Abstract
Since its initial identification as a HIV-1-inducible gene in 2002, astrocyte elevated gene-1 (AEG-1), subsequently cloned as metadherin (MTDH) and lysine-rich CEACAM1 coisolated (LYRIC), has emerged over the past 10 years as an important oncogene providing a valuable prognostic marker in patients with various cancers. Recent studies demonstrate that AEG-1/MTDH/LYRIC is a pleiotropic protein that can localize in the cell membrane, cytoplasm, endoplasmic reticulum (ER), nucleus, and nucleolus, and contributes to diverse signaling pathways such as PI3K–AKT, NF-κB, MAPK, and Wnt. In addition to tumorigenesis, this multifunctional protein is implicated in various physiological and pathological processes including development, neurodegeneration, and inflammation. The present review focuses on the discovery of AEG-1/MTDH/LYRIC and conceptualizes areas of future direction for this intriguing gene. We begin by describing how AEG-1, MTDH, and LYRIC were initially identified by different research groups and then discuss AEG-1 structure, functions, localization, and evolution. We conclude with a discussion of the expression profile of AEG-1/MTDH/LYRIC in the context of cancer, neurological disorders, inflammation, and embryogenesis, and discuss how AEG-1/MTDH/LYRIC is regulated. This introductory discussion of AEG-1/MTDH/LYRIC will serve as the basis for the detailed discussions in other chapters of the unique properties of this intriguing molecule.
1. INTRODUCTION
Astrocyte elevated gene-1 (AEG-1), also called metadherin (MTDH) and lysine-rich CEACAM1 coisolated (LYRIC), was first identified and cloned by subtraction hybridization of genes expressed at elevated levels in primary human fetal astrocytes (PHFAs) infected by human immunodeficiency virus-1 (HIV-1) (Su et al., 2002). Subsequently, three independent groups, in addition to ours, reported cloning and initial characterization of the full-length cDNA of AEG-1 between 2002 and 2005 (Britt et al., 2004; Brown & Ruoslahti, 2004; Kang et al., 2005; Su et al., 2002; Sutherland, Lam, Briers, Lamond, & Bickmore, 2004). AEG-1 is also referred to as MTDH, LYRIC, or 3D3/LYRIC in the literature depending on the context of its discovery and postulated function.
Association of AEG-1/MTDH/LYRIC with the cancer phenotype was evident from its initial characterization in which AEG-1/MTDH/LYRIC was shown to be overexpressed in various types of cancerous cell lines and a mediator of metastasis of murine breast cancer cells to the lungs (Brown & Ruoslahti, 2004; Kang et al., 2005). AEG-1/MTDH/LYRIC expression is elevated in almost all types of cancers and has been shown to enhance proliferation, survival, and metastatic capability of cancer cells through multiple mechanisms (Emdad et al., 2007; Sarkar et al., 2009; Ying, Li, & Li, 2011; Yoo, Emdad, et al., 2011). AEG-1/MTDH/LYRIC is also involved in glioma-associated neurodegeneration potentially through its ability to enhance glutamate excitotoxicity as seen in HIV-1-associated neuropathy (Lee et al., 2011). In addition to its tumor-promoting activity, AEG-1/MTDH/LYRIC is also implicated in migraine, inflammation, and development (Anttila et al., 2010; Jeon et al., 2010; Sarkar et al., 2008; Yoo, Emdad, et al., 2011).
AEG-1/MTDH/LYRIC participates in diverse signaling pathways culminating in multiple cellular responses. AEG-1/MTDH/LYRIC regulates signaling pathways including PI3K/AKT, NF-κB, MEK/ERK, and WNT/β-catenin and is regulated by c-Myc and vice versa, forming a positive feedback loop (Emdad et al., 2006; Lee, Su, Emdad, Sarkar, & Fisher, 2006; Yoo et al., 2009). It is hypothesized that the tumor-promoting activity of AEG-1/MTDH/LYRIC is a consequence of activation of defined signaling pathways and the positive feedback between AEG-1/MTDH/LYRIC and c-Myc. Moreover, AEG-1/MTDH/LYRIC appears to function in microRNA (miRNA) processing through interaction with SND1 (Staphylococcal nuclease and tudor domain containing 1), a component of RISC (RNA-induced silencing complex) (Yoo, Santhekadur, et al., 2011). What is very apparent is that AEG-1/MTDH/LYRIC associates with multiple biological phenomena and further research will undoubtedly identify a broad spectrum of processes correlated with and potentially regulated by AEG-1/MTDH/LYRIC.
Despite the canonical role of AEG-1/MTDH/LYRIC in tumor progression and potentially in development of other diseases, the molecular basis of the numerous functions of AEG-1/MTDH/LYRIC remains to be defined. Thus, this thematic volume is very timely to overview the current status of AEG-1/MTDH/LYRIC research in detail and to provide conceptual basis for new insights into AEG-1/MTDH/LYRIC functions. As a part of this undertaking, we are providing a brief chronological history and summary of the contexts of the initial clonings and characterization of AEG-1/MDTH/LYRIC. In addition, we describe the expression patterns of AEG-1/MTDH/LYRIC and its regulation, which are crucial for understanding the precise roles of AEG-1/MTDH/LYRIC in regulating normal and abnormal physiology.
2. INITIAL CLONING
Over the past two decades, transcriptome analysis has provided a robust method for expanding gene discovery. The advent of new methodologies, including microarrays and various forms of differential hybridization (including subtraction hybridization), has permitted the efficient identification of differentially expressed sequence tags (ESTs) in both normal and pathogenic conditions, as well as generating tissue-specific profiles. In conjunction with progress in various genome projects and full-length cDNA cloning projects, transcriptome analysis has expanded its scope from searches for ESTs to characterization and functional analysis of these ESTs. AEG-1/MTDH/LYRIC was reported as an outcome of successful application of the then novel function-based gene discovery tools including RaSH (rapid subtraction hybridization) (Jiang, Kang, Alexandre, & Fisher, 2000; Kang et al., 2005; Simm et al., 2001; Su et al., 2002), in vivo phage display screening (Brown & Ruoslahti, 2004), and gene-trap screening of localization-specific genes (Sutherland et al., 2004). Based on its differential expression in multiple contexts and its unique expression pattern, AEG-1/MTDH/LYRIC was chosen for further study. This first section focuses on the settings and methodologies of cloning AEG-1/MTDH/LYRIC and the initial characterization efforts in the context of our current knowledge on AEG-1/MTDH/LYRIC.
2.1. Astrocyte elevated gene-1
HIV-associated dementia (HAD) develops in about 20% of HIV-1-infected individuals (Gonzalez-Scarano & Martin-Garcia, 2005). HIV primarily infects microglia and rarely infects astrocytes but does not infect neurons in the central nervous system (Brack-Werner, 1999). Although HIV failed to efficiently infect astrocytes, infected astrocytes were still believed to participate in neuropathogenesis associated with HIV infection (Borjabad, Brooks, & Volsky, 2010; Wang et al., 2004). In an attempt to elucidate etiology of HIV-induced neurodegeneration, alteration of gene expression in HIV-infected astrocytes was analyzed by RaSH (Su, Chen, et al., 2003; Su et al., 2002). AEG-1 was among the 15 AEGs whose expression was upregulated in PHFA upon infection of HIV-1 or treatment with gp120 (Su et al., 2002).
Cloning AEGs and also astrocyte suppressed genes (ASGs) was accomplished by RaSH that was invented in 2000 for discovery of genes distinctly expressed between HIV-1-infected and uninfected PHFA (Jiang et al., 2000; Su, Chen, et al., 2003; Su et al., 2002; Su, Kang, et al., 2003). RaSH employs directional subtraction of PCR-amplified cDNA libraries and vector selection of uniquely expressing unsubtracted target fragments (Boukerche, Su, Kang, & Fisher, 2004; Kang, Jiang, Su, Volsky, & Fisher, 2002). RaSH is a very simple and efficient method and does not require large amounts of mRNA as does conventional subtractive cDNA library techniques. Since its first demonstration of proof of principle and successful application to cloning AEGs and ASGs, RaSH was also found to have utility in various experimental settings including gene discovery associated with HIV-resistance of T lymphocytes (Simm et al., 2001) and genes associated with metastasis (Boukerche et al., 2004).
AEG-1 is a late onset gene that is expressed 3 ~ 7 days after HIV-1 infection (Su, Chen, et al., 2003; Su et al., 2002). AEG-1 expression in PHFA is also increased by treatment with HIV envelope protein, gp120, and TNF-α and did not require productive infection of astrocytes (Su, Chen, et al., 2003; Su et al., 2002). The signaling pathways of AEG-1 induction in astrocytes by HIV-1 infection and treatment of gp120 or TNF-α requires clarification. Studies aimed to elucidate the signaling pathways may consider cross talk of CD4/CXCR4 and TNFR signaling as a starting point, since treatment of both gp120 and TNF-α increases AEG-1 expression in the astrocytes (Rehman & Wang, 2009; Xia et al., 2008).
Characterization of AEG-1 function was possible after AEG-1 full-length cDNA was cloned using the complete open reading frame (C-ORF) technique (Kang et al., 2005). The C-ORF technique was successfully applied to clone full-length cDNAs of unknown ESTs including mda-5 (melanoma differentiation associated gene-5) and hPNPaseold-35 (human polynucleotide phosphorylase) (Kang et al., 2002; Leszczyniecka et al., 2002). C-ORF utilizes a degenerate stem-loop annealing primer (dSLAP) that consists of a stem-loop structure and 3′ 12 random nucleotides (Kang & Fisher, 2005, 2007). The design of dSLAP is to promote annealing the primer to the 3′ end of reverse transcribed cDNAs and to provide primer site for second strand cDNA synthesis. Application of C-ORF yielded C-ORF of AEG-1, which could then be used for subsequent detailed functional characterization.
AEG-1 mRNA consists of 3611 nucleotides, excluding the poly A tail, and encodes a 582-amino acid protein with calculated molecular weight 64 kDa and pI 9.33 (Kang et al., 2005). Northern blotting indicated at least three transcripts for AEG-1 (9, 4, and 1.5 kb) that might be generated by alternative splicing and/or different transcription start sites. Antibody directed against whole AEG-1 protein detected two closely migrating bands of relative mobility ~86,000 in cell lysates from either untransfected or expression vector AEG-1-HA transfected cells. However, only the higher migrating band was detected in immunoblotting with anti-HA antibody. Since the HA-tag is located at the C-terminal end of the molecule, the lower migrating band is most probably generated by C-terminal truncation of AEG-1.
Membrane topology of AEG-1 is predicted as either type Ib or II and remains controversial. Although the topology of AEG-1 needs resolution, immunofluorescent microscopy with antibody against the whole AEG-1 protein clearly demonstrated colocalization of AEG-1 with the ER marker calreticulin and speckled distribution in the nucleus (Emdad et al., 2006; Kang et al., 2005). Localization on the plasma and the nuclear membranes was not obvious in these experiments.
The astrocytic glutamate transporter EAAT2 mediates uptake of the excitatory neurotransmitter glutamate that is responsible for neuronal transmission and is released in the synapses (Borjabad et al., 2010; Wang et al., 2004). Glutamate has the capacity to damage neurons by a process called glutamate excitotoxicity, if the glutamate remains at high levels in the synaptic cleft (Choi, 1988). HIV infection decreases EAAT2 expression in astrocytes followed by reduced uptake of glutamate (Wang et al., 2004). To investigate how glutamate transport is regulated by HIV-1 in astrocytes, we cloned the EAAT2 promoter (Su, Leszczyniecka, et al., 2003). We demonstrated that glutamate transport was regulated by HIV-1 at the EAAT2 promoter level and found that regulators of glutamate transport functioned by either enhancing or decreasing EAAT2 promoter activity (Lee et al., 2008; Rothstein et al., 2005; Su, Leszczyniecka, et al., 2003; Wang et al., 2004). To investigate the mechanism by which AEG-1 expression might affect EAAT2 promoter function, we used reporter assays. AEG-1 significantly decreased EAAT2 promoter activity, but not EAAT1 promoter activity, suggesting that AEG-1 association with HAD occurred by down-regulation of EAAT2 expression followed by reduction in glutamate transport (Kang et al., 2005; Lee et al., 2011).
AEG-1 expression was upregulated in glioma cells, which motivated us to examine its expression in additional types of cancer cells. AEG-1 was expressed higher in cancer cells in comparison with immortal normal counterparts (Kang et al., 2005). Subsequently, tumor-promoting activity of AEG-1 was examined by soft agar colony formation assay in immortalized melanocytes transfected with AEG-1 and/or T24 Ha-ras expression vectors (Kang et al., 2005). Expression of AEG-1 by itself and coexpression with T24 Ha-ras significantly increased soft agar colony formation of immortalized melanocytes, suggesting tumor-promoting activity of AEG-1 by itself and in collaboration with Ha-ras. Later, AEG-1 was found to be a downstream of oncogenic Ha-ras signaling pathway (Lee et al., 2006).
