Abstract
Microglia cells, the resident innate immune cells in the brain, are highly active, extending and retracting highly motile processes through which they continuously survey their microenvironment for ‘danger signals’ and interact dynamically with surrounding cells. Upon sensing changes in their central nervous system microenvironment, microglia become activated, undergoing morphological and functional changes. Microglia activation is not an ‘all-or-none’ process, but rather a continuum depending on encountered stimuli, which is expressed through a spectrum of molecular and functional phenotypes ranging from so-called ‘classically activated’, with a highly pro-inflammatory profile, to ‘alternatively activated’ associated with a beneficial, less inflammatory, neuroprotective profile. Microglia activation has been demonstrated in most neurological diseases of diverse aetiology and has been implicated as a contributor to neurodegeneration. The possibility to promote microglia’s neuroprotective phenotype has therefore become a therapeutic goal. We have focused our discussion on the role of microglia in multiple sclerosis, a prototype of inflammatory, demyelinating, neurodegenerative disease, and on the effect of currently approved or on-trial anti-inflammatory therapeutic strategies that might mediate neuroprotection at least in part through their effect on microglia by modifying their behaviour via a switch of their functional phenotype from a detrimental to a protective one. In addition to pharmaceutical approaches, such as treatment with glatiramer acetate, interferon-β, fingolimod or dimethyl fumarate, we address the alternative therapeutic approach of treatment with mesenchymal stem cells and their potential role in neuroprotection through their ‘calming’ effect on microglia.
Keywords: anti-inflammatory treatment, mesenchymal stem cells, microglia, neuroinflammation
Introduction
Microglia, the resident innate immune cells in the brain, represent the first line of defence against exogenous and endogenous threats to the central nervous system (CNS). Microglia are believed to derive from progenitors of mesodermal/mesenchymal origin migrated from the periphery in early postnatal development. In the normal healthy CNS, microglia display a so-called ‘resting’ phenotype, characterized by a typical ramified morphology, a slow turnover rate and low expression of surface molecules. However, microglia are not dormant, continually patrolling the CNS parenchyma through their fine processes to sense changes in the microenvironment, and dynamically interact with other neural cells, such as neurons, oligodendrocytes and astrocytes. Microglia contact synapses, ‘stripping’ dysfunctional ones, removing cell debris, and sensing and modulating neuronal activity. Hence, microglia contribute to CNS homeostasis and plasticity. Under pathological conditions, resting microglia sense activating ‘danger signals’, such as molecules expressed by infectious agents or released upon tissue damage, through diverse types of receptors, and respond rapidly towards injury displaying an alerted phenotype. Such a shift to an activated state is accompanied by dynamic morphological, molecular and functional alterations resulting from the balance between activating inputs and calming signals. While activated microglia have been observed in many neurological diseases of diverse aetiology, ‘activation’ does not reveal the functional state of the cells, which are often engaged in highly different roles. Indeed, microglia can play both detrimental and beneficial roles depending on inputs and feedback signals arising from the neural environment; such paradoxical roles are associated with phenotypes that range from ‘classically activated’, with highly pro-inflammatory features, to ‘alternatively activated’ associated with a repair-oriented profile.
Here, we review microglial phenotype and behaviour in health and disease and their impact on neurodegeneration; we discuss how therapeutic approaches to a neurodegenerative disease with a predominant inflammatory component, multiple sclerosis (MS), could modulate microglia activation towards an alternative phenotype favouring neuroprotection, with the potential to modify the outcome of neurological diseases.
Microglia phenotype in health and disease
From ‘resting’ to ‘activated’ phenotype: danger sensing, morphological changes and migration
Monitoring of microglial morphology in the intact brain by two-photon microscopy has shown that ‘resting’ microglia are highly active, extending and retracting motile processes through which they survey their microenvironment and interact dynamically with surrounding cells.1,2 Through this dynamic sensing of their environment, microglia perceive ‘danger signals’ upon changes of the CNS microenvironment or upon injury and become activated, undergoing morphological changes through an intermediate amoeboid form with several short, thickened processes to a round ‘over-activated’ profile. The functional role of the immediate microglial response upon injury has not been fully elucidated, but might be related to a shielding of the injured area, with the number of responding microglia apparently dependent upon the severity of the injury, to preserve a stable environment in the vicinity of nearby neurons and thereby minimize ensuing damage.2 Microglia activation is controlled by two classes of signals, ‘off’ signals that are constitutive to the brain environment and whose disappearance triggers a microglial response, regardless of the known or unknown nature of the danger sensed, and ‘on’ signals that are produced upon neuronal damage.3 ‘On’ signals act to attract activated microglia to the site of injury along a chemical gradient through activation of specific receptors. Among possible chemoattractants, release of ATP upon focal brain injury triggers the rapid response of microglial processes towards the site of injury,1 a process that involves purinergic (P2) receptors as demonstrated in vivo by the decrease in chemotactic microglial response upon application of various P2 receptor inhibitors directly to the cortex,1 or through experiments in P2Y12-deficient mice.4 Excessive neuronal glutamate release associated with neurodegenerative processes serves as a signal for differential activation of microglia, presumably through activation of different glutamate receptors, in particular α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid and metabotropic glutamate receptors, as shown by chemotactic experiments in cell culture and spinal cord slices where green fluorescent protein (GFP) -expressing microglia could be seen to respond to concentration gradients of glutamate.5 Chemokines released by endangered neurons, in particular CX3CL1 and CCL21, may also act as chemoattractants for microglia that up-regulate their constitutive expression of the relevant chemokine receptors under pathological conditions. A role for CX3CL1–CX3CR1 interaction in microglial migration was first demonstrated in vitro by Harrison et al.,6 and recently confirmed by ex vivo studies which showed that ablation of CX3CR1 signalling in transgenic CX3CR1GFP/GFP CX3CR1−/− mice did not abrogate dynamic motility of retinal microglia processes, but significantly reduced their rates of movement and microglial migration to laser-induced focal injury.7 Similar studies have also demonstrated the importance of CCL21–CXCR3 signalling in microglia migration.8
From ‘classically activated’ through to ‘alternatively activated’: microglia display a spectrum of phenotypes in parallel with a continuum of activation
Microglial activation is not an ‘all-or-none’ process; rather, activated microglia can have different functional states. They can shift from a functional state, mainly associated with the maintenance of CNS homeostasis and plasticity characterized by neuroprotective features, to a pro-inflammatory state often related to defence functions that may occur upon infections, or acute and chronic CNS injuries. In the latter case, ‘classical’ activation of microglia may lead to bystander damage of the CNS resulting in neurotoxicity. In general, the ‘classically activated’ status is associated with production of reactive oxygen species, through increased NADPH oxidase activity, and of pro-inflammatory cytokines, in particular tumour necrosis factor-α (TNF-α) and interleukin-1β (IL-1β), and with an increased level of inducible nitric oxide synthase expression. In contrast, ‘alternative activation’ of microglia is associated with secretion of high levels of anti-inflammatory cytokines and neurotrophic factors leading to inhibition of pro-inflammatory responses, neuroprotection and wound healing.9,10 It should, however, be noted that microglial activation is a continuum that depends on the stimulus encountered in their microenvironment.11
