phosphorylation of CREB in NMDA-induced retinal neurotoxicity. Brain Res. 2007;1184:306–315. doi: 10.1016/j.brainres.2007.09.055. [DOI] [PubMed] [Google Scholar]
- Weick JP, Groth RD, Isaksen AL, Mermelstein PG. Interactions with PDZ proteins are required for L-type calcium channels to activate cAMP response element-binding protein-dependent gene expression. J Neurosci. 2003;23:3446–3456. doi: 10.1523/JNEUROSCI.23-08-03446.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AE, Griffith EC, Greenberg ME. Regulation of transcription factors by neuronal activity. Nat Rev Neurosci. 2002;3:921–931. doi: 10.1038/nrn987. [DOI] [PubMed] [Google Scholar]
- Wheeler DG, Barrett CF, Groth RD, Safa P, Tsien RW. CaMKII locally encodes L-type channel activity to signal to nuclear CREB in excitation-transcription coupling. J Cell Biol. 2008;183:849–863. doi: 10.1083/jcb.200805048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler DG, Barrett CF, Tsien RW. L-type calcium channel ligands block nicotine-induced signaling to CREB by inhibiting nicotinic receptors. Neuropharmacology. 2006;51:27–36. doi: 10.1016/j.neuropharm.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Wheeler DG, Cooper E. Depolarization strongly induces human cytomegalovirus major immediate-early promoter/enhancer activity in neurons. J Biol Chem. 2001;276:31978–31985. doi: 10.1074/jbc.M103667200. [DOI] [PubMed] [Google Scholar]
- Zhang H, Maximov A, Fu Y, Xu F, Tang TS, Tkatch T, Surmeier DJ, Bezprozvanny I. Association of CaV1.3 L-type calcium channels with Shank. J Neurosci. 2005;25:1037–1049. doi: 10.1523/JNEUROSCI.4554-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]