Expression of AEG-1 in astrocytes infected with HIV or treated with TNF-α or gp120 suggested a potential association of AEG-1 upregulation with HAD (Kang et al., 2005; Su, Chen, et al., 2003). Three key features of AEG-1, that is, localization, relationship with different cancers and HAD-association were determined in the initial efforts to characterize the molecule (Kang et al., 2005). Although the relationship between AEG-1 and neurodegeneration requires further investigation, its tumor-promoting activity has been well established by numerous critical studies (Yoo, Emdad, et al., 2011).
2.2. Metadherin
Although AEG-1 was found to be associated with cancer serendipitously after screening for genes overexpressed in HIV-infected astrocytes, MTDH, the murine ortholog of AEG-1, was cloned in an attempt to identify cell surface molecules mediating metastasis of cancer cells (Brown & Ruoslahti, 2004). A domain of MDTH was involved in lung metastasis of mouse 4T1 mammary tumors and designated as a lung-homing domain (LHD) (a.a. 378–440). AEG-1/MDTH was subsequently found to be overexpressed in human breast cancer (Hu et al., 2009; Kornegoor et al., 2012; Li et al., 2008; Tokunaga et al., 2012) and other cancers (reviewed in Yoo, Emdad, et al., 2011).
MDTH, metastasis adhesion protein, was identified using in vivo phage screening (Brown & Ruoslahti, 2004). Breast cancer predominantly metastasizes to lung, bone, brain, and liver. In order to find proteins implicated in metastasis of breast cancer cells to the lung, a phage display library was prepared with cDNAs enriched for secreted and transmembrane proteins of 4T1. Phage that specifically localized to the 4T1 lung metastases was selected and cloned for further analysis (Brown & Ruoslahti, 2004). MTDH was predicted to be a type II membrane protein the C-terminal of which is located in the extracellular space. The authors defined the location and function of the LHD of MTDH (Brown & Ruoslahti, 2004).
Since MTDH was cloned as a mediator of metastasis, its LHD was predicted to be located in the extracellular region as anticipated by motif analysis. Location of MTDH in cancer cells was investigated with various methods including FACS and immunofluorescent microscopy (Brown & Ruoslahti, 2004). FACS demonstrated extracellular localization of the LHD tagged with the Myc epitope. In addition, MTDH was found in the edge of impermeabilized cells, whereas it was dispersed in the cytoplasm of permeabilized cells, suggesting that localization of MTDH LHD is extracellular.
Expression in cancer cells and biological functions of MTDH LHD were verified (Brown & Ruoslahti, 2004). Expressing MTDH in HEK293T cells enhanced lung localization of the cells. In addition, knockdown of MTDH or anti-MTDH antibody inhibited lung metastasis of 4T1 cells. These experiments clearly demonstrated that MTDH can mediate lung metastasis of 4T1 breast cancer cells.
Based on the functional data providing compelling evidence for MTDH as a lung metastasis-promoting gene, MTDH became the official nomenclature for AEG-1 and LYRIC. A separate study also demonstrated that MTDH overexpression enhanced lung metastasis of human breast cancer cells (Hu et al., 2009). However, there remains a controversy about the topology of MTDH. Metastasis association of MTDH mandates type II topology of the protein. LHD of MTDH was shown to be extracellular by FACS and immunofluorescent microscopy, which strongly supports type II topology (Brown & Ruoslahti, 2004). However, type Ib topology of MTDH was also predicted and intracellular localization of its orthologs was confirmed by immunofluorescent microscopy (Britt et al., 2004; Kang et al., 2005; Sutherland et al., 2004). Thus, controversies on the membrane topology, localization, and dual functionality of AEG-1/MTDH remain to be resolved.
2.3. Lysine-rich CEACAM1 coisolated
Two additional groups of researchers reported an originally novel protein which they named LYRIC that stands for “lysine-rich CEACAM1 coisolated” (Britt et al., 2004) and 3D3/LYRIC (Sutherland et al., 2004). Unlike function-based cloning in the previous two attempts (Brown & Ruoslahti, 2004; Kang et al., 2005), LYRIC and 3D3/LYRIC were discovered through identification of proteins localized at tight junction and the nuclear subcompartment, respectively.
Rat LYRIC was identified as an interacting protein with CEACAM1, and the cDNA was cloned by expression library screening (Britt et al., 2004). The existence of a transmembrane domain (TMD), a nuclear localization signal (NLS), and a potential ATP/GTP-binding site was predicted from the deduced protein sequence. Organ-specific expression of the transcript was examined by Northern blotting analysis, which revealed two transcripts (3.5 and 2.7 kb) with organ-specific distribution. Three splice variants, which lack either of the two or both exons, were also cloned.
Although interaction of LYRIC with CEACAM1 was not pursued further, its colocalization with tight junction was analyzed intensively in various cells and under different growth conditions with a monoclonal antibody MAb 52.15 (Britt et al., 2004). Endogenous LYRIC displayed membrane localization around the cell perimeter, while FLAG-tagged LYRIC displayed diffuse cytoplasmic and perinuclear distribution. LYRIC showed a honeycomb staining pattern in epithelial cells. LYRIC was found colocalized with tight junction component ZO-1 in cultured epithelial cells, tissues, and tumor grafts that formed tight junctions. The LYRIC staining pattern was lost by disruption of the tight junction upon calcium removal and restored by reformation of the junction, which is quite compelling evidence for colocalization of LYRIC with ZO-1 at tight junctions.
Mouse 3D3/LYRIC was cloned independently from AEG-1 and MTDH in an attempt to screen proteins located at distinct subcompartments of the nucleus using a gene trapping approach (Sutherland et al., 2004). A reporter gene with an upstream splice acceptor site was transfected into target cells and allowed to fuse with trapped genes through splicing. Then, localization of the trapped gene was screened by staining for the reporter gene. The N-terminal of LYRIC was trapped and found to localize at the nuclear membrane as discrete patches. Northern blot analysis also revealed existence of multiple transcripts, and TMD of type Ib topology and an NLS were also predicted from the amino acid sequence.
By contrast, mouse 3D3/LYRIC showed a different localization from the rat one and even from that of mouse MTDH (Sutherland et al., 2004). The mouse 3D3/LYRIC was found localized at the ER and nucleus by immunofluorescent microscopy. Endogenous 3D3/LYRIC localized in the nucleolus and in unidentified intranuclear suborganelles in a spotty pattern. Further analysis revealed existence of LYRIC in dense fibrillar components of the nucleolus. Western blotting of protein samples from fractionated organelles confirmed nucleolar localization of 3D3/LYRIC. Furthermore, the smaller of the two 3D3/LYRIC bands detected by Western blotting of endogenous protein predominantly existed in the nucleolus. Moreover, a 3D3/LYRIC mutant lacking the TMD was also localized in the nucleolus. 3D3/LYRIC was suggested to be modified based on these results, which might be necessary for locating a transmembrane protein such as 3D3/LYRIC to the nuclear suborganelles including the nucleolus.
2.4. Confirmation of initial observations relative to AEG-1/MTDH/LYRIC
We briefly overviewed the four articles reporting the initial characterization results on AEG-1/MTDH/LYRIC (Britt et al., 2004; Brown & Ruoslahti, 2004; Kang et al., 2005; Sutherland et al., 2004). All four laboratories reported similar physicochemical properties of AEG-1/MTDH/LYRIC, including amino acid composition, calculated molecular weight, and pI and TMD. Existence of predicted NLS was described in two of the papers (Britt et al., 2004; Sutherland et al., 2004). Although the number and sizes of the transcripts and their tissue distribution were different, a ~3.5 kb mRNA band was reported in the various studies. Mobility of AEG-1/MTDH/LYRIC in SDS-PAGE was slower than expected probably due to the high pI value of the protein. Doublets around ~85 kDa that showed distinct organelle distribution were demonstrated in two reports (Kang et al., 2005; Sutherland et al., 2004).
Localization and membrane topology of AEG-1/MTDH/LYRIC are critical factors for proper functioning of the molecule. However, with respect to the localization and membrane topology of AEG-1/MTDH/LYRIC, there are remaining unresolved issues. Both type Ib and II membrane topology of AEG-1/MTDH/LYRIC are predicted (Britt et al., 2004; Brown & Ruoslahti, 2004; Kang et al., 2005; Sutherland et al., 2004). Type Ib topology with ER/nucleus localization supports tumor-promoting activity of AEG-1/MTDH/LYRIC by stimulating AEG-1/MTDH/LYRIC-associated signaling processes (Kang et al., 2005; Sutherland et al., 2004), whereas type II is preferred for the proposed metastasis-mediating role of this molecule (Brown & Ruoslahti, 2004). The mechanism underlying nuclear localization of AEG-1/MTDH/LYRIC requires further clarification to explain the nuclear functions of AEG-1/MTDH/LYRIC. Further experimentation is required to understand the dual topology and multi-functionality of AEG-1/MTDH/LYRIC. Existence of transcript variants and their functions is another issue requiring clarification.
An association between AEG-1/MTDH/LYRIC and cancer was suggested during the early studies of AEG-1/MTDH (Brown & Ruoslahti, 2004; Kang et al., 2005) and this assumption has now been firmly established through numerous in-depth subsequent studies (Emdad et al., 2007; Sarkar et al., 2009; Ying et al., 2011; Yoo, Emdad, et al., 2011). AEG-1/MTDH/LYRIC has been documented to function as an oncogene (Emdad et al., 2009). Understanding how this unique gene functions as an oncogene, including defining and characterizing its many potential interactive partners, will undoubtedly provide further insights into the mechanism of action of AEG-1/MTDH/LYRIC. This unique oncogenic property of AEG-1/MTDH/LYRIC and those studies confirming that suppressing expression of this gene inhibits various transformation-associated properties supports the possibility of AEG-1/MTDH/LYRIC as a significant drug target and diagnostic/prognostic marker, which was not anticipated during its initial cloning (Su et al., 2002).
3. STRUCTURE OF AEG-1/MTDH/LYRIC
AEG-1/MTDH/LYRIC cDNAs encode 582, 581, and 579 a.a. proteins of molecular weights ~64 kDa and pI ~9.3 in human, rat, and mouse, respectively (Britt et al., 2004; Brown & Ruoslahti, 2004; Kang et al., 2005; Sutherland et al., 2004). Sequence homology at nucleotide level of human AEG-1/MTDH/LYRIC cDNA to mouse and rat are 90% and 89%, respectively. Reported length of rat lyric cDNA (2234 bps) is much shorter than those of mouse (3575 bps) and human (3611 bps) at the 3′-untranslated region (UTR). Sequence identity and homology at protein level of human AEG-1/MTDH/LYRIC to mouse are 91% and 94% and to rat are 90% and 94%, respectively (Fig. 1.1). AEG-1/MTDH/LYRIC is lysine-rich protein from which the name LYRIC was derived (Britt et al., 2004; Sutherland et al., 2004). The highest sequence mismatches are found at amino acids of 93–105, 135–155, and 515–531, and two of these areas of high sequence mismatches are located outside any known domains, although core sequences in the protein are conserved.
Figure 1.1.
Sequence alignment and posttranslational modification of AEG-1/MTDH/LYRIC. Amino acid sequences of AEG-1/MTDH/LYRIC of seven representative vertebrates were aligned by ClustralW2 (http://www.ebi.ac.uk/Tools/services/web/toolform.ebi? tool=clustalw2) and curated with BOXSHADE 3.21 (http://www.ch.embnet.org/soft ware/BOX_form.html). Identities in the alignment are shown in inverted characters and similarities in shaded ones. Running PhosphoSitePlus (http://www.phosphosite.org/homeAction.do) identified 13 posttranslational modification sites (*, phosphorylation; u, ubiquitination; a, acetylation). Transmembrane domain (TMD), nuclear localization signals (NLS-1, 2, and 3), SND1/NF-κB-interacting domain (SND1/NF-κB), and lung-homing domain (LHD) are demarked by boxes. Unmarked sites here for legibility are PLZF-interacting domains (a.a. 1–285 and 487–582) and a BCCIP-interacting domain (a.a. 72–169) of human AEG-1/MTDH/LYRIC.
Proteins with defined domains and motifs are readily amenable to functional analysis. When originally identified and cloned, AEG-1/MTDH/LYRIC was reported not to have any known functional domains and motifs except an N-terminal TMD, three NLSs, and a potential ATP/GTP-binding site (Britt et al., 2004; Brown & Ruoslahti, 2004; Kang et al., 2005; Sutherland et al., 2004). However, intensive functional studies on AEG-1/MTDH/LYRIC so far have defined at least five additional domains that participate in protein–protein interactions (Fig. 1.1) (Yoo, Emdad, et al., 2011).
AEG-1/MTDH/LYRIC was predicted to have a TMD at a.a. 51–72 in human and its membrane topology was considered as a type Ib or II (Britt et al., 2004; Brown & Ruoslahti, 2004; Kang et al., 2005; Sutherland et al., 2004). FACS analysis of MTDH tagged with Myc epitope at the LHD suggested extracellular distribution of the C-terminal, which supports type II topology (Brown & Ruoslahti, 2004). However, type Ib topology is supported by functional analysis of the molecule (Britt et al., 2004; Kang et al., 2005; Sutherland et al., 2004). Therefore, as indicated, the topology issue remains to be resolved. GFP-fusion protein of ΔTMD-LYRIC localized at the nucleolus and a protein smaller than full-length LYRIC was enriched in the nucleolus fraction, suggesting truncation of the molecule at around TMD for nuclear distribution (Sutherland et al., 2004). However, N-terminal including TMD (a.a. 1–71) was required for AEG-1/MTDH/LYRIC-induced NF-κB activation, although p65 subunit of NF-κB interacted at a.a. 101–205 of AEG-1/MTDH/LYRIC (Sarkar et al., 2008). In addition, a protease potentially targeting and releasing AEG-1/MTDH/LYRIC from the membrane has yet to be identified. Support for the nuclear function of AEG-1/MTDH/LYRIC, either in nucleolus or in nucleoplasm, requires additional experiments.
