Abstract
Loss of ζ-associated protein 70 (Zap70) results in severe immunodeficiency in humans and mice because of the critical role of Zap70 in T-cell receptor (TCR) signalling. Here we describe a novel mouse strain generated by N-ethyl-N-nitrosourea mutagenesis, with the reduced protein stability (rps) mutation in Zap70. The A243V rps mutation resulted in decreased Zap70 protein and a reduced duration of TCR-induced calcium responses, equivalent to that induced by a 50% decrease in catalytically active Zap70. The reduction of signalling through Zap70 was insufficient to substantially perturb thymic differentiation of conventional CD4 and CD8 T cells, although Foxp3+ regulatory T cells demonstrated altered thymic production and peripheral homeostasis. Despite the mild phenotype, the Zap70A243V variant lies just above the functional threshold for TCR signalling competence, as T cells relying on only a single copy of the Zap70rps allele for TCR signalling demonstrated no intracellular calcium response to TCR stimulation. This addition to the Zap70 allelic series indicates that a rate-limiting threshold for Zap70 protein levels exists at which signalling capacity switches from nearly intact to effectively null.
Keywords: Foxp3, N-ethyl-N-nitrosourea mutagenesis, protein stabilization, T cell, T-cell receptor, Zap70
Introduction
From the recent explosion of human exome sequencing experiments it is becoming increasingly apparent that every individual inherits a high mutational burden, with hundreds of rare mutations that may affect protein sequences and have a major effect on the incidence of human immune disorders.1 Within the rare mutation pool, far more common than ‘knockout’ mutations are point mutations that retain at least some protein function, and yet the effect of hypomorphs is poorly understood because of a primary focus on knockout mouse models. Additional complexity to the genetic context is created by combinatorial variation, because outside consanguineous families the probability of rare mutation compound heterozygosity is greater than rare mutation homozygosity. The potential importance of rare mutations to human health makes it essential to extend the phenotype analysis of mouse models to both hypomorphs and heterozygous interactions of multiple hypomorphic alleles.1,2
A major protein at the crossroads between immunity and tolerance is the tyrosine kinase ζ-associated protein 70 (Zap70), which plays an essential role in activating downstream signal transduction upon T-cell receptor (TCR) engagement. These Zap70-induced signalling pathways are central to T-cell differentiation, activation and proliferation, with a loss of Zap70 in mice resulting in the absence of CD4+ and CD8+ T cells and hence severe combined immunodeficiency (SCID).3–5 ZAP70-deficient patients present with a similar absence of CD8+ T cells, but CD4+ T cells are present in normal numbers, albeit of non-functional nature.6 The difference between the mouse and human Zap70/ZAP70-null phenotypes lies in the ability of SYK to partially compensate for ZAP70-deficiency during thymic differentiation of human CD4+ T cells;7,8 however, SYK cannot compensate for ZAP70 deficiency in peripheral T cells, leading to the same functional outcome of SCID in mice and humans.
Studies of a series of hypomorphic Zap70 mutant mice with graded defects in TCR signalling significantly extended the understanding of the fine-tuned balance between tolerogenic and immunogenic signalling in T cells by revealing phenotypes that would not be predicted based on the simple SCID phenotype of knockout mice. Indeed, in various hypomorphic mutant strains, immune deficiency was associated with immune hyper-activation and/or autoimmunity, ranging from hyper-IgE,9,10 anti-DNA9 and rheumatoid factor autoantibody production,11,12 to overt development of autoimmune arthritis.12 The presentation of immune dysregulation in these mutant mouse strains was associated with intermediate TCR signalling, showing that the degree of immune deficiency determines the paradoxical development of autoimmunity, i.e. by impairing negative selection and/or by disturbing regulatory T (Treg) cell function, disturbing the homeostatic equilibrium between immunogenic and tolerogenic T-cell functions.9,13,14
In the present study, we describe a novel hypomorphic Zap70 mutant mouse strain generated by N-ethyl-N-nitrosourea (ENU) mutagenesis, Zap70rps mice. The reduced protein stability mutation results in an A243V change, which causes a ˜ 55% reduction in Zap70 protein levels. Here we demonstrate that a 55% reduction in functional Zap70 protein results in only mild TCR signalling and cellular phenotypes, whereas a further 50% reduction in Zap70 protein levels generated by compound heterozygosity of hypomorphic alleles demonstrates near-normal positive selection but no peripheral intracellular calcium responses to TCR stimulation. These data demonstrate that combinatorial association of multiple hypomorphic alleles can generate unexpected phenotypes once the net genetic burden rises above set thresholds.
Materials and methods
Mice
Foxp3GFP mice15 were backcrossed to the C57BL/6 background. Zap70mrt mice are described in the accompanying paper16. To generate the Zap70rps strain, founder C57BL/6 male mice were treated with 100 mg/kg ENU and bred to Foxp3GFP females. First-generation male offspring were bred back to Foxp3GFP females to produce the second-generation offspring, which were in turn inter-crossed to produce the third generation for phenotype screening. Phenotypic screening involved flow cytometric analysis for CD4 (anti-CD4 antibody) and Foxp3 (GFP), and identification of individuals with values for the percentage of GFP+ cells within the CD4+ population that were more than two standard deviations from the norm. The identified variant offspring were inter-crossed to produce the C57BL/6.Foxp3GFP.Zap70rps mutant mouse strain. Experimental mice were age-matched and housed under specific pathogen-free conditions. All mice were used after ethics approval of the University of Leuven mouse facility.
