Abstract
Genistein (Gen), the primary isoflavone in soy, has been shown to adversely affect various endocrine-mediated endpoints in rodents and humans. Soy formula intake by human infants has been associated with early age at menarche and decreased female-typical behavior in girls. Adipose deposition and expansion are also hormonally regulated and Gen has been shown to alter these processes. However, little is known about the impact of early-life soy intake on metabolic homeostasis in adulthood. The current study examined the impact of early-life Gen exposure on adulthood body composition (by magnetic resonance imaging) and the molecular signals mediating adipose expansion. From postnatal day (PND) 1 to 22, rat pups were daily orally dosed with 50mg/kg Gen to mimic blood Gen levels in human infants fed soy formula. Female but not male Gen-exposed rats had increased fat/lean mass ratio, fat mass, adipocyte size and number, and decreased muscle fiber perimeter. PND22 Gen-exposed females, but not males, had increased expression of adipogenic factors, including CCAAT/enhancer binding protein alpha (Cebpα), CCAAT/enhancer binding protein beta (Cebpβ), and peroxisome proliferator-activated receptor gamma (Pparγ). Furthermore, Wingless-related MMTV integration site 10b (Wnt10b), a critical regulator of adipogenic cell fate determination, was hypermethylated and had decreased expression in adipose of PND22 Gen-exposed females. These data suggest that developmental Gen exposure in rats has gender-specific effects on adiposity that closely parallel the effects of a postweaning high-fat diet and underscore the importance of considering timing of exposure and gender when establishing safety recommendations for early-life dietary Gen intake.
Key Words: adipogenesis, body composition, genistein, methylation, obesity, soy infant formula.
Obesity, and specifically increased adiposity, is the primary risk factor for numerous adult-onset diseases, and the rates of obesity are alarmingly high, both in the developed and developing worlds (Misra et al., 2010). Body weight and fat mass homeostasis are tightly regulated processes (Lee et al., 2012; Ricci et al., 2010; Sathyapalan et al., 2011). Certain dietary botanical phytoestrogens, including genistein (Gen), can affect endocrine balance and energy metabolism by acting through estrogen receptors (ERs) to activate estrogen-stimulated signaling cascades (Kuiper et al., 1998). The intake of Gen has been implicated in modulating various physiological processes in humans (Cruz et al., 2006; Kreijkamp-Kaspers et al., 2004), and although several studies have shown that Gen affects adiposity and hepatic metabolism in adult animals (Kim et al., 2005; Penza et al., 2006), few studies are available demonstrating the impact and metabolic consequences of Gen exposure during early developmental windows.
The primary mode of early-life Gen exposure in humans occurs through the intake of infant soy-based formulas, which constitute approximately 13% of the infant formula market in the United States (2009 International Formula Council comment; McCarver et al., 2011). Gen is the primary isoflavone constituent within soy-based formulas, and plasma levels of Gen in U.S. infants that consume soy-based formulas have been shown to be higher than in Japanese men consuming a traditional soy-based diet and much higher than in omnivorous U.S. adults (684ng/ml vs 105 and 4.7ng/ml, respectively) (McCarver et al., 2011; Setchell et al., 1997). Developmental Gen dosing studies in rodents have clearly classified Gen as a reproductive toxicant and endocrine disruptor (Jefferson et al., 2009; Newbold et al., 2001), but despite the critical role of sex hormones in energy homeostasis, there is little research describing the long-term consequences of early-life Gen exposure on body composition or energy metabolism.
Adulthood adiposity can be programmed by developmental dietary exposures in experimental animal models and in humans. In rodents, both maternal and neonatal macronutrient manipulations have been shown to increase adiposity and metabolic syndrome in the offspring (Ainge et al., 2010; Bayol et al., 2005; Fuente-Martin et al., 2012; Nielsen et al., 2012). In humans, it is difficult to establish direct causality between early-life exposures and adulthood obesity, but strong associations of childhood body composition with maternal diet during gestation and lactation and with maternal breastfeeding versus formula feeding practices have been observed (Aaltonen et al., 2011; Cole et al., 2009; Wu et al., 2012; Yin et al., 2012). These studies demonstrate that the propensity for increased adipose tissue accretion or expansion has developmental origins, but the mechanisms for this programmed response remain unclear and likely involve alterations in numerous developmental and endocrine signaling pathways.
Studies classifying Gen as a developmental endocrine disruptor have primarily focused on reproductive outcomes, but the endocrine basis for energy metabolism warrants the investigation of Gen’s capacity to dysregulate early-life signals that establish adulthood energy metabolism and body composition. Therefore, the current study investigated the long-term impact of direct early developmental Gen exposure (from postnatal day [PND] 1 to 22) on body composition in male and female offspring. Furthermore, we focused on developmental markers of adiposity to characterize the potential molecular genetic and epigenetic basis for the morphological changes we observed. Overall, results from this study demonstrate that early-life Gen exposure has gender-specific effects on fat mass that persist into adulthood, and these changes are preceded by developmental changes to key molecular markers of adipogenesis at the level of gene expression and DNA methylation.
MATERIALS AND METHODS
Animals, genistein dosing, and dietary treatment.
Timed-pregnant Sprague Dawley female rats (n = 12) were obtained from Charles River (Wilmington, Massachusetts) on embryonic day 2 and were individually housed in ventilated cages in a temperature-controlled environment and fed ad libitum an AIN-93G diet, which was developed to provide suitable nutrition for growth, pregnancy, and lactation (Table 1). Immediately after birth, gender distributions and offspring weights were recorded, pups from all dams were mixed and randomized, and 4 male and 4 female pups were returned to each dam. After being allowed to acclimate for 36h, pups were orally dosed daily with genistein (n = 6 litters; Gen, 50mg/kg BW/day) diluted in tocopherol-stripped corn oil or corn oil alone (n = 6 litters; Oil, as a vehicle control) until weaning on PND22. The Gen dose was selected because it has been demonstrated in previous rodent studies to produce blood total Gen levels (approximately 3.0μM [270ng/ml] after 1h and approximately 1.0μM [270ng/ml] after 12h) similar to those in human infants consuming soy-based formulas (approximately 2.5μM [676ng/ml]) (Cimafranca et al., 2010; Doerge et al., 2002; Setchell et al., 1997). Oral dosing was performed using a 1–10 μl Eppendorf pipette and sterilized tips. Pups were gently held while they suckled on the tip. After weaning on PND22, 5 male and 5 female pups from each treatment group (Gen and Oil) were euthanized to collect the gastrocnemius muscle and white adipose tissues (WAT), which were snap frozen in liquid nitrogen and stored at −80°C for subsequent analyses. Uteri from female offspring were also collected and weighed at this time. For the remainder of the offspring, a subset of the Oil-treated offspring were either kept on the same modified AIN-93G diet as the Gen-dosed pups (Oil-C) or were fed a high-fat (HF) diet (Oil-HF, Table 1) to establish a standard model of diet-induced obesity for comparison to the Gen-exposed pups (Gen-C). The HF diet contained 45% kcal from fat and 4.8 kcal/g (compared with 16% and 4.0 kcal/g in the Oil-C rats fed the AIN-93G diet). The HF diet had increased saturated fat content and decreased corn starch. All other dietary components were matched across the 2 diets. Offspring remained on these diets until the end of the study on PND110, when the remaining animals were euthanized with CO2. Tissues were either snap frozen in liquid nitrogen and stored at −80°C (adipose and muscle) or fixed in 10% formalin for future analyses (liver, pancreas, adipose, and muscle), and serum was collected via vena cava puncture into glass tubes, allowed to clot, centrifuged, and stored at −80°C for the analysis of serum estradiol, insulin, glucose, leptin, and adiponectin.
TABLE 1.
Diet Compositiona
| % | Control (C) | High Fat (HF) | ||
|---|---|---|---|---|
| g | kcal | g | kcal | |
| Protein | 20 | 20 | 24 | 20 |
| Carbohydrate | 64 | 64 | 41 | 35 |
| Fat | 7 | 16 | 24 | 45 |
| kcal/g | 4.0 | 4.8 | ||
| Ingredients | ||||
| Casein | 200 | 800 | 200 | 800 |
| l-Cystine | 3 | 12 | 3 | 12 |
| Corn starch | 397.5 | 1590 | 105 | 420 |
| Maltodextrin | 132 | 528 | 132 | 528 |
| Sucrose | 100 | 400 | 100 | 400 |
| Cellulose | 50 | 0 | 50 | 0 |
| Soybean oil | 70 | 630 | 70 | 630 |
| Lard | 0 | 0 | 130 | 1170 |
| Mineral mix | 35 | 0 | 35 | 0 |
| Vitamin mix | 10 | 40 | 10 | 40 |
| Choline bitartrate | 2.5 | 0 | 2.5 | 0 |
aResearch Diets, Inc, New Brunswick, New Jersey.
Body weight, energy intake, and body composition.