Three NLSs and a potential ATP/GTP-binding site were predicted for AEG-1/MTDH/LYRIC (Britt et al., 2004; Sutherland et al., 2004). Although NLSs in AEG-1/MTDH/LYRIC were well characterized in subsequent studies, binding of ATP/GTP to the predicted sites remains to be confirmed. AEG-1/MTDH/LYRIC distributes both in the cytoplasm, specifically in the ER, and nucleus, and the three putative NLSs in AEG-1/MTDH/LYRIC (NLS-1, 2, and 3 at a.a. 79–91, 432–451, and 561–580, respectively) appear to function differently in the nuclear localization of AEG-1/MTDH/LYRIC. NLS-3 (a.a. 546–582) is a primary determinant of AEG-1/MTDH/LYRIC nuclear localization, while an extended NLS-1 (a.a. 78–130) regulates its nucleolar localization (Thirkettle, Girling, et al., 2009). AEG-1/MTDH/LYRIC is ubiquitinated at NLS-2 extension (a.a. 415–486), and the NLS-2 ubiquitination is postulated to direct its cytoplasmic distribution that occurs in cancer cells (Thirkettle, Girling, et al., 2009).
AEG-1/MTDH/LYRIC multifunctionality might be ascribed to the existence of multiple binding domains with diverse proteins (Yoo, Emdad, et al., 2011). LHD defined in AEG-1/MTDH/LYRIC (a.a. 378–440) that confers metastatic capabilities of breast cancer cells to the lung might promote interaction of the cells with lung microvasculature, although its interacting partner has not been defined (Brown & Ruoslahti, 2004). AEG-1/MTDH/LYRIC translocates to the nucleus and activates NF-κB by its interaction with p65 subunit of NF-κB through a.a. 101–205 region in TNF-α treated cells. By interacting with cyclic AMP-response element-binding protein (CREB)-binding protein (CBP), AEG-1/MTDH/LYRIC facilitates NF-κB-CBP complex on the IL-8 promoter in inflammatory responses (Emdad et al., 2006; Sarkar et al., 2008). AEG-1/MTDH/LYRIC also interacts with sumoylated PLZF (promyelocytic leukemia zinc finger protein) through two regions (a.a. 1–285 and 487–582) (Thirkettle, Mills, Whitaker, & Neal, 2009). PLZF is an inhibitor of c-Myc transcription and interaction of AEG-1/MTDH/LYRIC with PLZF prevents its recruitment to the c-Myc promoter, which could result in upregulation of c-Myc transcription. BRCA2- and CDKN1A-interacting protein alpha (BCCIPα) that interacts with p21mda-6/cip1-CDK complex and enhances p21mda-6/cip1 activity is another binding partner of AEG-1/MTDH/LYRIC (Ash, Yang, & Britt, 2008). Interaction of BCCIPα with AEG-1/MTDH/LYRIC facilitates degradation of BCCIPα. AEG-1/MTDH/LYRIC was also found to bind SND1 and participate in RISC in a yeast two-hybrid screening (Yoo, Santhekadur, et al., 2011). Interaction with SND1 increased RISC activity in degradation of tumor suppressor mRNAs (Wang et al., 2012; Yoo, Santhekadur, et al., 2011). These interactions of AEG-1/MTDH/LYRIC with PLZF, BCCIPα, and SND1 could promote cell proliferation, which could be translated into tumor-promoting activity of AEG-1/MTDH/LYRIC in addition to a metastasis-mediating role.
In addition to the experimentally verified domains, numerous posttranslational modification sites are predicted and reported via high-throughput proteomic analysis (Hornbeck et al., 2012; Xue et al., 2008). Among the posttranslational modification sites curated in PhosphoSitePlus (http://www.phosphosite.org/homeAction.do), 13 residues are reported to be phosphorylated (nine sites), ubiquitinated (three sites), or acetylated (one site) more than five times in high-throughput mass spectrometry as indicated in Fig. 1.1 (Hornbeck et al., 2012). Two phosphorylation sites (S568 and T582) are well conserved in vertebrates, and evolutionary conservation is also verified for two ubiquitination sites (K150 and K185) and one acetylation site (K314). However, biological significance of the posttranslational modification remains to be investigated.
4. EVOLUTION OF AEG-1/MTDH/LYRIC
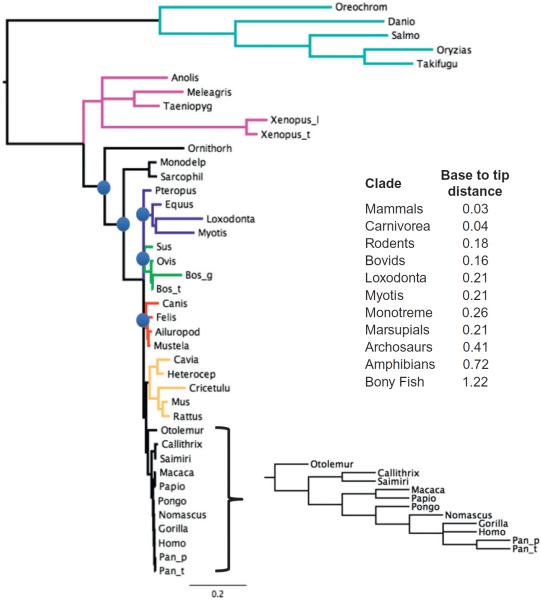
A phylogenetic analysis of DNA sequences for the AEG-1/MTDH/LYRIC gene from a wide range of vertebrates is shown in Fig. 1.2. This tree was generated by Bayesian inference using a GTR-Invariants-Gamma model with 1 million generations and 20% burn-in (Ronquist et al., 2012). There are three immediately obvious and important observations that can be made from this tree and a consideration of the phylogenetic occurrence of the AEG-1/MTDH/LYRIC gene in animals (Fig. 1.2). First, these are the only major animal taxa in the current whole genome sequence database where AEG-1/MTDH/LYRIC exists. This result indicates that AEG-1/MTDH/LYRIC arose in the common ancestor of all jawed vertebrates over 500 million years ago. The second striking result from this tree is the extremely long branches in fish (light blue group), amphibians and archosaurs (birds and reptiles—purple group), and in specific species scattered throughout the mammals. The only groups of mammals that do not show extremely long branches are the primates (black group) and the carnivores (red group). Usually, when this pattern emerges in phylogenetic analysis, it suggests a strong degree of purifying natural selection in the short branched clades in the tree. By quantifying the base to tip lengths of various clades (table inset in Fig 1.2), we suggest that the primates and carnivores have fourfold slower rates of change in their AEG-1/MTDH/LYRIC genes. Compared to archosaurs and bony fish, primates and carnivores have 10- to 30-fold differences in group depth. Some of these differences in rates of change are probably due to the age of the groups under consideration.
Figure 1.2.
A Bayesian phylogenetic tree showing the relationships of vertebrate AEG-1/MTDH/LYRIC genes. The model used in the analysis was a GTR with invariants gamma. One million generations were run, upon which convergence of chains was assessed to be adequate. The tree is the result of removing a burn-in of 20%. All nodes in the tree except for those marked by blue circles have posterior probabilities of 0.99 or better. The nodes marked by blue circles had posterior probabilities between 0.5 and 0.8, suggesting that these nodes are not particularly robust. The different colored branches in the tree indicate well established taxonomic groups except for the dark blue which contains elephants and bats and horse. The more conventionally accepted groups are as follows: light blue, bony fish; purple, archosaurs and amphibians; light green, bovids and close relatives; red, carnivores; orange, rodents; black, primates. The inset table shows the base to tip distance of the indicated groups in the tree.
5. EXPRESSION PROFILE
AEG-1/MTDH/LYRIC mRNA expression was ubiquitously detected in all human normal tissues with relatively higher expression in the heart, skeletal muscle, liver, and endocrine glands such as adrenal gland and thyroid (Kang et al., 2005). However, 10 years after its initial cloning, AEG-1/MTDH/LYRIC is now appreciated as an important oncogene overexpressed in various types of human cancers analyzed so far such as brain tumor, breast cancer, hepatocellular carcinoma (HCC), colorectal cancer, neuroblastoma, non-small cell lung cancer (NSCLC), etc., and its overexpression in tumor cells enhances characteristics of malignant aggressiveness including increased tumor growth, invasion and metastasis, angiogenesis, and chemoresistance (Yoo, Emdad, et al., 2011). In addition, recent studies have shown differential expression patterns of AEG-1/MTDH/LYRIC in other physiological and pathological processes (Yoo, Emdad, et al., 2011). In this section, we review the expression patterns of AEG-1/MTDH/LYRIC in various physiological and pathological circumstances.
5.1. Cancer
5.1.1 Brain tumors and neuroblastoma
Recent studies compared the expression of AEG-1/MTDH/LYRIC in various types of brain tumors (Emdad et al., 2010; Lee et al., 2011; Liu et al., 2010; Xia et al., 2010). Western blotting analysis in 98 patient samples of various brain tumors (25 glioblastoma multiforme (GBM), 18 astrocytoma, 18 meningioma, 19 oligodendroglioma, and 18 other types such as ependymoma, ganglioglioma, peripheral neural sheath tumor, etc.) compared to nine normal brain tissues indicated a 3- to 10-fold higher expression of AEG-1/MTDH/LYRIC in >90% cases (Emdad et al., 2010). Immunofluorescence staining in 33 GBM patient tissues, 2 grade III astrocytoma tissues, and 5 normal brain samples in a tissue microarray revealed higher expression of AEG-1/MTDH/LYRIC in the patient tissues (Lee et al., 2011). In addition, expression of AEG-1/MTDH/LYRIC in various glioma cell lines was elevated compared to that in primary normal astrocytes and oligodendroglial cells (Emdad et al., 2010; Liu et al., 2010; Xia et al., 2010). Immunohistochemical analysis of AEG-1/MTDH/LYRIC in 296 archived glioma patient tissues including 39 grade I cases, 121 grade II cases, 88 grade III cases, and 48 grade IV cases compared with that in normal tissues indicated that AEG-1/MTDH/LYRIC was upregulated in 89.5% of patients with a significant correlation with clinicopathologic grades of glioma (Liu et al., 2010). In oligodendroglioma, both mRNA and protein levels of AEG-1/MTDH/LYRIC were elevated in patient samples and oligodendroglioma cell lines compared to adjacent noncancerous brain tissues and primary oligodendroglial cells, and immunohistochemical analysis of 75 patient samples showed increased AEG-1/MTDH/LYRIC expression in 68% of cases with a significant correlation with histological grades of oligodendroglioma and with shorter survival time (Xia et al., 2010). Furthermore, the increased expression of AEG-1/MTDH/LYRIC played a critical role in invasion of glioma cells through activation of matrix metalloproteinases (MMP-2 and MMP-9) (Emdad et al., 2010; Liu et al., 2010). In neuroblastoma, the most common sympathetic nervous tumor, the increased expression of AEG-1/MTDH/LYRIC was observed in 6 of 10 patient samples and neuroblastoma cell lines compared to normal peripheral nerve tissues and normal cell counterparts (Lee et al., 2009). Analysis of AEG-1/MTDH/LYRIC in more archived neuroblastoma patient tissues (32 patients) indicated that AEG-1/MTDH/LYRIC expression was present in all samples with higher expression in 75% cases and was strongly correlated with various clinicopathologic stages and reduced survival of patients (Liu, Liu, Han, Zhang, & Sun, 2011). These studies have documented that AEG-1/MTDH/LYRIC expression is elevated in various neoplasms of the nervous system indicating its critical role in tumor invasion and suggest that it would be worthwhile systematically investigating its roles in other tumor types of the nervous system.
5.1.2 Head and neck tumors
Three independent recent studies revealed increased AEG-1/MTDH/LYRIC expression as a novel prognostic marker for salivary gland carcinoma and tongue carcinoma progression and patient survival (Deng & Feng, 2011; Ke et al., 2012; Liao et al., 2011). Immunohistochemical analysis of AEG-1/MTDH/LYRIC in tissues of 141 salivary gland carcinoma patients (Liao et al., 2011), 45 tongue squamous cell cancer patients (Deng & Feng, 2011), and 93 tongue squamous cell carcinoma patients (Ke et al., 2012) showed its higher expression than that in each peritumoral normal tissues, and the increased AEG-1/MTDH/LYRIC expression was strongly correlated with the clinicopathologic classifications and poor survival of the patients. In addition, the increased expression of AEG-1/MTDH/LYRIC was detected at the level of mRNA in the cancer patients (Ke et al., 2012; Liao et al., 2011).