Molecular biology
The rps mutation identified by all-exome sequencing was confirmed by Sanger sequencing. Using primers that flank the rps mutation (forward primer 5′-GAC AAG GCT GGC AAG TAC TGC ATC-3′ and reverse primer 5′-TGT CAC GTC TCA ACG CTG AGG TG-3′) a 400-bp fragment was amplified and cloned into the pCR®4-TOPO® vector (Invitrogen, Carlsbad, CA) for sequencing.
Zap70rps mice were genotyped using the Amplifluor SNP genotyping assay based on PCR amplification with tailed allele-specific primers, a common reverse primer and fluorescence-labelled (JOE-FAM) Amplifluor universal primers (Millipore, Billerica, MA). Primers were designed with the assayarchitect software (Millipore) and were 5′-GA AGG TCG GAG TCA ACG GAT TTG GAG TAC CTG AAG CTG AAG GC-3′ (wild-type), 5′-GAA GGT GAC CAA GTT CAT GCT GTG GAG TAC CTG AAG CTG AAG GT-3′ (rps) and the common reverse primer was 5′-CTG TTG GGA CAG ACC TCC TT-3′. Fluorescence end-point measurement was performed using the Taqman RT-PCR 7300 (Applied Biosystems, Foster City, CA).
CD4+ CD8− and CD4− CD8+ splenocytes were sorted after enrichment with an EasySep™ Mouse T Cell Isolation Kit (Stemcell Technologies, Vancouver, BC, Canada). RNA was purified using an RNeasy micro kit (Qiagen, Hilden, Germany) and was reverse transcribed with random hexamers (Life Technologies, Carlsbad, CA) and M-MLV (Invitrogen). Quantitative PCR was performed with the StepOnePlus (Life Technologies) and TaqMan Fast Universal PCR Master Mix (Life Technologies) for Zap70 (Mm.PT.56a.12463755, IDT DNA) and β-actin (Mm.PT.56a.33540333, IDT DNA). Relative gene expression was determined by the 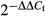 method17 and normalized to the average of wild-type CD4+ T cells.
method17 and normalized to the average of wild-type CD4+ T cells.
Flow cytometry
Cell surface staining and flow cytometric analysis were performed using the following antibodies: anti-B220-phycoerythrin (PE) -Cy5 (1/400) (RA3-6B2) or PE-Cy7 (1/400) (RA3-6B2), and anti-CD3e-allophycocyanin (APC) (1/400) (145-2c11), anti-CD4-APC (1/400) (GK1.5) or APC-H7 (1/400) (GK1.5) or Peridinin chlorophyll protein (PerCP)-Cy5.5 (1/400) (GK1.5) or eFluor450 (1/400) (GK1.5), anti-CD8-APC-eFluor780 (1/400) (53-6.7) or PerCP-Cy5.5 (1/400) (53-6.7), anti-CD25-APC (1/250) (PC61.5) or PE (1/250) (PC61.5), anti-CD62L-PE-Cy7 (1/250) (MEL-14) and anti-CD69-PE-Cy5.5 (1/250) (H1.2F3) or PE-Cy7 (1/250) (H1.2F3) (all eBioscience, San Diego, CA, unless indicated otherwise). Intracellular staining with anti-Foxp3-APC (1/100) (eBioscience), anti-Ki67-PE (1/50) (BD) and anti-Zap70-PE (1/400) (1E7.2; eBioscience) antibodies was performed following fixation and permeabilization using the reagents from the eBioscience Foxp3 staining kit. Data were collected by BD canto I and canto II. Cell sorting was performed on splenocytes stained for anti-CD4-PE-Cy7 (GK1.5, eBioscience) and anti-CD8α-APC-eFluor780 (eBioscience) on a BD FACS Aria III.
T-cell stimulation
Splenocytes were resuspended in 10% complete RPMI-1640 media at 1 × 107 cells/ml in round-bottomed 96-well plates (Greiner Bio-one, Frickenhausen, Germany). Cells were stimulated with plate-bound anti-CD3 (eBioscience) and anti-CD28 (eBioscience) antibodies at 37° for 24 hr before harvesting for flow cytometry as described above.
