Abstract
3,4-(±)-Methylenedioxymethamphetamine (MDMA) and 3,4-(±)-methylenedioxyamphetamine (MDA), a primary metabolite of MDMA, are phenylethylamine derivatives that cause serotonergic neurotoxicity. Although several phenylethylamine derivatives activate microglia, little is known about the effects of MDMA on glial cells, and evidence of MDMA-induced microglial activation remains ambiguous. We initially determined microglial occupancy status of the parietal cortex in rats at various time points following a single neurotoxic dose of MDMA (20mg/kg, SC). A biphasic microglial response to MDMA was observed, with peak microglial occupancy occurring 12- and 72-h post-MDMA administration. Because direct injection of MDMA into the brain does not produce neurotoxicity, the glial response to MDMA metabolites was subsequently examined in vivo and in vitro. Rats were treated with MDA (20mg/kg, SC) followed by ex vivo biopsy culture to determine the activation of quiescent microglia. A reactive microglial response was observed 72h after MDA administration that subsided by 7 days. In contrast, intracerebroventricular (ICV) administration of MDA failed to produce a microglial response. However, thioether metabolites of MDA derived from α-methyldopamine (α-MeDA) elicited a robust microglial response following icv injection. We subsequently determined the direct effects of various MDMA metabolites on primary cultures of E18 hippocampal mixed glial and neuronal cells. 5-(Glutathion-S-yl)-α-MeDA, 2,5-bis-(glutathion-S-yl)-α-MeDA, and 5-(N-acetylcystein-S-yl)-α-MeDA all stimulated the proliferation of glial fibrillary acidic protein–positive astrocytes at a dose of 10µM. The findings indicate that glial cells are activated in response to MDMA/MDA and support a role for thioether metabolites of α-MeDA in the neurotoxicity.
Key Words: 3,4-(±)-methylenedioxymethamphetamine; metabolites; microglial activation.
3,4-(±)-Methylenedioxymethamphetamine (MDMA) and its primary metabolite, 3,4-(±)-methylenedioxyamphetamine (MDA), are widely abused illicit amphetamine derivatives. The neurotoxic effects of MDMA/MDA on central dopaminergic (dopamine [DA]) and serotonergic (serotonin [5-HT]) neurons have been well studied (O’Hearn et al., 1988; Stone et al., 1986). 5-HT axons are widely distributed in the forebrain, where 2 morphologically distinct classes (fine and bead) of 5-HT axon terminals exist (Kosofsky and Molliver, 1987; Törk, 1990). Fine axons, the predominant 5-HT axons in most areas of the forebrain, are vulnerable to the neurotoxic effects of MDMA (Mamounas et al., 1991; Molliver, 1987). However, although the serotonergic neurotoxicity of MDMA/MDA is well established, the mechanisms by which MDMA/MDA cause neurotoxicity remain to be elucidated. Moreover, controversy still remains over whether the axonal damage caused by this class of drugs is sufficient to trigger a glial response.
Neuroglia (astrocytes, oligodendrocytes, and microglia) provide physical and trophic support for neurons and respond to local or remote tissue injury, which may either be protective against or potentiate tissue injury (Aschner et al., 1999). Reactive gliosis is a response to various central nervous system (CNS) injuries (Norton et al., 1992; Streit et al., 1999). Astrocytes and microglia are 2 major populations of reactive glial cells, while oligodendrocytes typically do not show reactive changes after CNS injury (Norton, 1999). Reactive microglial cells undergo transformation from ramified resting microglia to phagocytic microglia and alter their immunophenotype, as well as the pattern of cytokine and growth factor secretion (Streit et al., 1999). Reactive astrogliosis manifests as cell swelling, hypertrophy of cellular process and hyperplasia, and changes associated with increased expression of glial fibrillary acidic protein (GFAP) (Norton et al., 1992).
Although reactive gliosis is a universal response to many types of brain injury, little work has been conducted to thoroughly study the characteristics and dynamics of the glial response to amphetamine analogs. In addition, there remains debate over whether the degenerating 5-HT axons following exposure to amphetamine analogs is sufficient to trigger a glial response. para-Chloroamphetamine (PCA) causes selective ablation of serotonergic axons in the forebrain of rats and evokes a mild and delayed microglial response characterized by microglial cell hyper-ramification, an intermediate stage of microglia between the resting and the reactive forms, without affecting astrocytic GFAP expression (Wilson and Molliver, 1994). These data suggest that sensitive microglial markers can detect a subtle axonal lesion that provokes no detectable increase in GFAP expression by astrocytes. MDMA (20mg/kg, IP) increased hippocampal GFAP expression in rats, an effect prevented by prior administration of the antioxidant, α-lipoic acid (Aguirre et al., 1999). In contrast, Pubill et al. (2003) reported that MDMA (20mg/kg, SC, 2 times a day, for 4 days) failed to induce GFAP or Hsp27 immunoreactivity, or microglial activation (determined by assessing [3H]PK11195 binding and OX-6 immunostaining). In C57Bl/6J mice, (±)-MDA and (±)-MDMA (20mg/kg × 4, SC, at 2-h intervals) produce a large increase in GFAP, associated with damage to DA projections in the striatum (Johnson et al., 2002; Miller and O’Callaghan, 1995; O’Callaghan and Miller, 1994). This increase of GFAP is robust 3 days after drug administration and is resolved by 3 weeks (Miller and O’Callaghan, 1995; O’Callaghan and Miller, 1994). In addition, (±)-MDA and (±)-MDMA (20mg/kg × 4, SC, at 2-h intervals) also increase cortical GFAP in C57BL/6J mice, an effect, which parallels the transient decreases in cortical 5-HT (O’Callaghan and Miller, 1994). However, fenfluramine produces a prolonged decrease in cortical 5-HT in rats and mice, without corresponding glial reactions (O’Callaghan and Miller, 1994; Rowland et al., 1993).
Methamphetamine, a phenylethylamine derivative that shares structural similarities with MDMA/MDA, is neurotoxic (Carvalho et al., 2012) and activates microglia (Sekine et al., 2008; Thomas et al., 2004b). Microglial responses have been detected after treatment with a number of substituted amphetamine derivatives, leading some to posit that microglial activation could be used as a marker of amphetamine neurotoxicity (Thomas et al., 2004a). Given the contrasting reports on the ability of MDMA to activate microglia, and the advent of more selective microglial markers (eg, ionized calcium–binding adapter molecule 1 [Iba1]), we initiated an investigation of the degree of microglial activation in response to MDMA administration. Moreover, because direct injection of MDMA into brain does not produce neurotoxicity, we focused our studies on the ability of MDMA metabolites to activate a microglial response in vivo and in vitro.
MATERIALS AND METHODS
Chemicals.
(±)-MDMA-HCl and (±)-MDA-HCl (both listed at >95% purity) were kindly provided by the National Institute on Drug Abuse Drug Supply Program (Bethesda, Maryland), and identity of these compounds was verified by tandem mass spectrometry. α-Methyldopamine (α-MeDA) was a generous gift from Anthony Y. H. Lu (Merck Research Laboratories, Rahway, New Jersey). Glutathione and mushroom tyrosinase (5600U/mg) were obtained from Sigma Chemical Co (St Louis, Missouri). Acetylated low density lipoprotein labeled with 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindo-carbocyanine perchlorate (DiI-ac-LDL) was acquired from Biomedical Technologies, Inc (Cambridge, Massachusetts). Primary antibody against Iba1 was purchased from Wako Chemicals (Richmond, Virginia). MACH4 detection system and blocking reagent were purchased from BioCare Medical (Concord, California). Antibody diluent was purchased from MP Biomedicals (Solon, Ohio). Protease inhibitor cocktail and monoclonal antibody against GFAP were obtained from Roche Molecular Biochemicals (Germany). Hyperfilms used for the Western Blot were purchased from Amersham Life Science (United Kingdom). Rabbit monoclonal antibody against neuron-specific enolase (NSE) and mouse monoclonal antibody against tryptophan hydroxylase (TPH) were obtained from Chemicon International and Sigma Chemical Co, respectively. 5-(Glutathion-S-yl)-α-MeDA, 2,5-bis-(glutathion-S-yl)-α-MeDA, and 5-(N-acetylcystein-S-yl)-α-MeDA were synthesized and purified as previously described (Miller et al., 1995). For primary cell cultures, trypsin, DNase, and Ca2+/Mg2+-free Hank’s buffer were purchased from Sigma Chemical Co. Dulbecco’s modified eagle medium-F12 and minimum essential medium and N2 supplies were obtained from Gibco.