It has been suggested that under different pathological conditions, different stimuli act on different microglial receptors to orchestrate microglial response with a shift towards a more deleterious or a more neuroprotective phenotype.12 The dynamic microglia interacts with different types of cells in the inflammatory environment, both of neural and immune origin. In particular, T cells, a component of the neuroinflammatory reaction in CNS diseases, can modulate microglial activation through secretion of pro-inflammatory and anti-inflammatory cytokines.13 In this context, interferon-γ (IFN-γ) secreted by T helper type 1 T cells induces a classically activated phenotype in microglia upon binding to the IFN-γ receptors 1/2,14 with up-regulation of MHC class II and co-stimulatory molecules and enhancement of their function as antigen-presenting cells,13 possibly through microglia–T-cell cross-talk via the CD40–CD40 ligand interaction.11 In contrast, low doses of IFN-γ or the anti-inflammatory cytokine IL-4, which is released by T helper type 2 cells, promote an alternatively activated profile with a release of neurotrophic factors.15 In addition to Toll-like receptors (TLR) and other pattern recognition receptors through which they perceive, and react to, the presence of pathogens, microglia express a number of other receptors, whose up- or down-regulation depends on microglial activation status under pathological conditions. In vitro stimulation of mouse microglia with TLR agonists, including lipopolysaccharide (LPS) for TLR4 and CpG DNA for TLR9, leads to increased secretion of pro-inflammatory cytokines, such as TNF-α, IL-1β, IL-12, as well as nitric oxide, that in turn cause neuronal injury.16 Recently, microRNA let-7 was shown to activate microglia, acting as a signalling activator of TLR7.17 Activation of microglial TLR-signalling pathway(s) plays a role also in non-infectious CNS diseases, as a response to endogenous danger signals.16 For example, heat-shock protein 60 released from injured CNS cells binds microglia through TLR4 and triggers neuronal injury in a TLR4-dependent and myeloid differentiation factor 88-dependent manner, inducing release of neurotoxic nitric oxide from microglia.18 Maintenance of the interaction between CD200 expressed on neurons and its receptor CD200R expressed on microglia is an off signal that is essential for preventing the expression of a classically activated microglial profile with over-activation of microglia and subsequent neurotoxicity.19 Similarly, disruption of the CX3CL1–CX3CR1 interaction results in highly activated microglia with increased IL-1β production that may induce neurotoxicity.20
Engagement of the TREM-2 (triggering receptor expressed on myeloid cells-2) –DAP12 complex by apoptotic neurons through an as yet uncharacterized TREM-2 ligand appears to specifically promote an alternative microglial phenotype that enhances phagocytosis of apoptotic neurons,21 with decreased expression of inflammatory cytokines and nitric oxide synthase 2.22
The role of microglial phagocytosis
In neurodegenerative diseases, microglia exert an important role9 contributing to repair of the damaged tissue, resolution of the inflammatory process and disease recovery, through an efficient removal of apoptotic cells and cellular debris by phagocytosis.23 Upon sensing neurodegeneration, microglia become alternatively activated and enhance their phagocytic activity, regulated by P2 and other receptors.24 Classically activated phagocytosing microglia become highly detrimental, promoting the inflammatory process through over-production of pro-inflammatory and neurotoxic factors, which results in disease exacerbation,25 as exemplified in amyotrophic lateral sclerosis (ALS).26
Receptor–ligand interactions involved in microglial phagocytosis have not been fully elucidated. Recent investigations of interactions that trigger phagocytosis in microglia have focused on the role of TREM-2, involved in clearance of apoptotic neurons by microglia.21,27 In vitro studies have shown that TREM-2 is expressed by microglia in ‘resting’ state and that its expression is down-regulated by strong inflammatory signals.28 Signalling through TREM-2 regulates microglial phagocytosis, as demonstrated by studies in which increased expression of TREM-2 in microglia through genetic engineering enhanced phagocytosis and promoted an alternatively activated phenotype in these cells,27 whereas blockade of TREM-2 resulted in increased inflammation and neural damage in vivo.29
The importance of phagocytosis, and thereby of microglia, in the maintenance of a pro-regenerative environment in the CNS has been further demonstrated in the murine model for multiple sclerosis, where apoptotic cells and myelin debris were shown to inhibit axonal outgrowth and affect differentiation of oligodendrocyte progenitor cells into mature oligodendrocytes.30 More controversial is the role of phagocytosis in Alzheimer's disease in which the particular location of microglia surrounding plaques in human patients and murine models has suggested the hypothesis that these cells could be responsible for phagocytosing amyloid plaques and could contribute to their clearance.31 Although this has been demonstrated in vitro together with the ability of amyloid β to induce the migration of microglia,32,33 in vivo imaging showed no evidence of amyloid β phagocytosis by microglial cells. Investigation of microglial phagocytosis in an experimental mouse model of Parkinson's disease indicate that microglia can create complex intercellular interactions with neurons that lead to the phagocytosis of dopaminergic cell bodies.34
Triggering microglia activation in neurodegenerative diseases
Activation of microglia, accompanied or not by microgliosis, occurs in essentially all diseases of the CNS. Although alternatively activated microglia exert a beneficial role in early disease phase, continuous activation has been implicated as a contributor to neurodegeneration; indeed, microglial activation has been shown to correlate with neuronal degeneration in several neurodegenerative diseases, as demonstrated by positron emission tomography (PET) imaging,35 which enables monitoring of microglial activation in vivo,36 and classical activation of microglia through chronic local infusion of LPS was shown to trigger neurodegeneration in animal models.37 In primarily non-inflammatory neurodegenerative diseases, such as Alzheimer's disease, ALS and Parkinson's disease among others, misfolded proteins play a crucial role in the pathogenic process38 and their involvement in microglial activation has been demonstrated in several neurodegenerative diseases. Early activation of microglia was observed in mice transgenic for wild-type α-synuclein, an animal model of Parkinson's disease39,40 and in vitro and in vivo studies have suggested that transgenic expression of mutant superoxide dismutase 1 in models of ALS results in activated microglial phenotypes that are inherently neurotoxic.26 The importance of the role of glial cells in ALS was demonstrated in the animal model whereby conditional transgenic mice with simultaneous over-expression of mutant superoxide dismutase 1 in both neurons and microglia developed motor neuron degeneration,41 whereas selective motoneuronal expression was not pathogenic.42
Release of misfolded protein from damaged neurons is a possible trigger for microglia activation. Among non-mutually exclusive mechanisms that implicate release of misfolded protein by neurons in microglial activation in neurodegenerative diseases, a possible common mode of action has been postulated in Alzheimer's disease and Parkinson's disease whereby binding to the scavenger receptor CD36 mediates microglial inflammatory response to fibrillar amyloid β43 and α-synuclein,39,44 respectively. Other studies suggest another pathway triggering microglial inflammatory response to α-synuclein through binding to Mac-1 receptors, thereby signalling to activate reactive oxygen species production by NADPH oxidase.45 Signalling through TLR4 might also represent a common pathway for microglia activation to neurotoxic phenotype in Alzheimer's disease and ALS. Mutant superoxide dismutase 1, which is released from neurons and astrocytes through interaction with the neurosecretory proteins, chromogranin A and B,46 binds to the microglial pattern recognition receptor, CD14, signalling in conjunction with TLR2 and TLR4 to induce in vitro morphological and functional activation changes in microglia that lead to neurotoxicity through release of nitric oxide and superoxide.47 Genetic engineering of HE3K cells showed that a tri-molecular complex consisting of TLR4, MD-2 and CD14 is necessary for full microglial activation by aggregated amyloid β.48 However, the role of TLRs in Alzheimer's disease is complex, because amyloid β uptake and clearance by microglia is also stimulated through TLR, which may therefore also serve a protective role.49 A role for galectin-3, the expression of which correlates with microglial activation and microgliosis in ALS and animal models, was recently postulated. Based on their studies in Gal-3 knockout mice, Lerman et al.50 speculated that Gal-3 is involved in maintaining the trophic and reparative effects of an alternatively activated microglial phenotype.
Microglia activation in multiple sclerosis: when, where and how
It has been known for many years that classically activated microglia in MS and its animal model experimental autoimmune encephalomyelitis (EAE) contribute directly to CNS damage through several mechanisms, such as the production of pro-inflammatory and neurotoxic molecules as well as their possible role in presenting antigen to T cells in the CNS. Indeed, activation of CNS-resident microglia was shown to provide an inflammatory milieu critical for maintenance of T-cell encephalitogenicity within the CNS. In vivo evidence that minocycline, a semi-synthetic antibiotic with multiple anti-inflammatory properties, can ameliorate EAE through its effect on microglia,51 prompted investigations on how these cells contribute to the pathogenesis and progression of EAE and MS.
Microglial activation has been demonstrated in MS post-mortem tissue and implicated in lesion pathogenesis.52 To clarify the involvement of microglia in the pathogenesis of autoimmune demyelinating disease, Heppner et al.53 generated a pharmacogenetically inducible in vivo model of microglial paralysis, using transgenic CD11b-HSVTK mice, in which microglia activation is inhibited following treatment with ganciclovir. Such microglial paralysis resulted in a delay in EAE onset and reduced severity of clinical symptoms; histological analysis showed few inflammatory infiltrates (macrophages and T cells) and no significant myelin and axonal destruction,53 supporting the hypothesis that microglia are essential for the development of disease.
