Abstract
Objective
Little attention has been paid to the role of holding back sharing concerns in the psychological adaptation of women newly diagnosed with gynecological cancers. The goal of the present study was to evaluate the role of holding back concerns in psychosocial adjustment and quality of life, as well as a possible moderating role for emotional expressivity and perceived unsupportive responses from family and friends.
Method
Two hundred forty four women diagnosed with gynecological cancer in the past eight months completed measures of holding back, dispositional emotional expressivity, perceived unsupportive responses from family and friends, cancer-specific distress, depressive symptoms, and quality of life.
Results
Emotional expressivity moderated the association between holding back and cancer- specific distress and quality of life, but not depressive symptoms. Greater holding back was more strongly associated with higher levels of cancer-related distress among women who were more emotionally expressive than among women who were less expressive. Perceived unsupportive responses did not moderate the associations between holding back and psychosocial outcomes.
Conclusion
Holding back sharing concerns was more common in this patient population than other cancer populations. Dispositional expressivity played a role in how harmful holding back concerns was for women, while unsupportive responses from family and friends did not.
Keywords: Gynecological cancer, depressive symptoms, trajectories of change, coping
1. Introduction
Cancers of the reproductive organs are the fourth most common site of cancer in American women [1]. It is estimated that approximately 91,730 women will be diagnosed with ovarian, uterine, cervical, or vulvar cancers in the United States in 2013 [2]. Medical advances in the past 30 years have increased survival rates for some types of gynecological cancers: For example, the five year survival rate for cervical cancer is 68% [2]. Unfortunately, the five-year survival rate for women diagnosed with metastatic ovarian cancer is only 27% [2], and the five-year survival rate for women diagnosed with metastatic uterine cancer is only 16% [2]. In addition to facing a poor prognosis, women diagnosed with gynecological cancers undergo a difficult treatment regimen, followed by significant medical complications [3–13]. Furthermore, the probability of recurrent disease can be quite high. For ovarian cancer, recurrence rates in the first five years following diagnosis vary between 10% for Stage 1 disease to 95% for Stage 4 disease [14]. For women diagnosed with endometrial cancers, recurrence rates average about 46% within the first five years after diagnosis [14].
Given the challenges this patient population faces, it is not surprising that psychological distress is prevalent. Indeed, between 30% and 52% of women diagnosed with ovarian cancer report moderate to severe levels of anxiety [15–18] and up to 45% of women report clinically-relevant levels of depression [15–16]. Longitudinal studies have shown that some women experience persistent anxiety (22%) and depression (6%) [16]. Women diagnosed with cervical, endometrial, uterine, and vulvar cancers also report relatively high levels of psychological distress [6, 19–22]. Studies evaluating post-traumatic stress disorder (PTSD) indicate that between 36% and 45% of women diagnosed with ovarian cancer experience PTSD after diagnosis [23] and that there is a progressive increase in PTSD prevalence over time [23]. Quality of life can also be adversely impacted among women with gynecological cancers, particularly in the body image domain [24].
Despite the prevalence of psychological distress, there has been little attention paid to what factors place women with gynecological cancers at increased psychosocial risk. The few studies that have been conducted have primarily evaluated demographic and medical correlates of distress responses among women diagnosed with ovarian cancer [15, 17–18] and women with mixed gynecological cancer diagnoses [25]. Studies have suggested that younger age [15, 23, 26–27], a diagnosis of advanced disease [27], greater functional impairment [18], and a more recent diagnosis [27] contribute to higher levels of psychological distress. In terms of psychological factors, research has suggested that avoidant strategies such as wishful thinking, mental and behavioral disengagement, self-blame, and neuroticism are associated with greater distress [16]. Less emotional support has also been associated with distress in some studies [25, 27], but research has shown an inconsistent relationship between social support and distress [16].
According to Horowitz [28], Janoff-Bulman [29] and others, stressful events challenge individuals’ existing schemas about themselves and their world. Successful adaptation to these stressors involves actively assimilating or accommodating the experience into one’s “world view.” Individuals achieve this goal by using both cognitive and social processing strategies. Cognitive processing strategies include efforts to reduce the amount of threat by perceiving the experience as being less harmful. These cognitive strategies can include accepting that the event has happened and reappraising the experience as beneficial. Social processing by sharing concerns with others is also important: One’s social network can facilitate or interfere with effective cognitive processing [30]. Research suggests that sharing cancer-related concerns contributes to better psychological adjustment [31–32]. However, sharing with others may be compromised by internal (characteristics of the person) and external (characteristics of the social environment) barriers. One common internal barrier is dispositional emotional expressivity, or comfort with openly (or not openly) expressing emotions, both positive and negative. Indeed, dispositional emotional expressivity is associated with higher levels of emotionally expressive coping with cancer [33]. One common external barrier to disclosure is the perception that one’s friends and family will not be respond in a supportive manner. Research has supported this contention: Cancer patients are less likely to share their concerns when they perceive others are unsupportive [32].
The match between internal and external barriers to disclosure with how much the patient holds back may play a role in the effects of holding back on psychosocial adaptation. Social psychological theories of person-environment fit suggest that concordance between personal disposition and coping strategies chosen (such as holding back versus sharing concerns) is important [34]. For example, a less emotionally expressive person may find sharing with others to be unhelpful for understanding experiences because sharing is not congruent with his/her personality; however, sharing concerns may be a helpful strategy for a more emotionally expressive person. Research has supported this contention: Among women who are more dispositionally emotionally expressive, emotional expression is linked with less depression [33, 35]. Additional work has shown that dispositional emotional expressivity moderates associations between intrusive cognitions and distress: Intrusions are more likely to lead to distress among less emotionally expressive cancer patients [36–37]. The degree of match or concordance may also be important with regard to coping strategies chosen and one’s external environment. Thus, a mismatch between external factors, such as the perception that one’s social network is unsupportive, and a coping strategy chosen, such as sharing concerns, may also lead to greater distress. That is, holding back sharing concerns would be more detrimental when the person perceives that his or her social network is unsupportive than when the person holds back for personal reasons not related to one’s social environment (e.g., fear of being a burden, not feeling well). Prior work has not separated effects of holding back (one’s own behavior) on distress from the effects of perceptions of the level of supportiveness of one’s social network (the perceived response of others).
In the present study, we extended the examination of internal and external factors that may moderate the association between holding back sharing concerns and psychological adaptation to cancer in two ways. First, we focused on a population of patients facing challenging diagnosis and disease course - women diagnosed with gynecological cancer. This population of patients typically exhibits high levels of emotional distress. We also focused on a population of patients close to diagnosis, rather than long-term survivors. For these patients, the effects of holding back sharing concerns may be particularly detrimental when one is dealing with a newly diagnosed, aggressive form of cancer. Second, we evaluated holding back sharing concerns and perceived unsupportive behaviors separately from perceived constraints in sharing. Traditionally, social constraints measures assess both holding back sharing and the reason for holding back sharing and/or other person’s reaction in the same instrument. For example, Lepore’s [38] instrument asks both reasons for holding back (e.g., “How often did you have to keep your feelings to yourself because they made the other person uncomfortable?”) in the same instrument as perceived unsupportive responses (e.g., “How often did they avoid you?”)