Animals.
Adult male Sprague Dawley rats (~200g) were group housed and maintained on a 12-h light/dark cycle. Food and water were provided ad libitum. Rats were allowed 1 week to acclimate to their surroundings before initiation of experiments. All procedures were approved by the Institutional Animal Care and Use Committee either at the University of Arizona or the University of Texas. Animals were kept at 22°C ± 1°C for housing and all experiments. Embryos from pregnant female Holzman rats (Harlan Sprague Dawley) were obtained at the 18th day of pregnancy and were used for the isolation of cells from the hippocampus.
Drug administration.
Animals were injected with either MDMA (20mg/kg [104 µmol/kg], 1.4ml/kg, SC), MDA (20mg/kg [93 µmol/kg], 1.2ml/kg, SC), or saline. Animals were anesthetized with 3.5ml/kg of a 0.9% saline solution containing 9.4mg/ml sodium pentobarbital and 37.5mg/ml chloral hydrate and surgically implanted with intracerebroventricular (ICV) cannulae as previously described (Miller et al., 1995). Rats were allowed to recover from the surgery for 2 weeks before initiation of drug treatment. Animals were dosed every 12h for a total of 4 consecutive doses. 5-(Glutathion-S-yl)-α-MeDA (720 nmol × 1, 360 nmol × 3), 2,5-bis-(glutathion-S-yl)-α-MeDA (450 nmol × 4), or MDA (2 µmol × 1, 1 µmol × 3) were infused by ICV injection in 10-µl artificial cerebrospinal fluid into the left ventricle of awake, freely moving rats at a rate of 0.2 µl/min, 4 times at 12-h intervals, 14 days after implantation of the cannulae. MDA was administered SC (93 µmol/kg) as a positive control. Control animals experienced the same surgical and recovery procedures and received the same volume of vehicle. Because stab wounding of the rodent cerebrum is a common and well-established model of reactive gliosis (Fujita et al., 1998; Miyake et al., 1992), it is important to minimize the complications of such physical injury following the implantation of cannulae. Reactive astrogliosis can be detected within hours, and reaches a maximum three-seven days postoperation (Miyake et al., 1992). In the present study, all the rats were allowed to recover for 2 weeks from the cannula implantation surgery before drug administration, in order to avoid the reactive gliotic reaction due to the trauma of surgery. Because no phagocytic microglial cells were detected in the control rats following this protocol (Fig. 2A), the microglial response due to the surgery must have subsided by the time of drug administration. In addition, no changes in GFAP expression were observed in the striatum, cortex, and hippocampus of controls at all the sampling time points, indicating that astrocytes were also in a relatively stable status. Thus, we can assume that the changes in GFAP expression and the presence of phagocytic microglial cells in this study were due to toxicant exposure rather than physical damage arising as a consequence of surgery.
Rationale for dosage selection.
The single MDMA/MDA dose was selected based on work by Schmued (2003), where a single dose of MDMA was sufficient to elicit positive Fluoro-Jade B staining in regions of the rat brain including the parietal cortex, indicative of neurodegeneration. We therefore sought to determine whether a single dose of MDMA/MDA would be sufficient to elicit a microglial response on the assumption that if so, then a multiple-dosing regimen of MDMA/MDA would also elicit a similar response. However, with the ICV studies, our prior work revealed that multiple intracranial doses of MDMA/MDA metabolites are necessary to produce neurotoxicity without lethality (Bai et al., 1999; Jones et al., 2005; Miller et al., 1996, 1997). Therefore, we determined that intracranially administered MDA should also be delivered in a multiple-dose regimen.
Immunohistochemistry.
Rats were transcardially perfused under sodium pentobarbital anesthesia with approximately 200ml of 10mM PBS pH 7.2 followed by 200ml of 4% paraformaldehyde in 10mM PBS pH 7.2. Whole brains were then immersed in 4% paraformaldehyde and placed at 4°C for 48h for postfixation. Brains were then rinsed with Milli-Q water and immersed in 70% ethanol before submission to the Histology Service Laboratory at the University of Arizona for paraffin embedding and sectioning. Coronal tissue sections of 7 µm were taken beginning 5mm from the tip of the frontal cortex and mounted on slides for immunohistochemical analysis. The immunohistochemistry method was adapted from Hardwick et al. (2010). Tissue sections were deparaffinized in xylene and rehydrated in ethanol. Antigen retrieval was achieved using citrate buffer (10mM citric acid, 0.05% vol/vol Tween 20, pH 6.0) at 100°C for 6min. Endogenous peroxidase activity was blocked by immersing slides in 0.3% vol/vol H2O2 in methanol, for 20min. Sections were incubated in a 1:1000 dilution of Iba1 antibody for 2h at room temperature and then overnight at 4°C. Antibody binding was detected using the MACH4 detection system following manufacturer recommendations. Color development was achieved by immersion in a 3,3'-diaminobenzidine (DAB) solution (2.5-g nickel (II) sulfate and 20-mg DAB in 100ml 0.175M sodium acetate) for 15min. For quantitation of Iba1 stained area, slides were imaged using an Olympus IMT-2 microscope (Olympus America Inc, Center Valley, Pennsylvania) and a Hamamatsu Orca-100 camera (Hamamatsu Corp, Bridgewater, New Jersey). The digital images were analyzed with SimplePCI v6.5 software (Hamamatsu Corp, Sewickley, Pennsylvania) using a calibration file matched to the magnification used (×200). Images were digitally captured in a systematic fashion, and an intensity threshold was set to select all the stained cells and processes in the field. To discriminate in-plane objects from out-of-plane objects, a second step removed all threshold objects smaller than 2 µm2, as well as any objects that touched the edge of the image. The software then measured the area of the remaining threshold objects. Twelve images were captured per animal, and the average of the 12 images was considered the stained area from a single animal. Two-way ANOVA followed by Bonferroni post hoc tests were employed to determine statistically significant differences over both treatment group and time (GraphPad Prism Software version 5, La Jolla, CA).
Biopsy culture.
Animals were anesthetized with 200mg/kg sodium pentobarbital (IP) and subsequently euthanized by decapitation 72h and 7 days after MDA single administration (SC) or 3 days after multiple dosing with MDA, 5-(glutathion-S-yl)-α-MeDA, or 2,5-bis-(glutathion-S-yl)-α-MeDA (ICV). Tissue samples (2.0×1.0mm) from both experimental and control rats were isolated by punch microdissection from frontal cortex, hippocampus, striatum, cerebellum, and hypothalamus. These biopsies were incubated for 24h in chemically defined N2 medium (Bottenstein and Sato, 1979) with 10% fetal bovine serum (FBS). Samples were then incubated with DiI-ac-LDL for 6h and fixed in 3% formaldehyde in PBS. To determine the number of DiI-ac-LDL(+) microglia present, fixed tissue was pressed under glass coverslips and viewed by fluorescence microscopy (×200 magnification) (Giulian and Ingeman, 1988).
Cell isolation and identification.