5.1.3 Breast cancer
One of the first tumor types in which increased AEG-1/MTDH/LYRIC expression was appreciated was breast cancer (Brown & Ruoslahti, 2004). The group who cloned AEG-1/MTDH/LYRIC as MTDH demonstrated that AEG-1/MTDH/LYRIC is overexpressed in breast cancer cell lines, breast cancer patient tissues, and breast tumor xenografts, and that its overexpression is related with lung metastasis of breast cancer (Brown & Ruoslahti, 2004). Many subsequent studies with over 1000 breast cancer patient tissues from six independent groups including both female and male noticed that AEG-1/MTDH/LYRIC overexpression was detected in more than 40% of patient tissues compared to normal tissues and was strongly correlated with the clinical staging and various pathological classifications of breast cancer indicating its roles in aggressive tumor growth, chemoresistance, invasion and metastasis, and angiogenesis, as well as with poor survival of the patients (Hu et al., 2009; Kornegoor et al., 2012; Li et al., 2009, 2008; Li, Li, et al., 2011; Su, Zhang, & Yang, 2010; Tokunaga et al., 2012). Increased AEG-1/MTDH/LYRIC expression in clinical tissues was detected at the level of both mRNA and protein, and genomic amplification has been reported as one of the mechanisms by which AEG-1/MTDH/LYRIC is overexpressed in breast cancer (Hu et al., 2009; Kornegoor et al., 2012; Li et al., 2008; Tokunaga et al., 2012). Full-length sequencing of AEG-1/MTDH/LYRIC using 108 breast cancer patient tissues and 100 normal tissues discovered nine novel variants of AEG-1/MTDH/LYRIC, and two of them were associated with the susceptibility of breast cancer, even though further studies with the variants are required (Liu, Zhang, et al., 2011). These results clearly suggest that AEG-1/MTDH/LYRIC overexpression is a valuable marker of breast cancer progression associated with poor survival of the patients.
5.1.4 Non-small cell lung cancer
The clinical importance of AEG-1/MTDH/LYRIC was also determined in NSCLC. Overexpression of AEG-1/MTDH/LYRIC was detected in NSCLC cell lines and patient tissues at the levels of both mRNA and protein, and immunohistochemical staining of AEG-1/MTDH/LYRIC in 267 NSCLC patient tissues revealed the expression of AEG-1/MTDH/LYRIC mostly in the cytoplasm of the tissues, and a strong correlation of AEG-1/MTDH/LYRIC overexpression was evident with clinicopathologic characteristics of NSCLC and poor clinical outcome of the patients (Song et al., 2009; Sun et al., 2012). In addition, analyzing mRNA expression of 11 genes involved in the EGFR and NF-κB pathways in 60 metastatic NSCLC patients and lung cancer cell lines revealed that AEG-1/MTDH/LYRIC expression was correlated with BRCA1 expression and the overexpression of both genes was associated with poor survival of the patients, suggesting that the combination of AEG-1/MTDH/LYRIC and BRCA1 expression could serve as a potential prognostic marker for the disease (Santarpia et al., 2011).
5.1.5 Liver and gallbladder cancers
Expression of AEG-1/MTDH/LYRIC was significantly higher in HCC patient tissues including hepatitis B virus-related HCC patients and HCC cell lines than in normal hepatocytes at the levels of both mRNA and protein, where the protein was mostly localized at the perinuclear region of the HCC cells. Moreover, its overexpression was correlated with clinicopathologic characters including cell proliferation, invasion, metastasis, chemoresistance, and angiogenesis, as well as with poor clinical outcome of the patients (Gong et al., 2012; Yoo et al., 2009; Zhou, Deng, et al., 2012; Zhu et al., 2011). AEG-1/MTDH/LYRIC overexpression in HCC was associated with elevated copy number and was correlated with regulation of epithelial–mesenchymal transition (EMT) markers, suggesting a mechanism of AEG-1/MTDH/LYRIC overexpression in HCC and its role in HCC metastasis by inducing EMT (Yoo et al., 2009; Zhu et al., 2011). Two independent groups also investigated the expression of AEG-1/MTDH/LYRIC in gallbladder carcinoma (GBC) (Liu & Yang, 2011; Sun et al., 2011). They found that AEG-1/MTDH/LYRIC expression was higher in GBC patient tissues and GBC cell lines than in normal counterpart tissues and cells at the level of both mRNA and protein, and its overexpression in patients was strongly correlated with cancer progression including proliferation, differentiation, and metastasis, and with poor prognosis of the patients (Liu & Yang, 2011; Sun et al., 2011).
5.1.6 Renal cancer
Expression of AEG-1/MTDH/LYRIC in renal cell carcinoma (RCC) patient tissues and RCC cell lines was markedly higher than that in the paired normal tissues and normal kidney cells at both mRNA and protein levels, and immunohistochemical analysis in 106 cases of RCC patient tissues showed overexpression of AEG-1/MTDH/LYRIC in 96 cases (94.1%) (Chen, Ke, Shi, Yang, & Wang, 2010). Statistical analysis of the results indicated a significant correlation of AEG-1/MTDH/LYRIC expression with tumor grade, clinical staging, and tumor progression including metastasis as well as with poor clinical outcome of the patients.
5.1.7 Bladder and prostate cancers
Prostate cancer is another first tumor type in which overexpression of AEG-1/MTDH/LYRIC was examined (Kikuno et al., 2007). Immunohistochemical staining of AEG-1/MTDH/LYRIC revealed its overexpression in 16 of 20 prostate cancer patient samples compared to benign prostatic hyperplasia cases (Kikuno et al., 2007). Another independent study using a large cohort of 206 patients confirmed higher expression of AEG-1 in prostate cancer compared with benign tissue and found that AEG-1 predominantly localized in the nucleus of benign prostatic hyperplasia tissues as well as in thyroid and lung while its cytoplasmic expression was predominantly detected in prostate cancer tissues (Thirkettle, Girling, et al., 2009). In addition, when compared with normal bones, 9 of 11 prostate bone metastases showed increased AEG-1/MTDH/LYRIC expression, which was exclusively distributed in the cytoplasm and membrane (Thirkettle, Girling, et al., 2009). These results indicated that increased expression and significant changes in the distribution of AEG-1/MTDH/LYRIC can predict Gleason grade and patient survival of prostate cancer. A recent study also found that significant levels of AEG-1/MTDH/LYRIC expression was detected in 65% of bladder cancer patients, but not in normal bladder tissues, and that increased expression of AEG-1/MTDH/LYRIC in bladder cancer tissues was correlated with tumor grade, clinical staging, and tumor progression as well as with poor clinical outcome of the patients (Zhou, Li, Wang, Yin, & Zhang, 2012).
5.1.8 Ovary and endometrium cancers
Recent studies have also investigated the expression of AEG-1/MTDH/LYRIC in ovarian cancer, the most lethal gynecological cancer (Borley, Wilhelm-Benartzi, Brown, & Ghaem-Maghami, 2012; Li et al., 2012; Li, Liu, et al., 2011; Meng, Luo, Ma, Hu, & Lou, 2011). Immunohistochemical staining of AEG-1/MTDH/LYRIC in 81 ovarian cancer patient tissues revealed that high expression of AEG-1/MTDH/LYRIC was detected in 66.7% of ovarian cancer patients in association with clinicopathologic features of the cancer and poor overall survival of the patients when compared with patients with lower expression of AEG-1/MTDH/LYRIC (Meng et al., 2011). Another study using 157 epithelial ovarian cancer patient tissues found that AEG-1/MTDH/LYRIC overexpression was detected in 87 patients and 95.4% and 47.1% of the patients with AEG-1/MTDH/LYRIC overexpression presented, respectively, peritoneal dissemination and lymph node metastasis, indicating AEG-1/MTDH/LYRIC as a valuable clinical marker for predicting ovarian cancer metastasis (Li, Liu, et al., 2011). In addition, immunohistochemical study with 131 stage III-IV ovarian serous carcinoma patient tissues indicated that AEG-1/MTDH/LYRIC overexpression was associated with poor prognosis and cisplatin resistance in advanced serous ovarian cancer (Li et al., 2012). A systemic review using 30 studies from the Cancer Genome Atlas ovarian cancer data sets also noticed that expression of only AEG-1/MTDH/LYRIC and insulin-like growth factor-1 receptor was significantly correlated with surgical debulking of the ovarian cancer (Borley et al., 2012). Investigation of AEG-1 expression in 35 normal endometrium, 40 atypical endometrial hyperplasia tissues, and 274 endometrial cancer patient tissues showed that AEG-1/MTDH/LYRIC expression was gradually elevated in normal, atypical hyperplasia, and cancer tissues, and statistical analysis of the results indicated that overexpression of AEG-1/MTDH/LYRIC in the patients was significantly correlated with clinicopathological characteristics of endometrial cancer including stages, aggressive growth, invasion, and metastasis as well as with poor overall survival and disease-free survival compared with patients with lower AEG-1/MTDH/LYRIC expression (Song, Li, Lu, Zhang, & Geng, 2010). Together these results suggest that AEG-1/MTDH/LYRIC overexpression is a valuable prognostic marker for two types of cancers in the female genital tract.
5.1.9 Tumors in gastrointestinal tract
Overexpression of AEG-1/MTDH/LYRIC was associated with tumor progression and prognosis in cancers of the esophagus and stomach (Jian-bo et al., 2011; Yu et al., 2009). Expression of AEG-1/MTDH/LYRIC was markedly increased in esophageal cancer cell lines and surgical esophageal squamous cell carcinoma (ESCC) patient tissues compared with each normal counterpart at both transcriptional and translational levels (Yu et al., 2009). Immunohistochemical analysis and statistical analysis of AEG-1/MTDH/LYRIC expression in ESCC patient samples further revealed that over-expression of AEG-1/MTDH/LYRIC was detected in 47.6% cases in correlation with clinical stages, pathological characters, and poor survival of the patients (Yu et al., 2009). Overexpression of AEG-1/MTDH/LYRIC was also found in 66 of 106 gastric cancer patients in association with TNM stage and Ki-67 proliferation index, and AEG-1/MTDH/LYRIC was mainly localized in the cytoplasm of primary gastric cancer cells (Jian-bo et al., 2011). Furthermore, overexpression of AEG-1/MTDH/LYRIC was significantly associated with poor survival of the gastric cancer patients as an independent prognostic factor for the disease (Jian-bo et al., 2011). Colon cancer is another tumor type in the gastrointestinal tract associated with over-expression of AEG-1/MTDH/LYRIC (Yoo, Emdad, et al., 2011). Investigation of AEG-1/MTDH/LYRIC expression at both mRNA and protein levels using over 1000 colon cancer patient tissues including adenomas and carcinomas and colon cancer cell lines compared with distant normal mucosa and adjacent nontumor tissues revealed that increased expression of AEG-1/MTDH/LYRIC in colon cancer was significantly associated with the advanced stages of various clinicopathological staging systems such as UICC stage, TNM classification, Duke's stage, etc., as well as with poor survival of the patients (Gnosa et al., 2012; Jiang, Zhu, Zhu, & Piao, 2012; Song, Li, Li, & Geng, 2010; Wang et al., 2012; Zhang et al., 2012). AEG-1/MTDH/LYRIC was mainly detected in the cytoplasm of colon cancer cells, but nuclear staining of AEG-1/MTDH/LYRIC was more common in lesions with more advanced disease stages (Song, Li, Li, et al., 2010). In addition, a positive correlation in the context of increased expression or direct interaction of AEG-1/MTDH/LYRIC with b-catenin or SND1 in colon cancer patients indicated the mechanisms by which AEG-1/MTDH/LYRIC is involved in colon cancer progression (Wang et al., 2012; Zhang et al., 2012).
5.1.10 Bone tumors
Expression of AEG-1/MTDH/LYRIC in osteosarcoma, the most common histological form of primary bone cancer, was examined using 62 osteosarcoma patient tissues and 20 normal bones (Wang et al., 2011). Statistical analysis of the data with immunohistochemical staining of AEG-1/MTDH/LYRIC in the tissues found that AEG-1 was overexpressed in osteosarcoma tissues in strong correlation with gender, clinical stages, classification, metastasis, differentiation, and poor survival of the patients (Wang et al., 2011).
5.1.11 Lymphoid neoplasms
Elevated expression of AEG-1/MTDH/LYRIC at both mRNA and protein levels was also evident in 76.7% cases of diffuse large B-cell lymphoma (DLBCL) and 80.6% cases of T-cell non-Hodgkin's lymphoma (T-NHL) patient tissues (Ge et al., 2012; Yan, Zhang, Chen, & Zhang, 2012). Furthermore, overexpression of AEG-1/MTDH/LYRIC in DLBCL cells increased expression and nuclear translocation of β-catenin as seen in colon cancer, and AEG-1/MTDH/LYRIC in T-NHL was mostly detected in the cytoplasm (Ge et al., 2012; Yan, Zhang, et al., 2012; Zhang et al., 2012).