In the present study, we assessed the patient’s behavior (holding back) separately from perceived unsupportive responses from the person’s social environment. Rather, unsupportive responses from friends were assessed as a moderator of the effects of holding back.
The study had two aims. The first aim was to evaluate the prevalence of holding back sharing concerns in this patient population, characterize the types of concerns that gynecological cancer patients are most likely to hold back sharing with others, and compare these levels with other studies. The second aim was to evaluate the moderating role of emotional expressivity and perceived unsupportive response from family and friends in the association between holding back and cancer-specific distress, depressive symptoms, and cancer-specific emotional and physical quality of life. We predicted that holding back would be more detrimental to distress and quality of life among those patients who were more dispositionally emotionally expressive, and that holding back would be more detrimental among patients who perceived higher levels of unsupportive responses from friends and family.
2. Methods
2.1. Participants
Participants were drawn from the baseline data of a randomized clinical trial evaluating the efficacy of two psychological interventions for women with gynecological cancer (Manne, unpublished data). Eligible participants were women diagnosed with primary gynecological cancer (ovarian, endometrial, cervical, vulvar, fallopian tube, or uterine cancer). The larger study took place at four comprehensive cancer centers located in the New Jersey, New York, and Philadelphia areas, and two additional cancer treatment centers in New Jersey. In addition to having a diagnosis of primary gynecological cancer, criteria for study inclusion were as follows: a) at the time of recruitment (not survey completion) the patient was diagnosed six months or less; b) an Eastern Cooperative Oncology Group [ECOG; 39] score of 0 (“asymptomatic; fully active, able to carry on all pre-disease activities without restriction”) or 1 (“symptomatic but completely ambulatory; restricted in physically strenuous activity but ambulatory and able to carry out work of a light or sedentary nature. For example, light housework, office work”); c) lived within a two-hour commuting distance from the center being recruited from; d) 18 years of age or older; e) English speaking, and; f) no hearing impairment.
2.2. Procedure
Eligible women were identified and permission to contact granted by the attending physician. They were called by the research assistant or approached in person after a letter describing the study was sent, and the study was described. Participants were provided with a written informed consent and the questionnaire to complete. Participants signed an informed consent approved by an Institutional Review Board. Participants were paid $25 for a returned survey.
2.3. Measures
2.3.1. Medical variables
Data regarding current disease stage, date of diagnosis, recurrence status at baseline and over the course of the study, and ECOG status [39] were obtained from the medical chart.
2.3.2. Holding back sharing concerns
A 13-item scale adapted from Pistrang and Barker [40] and used by Porter et al. [32] was used. Participants rated the degree to which they held back sharing concerns in 13 specific areas (e.g., concerns about disease progression or death, finances, job, relationships, sexual functioning, physical functioning) with family and friends on a 6-point Likert scale (0 = not at all, 3 = somewhat, 5 = a lot). Because not all concerns were endorsed, an average across concerns endorsed was used. Internal consistency as estimated by Cronbach’s alpha was .92.
2.3.3. Moderator Measures
2.3.3.1. Emotional expressivity
Expression of emotions was measured by the Emotional Expressiveness Questionnaire [41]. Sixteen items were rated on a 5-point Likert scale (0= “never”, 4 = “always”). Higher scores indicated higher emotional expressivity. Internal consistency as calculated by Cronbach’s alpha was .78.
2.3.3.2. Perceived unsupportive responses of family and friends
The family and friends version of the 13 item Perceived Negative Behaviors Scale [42–43] was used to assess the negative or unsupportive behaviors of family and friends. Items assessed both overtly critical responses such as criticism of the patient’s response to the illness as well as avoidance (“Seemed uncomfortable talking to you about your illness”). Participants rated how their family and friends had responded during the past month on a scale of 1 (never responds this way) to 4 (often responds this way) with higher scores indicating a greater frequency of unsupportive behaviors. Internal consistency as calculated by Cronbach’s alpha was .90.
2.3.4. Outcome measures
2.3.4.1. Cancer-specific distress
The Impact of Events Scale (IES) [44] is a 15-item self-report measure focusing on intrusive and avoidant ideation associated with a stressor, in this case gynecological cancer and its treatment. The IES has been used in studies of women with cancer [e.g., 45] and in our previous work [46]. Using a 4-point Likert scale, participants rated how true each statement had been for them during the past week. Internal consistency as calculated by Cronbach’s alpha was .90.
2.3.4.2. Depressive symptoms
The 21-item Beck Depression Inventory [BDI; 47] was used to assess depressive symptoms. The BDI has been widely-used in studies incorporating cognitive-behavioral intervention. Internal consistency of the scale has been well-documented [48]. For the present study, Cronbach’s alpha was .85.
2.3.4.3. Quality of Life
The Functional Assessment of Cancer Therapy – General [FACT-G; 49] is comprised of 27 questions that assess well-being. For the present study, we focused on two domains: physical (PWB) and emotional well-being (EWB). PWB assesses physical symptoms (e.g., 7 items, “I have a lack of energy”) and EWB measures mood and emotional response to illness (e.g., 6 items, “I feel sad”, “I am satisfied with how I am coping with my illness,” “I am losing hope in the fight against my illness”). Each domain was summed. Both the total and domain scores have good internal consistency among cancer patients (alpha = .72 –.85) [49] and the instrument is well-validated [49]. For the present study, Cronbach’s alphas were .87 (physical) and .88 (emotional).
3. Results
3.1. Sample Description
Eight hundred seventy four eligible women were approached for this study. Two hundred forty four women accepted (27.9%). The most common reason for study refusal was that the patient felt she lived too far to commute (this was a psychological intervention study). Differences between study refusers and participants on available data (age, race, time since diagnosis, stage of disease, ECOG status) were examined. Results indicated that study participants were significantly younger (M = 55 years) than study refusers (M = 60 years) (t (872) = 6.1, p < .05).
The characteristics of participants are shown in Table 1. The majority of the sample was diagnosed with ovarian cancer and over half of the sample had advanced stage disease. Average time from diagnosis to completion of the survey was about four months. Approximately 88% of the sample was undergoing chemotherapy at the time of the assessment.
Table 1.