Mixed glial and neuronal primary cultures were prepared from E18 embryos. Briefly, the hippocampus was dissociated by trituration with Pasteur pipettes after 15min incubation with 0.2% trypsin (Sigma) and 96U/ml DNase (Sigma) in Ca2+/Mg2+-free Hank’s buffer (Hank’s). Cells were then washed twice with Hank’s, containing 5% FBS, and once with N2 culture media containing 5% FBS and 30mM KCl, followed by centrifugation at 800 × g for 10min. Cells were then resuspended in N2 media supplemented with 5% FBS and 30mM KCl and seeded on poly-l-lysine–coated glass coverslips in 24-well plates (0.25×106/ml/well). After 7 days in culture, half of the media was replaced with fresh N2 media supplemented with 5% FBS and 30mM KCl once a day for 3 days. The final media contained 0.6% FBS. 5-HT–containing neurons were identified in mixed cell preparations by indirect immunohistochemical double staining for NSE and TPH using rabbit monoclonal anti-NSE (1:150; Chemicon International Inc) and mouse monoclonal anti-TPH (1:1000; Sigma). Astroglia were stained to identify GFAP using mouse monoclonal anti-GFAP (1:40, 0.5mg/ml; Boehringer Mannheim Biochemicals).
RESULTS
MDMA Produces a Modest Increase in Microglial Occupancy in the Parietal Cortex
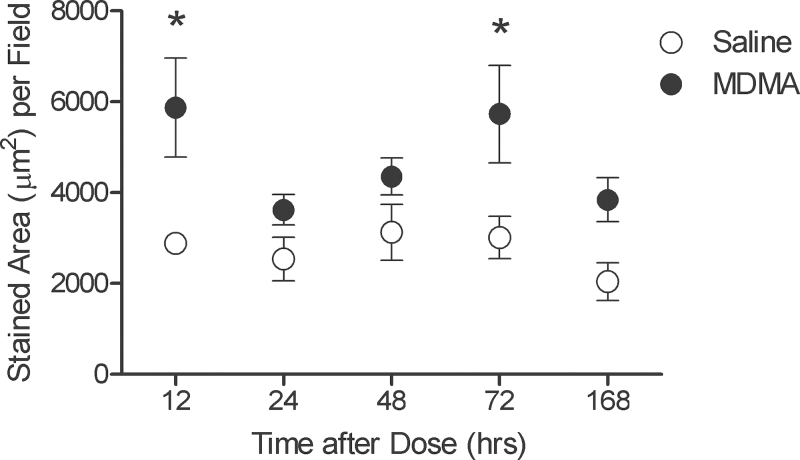
To initially determine the ability of systemically administered MDMA to induce a microglial response in vivo, we determined microglial occupancy, specifically of the parietal cortex, based on Schmued (2003), whose work indicated this region of the brain showed evidence of degenerating neurons following a systemic MDMA dose of 104 µmol/kg. Microglia from the parietal cortex of rats treated with either saline or MDMA, from 12h to 1 week after drug administration, revealed no readily apparent uniform phenotypic changes by this technique. However, there was a 1.73-fold increase in microglial occupancy (µm2/field) when the mean of all fields from MDMA-treated rats was compared against the mean of all fields from saline-treated rats (Fig. 1). Moreover, it appears there exists a biphasic increase in microglial occupancy after MDMA administration, with peak values reached at 12 and 72h after dosing. Two-way ANOVA analysis revealed that drug treatment effect (p < .001) was more significant than either the effect of time (p = .093) or the combined drug and time effect (p = .472). Post hoc Bonferroni tests revealed that only the elevations in stained area at the 12 and 72h time points were significant (p < .05).
FIG. 1.
MDMA increases microglial occupancy in the parietal cortex. Closed circles represent those animals treated with MDMA (104 µmol/kg, SC) and open circles represent those animals treated with saline. Each symbol represents the mean ± SEM of 4 animals. The value for each animal was the mean of stained area from 12 fields. *p < .05 compared with saline-treated animals of identical time point. Abbreviation: MDMA, 3,4-(±)-methylenedioxymethamphetamine.
MDA, 5-(Glutathion-S-yl)-α-MeDA, and 2,5-bis-(Glutathion-S-yl)-α-MeDA Stimulate a Glial Cell Response Following In Vivo Administration
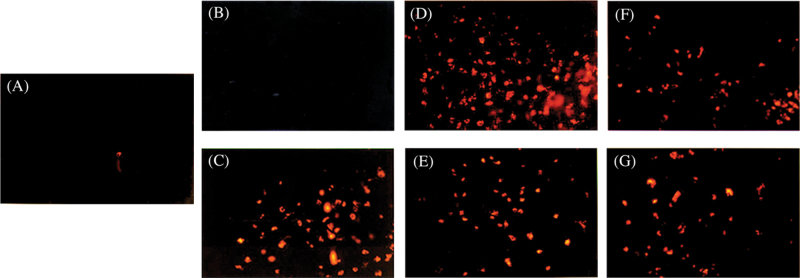
Because MDMA fails to induce neurotoxicity when injected directly into the brain, we subsequently examined the ability of various MDMA metabolites to activate microglial cells using a coupled in vivo/ex vivo assay. Using biopsy culture, a reactive microglial response was observed 72h after a single systemic administration of MDA (93 µmol/kg, SC; Fig. 2C), which subsided by 7 days (data not shown). Activated microglial cells exhibited a rounded, amoeboid morphology. These amoeboid microglia were phagocytic and expressed acetylated low-density lipoprotein (ac-LDL) receptors, as evidenced by the ability of these cells to phagocytize fluorescent DiI-ac-LDL (Fig. 2). However, direct injection of MDA (2 µmol × 1, 1 µmol × 3) into brain failed to reproduce the microglial response observed following systemic administration of MDA (Fig. 2B).
FIG. 2.
Transformation of quiescent microglia to phagocytic microglia following 3,4-(±)-methylenedioxyamphetamine (MDA) (SC), 5-(glutathion-S-yl)-α-MeDA (ICV), or 2,5-bis-(glutathion-S-yl)-α-MeDA (ICV) administration. Activated microglial cells were detected 72h after drug treatment using biopsy cultures coupled with the phagocytosis of fluorescent DiI-ac-LDL, shown as red phagocytic cells. A, control; B, MDA ICV; C, the cortex of MDA (SC)-treated rats; D, the cortex of 5-(glutathion-S-yl)-α-MeDA (ICV)–treated rats; E, the striatum of 5-(glutathion-S-yl)-α-MeDA (ICV)–treated rats; F, the striatum of 2,5-bis-(glutathion-S-yl)-α-MeDA (ICV)–treated rats; and G, the cortex of 2,5-bis-(glutathion-S-yl)-α-MeDA (ICV)–treated rats. Abbreviations: α-MeDA, α-methyldopamine; DiI-ac-LDL, acetylated low density lipoprotein labeled with 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindo-carbocyanine perchlorate.
Both 5-(glutathion-S-yl)-α-MeDA and 2,5-bis-(glutathion-S-yl)-α-MeDA also provoked reactive microgliosis, 3 days after direct ICV administration. The morphology of the DiI-ac-LDL(+) microglial cells present in rats treated with 5-(glutathion-S-yl)-α-MeDA (Fig. 2D and E) or 2,5-bis-(glutathion-S-yl)-α-MeDA (Fig. 2F and G) was identical to that seen in the rats treated peripherally with MDA (Fig. 2C). However, the degree of the microglial response differed between brain regions and was dependent upon the compound administered. In 5-(glutathion-S-yl)-α-MeDA–treated rats, large numbers of DiI-ac-LDL(+) microglia were present in the cortex and striatum, whereas fewer DiI-ac-LDL(+) microglia were observed in the hippocampus. In contrast, the numbers of DiI-ac-LDL(+) microglia were highest in the hippocampus of 2,5-bis-(glutathion-S-yl)-α-MeDA–treated rats, followed by the cortex. Only a few DiI-ac-LDL(+) microglia were observed in the striatum of 2,5-bis-(glutathion-S-yl)-α-MeDA–treated rats.