5.1.12 Serum anti-AEG-1 autoantibody
As described above, overexpression of AEG-1/MTDH/LYRIC has been observed in various types of human cancer in association with poor clinical outcome. Another intriguing study recently reported the existence of anti-AEG-1 autoantibodies in the serum samples of 49% (248 of 483) cancer patients including patients with breast cancer, HCC, rectal cancer, lung cancer, or gastric cancer while no detection of AEG-1/MTDH/LYRIC antibody in the serum of 230 normal individuals (Chen et al., 2012). These results suggest that AEG-1/MTDH/LYRIC autoantibody in the serum could be a potential diagnostic biomarker for various cancer patients even though further studies are required.
5.2. Brain with neurological disorders
5.2.1 HIV-associated dementia and glioma-induced neurodegeneration
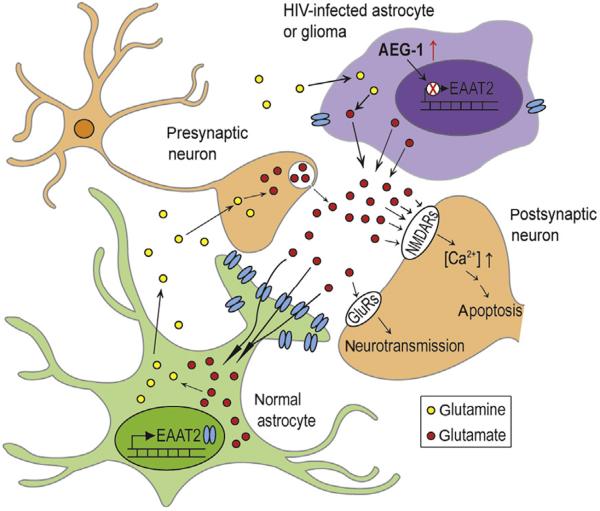
As described in the cloning section, AEG-1/MTDH/LYRIC was first identified as an elevated gene in PHFA infected with HIV-1, or treated with HIV-1 gp120 or TNF-α (Su, Chen, et al., 2003; Su et al., 2002). However, expression of AEG-1/MTDH/LYRIC in the HAD patients and the role of AEG-1/MTDH/LYRIC in HIV-related pathogenesis in the brain remain to be determined. Impaired glutamate uptake by aberrant EAAT2 expression in astrocytes induces glutamate excitotoxicity, which causes neuronal damage and various neurodegenerative diseases including HAD and glioma-induced neurodegeneration (Brack-Werner, 1999; Choi, 1988; Gonzalez-Scarano & Martin-Garcia, 2005; Kim et al., 2011; Su, Leszczyniecka, et al., 2003). Hyperactivation of glutamate receptors, specifically N-methyl-d-aspartate receptors (NMDARs), increases Ca2+ influx into the postsynaptic cell to activate apoptotic pathways (Choi, 1988; Duchen, 2012). Our groups reported that AEG-1/MTDH/LYRIC repressed the expression of EAAT2 which is an astroglial glutamate transporter responsible for glutamate clearance in the neuronal synapses, suggesting a potential role of AEG-1/MTDH/LYRIC in the disease by regulating glutamate excitotoxicity in the brain (Kang et al., 2005; Lee et al., 2011). In addition, expression of AEG-1/MTDH/LYRIC was increased in glioma patients, and the increased AEG-1/MTDH/LYRIC reduced EAAT2 expression and also caused a reduction of glutamate uptake by glial cells, resulting in neuronal cell death in the patients with glioma (Lee et al., 2011). Other groups also reported that glioma cells release excitotoxic levels of glutamate, which promotes neuronal cell death, peritumoral seizures and tumor-associated epilepsy, as well as malignant glioma progression (Watkins & Sontheimer, 2012). Together, these results suggest that overexpression of AEG-1/MTDH/LYRIC could be involved in HIV-associated dementia as well as in glioma-induced neurodegeneration by regulating EAAT2 expression as summarized in Fig. 1.3.
Figure 1.3.
Molecular mechanism of AEG-1 function in HAD- and glioma-induced neurodegeneration. The EAAT2 in normal astrocytes is responsible for clearing extracellular glutamate to minimize excitotoxicity, and astrocytes convert glutamate to glutamine via the glutamine synthetase. Increased AEG-1 in HIV-infected astrocytes and glioma reduces EAAT2 expression and boosts synaptic glutamate concentrations. Increased synaptic glutamate overactivates glutamate receptors (GluRs), especially NMDA receptors (NMDARs) in postsynaptic neurons, resulting in induction of high levels of Ca2+ influx and apoptotic cell death.
5.2.2 Huntington disease
Although role of AEG-1/MTDH/LYRIC was not determined in the pathogenesis of Huntington disease (HD), a recent study observed a significant increase of AEG-1/MTDH/LYRIC in the brain tissues of HD patients compared with normal brain tissues, its localization in the ER and nucleolus, and induction of AEG-1/MTDH/LYRIC by ER stress in neurons, suggesting a potential role of AEG-1/MTDH/LYRIC in HD-associated ER stress (Carnemolla et al., 2009). Further studies are required to confirm these observations and to define the mechanism involved in upregulation in HD.
5.2.3 Migraine
A recent genome-wide association study (GWAS) of migraine with 2731 cases from three European headache clinics and 10,747 population-matched controls established AEG-1/MTDH/LYRIC as the first genetic marker for the disease (Anttila et al., 2010). Another following GWAS with 2446 cases and 8534 controls in six population-based European cohorts revealed a modest gene-based significant association between migraine and AEG-1/MTDH/LYRIC (Ligthart et al., 2011). Although additional investigations are required to confirm, these results suggest that AEG-1/MTDH/LYRIC-mediated downregulation of EAAT2 may represent a mechanism by which AEG-1/MTDH/LYRIC contributes to the pathogenesis of migraine.
5.2.4 Reactive astrogliosis
A brain injury mouse model showed induction of AEG-1/MTDH/LYRIC in astrocytes at the wound sites, and the injury in human astrocytes induced specific compartmentalization into the nucleolus of AEG-1/MTDH/LYRIC (Vartak-Sharma & Ghorpade, 2012). In addition, the increased expression of AEG-1/MTDH/LYRIC was involved in the process of wound healing by inducing proliferation and migration of astrocytes. These results indicate a role of AEG-1/MTDH/LYRIC in mediating reactive astrogliosis as well as in regulating astrocyte responses to brain injury.
5.3. Inflammatory status
AEG-1/MTDH/LYRIC has been known to activate NF-κB, a key regulator of pro-inflammatory cytokines, suggesting a potential role of AEG-1/MTDH/LYRIC in inflammation, which is a crucial process in the pathogenesis of cancer progression and neurodegeneration (Sarkar et al., 2008; Yoo, Emdad, et al., 2011). A recent study found that AEG-1/MTDH/LYRIC was induced by lipopolysaccharide (LPS) via the NF-κB pathway in human promonocytic cells and also mediated the LPS-induced NF-κB activation and upregulation of TNFα and prostaglandin E2, suggesting a potential role of AEG-1/MTDH/LYRIC in LPS-induced inflammatory responses as a target gene of LPS (Khuda et al., 2009). In addition, AEG-1/MTDH/LYRIC was induced in the process of wound healing in response to brain injury, which pro-inflammatory cytokines and chemokines mediate during injury and the related disease progression (Vartak-Sharma & Ghorpade, 2012). Together these results suggest that AEG-1/MTDH/LYRIC could be a key factor inducing inflammatory responses for progression of various diseases.
5.4. Development
We recently analyzed the expression patterns of AEG-1/MTDH/LYRIC during mouse development (Jeon et al., 2010). AEG-1/MTDH/LYRIC was detected in mid-to-hindbrain, frontonasal processes, limbs, and pharyngeal arches in the early developmental period E9.5–10.5, and its expression was increased in the brain, olfactory and skeletal systems, skin, hair follicles, and the liver at specific stages during E12.5–18.5, indicating a prominent role of AEG-1/MTDH/LYRIC during normal development of the organs. Colocalization of AEG-1/MTDH/LYRIC with Ki-67 suggested its role in cell proliferation during embryogenesis.
6. REGULATION OF GENE EXPRESSION
6.1. Induction of AEG-1/MTDH/LYRIC
AEG-1/MTDH/LYRIC was originally identified as one of several elevated genes in PHFA infected with HIV-1 or treated with HIV-1 gp120 or TNF-α, suggesting that AEG-1/MTDH/LYRIC could be involved in HIV-mediated pathogenesis in the brain and that HIV-1, gp120, and TNF-α are inducers of AEG-1/MTDH/LYRIC (Su, Chen, et al., 2003; Su et al., 2002). In addition, TNF-α induced nuclear translocation of AEG-1/MTDH/LYRIC and its interaction with p65 resulted in NF-κB activation (Emdad et al., 2006). However, the mechanisms by which these inducers increase the expression of AEG-1/MTDH/LYRIC in astrocytes and its nuclear translocation have not been clarified yet. Another recent study using an in vivo mouse model of reactive astrogliosis using needle injection revealed increased AEG-1/MTDH/LYRIC expression in astrocytes comprising a glial scar tissue around the needle tract, indicating that brain injury/trauma induces AEG-1/MTDH/LYRIC expression in astrocytes during astrogliosis (Vartak-Sharma & Ghorpade, 2012). In an HD mouse model, tunicamycin, an ER stress inducer, increased AEG-1/MTDH/LYRIC expression in neurons, suggesting its role in the pathogenesis of HD (Carnemolla et al., 2009). Although interesting inducers of AEG-1/MTDH/LYRIC in astrocytes and neurons of the brain have been uncovered, further studies are required to clarify the mechanisms by which these inducers contribute to AEG-1/MTDH/LYRIC expression.
AEG-1/MTDH/LYRIC has been recognized as an emerging oncoprotein upregulated in various types of human cancers as described in Section 5.1. AEG-1/MTDH/LYRIC is upregulated in cancer by a variety of mechanisms. AEG-1/MTDH/LYRIC was uncovered as a downstream target of oncogenic H-ras, which is one of the most crucial proteins in cellular transformation, tumor progression, and metastasis (Lee et al., 2006). H-ras markedly induced AEG-1/MTDH/LYRIC through the PI3K–AKT signaling pathway augmenting binding of c-Myc to E-box elements in the AEG-1/MTDH/LYRIC promoter, resulting in regulation of AEG-1/MTDH/LYRIC transcription (Lee et al., 2006). These results suggested that AEG-1/MTDH/LYRIC is one of the crucial mediators of H-ras- and c-Myc-induced oncogenesis. Since H-ras, PI3K/AKT, and c-Myc function as oncogenes in a large variety of cancers, this regulatory mechanism might explain why AEG-1/MTDH/LYRIC is overexpressed in all types of cancers with gradual increase in its expression as the disease progresses. Another study found that hypoxia and glucose deprivation induced AEG-1/MTDH/LYRIC expression in glioma cells for cell survival (Noch, Bookland, & Khalili, 2011). The PI3K–AKT pathway mediated the hypoxic induction of AEG-1/MTDH/LYRIC through stabilization of HIF-1α. AEG-1/MTDH/LYRIC induction by glucose deprivation was dependent on reactive oxygen species (ROS), and the increased AEG-1/MTDH/LYRIC prevented ROS production. These results suggest that AEG-1/MTDH/LYRIC induction is necessary for cancer cell survival under stress conditions such as hypoxia and glucose deprivation (Noch et al., 2011).
As described in Section 5.3, LPS is another inducer of AEG-1/MTDH/LYRIC in human promonocytic cells (Khuda et al., 2009). LPS induced AEG-1/MTDH/LYRIC via the NF-κB pathway, and AEG-1/MTDH/LYRIC was also required for LPS-induced NF-κB activation, indicating a positive feedback loop between AEG-1/MTDH/LYRIC and NF-κB (Khuda et al., 2009). LPS-mediated AEG-1 induction and the positive feedback regulation between AEG-1/MTDH/LYRIC and NF-κB were also examined in breast cancer cells (Zhao et al., 2011). In addition, AEG-1/MTDH/LYRIC mediated the LPS-induced IL-8 and MMP-9 production, which are critical molecules in LPS-induced invasion and metastasis (Zhao et al., 2011). Since inflammation has been implicated in migration and metastasis of breast cancer cells, these results suggest that AEG-1/MTDH/LYRIC may be involved in inflammation-induced tumor progression (Zhao et al., 2011).
6.2. Repression of AEG-1/MTDH/LYRIC
Repression of AEG-1/MTDH/LYRIC has not been evident in any pathophysiological condition. Instead, increased AEG-1/MTDH/LYRIC in pathological conditions such as cancer indicates that AEG-1/MTDH/LYRIC could be a potential therapeutic target for these diseases. Two recent studies found that the potential anticancer agents ursolic acid and cryptotanshinone repressed expression of AEG-1/MTDH/LYRIC in ovarian cancer and prostate cancer, respectively (Lee et al., 2012; Song et al., 2012). The mechanism by which ursolic acid repressed AEG-1/MTDH/LYRIC expression was not clarified, but HIF-1α was a mediator in cryptotanshinone-induced AEG-1/MTDH/LYRIC repression (Lee et al., 2012; Song et al., 2012). In addition, cadmium chloride known to induce cell death in breast cancer cells reduced AEG-1/MTDH/LYRIC expression and NF-κB activity in breast cancer cells, suggesting AEG-1/MTDH/LYRIC downregulation as a mediator for cadmium chloride-induced breast cancer cell death (Luparello, Longo, & Vetrano, 2012). Although three repressors of AEG-1/MTDH/LYRIC were uncovered, more studies are required to find more repressors as potential anticancer drugs, and furthermore investigations to understand more detailed action mechanisms of these agents would be valuable for establishing a better screening system to find anticancer drugs targeting AEG-1/MTDH/LYRIC.