Body weight and food intake were measured every 4 days. Energy intake after weaning was calculated by weighing pellets remaining on the fourth day, subtracting from initial food supplied, and multiplying daily calculated intake by the caloric composition of each diet (4.0 kcal/g in Control and 4.8 kcal/g in HF). Body composition was measured on PND1, PND21, PND60, and PND90 using the EchoMRI-700 Body Composition Analyzer (Echo Medical Systems, Houston, Texas), which allows for the precise measurement of fat and lean mass in conscious and unrestrained animals using magnetic resonance imaging (MRI). Briefly, animals were placed into a plastic tube that had a stopper at one end and air holes at the other, which allowed for sufficient air flow but restricted movement. The tube with the rat was then inserted into the analyzer, and each scan lasted approximately 25 s. On PND1 and PND21, pups were scanned as a litter, but separated by gender, and on PND60 and PND90, all animals were scanned individually.
Histopathology.
At the end of the study (PND110), gastrocnemius muscle, WAT, liver, and pancreas samples from adult animals were fixed in 10% formalin, embedded in paraffin, and 3 µm histological slides were prepared and stained with hematoxylin & eosin. Histopathological evaluation was performed by a pathologist. All pathological findings were graded from 1 (minimal) to 5 (severe). For further evaluation and quantification of WAT and muscle, 2 independent representative fields were chosen for analysis from 5 male and 5 female offspring in each treatment group (Oil-C, Oil-HF, and Gen). Approximately 120–180 independent muscle fibers and 80–150 independent adipocytes were manually circled or counted per image to calculate the perimeter or cell number density (ImageJ Software, NIH, Bethesda, Maryland). Because initial histopathological examination of WAT clearly showed more tightly packed and irregularly shaped adipocytes in Gen-treated females compared with Oil-C females controls, a geometric approach was utilized for quantifying this phenomenon. This “adipose tissue cell density” was calculated by first determining cell area and the number of these cells that are expected to fit into each independent window view without overlap. Where cellular borders were not clearly obvious, circularity was predicted by finding the center of cells where at least half of the perimeter was visible, and using the circle feature within the software to predict the full cell perimeter (and area). The actual number of cells within each view was then divided by the calculated expected cell number, such that a higher ratio indicates an increase in adipose tissue cell density, which is also reflected in the representative images.
Oral glucose tolerance test.
On PND25 and PND97, offspring were fasted overnight, and their fasted blood glucose level was tested as the baseline the following morning. Immediately following the fasted measurement, each animal was orally gavaged with a 2g/kg BW bolus of d-glucose dissolved in water (50% wt/vol). Plasma glucose was checked after 30, 60, and 120min of the bolus. All measurements were taken using the Accu-Chek Glucometer and Comfort Curve strips (Roche, Indianapolis, Indiana) from a tail vein nick.
Serum measurements.
To determine unfasted circulating triglyceride or glucose levels, serum samples obtained from adult males and females on PND110 were thawed on ice and analyzed via either the Thermo Infinity Triglycerides Liquid Stable Reagent or the Glucose Oxidase Reagent (Thermo Fisher Scientific, Rockford, Illinois) following company protocol and using a commercially available standard reference kit (Verichem Laboratories, Providence, Rhode Island). ELISA kits were purchased and utilized per manufacturer’s instruction to measure serum 17β-estradiol (Enzo, Cat. no. ADI-900-174), adiponectin (Invitrogen, Cat. no. KRP0041), insulin (Mercodia, Cat. no. 10-1250-01), and leptin (Invitrogen, Cat. no. KRC2281) in the same serum samples collected from unfasted animals on PND110.
Adipose RNA isolation and RT-PCR analysis in PND22 pups.
To investigate the mRNA expression of adipogenic genes in WAT (CCAAT/enhancer binding protein alpha [Cebpα], CCAAT/enhancer binding protein beta [Cebpβ], peroxisome proliferator-activated receptor gamma [Pparγ], and Wingless-related MMTV integration site 10b [Wnt10b]) and myogenic genes in muscle (myogenic factor 5 [Myf5], myogenic differentiation 1 [MyoD1], and Wnt10b), frozen WAT or gastrocnemius muscle (100mg) from 5 male and 5 female PND22 pups from each treatment group (Oil or Gen) was ground in liquid nitrogen with mortar and pestle. Total RNA isolation, cDNA synthesis, and real-time PCR were performed as previously described (Strakovsky and Pan, 2011) with an additional spin-down step during RNA isolation to remove the lipid layer in adipose. A serial dilution was used to create a standard curve for quantification and a dissociation curve was analyzed following each reaction. All primers for real-time PCR analysis (Table 2) were designed using the VectorNTI software (Life Technologies, Grand Island, New York), analyzed using BLAST, and synthesized by IDT (Coralville, Iowa). All mRNA data were normalized to the housekeeping gene encoding ribosomal protein L7a (L7a).
TABLE 2.
Primer Information for mRNA and Methylation Analyses
| Gene for mRNA Analysis | Forward Sequence and Transcript Location | Reverse Sequence and Transcript Location | Transcript ID |
|---|---|---|---|
| Cebpα | AGTCGGTGGATAAGAACAGCAACG(+821) | GCTGTTTGGCTTTATCTCGGCTC(+910) | ENSRNOT00000014517 |
| Cebpβ | AGAACGAGCGGCTGCAGAAGA(+1220) | GAACAAGTTCCGCAGCGTGC(+1287) | ENSRNOT00000072673 |
| Myf5 | CATCCGAGTTGGCTCTTCAGGAC(+885) | TAAGTCTGGAACTGGAGGACCCG(+980) | ENSRNOT00000006453 |
| MyoD1 | CCTTTCCTCACAGTCCCTAG(+1521) | CAACAGGGATGTGGAAGG(+1589) | ENSRNOT00000015109 |
| Pparγ | GGTGCTCCAGAAGATGAC(+1431) | GGCTCATATCTGTCTCCG(+1530) | ENSRNOT00000051858 |
| Wnt10b | GCGTTCTCCTTCTCCATGCTGG(+683) | CAGCTTACCCAAGCTGCAGGCT(+754) | ENSRNOT00000019505 |
| L7a | GAGGCCAAAAAGGTGGTCAATCC(+64) | CCTGCCCAATGCCGAAGTTCT(+127) | ENSRNOT00000006754 |
| Wnt10b Methylation Analysis | Forward Sequence and DNA Location | Reverse Sequence and DNA Location | |
| ENSRNOT00000019505 | |||
| Upstream of promoter CpG methylated | TTTCGGAGGGTTTTAGTTGTTC(-247) | ACCCCTTAACTTCCAATATCTACGT(-173) | |
| Upstream of promoter CpG unmethylated | TTTTGGAGGGTTTTAGTTGTTTG(-247) | CCCCTTAACTTCCAATATCTACATT(-174) | |
| Downstream of promoter CpG methylated | AGGTTGGTGTTTTTAGAGTTTTAGC(+505) | AAAACAAATTTAACCCTTAAACGAT(+575) | |
| Downstream of promoter CpG unmethylated | TTTAGGTTGGTGTTTTTAGAGTTTTAGT(+502) | AAAACAAATTTAACCCTTAAACAAT(+575) | |
| Exon3 methylated (Set1) | TTATGTTGGTTGTTGGTGTTATGTAC(+2263) | GAAACCGATCCTACTCACCG(+2370) | |
| Exon3 unmethylated (Set1) | TGTTGGTTGTTGGTGTTATGTATG(+2266) | CAAAACCAATCCTACTCACCACT(+2371) | |
| Exon3 methylated (Set2) | AGTTGGTGAGTTGTGGTTGC(+2310) | ACAACTTAACCCGAAACCGAT(+2382) | |
| Exon3 unmethylated (Set2) | GTAAGTTGGTGAGTTGTGGTTGT(+2314) | TACAACAACTTAACCCAAAACCAAT(+2386) | |
| Exon3 methylated (Set3) | GAAGTTTTTCGGGATATTTAGGC(+2550) | ACCAAATAAACACACATACCTAACG(+2628) | |
| Exon3 unmethylated (Set3) | GAAGTTTTTTGGGATATTTAGGTGA(+2550) | CAAATAAACACACATACCTAACACC(+2626) | |
| Upstream of Exon4 CpG methylated (Set1) | TATTTTGATGTTGTAGGTGGTAATC(+3385) | ATACCATAACATTTACACTTCCGCT(+3444) | |
| Upstream of Exon4 CpG unmethylated (Set1) | GTTATTTTGATGTTGTAGGTGGTAATT(+3383) | ATACCATAACATTTACACTTCCACT(+3444) | |
| Upstream of Exon4 CpG methylated (Set2) | GTAGTTTTAGAGTTTCGGGTTATCG(+3479) | ATAAAAATAACTCTACCCAACCGCT(+3543) | |
| Upstream of Exon4 CpG unmethylated (Set2) | TAGTTTTAGAGTTTTGGGTTATTGG(+3480) | ATAAAAATAACTCTACCCAACCACT(+3543) | |
| First Exon4 CpG methylated | GAGAAGTTTTTTGATTTTTGTGAGC(+3622) | TATTACAAACCCGACCTCTCGTA(+3694) | |
| First Exon4 CpG unmethylated | GAAGTTTTTTGATTTTTGTGAGTGA(+3624) | CTTATTACAAACCCAACCTCTCATA(+3696) | |
| Second Exon4 CpG methylated | GGTCGGGTTTGTAATAAGATTAGTC(+3680) | GAAACACGTTATACCCACGAC(+3758) | |
| Second Exon4 CpG unmethylated | AGGTTGGGTTTGTAATAAGATTAGTTGT(+3679) | TCTACCAAAACACATTATACCCACA(+3764) |
Genomic DNA isolation and methylation analysis of Wnt10b using methylation-sensitive PCR in PND22 pups.