Demographic and Medical Data for Study Participants (N=244)
| Variable | M | SD | Range | n | % |
|---|---|---|---|---|---|
| Age (years) | 55.01 | 10.6 | 23–78 | ||
| Race | |||||
| Caucasian | 195 | 84.0 | |||
| African American | 29 | 10.0 | |||
| Asian/Pacific Island | 10 | 3.0 | |||
| Hispanic | 5 | 2.0 | |||
| Other | 3 | 1.2 | |||
| Missing | 2 | 1.6 | |||
| Education Level | |||||
| < High school | 8 | 3.2 | |||
| High school graduate | 34 | 13.9 | |||
| Trade or Business school | 6 | 2.5 | |||
| Some college | 34 | 13.9 | |||
| College graduate | 62 | 35.4 | |||
| Some graduate school | 18 | 7.4 | |||
| Graduate degree | 81 | 33.2 | |||
| Missing | 1 | 0.4 | |||
| Marital Status | |||||
| Married | 158 | 64.8 | |||
| Unmarried | 82 | 34.0 | |||
| Missing | 3 | 1.2 | |||
| Household income ($) | 144,001 | 264,382 | 2,000–750,000,000 | ||
| Primary Cancer | |||||
| Ovarian | 141 | 57.8 | |||
| Endometrial | 31 | 11 | |||
| Uterine | 26 | 11 | |||
| Fallopian | 23 | 8 | |||
| Cervical | 11 | 2 | |||
| Peritoneal | 5 | 2 | |||
| More than one | 7 | 2 | |||
| Time since diagnosis (months) | 4.3 | 1.6 | |||
| Stage | |||||
| I | 41 | 16.8 | |||
| II | 23 | 9.4 | |||
| III | 119 | 48.8 | |||
| IV | 53 | 21.7 | |||
| Missing | 8 | 3.3 | |||
| Metastatic Cancer | |||||
| Yes | 148 | 60.7 | |||
| No | 94 | 38.5 | |||
| Missing | 2 | 0.8 | |||
| CA-125 Level | 213.2 | 0–7788 | |||
| Tumor Debulking | |||||
| Yes | 137 | 56.0 | |||
| No | 69 | 28.2 | |||
| Data missing | 36 | 14.8 | |||
| Tumor Debulking, if yes | |||||
| Optimal | 114 | 89.8 | |||
| Suboptimal | 13 | 10.2 | |||
| Psych Medication | |||||
| Yes | 132 | 64 | |||
| No | 96 | 31 | |||
| Missing | 6 | 5 | |||
3.2. Descriptive Information Regarding Holding Back Sharing Concerns
Descriptive information is shown in Table 2. The average rating on holding back was 2.01, which corresponded to slightly below “somewhat” on the 6-point rating scale. Average ratings on each item ranged from 1.49 (0 = not at all) to 2.71 (3 = somewhat). The highest rated items on the scale were “Fear of disease progression or death” which was rated 2.71, “concerns about sexual functioning” which was rated 2.59, and “concerns about emotional reactions (fear, sadness)” which was rated 2.26. The lowest-rated item was “Concerns about your cancer treatment (e.g., medical or surgical treatments, medicines, interactions with doctors and nurses, being in the hospital)” which had an average rating of 1.49, followed by concerns about physical symptoms (M= 1.59) and concerns about job (M = 1.59).
Table 2.
Levels of Holding Back regarding Cancer Concerns with Family and Friends
| Concerns Item | M | SD |
|---|---|---|
| Cancer treatment | 1.49 | 1.7 |
| Physical symptoms | 1.59 | 1.6 |
| Financial concerns | 2.17 | 1.8 |
| Relationship with partner | 1.93 | 1.7 |
| Job-related concerns | 1.59 | 1.7 |
| Relationship with others | 1.67 | 1.7 |
| Body image | 1.69 | 1.8 |
| Sexual function | 2.59 | 2.1 |
| Emotional reactions | 2.26 | 1.7 |
| Disease progression/death | 2.71 | 1.8 |
| Well-being | 2.18 | 1.7 |
| Partner well-being | 2.10 | 1.6 |
3.3. Moderator Analyses for Dispositional Emotional Expressivity
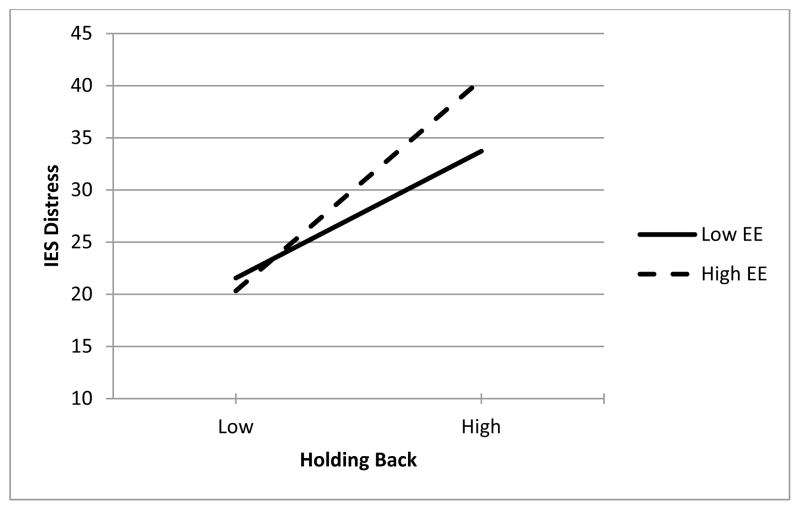
Correlations between variables included in the models are shown in Table 3. Moderated regression analyses were conducted to examine whether the effects of holding back were moderated by emotional expressivity (see Table 4). In these analyses predictors were grand-mean centered prior to forming the interaction. Two significant interactions between holding back and emotional expressivity emerged when considering the four outcome measures. In the first interaction, holding back and emotional expressivity interacted to predict cancer-specific distress. Simple slopes analyses (using plus/minus one SD) showed that when emotional expressivity was high, holding back was a very strong predictor of cancer-specific distress, b = 7.91, β = .62, t (234) = 7.64, p < .001, but when emotional expressivity was low, holding back was a significantly weaker, although still significant predictor of cancer-specific distress, b = 4.75, β = .37, t (234) = 4.62, p < .001. This interaction is graphed in Figure 1.
Table 3.
Means, Standard Deviations, and Correlations Among the Study Variables
| Variables | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
|
|
|||||||
| 1. Holding Back | |||||||
| 2. Emotional Expressivity | −.26** | ||||||
| 3. Perceived Unsupportive Behaviors | .31** | −0.1 | |||||
| 4. IES Distress | .46** | −0.04 | .24** | ||||
| 5. BDI Depression | .46** | −.19** | .40** | .58** | |||
| 6. FACT-G Physical | −.20** | 0.02 | −.24** | −.28** | −.57** | ||
| 7. FACT-G Emotional | −.34** | 0.11 | −.24** | −.56** | −.62** | .34** | |
| M | 2.01 | 40.76 | 17.11 | 28.47 | 13.75 | 20.54 | 17.28 |
| SD | 1.28 | 7.1 | 5.85 | 16.35 | 7.51 | 5.64 | 4.44 |
| N | 244 | 244 | 236 | 238 | 243 | 243 | 244 |
Note.
p < .05,
p < .01;
IES = Impact of Events Scale; BDK = Beck Depression Inventory
Table 4.