Discovery of the radiolabelled molecule (R)-PK11195,54 a ligand for the benzodiazepine receptor whose expression in the CNS is increased in activated microglia, has allowed monitoring of microglial activation in vivo,36 and a recent study showed correlation between clinical disability and PK11195 PET binding in the cortex of patients.35 Studies in both MS and EAE have shown a dramatic increase in bound radiolabel in inflamed white matter, but also in white matter with normal appearance on MRI where some increase in [11C](R)-PK11195 binding potential indicated subtle microglial activation,36,55 supporting the hypothesis that microglia activation reflects early tissue damage preceding demyelination and lesion formation.56 A possible trigger for microglia activation in white matter of normal appearance is leakage of fibrinogen upon even mild blood–brain barrier (BBB) damage, resulting in perivascular deposition of fibrin, which co-localizes with areas of activated microglia.57 Indeed, in vivo imaging has shown immediate and focal activation upon BBB disruption.2 Fibrinogen induces the activation of microglia to a phagocytic state through binding to the Mac-1 integrin receptor and abolishment of this interaction through pharmaceutical fibrin depletion upon administration of anti-coagulant or in Fibγ390-396A mice mutated in the fibrinogen-Mac-1 binding site resulted in EAE reversal or significantly decreased disease expression, respectively, together with reduced microglial activation and CNS inflammation.57 Recognition of fibrinogen as a danger signal and subsequent activation of microglia was shown in vivo to promote the formation of microglial clusters and subsequent axonal damage.58 Studies carried out on post-mortem brain tissues from MS patients have identified clusters of activated microglia not only within CNS inflammatory lesions but also in the white matter of normal appearance,56,59 supporting the hypothesis that these clusters may represent the earliest stage in MS lesion development. These so-called pre-active lesions have been observed in the absence of BBB damage or overt demyelination and are not apparently associated with leucocyte infiltration or astrogliosis,56,60 suggesting that a CNS endogenous, rather than exogenous, trigger for microglia activation is at play.56 In this context, it was suggested that axonal degeneration drives microglial activation and cluster formation in a mouse model of anterograde axonal damage,61 and Singh et al.62 described the association of microglial clusters with damaged axons in periplaque white matter of early MS biopsy samples. Early activation of microglia has been confirmed in EAE. Ponomarev et al.,63 using bone marrow chimera mice to distinguish between activated microglial cells and infiltrating peripheral macrophages, had demonstrated by flow cytometry and immunohistochemistry that activation and proliferation of microglia are evident before any clinical signs of EAE and precede the migration of peripheral monocytes/macrophages into the CNS. More recently, a two-photon in vivo microscopy study showed that in chronic EAE induced by myelin oligodendrocyte glycoprotein, microglial clusters start to form in proximity to the vasculature before the onset of clinical symptoms, increase in number through the acute phase, and decrease progressively in the chronic phase.58 In contrast, microglia activation persists after the first relapse in the relapsing–remitting EAE model induced by proteolipid protein.59 Mechanisms at play in microglia activation and role in MS have been studied at the functional level in EAE. Hence, interaction between microglia and infiltrating activated encephalitogenic T cells through CD40 and its ligand was studied by Ponomarev et al.64 using bone-marrow chimera mice in which microglia can be distinguished from peripheral macrophages and are deficient in CD40. Such an interaction has been shown to promote the activation of microglia in vitro65 and its genetically engineered or pharmaceutical abrogation results in amelioration of EAE expression.66 Ponomarev et al. described a two-step process for microglia activation in EAE. They proposed that the CD40-independent first step occurring just before EAE onset is mediated by pro-inflammatory cytokines released by encephalitogenic T cells, such as IFN-γ, and results in microglial cell proliferation and up-regulation of MHC class II, CD40 and CD86; the second step of activation, which is CD40-dependent, occurs at the peak of disease and is characterized by a further increase in expression of activation markers and a reduced proliferation.64 Upon full activation, microglia can act as antigen-presenting cells to present phagocytosed myelin antigen to encephalitogenic T cells leading to their expansion in the CNS and severe disease expression.64 However, antigen presentation is unlikely to be the main mechanism of damage mediated by microglia in EAE; rather, release of inflammatory cytokines and reactive oxygen species may be more relevant. A recent study identified Peli1, a family member of E3 ubiquitin ligases implicated in TLR and IL-1 receptor signalling in innate immune cells, as an essential regulator of microglia activation during EAE pathogenesis that is required for mitogen-activated protein kinase (MAPK)-dependent production of pro-inflammatory cytokines and chemokines.67 Peli1 knockout mice were refractory to EAE induction and showed reduced numbers of CNS-infiltrating cells and activated resident microglia. Peli1 was abundantly expressed in microglia and absolutely required for microglial activation during EAE, as shown by studies in Peli1-knockout GFP-expressing chimeric mice.67 In vitro studies showed that Peli1 affects MAPK activation through MyD88-dependent TLR regulation, and acts by promoting degradation of TNF receptor-associated factor 3, a potent inhibitor of MAPK activation and gene induction.67 Another molecule that plays an important role in microglia activation leading to neurotoxicity is macrophage migration inhibitory factor (MIF), a pro-inflammatory cytokine identified as a marker of clinical worsening in MS patients.68 A recent study has shown that MIF can drive the activation of microglia both in vitro in primary microglia cell cultures, and in vivo in EAE-affected mice or in the focal EAE model in MIF-deficient mice.69 Increasing concentrations of MIF induced dose-dependent changes in expression of inflammatory molecules, such as TNF-α, IL-1β, IL-6 and inducible nitric oxide synthase, in primary microglia in vitro, that were accompanied by morphological changes from resting to activated and/or phagocytic phenotype. Spinal cord microinjection of MIF in MIF-deficient mice, in which EAE is less severe and accompanied by reduced inflammation with microglia of resting-type phenotype, restored neuroinflammation with focal inflammatory responses associated with increased expression of inflammatory cytokines.69 Both in vitro and in vivo stimulation of microglial expression of inflammatory molecules by MIF was associated with up-regulated expression of CCAAT/enhancer binding protein-β (C/EBP-β) that participates in the regulation of inflammatory cytokines,70 suggesting a role for MIF in promoting microglia activation through induction of C/EBP-β, possibly through binding to CD74,71 a marker of activated microglia.72
Together these studies confirm a role for microglia in the pathogenesis and progression of EAE, with a beneficial effect on disease progression of inhibitors of microglial activation. However, microglia do not only contribute to the disease in an adverse manner, and the impact of microglial activation on disease outcome depends on the form and timing of activation. Indeed, evidence has accumulated indicating that microglia can also exert a neuroprotective role in EAE/MS. One of the most important beneficial roles of microglia in EAE is the phagocytic removal of apoptotic cells and myelin debris, without the induction of inflammation, which is crucial for the maintenance of a microenvironment that supports tissue regeneration. Indeed, myelin debris has an inhibitory effect on maturation of oligodendrocyte progenitor cells30 and on axonal regeneration.73 In this context, the role of TREM-2 in the control of excessive inflammation was recently demonstrated in EAE. TREM-2, which stimulates phagocytosis and down-regulates inflammatory signals in microglia via the signalling adaptor molecule DAP12,22 is up-regulated on microglia and macrophages, mainly in the spinal cord, during EAE27,29 and its blockade during the effector phase of EAE leads to disease exacerbation with more diffuse CNS inflammatory infiltrates and demyelination in the brain parenchyma.29 Intravenous treatment of EAE-affected mice at disease peak with TREM-2-transduced myeloid precursor cells, which migrated to the perivascular inflammatory lesions, led to increased phagocytosis of debris in these mice, together with a decrease in expression of inflammatory cytokines in the spinal cord, some diminution of the inflammatory infiltrate, and a clear reduction of axonal damage and demyelination. These effects were associated with a marked amelioration of the clinical course in mice treated at disease peak, with early and almost complete recovery from clinical symptoms.27
More recently, microRNA-124 (miR-124) was identified through EAE studies as a key regulator of microglia quiescence. In healthy mice, CNS-resident microglia, but not peripheral macrophages, were found to express high levels of miR-124, and EAE studies with chimeric mice showed that miR-124 expression by microglia decreased by ∼ 70% during the course of the disease. Highest levels were observed in resting CD45low MHC class II− microglia, whereas levels were 60% lower in activated MHC class II+ microglia at disease onset; in vitro experiments showed that miR-124 is similarly down-regulated in LPS-activated microglia.74 Intravenous administration of miR-124 at the effector phase of disease ameliorated EAE and reduced neuroinflammation probably through its effect on macrophages, whereby miR-124 is able to promote a phenotypic switch from classically to alternatively activated macrophage, through indirect down-regulation of transcription factor PU.1, and thereby decreased expression of activation markers CD45, MHC class II and CD86, via inhibition of C/EBP-α.74 Such a function is probably also at play in the maintenance of a quiescent microglial phenotype in the normal CNS.
Alternatively activated microglia can secrete a wide range of molecules that can have a neuroprotective effect in MS/EAE, either directly, such as insulin-like growth factor 1, which promotes proliferation and differentiation of neural progenitor cells,75,76 or indirectly through their anti-inflammatory effect, such as the anti-inflammatory cytokines IL-4, IL-10 and TGF-β. In vitro studies have shown that IL-4-stimulated microglia are able to instruct neural progenitor cells to differentiate into oligodendrocytes, at least in part through release of insulin-like growth factor 1.75
Does the therapeutic effect of currently approved disease-modifying drugs for MS also reflect their impact on microglia?
A number of disease-modifying drugs that have been, or are in the process of being, approved for MS, can potentially affect microglial phenotype directly or indirectly. We shall address this issue for the two most used first-line treatments for relapsing–remitting MS, IFN-β and glatiramer acetate (GA), and for the recently approved fingolimod and dimethyl fumarate (DMF).