MDMA Metabolites Stimulate GFAP Expression in Primary Hippocampal Mixed Neuronal/Glial Cell Cultures
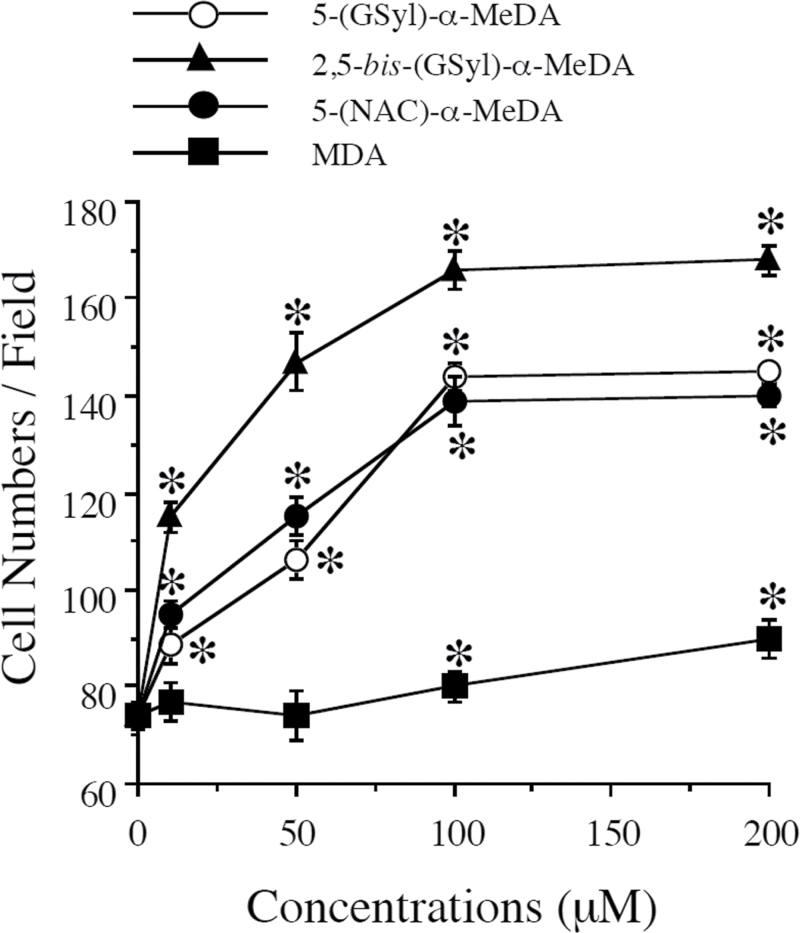
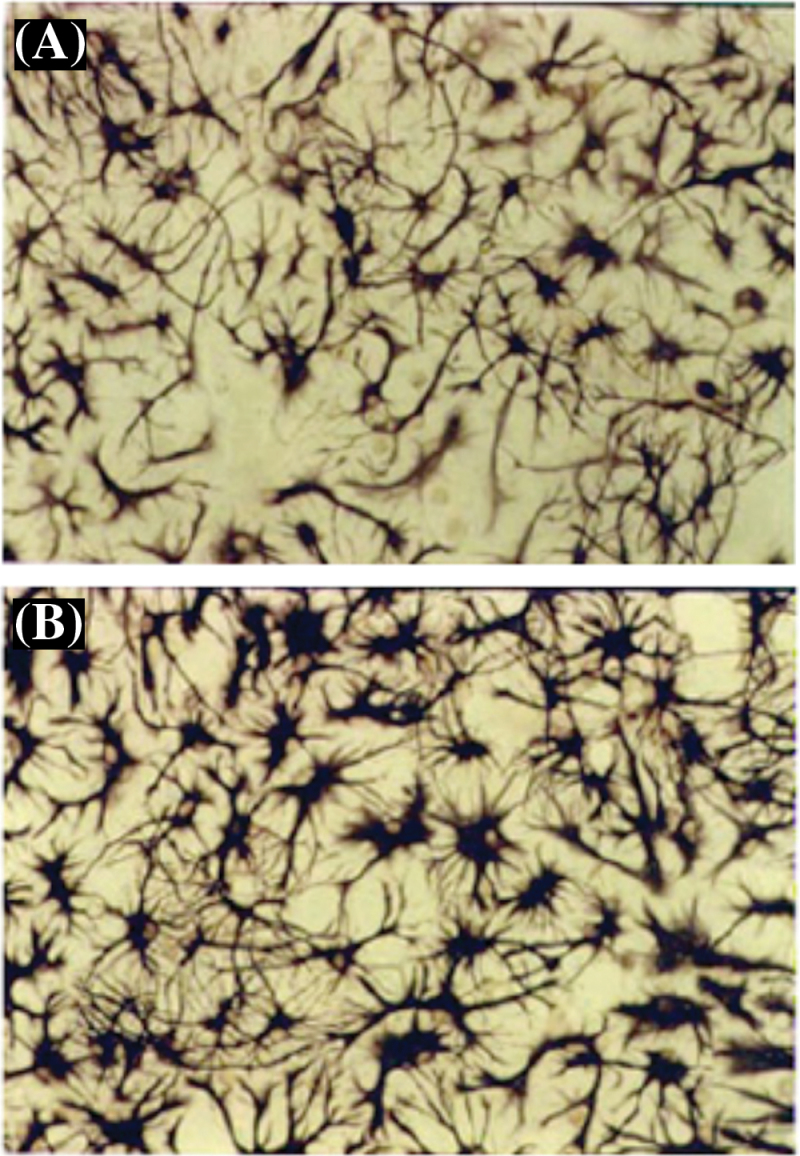
5-(Glutathion-S-yl)-α-MeDA, 5-(N-acetylcystein-S-yl)-α-MeDA, and 2,5-bis-(glutathion-S-yl)-α-MeDA stimulated the dose-dependent proliferation of GFAP(+) astrocytes in E18 primary cell cultures 24h following drug treatment (Fig. 3). Each of the MDMA metabolites produced significant increases in the number of GFAP(+) cells at the lowest dose tested (10µM). The number of GFAP(+) astrocytes increased 2.4-fold in the presence of 100µM 5-(glutathion-S-yl)-α-MeDA compared with controls. Astrocytes in 5-(glutathion-S-yl)-α-MeDA–, 5-(N-acetylcystein-S-yl)-α-MeDA–, or 2,5-bis-(glutathion-S-yl)-α-MeDA–treated mixed primary cell cultures became hyperplastic and hypertrophic (Fig. 4B). Cells labeled with antibodies against GFAP showed darker staining (Fig. 4B) compared with controls (Fig. 4A). GFAP(+) cells were labeled with monoclonal antibody against GFAP and quantified by counting GFAP(+) cells in 10 random selected fields under the bright field microscope. A one-way ANOVA followed by Student Newman-Keuls tests were conducted on the data. GFAP(+) cells were significantly increased following 5-(glutathion-S-yl)-α-MeDA [F(4,45) = 124.695, p = .0001], 5-(N-acetylcystein-S-yl)-α-MeDA [F(4,45) = 86.782, p = .0001], and 2,5-bis-(glutathion-S-yl)-α-MeDA [F(4,45) = 106.686, p = .0001] treatment. GFAP(+) cells were also significantly increased following MDA (100 and 200µM) treatment [F(4,45) = 7.147, p = .0002].
FIG. 3.
Increases in the number of glial fibrillary acidic protein (GFAP)(+) cells following α-MeDA-thioethers or MDA treatment in E18 mixed glial and neuronal primary cultures. E18 mixed glial and neuronal primary cells were treated with 5-(glutathion-S-yl)-α-MeDA, 5-(N-acetylcystein-S-yl)-α-MeDA, 2,5-bis-(glutathion-S-yl)-α-MeDA, or MDA for 24h. The number of GFAP(+) cells was expressed as the mean ± SEM (n = 10 for each treatment). *p < .05 compared with control. Abbreviations: α-MeDA, α-methyldopamine; MDA, 3,4-(±)-methylenedioxyamphetamine.
FIG. 4.
