Abstract
Gold colloids have fascinated scientists for over a century and are now heavily utilized in chemistry, biology, engineering, and medicine. Today these materials can be synthesized reproducibly, modified with seemingly limitless chemical functional groups, and, in certain cases, characterized with atomic-level precision. This Review highlights recent advances in the synthesis, bioconjugation, and cellular uses of gold nanoconjugates. There are now many examples of highly sensitive and selective assays based upon gold nanoconjugates. In recent years, focus has turned to therapeutic possibilities for such materials. Structures which behave as gene-regulating agents, drug carriers, imaging agents, and photoresponsive therapeutics have been developed and studied in the context of cells and many debilitating diseases. These structures are not simply chosen as alternatives to molecule-based systems, but rather for their new physical and chemical properties, which confer substantive advantages in cellular and medical applications.
Keywords: cytotoxicity, DNA, drug delivery, gold, nanoparticles
1. Introduction
Gold nanoparticles (AuNPs) have a rich history in chemistry, dating back to ancient Roman times where they were used to stain glasses for decorative purposes. The modern era of AuNP synthesis began over 150 years ago with the work of Michael Faraday, who was possibly the first to observe that colloidal gold solutions have properties that differ from bulk gold.[1,2] Reliable and high-yielding methods for the synthesis of AuNPs, including those with spherical and nonspherical shapes, have been developed over the last half-century.[3] The resulting AuNPs have unique properties, such as size- and shape-dependent optical and electronic features, a high surface area to volume ratio, and surfaces that can be readily modified with ligands containing functional groups such as thiols, phosphines, and amines, which exhibit affinity for gold surfaces.[3] By using these functional groups to anchor the ligands, additional moieties such as oligonucleotides, proteins, and antibodies can be used to impart even greater functionality. The realization of such gold nanoconjugates has enabled a broad range of investigations, including programmed assembly and crystallization of materials,[4,5] arrangement of nanoparticles into dimers and trimers onto DNA templates,[6] bioelectronics,[7–9] and detection methods.[10,11] The application of gold nanoconjugates for biodetection and biodiagnostics have been reviewed elsewhere.[12–14]
In recent years, gold nanoconjugates and their properties have led to new and exciting developments with enormous potential in biology and medicine. These investigations represent a new direction that greatly deviates from the more established use of gold nanoconjugates as labels for electron microscopy.[15] Our recent studies, as well as those of several other research groups, have shown that gold nanoconjugates, when functionalized with appropriate surface moieties, can readily enter living cells. These developments have forged a new frontier in nanoparticle research, including the broader use of gold nanoconjugates in cellular biology and the promise for their eventual use as therapeutic agents.
In this Review we describe the current status of gold nanoconjugates for cellular and therapeutic uses. As surface chemistry is one of the key features that controls the properties and functionality, we have divided this Review into sections based on the type of surface functionalization, including citrate, amine, nucleic acid, peptide, antibody, and lipid ligands (Table 1). In each section, our discussion focuses on chemical synthesis, physical and chemical properties, as well as investigations and applications in cells. In Section 8, we also propose key opportunities and open questions that have yet to be addressed by the scientific community. These questions should inspire future investigations and lead to discoveries that continue the development of the rich chemistry of gold nanoparticles.
Table 1.
Au NP surface functionalites.
| Surface functionality | Application | Reference |
|---|---|---|
| citrate | cell uptake | [18, 19] |
| transferrin | cell uptake | [20, 21] |
| CTAB | cell uptake | [14, 94] |
| amine | gene transfection | [26, 30, 31] |
| antiviral activity | [34] | |
| drug delivery | [34] | |
| oligonucleotide transfection | [36] | |
| oligonucleotide | antisense gene regulation | [25, 77, 88, 102] |
| mRNA detection | [87, 88] | |
| small-molecule detection | [89] | |
| RNA interference | [90] | |
| cancer cell detection | [93] | |
| peptide | nuclear translocation | [23, 100] |
| antisense gene regulation | [102] | |
| antibody | imaging | [15, 106, 107, 110] |
| photothermal therapy | [108, 109, 110] | |
| lipid | imaging | [112] |
| cholesterol binding | [111] |
2. Citrate and Transferrin
Citrate-functionalized gold nanoparticles can be prepared on a relatively large scale and with a high degree of monodispersity by using the methods of Frens[16] as well as Enustun and Turkevich.[17] These methods allow for the synthesis of citrate-capped spherical nanoparticles with diameters ranging from 5 to 250 nm.[16,17] This well-established synthesis and the ability to finely control size has contributed to citrate-functionalized nanoconjugates forming the basis of recent investigations of the uptake of gold nanoparticles by cells.[18] In one such study, Chan and co-workers determined how the size and shape of the particles influence their ability to be internalized by cells.[19] Their study demonstrates that, in a HeLa cell model, the amount of time that the citrate particles remain internalized is independent of the particle size when they have diameters between 14 and 74 nm. However, the size does affect the total number of nanoparticle conjugates internalized during the experiment. By using inductively coupled plasma atomic emission spectroscopy (ICP-AES) to determine the intracellular gold content, these researchers determined that citrate-capped gold nanoconjugates with diameters of 50 nm are most readily internalized by HeLa cells (Figure 1). They found that the maximum number of citrate-stabilized gold nanoconjugates taken up by a HeLa cell is 3000, 6160, and 2988 for gold nanoconjugates with diameters of 14, 50, and 74 nm, respectively.
Figure 1.
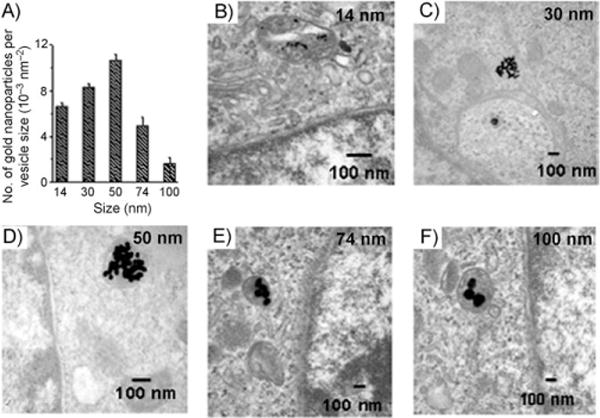
Transmission electron microscopy imaging and measurements of gold nanoparticles in cells. A) Graph of number of gold nanoparticles per vesicle diameter for various nanoparticle sizes. B–F) TEM images of gold nanoparticles with sizes of 14, 30, 50, 74, and 100 nm, respectively, trapped inside vesicles of a HeLa cell. Adapted from Ref. [19], with permission from the American Chemical Society; Copyright 2006.
The mechanism by which the citrate-capped gold nanoconjugates enter cells has been the subject of investigation. Chan and co-workers recorded transmission electron microscopy images of internalized “bare” citrate nanoconjugates and showed that the particles were mainly localized within vesicles inside of the cells.[19] They correlated cell uptake with the nonspecific adsorption of proteins to the citrate-capped nanoparticle surfaces.
The negatively charged citrate surface provides a convenient scaffold to attach positively charged proteins such as transferrin, which is expected to facilitate and improve entry into cells. In one study, atomic force microscopy was used to image transferrin-coated citrate-functionalized gold nanoconjugates on the cell surface.[20] The images obtained suggest vesicle formation at the cell surface and nanoconjugate internalization through endocytosis. A series of experiments by Chithrani and Chan further determined that transferrin-coated citrate-functionalized gold nanoconjugates enter cells through the clathrin-mediated endocytosis pathway.[21]
Many investigations in cells use citrate-capped AuNPs as important precursors of covalent conjugates with additional functionality, because further derivatization has been shown to increase uptake ability,[22] alter intracellular localization,[23,24] or impart functionality that can be used to affect a cellular response.[25,26] Indeed, citrate-coated particles are generally not ideal structures for investigations and internalization studies on cells. They are susceptible to environmentally induced aggregation and can be quite difficult to work with. In the next sections we describe the major classes of gold nanoconjugates that are functionalized with designer ligands, which have been developed and used for experiments on cells.
3. Amines
In addition to the methods of Enustun and Turkevich and of Frens, alternative methods for the synthesis of gold nanoparticles have been developed. The Brust–Schiffrin method allows for the synthesis of monodisperse gold nanoparticles ranging from 1 to 3 nm in diameter.[27] The resultant nanoparticles are stabilized by a monolayer of alkanethiolates. The composition of the monolayer can be changed through a substitution reaction to include specific functionalities, depending on the intended use of the nanoparticles.[28] Accordingly, gold nanoconjugates functionalized with a monolayer of amine-terminated alkanethiolates (hereafter referred to as amine-functionalized) have been prepared for various biological applications.
3.1. Gene Transfection
The ability to induce control over biological systems at the genetic level is a fundamental concept in experimental biology, and holds great promise for developing new treatments of disease.[29] The search for the best method for controlling gene expression is ongoing. Their straightforward synthesis and high-degree of chemical tunability has resulted in amine-functionalized nanoparticles having been developed as a means to transfer genetic material into cell models.[26,30]
Amine surface groups are positively charged at physiological pH values, and thus amine-functionalized nanoconjugates electrostatically interact with negatively charged nucleic acids. Studies by Rotello and co-workers have demonstrated that 2 nm gold nanoparticles functionalized with a mixed monolayer containing quaternary amines and uncharged surface groups are able to bind DNA plasmids and deliver them efficiently to 293T cells.[26] In fact, these nanoconjugates are able to transfect these cells with a greater efficiency than the commonly used cationic polymer transfection agent polyethylenimine (PEI, 60 kDa). These researchers also found that the efficiency of the nanoparticle-mediated gene transfection was affected by the ratio of positively charged quaternary amines to negatively charged phosphate groups on the DNA, as well as the relative amount and length of the surface-bound uncharged thiol chain. Building on these observations, these researchers have recently shown that gold nanoparticles functionalized with lysine moieties are highly efficacious at delivering DNA plasmids, and outperform a commercial vector by a factor of 28.[31]
The utility of amine-functionalized nanoconjugates for gene delivery was also demonstrated by Thomas and Klibanov.[30] In this study, combinations of thiol-modified PEI (2 kDa) and dodecyl-PEI (2 kDa) were used as surfactants or complexing agents during AuNP synthesis. The concentration of PEI was used to control the size of the functionalized nanoparticles from 2.3 to 4.1 nm in diameter. The resultant nanoconjugates deliver plasmid DNA to COS-7 cells more efficiently than PEI alone.