The precise mechanisms through which IFN-β exerts its immunomodulatory effect in MS are still uncertain, but generally include inhibition and apoptosis of autoreactive T cells, induction of regulatory T cells, inhibition of leucocyte extravasation through the BBB, and modulation of cytokine expression.77 Its effect on microglia has, as yet, been poorly investigated, with only scant in vitro studies reported. Kim et al.78 showed that IFN-β induced the expression of chemokines such as RANTES and MIP-1b in primary human microglia, through activation of at least three different partially interconnected signalling cascades including nuclear factor-κB, activator protein-1 and Janus kinase/signal transducer and activator of transcription. Kawanokuchi et al.79 addressed the effect of IFN-β on murine microglial functions such as antigen presentation and secretion of inflammatory mediators; they showed that IFN-β inhibits the antigen-presenting function of microglia through suppression of IFN-γ-induced MHC class II expression and down-regulation of the co-stimulatory molecule B7-1, and suppresses differentiation of pathogenic autoreactive T helper type 1 T cells through down-regulation of microglial IL-12 production. Surprisingly, and in accordance with the study of Dasgupta et al.,80 IFN-β dose-dependently enhanced the production of the inflammatory mediators TNF-α, IL-1β, IL-6 and NO, an effect they suggested as being related to the frequent flu-like syndrome side-effect of IFN-β treatment. More recently, Jin et al.81 studied the possible neuroprotective action of IFN-β against the toxicity induced by LPS-activated microglia on cortical neurons in vitro. They report that IFN-β drastically suppressed the neurotoxic production of superoxide and glutamate by activated microglia, and thereby prevented microglia-induced neuronal cell death.81
In contrast, there are many studies on the effect of GA on microglia. GA was developed to mimic a major component of the myelin sheath, myelin basic protein, and its beneficial immunomodulatory effects are not completely understood, albeit apparently related to modulation of antigen-presenting cells that affect effector T-cell and B-cell responses, as well as regulatory T cells.82 Although the exact mechanism of GA is not clear, the many studies conducted both in EAE and MS indicate that GA modulates the function of both adaptive and innate immune system cells directly or indirectly, promoting a less pro-inflammatory environment. Kim et al.83 postulated that GA exerts its effect also through the induction of type 2 antigen-presenting cells, which preferentially mediate T helper type 2 cell differentiation, and showed in an ex vivo study that GA-reactive T cells isolated from GA-treated MS patients promote an alternatively activated phenotype in human microglia. Exposure to the supernatant of GA-reactive T cells before or after initiation of GA therapy modulated human microglia differentially, promoting a classically or alternatively activated phenotype, respectively.83 In contrast, Pul et al.84 addressed the possibility that GA also has a direct effect on microglia in vitro. They observed an induction of the alternatively activated phenotype in primary LPS-activated rat microglia cultures exposed to GA, with down-regulation of TNF-α and up-regulation of IL-10, together with an increase in phagocytic activity perhaps mediated through an IL-10 autocrine loop.84 Gentile et al.85 showed through in vivo and ex vivo electrophysiological studies and confocal microscopy analysis that the beneficial effect of GA on EAE-induced glutamate synapse dysfunction is related to a direct effect on microglia, promoting the alternatively activated phenotype in these cells, with inhibition of TNF-α release, which has been shown to exert a direct detrimental effect on synapses.86 They report that GA treatment led to a reduction in microglia proliferation and to a modulation of the classically activated phenotype, with microglial cells of a resting morphology being observed in the striatum of EAE-affected GA-treated mice.85 Electrophysiological analyses of striatal slices from control mice exposed to activated microglial cells stimulated with GA in vitro reproduced the anti-glutamatergic effect observed in vivo, whereby the TNF-α-induced alterations of striatal glutamate-mediated excitatory postsynaptic currents were reversed in GA-treated EAE mice.85 In a recent, albeit limited, pilot clinical study, Ratchford et al.87 measured the level of microglial activation in MS patients treated with GA by PK11195 PET binding and observed a significant reduction in levels of microglial activation, consistent with a reduction in neuroinflammation. Taken together, these studies seem to indicate that the action of GA on microglia is likely to play a significant role in the immunomodulatory effect of this drug, contributing with several mechanisms to its well-known promotion of a less pro-inflammatory environment.
Fingolimod phosphate (FTY720), the first oral disease-modifying therapy approved for the treatment of MS, is a sphingosine 1-phosphate (S1P) receptor agonist. It acts through binding to S1P receptors expressed on lymphocytes and on resident CNS cells88; at lymphocyte level, fingolimod is believed to inhibit egress of chemokine receptor 7-positive T cells from lymph nodes,89 thereby preventing their passage to the blood and reducing the possibility of their infiltration into the CNS.90 However, emerging evidence suggests that the mechanism of action of fingolimod might not only be primarily immunomodulatory as first considered, but might also involve direct effects in the CNS. Being highly lipophilic, FTY720 easily crosses the BBB and can reach physiologically meaningful concentrations in the CNS; it is thought to act directly on CNS cells, including microglia, albeit through mechanisms that are still unclear. Jackson et al.91 used a rat CNS reaggregate spheroid cell culture model that is devoid of classical blood-borne immune cells, but contains microglia (5–10% of total cell population), to study the effect of fingolimod on remyelination in a CNS environment devoid of immune system effects. Upon lysophosphatidyl choline-induced transient demyelination and recovery period in the presence of fingolimod, Jackson et al.91 observed an increase in remyelination, as per myelin basic protein levels, at a fingolimod concentration based on that observed in the brain of EAE-affected rats upon treatment with fingolimod; increased remyelination was associated with a partial inhibition of microglial activation as measured by ferritin levels, with reduction in TNF-α and IL-1β. Noda et al.92 evaluated the production of pro-inflammatory cytokines in LPS-activated mouse microglia treated with FTY720 and observed a dose-dependent down-regulation of TNF-α, IL-1β and IL-6 expression, which they confirmed to be mediated via FTY7120 binding to S1P receptor 1, similarly to what is observed for lymphocytes, using receptor-specific antagonists. Most importantly, FTY720 enhanced the production of neurotrophic factors, brain-derived nerve factor and glial-derived nerve factor, by LPS-activated microglia, further promoting their neuroprotective phenotype.92
Oral DMF (BG-12) was very recently approved for relapsing–remitting MS; it has been shown to have multiple immunomodulatory and neuroprotective effects, but its exact mechanism of action is still not fully understood. In vitro experiments have revealed that DMF, as well as its primary metabolite monomethyl fumarate (MMF), can exert immunomodulatory effects on T-cell subsets as well as on antigen-presenting cells,93,94 and experiments in EAE have demonstrated that DMF is effective in both preventive and therapeutic applications, albeit marginal in chronic EAE, promoting myelin and axonal preservation and reducing astrocyte activation.95,96 It has been speculated that part of the effect of DMF could be mediated through modulation of microglia phenotype. Histological studies demonstrated that, during the acute phase of EAE, Mac-3-positive cells (microglia and macrophages) are significantly reduced in the spinal cord of DMF-treated animals.95 Such an observation is also supported by in vitro studies in which pre-treatment with DMF can inhibit LPS-induced activation of microglial cells by reducing the expression of NO, TNF-α, IL-1β and IL-6, possibly through an inhibition of the extracellular-signal regulated kinase pathway and an activation of the nuclear factor erythroid 2-related factor 2 (Nrf2) pathway.97 While in vitro data prompted the hypothesis that DMF and MMF could affect microglia activation through Nrf2, a pathway involved in the expression of proteins critical in the detoxification of reactive oxygen and reactive nitrogen species,97,98 this has not been demonstrated in vivo. Indeed, although Linker et al.96 showed that Nrf2 is required for the therapeutic effect of DMF, double-labelling of Nrf2 with a marker for microglia did not reveal an increase of its expression in those cells after DMF treatment in EAE-affected mice. Further in vitro and in vivo studies are needed to dissect the pathways through which DMF promote an alternative neuroprotective phenotype in microglia.