Frozen WAT (100mg) from 5 male and 5 female PND22 pups from each group (Oil or Gen) was ground in liquid nitrogen with a mortar and pestle. Genomic DNA (gDNA) was isolated using the GenElute Mammalian Genomic DNA Purification Kit (Sigma-Aldrich) per manufacturer’s instruction with an additional initial spin-down to remove the lipid layer. gDNA (20ng/µl in a 20-µl reaction volume) was bisulfite converted using the EZ DNA Methylation-Gold Kit (Zymo Research, Irvine, California), following the manufacturer’s instructions. Real-time PCR was performed using 20ng gDNA as the template, SYBR Green PCR Master Mix (Quanta Biosciences, Gaithersburg, Maryland), and 5μM of each forward and reverse primer (Table 2) designed using the methprimer website (http://www.urogene.org/methprimer/index.html) (Li and Dahiya, 2002) to specifically amplify either the methylated or unmethylated templates in the 7300 Real-Time PCR System (Applied Biosystem) as for mRNA analysis, but using 45 cycles. A serial dilution from a reaction combining 1 sample from each experimental group was used to create an internal standard curve for quantification. Primers covered 1 region upstream of the promoter-spanning CpG island, 1 CpG island and “shore” downstream of the promoter, 3 regions within a non-CpG island containing exon, 2 regions upstream of the CpG islands in exon 4, 1 region within the first CpG island in exon 4, and 1 within the second CpG island in exon 4. Data are presented as the ratio of methylated values divided by the sum of methylated and unmethylated values.
Statistical analysis.
Body weight and energy intake were analyzed from n = 5 male and n = 5 female offspring per treatment group using 1-way repeated-measures ANOVA to determine differences between treatment groups. Because we had an a priori interest in investigating gender differences in their response to Gen, all statistical analyses were performed separately for males and females. The analyses were also performed separately for the PND22/26–50 and PND62/69–97 growth curves. Statistical analysis of the fat/lean mass ratio data at birth and weaning was performed in n = 5 litters per treatment group (Gen or Oil) using Student’s t test with p < .05. At PND60 and 97, the fat/lean mass ratio was analyzed in n = 5 male and n = 5 female offspring from each of the 3 postweaning treatment groups (Gen-C, Oil-C, and Oil-HF) using repeated-measures ANOVA. The measures of adulthood body composition as well as adipocyte and muscle morphology were analyzed using 1-way ANOVA from n = 5 male and n = 5 female offspring from each of the 3 postweaning treatment groups (Gen-C, Oil-C, and Oil-HF). For morphology quantification, 2 fields from each animal were counted, but SEM was calculated from n = 5 male and n = 5 female offspring per treatment group using the average values from the 2 fields. mRNA expression and DNA methylation at PND22 were analyzed in n = 5 male and n = 5 female offspring per treatment group (Gen and Oil) using Student’s t test, and organ pathology findings were analyzed using Wilcoxon rank-sum test, with significance set at p < .05 or p < .01. Glucose area under the curve (AUC) at weaning and adulthood was analyzed in n = 4 male and n = 4 female offspring from each treatment group (Gen and Oil at weaning; Gen-C, Oil-C, and Oil-HF in adulthood) using the following equation: AUC = (0.25 × fasted) + (0.5×30min) + (0.75×60min) + (0.5×120min). All ANOVA analyses were performed in SAS (Chicago, Illinois) and Student’s t test was performed in Microsoft Excel.
RESULTS
Body Weight and Energy Intake
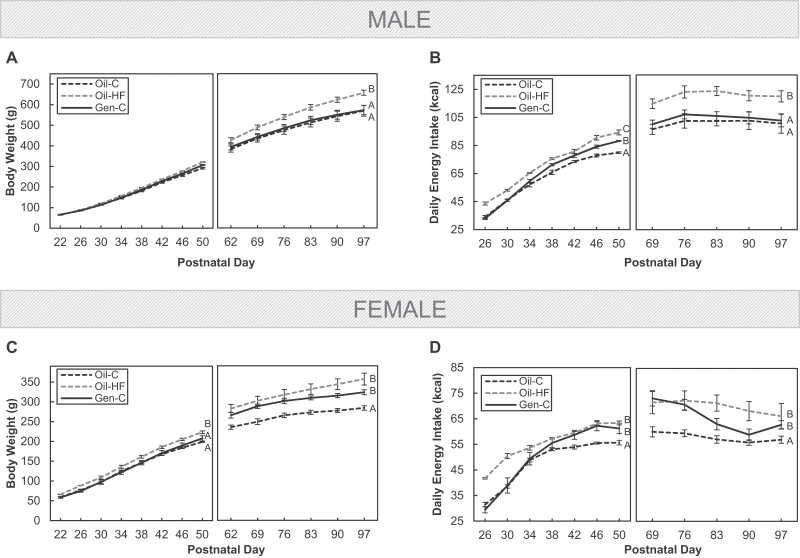
In males, preweaning Gen exposure (Gen-C) had no effect on body weight when compared with Oil-dosed controls (Oil-C), whereas a postweaning HF diet increased body weight when compared with both Oil-C and Gen-C groups (p < .05; Fig. 1A). Similarly, HF diet increased energy intake in males when compared with Oil-C (p < .05), and although Gen increased energy intake during the postweaning period, this effect was no longer present in adulthood (Fig. 1B). In females, both preweaning Gen and a postweaning HF diet increased body weight when compared with Oil-C (p < .05; Fig. 1C). Overall, both Gen and HF also significantly increased energy intake in females when compared with Oil-C (p < .05), but the pattern of increase appeared to be different between Oil-HF and Gen females (Fig. 1D).
FIG. 1.
Body weights from postnatal day (PND) 22 until PND97 in males (A) and females (C), as well as energy intake (kcal/day) in male (B) and female (D) offspring. Offspring were dosed with oil (vehicle control) or genistein (Gen; 50mg/kg/day in oil) from PND2 until PND22. The Gen-exposed animals then consumed a postnatal control (C) diet, whereas the oil controls either consumed the C or high-fat (HF) diet. The PND22/26–50 and PND62/69–97 curves are shown in separate panels (left or right). Values are means ± SEM. n = 5 males or females. Curves with different letters during the 2 periods differ at p < .05 by repeated-measures ANOVA.
Body Composition
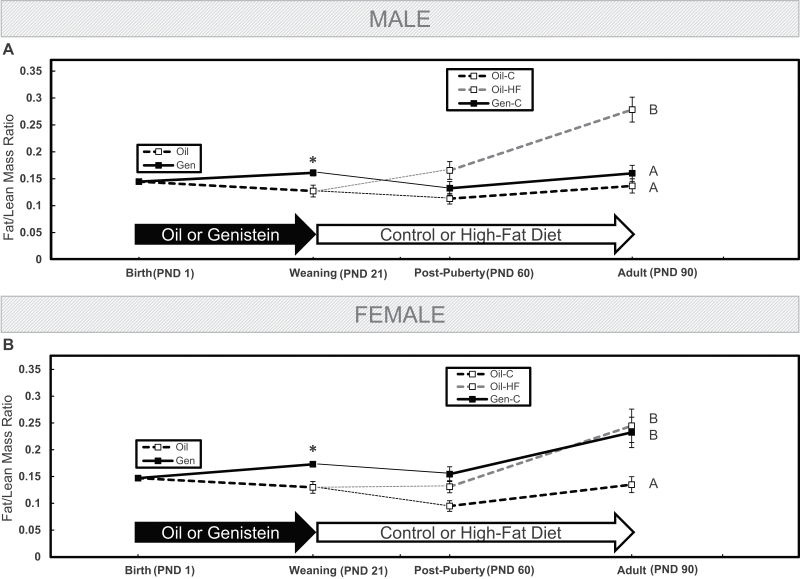
Figure 2 represents the fat/lean mass ratio (a measure of body composition) in male (Fig. 2A) and female (Fig. 2B) rats throughout the study using MRI technology. The ratio was not different in offspring at birth, but by weaning, preweaning Gen exposure increased the ratio in both male and female pups (p < .05). After the onset of puberty, a postweaning HF diet increased the fat/lean mass ratio in males when compared with Oil-C and Gen (p < .05), but Gen-exposed males now had a similar ratio to the Oil-C group, and this pattern continued into adulthood (Fig. 2A). However, after the onset of puberty in females, a postweaning HF diet increased the fat/lean mass ratio (p < .05). Importantly, preweaning Gen exposure increased the fat/lean mass ratio in females to the same degree as in females fed a HF diet from weaning through adulthood. Both groups had fat/lean mass ratios that were significantly higher than in the Oil-C group (p < .05), and this pattern of increased fat/lean mass ratio continued into adulthood (Fig. 2B).
FIG. 2.