6.3. AEG-1/MTDH/LYRIC as a target of miRNAs
miRNAs are short noncoding RNAs of 20–24 nucleotides that play important roles in normal development, differentiation, growth control, and human diseases, and especially in the context of cancer pathogenesis, where miRNAs are generally working as tumor suppressors and are downregulated in cancer cells (Jansson & Lund, 2012). Five miRNAs have been recognized as repressors of AEG-1/MTDH/LYRIC expression. The first miRNA identified as a regulator of AEG-1/MTDH/LYRIC expression was miR-26a in breast cancer (Zhang et al., 2011). miR-26a repressed AEG-1/MTDH/LYRIC expression by directly targeting 3′ UTR of AEG-1/MTDH/LYRIC mRNA, and moreover, a similar inverse correlation between the expression levels of miR-26a and AEG-1/MTDH/LYRIC was shown in breast cancer patient samples and breast cancer cell lines (Zhang et al., 2011). Second, two miRNAs, miR-203 and miR-22, suppressed expression of AEG-1/MTDH/LYRIC in p53-mutated colon cancer cells through the PI3K–AKT pathway via negative regulation of AKT2 and activation of PTEN, respectively, resulting in alleviation of chemoresistance of cancer cells, but not in the p53 wild-type cells (Li, Chen, Zhao, Kong, & Zhang, 2011; Li, Zhang, Zhao, Kong, & Chen, 2011). Another study found that one of the most well-known tumor suppressors miR-375 directly downregulated expression of AEG-1/MTDH/LYRIC by its binding to the 3′-UTR of AEG-1/MTDH/LYRIC mRNA, and similar expression patterns of decreased miR-375 and increased AEG-1/MTDH/LYRIC were observed in patient samples of several tumor types including HNSCC, liver cancer, breast cancer, and ESCC (He et al., 2012; Hui et al., 2011; Isozaki et al., 2012; Nohata et al., 2011; Ward et al., 2012). In addition, AEG-1/MTDH/LYRIC was identified as a target of miR-136 in glioma cells (Yang, Wu, et al., 2012). Furthermore, increased expression of AEG-1/MTDH/LYRIC by the suppressed miRNAs in cancer cells was significantly involved in tumorigenesis. These results clearly indicate that overexpression of miRNAs targeting AEG-1/MTDH/LYRIC might be a potential therapeutic strategy for inhibiting cancer cell growth.
7. CONCLUDING REMARKS
Between 2002 and 2004, four independent groups identified AEG-1/MTDH/LYRIC with different names. We first identified AEG-1 as a HIV-1-and HIV-1 gp120-inducible gene from PHFA in 2002, and as a TNF-α-inducible gene. In 2004, three other groups identified 3D3/LYRIC as a transmembrane protein of the ER, nuclear envelope, and nucleolus in mouse cells using a gene-trap screening system, MTDH as a cell surface protein involved in breast cancer metastasis to the lung using a phage display strategy in a mouse model of breast cancer, and LYRIC as a tight junction-associated protein in polarized epithelial cells, respectively. These results suggested that AEG-1/MTDH/LYRIC could be important in both pathological and normal physiological processes. A recent study indicating that AEG-1/MTDH/LYRIC can induce protective autophagy supports a potential role in promoting cell survival (Bhutia et al., 2010). However, until now most researchers have focused on its oncogenic functions in cancer, even though some other biological functions and biochemical characteristics of AEG-1/MTDH/LYRIC including its amino acid sequences, localization, and posttranslational modification such as ubiquitination have been clarified. Especially, AEG-1/MTDH/LYRIC has been recognized as a potential prognostic marker for patients with various types of human cancer such as breast cancer, HCC, prostate cancer, glioma, etc. In addition, increased insights into the detailed mechanism on oncogenic functions of AEG-1/MTDH/LYRIC involved in cell proliferation, chemoresistance, angiogenesis, invasion, and metastasis clearly indicate that AEG-1/MTDH/LYRIC is a potentially good target for cancer treatment. Some recent results have already suggested that AEG-1/MTDH/LYRIC could be a target of therapeutic reagents and miRNAs with therapeutic potential for directly suppressing the cancer phenotype. Recent studies also reveal the importance of AEG-1/MTDH/LYRIC in other pathological processes such as neurodegeneration including migraine and HD, and inflammation. These results have aroused enhanced interests in AEG-1/MTDH/LYRIC, and a greater understanding of AEG-1/MTDH/LYRIC's normal and abnormal biological functions and basic biochemical characteristics including its structure will extend its roles in various other pathological and normal physiological processes. Additional reviews in this thematic issue focus on various aspects of AEG-1/MTDH/LYRIC and future research directions. There has been a decade of research on AEG-1/MTDH/LYRIC since its original identification as an AEG. We have made significant progress and learned a lot in a short time about this interesting molecule. Expanded research is justified and should usher in a greater understanding of the normal and abnormal functions of this protein which may serve as a viable target for targeting various pathogenic diseases, including cancer and neurodegeneration.
ACKNOWLEDGMENTS
We are indebted to the National Institutes of Health, including the National Cancer Institute Grant 1 R01 CA134721, National Institute of Neurological Disease and Stroke, 5 P01 NS31492, and the Thelma Newmeyer Corman Endowment to P. B. F., by National Cancer Institute Grant R01 CA138540 to D. S., by HRF-G-2012-6 to D. K. from Hallym University, and by a National Research Foundation of Korea grant (No. 20120005755) to S.-G. L. D. S. is a Harrison Scholar in the VCU Massey Cancer Center. P. B. F. holds the Thelma Newmeyer Corman Endowed Chair in Cancer Research at the VCU Massey Cancer Center.
REFERENCES
- Anttila V, Stefansson H, Kallela M, Todt U, Terwindt GM, Calafato MS, et al. Genome-wide association study of migraine implicates a common susceptibility variant on 8q22.1. Nature Genetics. 2010;42(10):869–873. doi: 10.1038/ng.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ash SC, Yang DQ, Britt DE. LYRIC/AEG-1 overexpression modulates BCCIPalpha protein levels in prostate tumor cells. Biochemical and Biophysical Research Communications. 2008;371(2):333–338. doi: 10.1016/j.bbrc.2008.04.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhutia SK, Kegelman TP, Das SK, Azab B, Su ZZ, Lee SG, et al. Astrocyte elevated gene-1 induces protective autophagy. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(51):22243–22248. doi: 10.1073/pnas.1009479107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borjabad A, Brooks AI, Volsky DJ. Gene expression profiles of HIV-1-infected glia and brain: Toward better understanding of the role of astrocytes in HIV-1-associated neurocognitive disorders. Journal of Neuroimmune Pharmacology. 2010;5(1):44–62. doi: 10.1007/s11481-009-9167-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borley J, Wilhelm-Benartzi C, Brown R, Ghaem-Maghami S. Does tumour biology determine surgical success in the treatment of epithelial ovarian cancer? A systematic literature review. British Journal of Cancer. 2012;107(7):1069–1074. doi: 10.1038/bjc.2012.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukerche H, Su ZZ, Kang DC, Fisher PB. Identification and cloning of genes displaying elevated expression as a consequence of metastatic progression in human melanoma cells by rapid subtraction hybridization. Gene. 2004;343(1):191–201. doi: 10.1016/j.gene.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Brack-Werner R. Astrocytes: HIV cellular reservoirs and important participants in neuropathogenesis. AIDS. 1999;13(1):1–22. doi: 10.1097/00002030-199901140-00003. [DOI] [PubMed] [Google Scholar]
- Britt DE, Yang DF, Yang DQ, Flanagan D, Callanan H, Lim YP, et al. Identification of a novel protein, LYRIC, localized to tight junctions of polarized epithelial cells. Experimental Cell Research. 2004;300(1):134–148. doi: 10.1016/j.yexcr.2004.06.026. [DOI] [PubMed] [Google Scholar]
- Brown DM, Ruoslahti E. Metadherin, a cell surface protein in breast tumors that mediates lung metastasis. Cancer Cell. 2004;5(4):365–374. doi: 10.1016/s1535-6108(04)00079-0. [DOI] [PubMed] [Google Scholar]
- Carnemolla A, Fossale E, Agostoni E, Michelazzi S, Calligaris R, De Maso L, et al. Rrs1 is involved in endoplasmic reticulum stress response in Huntington disease. The Journal of Biological Chemistry. 2009;284(27):18167–18173. doi: 10.1074/jbc.M109.018325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Dong K, Long M, Lin F, Wang X, Wei J, et al. Serum anti-AEG-1 autoantibody is a potential novel biomarker for malignant tumors. Oncology Letters. 2012;4(2):319–323. doi: 10.3892/ol.2012.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Ke Z, Shi H, Yang S, Wang L. Overexpression of AEG-1 in renal cell carcinoma and its correlation with tumor nuclear grade and progression. Neoplasma. 2010;57(6):522–529. doi: 10.4149/neo_2010_06_522. [DOI] [PubMed] [Google Scholar]
- Choi DW. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988;1(8):623–634. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- Deng N, Feng Y. Expression of EphA7 and MTDH and clinicopathological significance in the squamous cell cancer of the tongue. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2011;36(12):1195–1198. doi: 10.3969/j.issn.1672-7347.2011.12.012. [DOI] [PubMed] [Google Scholar]
- Duchen MR. Mitochondria, calcium-dependent neuronal death and neurodegenerative disease. Pfl ü gers Archiv. 2012;464(1):111–121. doi: 10.1007/s00424-012-1112-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emdad L, Lee SG, Su ZZ, Jeon HY, Boukerche H, Sarkar D, et al. Astrocyte elevated gene-1 (AEG-1) functions as an oncogene and regulates angiogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(50):21300–21305. doi: 10.1073/pnas.0910936106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emdad L, Sarkar D, Lee SG, Su ZZ, Yoo BK, Dash R, et al. Astrocyte elevated gene-1: A novel target for human glioma therapy. Molecular Cancer Therapeutics. 2010;9(1):79–88. doi: 10.1158/1535-7163.MCT-09-0752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emdad L, Sarkar D, Su ZZ, Lee SG, Kang DC, Bruce JN, et al. Astroeyte elevated gene-1: Recent insights into a novel gene involved in tumor progression, metastasis and neurodegeneration. Pharmacology and Therapeutics. 2007;114(2):155–170. doi: 10.1016/j.pharmthera.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emdad L, Sarkar D, Su ZZ, Randolph A, Boukerche H, Valerie K, et al. Activation of the nuclear factor kappaB pathway by astrocyte elevated gene-1: Implications for tumor progression and metastasis. Cancer Research. 2006;66(3):1509–1516. doi: 10.1158/0008-5472.CAN-05-3029. [DOI] [PubMed] [Google Scholar]
- Ge X, Lv X, Feng L, Liu X, Gao J, Chen N, et al. Metadherin contributes to the pathogenesis of diffuse large B-cell lymphoma. PLoS One. 2012;7(6):e39449. doi: 10.1371/journal.pone.0039449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnosa S, Shen YM, Wang CJ, Zhang H, Stratmann J, Arbman G, et al. Expression of AEG-1 mRNA and protein in colorectal cancer patients and colon cancer cell lines. Journal of Translational Medicine. 2012;10:109. doi: 10.1186/1479-5876-10-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Z, Liu W, You N, Wang T, Wang X, Lu P, et al. Prognostic significance of metadherin overexpression in hepatitis B virus-related hepatocellular carcinoma. Oncology Reports. 2012;27(6):2073–2079. doi: 10.3892/or.2012.1749. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nature Reviews Immunology. 2005;5(1):69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- He XX, Chang Y, Meng FY, Wang MY, Xie QH, Tang F, et al. Micro-RNA-375 targets AEG-1 in hepatocellular carcinoma and suppresses liver cancer cell growth in vitro and in vivo. Oncogene. 2012;31(28):3357–3369. doi: 10.1038/onc.2011.500. [DOI] [PubMed] [Google Scholar]