3.2. Drug Delivery
Site-specific delivery, stability, and the programmed release of the drugs to physiological targets have been major challenges for molecular and macromolecular therapeutics.[32] The highly tunable and multivalent surface architecture of gold nanoconjugates offers the potential to incorporate multiple therapeutic agents as well as to target and protect molecules on the surface of a single nanoparticle, and thus are expected to improve the delivery and efficacy of therapeutic payloads. New generations of novel nanoconjugates with AuNPs as their cores have been designed and synthesized.[33] A recent study by Feldheim and co-workers has shown how multivalent AuNPs functionalized with derivatives of an important HIV antagonist are highly effective at silencing viral production in a cell model.[34]
Rotello and co-workers have developed a cationic 2 nm gold nanoconjugate functionalized with thiol-modified alkyl amines that possess photoactive o-nitrobenzyl ester linkages, which can be cleaved with near-UV irradiation (Figure 2).[35] Irradiation releases the positively charged alkyl amine from the particle, thereby resulting in a net negatively charged carboxylate-functionalized nanoparticle. The reversal in charge provides an effective means of releasing a negatively charged payload such as an oligonucleotide from the nanoparticle surface. These cationic nanoparticles with photocleavable ligands were shown to inhibit transcription of the bound oligonucleotide; however, the transcription activity can be recovered following the cleavage reaction. Intracellular delivery of the bound oligonucleotide was also demonstrated in MEF cells. Fluorescence-based experiments show that, upon photoinduced cleavage, the bound DNA is released from the nanoparticle surface to the intracellular environment where it then localizes in the nucleus. A similar strategy has been developed to deliver anticancer drugs.[36]
Figure 2.
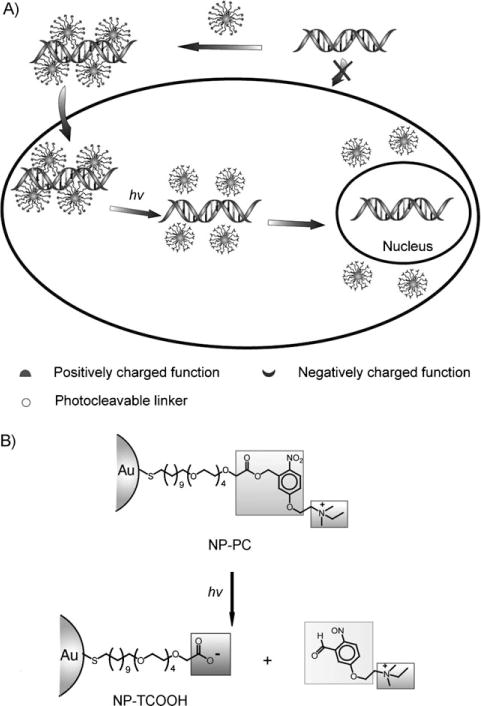
A) Schematic illustration of the release of DNA from a photocleavable AuNP complex (NP-PC) upon UV irradiation within the cell. B) Schematic presentation of light-induced surface transformation of NP-PC. Adapted from Ref. [35].
Another study by Rotello and co-workers demonstrates an alternative method of releasing molecules from gold nanoparticle drug carriers. In this method, gold nanoparticles functionalized with a mixed monolayer of amine-terminated and fluorophore-labeled alkyl thiol ligands were internalized by either HepG2 or MEF cells. Exposure to intracellular environments containing an elevated glutathione concentration (a thiol-possessing peptide) results in substitution and the passive release of the nanoconjugate ligands.[37]
3.3. Stability
In addition to providing functional groups, surface-bound ligands also contribute to the stability of the AuNPs. The stability of the nanoconjugates is an important consideration for their potential use as therapeutic agents because they must maintain their stability under harsh conditions such as in the cell or in the bloodstream. In a study by Rotello and co-workers, the effect of surface charge on the stability of amine-functionalized gold nanoparticle was characterized.[38] In this study, 2 nm gold nanoparticles functionalized with combinations of positively charged amines, negatively charged carboxylates, and fluorescent ligands were used. Various thiol species were tested for their ability to displace ligands bound to the nanoparticle surface. It was found that increasing the net positive charge on the nanoparticle surface caused a more rapid displacement of ligands, whereas more negatively charged nanoconjugates did not display measurable displacement of surface-bound ligands.[38] This result is consistent with studies by our research group on the stability of 13 nm oligonucleotide/gold nanoparticle conjugates which found that the negatively charged thiolated oligonucleotide ligands are not easily displaced in intracellular environments or by small molecules such as glutathione.[25]
4. Oligonucleotides
Over the past decade, our research group and others have synthesized, characterized, and applied polyvalent DNA-functionalized gold nanoconjugates (DNA-AuNPs).[4] This unique class of nanomaterial consists of a gold nanoparticle core that is functionalized with a dense shell of synthetic oligonucleotides. DNA-AuNPs exhibit cooperative properties that result from their polyvalent surfaces,[39–43] and these properties have been applied to areas such as programmable crystallization[44–46] and enzyme-free biodiagnostic assays.[47,48] Indeed, the optical, catalytic, and binding properties of DNA-AuNPs have been used for a variety of colorimetric,[11,49,50] electronic,[7] scanometric,[51] and Raman-based[52] detection strategies, some of which have recently been commercialized and approved by the American Food and Drug Administration.[51]
4.1. Synthesis
Nanoconjugates densely functionalized with synthetic oligonucleotides are prepared by mixing alkanethiol-terminated oligonucleotides and citrate-capped AuNPs. Oligonucleotide ligands displace the citrate from the AuNPs through formation of a gold–thiol bond. NaCl is added to the reaction mixture to shield charge repulsion, thus allowing a greater number of oligonucleotides to chemically adsorb to the nanoparticle surface, thereby resulting in a dense monolayer of oligonucleotides (Figure 3). Approximately 250 oligonucleotides can be chemisorbed to the surface of 15 nm diameter AuNPs, thus creating polyvalent structures.[53] Methods have been optimized for functionalizing particles with diameters ranging from 2 to 250 nm.[54,55] This polyvalent material has a number of emergent properties that are unique from the properties of the oligonucleotides or the AuNPs alone.
Figure 3.
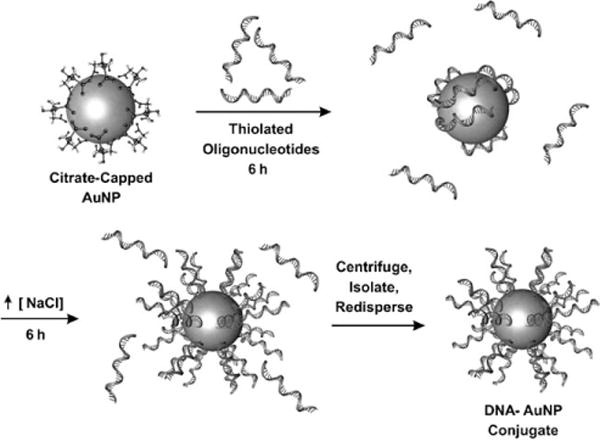
The synthesis of the oligonucleotide gold nanoconjugates: Alkanethiol-terminated oligonucleotides are added to citrate-stabilized AuNPs, thereby displacing the capping citrate ligands through formation of a gold–thiol bond. Subsequent addition of a salt shields repulsion between the strands, thus leading to a dense monolayer of oligonucleotides.
4.2. Properties
One unusual but now fairly well understood property of DNA-AuNPs is their ability to bind complementary nucleic acids with a high affinity.[56] In fact, polyvalent particles exhibit binding constants as large as two orders of magnitude greater than the analogous molecular oligonucleotides of the same sequence.[40] Experimental data and later theoretical models show that this property likely arises from the dense packing and high local concentration of oligonucleotides on the gold surface.[41,57] Additionally, the oligonucleotides on the AuNP surface are close enough such that the counterions associated with one oligonucleotide also act to screen negative charges on adjacent oligonucleotides. This additional charge screening causes increased stabilization of the oligonucleotide duplex, thereby increasing the effective binding constants associated with the DNA-AuNP compared with molecular oligonucleotides. Consistent with this observation, larger particles that have more DNA per particle, but less DNA per unit area exhibit affinities comparable to the molecular system and lower than the gold nanoconjugate structures.[58] In the context of cellular applications, it was hypothesized and subsequently demonstrated that the higher binding constant of the DNA-AuNP would lead to better intracellular binding of the target molecule, thereby increasing the effectiveness of antisense gene regulation (see Section 4.4.1).[25]
Nucleic acids are often hampered in biological investigations by enzymatic hydrolysis, which leads to degradation and renders them inactive.[59,60] Another emergent property of DNA-AuNPs is resistance to degradation by enzymes such as DNase I.[25] Two explanations have been proposed as the origin of this enhanced stability: First, the dense packing of DNA on the surface of the particle could result in steric inhibition of enzyme binding, so that the inaccessible, particle-bound DNA would not be engaged or cleaved by the enzyme. An alternate hypothesis is that the high local ion concentration associated with the densely packed DNA inhibits enzyme activity, since it is known that high concentrations of Na+ ions result in a reduction of enzymatic activity.[61,62] Experiments elucidating these two possibilities have recently been carried out.[63] Molecular DNA and DNA-AuNPs have similar enzymatic degradation rates under conditions where salt concentrations do not affect the enzymatic activity. However, the DNA-AuNP reaction rate is greatly slowed relative to that of molecular DNA under conditions where the salt concentrations affect enzymatic activity. The study concluded that the local Na+ concentration is the dominant factor that contributes to the enhanced stability of DNA. The resistance of DNA-AuNPs to enzymatic degradation is an important property that renders these structures extremely promising candidates for introducing nucleic acids into cells, where oligonucleotide degradation has historically been a major challenge.