The fat/lean mass ratio was calculated as a measure of body composition throughout the study in males (A) and females (B) using EchoMRI technology at birth, weaning, after the onset of puberty, and at adulthood. Offspring were dosed with oil (vehicle control) or genistein (Gen; 50mg/kg/day in oil) from postnatal day (PND) 2 until PND22. The Gen-exposed animals then consumed a postnatal control (C) diet, whereas the oil controls either consumed the C or high-fat (HF) diet. Values are means ± SEM. n = 5 male or female litters at birth and weaning, and n = 5 males or females at postpuberty and at adulthood. *p < .05 at weaning. Curves with different letters differ at p < .05 from postpuberty until adulthood by repeated-measures ANOVA.
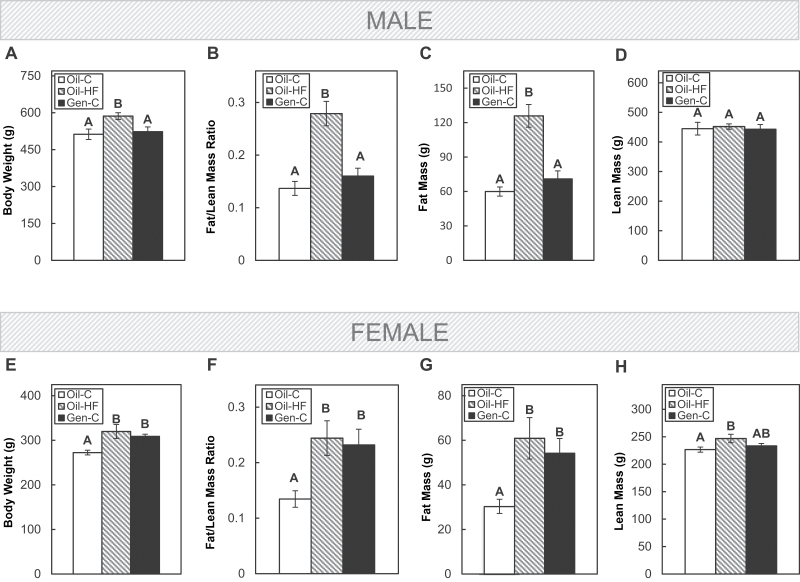
Further characterizing adulthood body composition showed that in males, a postweaning HF diet, but not preweaning Gen, increased body weight (p < .05; Fig. 3A), the fat/lean mass ratio (p < .05; Fig. 3B), and fat mass (p < .05; Fig. 3C), whereas neither HF diet nor Gen affected lean mass in males when compared with the Oil-C group (Fig. 3D). However, in females, both postweaning HF diet and preweaning Gen increased body weight (p < .05; Fig. 3E), the fat/lean mass ratio (p < .05; Fig. 3F), and fat mass (p < .05; Fig. 3G), whereas only the HF diet increased lean mass in females when compared with the Oil-C group (p < .05; Fig. 3H).
FIG. 3.
Body weight in adult males (A) and females (E), the fat/lean mass ratio in males (B) and females (F), the fat mass in males (C) and females (G), and the lean mass in males (D) and females (H) were assessed using EchoMRI technology. Offspring were dosed with oil (vehicle control) or genistein (Gen; 50mg/kg/day in oil) from postnatal day (PND) 2 until PND22. The Gen-exposed animals then consumed a postnatal control (C) diet, whereas the oil controls either consumed the C or high-fat (HF) diet. Values are means ± SEM. n = 5 male or female adults. Values with different letters differ at p < .05.
Adipocyte and Muscle Morphology in Adults
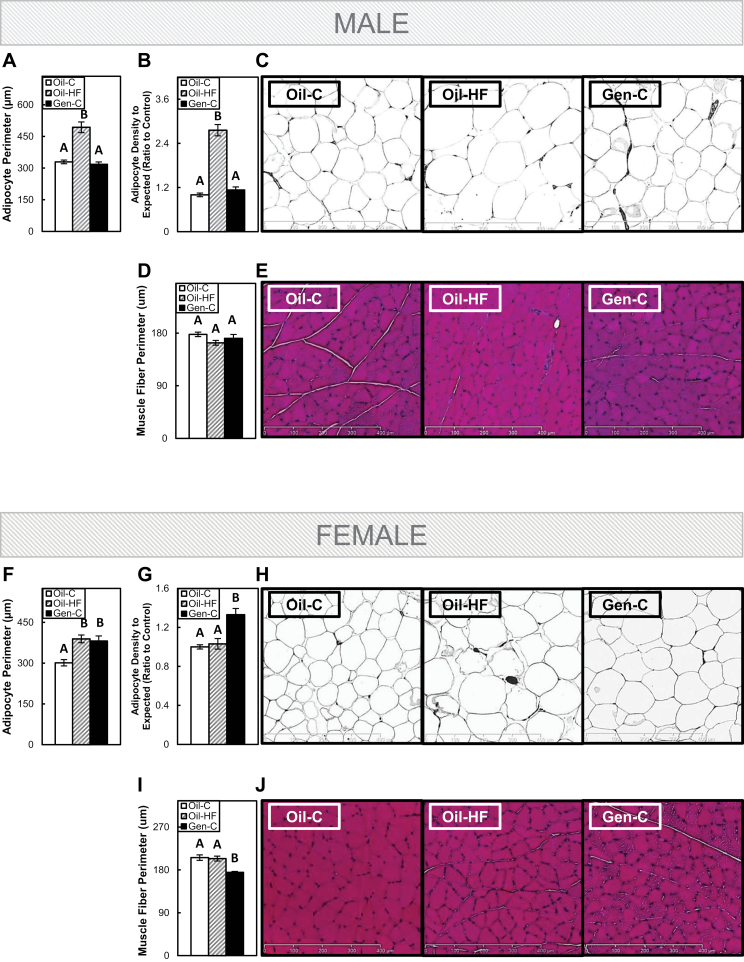
In males, a postweaning HF diet increased adipocyte perimeter (size) (Fig. 4A) and a measure of adipocyte number or adipose tissue cell density (Fig. 4B) when compared with Oil-C group (p < .05), whereas preweaning Gen had no impact on either adipocyte size or cell density (and extrapolated cell number) (with representative images in Fig. 4C). Neither HF diet nor Gen had a significant effect on muscle fiber perimeter in males when compared with the Oil-C group (Fig. 4D with representative images in Fig. 4E).
FIG. 4.
White adipose tissue and gastrocnemius muscle morphology in adult males and females. Adipocyte perimeter in males (A) and females (F), adipose tissue cell density in males (B) and females (F), representative images of adipose tissue in males (C) and females (H), muscle fiber perimeter in males (D) and females (I), and representative images of muscle in males (E) and females (J). Offspring were dosed with oil (vehicle control) or genistein (Gen; 50mg/kg/day in oil) from postnatal day (PND) 2 until PND22. The Gen-exposed animals then consumed a postnatal control (C) diet, whereas the oil controls either consumed the C or high-fat (HF) diet. Quantification was performed using ImageJ software, and representative images are 3 µm hematoxylin & eosin–stained sections under ×10 magnification. Values are means ± SEM. n = 5 male or female adults with 2 independent fields counted. Values with different letters differ at p < .05.
In females, both a postweaning HF diet and preweaning Gen exposure increased adipocyte size (Fig. 4F) when compared with the Oil-C group (p < .05), but only Gen increased the measure of adipocyte cell density and number when compared with the Oil-C group (p < .05; Fig. 4G with representative images in Fig. 4H). Muscle fiber perimeter was not affected by a postweaning HF diet in females, but early-life Gen exposure decreased muscle fiber perimeter when compared with the Oil-C group (p < .05; Fig. 4I with representative images in Fig. 4J).
Serum Measurements
Early-life Gen exposure significantly increased estradiol in adult males (p < .05, 68% increase compared with Oil-C males) and significantly decreased estradiol in adult females (p < .05, 31% decrease compared with Oil-C females) (data not shown). Gen had no effect on unfasted insulin, glucose, leptin, or adiponectin in adult males or females or on the glucose AUC in PND25 or PND97 males or females (data not shown).
Organ Pathology Findings in Adults
On PND22, Gen-exposed females had a significant (p < .05) increase in uterine wet weights, either as raw data (19.8±3.48 vs 40.46±4.53) or after normalization to body weight (0.44±0.06 vs 0.81±0.11). On PND110 in males, Gen had no effect on the number of microfoci of inflammation in WAT, but significantly (p < .05) increased the number of inflammatory foci in females (1.0±0.37 vs 3.2±0.86). Gen exposure had no effect on hepatocellular vacuolation or on inflammation/fibrosis and/or hypertrophy within the Islets of Langerhans, in either males or females (data not shown).
Muscle and Adipose mRNA Analysis in PND22 Pups
Early-life Gen exposure had no effect on the expression of muscle Myf5, MyoD1, or Wnt10b in males, whereas Gen decreased the expression of muscle Myf5, MyoD1, and Wnt10b in females (p < .05; Table 3).