Moderated Regression Results Predicting Outcomes as a Function of Holding Back, Emotional Expressivity, and their Interaction
| Predictors
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Holding Back (HB) | Emotional Expressivity (EE) | HB by EE Interaction | |||||||
|
| |||||||||
| Outcome Variables | B | β | t | b | β | t | b | β | t |
| IES Distress | 6.33 | 0.49 | 8.32** | 0.2 | 0.09 | 1.46 | 0.22 | 0.12 | 2.27* |
| BDI Depression | 2.58 | 0.44 | 7.44** | −0.08 | −0.07 | 1.2 | −0.04 | −0.05 | 0.87 |
| FACT-G Physical | −0.9 | −0.2 | 3.13** | −0.03 | −0.04 | 0.6 | 0.04 | 0.07 | 1.21 |
| FACT-G Emotional | −1.17 | −0.34 | 5.43** | 0.02 | 0.03 | 0.42 | −0.07 | −0.15 | 2.58* |
Note.
p < .05,
p < .01;
IES = Impact of Events Scale; BDI = Beck Depression Inventory
Figure 1.
Interaction between Holding Back Concerns and Dispositional Emotional Expressivity (EE) Predicting Impact of Events (IES) Distress.
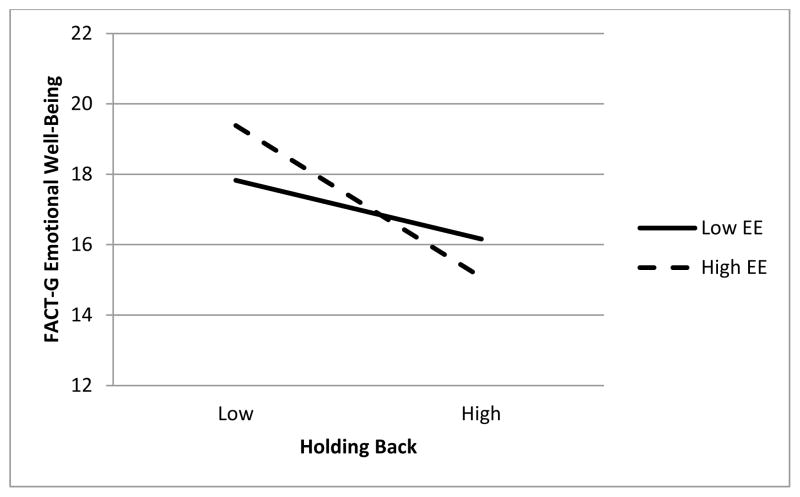
Emotional expressivity and holding back also interacted to predict FACT-G emotional well-being. As with cancer-specific distress, although both simple slopes for holding back were significant at high and low emotional expressivity, the effect of holding back was considerably stronger when emotional expressivity was high, b = −1.68, β = −.48, t (240) = 5.77, p < .001 rather than low, b = −.66, β = −.19, t (240) = 2.22, p = .028. This interaction is graphed in Figure 2.
Figure 2.
Interaction between Holding Back Concerns and Dispositional Emotional Expressivity (EE) Predicting FACT-G Emotional Well-Being
3.4. Moderator Analyses for Perceived Unsupportive Behaviors
As can be seen in Table 5, although greater holding back and greater perceived unsupportive behavior on the part of family and friends predicted distress and depressive symptoms, there were no moderator effects for unsupportive behaviors on the association between holding back and distress or emotional and physical well-being.
Table 5.
Moderated Regression Results Predicting Outcomes as a Function of Holding Back, Perceived Unsupportive Behavior from Family and Friends, and their Interaction
| Predictors
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Holding Back | Unsupportive Family/Friends | HB by Unsupp F/F Interaction | |||||||
|
| |||||||||
| Outcome Variables | B | β | t | b | β | T | b | β | t |
| IES Distress | 5.56 | 0.41 | 6.57** | 0.42 | 0.15 | 2.24* | −0.18 | −0.08 | 1.37 |
| BDI Depression | 2.05 | 0.35 | 5.92** | 0.38 | 0.3 | 4.53** | 0 | 0 | 0.03 |
| FACT-G Physical | −0.57 | −0.13 | 1.95 | −0.18 | −0.19 | 2.55* | −0.02 | −0.03 | 0.39 |
| FACT-G Emotional | 1.05 | −0.3 | 4.66** | −0.09 | −0.12 | 1.73 | −0.04 | −0.07 | 1.04 |
Note.
p < .05,
p < .01;
IES = Impact of Events Scale; BDI = Beck Depression Inventory
4. Discussion
The present study examined the prevalence of holding back sharing concerns with family and friends among women newly diagnosed with gynecological cancer and evaluated whether the associations between holding back sharing concerns and psychological adaptation to gynecological cancer were moderated by dispositional emotional expressivity and perceived unsupportive behavior on the part of family and friends. Results indicated that holding back disclosing to family and friends was relatively common – across domains, the average rating was slightly below “sometimes”. Concerns about disease progression/death and sexual functioning were most likely to be held back, and concerns about cancer treatment and job-related concerns were less likely to be held back. Our results are partially consistent with Porter and colleagues [32], who also reported that patients held back more from disclosing concerns related to disease progression and death as compared with job-related concerns. Our results are also consistent with our previous work with men with localized prostate cancer which suggests that patients were most likely to hold back from sharing concerns about sexual functioning and about their worries about disease progression/death [50].
Given that concerns about disease progression and death are the highest-rated fears among women with gynecological cancers (M = 3.4 on a 5 point scale; Manne, unpublished data) and many studies suggest that fears of disease recurrence and death are common amongst ovarian cancer patients [51–55]. It is particularly important to document that patients hold back sharing these concerns so that oncology health care professionals are aware that patients experience these worries but do not share them with close others. Given the discomfort patients experience about discussing sexual functioning [56], it is not surprising that patients held back sharing their concerns about sexuality. However, given the fact that these concerns are also relatively prevalent in this population (M= 2.3; 3 = “ somewhat a concern”; Manne, unpublished data), oncology health care professionals may wish to be aware that these concerns are not being shared. However, it should be noted that oncology health care providers also do not discuss sexuality due to barriers such as discomfort or lack of resources [57].