- Hornbeck PV, Kornhauser JM, Tkachev S, Zhang B, Skrzypek E, Murray B, et al. PhosphoSitePlus: A comprehensive resource for investigating the structure and function of experimentally determined post-translational modifications in man and mouse. Nucleic Acids Research. 2012;40(Database issue):D261–D270. doi: 10.1093/nar/gkr1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G, Chong RA, Yang Q, Wei Y, Blanco MA, Li F, et al. MTDH activation by 8q22 genomic gain promotes chemoresistance and metastasis of poor-prognosis breast cancer. Cancer Cell. 2009;15(1):9–20. doi: 10.1016/j.ccr.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui AB, Bruce JP, Alajez NM, Shi W, Yue S, Perez-Ordonez B, et al. Significance of dysregulated metadherin and microRNA-375 in head and neck cancer. Clinical Cancer Research. 2011;17(24):7539–7550. doi: 10.1158/1078-0432.CCR-11-2102. [DOI] [PubMed] [Google Scholar]
- Isozaki Y, Hoshino I, Nohata N, Kinoshita T, Akutsu Y, Hanari N, et al. Identification of novel molecular targets regulated by tumor suppressive miR-375 induced by histone acetylation in esophageal squamous cell carcinoma. International Journal of Oncology. 2012;41(3):985–994. doi: 10.3892/ijo.2012.1537. [DOI] [PubMed] [Google Scholar]
- Jansson MD, Lund AH. MicroRNA and cancer. Molecular Oncology. 2012;6(6):590–610. doi: 10.1016/j.molonc.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon HY, Choi M, Howlett EL, Vozhilla N, Yoo BK, Lloyd JA, et al. Expression patterns of astrocyte elevated gene-1 (AEG-1) during development of the mouse embryo. Gene Expression Patterns. 2010;10(7–8):361–367. doi: 10.1016/j.gep.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian-bo X, Hui W, Yu-long H, Chang-hua Z, Long-juan Z, Shi-rong C, et al. Astrocyte-elevated gene-1 overexpression is associated with poor prognosis in gastric cancer. Medical Oncology. 2011;28(2):455–462. doi: 10.1007/s12032-010-9475-6. [DOI] [PubMed] [Google Scholar]
- Jiang H, Kang DC, Alexandre D, Fisher PB. RaSH, a rapid subtraction hybridization approach for identifying and cloning differentially expressed genes. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(23):12684–12689. doi: 10.1073/pnas.220431297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T, Zhu A, Zhu Y, Piao D. Clinical implications of AEG-1 in liver metastasis of colorectal cancer. Medical Oncology. 2012;29(4):2858–2863. doi: 10.1007/s12032-012-0186-z. [DOI] [PubMed] [Google Scholar]
- Kang DC, Fisher PB. Complete open reading frame (C-ORF) technology: Simple and efficient technique for cloning full-length protein-coding sequences. Gene. 2005;353(1):1–7. doi: 10.1016/j.gene.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Kang DC, Fisher PB. Complete open reading frame (C-ORF) technique: Rapid and efficient method for obtaining complete protein coding sequences. Methods in Molecular Biology. 2007;383:123–133. doi: 10.1007/978-1-59745-335-6_8. [DOI] [PubMed] [Google Scholar]
- Kang DC, Gopalkrishnan RV, Wu Q, Jankowsky E, Pyle AM, Fisher PB. mda-5: An interferon-inducible putative RNA helicase with double-stranded RNA-dependent ATPase activity and melanoma growth-suppressive properties. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(2):637–642. doi: 10.1073/pnas.022637199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang DC, Jiang H, Su ZZ, Volsky DJ, Fisher PB. RaSH-Rapid subtraction hybridization. In: Lorkowski S, Cullen P, editors. Analysig gene expression. Wiley-VCH; Germany: 2002. pp. 206–214. [Google Scholar]
- Kang DC, Su ZZ, Sarkar D, Emdad L, Volsky DJ, Fisher PB. Cloning and characterization of HIV-1-inducible astrocyte elevated gene-1, AEG-1. Gene. 2005;353(1):8–15. doi: 10.1016/j.gene.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Ke ZF, He S, Li S, Luo D, Feng C, Zhou W. Expression characteristics of astrocyte elevated gene-1 (AEG-1) in tongue carcinoma and its correlation with poor prognosis. Cancer Epidemiology. 2012;37(2):179–185. doi: 10.1016/j.canep.2012.10.007. [DOI] [PubMed] [Google Scholar]
- Khuda II, Koide N, Noman AS, Dagvadorj J, Tumurkhuu G, Naiki Y, et al. Astrocyte elevated gene-1 (AEG-1) is induced by lipopolysaccharide as toll-like receptor 4 (TLR4) ligand and regulates TLR4 signalling. Immunology. 2009;128(1 Suppl):e700–e706. doi: 10.1111/j.1365-2567.2009.03063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuno N, Shiina H, Urakami S, Kawamoto K, Hirata H, Tanaka Y, et al. Knockdown of astrocyte-elevated gene-1 inhibits prostate cancer progression through upregulation of FOXO3a activity. Oncogene. 2007;26(55):7647–7655. doi: 10.1038/sj.onc.1210572. [DOI] [PubMed] [Google Scholar]
- Kim K, Lee SG, Kegelman TP, Su ZZ, Das SK, Dash R, et al. Role of excitatory amino acid transporter-2 (EAAT2) and glutamate in neurodegeneration: Opportunities for developing novel therapeutics. Journal of Cellular Physiology. 2011;226(10):2484–2493. doi: 10.1002/jcp.22609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornegoor R, Moelans CB, Verschuur-Maes AH, Hogenes MC, de Bruin PC, Oudejans JJ, et al. Oncogene amplification in male breast cancer: Analysis by multiplex ligation-dependent probe amplification. Breast Cancer Research and Treatment. 2012;135(1):49–58. doi: 10.1007/s10549-012-2051-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SG, Jeon HY, Su ZZ, Richards JE, Vozhilla N, Sarkar D, et al. Astrocyte elevated gene-1 contributes to the pathogenesis of neuroblastoma. Oncogene. 2009;28(26):2476–2484. doi: 10.1038/onc.2009.93. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Jung DB, Sohn EJ, Kim HH, Park MN, Lew JH, et al. Inhibition of hypoxia inducible factor alpha and astrocyte-elevated gene-1 mediates cryptotanshinone exerted antitumor activity in hypoxic PC-3 cells. Evidence-Based Complementary and Alternative Medicine: eCAM. 2012;2012:13. doi: 10.1155/2012/390957. Research Article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SG, Kim K, Kegelman TP, Dash R, Das SK, Choi JK, et al. Oncogene AEG-1 promotes glioma-induced neurodegeneration by increasing glutamate excitotoxicity. Cancer Research. 2011;71(20):6514–6523. doi: 10.1158/0008-5472.CAN-11-0782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SG, Su ZZ, Emdad L, Gupta P, Sarkar D, Borjabad A, et al. Mechanism of ceftriaxone induction of excitatory amino acid transporter-2 expression and glutamate uptake in primary human astrocytes. The Journal of Biological Chemistry. 2008;283(19):13116–13123. doi: 10.1074/jbc.M707697200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SG, Su ZZ, Emdad L, Sarkar D, Fisher PB. Astrocyte elevated gene-1 (AEG-1) is a target gene of oncogenic Ha-ras requiring phosphatidylinositol 3-kinase and c-Myc. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(46):17390–17395. doi: 10.1073/pnas.0608386103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leszczyniecka M, Kang DC, Sarkar D, Su ZZ, Holmes M, Valerie K, et al. Identification and cloning of human polynucleotide phosphorylase, hPNPase old-35, in the context of terminal differentiation and cellular senescence. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(26):16636–16641. doi: 10.1073/pnas.252643699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Chen Y, Zhao J, Kong F, Zhang Y. miR-203 reverses chemoresistance in p53-mutated colon cancer cells through downregulation of Akt2 expression. Cancer Letters. 2011;304(1):52–59. doi: 10.1016/j.canlet.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Li C, Li R, Song H, Wang D, Feng T, Yu X, et al. Significance of AEG-1 expression in correlation with VEGF, microvessel density and clinicopathological characteristics in triple-negative breast cancer. Journal of Surgical Oncology. 2011;103(2):184–192. doi: 10.1002/jso.21788. [DOI] [PubMed] [Google Scholar]
- Li C, Li Y, Wang X, Wang Z, Cai J, Wang L, et al. Elevated expression of astrocyte elevated gene-1 (AEG-1) is correlated with cisplatin-based chemoresistance and shortened outcome in patients with stages III–IV serous ovarian carcinoma. Histopathology. 2012;60(6):953–963. doi: 10.1111/j.1365-2559.2012.04182.x. [DOI] [PubMed] [Google Scholar]
- Li C, Liu J, Lu R, Yu G, Wang X, Zhao Y, et al. AEG -1 overexpression: A novel indicator for peritoneal dissemination and lymph node metastasis in epithelial ovarian cancers. International Journal of Gynecological Cancer. 2011;21(4):602–608. doi: 10.1097/IGC.0b013e3182145561. [DOI] [PubMed] [Google Scholar]
- Li J, Yang L, Song L, Xiong H, Wang L, Yan X, et al. Astrocyte elevated gene-1 is a proliferation promoter in breast cancer via suppressing transcriptional factor FOXO1. Oncogene. 2009;28(36):3188–3196. doi: 10.1038/onc.2009.171. [DOI] [PubMed] [Google Scholar]
- Li J, Zhang N, Song LB, Liao WT, Jiang LL, Gong LY, et al. Astrocyte elevated gene-1 is a novel prognostic marker for breast cancer progression and overall patient survival. Clinical Cancer Research. 2008;14(11):3319–3326. doi: 10.1158/1078-0432.CCR-07-4054. [DOI] [PubMed] [Google Scholar]
- Li J, Zhang Y, Zhao J, Kong F, Chen Y. Overexpression of miR-22 reverses paclitaxel-induced chemoresistance through activation of PTEN signaling in p53-mutated colon cancer cells. Molecular and Cellular Biochemistry. 2011;357(1–2):31–38. doi: 10.1007/s11010-011-0872-8. [DOI] [PubMed] [Google Scholar]
- Liao WT, Guo L, Zhong Y, Wu YH, Li J, Song LB. Astrocyte elevated gene-1 (AEG-1) is a marker for aggressive salivary gland carcinoma. Journal of Translational Medicine. 2011;9:205. doi: 10.1186/1479-5876-9-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligthart L, de Vries B, Smith AV, Ikram MA, Amin N, Hottenga JJ, et al. Meta-analysis of genome-wide association for migraine in six population-based European cohorts. European Journal of Human Genetics. 2011;19(8):901–907. doi: 10.1038/ejhg.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HY, Liu CX, Han B, Zhang XY, Sun RP. AEG-1 is associated with clinical outcome in neuroblastoma patients. Cancer Biomarkers. 2011;11(2):115–121. doi: 10.3233/CBM-2012-0268. [DOI] [PubMed] [Google Scholar]
- Liu L, Wu J, Ying Z, Chen B, Han A, Liang Y, et al. Astrocyte elevated gene-1 upregulates matrix metalloproteinase-9 and induces human glioma invasion. Cancer Research. 2010;70(9):3750–3759. doi: 10.1158/0008-5472.CAN-09-3838. [DOI] [PubMed] [Google Scholar]
- Liu DC, Yang ZL. MTDH and EphA7 are markers for metastasis and poor prognosis of gallbladder adenocarcinoma. Diagnostic Cytopathology. 2011;41(3):199–205. doi: 10.1002/dc.21821. [DOI] [PubMed] [Google Scholar]
- Liu X, Zhang N, Li X, Moran MS, Yuan C, Yan S, et al. Identification of novel variants of metadherin in breast cancer. PLoS One. 2011;6(3):e17582. doi: 10.1371/journal.pone.0017582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luparello C, Longo A, Vetrano M. Exposure to cadmium chloride influences astrocyte-elevated gene-1 (AEG-1) expression in MDA-MB231 human breast cancer cells. Biochimie. 2012;94(1):207–213. doi: 10.1016/j.biochi.2011.10.009. [DOI] [PubMed] [Google Scholar]
- Meng F, Luo C, Ma L, Hu Y, Lou G. Clinical significance of astrocyte elevated gene-1 expression in human epithelial ovarian carcinoma. International Journal of Gynecological Pathology. 2011;30(2):145–150. doi: 10.1097/PGP.0b013e3181ffd2f7. [DOI] [PubMed] [Google Scholar]
- Noch E, Bookland M, Khalili K. Astrocyte-elevated gene-1 (AEG-1) induction by hypoxia and glucose deprivation in glioblastoma. Cancer Biology & Therapy. 2011;11(1):32–39. doi: 10.4161/cbt.11.1.13835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nohata N, Hanazawa T, Kikkawa N, Mutallip M, Sakurai D, Fujimura L, et al. Tumor suppressive microRNA-375 regulates oncogene AEG-1/MTDH in head and neck squamous cell carcinoma (HNSCC) Journal of Human Genetics. 2011;56(8):595–601. doi: 10.1038/jhg.2011.66. [DOI] [PubMed] [Google Scholar]