TABLE 3.
mRNA Expression in Adipose and Muscle of PND22 Males and Females
| Tissue | Oil Control | Genistein | |
|---|---|---|---|
| (mRNA amount relative to L7a) | |||
| Muscle | Myf5 | ||
| Male | 1.24±0.08 | 1.16±0.01 | |
| Female | 1.50±0.06 | 1.08±0.04** | |
| MyoD1 | |||
| Male | 0.96±0.01 | 1.08±0.13 | |
| Female | 1.58±0.16 | 1.02±0.06* | |
| Wn10b | |||
| Male | 1.19±0.10 | 1.14±0.14 | |
| Female | 1.43±0.22 | 0.85±0.15* | |
| Adipose | Cebpα | ||
| Male | 1.03±0.09 | 0.89±0.14 | |
| Female | 0.47±0.05 | 0.84±0.06** | |
| Cebpβ | |||
| Male | 1.03±0.04 | 0.97±0.04 | |
| Female | 0.41±0.18 | 0.89±0.11* | |
| Pparγ | |||
| Male | 1.14±0.16 | 0.96±0.17 | |
| Female | 0.47±0.08 | 0.80±0.02* | |
| Wnt10b | |||
| Male | 0.55±0.02 | 0.81±0.05* | |
| Female | 2.43±0.53 | 0.84±0.11* | |
n = 5. PND: postnatal day.
*p < .05 and **p < .01 comparing oil control versus genistein within the same gender.
Gen did not affect the expression of WAT Cebpα, Cebpβ, or Pparγ and slightly, but significantly increased Wnt10b in males (p < .05). However, early-life Gen exposure increased Cebpα, Cebpβ, and Pparγ and significantly decreased Wnt10b in females (p < .05; Table 3).
Adipose Wnt10b Methylation Analysis in PND22 Pups
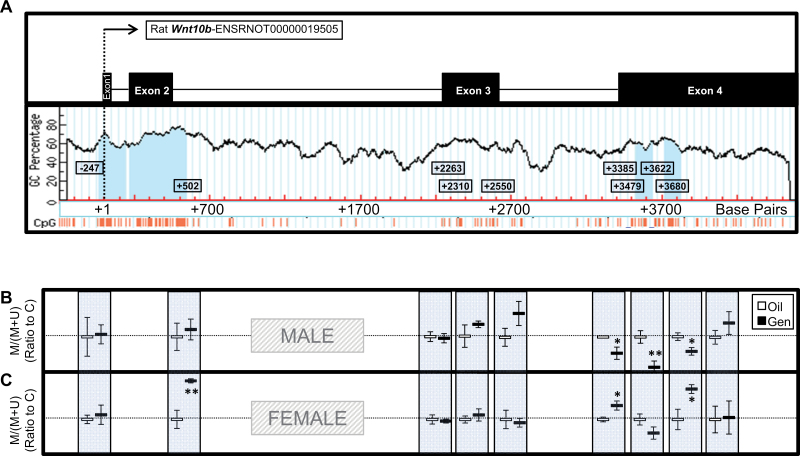
The methylation status of 9 regions was analyzed within the rat adipose Wnt10b gene in response to early-life Gen exposure (Fig. 5A, with CpG islands signified by light blue columns and individual CpGs by orange lines below). In males, Gen exposure had no effect on the methylation status of the 2 regions located near the promoter or within the 3 regions within the non-CpG-containing exon. However, Gen significantly decreased methylation within 3 of the 4 regions near or within the CpG islands in exon 4 (p < .05; Fig. 5B). In females, Gen had no effect on the methylation of the region upstream of the promoter but significantly increased methylation within the CpG island just downstream of the promoter (p < .05). In females, Gen also had no effect on the 3 regions within the non-CpG-containing exon but increased methylation within 2 of the 4 regions near or within the CpG island in exon 4 (p < .05; Fig. 5C).
FIG. 5.
Wnt10b (A) methylation analysis in white adipose tissue of postnatal day (PND) 22 males (B) and females (C). Nine regions within the Wnt10b gene were selected for methylation-sensitive PCR analysis: 1 upstream of the promoter-spanning CpG island, 1 covering the CpG island and “shore” downstream of the promoter, 3 within a non-CpG island containing exon, 2 upstream of the CpG islands in exon 4, 1 within the first CpG island in exon 4, and 1 within the second CpG island in exon 4 (with CpG islands signified by light blue columns and individual CpGs by orange lines below). Offspring were dosed with oil (vehicle control) or genistein (Gen; 50mg/kg/day in oil) from PND2 until PND22. Values are means ± SEM. n = 5 males or females. *p < .05 or **p < .01 for the ratio of (methylated) to (methylated + unmethylated) values after normalization to control.
DISCUSSION
To our knowledge, the current study is the first to demonstrate that early-life exposure to the soy isoflavone, genistein, increases adiposity in female, but not male adult rats. Importantly, these effects were associated with early-life epigenetic regulation of Wnt10b, a key adipogenic gene in adipose tissue. The data regarding the effect of soy-based formula intake in human infants on adiposity have thus far been inconclusive, and no studies have assessed longitudinal changes in body composition in humans exposed to soy-based formula as infants. The 2010 National Toxicology Program report on the potential safety or toxicity of soy-based formulas concluded that there was “minimal concern for adverse effects” due to insufficient data. The report stated that (1) “there is insufficient evidence to reach a conclusion on whether soy-based formula causes, or does not cause developmental toxicity in animal models,” (2) “the literature is considered insufficient to reach a conclusion on whether the use of soy-based formula adversely affects human development…,” and (3) “…the possibility that soy-based formulas may adversely affect human development cannot be dismissed” (McCarver et al., 2011). Therefore, additional studies are needed to more thoroughly assess the risks of isoflavone-containing infant formulas on human development. Data from the current study suggest that in addition to reproductive outcomes, metabolic endpoints must also be considered in future studies.
Early-Life Genistein Exposure As a Metabolic Disruptor
Because few (if any) animal studies have specifically focused on the life-long metabolic consequences of early-life Gen exposure, it is difficult to compare or contrast our metabolic findings with previous outcomes. On PND22 in our study, body weight (the typical measure of metabolic homeostasis) was not affected by Gen. Body weight differences in our study were not apparent until after the onset of puberty, which is after the final collection point for many previous reproductive studies. However, MRI analysis demonstrated that Gen-exposed animals already had altered body composition compared with the Oil-C group on PND22. In a study where Long-Evans dams consumed soymilk during lactation, body weights of pups were increased at weaning in females and not males when compared with offspring of dams not receiving soymilk (Hughes et al., 2004). Although this observed gender difference is similar to what was observed in the current study, the mixture of isoflavones in soymilk makes it difficult to separate out the direct effects of Gen, and the maternal dosing versus direct exposure to pups used in our study does not allow for a clear comparison between this study and ours. In another study in which 4-week-old mice were dosed with various amounts of Gen, doses of 50mg/kg/day or less increased fat pad weights in males, but not in females, and this was associated with increased adipocyte size and altered expression of adipogenic factors (Penza et al., 2006). Although suggesting that Gen has gender-specific effects on adipose metabolism, differences in the window of exposure between this previous study and ours may account for the differences in outcomes.
Importance of Adipose Tissue in Metabolic Homeostasis
A likely reason that previous developmental Gen studies have focused primarily on reproductive toxicity and not on energy metabolism is the lack of overt metabolic syndrome observed in response to Gen. Even in our study, Gen-exposed overweight females did not exhibit any metabolic abnormalities. Although adiposity is often associated with diabetes (the most common clinical outcome measured in animal models of diet-induced obesity), diabetes does not occur in all overweight/obese individuals—referred to as “metabolically healthy” obesity, or the “fat, but fit” phenotype, and according to the 1999–2004 National Health and Nutrition Examination Survey (NHANES), this classification constitutes approximately one-third of all obese Americans (Wildman et al., 2008). However, despite the lack of explicit metabolic disease in this population, it is dangerous to presume that increased adiposity will not lead to metabolic disease or to other detrimental health outcomes with age. In the current study, animals were euthanized in early adulthood (PND110), so it is possible that metabolic abnormalities associated with early-life Gen-induced adiposity would emerge in middle or old age. Adipose tissue, which was once considered a simple storage depot for excess lipids, is now known to have numerous endocrine and inflammatory functions (Trayhurn and Wood, 2004). Females in the current study were heavier and had higher adiposity in response to early-life Gen exposure when compared to Oil-C females, and also had increased WAT inflammation, which has previously been associated with several conditions not related to energy metabolism-related conditions, including depression (Daly, 2013), dementia (Solfrizzi et al., 2011), sarcopenia (Kohara et al., 2011), and altered bone density (Zhuo et al., 2012). Recent studies also suggest that adiposity may be associated with increased cancer risk or the risk for a more aggressive metastasizing cancer phenotype (Kwan et al., 2012; Zhang et al., 2012). Therefore, adiposity, as a risk factor for these conditions, should be considered in future studies and when establishing safety recommendations for the intake of isoflavones during the earliest developmental periods.