It is interesting to note that when levels of holding back were compared with Porter et al.’s sample of gastrointestinal cancer patients [32] and with our prior work with men diagnosed with localized prostate cancer (Manne, unpublished data), our averages were higher. Porter and colleagues’ [32] average item means ranged from .75 to 1.76 and Manne and colleagues’ (unpublished data) average item means ranged from .76 to 1.57. It is possible that the difference between the present study and our work with localized prostate cancer patients (Manne, unpublished data) is due to the fact that the ovarian cancer is much more likely to recur than localized prostate cancer. However, a similar proportion of Porter and colleagues’ [32] sample was diagnosed with advanced disease and thus it is not clear whether this explains the difference. It is possible gender differences explain the difference; the current sample was comprised of women, whereas Porter and colleagues’ sample [32] was primarily comprised of men.
The finding that women who held back sharing cancer concerns and were more dispositionally emotionally expressive reported more cancer-specific distress and lower cancer-related well-being supports our prediction, and the hypothesis that a match between relevant personal characteristics, such as dispositional expressivity, and social processing strategies is important was confirmed. This work is consistent with other studies evaluating the moderating role of dispositional emotional expressivity and cancer–related emotional expression. However, one difference between the present study and other studies is that these studies evaluated cancer-related emotional expression [33] or perceived social constraints [58], and the present study examined holding back sharing cancer concerns. Agustdottir and colleagues [58] evaluated the role of emotional expressivity as a moderator of the associations between perceived social constraints and distress among prostate cancer survivors, and found that greater constraints were associated with greater distress among survivors with higher levels of dispositional emotional expressivity.
It is interesting to note that the moderator effects were not consistent across all outcomes. We did not find a moderating effect when depressive symptoms and physical quality of life were evaluated, suggesting that the moderating effects for dispositional expressivity on psychosocial outcomes may be specific to emotional adjustment to cancer. This finding is in contrast to prior work by Stanton and Low [33] and Agustdottir and colleagues [58] who reported a moderating effect for dispositional expressivity and emotional expression or social constraints on depressive and anxiety symptoms. Future research should evaluate possible differences in moderating effects across domains of psychosocial and physical functioning and explore possible reasons for such differences.
Although holding back was associated with greater distress, as well as higher levels of friend and family unsupportive responses, holding back was not more strongly associated with distress when patients felt their social network was unsupportive. Holding back was associated with greater global and cancer-specific distress even when friend and family unsupportive responses were low. Thus, our contention that this environmental/social barrier for holding back may play a role in the effects of this processing activity was not supported. One possible explanation is that perceived negative responses are not the primary motivator of holding back. One relevant construct is protective buffering, which is defined as holding back disclosing concerns to shield others from worry or avoid topics that may cause upset [59–63]. Studies suggest that protective buffering is associated with greater psychological distress among female cancer patients [43, 64–65]. Patients in this study may have held back sharing because they did not want to burden their family and friends. Holding back sharing, even when one’s network may be receptive to such disclosure (or at least not unreceptive), may be detrimental to adaptation to cancer. Future research might evaluate other social network responses, such as the holding back and self-disclosure on the part of family and friends. It is possible that the match between patient and social network may be important for these two variables. For example, Hagedoorn and colleagues [66] reported that high levels of colorectal cancer patients’ partners’ self-disclosure to patients who disclosed few emotions and concerns was associated with more distress for both patient and partner. Thus, the match between the patients’ holding back and social responses as assessed from network members (whether others respond with mutual disclosure or hold back) could be an important consideration. Overall, our results suggest that holding back was associated with greater distress, depression, and lower emotional and physical quality of life, which is consistent with the limited prior work assessing this construct in other cancer populations [gastrointestinal cancer; 32; localized prostate cancer; 50].
There are several limitations to our study. First, this is a cross-sectional study, and thus causality cannot be determined. It is possible that emotional distress resulted in greater holding back. Future research should evaluate these associations using longitudinal data. Second, most of the participants were white and well-educated and the majority was diagnosed with ovarian cancer and had metastatic disease. It is possible that the role of dispositional emotional expressivity on associations between holding back and distress and well-being may differ among minority women and women with less education, as well as among women with early stage cancers. Third, all measures were self-report, which has inherent biases, particularly with regard to holding back. Supplementing objective assessments of holding back using an experimental paradigm where participants have a discussion with a close other and subsequently rate the degree to which they held back sharing during that discussion with objective assessments of disclosure of concerns would enable the validation of self-report with actual interactions. Finally, the role of marital-specific variables such as holding back sharing from one’s spouse, unsupportive partner behaviors, and marital satisfaction were not evaluated. Spouse unsupportive responses have been shown to play a more important role than unsupportive responses from family and friends [67]. Further, holding back sharing concerns with one’s partner could be a sign of marital distress, and thus future work may wish to evaluate the role of marital distress.
In terms of clinical implications, it is important to consider individual differences in emotional expressivity when working with cancer patients. Emotion-based interventions, such as supportive expressive therapy, may be more effective for patients who naturally use expressive skills to deal with difficult life situations [68–69]. Encouraging inexpressive patients to express rather than hold back may not be a fit for the person’s natural coping style. Cognitive-behavioral interventions may be more effective for such patients, because emotional expression is not a significant aspect of this type of treatment. However, it should be noted that holding back was associated with distress even among those patients who were emotionally expressive, suggesting that for all patients, holding back may not be a beneficial processing strategy. Our findings highlight the importance of considering dispositional characteristics when considering how patients cope with cancer and suggest that it is important to assess self-reported holding back separately from perceived responses from one’s social environment.