4.3. Cellular Uptake
Perhaps the most surprising property of DNA-AuNPs is their ability to enter a wide variety of cell types. The facile uptake of these structures into cells was not predicted, given that these structures contain a densely functionalized shell of polyanionic DNA (ca. 100 DNAs on the surface of each 13 nm gold particle), and that strategies for the introduction of oligonucleotides typically require that DNAs are complexed with positively charged agents to effect cellular internalization. Indeed, because of their high negative charge, most researchers at the time would have predicted that the nanoparticles would not enter cells.[64] Remarkably, it has been shown in all the cell types examined to date (which include over 30 cell lines, primary cells, and neurons, Table 2) that DNA-AuNPs can be added directly to cell culture media and are subsequently taken up by cells in high numbers (Figure 4). Quantification of uptake using ICP-MS shows that while the number of internalized particles varies as a function of cell type, concentration, and incubation time, the cellular internalization of DNA-AuNPs is a general property of these materials. Importantly, the density of DNA on the particle surface was found to be the deciding factor of DNA-AuNP uptake. At DNA surface loadings of greater than about 18 pmolcm−2, cellular uptake can exceed one million DNA-AuNPs per cell.[65] The importance of the polyvalent arrangement of oligonucleotides to cellular uptake can be further emphasized when comparing DNA-AuNPs to other types of AuNPs. For example, HeLa cells internalize only a few thousand citrate-coated gold particles,[19] compared to over one million DNA-AuNPs under nearly identical conditions.[65] Importantly, fluorescence spectroscopy studies reveal that the thiolated oligonucleotides remain bound to the AuNPs after cellular internalization (Figure 4).
Table 2.
Cell types that internalize polyvalent DNA gold nanoconjugates. Cellular internalization was determined using mass spectrometry and cell-associated fluorescence measurements.
| Cell type | Designation or source |
|---|---|
| breast | SKBR3, MDA-MB-321, AU-565 |
| brain | U87, LN229 |
| bladder | HT-1376, 5637, T24 |
| colon | LS513 |
| cervix | HeLa, SiHa |
| skin | C166, KB, MCF, 10 A |
| kidney | MDCK |
| blood | Sup T1, Jurkat |
| leukemia | K562 |
| liver | HepG2 |
| kidney | 293T |
| ovary | CHO |
| macrophage | RAW 264.7 |
| hippocampus neurons | primary, rat |
| astrocytes | primary, rat |
| glial cells | primary, rat |
| bladder | primary, human |
| erythrocytes | primary, mouse |
| peripheral blood mononuclear cell | primary, mouse |
| T cells | primary, human |
| beta islets | primary, mouse |
| skin | primary, mouse |
Figure 4.
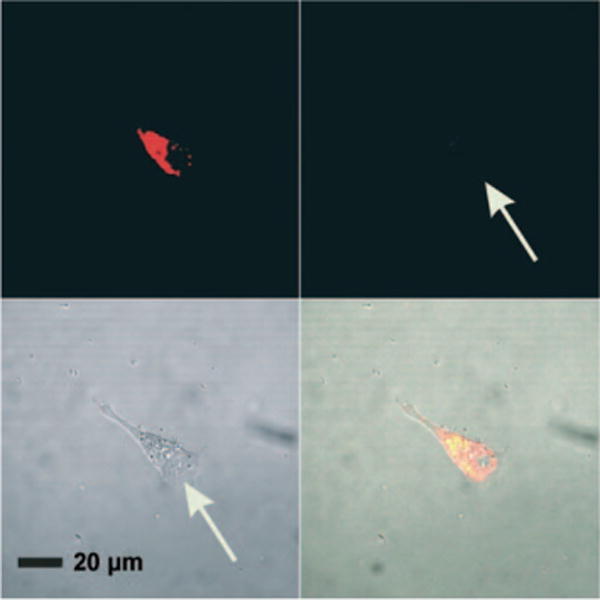
Fluorescent microscopy images of C166-EGFP cells incubated for 48 h with gold nanoconjugates functionalized with dual-fluorophore-labeled oligonucleotides (3′-Cy3 and 5′-Cy5.5) only reveal fluorescence from Cy5.5 (706–717 nm, upper left). Negligible fluorescence is observed in the emission range of Cy3 (565–615 nm, upper right). Transmission and composite overlay images are shown in the lower left and lower right quadrants, respectively. The arrows indicate the location of the cell. Adapted from Ref. [25], with permission from the American Association for the Advancement of Science; Copyright 2006.
Given the surprising ability of DNA-AuNPs to enter cells, the mechanism of uptake is of great interest. Interestingly, biophysical characterization of DNA-AuNPs after exposure to serum-containing media reveals changes in the charge and size of the nanoconjugates. Exposure to cell culture conditions results in greater positive charge and larger nanoparticle diameter (as measured by zeta potential and light scattering), which was further shown to be caused by the adsorption of proteins.[65] The interaction of polyvalent nanoparticle conjugates with proteins provides a possible mechanism of recognition and subsequent internalization of these highly negatively charged particles, the details of which are still under intensive investigation.
4.4. Applications in Cells
Methods based on nucleic acids for detecting and controlling gene expression have had a significant impact on fundamental studies of gene pathways and functions.[29] Methods for controlling gene expression include the use of antisense oligonucleotides[66] and small interfering RNA (siRNA),[67] which can be directed against messenger RNA (mRNA) through Watson–Crick pairing. While the promise of “gene therapy” based on nucleic acids was recognized over 20 years ago, its development has faced challenges with regard to entry into cells, delivery of intact oligonucleotides, and efficacy.[68] Various transfection agents, such as cationic lipids and polymers,[69] modified viruses,[70] dendrimers,[71] liposomes,[72] and nanoparticles,[26,73] have thus been developed to shuttle nucleic acids into cells. Despite the use of these materials, the toxicity of these agents and their offtarget effects limit the amount of oligonucleotides that can be delivered safely. An ideal gene regulation system—from a research standpoint—should feature high uptake efficiencies across all cell types, high intracellular stability, strong binding affinity for target nucleic acids, and very low toxicity. Recently DNA-AuNPs were used as agents to alleviate several of the challenges that are commonly associated with the application of nucleic acids in cells.[25]
4.4.1. Antisense Gene Control
We hypothesized that, because of their enhanced binding properties, DNA-AuNPs could act as potent “sponges” for binding mRNA and preventing translation into proteins. As a demonstration of this concept, we developed DNA-AuNPs that target the mRNA sequences that code for enhanced green fluorescent protein (eGFP) expressed in mouse endothelial cells. An antisense sequence complementary to an internal coding region of the mRNA for eGFP was used in the design and synthesis of “antisense nanoparticles”.[25] Quantitative measurement of expression by using fluorescence assays demonstrates that these particles outperform lipid-complexed DNA used in a direct comparison. Initial experiments demonstrate a silencing of approximately 20%, but further optimization of the experimental parameters and conjugate structure has increased the gene silencing ability to greater than 75% (Figure 5).
Figure 5.
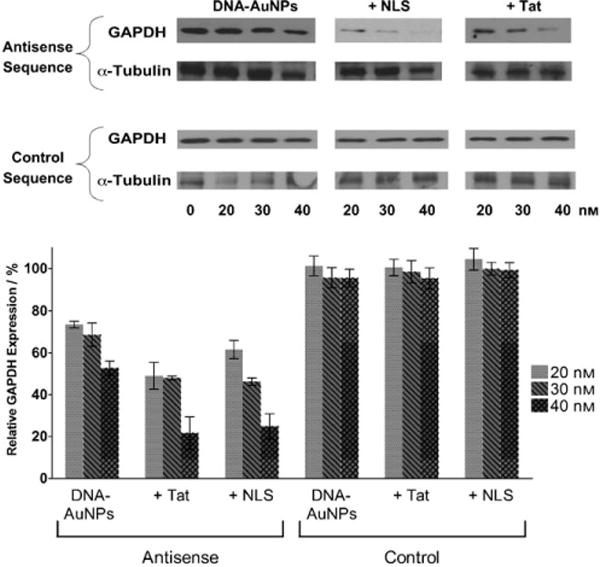
A) Representative Western blots showing the expression of glyceradlehyde 3-phosphate dehydrogenase (GAPDH) in HeLa cells treated with various concentrations and compositions of the gold nanoconjugates. GAPDH expression is reduced in a dose- and sequence-dependent manner. α-Tubulin is shown as the loading control. B) Relative decrease in GAPDH expression in HeLa cells. α-Tubulin was used as a loading control and for subsequent normalization of GAPDH knockdown. The error bars represent the standard deviation from at least three Western blots. Adapted from Ref. [102], with permission from the National Academy of Sciences; Copyright 2008.
Although more than a decade of studies have been dedicated to the synthesis and characterization of DNA-AuNPs, functionalization is not limited to DNA-type oligomers. Indeed, AuNPs can be encoded with a suite of designer oligonucleotides that confer enhanced properties, ranging from increased target specificity to catalytically enhanced biological processing.[74,75] In a recent example, locked nucleic acid (LNA) nanoparticle conjugates have been synthesized and investigated.[76,77] LNAs incorporate bridged sugars in their backbones, which have been shown to increase binding affinity and increase duplex stability.[78] AuNPs densely functionalized with LNA form remarkably stable duplexes with complementary nucleic acids, and can be easily handled and manipulated under biologically relevant conditions. For application in cells, the use of LNA-modified AuNPs increases the effectiveness of gene knockdown compared to analogous DNA-modified AuNPs.[77]
4.4.2. Intracellular Detection and Imaging
Oligonucleotide-based probes to visualize and detect intracellular RNA, including those used for in situ staining,[79,80] molecular beacons,[81,82] and fluorescence resonance energy transfer (FRET) probes[83,84] are important biological tools to measure and quantify biological activity in living systems. However, cells do not readily internalize molecular probes, they require the use of transfection agents or microinjection for uptake. In addition, as a consequence of their oligonucleotide structure, such imaging agents can have limited stability to nuclease degradation, which can lead to a high background signal and decreased ability to specifically detect target structures.