Gender Differences in the Response to Early-Life Genistein Exposure
The observation that female—but not male—rats directly exposed to Gen during early life had the same level of adiposity in adulthood as those consuming a HF diet from weaning until adulthood suggests that in females, early-life Gen exposure acts as an obesogen. Additionally, the apparent differences between males and females in their response to Gen suggest that sex hormones may play a role in these stark metabolic differences. Adiposity in women is known to be associated with altered levels of circulating estradiol levels, as is most often demonstrated in postmenopausal women who tend to gain central adiposity with declining serum estradiol (Toth et al., 2000). Estradiol is also associated with food intake in humans (Farage et al., 2008) and rats (Jiang et al., 2008), which is consistent with our results showing a significant decrease in circulating estradiol levels and a corresponding increase in adiposity and food intake in adult females exposed to Gen during early development. Although we did not investigate the precise mechanism behind this decrease in circulating estradiol in the current study, previous studies have suggested that at high enough levels, Gen can bind to both ERs (Chang et al., 2008), thereby acting as a weak estrogen. This appears to be the case in the current study because consistent with previous reports in rodents, uterine weights (as a parameter of estrogenicity) were significantly increased in Gen-exposed females compared with controls on PND22. The early overstimulation of the ERs by an exogenous estrogen, like Gen, during a developmental period when estrogen is normally low, likely creates an altered feedback loop that results in decreased endogenous estradiol production in adulthood. The increase in serum estradiol levels in adult males in the current study was not associated with metabolic changes. Although increased estrogen in males has sometimes been associated with “feminization” and several chronic diseases, future long-term studies are needed to confirm that this is also the case with early-life Gen exposure.
The Critical Role of WNT Signaling in Adipogenesis and Myogenesis
Numerous cancer-related studies have classified Gen as a potent modulator of the WNT signaling pathway (reviewed in Kim et al., 2012). However, Wnt is critical during all aspects of cell differentiation, and WNT10B, a canonical WNT ligand that is highly expressed during development, can also block adipocyte differentiation (Bennett et al., 2002). In rodents, an overexpression of Wnt10b has been shown to inhibit obesity in ob/ob mice (Wright et al., 2007) and decrease adiposity in obese rats (Aslanidi et al., 2007), whereas in humans, WNT10B polymorphisms have been associated with obesity/adiposity in males and females (Kim et al., 2011; Van Camp et al., 2012, 2013). In the current study, a decrease in the mRNA expression of Wnt10b in PND22 female WAT directly following Gen exposure corresponded with increased adiposity both at PND22 and in adulthood. However, although PND22 males also had increased adiposity, they were lean adults, and this corresponded to an increase in the expression of Wnt10b on PND22. The decrease in the expression of Wnt10b in females was further accompanied by a concurrent increase in other markers of the adipogenic cascade, including Pparγ, Cebpα, and Cebpβ. These additional factors have previously been shown to be increased during adipogenesis together with decreased Wnt10b (Chung et al., 2012; Hossain et al., 2010; Rathod et al., 2009), suggesting that the decrease in Wnt10b expression in the current study could be in part responsible for the increased adiposity observed in adult females. Furthermore, compared with the standard model of diet-induced obesity, Gen-exposed females had an increase in both cell size and number, whereas HF-fed females only showed increased cell size but not number, suggesting an innate difference in the adipose tissue of these animals, potentially related to the adipogenic “cell fate commitment” role of Wnt10b in Gen-exposed females. In males, the increase in Wnt10b did not appear to be associated with changes in the adipogenic cascade, and additional analyses will be needed to determine whether an increase in Wnt10b results in the inhibition of adipogenesis, thus allowing PND22 males to “lean out” during puberty and adulthood.
The morphological changes in adipose tissue of females were accompanied by decreased muscle fiber perimeter, decreased expression of myogenic factors, and a trend toward a decrease in muscle Wnt10b. These observations in muscle could also be related to the role of Wnt10b in myogenesis (Vertino et al., 2005) and are also consistent with a study showing that Wnt10b reduction leads to the adipogenic conversion of muscle cells in rats (Scarda et al., 2010). Although the decrease in muscle fiber perimeter was not associated with an overall decrease in muscle mass in adults in the current study, changes in fiber morphology can indicate major alterations in fiber-type distribution (van Wessel et al., 2010), which has been linked to muscle contractility (Blijham et al., 2006) and muscular senescence (Rowan et al., 2012).
The Regulation of Wnt10b Expression by DNA Methylation
As the importance of epigenetics in regulating the genotype during disease development and progression has become increasingly clear, several studies have demonstrated that adipose tissue metabolism is regulated via epigenetic modifications (Bouchard et al., 2010; Fujimoto et al., 2011; Kamei et al., 2010; Wheatley et al., 2011). As a regulator of adipogenesis, Wnt10b expression was shown to be regulated by promoter methylation that led to altered transcription factor binding in cell culture (Fox et al., 2008). However, the present study is the first, to our knowledge, to demonstrate a clear correlation between in vivo mRNA expression of Wn10b and its methylation at several regions within the gene and in response to a botanical compound. The methylation analysis suggests that in obese females, downregulated Wnt10b transcription in response to early postnatal Gen exposure was associated with increased methylation of a CpG island near the promoter as well as within a CpG island located relatively far downstream from the promoter. In males, however, decreased methylation, which corresponded to increased transcription (although the scale of change was less than in females), only occurred in the downstream CpG island. The previous report showing the importance of promoter methylation in adipogenic Wnt10b transcription suggests that in the current study, early-life Gen exposure in females likely decreased Wnt10b transcription by actively methylating the promoter, with probable inhibition of transcription factor binding. Although it is not possible to state precisely which hypermethylated regions contribute most to the inhibition of Wnt10b transcription, the critical role that promoter hypermethylation has been shown to have in cancer models suggests that perhaps the downstream methylation marks play a supporting role for the transcriptional regulation by promoter hypermethylation. Although Gen has been shown to modify promoter methylation in embryonic stem cells (Sato et al., 2011) and cancer models have shown that Gen regulates gene transcription through both DNA methylation and histone modifications (Matsukura et al., 2011; Wang et al., 2012; Zhang and Chen, 2011), additional binding studies will be necessary to further identify the precise mechanism by which Gen can gender specifically regulate Wnt10b transcription in adipose tissue. Furthermore, because recent cell culture studies have proposed a relationship between ERs and WNT signaling (Bhukhai et al., 2012; Galea et al., 2013) and because of the increased adiposity observed in ER knockout animals and humans with ER gene polymorphisms (Geary et al., 2001; Heine et al., 2000; Okura et al., 2003), future mechanistic studies may help to explain the apparent correlation between the gender-specific Wnt10b methylation and expression, circulating estradiol, and adiposity in response to early-life Gen exposure.
In conclusion, few studies have considered the safety of soy or its isolates during the earliest developmental windows when exogenous endocrine signals can reprogram the adult phenotype. Although there are developmental differences between rodents and humans that also include the timing of adipogenesis, the early postnatal period is a critical window for establishing the adult adipose phenotype in rodents and humans, and this study suggests that developmental exposure to Gen can alter body composition in female rats by disrupting and reprogramming the signals dictating adipose tissue expansion. Additionally, although there are likely notable differences in compound structure, solubility, and metabolism between our exposure approach (the dilution of Gen alone in a corn oil vehicle) versus the intake of Gen and other isoflavones in infant soy-based formulas, these data underscore the importance of considering gender as well as window of exposure when creating recommendations about the safety of isoflavone-containing diets.
FUNDING
R. S. Strakovsky was supported by the National Institute of Environmental Health Sciences (NIEHS) training grant T32 ES007326; College of ACES pilot grant; National Center for Complementary and Alternative Medicines (P50AT006268); Office of Dietary Supplements; National Cancer Institute.
ACKNOWLEDGMENTS
The authors thank Dr Anna Dilger for the EchoMri use and training and Dr Hong Chen for her technical expertise. The authors declare no financial conflict of interest. We certify that all applicable institutional and governmental regulations regarding the ethical use of animals were followed during this research (University of Illinois Institutional Animal Care and Use Committee approval no. 11191). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Center for Complementary and Alternative Medicines, Office of Dietary Supplements, National Cancer Institute, or the National Institutes of Health.