In vitro activation of glia following 5-(glutathion-S-yl)-α-MeDA MDA treatment in E18 mixed glial and neuronal primary cell cultures. Astrocytes in E18 mixed glial and neuronal primary cell cultures are evident as purple colored, star-shaped cells (A). Hypertrophy and overexpression of glial fibrillary acidic protein (GFAP) occurs in GFAP(+) cells when the primary cells are treated with 5-(glutathion-S-yl)-α-MeDA (100µM) (B) for 24h. Similar morphological changes of astrocytes were also observed in primary cells treated with 2,5-bis-(glutathion-S-yl)-α-MeDA (100µM) or 5-(N-acetylcystein-S-yl)-α-MeDA (100µM). Abbreviations: α-MeDA, α-methyldopamine; MDA, 3,4-(±)-methylenedioxyamphetamine.
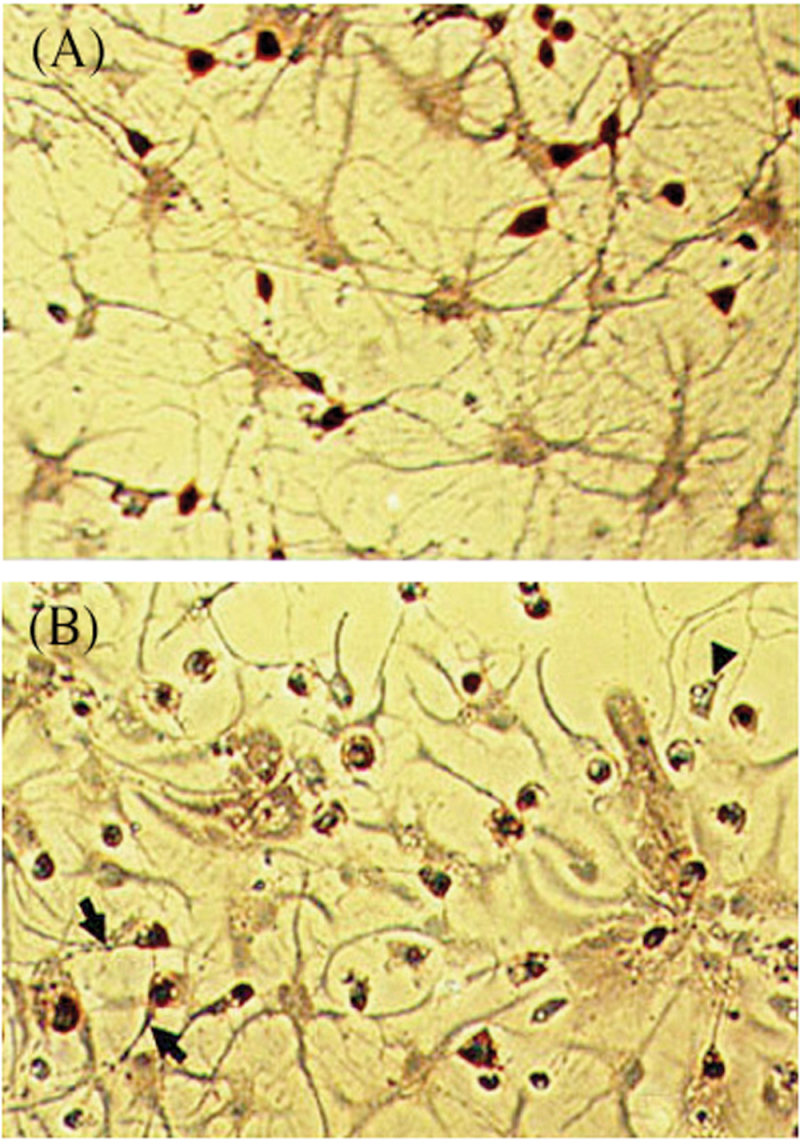
Immunohistochemical double labeling of E18-derived primary hippocampal mixed neuronal/glial cell cultures revealed a clear enrichment in 5-HT–positive staining cells (Fig. 5A). Exposure of the mixed neuronal/glial cell cultures to 5-(glutathion-S-yl)-α-MeDA (100µM) for 24h caused a loss of axons in 5-HT–positive neurons, increased the caliber of 5-HT–positive axons, and induced the formation of large varicosities in these same neurons (Fig. 5B).
FIG. 5.
Damage to serotonergic neurons following 5-(glutathion-S-yl)- a-MeDA treatment in E18 mixed glial and neuronal primary cultures. Serotonin (5-HT)-enriched neurons were labeled using immunohistochemistry double staining for neuron-specific enolase and TPH. A, 5-HT neuronal cell bodies in the control cells exhibit a dark orange color, with fine axonal projections. B, Swelling of the axons (arrows), loss of axons (arrowhead), and vacuolization in the cytoplasm (arrowhead) occurs in 5-HT neurons treated with 5-(GSyl)-α-MeDA (100µM) for 24h. Abbreviation: α-MeDA, α-methyldopamine.
DISCUSSION
Whether or not degenerating 5-HT axons trigger gliosis following exposure to analogs of amphetamine is the subject of debate. (±)- or (+)-Fenfluramine produces a dose-dependent loss of 5-HT uptake and swelling of 5-HT axons, in the absence of concomitant astrocytic reaction in vivo (Rowland et al., 1993). We now show that systemic MDMA administration produces a significant increase in stained microglial area in the parietal cortex (Fig. 1). However, metabolism of MDMA is required to produce neurotoxicity, and although SC injection of MDA, a primary metabolite of MDMA, also produced microglial activation (Fig. 2C), direct injection of MDA into rat brain failed to reproduce this effect (Fig. 2B). The onset of the microglial response to MDMA/MDA (SC) started as early as 12h after administration (Fig. 1), reached a maximum by 3 days (Figs. 1 and 2), and subsided by 7 days (Fig. 1). We speculate that the initial pharmacologic effects of MDMA (hyperthermia) produce the first wave of the microglial response, with the subsequent developing neurodegeneration accounting for the second wave of microglial occupation. Hyperthermia is likely the most important acute MDMA-induced physiological event, because microglial activation is minimal in mice administered methamphetamine in the absence of hyperthermia (Bowyer et al., 2008). As nerve terminals begin to degenerate between 1 and 3 days (Molliver et al., 1990), a secondary microglial response to this neuronal damage is observed. In another study of acute methamphetamine exposure, a significant increase in active microglia was observed 72h following administration (Bowyer et al., 2008).
Glial cell activation and/or proliferation occurs after CNS injury and involves both activated astroglia and microglia (Hatten et al., 1991). Microglia of the normal brain are maintained in a “surveillance state,” in which cells are characterized by multiple ramifications, cellular processes withdrawn and rebuilt to scan a clearly defined territory of brain parenchyma, low proliferative activity, and exhibit no phagocytic activity (Hanisch and Kettenmann, 2007). Ramified microglia, when activated, display an enhanced expression of certain cell surface markers, retract processes, migrate to areas of tissue damage, and engage in vigorous phagocytosis (Wilson and Molliver, 1994). MDA elicited the transformation of quiescent microglia to fully activated phagocytic microglia (Fig. 2C), in contrast to the hyper-ramified intermediate stage present following PCA treatment (Streit et al., 1999; Wilson and Molliver, 1994). It is noteworthy that ICV administration of MDA failed to trigger a microglial response (Fig. 2B), suggesting that MDMA metabolites further downstream of MDA are contributing to the microglial response. Consistent with this view, direct injection of MDA or MDMA into the brain fails to reproduce the acute or long-term neurotoxic effects observed after systemic administration (Esteban et al., 2001; O’Shea et al., 1998).