Much work has thus gone into the development of structures that overcome these limitations, including chemically modified molecular beacons[85] or their corresponding peptide conjugates.[86] Recently, our research group has developed novel intracellular detection probes termed “nanoflares” that take advantage of the properties of DNA-AuNPs.[87–89] Nanoflares are oligonucleotide-functionalized gold nanoparticles that are hybridized to short, fluorophore-labeled complements designed to provide an intracellular fluorescence signal that correlates with the concentration of a specific nucleic acid or molecular target. In the absence of a target, the fluorophore is close to the nanoparticle surface, which quenches its fluorescence. Target binding releases the fluorophore, thereby generating a signal that can be detected inside a live cell. Nanoflares can distinguish between different cell types on the basis of the expression profile, and give a semiquantitative real-time readout of gene expression in a living sample (Figure 6).
Figure 6.
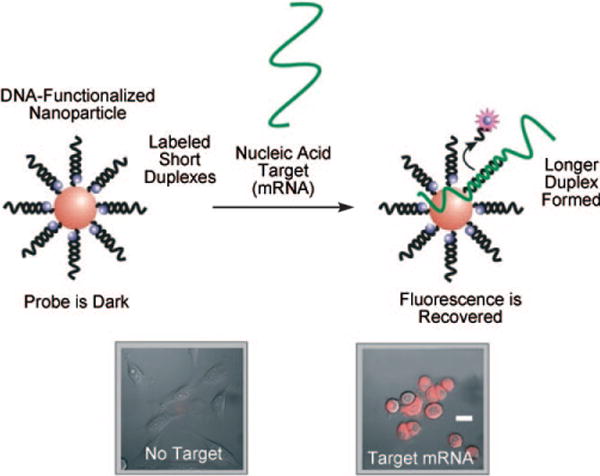
“Nanoflares” are gold nanoconjugates functionalized with oligonucleotide sequences complementary to a specific nucleic acid target (messenger RNA) hybridized to short fluorescent sequences. In the absence of a target the nanoflares are dark, because of quenching by the gold nanoparticle. In the presence of a target binding displaces the short flare through the formation of a longer (more energetically favorable) duplex. The result is a fluorescence signal inside the cell, which indicates the target has been detected. Scale bar: 20 μm. Adapted from Ref. [87], with permission from the American Chemical Society; Copyright 2007.
Several problems commonly associated with intracellular RNA detection, including the difficulty associated with cell entry, toxicity, and intracellular instability, are obviated as these nanoparticles are densely functionalized with oligonucleotides. These probes do not require microinjection or auxiliary reagents to enter cells and are more resistant than molecular nucleic acids towards enzymatic degradation, thus lowering background signal and improving detection ability.
4.4.3. RNA Interference
Additional work is now underway on conjugates functionalized with RNA-capping ligands that are capable of acting in the highly potent RNA interference (RNAi) pathway. Recently, we determined that RNA-AuNPs can be synthesized and subsequently introduced into cells without the use of transfection agents.[90] Traditional RNAi uses molecular RNAs, which have extremely short half-lives as a result of the instability of ribonucleotides to RNase-type enzymes, thus limiting their efficacy.[91,92] In the case of RNA-gold nanoconjugates, a dense monolayer of surface-immobilized RNA increases the protection from nonspecific degradation both in cell culture media and in the intracellular environment. These structures are over six times more stable than molecular RNA in serum-containing media, and this enhanced stability does not rely on chemical modifications to the RNA molecular structure. We have further shown that the RNA-gold nanoconjugates have a more persistent ability to silence genes. The enhanced stability and high cellular uptake should result in these structures playing an important role in future fundamental studies as well as in the therapeutic application of RNAi.
4.4.4. Cellular Detection
In addition to intracellular applications, Tan and co-workers have developed a colorimetric assay that uses DNA-AuNPs for the detection of cancer cells. Specifically, AuNPs were functionalized with a monolayer of aptamers selected to have a high affinity for surface receptors expressed by a cancer cell line (CCRF-CEM).[93] The aptamer-functionalized nanoconjugates assemble on the cell surfaces, which causes their surface plasmon resonances to interact. This results in a red shift in the extinction spectra, thus providing a direct readout of target binding. The strong extinction of AuNPs means that the presence of cancer cells can be detected by the naked eye or by using a spectrometer, which eliminates the need for expensive and complicated instrumentation and makes the assay potentially useful for cancer diagnosis or disease screening.
5. Peptides
The targeting portions of many proteins are short stretches of oligopeptides. Peptide-based nuclear localization signals have been used to alter the intracellular localization and increase efficacy of conjugated biomolecules.[94] Such peptide signaling sequences are often composed of a stretch of positively charged amino acids such as arginine and lysine, which interact with Importin A for transport across the nuclear envelope.[95] Sequences derived from the HIV Tat protein (CYGRKKRRQRRR) and integrin binding domain (CKKKKKKGGRGDMFG) have been studied extensively for delivery of exogenous proteins and synthetic materials to the nucleus.[23,96–99]
5.1. Peptide Nanoconjugates
Recently, examples of peptide–gold nanoparticle conjugates have been reported. Feldheim, Franzen, and co-workers conjugated peptides to gold nanoparticles through attachment to bovine serum albumin (BSA) and subsequent electrostatic association.[23,100] The resulting nanoconjugates enter the nucleus of HepG2 cells in culture. Interestingly, only nanoconjugates functionalized with peptides containing both a receptor-mediated endocytosis (RME) and nuclear localization signal (NLS) are able to enter the nucleus of these cells (Figure 7). The same researchers recently investigated the ability of AuNPs modified with both peptides and polyethylene glycol (PEG) to enter cells. Interestingly, the particles are actively internalized even if the PEG molecule within the monolayer is large (molecular weight: 5000).[101] These studies point to exciting opportunities in the design of multifunctional conjugates.
Figure 7.
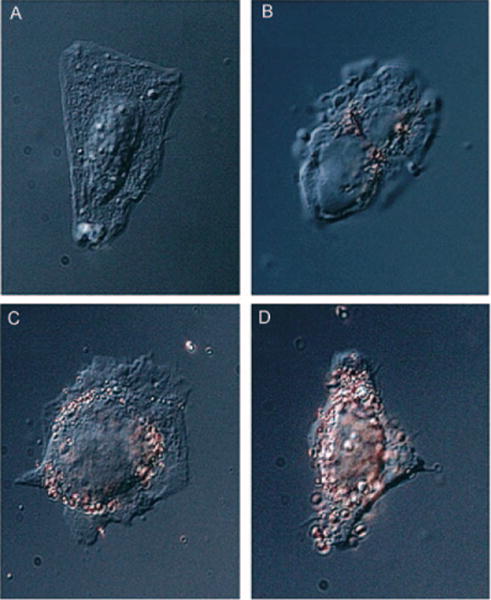
Images of nanoparticle–peptide complexes incubated with HepG2 cells for 2 h. Complexes were: A) nuclear localization peptide, B) receptor-mediated endocytosis peptide, C) adenoviral fiber protein, and D) both nuclear localization and receptor-mediated endocytosis peptides. Adapted from Ref. [23], with permission from the American Chemical Society; Copyright 2003.
5.2. Peptide/DNA-Gold Nanoparticle Conjugates
We recently prepared gold nanoconjugates functionalized with both antisense oligonucleotides and NLS or HIV Tat peptides.[102] Our synthetic strategy uses thiolated oligonucleotides and cysteine-terminated peptides to functionalize the AuNP surfaces. As the oligopeptides and oligonucleotides are oppositely charged, the addition of salt is required to screen oppositely charged biomolecules during synthesis. When tested in cell culture, the resultant conjugates are internalized and localized in the perinuclear region. Consequently, these particles have a high gene silencing ability (>75% decrease in expression of the target protein).
5.3. Multifunctional and Multicomponent DNA Nanoconjugates
The versatility of nanoconjugates can be increased by incorporating multiple functional groups into each construct, or by rationally designing it to have multiple functions. Recently, our research group has demonstrated that nanoflares (see Section 4.4.2) can be adapted for both intracellular mRNA detection and gene knockdown.[88] These nanoflares enter cells and bind mRNA in a location suitable for gene knockdown, thereby decreasing the relative abundance of mRNA, while simultaneously releasing a fluorescent flare. Here, the nanoflare provides a read-out of gene regulation inside the cell. Such capabilities will provide valuable feedback, as the results of manipulating a cellular system can be observed in real time. In addition, one can, in principle, create all sorts of cell-sorting genetic screening asays by using the nanoflare approach.
Other therapeutic nanoconstructs have been designed to take advantage of the uptake of DNA-AuNPs by cells. For example, PtIV complexes are being explored for chemotherapy in an effort to reduce the side effects of cisplatin. Studies by the research groups of Lippard and Mirkin have shown that AuNPs can be modified with both oligonucleotides and cisplatin prodrugs. These constructs, similar to their cannonical DNA counterparts, deliver the drug payload effectively to cells.[127] The prodrug consists of a PtIV complex designed to be reduced and released as active cisplatin in the acidic endo-somes of cells. In addition, synthetic handles (in this case, a carboxylic acid) can be added to the cisplatin precursor to allow for straightforward conjugation to the oligonucleotides through amide linkages. Future work in this area will examine regulating gene expression to chemosensitize the cells while delivering drugs. Such multicomponent conjugates should decrease the amount of chemotherapeutic agent needed for therapeutic efficacy while simultaneously reducing systemic toxicity.