REFERENCES
- Aaltonen J., Ojala T., Laitinen K., Poussa T., Ozanne S., Isolauri E. (2011). Impact of maternal diet during pregnancy and breastfeeding on infant metabolic programming: A prospective randomized controlled study. Eur. J. Clin. Nutr. 65, 10–19 [DOI] [PubMed] [Google Scholar]
- Ainge H., Thompson C., Ozanne S. E., Rooney K. B. (2010). A systematic review on animal models of maternal high fat feeding and offspring glycaemic control. Int. J. Obes. (Lond.). 35, 325–335 [DOI] [PubMed] [Google Scholar]
- Aslanidi G., Kroutov V., Philipsberg G., Lamb K., Campbell-Thompson M., Walter G. A., Kurenov S., Ignacio Aguirre J., Keller P., Hankenson K., et al. (2007). Ectopic expression of Wnt10b decreases adiposity and improves glucose homeostasis in obese rats. Am. J. Physiol. Endocrinol. Metab. 293, E726–E736 [DOI] [PubMed] [Google Scholar]
- Bayol S. A., Simbi B. H., Stickland N. C. (2005). A maternal cafeteria diet during gestation and lactation promotes adiposity and impairs skeletal muscle development and metabolism in rat offspring at weaning. J. Physiol. 567(Pt 3), 951–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett C. N., Ross S. E., Longo K. A., Bajnok L., Hemati N., Johnson K. W., Harrison S. D., MacDougald O. A. (2002). Regulation of Wnt signaling during adipogenesis. J. Biol. Chem. 277, 30998–31004 [DOI] [PubMed] [Google Scholar]
- Bhukhai K., Suksen K., Bhummaphan N., Janjorn K., Thongon N., Tantikanlayaporn D., Piyachaturawat P., Suksamrarn A., Chairoungdua A. (2012). A phytoestrogen diarylheptanoid mediates estrogen receptor/Akt/glycogen synthase kinase 3β protein-dependent activation of the Wnt/β-catenin signaling pathway. J. Biol. Chem. 287, 36168–36178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blijham P. J., ter Laak H. J., Schelhaas H. J., van Engelen B. G., Stegeman D. F., Zwarts M. J. (2006). Relation between muscle fiber conduction velocity and fiber size in neuromuscular disorders. J. Appl. Physiol. 100, 1837–1841 [DOI] [PubMed] [Google Scholar]
- Bouchard L., Rabasa-Lhoret R., Faraj M., Lavoie M. E., Mill J., Pérusse L., Vohl M. C. (2010). Differential epigenomic and transcriptomic responses in subcutaneous adipose tissue between low and high responders to caloric restriction. Am. J. Clin. Nutr. 91, 309–320 [DOI] [PubMed] [Google Scholar]
- Chang E. C., Charn T. H., Park S. H., Helferich W. G., Komm B., Katzenellenbogen J. A., Katzenellenbogen B. S. (2008). Estrogen receptors alpha and beta as determinants of gene expression: Influence of ligand, dose, and chromatin binding. Mol. Endocrinol. 22, 1032–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S. S., Lee J. S., Kim M., Ahn B. Y., Jung H. S., Lee H. M., Kim J. W., Park K. S. (2012). Regulation of Wnt/β-catenin signaling by CCAAT/enhancer binding protein β during adipogenesis. Obesity (Silver Spring) 20, 482–487 [DOI] [PubMed] [Google Scholar]
- Cimafranca M. A., Davila J., Ekman G. C., Andrews R. N., Neese S. L., Peretz J., Woodling K. A., Helferich W. G., Sarkar J., Flaws J. A., et al. (2010). Acute and chronic effects of oral genistein administration in neonatal mice. Biol. Reprod. 83, 114–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole Z. A., Gale C. R., Javaid M. K., Robinson S. M., Law C., Boucher B. J., Crozier S. R., Godfrey K. M., Dennison E. M., Cooper C. (2009). Maternal dietary patterns during pregnancy and childhood bone mass: A longitudinal study. J. Bone Miner. Res. 24, 663–668 [DOI] [PubMed] [Google Scholar]
- Cruz M. N., Luksha L., Logman H., Poston L., Agewall S., Kublickiene K. (2006). Acute responses to phytoestrogens in small arteries from men with coronary heart disease. Am. J. Physiol. Heart Circ. Physiol. 290, H1969–H1975 [DOI] [PubMed] [Google Scholar]
- Daly M. (2013). The relationship of C-reactive protein to obesity-related depressive symptoms: A longitudinal study. Obesity (Silver Spring). 21, 248–250 [DOI] [PubMed] [Google Scholar]
- Doerge D. R., Twaddle N. C., Banks E. P., Jefferson W. N., Newbold R. R. (2002). Pharmacokinetic analysis in serum of genistein administered subcutaneously to neonatal mice. Cancer Lett. 184, 21–27 [DOI] [PubMed] [Google Scholar]
- Farage M. A., Osborn T. W., MacLean A. B. (2008). Cognitive, sensory, and emotional changes associated with the menstrual cycle: A review. Arch. Gynecol. Obstet. 278, 299–307 [DOI] [PubMed] [Google Scholar]
- Fox K. E., Colton L. A., Erickson P. F., Friedman J. E., Cha H. C., Keller P., MacDougald O. A., Klemm D. J. (2008). Regulation of cyclin D1 and Wnt10b gene expression by cAMP-responsive element-binding protein during early adipogenesis involves differential promoter methylation. J. Biol. Chem. 283, 35096–35105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuente-Martín E., García-Cáceres C., Granado M., Sánchez-Garrido M. A., Tena-Sempere M., Frago L. M., Argente J., Chowen J. A. (2012). Early postnatal overnutrition increases adipose tissue accrual in response to a sucrose-enriched diet. Am. J. Physiol. Endocrinol. Metab. 302, E1586–E1598 [DOI] [PubMed] [Google Scholar]
- Fujimoto S., Goda T., Mochizuki K. (2011). In vivo evidence of enhanced di-methylation of histone H3 K4 on upregulated genes in adipose tissue of diabetic db/db mice. Biochem. Biophys. Res. Commun. 404, 223–227 [DOI] [PubMed] [Google Scholar]
- Galea G. L., Meakin L. B., Sugiyama T., Zebda N., Sunters A., Taipaleenmaki H., Stein G. S., van Wijnen A. J., Lanyon L. E., Price J. S. (2013). Estrogen receptor α mediates proliferation of osteoblastic cells stimulated by estrogen and mechanical strain, but their acute down-regulation of the Wnt antagonist Sost is mediated by estrogen receptor β. J. Biol. Chem. 288, 9035–9048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geary N., Asarian L., Korach K. S., Pfaff D. W., Ogawa S. (2001). Deficits in E2-dependent control of feeding, weight gain, and cholecystokinin satiation in ER-alpha null mice. Endocrinology 142, 4751–4757 [DOI] [PubMed] [Google Scholar]
- Heine P. A., Taylor J. A., Iwamoto G. A., Lubahn D. B., Cooke P. S. (2000). Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc. Natl. Acad. Sci. U.S.A. 97, 12729–12734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain M. G., Iwata T., Mizusawa N., Shima S. W., Okutsu T., Ishimoto K., Yoshimoto K. (2010). Compressive force inhibits adipogenesis through COX-2-mediated down-regulation of PPARgamma2 and C/EBPalpha. J. Biosci. Bioeng. 109, 297–303 [DOI] [PubMed] [Google Scholar]
- Hughes C. L., Liu G., Beall S., Foster W. G., Davis V. (2004). Effects of genistein or soy milk during late gestation and lactation on adult uterine organization in the rat. Exp. Biol. Med. (Maywood) 229, 108–117 [DOI] [PubMed] [Google Scholar]
- Jefferson W. N., Padilla-Banks E., Goulding E. H., Lao S. P., Newbold R. R., Williams C. J. (2009). Neonatal exposure to genistein disrupts ability of female mouse reproductive tract to support preimplantation embryo development and implantation. Biol. Reprod. 80, 425–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J. M., Sacco S. M., Ward W. E. (2008). Ovariectomy-induced hyperphagia does not modulate bone mineral density or bone strength in rats. J. Nutr. 138, 2106–2110 [DOI] [PubMed] [Google Scholar]
- Kamei Y., Suganami T., Ehara T., Kanai S., Hayashi K., Yamamoto Y., Miura S., Ezaki O., Okano M., Ogawa Y. (2010). Increased expression of DNA methyltransferase 3a in obese adipose tissue: Studies with transgenic mice. Obesity (Silver Spring) 18, 314–321 [DOI] [PubMed] [Google Scholar]
- Kim I. C., Cha M. H., Kim D. M., Lee H., Moon J. S., Choi S. M., Kim K. S., Yoon Y. (2011). A functional promoter polymorphism −607G>C of WNT10B is associated with abdominal fat in Korean female subjects. J. Nutr. Biochem. 22, 252–258 [DOI] [PubMed] [Google Scholar]
- Kim S., Sohn I., Lee Y. S. (2005). Hepatic gene expression profiles are altered by genistein supplementation in mice with diet-induced obesity. J. Nutr. 135, 33–41 [DOI] [PubMed] [Google Scholar]
- Kim Y. S., Farrar W., Colburn N. H., Milner J. A. (2012). Cancer stem cells: Potential target for bioactive food components. J. Nutr. Biochem. 23, 691–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohara K., Ochi M., Tabara Y., Nagai T., Igase M., Miki T. (2011). Leptin in sarcopenic visceral obesity: Possible link between adipocytes and myocytes. PLoS One 6, e24633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreijkamp-Kaspers S., Kok L., Grobbee D. E., de Haan E. H., Aleman A., Lampe J. W., van der Schouw Y. T. (2004). Effect of soy protein containing isoflavones on cognitive function, bone mineral density, and plasma lipids in postmenopausal women: A randomized controlled trial. JAMA 292, 65–74 [DOI] [PubMed] [Google Scholar]
- Kuiper G. G., Lemmen J. G., Carlsson B., Corton J. C., Safe S. H., van der Saag P. T., van der Burg B., Gustafsson J. A. (1998). Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology 139, 4252–4263 [DOI] [PubMed] [Google Scholar]