Acknowledgments
We would like to acknowledge Tina Gadja, Sara Worhach, and Shira Hichenberg for study management, Joanna Crincoli, Katie O’Neill, Danielle Ryan, Arielle Schwerd, Kaitlyn Smith, Kristen Sorice, Amanda Viner, Rebecca Henderson, and Sloan Harrison for collection of study data. We thank the oncologists at all five centers for allowing their patients to participate as well as the nurses working in these practices. Finally, we thank the study participants for their time. This work was supported by grant #CA855066 from the National Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Sharon Manne, Cancer Institute of New Jersey
Shannon Myers, Cancer Institute of New Jersey
Melissa Ozga, Memorial Sloan Kettering Cancer Center
David Kissane, Memorial Sloan Kettering Cancer Center
Debby Kashy, Michigan State University
Stephen Rubin, University of Pennsylvania School of Medicine
Carolyn Heckman, Fox Chase Cancer Center
Norm Rosenblum, Jefferson Medical College of Thomas Jefferson University
References
- 1.American Cancer Society. Cancer Facts and Figures. Atlanta: American Cancer Society; 2007. http://www.cancer.org/acs/groups/content/@nho/documents/document/caff2007pwsecuredpdf.pdf. [Google Scholar]
- 2.American Cancer Society. Cancer Facts and Figures. Atlanta: American Cancer Society; 2007. http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-036845.pdf. [Google Scholar]
- 3.Aletti GD, Gallenberg MM, Cliby WA, Jatoi A, Hartmann LC. Current management strategies for ovarian cancer. Mayo Clin Proc. 2007 Jun;82(6):751–70. doi: 10.4065/82.6.751. [DOI] [PubMed] [Google Scholar]
- 4.Barton DP. The prevention and management of treatment related morbidity in vulval cancer. Best Pract Res Clin Obstet Gynaecol. 2003 Aug;17(4):683–701. doi: 10.1016/s1521-6934(03)00045-2. [DOI] [PubMed] [Google Scholar]
- 5.Bruner DW, Lanciano R, Keegan M, Corn B, Martin E, Hanks GE. Vaginal stenosis and sexual function following intracavitary radiation for the treatment of cervical and endometrial carcinoma. Int J Radiat Oncol Biol Phys. 1993 Nov 15;27(4):825–30. doi: 10.1016/0360-3016(93)90455-5. [DOI] [PubMed] [Google Scholar]
- 6.Corney RH, Everett H, Howells A, Crowther ME. Psychosocial adjustment following major gynaecological surgery for carcinoma of the cervix and vulva. J Psychosom Res. 1992 Sep;36(6):561–8. doi: 10.1016/0022-3999(92)90041-y. [DOI] [PubMed] [Google Scholar]
- 7.Del Priore G, Taylor SY, Esdaile BA, Masch R, Martas Y, Wirth J. Urinary incontinence in gynecological oncology patients. Int J Gynecol Cancer. 2005 Sep-Oct;15(5):911–4. doi: 10.1111/j.1525-1438.2005.00153.x. [DOI] [PubMed] [Google Scholar]
- 8.Frumovitz M, Sun CC, Schover LR, Munsell MF, Jhingran A, Wharton JT, et al. Quality of life and sexual functioning in cervical cancer survivors. J Clin Oncol. 2005 Oct 20;23(30):7428–36. doi: 10.1200/JCO.2004.00.3996. [DOI] [PubMed] [Google Scholar]
- 9.Huizing MT, van Warmerdam LJ, Rosing H, Schaefers MC, Lai A, Helmerhorst TJ, et al. Phase I and pharmacologic study of the combination paclitaxel and carboplatinas first -line chemotherapy in stage III and IV ovarian cancer. J Clin Oncol. 1997 May;15(5):1953–64. doi: 10.1200/JCO.1997.15.5.1953. [DOI] [PubMed] [Google Scholar]
- 10.Markman M, Kennedy A, Webster K, Peterson G, Kulp B, Belinson J. Combination chemotherapy with carboplatin and docetaxel in the treatment of cancers of the ovary and fallopian tube and primary carcinoma of the peritoneum. J Clin Oncol. 2001 Apr 1;19(7):1901–5. doi: 10.1200/JCO.2001.19.7.1901. [DOI] [PubMed] [Google Scholar]
- 11.Magrina JF, Goodrich MA, Weaver AL, Podratz KC. Modified radical hysterectomy: morbidity and mortality. Gynecol Oncol. 1995 Nov;59(2):277–82. doi: 10.1006/gyno.1995.0022. [DOI] [PubMed] [Google Scholar]
- 12.Michalas S, Rodolakis A, Voulgaris Z, Vlachos G, Giannakoulis N, Diakomanolis E. Management of early-stage cervical carcinoma by modified (Type II) radical hysterectomy. Gynecol Oncol. 2002 Jun;85(3):415–22. doi: 10.1006/gyno.2002.6633. [DOI] [PubMed] [Google Scholar]
- 13.Trimble EL, Adams JD, Vena D, Hawkins MJ, Friedman MA, Fisherman JS, et al. Paclitaxel for platinum-refractory ovarian cancer: results from the first 1,000 patients registered to National Cancer Institute Treatment Referral Center 9103. J Clin Oncol. 1993 Dec;11(12):2405–10. doi: 10.1200/JCO.1993.11.12.2405. [DOI] [PubMed] [Google Scholar]
- 14.Ries LAG, Melbert D, Krapcho M, Stinchcomb DG, Howlader N, Horner MJ, et al., editors. SEER Cancer Statistics Review, 1975–2005. Bethesda, MD: National Cancer Institute; 2008. Based on November 2007 SEER data submission, posted to the SEER web site. http://seer.cancer.gov/csr/1975_2005/ [Google Scholar]
- 15.Bodurka-Bevers D, Basen-Engquist K, Carmack CL, Fitzgerald MA, Wolf JK, de Moor C, et al. Depression, anxiety, and quality of life in patients with epithelial ovarian cancer. Gynecol Oncol. 2000 Sep;78(3 Pt 1):302–8. doi: 10.1006/gyno.2000.5908. [DOI] [PubMed] [Google Scholar]
- 16.Goncalves V, Jayson G, Tarrier N. A longitudinal investigation of psychological morbidity in patients with ovarian cancer. Br J Cancer. 2008 Dec 2;99(11):1794–801. doi: 10.1038/sj.bjc.6604770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kornblith AB, Thaler HT, Wong G, Vlamis V, Lepore JM, Loseth DB, et al. Quality of life of women with ovarian cancer. Gynecol Oncol. 1995 Nov;59(2):231–42. doi: 10.1006/gyno.1995.0014. [DOI] [PubMed] [Google Scholar]
- 18.Portenoy RK, Kornblith AB, Wong G, Vlamis V, Lepore JM, Loseth DB, et al. Pain in ovarian cancer patients. Prevalence, characteristics, and associated symptoms. Cancer. 1994 Aug 1;74(3):907–15. doi: 10.1002/1097-0142(19940801)74:3<907::aid-cncr2820740318>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 19.Boscaglia N, Clarke DM, Jobling TW, Quinn MA. The contribution of spirituality and spiritual coping to anxiety and depression in women with a recent diagnosis of gynecological cancer. Int J Gynecol Cancer. 2005 Sep-Oct;15(5):755–61. doi: 10.1111/j.1525-1438.2005.00248.x. [DOI] [PubMed] [Google Scholar]