6. Antibodies
Antibody-labeled gold nanoconjugates have been used in immunohistochemistry for almost 40 years.[15] Recently, however, there has been a resurgence in their use as a consequence of the development of gold nanoconjugates for live cell studies. Synthetic methods to produce antibody-gold nanoconjugates include adsorption,[15] N-hydroxysuccinimide (NHS) ester chemistry,[103] and oligonucleotide-directed immobilization.[104] Antibodies can adsorb to AuNPs through hydrophobic and ionic interactions, or through chemisorption of native thiol groups present in their chemical structure.[105] However, conjugates synthesized with this method have limited stability because the proteins are easily desorbed.[106] AuNPs functionalized with monolayers containing NHS esters can be reacted with the primary amine groups of the antibody to form more stable structures. Alternatively, DNA-AuNPs can be hybridized with antibodies that have been conjugated to complementary oligonucleotides.[106]
6.1. Imaging
AuNPs modified with antibodies specific to cancer-associated proteins have been used to image cancerous cells. In one example, conjugates with antibodies to epithelial growth factor receptor (EGFR) were incubated with oral epithelial cancerous and noncancerous epithelial cells. Light microscopy experiments show that conjugates bind to cancerous cells with a six times greater affinity than the noncancerous controls, thus making this technique potentially useful for the detection of cancer cells.[107]
6.2. Photothermal Therapy
Gold nanorods[108] and nanoshells[109] conjugated with antibodies are being developed as photothermal therapy agents that use antibody-coated surfaces to hone in on cancerous cells. For example, nanoshells conjugated to antibodies against human epidermal growth factor receptor 2 (HER2) were incubated with cancerous cells over-expressing HER2 receptors. These cells were then irradiated with near-IR light at a frequency that is resonant with the surface plasmon resonance of the nanoshell. Light absorption leads to heating, which causes cell death.[110] Nanoshells conjugated to control antibodies did not display this affect, because of the lack of nanoshell binding on the cell surfaces. These conjugates are also being developed as materials that combine photothermal therapy with near-IR imaging capabilities.[107,110]
7. Lipids
Recently, lipids have joined oligonucleotides, peptides, and antibodies as biomolecules used to modify AuNPs. Our research group and others have synthesized biomimetic high density lipoprotein (HDL) nanostructures by adsorbing lipids and proteins to the surface of AuNPs.[111] In this synthesis, thiolated lipids or alkanethiols along with apolipoprotein A1 (APOA1), a protein component of HDL, are adsorbed onto the surface of AuNPs. Next, a second lipid is adsorbed onto the AuNP surface through hydrophobic interactions between the lipid tails and thiolated species. Simple methods for synthesizing HDL with control over the size, shape, and composition had not been demonstrated prior to these studies. It is being increasingly appreciated that size, shape, and chemistry of HDL has an impact on its in vivo physiology, and these structures may prove useful as therapeutics and imaging agents.[111,112]
7.1. Therapeutics
Natural HDL is critical for transporting cholesterol from macrophages in atherosclerotic plaques and from the body, and increasing the HDL levels may provide an approach to preventing or reversing atherosclerosis. To that end, our research group synthesized HDL mimics called HDL AuNPs whose size as well as protein and lipid contents are similar to those of natural HDL (Figure 8). Importantly, these nanostructures can be used to determine the strength of interactions between HDL and cholesterol. In our first example using these conjugates we showed that HDL AuNPs are capable of binding a fluorescent cholesterol analogue with a high binding affinity (Kd = 4 nm).[111] To the best of our knowledge, this is the first measured binding constant for any form of HDL and a cholesterol derivative. This is important as it provides a key data point from which to evaluate future constructs and their ability to bind cholesterol as well as their potential as new therapeutic candidates.
Figure 8.
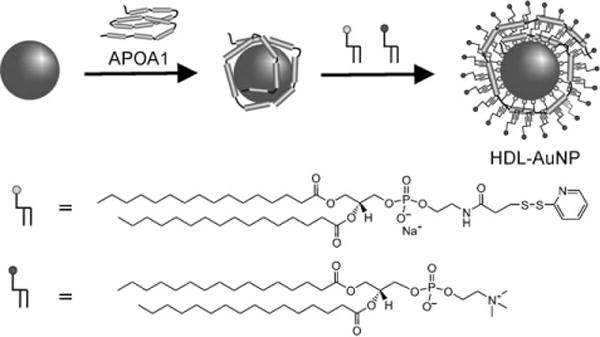
Templated synthesis of spherical HDL nanoparticles through use of thiol-terminated peptides and the protein (APOA1). Adapted from Ref. [111], with permission from the American Chemical Society; Copyright 2009.
7.2. Imaging
In addition to cholesterol transport, HDL-AuNP mimics have been used to image macrophage cells in vivo.[112] Macrophage density is indicative of high-risk atherosclerotic plaque, thus making it an attractive imaging target. Mice fed high cholesterol diets, an established model for atherosclerosis, were injected with HDL-AuNPs. Tomography images of the mice aortas showed a build-up of HDL-AuNPs, thereby indicating that the nanoparticles could be applied to atherosclerotic imaging.
8. Summary and Outlook
Gold nanoconjugates are an important class of materials that have already proven useful in fundamental cell biology applications. As is the case with all nanomaterials, little is known about the interactions of gold nanoconjugates and cells at the molecular level, and the design criteria for research and therapeutic usage are still being formulated. In the next sections, we discuss emerging challenges in the field. In our opinion, these questions will be the key towards the further development of gold nanoconjugates into viable therapeutic agents.
8.1. Mechanism of Uptake in Cells
Several research groups have now confirmed the internalization of gold nanoconjugates in common cell-line models. The mechanism of cellular internalization is likely to differ for different classes of gold nanoconjugates because of differences in their surface chemistry, size, and charge. Indeed, substitution reactions can be used to modulate the ability of an AuNP to be internalized by a cell.[24,113] In the case of AuNPs functionalized with positively charged amines or peptides, the mechanism likely involves the interaction of these positive moieties with the negatively charged cell surface.[26] In the case of antibody conjugates or those that possess peptidic internalization signals, interactions between specific cell-surface antigens are likely mechanistic steps.[23] Negatively charged gold nanoconjugates likely follow yet another uptake pathway. Studies by our research group and others suggest that internalization in the cell may involve the interaction of proteins with the nanoparticle surfaces.[21,65] Identifying the proteins that allow the negatively charged gold nanoconjugates to penetrate cells stands as a formidable challenge.
8.2. Targeting
The use of gold nanoconjugates provides a highly effective method for introducing substances into cells. We have described how the unique ensemble properties of these materials allow for multivalent drug and antisense agents. These agents can be used to control cellular function, regulate gene expression, and detect intracellular analytes with greater efficiency than molecular systems, which is in part due to composite properties and proven cellular uptake ability across diverse cell types. An important challenge for the continued development of these materials as therapeutics is to target specific cells and eventually tissues and organs. Strategies for targeted delivery may include the use of biomolecules such as antibodies,[108] aptamers,[114] peptides,[23] or small molecule ligands.[115]
Targeting strategies need to be integrated with functionality to create multifunctional particles for delivering oligonucleotides or other therapeutic cargos to target cells. For example, antibodies targeted against surface receptors for appropriate cellular targets should be able to effect cell-specific uptake and limit nonspecific uptake, but they must also maintain the other desired activity and properties of nanoconjugates. In the case of polyvalent DNA-AuNPs, moieties such as antibodies must be attached in a manner that does not limit the degree of DNA functionalization or the properties that result from the density of DNA. While this is not trivial, it is noteworthy that cofunctionalized AuNPs have already been synthesized and preliminarily studied, including structures which successfully incorporate peptides without compromising complementary binding to nucleic acids.[102] These results are promising steps towards the next generation of targeted polyvalent nanoconjugate therapeutics.
8.3. Toxicity
The toxicity of several types and sizes of gold nanoconjugates has been investigated by a number of independent research groups. Although results have varied to date, several important conclusions can be drawn from these studies. Perhaps the most salient is that the toxicity of gold nanoconjugates is dependent on the chemical composition of the surface ligands. In fact, it is often the surface group itself that leads to toxicity. For example, although gold nanoconjugates functionalized with cetyltrimethylammonium bromide (CTAB) were initially thought to be toxic, it was subsequently determined that the particles do not cause cytotoxicity if they are washed to remove excess ligand.[18] Additional work in this area, has shown how the toxicity of a ligand such as CTAB is reduced when complexed with an AuNP,[116] presumably because of an alteration of the cellular localization of the toxic agent. Rotello and co-workers have also shown how the chemical functionality and charge of nanoconjugate surface ligands influence toxicity. These researchers found that while amine-functionalized particles were only mildly toxic, particles functionalized with carboxylic acids were nontoxic under all the conditions examined.[117]
Several recent studies have focused on the toxicity of citrate-capped nanoconjugates. One study investigating human dermal fibroblasts determined that the rate of cell proliferation, spreading, and adhesion is slowed by the presence of citrate-capped nanoconjugates.[118] The authors presented evidence that actin stress is the cause of these effects. A second, independent study also reports decreased cell growth in the presence of citrate-capped nanoconjugates, and in this case, the authors present evidence that this is the result of oxidative damage.[119] Similar results have also been reported when similar particles were used in myeloma cells.[120] Although acute and gross toxicity was not observed in these cases, the adverse effects of citrate-capped nanoconjugates merit further attention.
Intriguing recent investigations demonstrate that the size of the conjugate also determines its toxicity. In a recent study, Simon, Jahnen-Dechent, and co-workers examined a panel of phosphine-functionalized AuNPs with diameters ranging from 0.8 to 15 nm. These researchers found that 1.4 nm diameter particles were toxic, whereas 15 nm diameter particles were nontoxic, even at up to 100-fold higher concentrations.[121] In the case of these 1.4 nm diameter particles, evidence is presented that toxicity results from necrosis; however, neither 1.2 nor 1.8 nm diameter particles display this effect. Chan and co-workers have recently investigated the cell response to herceptin-coated gold nanoparticles within the 2–100 nm size range and found that 40 and 50 nm particles have the greatest effect on cell signaling functions.[122] Clearly, these are important findings that need to be explored further. The challenge will be preparing a range of particle sizes by using a common synthetic strategy and ensuring exact chemical surface functionality for accurate comparison.