- Rehman AO, Wang CY. CXCL12/SDF-1 alpha activates NF-kappaB and promotes oral cancer invasion through the Carma3/Bcl10/Malt1 complex. International Journal of Oral Science. 2009;1(3):105–118. doi: 10.4248/IJOS.09059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Hohna S, et al. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Systems Biology. 2012;61(3):539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, et al. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433(7021):73–77. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- Santarpia M, Magri I, Sanchez-Ronco M, Costa C, Molina-Vila MA, Gimenez-Capitan A, et al. mRNA expression levels and genetic status of genes involved in the EGFR and NF-kappaB pathways in metastatic non-small-cell lung cancer patients. Journal of Translational Medicine. 2011;9:163. doi: 10.1186/1479-5876-9-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar D, Emdad L, Lee SG, Yoo BK, Su ZZ, Fisher PB. Astrocyte elevated gene-1: Far more than just a gene regulated in astrocytes. Cancer Research. 2009;69(22):8529–8535. doi: 10.1158/0008-5472.CAN-09-1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar D, Park ES, Emdad L, Lee SG, Su ZZ, Fisher PB. Molecular basis of nuclear factor-kappa B activation by astrocyte elevated gene-1. Cancer Research. 2008;68(5):1478–1484. doi: 10.1158/0008-5472.CAN-07-6164. [DOI] [PubMed] [Google Scholar]
- Simm M, Su Z, Huang EY, Chen Y, Jiang H, Volsky DJ, et al. Cloning of differentially expressed genes in an HIV-1 resistant T cell clone by rapid subtraction hybridization, RaSH. Gene. 2001;269(1–2):93–101. doi: 10.1016/s0378-1119(01)00456-5. [DOI] [PubMed] [Google Scholar]
- Song YH, Jeong SJ, Kwon HY, Kim B, Kim SH, Yoo DY. Ursolic acid from Oldenlandia diffusa induces apoptosis via activation of caspases and phosphor-ylation of glycogen synthase kinase 3 beta in SK-OV-3 ovarian cancer cells. Biological and Pharmaceutical Bulletin. 2012;35(7):1022–1028. doi: 10.1248/bpb.b110660. [DOI] [PubMed] [Google Scholar]
- Song H, Li C, Li R, Geng J. Prognostic significance of AEG-1 expression in colorectal carcinoma. International Journal of Colorectal Disease. 2010;25(10):1201–1209. doi: 10.1007/s00384-010-1009-3. [DOI] [PubMed] [Google Scholar]
- Song H, Li C, Lu R, Zhang Y, Geng J. Expression of astrocyte elevated gene-1: A novel marker of the pathogenesis, progression, and poor prognosis for endometrial cancer. International Journal of Gynecological Cancer. 2010;20(7):1188–1196. doi: 10.1111/igc.0b013e3181ef8e21. [DOI] [PubMed] [Google Scholar]
- Song L, Li W, Zhang H, Liao W, Dai T, Yu C, et al. Over-expression of AEG-1 significantly associates with tumour aggressiveness and poor prognosis in human non-small cell lung cancer. The Journal of Pathology. 2009;219(3):317–326. doi: 10.1002/path.2595. [DOI] [PubMed] [Google Scholar]
- Su ZZ, Chen Y, Kang DC, Chao W, Simm M, Volsky DJ, et al. Customized rapid subtraction hybridization (RaSH) gene microarrays identify overlapping expression changes in human fetal astrocytes resulting from human immunodeficiency virus-1 infection or tumor necrosis factor-alpha treatment. Gene. 2003;306:67–78. doi: 10.1016/s0378-1119(03)00404-9. [DOI] [PubMed] [Google Scholar]
- Su ZZ, Kang DC, Chen Y, Pekarskaya O, Chao W, Volsky DJ, et al. Identification and cloning of human astrocyte genes displaying elevated expression after infection with HIV-1 or exposure to HIV-1 envelope glycoprotein by rapid subtraction hybridization, RaSH. Oncogene. 2002;21(22):3592–3602. doi: 10.1038/sj.onc.1205445. [DOI] [PubMed] [Google Scholar]
- Su ZZ, Kang DC, Chen Y, Pekarskaya O, Chao W, Volsky DJ, et al. Identification of gene products suppressed by human immunodeficiency virus type 1 infection or gp120 exposure of primary human astrocytes by rapid subtraction hybridization. Journal of Neurovirology. 2003;9(3):372–389. doi: 10.1080/13550280390201263. [DOI] [PubMed] [Google Scholar]
- Su ZZ, Leszczyniecka M, Kang DC, Sarkar D, Chao W, Volsky DJ, et al. Insights into glutamate transport regulation in human astrocytes: Cloning of the promoter for excitatory amino acid transporter 2 (EAAT2) Proceedings of the National Academy of Sciences of the United States of America. 2003;100(4):1955–1960. doi: 10.1073/pnas.0136555100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su P, Zhang Q, Yang Q. Immunohistochemical analysis of Metadherin in proliferative and cancerous breast tissue. Diagnostic Pathology. 2010;5:38. doi: 10.1186/1746-1596-5-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Fan YZ, Xi H, Lu XS, Ye C, Zhang JT. Astrocyte elevated gene-1 overexpression in human primary gallbladder carcinomas: An unfavorable and independent prognostic factor. Oncology Reports. 2011;26(5):1133–1142. doi: 10.3892/or.2011.1387. [DOI] [PubMed] [Google Scholar]
- Sun S, Ke Z, Wang F, Li S, Chen W, Han A, et al. Overexpression of astrocyte-elevated gene-1 is closely correlated with poor prognosis in human nonsmall cell lung cancer and mediates its metastasis through up-regulation of matrix metalloproteinase-9 expression. Human Pathology. 2012;43(7):1051–1060. doi: 10.1016/j.humpath.2011.08.017. [DOI] [PubMed] [Google Scholar]
- Sutherland HG, Lam YW, Briers S, Lamond AI, Bickmore WA. 3D3/lyric: A novel transmembrane protein of the endoplasmic reticulum and nuclear envelope, which is also present in the nucleolus. Experimental Cell Research. 2004;294(1):94–105. doi: 10.1016/j.yexcr.2003.11.020. [DOI] [PubMed] [Google Scholar]
- Thirkettle HJ, Girling J, Warren AY, Mills IG, Sahadevan K, Leung H, et al. LYRIC/AEG-1 is targeted to different subcellular compartments by ubiquitinylation and intrinsic nuclear localization signals. Clinical Cancer Research. 2009;15(9):3003–3013. doi: 10.1158/1078-0432.CCR-08-2046. [DOI] [PubMed] [Google Scholar]
- Thirkettle HJ, Mills IG, Whitaker HC, Neal DE. Nuclear LYRIC/AEG-1 interacts with PLZF and relieves PLZF-mediated repression. Oncogene. 2009;28(41):3663–3670. doi: 10.1038/onc.2009.223. [DOI] [PubMed] [Google Scholar]
- Tokunaga E, Nakashima Y, Yamashita N, Hisamatsu Y, Okada S, Akiyoshi S, et al. Overexpression of metadherin/MTDH is associated with an aggressive phenotype and a poor prognosis in invasive breast cancer. Breast Cancer. 2012 doi: 10.1007/s12282-012-0398-2. http://dx.doi.org/10.1007/s12282-012-0398-2. [DOI] [PubMed]
- Vartak-Sharma N, Ghorpade A. Astrocyte elevated gene-1 regulates astrocyte responses to neural injury: Implications for reactive astrogliosis and neurodegeneration. Journal of Neuroinflammation. 2012;9:195. doi: 10.1186/1742-2094-9-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Du X, Zang L, Song N, Yang T, Dong R, et al. Prognostic impact of Metadherin-SND1 interaction in colon cancer. Molecular Biology Reports. 2012;39(12):10497–10504. doi: 10.1007/s11033-012-1933-0. [DOI] [PubMed] [Google Scholar]
- Wang F, Ke ZF, Sun SJ, Chen WF, Yang SC, Li SH, et al. Oncogenic roles of astrocyte elevated gene-1 (AEG-1) in osteosarcoma progression and prognosis. Cancer Biology & Therapy. 2011;12(6):539–548. doi: 10.4161/cbt.12.6.16301. [DOI] [PubMed] [Google Scholar]
- Wang Z, Trillo-Pazos G, Kim SY, Canki M, Morgello S, Sharer LR, et al. Effects of human immunodeficiency virus type 1 on astrocyte gene expression and function: Potential role in neuropathogenesis. Journal of Neurovirology. 2004;10(Suppl. 1):25–32. doi: 10.1080/753312749. [DOI] [PubMed] [Google Scholar]
- Ward A, Balwierz A, Zhang JD, Kublbeck M, Pawitan Y, Hielscher T, et al. Re-expression of microRNA-375 reverses both tamoxifen resistance and accompanying EMT-like properties in breast cancer. Oncogene. 2012;32(9):1173–1182. doi: 10.1038/onc.2012.128. [DOI] [PubMed] [Google Scholar]
- Watkins S, Sontheimer H. Unique biology of gliomas: Challenges and opportunities. Trends in Neurosciences. 2012;35(9):546–556. doi: 10.1016/j.tins.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Dai J, Lu P, Huang Y, Zhu Y, Zhang X. Distinct effect of CD40 and TNF-signaling on the chemokine/chemokine receptor expression and function of the human monocyte-derived dendritic cells. Cellular and Molecular Immunology. 2008;5(2):121–131. doi: 10.1038/cmi.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Z, Zhang N, Jin H, Yu Z, Xu G, Huang Z. Clinical significance of astrocyte elevated gene-1 expression in human oligodendrogliomas. Clinical Neurology and Neurosurgery. 2010;112(5):413–419. doi: 10.1016/j.clineuro.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Xue Y, Ren J, Gao X, Jin C, Wen L, Yao X. GPS 2.0, a tool to predict kinase-specific phosphorylation sites in hierarchy. Molecular & Cellular Proteomics. 2008;7(9):1598–1608. doi: 10.1074/mcp.M700574-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Zhang M, Chen Q, Zhang X. Expression of AEG-1 in human T-cell lymphoma enhances the risk of progression. Oncology Reports. 2012;28(6):2107–2114. doi: 10.3892/or.2012.2055. [DOI] [PubMed] [Google Scholar]
- Yang Y, Wu J, Guan H, Cai J, Fang L, Li J, et al. MiR-136 promotes apoptosis of glioma cells by targeting AEG-1 and Bcl-2. FEBS Letters. 2012;586(20):3608–3612. doi: 10.1016/j.febslet.2012.08.003. [DOI] [PubMed] [Google Scholar]
- Ying Z, Li J, Li M. Astrocyte elevated gene 1: Biological functions and molecular mechanism in cancer and beyond. Cell and Bioscience. 2011;1(1):36. doi: 10.1186/2045-3701-1-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo BK, Emdad L, Lee SG, Su ZZ, Santhekadur P, Chen D, et al. Astrocyte elevated gene-1 (AEG-1): A multifunctional regulator of normal and abnormal physiology. Pharmacology and Therapeutics. 2011;130(1):1–8. doi: 10.1016/j.pharmthera.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo BK, Emdad L, Su ZZ, Villanueva A, Chiang DY, Mukhopadhyay ND, et al. Astrocyte elevated gene-1 regulates hepatocellular carcinoma development and progression. The Journal of Clinical Investigation. 2009;119(3):465–477. doi: 10.1172/JCI36460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo BK, Santhekadur PK, Gredler R, Chen D, Emdad L, Bhutia S, et al. Increased RNA-induced silencing complex (RISC) activity contributes to hepatocellular carcinoma. Hepatology. 2011;53(5):1538–1548. doi: 10.1002/hep.24216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, Chen K, Zheng H, Guo X, Jia W, Li M, et al. Overexpression of astrocyte elevated gene-1 (AEG-1) is associated with esophageal squamous cell carcinoma (ESCC) progression and pathogenesis. Carcinogenesis. 2009;30(5):894–901. doi: 10.1093/carcin/bgp064. [DOI] [PubMed] [Google Scholar]
- Zhang B, Liu XX, He JR, Zhou CX, Guo M, He M, et al. Pathologically decreased miR-26a antagonizes apoptosis and facilitates carcinogenesis by targeting MTDH and EZH2 in breast cancer. Carcinogenesis. 2011;32(1):2–9. doi: 10.1093/carcin/bgq209. [DOI] [PubMed] [Google Scholar]
- Zhang F, Yang Q, Meng F, Shi H, Li H, Liang Y, et al. Astrocyte elevated gene-1 interacts with beta-catenin and increases migration and invasion of colorectal carcinoma. Molecular Carcinogenesis. 2012 doi: 10.1002/mc.21894. http://dx.doi.org/10.1002/mc.21894. [DOI] [PubMed]
- Zhao Y, Kong X, Li X, Yan S, Yuan C, Hu W, et al. Metadherin mediates lipopolysaccharide-induced migration and invasion of breast cancer cells. PLoS One. 2011;6(12):e29363. doi: 10.1371/journal.pone.0029363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Deng H, Yan W, Huang H, Deng Y, Li Y, et al. Expression of metadherin/AEG-1 gene is positively related to orientation chemotaxis and adhesion of human hepatocellular carcinoma cell lines of different metastatic potentials. Journal of Huazhong University of Science and Technology. Medical Sciences. 2012;32(3):353–357. doi: 10.1007/s11596-012-0061-3. [DOI] [PubMed] [Google Scholar]
- Zhou J, Li J, Wang Z, Yin C, Zhang W. Metadherin is a novel prognostic marker for bladder cancer progression and overall patient survival. Asia-Pacific Journal of Clinical Oncology. 2012;8(3):e42–e48. doi: 10.1111/j.1743-7563.2012.01541.x. [DOI] [PubMed] [Google Scholar]
- Zhu K, Dai Z, Pan Q, Wang Z, Yang GH, Yu L, et al. Metadherin promotes hepatocellular carcinoma metastasis through induction of epithelial-mesenchymal transition. Clinical Cancer Research. 2011;17(23):7294–7302. doi: 10.1158/1078-0432.CCR-11-1327. [DOI] [PubMed] [Google Scholar]