Mesenchymal stem cells promote an alternatively activated neuroprotective phenotype in microglia: implications for their therapeutic use in MS
Mesenchymal stem cells (MSC) are currently being investigated as an alternative therapeutic approach for MS.99 The potential therapeutic use of MSC for neurodegenerative diseases was originally considered as related to their possible regenerative function through their ability to differentiate into mesodermal tissues and perhaps into other embryonic lineages. However, recent observations have indicated that, upon systemic administration, most MSC are rapidly entrapped in the lungs, and only a few engraft into injured CNS, where they display negligible transdifferentiation capacity.100–102 In vitro studies demonstrating that MSC can modulate several effector functions of cells of both the adaptive and innate immune systems introduced the possibility that MSC might be effective in EAE. Indeed, Zappia et al.103 demonstrated that MSC injected intravenously ameliorate the clinical course of EAE, reducing demyelination, axonal loss, leucocyte infiltration and macrophage/microglia activation, an effect that has been reproduced in numerous subsequent studies.99 MSC show neuroprotective capacity due to a wide range of bystander effects on target tissues. It has been shown that MSC can rescue neurons from apoptosis and promote their long-term survival and maturation not only through their paracrine release of neuroprotective factors,104 but also through indirect effects mediated by their interaction with glial/local cells. In particular, MSC are able to modulate the activation of microglia induced by LPS, reducing the production of TNF and NO by microglial cells both in co-cultures and in transwell cultures, possibly by down-regulating the activation of p38 MAPK, which is critical for TLR4-induced microglia activation.105,106 Recently, we showed that cross-talk with MSC promotes an alternatively activated phenotype in microglia, associated with a significant up-regulation of surface molecules associated with a neuroprotective phenotype, such as CX3CR1, CD200R and nuclear orphan receptor NURR1, which suppresses the potentially neurotoxic inflammatory profile in microglia,107 and with a reversal in expression of TNF, inducible nitric oxide synthase and oxidative stress-associated proteins induced by LPS and other pro-inflammatory molecules.108 We observed that MSC impacted the microglia activation phenotype also at the functional level; while MSC did not affect the proliferation of LPS-activated microglia, the basal Ca2+ concentration of LPS-activated microglia and their phagocytic activity were significantly enhanced, an observation confirmed by the up-regulated expression of TREM2, which facilitates debris clearance in the absence of inflammation.108 These studies suggest that MSC act on the ability of microglia to reach an activated state and subsequently enter their ‘executive phase’ upon LPS triggering, by dissociating their capacity to release pro-inflammatory molecules from their phagocytic activity. Through blockade of CX3CL1 by siRNA silencing or antibody treatment, or by interference between CX3CL1 binding to its receptor on microglia with exogenous CX3CL1, we showed that MSC promote a switch in LPS-activated microglia from a detrimental phenotype to a beneficial, neuroprotective phenotype through release of CX3CL1.108 It is interesting to note similar results in a recent study whereby MSC were shown to alternatively activate microglia, promoting their migration towards Alzheimer's disease lesions through the release of CCL5.109
Conclusions
It is clear that microglia upon CNS injury can acquire unexpected neurotoxic features depending on the type and timing of activation. However, in vitro and in vivo experimental data support the possibility of modulating microglia activation towards an alternative phenotype reverting its functional state to its neuroprotective physiological role involved in CNS homeostasis and prone to injury healing. On this line, still pioneer experimental reports suggest that current and future treatments for MS may have some effects on microglia (Fig. 1). However, little is known of their mode of action on microglia in disease and, in view of their phenotypic spectrum, it would seem relevant to define and monitor specific windows of therapeutic opportunity. While PET imaging of microglia ligands has afforded meaningful insights into the evolution of microglial activation in neurodegenerative diseases in vivo, further studies are needed to define markers of increased specificity for microglial activation states that would enable monitoring of drugs that affect microglial activation in the CNS.
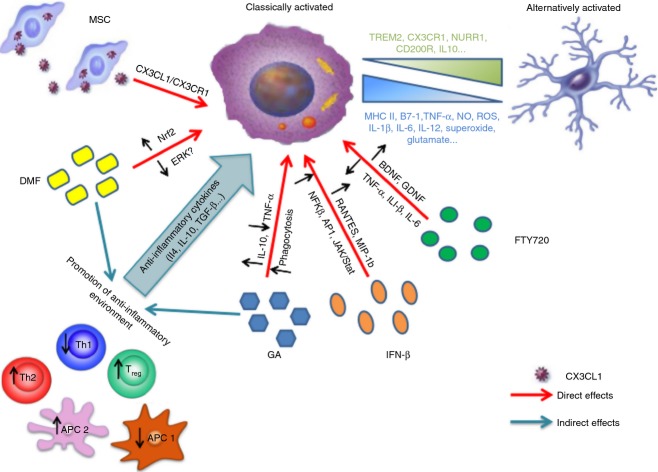
Figure 1.
How therapeutic approaches to multiple sclerosis (MS) promote an alternatively activated phenotype in microglia. Both direct and/or indirect effects on microglia have been demonstrated for first-line treatments and for more recent therapeutic approaches to MS. Mechanisms underlying direct effects are mostly unknown, albeit likely to occur through receptor binding such as S1P for fingolimod and CX3CL1/CX3CR1 interaction for mesnechymal stem cells (MSC), and result in modulation of expression and release of anti-inflammatory agents and neuroprotective molecules through signalling via multiple pathways. Indirect effects are mostly related to promotion of an anti-inflammatory environment through down-regulation of antigen-presenting cells (APC) type 1 (pro-inflammatory; APC1) and thereby T helper type 1 (Th1) cells [e.g. dimethyl fumarate (DMF)] and/or fostering APC type 2 (anti-inflammatory; APC2) and thereby Th2 cells [e.g. glatiramer acetate (GA) -specific Th2 cells] that secrete anti-inflammatory cytokines which induce a shift towards an alternatively activated microglial phenotype.
Acknowledgments
We gratefully acknowledge the financial support of the Italian MS Foundation, the Italian Ministry of Health, the Italian Ministry of the University and Scientific Research, the Liguria Region and the CARIGE Foundation.
Glossary
- ALS
amyotrophic lateral sclerosis
- BBB
blood-brain barrier
- C/EBP-β
CCAAT/enhancer binding protein-β
- CNS
central nervous system
- DMF
dimethyl fumarate
- EAE
experimental autoimmune encephalomyelitis
- GA
glatiramer acetate
- GFP
green fluorescent protein
- IFN
interferon
- IL
interleukin
- LPS
lipopolysaccharide
- MAPK
mitogen-activated protein kinase
- MIF
macrophage migration inhibitory factor
- miR
microRNA
- MMF
monomethyl fumarate
- MS
multiple sclerosis
- MSC
mesenchymal stem cells
- NrF2
nuclear factor erythroid 2-related factor 2
- PET
positron emission tomography
- S1P
sphingosine 1-phosphate
- TLR
Toll-like receptor
- TNF
tumour necrosis factor
- TREM-2
triggering receptor expressed on myeloid cells-2
Disclosure
The authors have no financial disclosures or competing interests.
References
- 1.Davalos D, Grutzendler J, Yang G, et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–8. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 2.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–8. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 3.Biber K, Neumann H, Inoue K, Boddeke HW. Neuronal ‘On’ and ‘Off’ signals control microglia. Trends Neurosci. 2007;30:596–602. doi: 10.1016/j.tins.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 4.Haynes SE, Hollopeter G, Yang G, Kurpius D, Dailey ME, Gan WB, Julius D. The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat Neurosci. 2006;9:1512–9. doi: 10.1038/nn1805. [DOI] [PubMed] [Google Scholar]
- 5.Liu GJ, Nagarajah R, Banati RB, Bennett MR. Glutamate induces directed chemotaxis of microglia. Eur J Neurosci. 2009;29:1108–18. doi: 10.1111/j.1460-9568.2009.06659.x. [DOI] [PubMed] [Google Scholar]
- 6.Harrison JK, Jiang Y, Chen S, et al. Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc Natl Acad Sci USA. 1998;95:10896–901. doi: 10.1073/pnas.95.18.10896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liang KJ, Lee JE, Wang YD, Ma W, Fontainhas AM, Fariss RN, Wong WT. Regulation of dynamic behavior of retinal microglia by CX3CR1 signaling. Invest Ophthalmol Vis Sci. 2009;50:4444–51. doi: 10.1167/iovs.08-3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Jong EK, Dijkstra IM, Hensens M, Brouwer N, van Amerongen M, Liem RS, Nitsch R, Weber JR. Vesicle-mediated transport and release of CCL21 in endangered neurons: a possible explanation for microglia activation remote from a primary lesion. J Neurosci. 2005;25:7548–57. doi: 10.1523/JNEUROSCI.1019-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gomes-Leal W. Microglial physiopathology: how to explain the dual role of microglia after acute neural disorders? Brain Behav. 2012;2:345–56. doi: 10.1002/brb3.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boche D, Perry VH, Nicoll JAR. Review: activation patterns of microglia and their identification in the human brain. Neuropathol Appl Neurobiol. 2013;39:3–18. doi: 10.1111/nan.12011. [DOI] [PubMed] [Google Scholar]