Gold nanorods and nanoshells have recently been tested in mouse models. Halas, West, and co-workers have evaluated the photothermal efficacy of PEG-coated nanoshells injected into tumors in a mouse model. These researchers found that tumors could be ablated by treatment with light, and the animals remained healthy after more than 90 days, thus pointing to a low toxicity of nanoconjugates in vivo.[123] A research group investigating the use of CTAB-functionalized gold nanorods as imaging agents found that the particles were rapidly cleared from the blood after injection into the tail vein.[124] Another study on very similar nanorod particles found that they are accumulated in the liver after 72 h.[125] Interestingly, however, when the surface groups were changed to PEG, very few particles remained in the liver after 72 h, and most were cleared. These initial animal studies are indeed promising, and should motivate future studies that investigate the biodistribution of gold nanoconjugates as a function of size, shape, and chemical properties of the ligands.
To date, no cytotoxicity of the DNA-AuNPs has been observed.[25] It is again important to note that these nanoconjugates have unique size, charge, and surface functionality, with properties derived from the combination of the DNA and the AuNP. Extensive toxicology screening of these unique materials will be a necessity, and determining what component or components of the structure contribute to a biological response will be an exciting endeavor. Preliminary work in our research group on the innate immune response, (as characterized by interferon production, one of the first pathways activated in an innate immune response) has shown little interferon-β production caused by the DNA-AuNPs compared to analogous molecular DNA.[126] Further work is required to examine any changes in the gene expression profile that may result from the introduction of these structures. In addition to in vitro assays, preliminary work to examine biodistribution and toxicity in vivo is now underway. While polyvalent DNA-AuNPs have already shown utility in cell culture assays, such animal studies will be required to assess the feasibility of these nanomaterials becoming possible therapeutic agents.
8.4. Conclusion
Although the properties of colloidal gold have been investigated for over a century, their application as intracellular agents in living cells emerged only prominently a few years ago. These investigations have demonstrated that multivalent and/or composite nanomaterials can provide significant advantages over molecular systems in terms of uptake and efficacy in cellular models. More fundamentally, these studies have reinforced the underlying concept in nanotechnology that composition, surface derivatization, charge, size, and shape are all critical to materials properties, and that this translates into a unique ability to interact with a biological system such as a cell. The highlighted classes of gold nanoconjugates represent a small but important sample of possible conjugate materials. The study of these classes highlights one very important conclusion: Namely, unique nanomaterials must be investigated and evaluated individually. This is exemplified in the studies of nanoparticle toxicity, where surface functionalization has repeatedly been shown to be a key parameter that influences toxicity. If one were to conclude from earlier work using CTAB-functionalized nanoconjugates that all gold nanoconjugates were toxic, then important opportunities would have been missed, for example the use of DNA-AuNPs for genetic regulation[25] or amine-functionalized conjugates for drug delivery,[36] where toxicity has been shown to be lower than polymer delivery systems.[25] As such, we encourage investigators to study and evaluate nanoconjugates on a case-by-case basis and avoid generalization wherever possible.
The preparation and use of functionalized gold nanoconjugates continues to be an extremely active and important area of research. This field continues to tantalize the chemical research community with major discoveries as well as new scientific challenges, while also involving cross-disciplinary investigators including materials scientists, biologists, engineers, and clinicians. The work carried out thus far provides only a glimpse of the wide range of potential applications for gold nanoparticles in biology and medicine.
Acknowledgments
C.A.M. acknowledges a Cancer Center for Nanotechnology Excellence (CCNE) award, the NSF-NSEC, and a U.S. Army Medical Research and Material Command Grant W81XWH-08-1-0766 for support of this work. C.A.M. is also grateful for a NIH Director’s Pioneer Award. D.S.S. was supported by the LUNGevity Foundation—American Cancer Society Postdoctoral Fellowship in Lung Cancer. P.C.P. was supported by a Ryan Fellowship.
Biographies

Chad A. Mirkin is the Director of the Northwestern University International Institute for Nanotechnology, the George B. Rathmann Prof. of Chemistry, Prof. of Chemical and Biological Engineering, Prof. of Biomedical Engineering, Prof. of Materials Science and Engineering, and Prof. of Medicine. He has authored over 400 manuscripts and has over 350 patents and applications. He is the founder of the companies Nanosphere, NanoInk, and AuraSense, and he cofounded the journal Small. He has received over 60 national and international awards for his contributions to chemistry, materials science, and nanoscience.

David Giljohann studied at Northwestern University and obtained his Bachelor’s Degree in 2003. He completed his PhD there under the mentorship of Chad Mirkin in 2009. His research is focused on the development of oligonucleotide-modified nanoparticles, including nanoflares as well as antisense DNA and RNA gold nanoparticles.

Weston Daniel studied chemistry at the University of Minnesota, Twin Cities and graduated in 2005. He is now conducting PhD research at Northwestern University under the mentorship of Chad Mirkin. His research focuses on developing detection schemes by using biomolecule-functionalized gold nanoparticles.

Pinal Patel completed his undergraduate studies at Towson University in 1999. He then worked at the United States Department of Defense before returning to academia. He is currently pursuing PhD research at Northwestern University under the guidance of Chad Mirkin. His research is focused on determining the cellular uptake and intracellular localization of oligonucleotide-modified gold nanoparticles.

Dwight Seferos completed his PhD at the University of California, Santa Barbara in 2006 under the guidance of Guillermo Bazan, and then carried out postdoctoral research at Northwestern University in the laboratory of Chad Mirkin. He is currently an Assistant Professor in the Department of Chemistry at the University of Toronto.

Matthew Massich completed his undergraduate studies in 2004 at the University of St. Thomas. He is currently carrying out PhD research at Northwestern University with Chad Mirkin. His research investigates the biological response to the use of oligonucleotide-functionalized gold nanoparticles for therapeutic and diagnostic applications.
References
- 1.Hayat MA. Colloidal gold: principles, methods, and applications. Academic Press; San Diego: 1989. [Google Scholar]
- 2.Edwards PP, Thomas JM. Angew Chem. 2007;119:5576. doi: 10.1002/anie.200700428. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2007;46:5480. [Google Scholar]
- 3.Daniel MC, Astruc D. Chem Rev. 2004;104:293. doi: 10.1021/cr030698+. [DOI] [PubMed] [Google Scholar]
- 4.Mirkin CA, Letsinger RL, Mucic RC, Storhoff JJ. Nature. 1996;382:607. doi: 10.1038/382607a0. [DOI] [PubMed] [Google Scholar]
- 5.Park SY, Lytton-Jean AKR, Lee B, Weigand S, Schatz GC, Mirkin CA. Nature. 2008;451:553. doi: 10.1038/nature06508. [DOI] [PubMed] [Google Scholar]
- 6.Alivisatos AP, Johnsson KP, Peng XG, Wilson TE, Loweth CJ, Bruchez MP, Schultz PG. Nature. 1996;382:609. doi: 10.1038/382609a0. [DOI] [PubMed] [Google Scholar]
- 7.Park SJ, Taton TA, Mirkin CA. Science. 2002;295:1503. doi: 10.1126/science.1067003. [DOI] [PubMed] [Google Scholar]
- 8.Wang J, Liu GD, Merkoci A. J Am Chem Soc. 2003;125:3214. doi: 10.1021/ja029668z. [DOI] [PubMed] [Google Scholar]
- 9.Xiao Y, Patolsky F, Katz E, Hainfeld JF, Willner I. Science. 2003;299:1877. doi: 10.1126/science.1080664. [DOI] [PubMed] [Google Scholar]