- 20.Cain EN, Kohorn EI, Quinlan DM, Schwartz PE, Latimer K, Rogers L. Psychosocial reactions to the diagnosis of gynecologic cancer. Obstet Gynecol. 1983 Nov;62(5):635–41. [PubMed] [Google Scholar]
- 21.Lalos A, Eisemann M. Social interaction and support related to mood and locus of control in cervical and endometrial cancer patients and their spouses. Support Care Cancer. 1999 Mar;7(2):75–8. doi: 10.1007/s005200050230. [DOI] [PubMed] [Google Scholar]
- 22.Mantegna G, Petrillo M, Fuoco G, Venditti L, Terzano S, Anchora LP, et al. Long-term prospective longitudinal evaluation of emotional distress and quality of life in cervical cancer patients who remained disease-free 2-years from diagnosis. BMC Cancer. 2013;13:127. doi: 10.1186/1471-2407-13-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goncalves V, Jayson G, Tarrier N. A longitudinal investigation of posttraumatic stress disorder in patients with ovarian cancer. J Psychosom Res. 2011 May;70(5):422–31. doi: 10.1016/j.jpsychores.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 24.Yavas G, Dogan NU, Yavas C, Benzer N, Yuce D, Celik C. Prospective assessment of quality of life and psychological distress in patients with gynecologic malignancy: a 1-year prospective study. Int J Gynecol Cancer. 2012 Jul;22(6):1096–101. doi: 10.1097/IGC.0b013e3182559c03. [DOI] [PubMed] [Google Scholar]
- 25.Norton TR, Manne SL, Rubin S, Hernandez E, Carlson J, Bergman C, et al. Ovarian cancer patients’ psychological distress: the role of physical impairment, perceived unsupportive family and friend behaviors, perceived control, and self-esteem. Health Psychol. 2005 Mar;24(2):143–52. doi: 10.1037/0278-6133.24.2.143. [DOI] [PubMed] [Google Scholar]
- 26.Hipkins J, Whitworth M, Tarrier N, Jayson G. Social support, anxiety and depression after chemotherapy for ovarian cancer: a prospective study. Br J Health Psychol. 2004 Nov;9(Pt 4):569–81. doi: 10.1348/1359107042304542. [DOI] [PubMed] [Google Scholar]
- 27.Norton TR, Manne SL, Rubin S, Carlson J, Hernandez E, Edelson MI, et al. Prevalence and predictors of psychological distress among women with ovarian cancer. J Clin Oncol. 2004 Mar 1;22(5):919–26. doi: 10.1200/JCO.2004.07.028. [DOI] [PubMed] [Google Scholar]
- 28.Horowitz MJ. Stress response syndromes. 2. New York: Jason Aronson; 1986. [Google Scholar]
- 29.Janoff-Bulman R. Assumptive worlds and the stress of traumatic events: Applications of the schema construct. Soc Cog, Special Issue: Soc Cog and Stress. 1989;7:113–136. [Google Scholar]
- 30.Clark LF. Stress and the cognitive-conversational benefits of social interaction. J Soc Clin Psychol. 1993;12:25–55. [Google Scholar]
- 31.Harrison J, Maguire P, Pitceathly C. Confiding in crisis: gender differences in pattern of confiding among cancer patients. Soc Sci Med. 1995 Nov;41(9):1255–60. doi: 10.1016/0277-9536(94)00411-l. [DOI] [PubMed] [Google Scholar]
- 32.Porter LS, Keefe FJ, Hurwitz H, Faber M. Disclosure between patients with gastrointestinal cancer and their spouses. Psychooncology. 2005 Dec;14(12):1030–42. doi: 10.1002/pon.915. [DOI] [PubMed] [Google Scholar]
- 33.Stanton AL, Low CA. Dispositional and stressor-related emotion regulation in the context of a chronic, life-limiting stressor. J Pers. 2012 Apr;80(2):287–311. doi: 10.1111/j.1467-6494.2011.00732.x. [DOI] [PubMed] [Google Scholar]
- 34.Lazarus RS, Folkman S. Stress, appraisal, and coping. New York: Springer; 1984. [Google Scholar]
- 35.Cutrona CE, Russell D. Type of social support and specific stress: Toward a theory of optimal matching. In: Sarason IG, Sarason BR, Pierce GR, editors. Social Support: An Interactional View. New York: Wiley; 1990. pp. 319–366. [Google Scholar]
- 36.Quartana PJ, Laubmeier KK, Zakowski SG. Psychological adjustment following diagnosis and treatment of cancer: an examination of the moderating role of positive and negative emotional expressivity. J Behav Med. 2006 Oct;29(5):487–98. doi: 10.1007/s10865-006-9069-0. [DOI] [PubMed] [Google Scholar]
- 37.Zakowski SG, Valdimarsdottir HB, Bovbjerg DH. Emotional expressivity and intrusive cognitions in women with family histories of breast cancer: application of a cognitive processing model. Br J Health Psychol. 2001 May;6(Pt 2):151–65. doi: 10.1348/135910701169124. [DOI] [PubMed] [Google Scholar]
- 38.Lepore SJ, Silver RC, Wortman CB, Wayment HA. Social constraints, intrusive thoughts, and depressive symptoms among bereaved mothers. J Pers Soc Psychol. 1996;70(2):271–82. doi: 10.1037//0022-3514.70.2.271. [DOI] [PubMed] [Google Scholar]
- 39.Zubrod CG, Schneiderman M, Frei E, Brindley C, Gold GG, Dederick M, et al. Appraisal of methods for the study of chemotherapy of cancer in man: comparative therapeutic trial of nitrogen mustard and triethylene thiophosphoramide. J Chronic Diseases. 1960;11:17–33. [Google Scholar]
- 40.Pistrang N, Barker C. The partner relationship in psychological response to breast cancer. Soc Sci Med. 1995 Mar;40(6):789–97. doi: 10.1016/0277-9536(94)00136-h. [DOI] [PubMed] [Google Scholar]
- 41.King LA, Emmons RA. Conflict over emotional expression: psychological and physical correlates. J Pers Soc Psychol. 1990 May;58(5):864–77. doi: 10.1037//0022-3514.58.5.864. [DOI] [PubMed] [Google Scholar]
- 42.Manne S, Glassman M. Perceived control, coping efficacy, and avoidance coping as mediators between spouses’ unsupportive behaviors and cancer patients’ psychological distress. Health Psychol. 2000 Mar;19(2):155–64. doi: 10.1037//0278-6133.19.2.155. [DOI] [PubMed] [Google Scholar]
- 43.Manne SL, Pape SJ, Taylor KL, Dougherty J. Spouse support, coping, and mood among individuals with cancer. Ann Behav Med. 1999 Spring;21(2):111–21. doi: 10.1007/BF02908291. [DOI] [PubMed] [Google Scholar]
- 44.Horowitz M, Wilner N, Alvarez W. Impact of Event Scale: a measure of subjective stress. Psychosom Med. 1979 May;41(3):209–18. doi: 10.1097/00006842-197905000-00004. [DOI] [PubMed] [Google Scholar]