5-(Glutathion-S-yl)-α-MeDA and 2,5-bis-(glutathion-S-yl)-α-MeDA produce long-term depletions in 5-HT following intrastriatal or intracortical administration (Bai et al., 1999), and potential pathways for their uptake into brain following systemic administration of MDMA/MDA have been described (Monks and Lau, 1997). We have now demonstrated that these serotonergic neurotoxicants are also capable of eliciting reactive microgliosis. In CNS lesions, the presence of activated astrocytes and microglia are most pronounced at the site of injured neurons and their processes (Streit et al., 1999). The microglial response to 5-(glutathion-S-yl)-α-MeDA and 2,5-bis-(glutathion-S-yl)-α-MeDA occurred in brain regions enriched in the 5-HT nerve terminals, the targets of MDMA neurotoxicity. The temporal, anatomical, and morphological characteristics of the reactive gliosis produced by 5-(glutathion-S-yl)-α-MeDA and 2,5-bis-(glutathion-S-yl)-α-MeDA were also comparable with those seen following systemic administration of MDA (Fig. 2C). Moreover, it is important to note that the doses of the various MDA metabolites used in these studies are within the range of amounts formed in vivo following systemic administration of MDA (Miller et al., 1996).
The morphology of activated microglia may be quite plastic and varies depending upon the pathological conditions. Activated microglia may appear as rod cells, perineuronal satellites, or foamy macrophages (Kreutzberg, 1996) and display an increase in expression of a number of cell surface receptors when responding to certain lesions. In particular, scavenger receptors responsible for endocytosis of modified low-density lipoprotein, such as LOX-1 and LRP-1, are among those that may be upregulated (Hendrickx et al., 2013). The activated microglial cells present in the striatum, cortex, hippocampus, and hypothalamus following MDA, 5-(glutathion-S-yl)-α-MeDA and 2,5-bis-(glutathion-S-yl)-α-MeDA administration were rounded, ameboid, phagocytic microglia, expressing scavenger receptors, as evidenced by the ability of these cells to phagocytize the fluorescent probe, DiI-ac-LDL (Fig. 2). The axonal injury produced by MDA, 5-(glutathion-S-yl)-α-MeDA and 2,5-bis-(glutathion-S-yl)-α-MeDA may provide neuron-derived stress signals to glial cells, which subsequently attempt to clear potential deleterious cell debris through phagocytosis. Thus, in instances where mixed glial and neuronal cultures are exposed to either MDA or various thioether metabolites (Fig. 3), the metabolites increase the number of GFAP-positive cells without any observable adverse effects on the glial cells (Fig. 4) while simultaneously causing axonal swelling, cytosolic vacuolization, and axonal loss in 5-HT neurons (Fig. 5). Consistent with these observations, axotomy elicits microglial activation (Kalla et al., 2001). However, signaling mechanisms facilitating this process remain to be established, and we cannot rule out the possibility that the metabolites also directly activate the microglial cells.
Microglia-derived cytokines and growth factors can play either beneficial or harmful roles in response to tissue injury (Giulian et al., 1994; Streit et al., 1999). Subtle, reversible neuronal injury tends to signal the production of neurotrophic factors such as nerve growth factor, neurotrophin-3, and brain-derived neurotrophic factor, all of which participate in both neuronal regeneration and the prevention of neuronal death (Elkabes et al., 1996). In contrast, severe, “irreversible” neuronal damage also signals the production of glial-derived neurotoxins that accelerate degeneration and the disposal of the dying neurons (Giulian et al., 1993). For example, microglia-derived proinflammatory cytokines, reactive oxygen species, nitric oxide, and various proteases are either directly or indirectly capable of contributing to neuronal degeneration (Kraft and Harry, 2011). Elevations in both proinflammatory cytokines (Neri et al., 2010; Torres et al., 2011) and reactive oxygen species/reactive nitrogen species (Darvesh et al., 2005) occur following MDMA treatment, providing a mechanism by which microglia may contribute to MDMA-induced neurotoxicity. In particular, incubation of DA quinones with cultured murine microglial cells proved sufficient to activate these cells (Kuhn et al., 2006). Furthermore, dopaminergic cell membranes modified with DA quinones are also capable of activating cultured microglia that can subsequently damage dopaminergic cells (Le et al., 2001). These findings are particularly relevant to the ability of MDMA metabolites to activate microglia, since the various thioether metabolites are capable of redox cycling (Felim et al., 2007) and accumulate in the rat brain following systemic MDMA administration (Erives et al., 2008).
Interestingly, microglial activation seems to play a larger role in methamphetamine-induced neurotoxicity than in MDMA-induced neurotoxicity, perhaps in part a consequence of the role of DA in the activation of microglia, as evidenced by the observation that elevations in extracellular DA concentrations were accompanied by greater microglial activation (Thomas et al., 2008). Methamphetamine produces a greater magnitude of DA release than does MDMA (Rothman et al., 2001), which may account for this observed difference in microglial response between these phenylethylamine derivatives. However, MDMA does stimulate DA release, albeit to a lesser extent than methamphetamine. Additionally, thioether metabolites of MDMA stimulate uptake of DA via serotonin reuptake transporter into serotonergic cells (Jones et al., 2004). Thus, elevations in extracellular DA concentrations as well as neuronal injury due to DA oxidation within serotonergic terminals likely promote the activation of microglia following MDMA administration.
Serotonergic regeneration occurs 2–8 months after MDA, MDMA, and PCA treatment (Molliver et al., 1990), likely in a process utilizing glia-derived neurotrophic factors. Intracortical infusion of brain-derived neurotrophic factor completely protects rats from PCA-induced loss of 5-HT axons, whereas neurotrophin-3 produces only a modest attenuation in the loss of 5-HT innervation in PCA-treated rats (Mamounas et al., 1995). Microglia also communicate with astrocytes and coordinate response to the neuronal damage. For example, microglia-derived growth factor (interleukin-1) is an astroglial mitogen (Giulian and Lachman, 1985). In turn, microglial proliferation may be regulated via macrophage colony-stimulating factor released from astrocytes (Giulian and Ingeman, 1988; Kloss et al., 1997). An increased presence of microglial cells is observed as early as 12h after MDMA administration but diminishes by 7 days. In contrast, GFAP expression was still elevated 7 days after combined acivicin and MDA (10mg/kg, SC) treatment (Bai et al., 2001). These temporal differences in the onset and recovery of the reactive microgliosis and reactive astrogliosis may reflect the sequential signal exchange between the neurons, microglia, and astrocytes.
In conclusion, MDMA and its metabolites, MDA, 5-(glutathion-S-yl)-α-MeDA, and 2,5-bis-(glutathion-S-yl)-α-MeDA, elicit a modest, but significant, microglial response that subsides 7 days post treatment. Detailed studies are needed to identify the complex signaling pathways involved in the intercellular cross-talk between microglia, astrocytes, and neurons during axonal degeneration and regeneration processed following amphetamine treatment. In particular, further studies are warranted to assess the activation state of the microglia, as well as chemical factors released by these cells following MDMA administration.
FUNDING
National Institute on Drug Abuse (DA023525); the National Institute of Environmental Health Sciences Training in Environmental Toxicology of Complex Diseases Training Grant (5T32ES007091 to J.M.H.); and the Southwest Environmental Health Sciences Center (P30 ES006694).
ACKNOWLEDGMENTS
We thank the NIDA Drug Supply Program for providing MDMA and MDA, as well as Dr A. Y. H. Lu for the generous gift of α-MeDA. We also thank Dr Xia Li and Dr Fengju Bai for work performed at the University of Texas at Austin.