- 10.He L, Musick MD, Nicewarner SR, Salinas FG, Benkovic SJ, Natan MJ, Keating CD. J Am Chem Soc. 2000;122:9071. [Google Scholar]
- 11.Liu J, Lu Y. J Am Chem Soc. 2003;125:6642. doi: 10.1021/ja034775u. [DOI] [PubMed] [Google Scholar]
- 12.Rosi NL, Mirkin CA. Chem Rev. 2005;105:1547. doi: 10.1021/cr030067f. [DOI] [PubMed] [Google Scholar]
- 13.Katz E, Willner I. Angew Chem. 2004;116:6166. [Google Scholar]; Angew Chem Int Ed. 2004;43:6042. [Google Scholar]
- 14.Penn SG, He L, Natan MJ. Curr Opin Chem Biol. 2003;7:609. doi: 10.1016/j.cbpa.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 15.Faulk WP, Taylor GM. Immunochemistry. 1971;8:1081. doi: 10.1016/0019-2791(71)90496-4. [DOI] [PubMed] [Google Scholar]
- 16.Frens G. Nat Phys Sci. 1973;241:20. [Google Scholar]
- 17.Enustun BV, Turkevich J. J Am Chem Soc. 1963;85:3317. [Google Scholar]
- 18.Connor EE, Mwamuka J, Gole A, Murphy CJ, Wyatt MD. Small. 2005;1:325. doi: 10.1002/smll.200400093. [DOI] [PubMed] [Google Scholar]
- 19.Chithrani BD, Ghazani AA, Chan WCW. Nano Lett. 2006;6:662. doi: 10.1021/nl052396o. [DOI] [PubMed] [Google Scholar]
- 20.Yang PH, Sun XS, Chiu JF, Sun HZ, He QY. Bioconjugate Chem. 2005;16:494. doi: 10.1021/bc049775d. [DOI] [PubMed] [Google Scholar]
- 21.Chithrani BD, Chan WCW. Nano Lett. 2007;7:1542. doi: 10.1021/nl070363y. [DOI] [PubMed] [Google Scholar]
- 22.Cho EC, Xie JW, Wurm PA, Xia YN. Nano Lett. 2009;9:1080. doi: 10.1021/nl803487r. [DOI] [PubMed] [Google Scholar]
- 23.Tkachenko AG, Xie H, Coleman D, Glomm W, Ryan J, Anderson MF, Franzen S, Feldheim DL. J Am Chem Soc. 2003;125:4700. doi: 10.1021/ja0296935. [DOI] [PubMed] [Google Scholar]
- 24.Nativo P, Prior IA, Brust M. ACS Nano. 2008;2:1639. doi: 10.1021/nn800330a. [DOI] [PubMed] [Google Scholar]
- 25.Rosi NL, Giljohann DA, Thaxton CS, Lytton-Jean AKR, Han MS, Mirkin CA. Science. 2006;312:1027. doi: 10.1126/science.1125559. [DOI] [PubMed] [Google Scholar]
- 26.Sandhu KK, McIntosh CM, Simard JM, Smith SW, Rotello VM. Bioconjugate Chem. 2002;13:3. doi: 10.1021/bc015545c. [DOI] [PubMed] [Google Scholar]
- 27.Brust M, Walker M, Bethell D, Schiffrin DJ, Whyman R. J Chem Soc Chem Commun. 1994;801 [Google Scholar]
- 28.Hostetler MJ, Templeton AC, Murray RW. Langmuir. 1999;15:3782. [Google Scholar]
- 29.Patil SD, Rhodes DG, Burgess DJ. AAPS J. 2005;7:E61. doi: 10.1208/aapsj070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomas M, Klibanov AM. Proc Natl Acad Sci USA. 2003;100:9138. doi: 10.1073/pnas.1233634100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghosh PS, Kim CK, Han G, Forbes NS, Rotello VM. ACS Nano. 2008;2:2213. doi: 10.1021/nn800507t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Torchilin VP. Adv Drug Delivery Rev. 2006;58:1532. doi: 10.1016/j.addr.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 33.Gibson JD, Khanal BP, Zubarev ER. J Am Chem Soc. 2007;129:11653. doi: 10.1021/ja075181k. [DOI] [PubMed] [Google Scholar]
- 34.Bowman MC, Ballard TE, Ackerson CJ, Feldheim DL, Margolis DM, Melander C. J Am Chem Soc. 2008;130:6896. doi: 10.1021/ja710321g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han G, You CC, Kim BJ, Turingan RS, Forbes NS, Martin CT, Rotello VM. Angew Chem. 2006;118:3237. doi: 10.1002/anie.200600214. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2006;45:3165. [Google Scholar]
- 36.Kim CK, Ghosh P, Pagliuca C, Zhu ZJ, Menichetti S, Rotello VM. J Am Chem Soc. 2009;131:1360. doi: 10.1021/ja808137c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hong R, Han G, Fernandez JM, Kim BJ, Forbes NS, Rotello VM. J Am Chem Soc. 2006;128:1078. doi: 10.1021/ja056726i. [DOI] [PubMed] [Google Scholar]
- 38.Chompoosor A, Han G, Rotello VM. Bioconjugate Chem. 2008;19:1342. doi: 10.1021/bc8000694. [DOI] [PubMed] [Google Scholar]
- 39.Storhoff JJ, Lazarides AA, Mucic RC, Mirkin CA, Letsinger RL, Schatz GC. J Am Chem Soc. 2000;122:4640. [Google Scholar]
- 40.Lytton-Jean AK, Mirkin CA. J Am Chem Soc. 2005;127:12754. doi: 10.1021/ja052255o. [DOI] [PubMed] [Google Scholar]
- 41.Jin RC, Wu GS, Li Z, Mirkin CA, Schatz GC. J Am Chem Soc. 2003;125:1643. doi: 10.1021/ja021096v. [DOI] [PubMed] [Google Scholar]
- 42.Hurst SJ, Hill HD, Mirkin CA. J Am Chem Soc. 2008;130:12192. doi: 10.1021/ja804266j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Demers LM, Mirkin CA, Mucic RC, Reynolds RA, III, Letsinger RL, Elghanian R, Viswanadham G. Anal Chem. 2000;72:5535. doi: 10.1021/ac0006627. [DOI] [PubMed] [Google Scholar]
- 44.Nykypanchuk D, Maye MM, van der Lelie D, Gang O. Nature. 2008;451:549. doi: 10.1038/nature06560. [DOI] [PubMed] [Google Scholar]
- 45.Park SY, Lytton-Jean AK, Lee B, Weigand S, Schatz GC, Mirkin CA. Nature. 2008;451:553. doi: 10.1038/nature06508. [DOI] [PubMed] [Google Scholar]
- 46.Hill HD, Macfarlane RJ, Senesi AJ, Lee B, Park SY, Mirkin CA. Nano Lett. 2008;8:2341. doi: 10.1021/nl8011787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nam JM, Thaxton CS, Mirkin CA. Science. 2003;301:1884. doi: 10.1126/science.1088755. [DOI] [PubMed] [Google Scholar]
- 48.Stoeva SI, Lee JS, Smith JE, Rosen ST, Mirkin CA. J Am Chem Soc. 2006;128:8378. doi: 10.1021/ja0613106. [DOI] [PubMed] [Google Scholar]
- 49.Elghanian R, Storhoff JJ, Mucic RC, Letsinger RL, Mirkin CA. Science. 1997;277:1078. doi: 10.1126/science.277.5329.1078. [DOI] [PubMed] [Google Scholar]
- 50.Storhoff JJ, Elghanian R, Mucic RC, Mirkin CA, Letsinger RL. J Am Chem Soc. 1998;120:1959. [Google Scholar]
- 51.Taton TA, Mirkin CA, Letsinger RL. Science. 2000;289:1757. doi: 10.1126/science.289.5485.1757. [DOI] [PubMed] [Google Scholar]
- 52.Cao YWC, Jin RC, Mirkin CA. Science. 2002;297:1536. doi: 10.1126/science.297.5586.1536. [DOI] [PubMed] [Google Scholar]
- 53.Hurst SJ, Lytton-Jean AK, Mirkin CA. Anal Chem. 2006;78:8313. doi: 10.1021/ac0613582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee JS, Seferos DS, Giljohann DA, Mirkin CA. J Am Chem Soc. 2008;130:5430. doi: 10.1021/ja800797h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hurst SJ, Lytton-Jean AKR, Mirkin CA. Anal Chem. 2006;78:8313. doi: 10.1021/ac0613582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bao YP, Huber M, Wei TF, Marla SS, Storhoff JJ, Muller UR. Nucleic Acids Res. 2005;33:7. doi: 10.1093/nar/gni017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Long H, Kudlay A, Schatz GC. J Phys Chem B. 2006;110:2918. doi: 10.1021/jp0556815. [DOI] [PubMed] [Google Scholar]
- 58.Lytton-Jean AKR, Mirkin CA. J Am Chem Soc. 2005;127:12754. doi: 10.1021/ja052255o. [DOI] [PubMed] [Google Scholar]
- 59.Cazenave C, Chevrier M, Thuong NT, Helene C. Nucleic Acids Res. 1987;15:10507. doi: 10.1093/nar/15.24.10507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Woolf TM, Jennings CGB, Rebagliati M, Melton DA. Nucleic Acids Res. 1990;18:1763. doi: 10.1093/nar/18.7.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pan CQ, Lazarus RA. Biochemistry. 1997;36:6624. doi: 10.1021/bi962960x. [DOI] [PubMed] [Google Scholar]
- 62.Shack J. J Biol Chem. 1959;234:3003. [PubMed] [Google Scholar]
- 63.Seferos DS, Prigodich AE, Giljohann DA, Patel PC, Mirkin CA. Nano Lett. 2009;9:308. doi: 10.1021/nl802958f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Niidome T, Huang L. Gene Ther. 2002;9:1647. doi: 10.1038/sj.gt.3301923. [DOI] [PubMed] [Google Scholar]
- 65.Giljohann DA, Seferos DS, Patel PC, Millstone JE, Rosi NL, Mirkin CA. Nano Lett. 2007;7:3818. doi: 10.1021/nl072471q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Uhlmann E, Peyman A. Chem Rev. 1990;90:543. [Google Scholar]
- 67.Dorsett Y, Tuschl T. Nat Rev Drug Discovery. 2004;3:318. doi: 10.1038/nrd1345. [DOI] [PubMed] [Google Scholar]
- 68.Lebedeva I, Stein CA. Annu Rev Pharmacol Toxicol. 2001;41:403. doi: 10.1146/annurev.pharmtox.41.1.403. [DOI] [PubMed] [Google Scholar]
- 69.Hwang SJ, Davis ME. Curr Opin Mol Ther. 2001;3:183. [PubMed] [Google Scholar]
- 70.Kamiya H, Tsuchiya H, Yamazaki J, Harashima H. Adv Drug Delivery Rev. 2001;52:153. doi: 10.1016/s0169-409x(01)00216-2. [DOI] [PubMed] [Google Scholar]
- 71.Braun CS, Vetro JA, Tomalia DA, Koe GS, Koe JG, Middaugh CR. J Pharm Sci. 2005;94:423. doi: 10.1002/jps.20251. [DOI] [PubMed] [Google Scholar]
- 72.Hughes MD, Hussain M, Nawaz Q, Sayyed P, Akhtar S. Drug Discovery Today. 2001;6:303. doi: 10.1016/s1359-6446(00)00326-3. [DOI] [PubMed] [Google Scholar]