- 11.Town T, Nikolic V, Tan J. The microglial “activation” continuum: from innate to adaptive responses. J Neuroinflammation. 2005;2:24. doi: 10.1186/1742-2094-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwartz M, Butovsky O, Bruck W, Hanisch UK. Microglial phenotype: is the commitment reversible? Trends Neurosci. 2006;29:68–74. doi: 10.1016/j.tins.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 13.McQuillan K, Lynch MA, Mills KH. Activation of mixed glia by Aβ-specific Th1 and Th17 cells and its regulation by Th2 cells. Brain Behav Immun. 2010;24:598–607. doi: 10.1016/j.bbi.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Merson TD, Binder MD, Kilpatrick TJ. Role of cytokines as mediators and regulators of microglial activity in inflammatory demyelination of the CNS. Neuromolecular Med. 2010;12:99–132. doi: 10.1007/s12017-010-8112-z. [DOI] [PubMed] [Google Scholar]
- 15.Shaked I, Porat Z, Gersner R, Kipnis J, Schwartz M. Early activation of microglia as antigen-presenting cells correlates with T cell-mediated protection and repair of the injured central nervous system. J Neuroimmunol. 2004;146:84–93. doi: 10.1016/j.jneuroim.2003.10.049. [DOI] [PubMed] [Google Scholar]
- 16.Lehnardt S. Innate immunity and neuroinflammation in the CNS: the role of microglia in Toll-like receptor-mediated neuronal injury. Glia. 2010;58:253–63. doi: 10.1002/glia.20928. [DOI] [PubMed] [Google Scholar]
- 17.Lehmann SM, Kruger C, Park B, et al. An unconventional role for miRNA: let-7 activates Toll-like receptor 7 and causes neurodegeneration. Nat Neurosci. 2012;15:827–35. doi: 10.1038/nn.3113. [DOI] [PubMed] [Google Scholar]
- 18.Lehnardt S, Schott E, Trimbuch T, Laubisch D, Krueger C, Wulczyn G, Nitsch R, Weber JR. A vicious cycle involving release of heat shock protein 60 from injured cells and activation of toll-like receptor 4 mediates neurodegeneration in the CNS. J Neurosci. 2008;28:2320–31. doi: 10.1523/JNEUROSCI.4760-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang S, Wang XJ, Tian LP, Pan J, Lu GQ, Zhang YJ, Ding JQ, Chen SD. CD200-CD200R dysfunction exacerbates microglial activation and dopaminergic neurodegeneration in a rat model of Parkinson's disease. J Neuroinflammation. 2011;8:154. doi: 10.1186/1742-2094-8-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cardona AE, Pioro EP, Sasse ME, et al. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci. 2006;9:917–24. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- 21.Hsieh CL, Koike M, Spusta SC, Niemi EC, Yenari M, Nakamura MC, Seaman WE. A role for TREM2 ligands in the phagocytosis of apoptotic neuronal cells by microglia. J Neurochem. 2009;109:1144–56. doi: 10.1111/j.1471-4159.2009.06042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takahashi K, Rochford CD, Neumann H. Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2. J Exp Med. 2005;201:647–57. doi: 10.1084/jem.20041611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2:965–75. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- 24.Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiol Rev. 2011;91:461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- 25.Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- 26.Philips T, Robberecht W. Neuroinflammation in amyotrophic lateral sclerosis: role of glial activation in motor neuron disease. Lancet Neurol. 2011;10:253–63. doi: 10.1016/S1474-4422(11)70015-1. [DOI] [PubMed] [Google Scholar]
- 27.Takahashi K, Prinz M, Stagi M, Chechneva O, Neumann H. TREM2-transduced myeloid precursors mediate nervous tissue debris clearance and facilitate recovery in an animal model of multiple sclerosis. PLoS Med. 2007;4:e124. doi: 10.1371/journal.pmed.0040124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmid CD, Melchior B, Masek K, et al. Differential gene expression in LPS/IFNγ activated microglia and macrophages: in vitro versus in vivo. J Neurochem. 2009;109(Suppl. 1):117–25. doi: 10.1111/j.1471-4159.2009.05984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piccio L, Buonsanti C, Mariani M, Cella M, Gilfillan S, Cross AH, Colonna M, Panina-Bordignon P. Blockade of TREM-2 exacerbates experimental autoimmune encephalomyelitis. Eur J Immunol. 2007;37:1290–301. doi: 10.1002/eji.200636837. [DOI] [PubMed] [Google Scholar]
- 30.Kotter MR, Li WW, Zhao C, Franklin RJ. Myelin impairs CNS remyelination by inhibiting oligodendrocyte precursor cell differentiation. J Neurosci. 2006;26:328–32. doi: 10.1523/JNEUROSCI.2615-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paresce DM, Ghosh RN, Maxfield FR. Microglial cells internalize aggregates of the Alzheimer's disease amyloid β-protein via a scavenger receptor. Neuron. 1996;17:553–65. doi: 10.1016/s0896-6273(00)80187-7. [DOI] [PubMed] [Google Scholar]
- 32.Huang WC, Yen FC, Shie FS, et al. TGF-β1 blockade of microglial chemotaxis toward Aβ aggregates involves SMAD signaling and down-regulation of CCL5. J Neuroinflammation. 2010;7:28. doi: 10.1186/1742-2094-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu S, Liu Y, Hao W, et al. TLR2 is a primary receptor for Alzheimer's amyloid β peptide to trigger neuroinflammatory activation. J Immunol. 2012;188:1098–107. doi: 10.4049/jimmunol.1101121. [DOI] [PubMed] [Google Scholar]
- 34.Barcia C, Ros CM, Annese V, et al. ROCK/Cdc42-mediated microglial motility and gliapse formation lead to phagocytosis of degenerating dopaminergic neurons in vivo. Sci Rep. 2012;2:809. doi: 10.1038/srep00809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Politis M, Giannetti P, Su P, et al. Increased PK11195 PET binding in the cortex of patients with MS correlates with disability. Neurology. 2012;79:523–30. doi: 10.1212/WNL.0b013e3182635645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cagnin A, Kassiou M, Meikle SR, Banati RB. Positron emission tomography imaging of neuroinflammation. Neurotherapeutics. 2007;4:443–52. doi: 10.1016/j.nurt.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gao HM, Jiang J, Wilson B, Zhang W, Hong JS, Liu B. Microglial activation-mediated delayed and progressive degeneration of rat nigral dopaminergic neurons: relevance to Parkinson's disease. J Neurochem. 2002;81:1285–97. doi: 10.1046/j.1471-4159.2002.00928.x. [DOI] [PubMed] [Google Scholar]
- 38.Winklhofer KF, Tatzelt J, Haass C. The two faces of protein misfolding: gain- and loss-of-function in neurodegenerative diseases. EMBO J. 2008;27:336–49. doi: 10.1038/sj.emboj.7601930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Su X, Maguire-Zeiss KA, Giuliano R, Prifti L, Venkatesh K, Federoff HJ. Synuclein activates microglia in a model of Parkinson's disease. Neurobiol Aging. 2008;29:1690–701. doi: 10.1016/j.neurobiolaging.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Watson MB, Richter F, Lee SK, Gabby L, Wu J, Masliah E, Effros RB, Chesselet MF. Regionally specific microglial activation in young mice over-expressing human wildtype α-synuclein. Exp Neurol. 2012;237:318–34. doi: 10.1016/j.expneurol.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boillee S, Yamanaka K, Lobsiger CS, Copeland NG, Jenkins NA, Kassiotis G, Kollias G, Cleveland DW. Onset and progression in inherited ALS determined by motor neurons and microglia. Science. 2006;312:1389–92. doi: 10.1126/science.1123511. [DOI] [PubMed] [Google Scholar]
- 42.Lino MM, Schneider C, Caroni P. Accumulation of SOD1 mutants in postnatal motoneurons does not cause motoneuron pathology or motoneuron disease. J Neurosci. 2002;22:4825–32. doi: 10.1523/JNEUROSCI.22-12-04825.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.El Khoury JB, Moore KJ, Means TK, Leung J, Terada K, Toft M, Freeman MW, Luster AD. CD36 mediates the innate host response to β-amyloid. J Exp Med. 2003;197:1657–66. doi: 10.1084/jem.20021546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alvarez-Erviti L, Couch Y, Richardson J, Cooper JM, Wood MJ. α-synuclein release by neurons activates the inflammatory response in a microglial cell line. Neurosci Res. 2011;69:337–42. doi: 10.1016/j.neures.2010.12.020. [DOI] [PubMed] [Google Scholar]