REFERENCES
- Aguirre N., Barrionuevo M., Ramírez M. J., Del Río J., Lasheras B. (1999). Alpha-lipoic acid prevents 3,4-methylenedioxy-methamphetamine (MDMA)-induced neurotoxicity. Neuroreport 10, 3675–3680 [DOI] [PubMed] [Google Scholar]
- Aschner M., Allen J. W., Kimelberg H. K., LoPachin R. M., Streit W. J. (1999). Glial cells in neurotoxicity development. Annu. Rev. Pharmacol. Toxicol. 39, 151–173 [DOI] [PubMed] [Google Scholar]
- Bai F., Jones D. C., Lau S. S., Monks T. J. (2001). Serotonergic neurotoxicity of 3,4-(+/-)-methylenedioxyamphetamine and 3,4-(+/-)-methylendioxymethamphetamine (ecstasy) is potentiated by inhibition of gamma-glutamyl transpeptidase. Chem. Res. Toxicol. 14, 863–870 [DOI] [PubMed] [Google Scholar]
- Bai F., Lau S. S., Monks T. J. (1999). Glutathione and N-acetylcysteine conjugates of alpha-methyldopamine produce serotonergic neurotoxicity: Possible role in methylenedioxyamphetamine-mediated neurotoxicity. Chem. Res. Toxicol. 12, 1150–1157 [DOI] [PubMed] [Google Scholar]
- Bottenstein J. E., Sato G. H. (1979). Growth of a rat neuroblastoma cell line in serum-free supplemented medium. Proc. Natl. Acad. Sci. U. S. A. 76, 514–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowyer J. F., Robinson B., Ali S., Schmued L. C. (2008). Neurotoxic-related changes in tyrosine hydroxylase, microglia, myelin, and the blood-brain barrier in the caudate-putamen from acute methamphetamine exposure. Synapse 62, 193–204 [DOI] [PubMed] [Google Scholar]
- Carvalho M., Carmo H., Costa V. M., Capela J. P., Pontes H., Remião F., Carvalho F., Bastos M. d. e. L. (2012). Toxicity of amphetamines: An update. Arch. Toxicol. 86, 1167–1231 [DOI] [PubMed] [Google Scholar]
- Darvesh A. S., Yamamoto B. K., Gudelsky G. A. (2005). Evidence for the involvement of nitric oxide in 3,4-methylenedioxymethamphetamine-induced serotonin depletion in the rat brain. J. Pharmacol. Exp. Ther. 312, 694–701 [DOI] [PubMed] [Google Scholar]
- Elkabes S., DiCicco-Bloom E. M., Black I. B. (1996). Brain microglia/macrophages express neurotrophins that selectively regulate microglial proliferation and function. J. Neurosci. 16, 2508–2521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erives G. V., Lau S. S., Monks T. J. (2008). Accumulation of neurotoxic thioether metabolites of 3,4-(+/-)-methylenedioxymethamphetamine in rat brain. J. Pharmacol. Exp. Ther. 324, 284–291 [DOI] [PubMed] [Google Scholar]
- Esteban B., O’Shea E., Camarero J., Sanchez V., Green A. R., Colado M. I. (2001). 3,4-Methylenedioxymethamphetamine induces monoamine release, but not toxicity, when administered centrally at a concentration occurring following a peripherally injected neurotoxic dose. Psychopharmacology (Berl). 154, 251–260 [DOI] [PubMed] [Google Scholar]
- Felim A., Urios A., Neudörffer A., Herrera G., Blanco M., Largeron M. (2007). Bacterial plate assays and electrochemical methods: An efficient tandem for evaluating the ability of catechol-thioether metabolites of MDMA (“ecstasy”) to induce toxic effects through redox-cycling. Chem. Res. Toxicol. 20, 685–693 [DOI] [PubMed] [Google Scholar]
- Fujita T., Yoshimine T., Maruno M., Hayakawa T. (1998). Cellular dynamics of macrophages and microglial cells in reaction to stab wounds in rat cerebral cortex. Acta Neurochir. (Wien). 140, 275–279 [DOI] [PubMed] [Google Scholar]
- Giulian D., Ingeman J. E. (1988). Colony-stimulating factors as promoters of ameboid microglia. J. Neurosci. 8, 4707–4717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulian D., Lachman L. B. (1985). Interleukin-1 stimulation of astroglial proliferation after brain injury. Science 228, 497–499 [DOI] [PubMed] [Google Scholar]
- Giulian D., Li J., Li X., George J., Rutecki P. A. (1994). The impact of microglia-derived cytokines upon gliosis in the CNS. Dev. Neurosci. 16, 128–136 [DOI] [PubMed] [Google Scholar]
- Giulian D., Vaca K., Corpuz M. (1993). Brain glia release factors with opposing actions upon neuronal survival. J. Neurosci. 13, 29–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanisch U. K., Kettenmann H. (2007). Microglia: Active sensor and versatile effector cells in the normal and pathologic brain. Nat. Neurosci. 10, 1387–1394 [DOI] [PubMed] [Google Scholar]
- Hardwick R. N., Fisher C. D., Canet M. J., Lake A. D., Cherrington N. J. (2010). Diversity in antioxidant response enzymes in progressive stages of human nonalcoholic fatty liver disease. Drug Metab. Dispos. 38, 2293–2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatten M. E., Liem R. K., Shelanski M. L., Mason C. A. (1991). Astroglia in CNS injury. Glia 4, 233–243 [DOI] [PubMed] [Google Scholar]
- Hendrickx D. A., Koning N., Schuurman K. G., van Strien M. E., van Eden C. G., Hamann J., Huitinga I. (2013). Selective upregulation of scavenger receptors in and around demyelinating areas in multiple sclerosis. J. Neuropathol. Exp. Neurol. 72, 106–118 [DOI] [PubMed] [Google Scholar]
- Johnson E. A., Shvedova A. A., Kisin E., O’Callaghan J. P., Kommineni C., Miller D. B. (2002). d-MDMA during vitamin E deficiency: Effects on dopaminergic neurotoxicity and hepatotoxicity. Brain Res. 933, 150–163 [DOI] [PubMed] [Google Scholar]
- Jones D. C., Duvauchelle C., Ikegami A., Olsen C. M., Lau S. S., de la Torre R., Monks T. J. (2005). Serotonergic neurotoxic metabolites of ecstasy identified in rat brain. J. Pharmacol. Exp. Ther. 313, 422–431 [DOI] [PubMed] [Google Scholar]
- Jones D. C., Lau S. S., Monks T. J. (2004). Thioether metabolites of 3,4-methylenedioxyamphetamine and 3,4-methylenedioxymethamphetamine inhibit human serotonin transporter (hSERT) function and simultaneously stimulate dopamine uptake into hSERT-expressing SK-N-MC cells. J. Pharmacol. Exp. Ther. 311, 298–306 [DOI] [PubMed] [Google Scholar]
- Kalla R., Liu Z., Xu S., Koppius A., Imai Y., Kloss C. U., Kohsaka S., Gschwendtner A., Möller J. C., Werner A., et al. (2001). Microglia and the early phase of immune surveillance in the axotomized facial motor nucleus: Impaired microglial activation and lymphocyte recruitment but no effect on neuronal survival or axonal regeneration in macrophage-colony stimulating factor-deficient mice. J. Comp. Neurol. 436, 182–201 [PubMed] [Google Scholar]
- Kloss C. U., Kreutzberg G. W., Raivich G. (1997). Proliferation of ramified microglia on an astrocyte monolayer: Characterization of stimulatory and inhibitory cytokines. J. Neurosci. Res. 49, 248–254 [PubMed] [Google Scholar]
- Kosofsky B. E., Molliver M. E. (1987). The serotoninergic innervation of cerebral cortex: Different classes of axon terminals arise from dorsal and median raphe nuclei. Synapse 1, 153–168 [DOI] [PubMed] [Google Scholar]
- Kraft A. D., Harry G. J. (2011). Features of microglia and neuroinflammation relevant to environmental exposure and neurotoxicity. Int. J. Environ. Res. Public Health 8, 2980–3018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutzberg G. W. (1996). Microglia: A sensor for pathological events in the CNS. Trends Neurosci. 19, 312–318 [DOI] [PubMed] [Google Scholar]
- Kuhn D. M., Francescutti-Verbeem D. M., Thomas D. M. (2006). Dopamine quinones activate microglia and induce a neurotoxic gene expression profile: Relationship to methamphetamine-induced nerve ending damage. Ann. N. Y. Acad. Sci. 1074, 31–41 [DOI] [PubMed] [Google Scholar]