- 73.Bharali DJ, Klejbor I, Stachowiak EK, Dutta P, Roy I, Kaur N, Bergey EJ, Prasad PN, Stachowiak MK. Proc Natl Acad Sci USA. 2005;102:11539. doi: 10.1073/pnas.0504926102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kubo T, Zhelev Z, Ohba H, Bakalova R. Biochem Biophys Res Commun. 2008;365:54. doi: 10.1016/j.bbrc.2007.10.116. [DOI] [PubMed] [Google Scholar]
- 75.Singh SK, Nielsen P, Koshkin AA, Wengel J. Chem Commun. 1998:455. [Google Scholar]
- 76.McKenzie F, Faulds K, Graham D. Small. 2007;3:1866. doi: 10.1002/smll.200700225. [DOI] [PubMed] [Google Scholar]
- 77.Seferos DS, Giljohann DA, Rosi NL, Mirkin CA. ChemBioChem. 2007;8:1230. doi: 10.1002/cbic.200700262. [DOI] [PubMed] [Google Scholar]
- 78.Koshkin AA, Nielsen P, Meldgaard M, Rajwanshi VK, Singh SK, Wengel J. J Am Chem Soc. 1998;120:13252. doi: 10.1016/s0960-894x(98)00366-7. [DOI] [PubMed] [Google Scholar]
- 79.Femino AM, Fay FS, Fogarty K, Singer RH. Science. 1998;280:585. doi: 10.1126/science.280.5363.585. [DOI] [PubMed] [Google Scholar]
- 80.Kloosterman WP, Wienholds E, de Bruijn E, Kauppinen S, Plasterk RH. Nat Methods. 2006;3:27. doi: 10.1038/nmeth843. [DOI] [PubMed] [Google Scholar]
- 81.Tyagi S, Kramer FR. Nat Biotechnol. 1996;14:303. doi: 10.1038/nbt0396-303. [DOI] [PubMed] [Google Scholar]
- 82.Sokol DL, Zhang X, Lu P, Gewirtz AM. Proc Natl Acad Sci USA. 1998;95:11538. doi: 10.1073/pnas.95.20.11538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sando S, Kool ET. J Am Chem Soc. 2002;124:9686. doi: 10.1021/ja026649g. [DOI] [PubMed] [Google Scholar]
- 84.Santangelo PJ, Nix B, Tsourkas A, Bao G. Nucleic Acids Res. 2004;32:e57. doi: 10.1093/nar/gnh062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang L, Yang CYJ, Medley CD, Benner SA, Tan WH. J Am Chem Soc. 2005;127:15664. doi: 10.1021/ja052498g. [DOI] [PubMed] [Google Scholar]
- 86.Nitin N, Santangelo PJ, Kim G, Nie S, Bao G. Nucleic Acids Res. 2004;32:e58. doi: 10.1093/nar/gnh063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Seferos DS, Giljohann DA, Hill HD, Prigodich AE, Mirkin CA. J Am Chem Soc. 2007;129:15477. doi: 10.1021/ja0776529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Prigodich AE, Seferos DS, Massich MD, Giljohann DA, Lane BC, Mirkin CA. ACS Nano. 2009;3:2147. doi: 10.1021/nn9003814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zheng D, Seferos DS, Giljohann DA, Patel PC, Mirkin CA. Nano Lett. 2009;9:3258. doi: 10.1021/nl901517b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Giljohann DA, Seferos DS, Prigodich AE, Patel PC, Mirkin CA. J Am Chem Soc. 2009;131:2072. doi: 10.1021/ja808719p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chiu YL, Rana TM. RNA. 2003;9:1034. doi: 10.1261/rna.5103703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Soutschek J, Akinc A, Bramlage B, Charisse K, Constien R, Donoghue M, Elbashir S, Geick A, Hadwiger P, Harborth J, John M, Kesavan V, Lavine G, Pandey RK, Racie T, Rajeev KG, Rohl I, Toudjarska I, Wang G, Wuschko S, Bumcrot D, Koteliansky V, Limmer S, Manoharan M, Vornlocher HP. Nature. 2004;432:173. doi: 10.1038/nature03121. [DOI] [PubMed] [Google Scholar]
- 93.Medley CD, Smith JE, Tang Z, Wu Y, Bamrungsap S, Tan WH. Anal Chem. 2008;80:1067. doi: 10.1021/ac702037y. [DOI] [PubMed] [Google Scholar]
- 94.Vives E, Schmidt J, Pelegrin A. Biochim Biophys Acta Rev Cancer. 2008;1786:126. doi: 10.1016/j.bbcan.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 95.Goldfarb DS, Gariepy J, Schoolnik G, Kornberg RD. Nature. 1986;322:641. doi: 10.1038/322641a0. [DOI] [PubMed] [Google Scholar]
- 96.Biju V, Muraleedharan D, Nakayama K, Shinohara Y, Itoh T, Baba Y, Ishikawa M. Langmuir. 2007;23:10254. doi: 10.1021/la7012705. [DOI] [PubMed] [Google Scholar]
- 97.Derfus AM, Chen AA, Min DH, Ruoslahti E, Bhatia SN. Bioconjugate Chem. 2007;18:1391. doi: 10.1021/bc060367e. [DOI] [PubMed] [Google Scholar]
- 98.Lanford RE, Kanda P, Kennedy RC. Cell. 1986;46:575. doi: 10.1016/0092-8674(86)90883-4. [DOI] [PubMed] [Google Scholar]
- 99.Oyelere AK, Chen PC, Huang X, El-Sayed IH, El-Sayed MA. Bioconjugate Chem. 2007;18:1490. doi: 10.1021/bc070132i. [DOI] [PubMed] [Google Scholar]
- 100.Tkachenko AG, Xie H, Liu YL, Coleman D, Ryan J, Glomm WR, Shipton MK, Franzen S, Feldheim DL. Bioconjugate Chem. 2004;15:482. doi: 10.1021/bc034189q. [DOI] [PubMed] [Google Scholar]
- 101.Liu YL, Shipton MK, Ryan J, Kaufman ED, Franzen S, Feldheim DL. Anal Chem. 2007;79:2221. doi: 10.1021/ac061578f. [DOI] [PubMed] [Google Scholar]
- 102.Patel PC, Giljohann DA, Seferos DS, Mirkin CA. Proc Natl Acad Sci USA. 2008;105:17222. doi: 10.1073/pnas.0801609105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Qian X, Peng XH, Ansari DO, Yin-Goen Q, Chen GZ, Shin DM, Yang L, Young AN, Wang MD, Nie S. Nat Biotechnol. 2008;26:83. doi: 10.1038/nbt1377. [DOI] [PubMed] [Google Scholar]
- 104.Niemeyer CM, Ceyhan B. Angew Chem. 2001;113:3798. doi: 10.1002/1521-3773(20011001)40:19<3685::aid-anie3685>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2001;40:3685. [Google Scholar]
- 105.Hermanson GT. Bioconjugate Techniques. Academic Press; San Diego, CA: 1996. [Google Scholar]
- 106.Nitin N, Javier DJ, Richards-Kortum R. Bioconjugate Chem. 2007;18:2090. doi: 10.1021/bc0701242. [DOI] [PubMed] [Google Scholar]
- 107.El-Sayed IH, Huang XH, El-Sayed MA. Nano Lett. 2005;5:829. doi: 10.1021/nl050074e. [DOI] [PubMed] [Google Scholar]
- 108.El-Sayed IH, Huang X, El-Sayed MA. Cancer Lett. 2006;239:129. doi: 10.1016/j.canlet.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 109.Hirsch LR, Stafford RJ, Bankson JA, Sershen SR, Rivera B, Price RE, Hazle JD, Halas NJ, West JL. Proc Natl Acad Sci USA. 2003;100:13549. doi: 10.1073/pnas.2232479100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Loo C, Lowery A, Halas N, West J, Drezek R. Nano Lett. 2005;5:709. doi: 10.1021/nl050127s. [DOI] [PubMed] [Google Scholar]
- 111.Thaxton CS, Daniel WL, Giljohann DA, Thomas AD, Mirkin CA. J Am Chem Soc. 2009;131:1384. doi: 10.1021/ja808856z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cormode DP, Skajaa T, van Schooneveld MM, Koole R, Jarzyna P, Lobatto ME, Calcagno C, Barazza A, Gordon RE, Zanzonico P, Fisher EA, Fayad ZA, Mulder WJ. Nano Lett. 2008;8:3715. doi: 10.1021/nl801958b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Huff TB, Hansen MN, Zhao Y, Cheng JX, Wei A. Langmuir. 2007;23:1596. doi: 10.1021/la062642r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Javier DJ, Nitin N, Levy M, Ellington A, Richards-Kortum R. Bioconjugate Chem. 2008;19:1309. doi: 10.1021/bc8001248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dixit V, Van den Bossche J, Sherman DM, Thompson DH, Andres RP. Bioconjugate Chem. 2006;17:603. doi: 10.1021/bc050335b. [DOI] [PubMed] [Google Scholar]
- 116.Hauck TS, Ghazani AA, Chan WCW. Small. 2008;4:153. doi: 10.1002/smll.200700217. [DOI] [PubMed] [Google Scholar]
- 117.Goodman CM, McCusker CD, Yilmaz T, Rotello VM. Bioconjugate Chem. 2004;15:897. doi: 10.1021/bc049951i. [DOI] [PubMed] [Google Scholar]
- 118.Pernodet N, Fang XH, Sun Y, Bakhtina A, Ramakrishnan A, Sokolov J, Ulman A, Rafailovich M. Small. 2006;2:766. doi: 10.1002/smll.200500492. [DOI] [PubMed] [Google Scholar]
- 119.Li JJ, Zou L, Hartono D, Ong CN, Bay BH, Yung LYL. Adv Mater. 2008;20:138. [Google Scholar]
- 120.Bhattacharya R, Patra CR, Verma R, Kumar S, Greipp PR, Mukherjee P. Adv Mater. 2007;19:711. [Google Scholar]
- 121.Pan Y, Neuss S, Leifert A, Fischler M, Wen F, Simon U, Schmid G, Brandau W, Jahnen-Dechent W. Small. 2007;3:1941. doi: 10.1002/smll.200700378. [DOI] [PubMed] [Google Scholar]
- 122.Jiang W, Kim BYS, Rutka JT, Chan WCW. Nat Nanotechnol. 2008;3:145. doi: 10.1038/nnano.2008.30. [DOI] [PubMed] [Google Scholar]
- 123.O’Neal DP, Hirsch LR, Halas NJ, Payne JD, West JL. Cancer Lett. 2004;209:171. doi: 10.1016/j.canlet.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 124.Wang HF, Huff TB, Zweifel DA, He W, Low PS, Wei A, Cheng JX. Proc Natl Acad Sci USA. 2005;102:15752. doi: 10.1073/pnas.0504892102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Niidome T, Yamagata M, Okamoto Y, Akiyama Y, Takahashi H, Kawano T, Katayama Y, Niidome Y. J Controlled Release. 2006;114:343. doi: 10.1016/j.jconrel.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 126.Massich MD, Giljohann DA, Seferos DS, Ludlow LE, Horvath CM, Mirkin CA. Mol Pharm. 2009;6:1934. doi: 10.1021/mp900172m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dhar S, Daniel WL, Giljohann DA, Mirkin CA, Lippard SJ. J Am Chem Soc. 2009;131:14652. doi: 10.1021/ja9071282. [DOI] [PMC free article] [PubMed] [Google Scholar]