- 45.Zhang P, Hatter A, Liu B. Manganese chloride stimulates rat microglia to release hydrogen peroxide. Toxicol Lett. 2007;173:88–100. doi: 10.1016/j.toxlet.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Urushitani M, Sik A, Sakurai T, Nukina N, Takahashi R, Julien JP. Chromogranin-mediated secretion of mutant superoxide dismutase proteins linked to amyotrophic lateral sclerosis. Nat Neurosci. 2006;9:108–18. doi: 10.1038/nn1603. [DOI] [PubMed] [Google Scholar]
- 47.Zhao W, Beers DR, Henkel JS, Zhang W, Urushitani M, Julien JP, Appel SH. Extracellular mutant SOD1 induces microglial-mediated motoneuron injury. Glia. 2010;58:231–43. doi: 10.1002/glia.20919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walter S, Letiembre M, Liu Y, et al. Role of the toll-like receptor 4 in neuroinflammation in Alzheimer's disease. Cell Physiol Biochem. 2007;20:947–56. doi: 10.1159/000110455. [DOI] [PubMed] [Google Scholar]
- 49.Okun E, Griffioen KJ, Lathia JD, Tang SC, Mattson MP, Arumugam TV. Toll-like receptors in neurodegeneration. Brain Res Rev. 2009;59:278–92. doi: 10.1016/j.brainresrev.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lerman BJ, Hoffman EP, Sutherland ML, Bouri K, Hsu DK, Liu FT, Rothstein JD, Knoblach SM. Deletion of galectin-3 exacerbates microglial activation and accelerates disease progression and demise in a SOD1(G93A) mouse model of amyotrophic lateral sclerosis. Brain Behav. 2012;2:563–75. doi: 10.1002/brb3.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Popovic N, Schubart A, Goetz BD, Zhang SC, Linington C, Duncan ID. Inhibition of autoimmune encephalomyelitis by a tetracycline. Ann Neurol. 2002;51:215–23. doi: 10.1002/ana.10092. [DOI] [PubMed] [Google Scholar]
- 52.Lassmann H. Mechanisms of inflammation induced tissue injury in multiple sclerosis. J Neurol Sci. 2008;274:45–7. doi: 10.1016/j.jns.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 53.Heppner FL, Greter M, Marino D, et al. Experimental autoimmune encephalomyelitis repressed by microglial paralysis. Nat Med. 2005;11:146–52. doi: 10.1038/nm1177. [DOI] [PubMed] [Google Scholar]
- 54.McMahon EJ, Bailey SL, Castenada CV, Waldner H, Miller SD. Epitope spreading initiates in the CNS in two mouse models of multiple sclerosis. Nat Med. 2005;11:335–9. doi: 10.1038/nm1202. [DOI] [PubMed] [Google Scholar]
- 55.Politis M, Su P, Piccini P. Imaging of microglia in patients with neurodegenerative disorders. Front Pharmacol. 2012;3:96. doi: 10.3389/fphar.2012.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van Horssen J, Singh S, van der Pol S, et al. Clusters of activated microglia in normal-appearing white matter show signs of innate immune activation. J Neuroinflammation. 2012;9:156. doi: 10.1186/1742-2094-9-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Adams RA, Bauer J, Flick MJ, Sikorski SL, Nuriel T, Lassmann H, Degen JL, Akassoglou K. The fibrin-derived γ377–395 peptide inhibits microglia activation and suppresses relapsing paralysis in central nervous system autoimmune disease. J Exp Med. 2007;204:571–82. doi: 10.1084/jem.20061931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Davalos D, Ryu JK, Merlini M, et al. Fibrinogen-induced perivascular microglial clustering is required for the development of axonal damage in neuroinflammation. Nat Commun. 2012;3:1227. doi: 10.1038/ncomms2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rasmussen S, Wang Y, Kivisakk P, Bronson RT, Meyer M, Imitola J, Khoury SJ. Persistent activation of microglia is associated with neuronal dysfunction of callosal projecting pathways and multiple sclerosis-like lesions in relapsing–remitting experimental autoimmune encephalomyelitis. Brain. 2007;130:2816–29. doi: 10.1093/brain/awm219. [DOI] [PubMed] [Google Scholar]
- 60.van der Valk P, Amor S. Preactive lesions in multiple sclerosis. Curr Opin Neurol. 2009;22:207–13. doi: 10.1097/WCO.0b013e32832b4c76. [DOI] [PubMed] [Google Scholar]
- 61.Dissing-Olesen L, Ladeby R, Nielsen HH, Toft-Hansen H, Dalmau I, Finsen B. Axonal lesion-induced microglial proliferation and microglial cluster formation in the mouse. Neuroscience. 2007;149:112–22. doi: 10.1016/j.neuroscience.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 62.Singh S, Metz I, Amor S, van der Valk P, Stadelmann C, Bruck W. Microglial nodules in early multiple sclerosis white matter are associated with degenerating axons. Acta Neuropathol. 2013;125:595–608. doi: 10.1007/s00401-013-1082-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ponomarev ED, Shriver LP, Maresz K, Dittel BN. Microglial cell activation and proliferation precedes the onset of CNS autoimmunity. J Neurosci Res. 2005;81:374–89. doi: 10.1002/jnr.20488. [DOI] [PubMed] [Google Scholar]
- 64.Ponomarev ED, Shriver LP, Dittel BN. CD40 expression by microglial cells is required for their completion of a two-step activation process during central nervous system autoimmune inflammation. J Immunol. 2006;176:1402–10. doi: 10.4049/jimmunol.176.3.1402. [DOI] [PubMed] [Google Scholar]
- 65.Matyszak MK, Denis-Donini S, Citterio S, Longhi R, Granucci F, Ricciardi-Castagnoli P. Microglia induce myelin basic protein-specific T cell anergy or T cell activation, according to their state of activation. Eur J Immunol. 1999;29:3063–76. doi: 10.1002/(SICI)1521-4141(199910)29:10<3063::AID-IMMU3063>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 66.Chitnis T, Khoury SJ. Role of costimulatory pathways in the pathogenesis of multiple sclerosis and experimental autoimmune encephalomyelitis. J Allergy Clin Immunol. 2003;112:837–49. doi: 10.1016/j.jaci.2003.08.025. quiz 50. [DOI] [PubMed] [Google Scholar]
- 67.Xiao Y, Jin J, Chang M, et al. Peli1 promotes microglia-mediated CNS inflammation by regulating Traf3 degradation. Nat Med. 2013;19:595–602. doi: 10.1038/nm.3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hagman S, Raunio M, Rossi M, Dastidar P, Elovaara I. Disease-associated inflammatory biomarker profiles in blood in different subtypes of multiple sclerosis: prospective clinical and MRI follow-up study. J Neuroimmunol. 2011;234:141–7. doi: 10.1016/j.jneuroim.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 69.Cox GM, Kithcart AP, Pitt D, et al. Macrophage migration inhibitory factor potentiates autoimmune-mediated neuroinflammation. J Immunol. 2013;191:1043–54. doi: 10.4049/jimmunol.1200485. [DOI] [PubMed] [Google Scholar]
- 70.Cloutier A, Guindi C, Larivee P, Dubois CM, Amrani A, McDonald PP. Inflammatory cytokine production by human neutrophils involves C/EBP transcription factors. J Immunol. 2009;182:563–71. doi: 10.4049/jimmunol.182.1.563. [DOI] [PubMed] [Google Scholar]
- 71.Leng L, Metz CN, Fang Y, et al. MIF signal transduction initiated by binding to CD74. J Exp Med. 2003;197:1467–76. doi: 10.1084/jem.20030286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Beutner C, Linnartz-Gerlach B, Schmidt SV, Beyer M, Mallmann MR, Staratschek-Jox A, Schultze JL, Neumann H. Unique transcriptome signature of mouse microglia. Glia. 2013;61:1429–42. doi: 10.1002/glia.22524. [DOI] [PubMed] [Google Scholar]
- 73.David S, Lacroix S. Molecular approaches to spinal cord repair. Annu Rev Neurosci. 2003;26:411–40. doi: 10.1146/annurev.neuro.26.043002.094946. [DOI] [PubMed] [Google Scholar]
- 74.Ponomarev ED, Veremeyko T, Barteneva N, Krichevsky AM, Weiner HL. MicroRNA-124 promotes microglia quiescence and suppresses EAE by deactivating macrophages via the C/EBP-α-PU.1 pathway. Nat Med. 2011;17:64–70. doi: 10.1038/nm.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Butovsky O, Ziv Y, Schwartz A, Landa G, Talpalar AE, Pluchino S, Martino G, Schwartz M. Microglia activated by IL-4 or IFN-γ differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Mol Cell Neurosci. 2006;31:149–60. doi: 10.1016/j.mcn.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 76.Thored P, Heldmann U, Gomes-Leal W, et al. Long-term accumulation of microglia with proneurogenic phenotype concomitant with persistent neurogenesis in adult subventricular zone after stroke. Glia. 2009;57:835–49. doi: 10.1002/glia.20810. [DOI] [PubMed] [Google Scholar]
- 77.Dhib-Jalbut S, Marks S. Interferon-β mechanisms of action in multiple sclerosis. Neurology. 2010;74(Suppl. 1):S17–24. doi: 10.1212/WNL.0b013e3181c97d99. [DOI] [PubMed] [Google Scholar]