- Le W., Rowe D., Xie W., Ortiz I., He Y., Appel S. H. (2001). Microglial activation and dopaminergic cell injury: An in vitro model relevant to Parkinson’s disease. J. Neurosci. 21, 8447–8455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamounas L. A., Blue M. E., Siuciak J. A., Altar C. A. (1995). Brain-derived neurotrophic factor promotes the survival and sprouting of serotonergic axons in rat brain. J. Neurosci. 15, 7929–7939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamounas L. A., Mullen C. A., O’Hearn E., Molliver M. E. (1991). Dual serotoninergic projections to forebrain in the rat: Morphologically distinct 5-HT axon terminals exhibit differential vulnerability to neurotoxic amphetamine derivatives. J. Comp. Neurol. 314, 558–586 [DOI] [PubMed] [Google Scholar]
- Miller D. B., O’Callaghan J. P. (1995). The role of temperature, stress, and other factors in the neurotoxicity of the substituted amphetamines 3,4-methylenedioxymethamphetamine and fenfluramine. Mol. Neurobiol. 11, 177–192 [DOI] [PubMed] [Google Scholar]
- Miller R. T., Lau S. S., Monks T. J. (1995). Metabolism of 5-(glutathion-S-yl)-alpha-methyldopamine following intracerebroventricular administration to male Sprague-Dawley rats. Chem. Res. Toxicol. 8, 634–641 [DOI] [PubMed] [Google Scholar]
- Miyake T., Okada M., Kitamura T. (1992). Reactive proliferation of astrocytes studied by immunohistochemistry for proliferating cell nuclear antigen. Brain Res. 590, 300–302 [DOI] [PubMed] [Google Scholar]
- Molliver M. E. (1987). Serotonergic neuronal systems: What their anatomic organization tells us about function. J. Clin. Psychopharmacol. 7(Suppl. 6), 3S–23S [PubMed] [Google Scholar]
- Miller R. T., Lau S. S., Monks T. J. (1996). Effects of intracerebroventricular administration of 5-(glutathion-S-yl)-alpha-methyldopamine on brain dopamine, serotonin, and norepinephrine concentrations in male Sprague-Dawley rats. Chem. Res. Toxicol. 9, 457–465 [DOI] [PubMed] [Google Scholar]
- Miller R. T., Lau S. S., Monks T. J. (1997). 2,5-Bis-(glutathion-S-yl)-alpha-methyldopamine, a putative metabolite of (+/-)-3,4-methylenedioxyamphetamine, decreases brain serotonin concentrations. Eur. J. Pharmacol. 323, 173–180 [DOI] [PubMed] [Google Scholar]
- Molliver M. E., Berger U. V., Mamounas L. A., Molliver D. C., O’Hearn E., Wilson M. A. (1990). Neurotoxicity of MDMA and related compounds: Anatomic studies. Ann. N. Y. Acad. Sci. 600, 649–61; discussion 661 [DOI] [PubMed] [Google Scholar]
- Monks T. J., Lau S. S. (1997). Biological reactivity of polyphenolic-glutathione conjugates. Chem. Res. Toxicol. 10, 1296–1313 [DOI] [PubMed] [Google Scholar]
- Neri M., Bello S., Bonsignore A., Centini F., Fiore C., Földes-Papp Z., Turillazzi E., Fineschi V. (2010). Myocardial expression of TNF-alpha, IL-1beta, IL-6, IL-8, IL-10 and MCP-1 after a single MDMA dose administered in a rat model. Curr. Pharm. Biotechnol. 11, 413–420 [DOI] [PubMed] [Google Scholar]
- Norton W. T. (1999). Cell reactions following acute brain injury: A review. Neurochem. Res. 24, 213–218 [DOI] [PubMed] [Google Scholar]
- Norton W. T., Aquino D. A., Hozumi I., Chiu F. C., Brosnan C. F. (1992). Quantitative aspects of reactive gliosis: A review. Neurochem. Res. 17, 877–885 [DOI] [PubMed] [Google Scholar]
- O’Callaghan J. P., Miller D. B. (1994). Neurotoxicity profiles of substituted amphetamines in the C57BL/6J mouse. J. Pharmacol. Exp. Ther. 270, 741–751 [PubMed] [Google Scholar]
- O’Hearn E., Battaglia G., De Souza E. B., Kuhar M. J., Molliver M. E. (1988). Methylenedioxyamphetamine (MDA) and methylenedioxymethamphetamine (MDMA) cause selective ablation of serotonergic axon terminals in forebrain: Immunocytochemical evidence for neurotoxicity. J. Neurosci. 8, 2788–2803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shea E., Granados R., Esteban B., Colado M. I., Green A. R. (1998). The relationship between the degree of neurodegeneration of rat brain 5-HT nerve terminals and the dose and frequency of administration of MDMA (‘ecstasy’). Neuropharmacology 37, 919–926 [DOI] [PubMed] [Google Scholar]
- Pubill D., Canudas A. M., Pallàs M., Camins A., Camarasa J., Escubedo E. (2003). Different glial response to methamphetamine- and methylenedioxymethamphetamine-induced neurotoxicity. Naunyn. Schmiedebergs. Arch. Pharmacol. 367, 490–499 [DOI] [PubMed] [Google Scholar]
- Rothman R. B., Baumann M. H., Dersch C. M., Romero D. V., Rice K. C., Carroll F. I., Partilla J. S. (2001). Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse 39, 32–41 [DOI] [PubMed] [Google Scholar]
- Rowland N. E., Kalehua A. N., Li B. H., Semple-Rowland S. L., Streit W. J. (1993). Loss of serotonin uptake sites and immunoreactivity in rat cortex after dexfenfluramine occur without parallel glial cell reactions. Brain Res. 624, 35–43 [DOI] [PubMed] [Google Scholar]
- Schmued L. C. (2003). Demonstration and localization of neuronal degeneration in the rat forebrain following a single exposure to MDMA. Brain Res. 974, 127–133 [DOI] [PubMed] [Google Scholar]
- Sekine Y., Ouchi Y., Sugihara G., Takei N., Yoshikawa E., Nakamura K., Iwata Y., Tsuchiya K. J., Suda S., Suzuki K., et al. (2008). Methamphetamine causes microglial activation in the brains of human abusers. J. Neurosci. 28, 5756–5761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone D. M., Stahl D. C., Hanson G. R., Gibb J. W. (1986). The effects of 3,4-methylenedioxymethamphetamine (MDMA) and 3,4-methylenedioxyamphetamine (MDA) on monoaminergic systems in the rat brain. Eur. J. Pharmacol. 128, 41–48 [DOI] [PubMed] [Google Scholar]
- Streit W. J., Walter S. A., Pennell N. A. (1999). Reactive microgliosis. Prog. Neurobiol. 57, 563–581 [DOI] [PubMed] [Google Scholar]
- Thomas D. M., Dowgiert J., Geddes T. J., Francescutti-Verbeem D., Liu X., Kuhn D. M. (2004a). Microglial activation is a pharmacologically specific marker for the neurotoxic amphetamines. Neurosci. Lett. 367, 349–354 [DOI] [PubMed] [Google Scholar]
- Thomas D. M., Francescutti-Verbeem D. M., Kuhn D. M. (2008). The newly synthesized pool of dopamine determines the severity of methamphetamine-induced neurotoxicity. J. Neurochem. 105, 605–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. M., Walker P. D., Benjamins J. A., Geddes T. J., Kuhn D. M. (2004b). Methamphetamine neurotoxicity in dopamine nerve endings of the striatum is associated with microglial activation. J. Pharmacol. Exp. Ther. 311, 1–7 [DOI] [PubMed] [Google Scholar]
- Törk I. (1990). Anatomy of the serotonergic system. Ann. N. Y. Acad. Sci. 600, 9–34; discussion 34 [DOI] [PubMed] [Google Scholar]
- Torres E., Gutierrez-Lopez M. D., Mayado A., Rubio A., O’Shea E., Colado M. I. (2011). Changes in interleukin-1 signal modulators induced by 3,4-methylenedioxymethamphetamine (MDMA): Regulation by CB2 receptors and implications for neurotoxicity. J. Neuroinflammation 8, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M. A., Molliver M. E. (1994). Microglial response to degeneration of serotonergic axon terminals. Glia 11, 18–34 [DOI] [PubMed] [Google Scholar]