- 45.Baider L, Andritsch E, Uziely B, Goldzweig G, Ever-Hadani P, Hofman G, et al. Effects of age on coping and psychological distress in women diagnosed with breast cancer: review of literature and analysis of two different geographical settings. Crit Rev Oncol Hematol. 2003 Apr;46(1):5–16. doi: 10.1016/s1040-8428(02)00134-8. [DOI] [PubMed] [Google Scholar]
- 46.Manne S, Badr H. Intimacy and relationship processes in couples’ psychosocial adaptation to cancer. Cancer. 2008 Jun 1;112(11 Suppl):2541–55. doi: 10.1002/cncr.23450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961 Jun;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 48.Beck AT, Rush AJ, Shaw BF, Emery G. Cognitive Therapy of Depression. New York: Guilford; 1979. [Google Scholar]
- 49.Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993 Mar;11(3):570–9. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 50.Manne SL, Kissane DW, Nelson CJ, Mulhall JP, Winkel G, Zaider T. Intimacy-enhancing psychological intervention for men diagnosed with prostate cancer and their partners: a pilot study. J Sex Med. 2011 Apr;8(4):1197–209. doi: 10.1111/j.1743-6109.2010.02163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bodurka-Bevers D, Basen-Engquist K, Carmack CL, Fitzgerald MA, Wolf JK, de Moor C, et al. Depression, anxiety, and quality of life in patients with epithelial ovarian cancer. Gynecol Oncol. 2000;78(3 Pt 1):302–8. doi: 10.1006/gyno.2000.5908. [DOI] [PubMed] [Google Scholar]
- 52.Ferrell B, Ervin K, Smith S, Marek T, Melancon C. Family perspectives of ovarian cancer. Cancer Pract. 2002;10(6):269–76. doi: 10.1046/j.1523-5394.2002.106001.x. [DOI] [PubMed] [Google Scholar]
- 53.Kornblith AB, Mirabeau-Beale K, Lee H, Goodman AK, Penson RT, Pereira L, et al. Long-term adjustment of survivors of ovarian cancer treated for advanced-stage disease. J Psychosoc Oncol. 2010;28(5):451–69. doi: 10.1080/07347332.2010.498458. [DOI] [PubMed] [Google Scholar]
- 54.Parker PA, Kudelka A, Basen-Engquist K, Kavanagh J, de Moor J, Cohen L. The associations between knowledge, CA125 preoccupation, and distress in women with epithelial ovarian cancer. Gynecol Oncol. 2006;100(3):495–500. doi: 10.1016/j.ygyno.2005.08.057. [DOI] [PubMed] [Google Scholar]
- 55.Wenzel LB, Donnelly JP, Fowler JM, Habbal R, Taylor TH, Aziz N, et al. Resilience, reflection, and residual stress in ovarian cancer survivorship: a gynecologic oncology group study. Psychooncology. 2002;11(2):142–53. doi: 10.1002/pon.567. [DOI] [PubMed] [Google Scholar]
- 56.McCallum M, Lefebvre M, Jolicoeur L, Maheu C, Lebel S. Sexual health and gynecological cancer: conceptualizing patient needs and overcoming barriers to seeking and accessing services. J Psychosom Obstet Gynaecol. 2012;33(3):135–42. doi: 10.3109/0167482X.2012.709291. [DOI] [PubMed] [Google Scholar]
- 57.Stead ML, Brown JM, Fallowfield L, Selby P. Lack of communication between healthcare professionals and women with ovarian cancer about sexual issues. Br J Cancer. 2003;88(5):666–71. doi: 10.1038/sj.bjc.6600799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Agustsdottir S, Kristinsdottir A, Jonsdottir K, Larusdottir SO, Smari J, Valdimarsdottir HB. The impact of dispositional emotional expressivity and social constraints on distress among prostate cancer patients in Iceland. Br J Health Psychol. 2010 Feb;15(Pt 1):51–61. doi: 10.1348/135910709X426148. [DOI] [PubMed] [Google Scholar]
- 59.Coyne JC, Smith DA. Couples coping with a myocardial infarction: a contextual perspective on wives’ distress. J Pers Soc Psychol. 1991 Sep;61(3):404–12. doi: 10.1037//0022-3514.61.3.404. [DOI] [PubMed] [Google Scholar]
- 60.Hinton J. Sharing or withholding awareness of dying between husband and wife. J Psychosom Res. 1981;25(5):337–43. doi: 10.1016/0022-3999(81)90045-3. [DOI] [PubMed] [Google Scholar]
- 61.Jamison KR, Wellisch DK, Pasnau RO. Psychosocial aspects of mastectomy: I. the women’s perspective. Am J Psychiatry. 1978 Apr;135(4):432–6. doi: 10.1176/ajp.135.4.432. [DOI] [PubMed] [Google Scholar]
- 62.Krant MJ. Caring for the terminally ill: expectations of patient and family. Hosp Med Staff. 1978 Feb;7(2):1–6. [PubMed] [Google Scholar]
- 63.Vess JD, Moreland JR, Schwebel AI, Kraut E. Psychosocial needs of cancer patients: learning from patients and their spouses. J Psychosoc Onc. 1989;6(1–2):31–51. [Google Scholar]
- 64.Manne S, Dougherty J, Veach S, Kless R. Hiding worries from one’s spouse: Protective buffering among Cancer patients and their spouses. Cancer Res Ther Control. 1999;8:175–88. [Google Scholar]
- 65.Manne S, Ostroff J, Winkel G, Grana G, Fox K. Partner Unsupportive Responses, Avoidance and Distress among Women with Early Stage Breast Cancer: Patient and Partner Perspectives. Health Psychol. 2005;24(6):635–41. doi: 10.1037/0278-6133.24.6.635. [DOI] [PubMed] [Google Scholar]
- 66.Hagedoorn M, Puterman E, Sanderman R, Wiggers T, Baas PC, van Haastert M, et al. Is self-disclosure in couples coping with cancer associated with improvement in depressive symptoms? Health Psychol. 2011 Nov;30(6):753–62. doi: 10.1037/a0024374. [DOI] [PubMed] [Google Scholar]
- 67.Manne S, Ostroff J, Sherman M, Glassman M, Ross S, Goldstein L, et al. Buffering effects of family and friend support on associations between partner unsupportive behaviors and coping among women with breast cancer. J Soc Pers Relat. 2003;20(6):771–92. [Google Scholar]
- 68.Manne S, Ostroff JS, Winkel G. Social-cognitive processes as moderators of a couple-focused group intervention for women with early stage breast cancer. Health Psychol. 2007 Nov;26(6):735–44. doi: 10.1037/0278-6133.26.6.735. [DOI] [PubMed] [Google Scholar]
- 69.Stanton AL, Kirk SB, Cameron CL, Danoff-Burg S. Coping through emotional approach: scale construction and validation. J Pers Soc Psychol. 2000 Jun;78(6):1150–69. doi: 10.1037//0022-3514.78.6.1150. [DOI] [PubMed] [Google Scholar]