- 78.Kim MO, Si Q, Zhou JN, Pestell RG, Brosnan CF, Locker J, Lee SC. Interferon-β activates multiple signaling cascades in primary human microglia. J Neurochem. 2002;81:1361–71. doi: 10.1046/j.1471-4159.2002.00949.x. [DOI] [PubMed] [Google Scholar]
- 79.Kawanokuchi J, Mizuno T, Kato H, Mitsuma N, Suzumura A. Effects of interferon-β on microglial functions as inflammatory and antigen presenting cells in the central nervous system. Neuropharmacology. 2004;46:734–42. doi: 10.1016/j.neuropharm.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 80.Dasgupta S, Jana M, Liu X, Pahan K. Myelin basic protein-primed T cells induce nitric oxide synthase in microglial cells. Implications for multiple sclerosis. J Biol Chem. 2002;277:39327–33. doi: 10.1074/jbc.M111841200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jin S, Kawanokuchi J, Mizuno T, Wang J, Sonobe Y, Takeuchi H, Suzumura A. Interferon-β is neuroprotective against the toxicity induced by activated microglia. Brain Res. 2007;1179:140–6. doi: 10.1016/j.brainres.2007.08.055. [DOI] [PubMed] [Google Scholar]
- 82.Saidha S, Eckstein C, Calabresi PA. New and emerging disease modifying therapies for multiple sclerosis. Ann N Y Acad Sci. 2012;1247:117–37. doi: 10.1111/j.1749-6632.2011.06272.x. [DOI] [PubMed] [Google Scholar]
- 83.Kim HJ, Ifergan I, Antel JP, Seguin R, Duddy M, Lapierre Y, Jalili F, Bar-Or A. Type 2 monocyte and microglia differentiation mediated by glatiramer acetate therapy in patients with multiple sclerosis. J Immunol. 2004;172:7144–53. doi: 10.4049/jimmunol.172.11.7144. [DOI] [PubMed] [Google Scholar]
- 84.Pul R, Moharregh-Khiabani D, Skuljec J, Skripuletz T, Garde N, Voss EV, Stangel M. Glatiramer acetate modulates TNF-α and IL-10 secretion in microglia and promotes their phagocytic activity. J Neuroimmune Pharmacol. 2011;6:381–8. doi: 10.1007/s11481-010-9248-1. [DOI] [PubMed] [Google Scholar]
- 85.Gentile A, Rossi S, Studer V, et al. Glatiramer acetate protects against inflammatory synaptopathy in experimental autoimmune encephalomyelitis. J Neuroimmune Pharmacol. 2013;8:651–63. doi: 10.1007/s11481-013-9436-x. [DOI] [PubMed] [Google Scholar]
- 86.Centonze D, Muzio L, Rossi S, et al. Inflammation triggers synaptic alteration and degeneration in experimental autoimmune encephalomyelitis. J Neurosci. 2009;29:3442–52. doi: 10.1523/JNEUROSCI.5804-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ratchford JN, Endres CJ, Hammoud DA, Pomper MG, Shiee N, McGready J, Pham DL, Calabresi PA. Decreased microglial activation in MS patients treated with glatiramer acetate. J Neurol. 2012;259:1199–205. doi: 10.1007/s00415-011-6337-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Foster CA, Howard LM, Schweitzer A, et al. Brain penetration of the oral immunomodulatory drug FTY720 and its phosphorylation in the central nervous system during experimental autoimmune encephalomyelitis: consequences for mode of action in multiple sclerosis. J Pharmacol Exp Ther. 2007;323:469–75. doi: 10.1124/jpet.107.127183. [DOI] [PubMed] [Google Scholar]
- 89.Groves A, Kihara Y, Chun J. Fingolimod: direct CNS effects of sphingosine 1-phosphate (S1P) receptor modulation and implications in multiple sclerosis therapy. J Neurol Sci. 2013;328:9–18. doi: 10.1016/j.jns.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Matloubian M, Lo CG, Cinamon G, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–60. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 91.Jackson SJ, Giovannoni G, Baker D. Fingolimod modulates microglial activation to augment markers of remyelination. J Neuroinflammation. 2011;8:76. doi: 10.1186/1742-2094-8-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Noda H, Takeuchi H, Mizuno T, Suzumura A. Fingolimod phosphate promotes the neuroprotective effects of microglia. J Neuroimmunol. 2013;256:13–8. doi: 10.1016/j.jneuroim.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 93.Linker RA, Kieseier BC, Gold R. Identification and development of new therapeutics for multiple sclerosis. Trends Pharmacol Sci. 2008;29:558–65. doi: 10.1016/j.tips.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 94.Ghoreschi K, Bruck J, Kellerer C, et al. Fumarates improve psoriasis and multiple sclerosis by inducing type II dendritic cells. J Exp Med. 2011;208:2291–303. doi: 10.1084/jem.20100977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schilling S, Goelz S, Linker R, Luehder F, Gold R. Fumaric acid esters are effective in chronic experimental autoimmune encephalomyelitis and suppress macrophage infiltration. Clin Exp Immunol. 2006;145:101–7. doi: 10.1111/j.1365-2249.2006.03094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Linker RA, Lee DH, Ryan S, et al. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain. 2011;134:678–92. doi: 10.1093/brain/awq386. [DOI] [PubMed] [Google Scholar]
- 97.Wilms H, Sievers J, Rickert U, Rostami-Yazdi M, Mrowietz U, Lucius R. Dimethylfumarate inhibits microglial and astrocytic inflammation by suppressing the synthesis of nitric oxide, IL-1β, TNF-α and IL-6 in an in-vitro model of brain inflammation. J Neuroinflammation. 2010;7:30. doi: 10.1186/1742-2094-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Motohashi H, Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol Med. 2004;10:549–57. doi: 10.1016/j.molmed.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 99.Laroni A, Novi G, Kerlero de Rosbo N, Uccelli A. Towards clinical application of mesenchymal stem cells for treatment of neurological diseases of the central nervous system. J Neuroimmune Pharmacol. 2013 doi: 10.1007/s11481-013-9456-6. doi 10.1007/s11481-013-9456-6. [DOI] [PubMed] [Google Scholar]
- 100.Uccelli A, Laroni A, Freedman MS. Mesenchymal stem cells for the treatment of multiple sclerosis and other neurological diseases. Lancet Neurol. 2011;10:649–56. doi: 10.1016/S1474-4422(11)70121-1. [DOI] [PubMed] [Google Scholar]
- 101.Uccelli A. Mesenchymal stem cells exert a remarkable regenerative effect requiring minimal CNS integration: commentary on: “Mesenchymal stem cells protect CNS neurons against glutamate excitotoxicity by inhibiting glutamate receptor expression and function” by Voulgari-Kokota et al. Exp Neurol. 2013;247:292–5. doi: 10.1016/j.expneurol.2013.01.028. [DOI] [PubMed] [Google Scholar]
- 102.Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair – current views. Stem Cells. 2007;25:2896–902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- 103.Zappia E, Casazza S, Pedemonte E, et al. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005;106:1755–61. doi: 10.1182/blood-2005-04-1496. [DOI] [PubMed] [Google Scholar]
- 104.Crigler L, Robey RC, Asawachaicharn A, Gaupp D, Phinney DG. Human mesenchymal stem cell subpopulations express a variety of neuro-regulatory molecules and promote neuronal cell survival and neuritogenesis. Exp Neurol. 2006;198:54–64. doi: 10.1016/j.expneurol.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 105.Zhou C, Zhang C, Chi S, Xu Y, Teng J, Wang H, Song Y, Zhao R. Effects of human marrow stromal cells on activation of microglial cells and production of inflammatory factors induced by lipopolysaccharide. Brain Res. 2009;1269:23–30. doi: 10.1016/j.brainres.2009.02.049. [DOI] [PubMed] [Google Scholar]
- 106.Pathak SK, Basu S, Bhattacharyya A, Pathak S, Banerjee A, Basu J, Kundu M. TLR4-dependent NF-κB activation and mitogen- and stress-activated protein kinase 1-triggered phosphorylation events are central to Helicobacter pylori peptidyl prolyl cistrans-isomerase (HP0175)-mediated induction of IL-6 release from macrophages. J Immunol. 2006;177:7950–8. doi: 10.4049/jimmunol.177.11.7950. [DOI] [PubMed] [Google Scholar]
- 107.Saijo K, Winner B, Carson CT, Collier JG, Boyer L, Rosenfeld MG, Gage FH, Glass CK. A Nurr1/CoREST pathway in microglia and astrocytes protects dopaminergic neurons from inflammation-induced death. Cell. 2009;137:47–59. doi: 10.1016/j.cell.2009.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Giunti D, Parodi B, Usai C, et al. Mesenchymal stem cells shape microglia effector functions through the release of CX3CL1. Stem Cells. 2012;30:2044–53. doi: 10.1002/stem.1174. [DOI] [PubMed] [Google Scholar]
- 109.Lee JK, Schuchman EH, Jin HK, Bae JS. Soluble CCL5 derived from bone marrow-derived mesenchymal stem cells and activated by amyloid β ameliorates Alzheimer's disease in mice by recruiting bone marrow-induced microglia immune responses. Stem Cells. 2012;30:1544–55. doi: 10.1002/stem.1125. [DOI] [PubMed] [Google Scholar]