- Kwan M. L., Chen W. Y., Kroenke C. H., Weltzien E. K., Beasley J. M., Nechuta S. J., Poole E. M., Lu W., Holmes M. D., Quesenberry C. P., Jr, et al. (2012). Pre-diagnosis body mass index and survival after breast cancer in the After Breast Cancer Pooling Project. Breast Cancer Res. Treat. 132, 729–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. C., Bloem C. J., Kasa-Vubu J. Z., Liang L. J. (2012). Effect of oral phytoestrogen on androgenicity and insulin sensitivity in postmenopausal women. Diabetes Obes. Metab. 14, 315–319 [DOI] [PubMed] [Google Scholar]
- Li L. C., Dahiya R. (2002). MethPrimer: Designing primers for methylation PCRs. Bioinformatics 18, 1427–1431 [DOI] [PubMed] [Google Scholar]
- Matsukura H., Aisaki K., Igarashi K., Matsushima Y., Kanno J., Muramatsu M., Sudo K., Sato N. (2011). Genistein promotes DNA demethylation of the steroidogenic factor 1 (SF-1) promoter in endometrial stromal cells. Biochem. Biophys. Res. Commun. 412, 366–372 [DOI] [PubMed] [Google Scholar]
- McCarver G., Bhatia J., Chambers C., Clarke R., Etzel R., Foster W., Hoyer P., Leeder J. S., Peters J. M., Rissman E., et al. (2011). NTP-CERHR expert panel report on the developmental toxicity of soy infant formula. Birth Defects Res. B Dev. Reprod. Toxicol. 92, 421–468 [DOI] [PubMed] [Google Scholar]
- Misra A., Singhal N., Khurana L. (2010). Obesity, the metabolic syndrome, and type 2 diabetes in developing countries: Role of dietary fats and oils. J. Am. Coll. Nutr. 29(3 Suppl.), 289S–301S [DOI] [PubMed] [Google Scholar]
- Newbold R. R., Banks E. P., Bullock B., Jefferson W. N. (2001). Uterine adenocarcinoma in mice treated neonatally with genistein. Cancer Res. 61, 4325–4328 [PubMed] [Google Scholar]
- Nielsen M. O., Kongsted A. H., Thygesen M. P., Strathe A. B., Caddy S., Quistorff B., Jorgensen W., Christensen V. G., Husted S., Chwalibog A., et al. (2012). Late gestation undernutrition can predispose for visceral adiposity by altering fat distribution patterns and increasing the preference for a high-fat diet in early postnatal life. Br. J. Nutr., 109, 2098–2110 [DOI] [PubMed] [Google Scholar]
- Okura T., Koda M., Ando F., Niino N., Tanaka M., Shimokata H. (2003). Association of the mitochondrial DNA 15497G/A polymorphism with obesity in a middle-aged and elderly Japanese population. Hum. Genet. 113, 432–436 [DOI] [PubMed] [Google Scholar]
- Penza M., Montani C., Romani A., Vignolini P., Pampaloni B., Tanini A., Brandi M. L., Alonso-Magdalena P., Nadal A., Ottobrini L., et al. (2006). Genistein affects adipose tissue deposition in a dose-dependent and gender-specific manner. Endocrinology 147, 5740–5751 [DOI] [PubMed] [Google Scholar]
- Rathod M. A., Rogers P. M., Vangipuram S. D., McAllister E. J., Dhurandhar N. V. (2009). Adipogenic cascade can be induced without adipogenic media by a human adenovirus. Obesity (Silver Spring) 17, 657–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci E., Cipriani S., Chiaffarino F., Malvezzi M., Parazzini F. (2010). Effects of soy isoflavones and genistein on glucose metabolism in perimenopausal and postmenopausal non-Asian women: A meta-analysis of randomized controlled trials. Menopause 17, 1080–1086 [DOI] [PubMed] [Google Scholar]
- Rowan S. L., Rygiel K., Purves-Smith F. M., Solbak N. M., Turnbull D. M., Hepple R. T. (2012). Denervation causes fiber atrophy and myosin heavy chain co-expression in senescent skeletal muscle. PLoS One 7, e29082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathyapalan T., Manuchehri A. M., Thatcher N. J., Rigby A. S., Chapman T., Kilpatrick E. S., Atkin S. L. (2011). The effect of soy phytoestrogen supplementation on thyroid status and cardiovascular risk markers in patients with subclinical hypothyroidism: A randomized, double-blind, crossover study. J. Clin. Endocrinol. Metab. 96, 1442–1449 [DOI] [PubMed] [Google Scholar]
- Sato N., Yamakawa N., Masuda M., Sudo K., Hatada I., Muramatsu M. (2011). Genome-wide DNA methylation analysis reveals phytoestrogen modification of promoter methylation patterns during embryonic stem cell differentiation. PLoS One 6, e19278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarda A., Franzin C., Milan G., Sanna M., Dal Prà C., Pagano C., Boldrin L., Piccoli M., Trevellin E., Granzotto M., et al. (2010). Increased adipogenic conversion of muscle satellite cells in obese Zucker rats. Int. J. Obes. (Lond.) 34, 1319–1327 [DOI] [PubMed] [Google Scholar]
- Setchell K. D., Zimmer-Nechemias L., Cai J., Heubi J. E. (1997). Exposure of infants to phyto-oestrogens from soy-based infant formula. Lancet 350, 23–27 [DOI] [PubMed] [Google Scholar]
- Solfrizzi V., Scafato E., Capurso C., D’Introno A., Colacicco A. M., Frisardi V., Vendemiale G., Baldereschi M., Crepaldi G., Di Carlo A., et al. (2011). Metabolic syndrome, mild cognitive impairment, and progression to dementia. The Italian Longitudinal Study on Aging. Neurobiol. Aging 32, 1932–1941 [DOI] [PubMed] [Google Scholar]
- Strakovsky R. S., Pan Y.-X. (2011). A decrease in DKK1, a WNT inhibitor, contributes to placental lipid accumulation in an obesity-prone rat model. Biol. Reprod. 86, 81. 10.1095/biolreprod.111.094482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth M. J., Tchernof A., Sites C. K., Poehlman E. T. (2000). Effect of menopausal status on body composition and abdominal fat distribution. Int. J. Obes. Relat. Metab. Disord. 24, 226–231 [DOI] [PubMed] [Google Scholar]
- Trayhurn P., Wood I. S. (2004). Adipokines: Inflammation and the pleiotropic role of white adipose tissue. Br. J. Nutr. 92, 347–355 [DOI] [PubMed] [Google Scholar]
- Van Camp J. K., Beckers S., Zegers D., Boudin E., Nielsen T. L., Andersen M., Roef G., Taes Y., Brixen K., Van Hul W. (2013). Genetic association study of WNT10B polymorphisms with BMD and adiposity parameters in Danish and Belgian males. Endocrine. 44, 247–254 [DOI] [PubMed] [Google Scholar]
- Van Camp J. K., Beckers S., Zegers D., Verrijken A., Van Gaal L. F., Van Hul W. (2012). Genetic association between WNT10B polymorphisms and obesity in a Belgian case-control population is restricted to males. Mol. Genet. Metab. 105, 489–493 [DOI] [PubMed] [Google Scholar]
- van Wessel T., de Haan A., van der Laarse W. J., Jaspers R. T. (2010). The muscle fiber type-fiber size paradox: Hypertrophy or oxidative metabolism? Eur. J. Appl. Physiol. 110, 665–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertino A. M., Taylor-Jones J. M., Longo K. A., Bearden E. D., Lane T. F., McGehee R. E., Jr, MacDougald O. A., Peterson C. A. (2005). Wnt10b deficiency promotes coexpression of myogenic and adipogenic programs in myoblasts. Mol. Biol. Cell 16, 2039–2048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Li Q., Chen H. (2012). Genistein affects histone modifications on Dickkopf-related protein 1 (DKK1) gene in SW480 human colon cancer cell line. PLoS One 7, e40955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheatley K. E., Nogueira L. M., Perkins S. N., Hursting S. D. (2011). Differential effects of calorie restriction and exercise on the adipose transcriptome in diet-induced obese mice. J. Obes. 2011, 265417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildman R. P., Muntner P., Reynolds K., McGinn A. P., Rajpathak S., Wylie-Rosett J., Sowers M. R. (2008). The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: Prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004). Arch. Intern. Med. 168, 1617–1624 [DOI] [PubMed] [Google Scholar]
- Wright W. S., Longo K. A., Dolinsky V. W., Gerin I., Kang S., Bennett C. N., Chiang S. H., Prestwich T. C., Gress C., Burant C. F., et al. (2007). Wnt10b inhibits obesity in ob/ob and agouti mice. Diabetes 56, 295–303 [DOI] [PubMed] [Google Scholar]
- Wu G., Imhoff-Kunsch B., Girard A. W. (2012). Biological mechanisms for nutritional regulation of maternal health and fetal development. Paediatr. Perinat. Epidemiol. 26(Suppl. 1), 4–26 [DOI] [PubMed] [Google Scholar]
- Yin J., Quinn S., Dwyer T., Ponsonby A. L., Jones G. (2012). Maternal diet, breastfeeding and adolescent body composition: A 16-year prospective study. Eur. J. Clin. Nutr. 66, 1329–1334 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Chen H. (2011). Genistein, an epigenome modifier during cancer prevention. Epigenetics 6, 888–891 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Daquinag A. C., Amaya-Manzanares F., Sirin O., Tseng C., Kolonin M. G. (2012). Stromal progenitor cells from endogenous adipose tissue contribute to pericytes and adipocytes that populate the tumor microenvironment. Cancer Res. 72, 5198–5208 [DOI] [PubMed] [Google Scholar]
- Zhuo Q., Yang W., Chen J., Wang Y. (2012). Metabolic syndrome meets osteoarthritis. Nat. Rev. Rheumatol. 8, 729–737 [DOI] [PubMed] [Google Scholar]