Calcium flux assays were performed on freshly isolated splenocytes. Before analysis cells were loaded with Fluo-4 (0·8 μg/ml; Invitrogen) and Indo-1 (2 μg/ml; Invitrogen), and stained with anti-B220-APC (1/400) (RA3-6B2), anti-CD3-biotin (145-2c11), anti-CD11b-PE (1/400) (M1/70), anti-Ly-6G-APC (1/400) (Gr-1), anti-MHC class II-PE (1/400), anti-NK1.1-APC (1/400) (PK136), anti-TER-199-PE (1/400) (TER-119), and either anti-CD4-biotin (1/200) (RM4-5) and anti-CD8-APC-Alexa780 (1/400) (53–6·7) or anti-CD4-APC-Cy7 (1/400) (GK1.5) and anti-CD8-biotin (1/200) (53–6·7) (all eBioscience unless indicated otherwise). A base line level of Fluo-4 and Indo-1 fluorescence was acquired at 37° on a BD Aria III before the addition of streptavidin (Sigma, St Louis, MO) to cross-link anti-CD3 and anti-CD4. Immediately following streptavidin addition, acquisition of flow data was reinitiated for an additional 500 seconds.
Statistics and modelling
Statistical analysis was performed using prism (Graphpad, San Diego, CA). A significance threshold of 5% in a Mann–Whitney U-test or a Kruskal–Wallis test (with Dunn's correction for multiple comparisons) was maintained throughout the study. P-values were obtained by comparison of the Zap70 mutant with wild-type control values (and between Zap70rps and Zap70mrt/wt in Fig. 5). Homology alignment was performed using clustal omega, BLAST and jalview.18 pymol Molecular Graphics System (Version 1.5.0.4 Schrödinger, LLC, Portland, OR) was used for the modelling of the Zap70 structure.
Results
Identification of the reduced protein stability mutation in Zap70 and the resulting alteration in regulatory T-cell differentiation
The ENU-exposed C57BL/6 mice were bred to Foxp3GFP females to generate a standard F2 intercross pedigree19 allowing screening of ENU mutants with altered numbers of Foxp3+ Treg cells in the blood. Within one pedigree, individuals were identified with an increased number of peripheral blood GFP+ Treg cells. Inter-crossing of affected individuals resulted in the reduced protein stability (rps) mutant strain, which consistently demonstrated increased numbers of Treg cells in the blood by 5–6 weeks of age (Fig. 1a). All-exon sequencing of affected individuals identified a C→T nucleotide substitution at nucleotide 36778405, which was confirmed by Sanger sequencing (Fig. 1b). This mutation resulted in an alanine to valine change at amino acid 243 in a region of the Zap70 protein sequence highly conserved across mammals (Fig. 1c) and partially conserved across related kinases in mice (Fig. 1d). The A243V change is located within the C-terminal SH2 domain, one of the two domains involved in the docking of Zap70 to phosphorylated immunoreceptor tyrosine-based activation motif on the TCR-ζ peptide (Fig. 1e–g).
Figure 1.
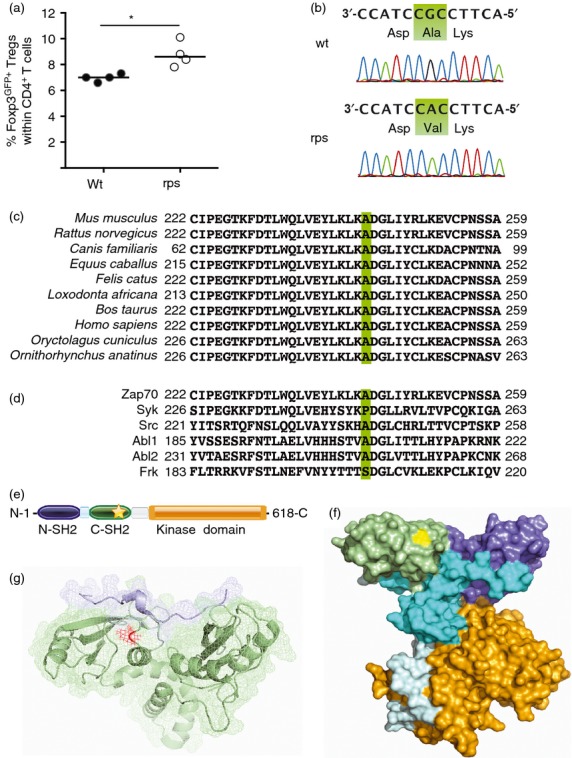
Increased peripheral regulatory T (Treg) cell frequencies in Zap70rps mice with an A243V in the C-terminal SH2-domain of Zap70. (a) Peripheral blood lymphocytes from wild-type and rps mutant mice were assessed for the percentage of Foxp3GFP+ Treg cells within the CD4+ T-cell compartment at 5–6 weeks of age. (b) Sanger sequencing of Zap70 in wild-type and rps mutant mice confirmed a C to T mutation at location 36778405, resulting in an alanine to valine change at amino acid 243. (c) Conservation of the rps mutation site between the mouse, rat, dog, horse, cat, elephant, bovine, human, rabbit and platypus homologous mammal sequences. (d) Partial conservation of the rps mutation site between related murine kinases, Zap-70, Syk, Src, Abl1, Abl2 and Frk. (e) Location of the A243V amino acid change (star) within the domain structure of Zap70. Identified are the N-terminal SH2 domain (purple), interdomain A (blue), C-terminal SH2 domain (green), interdomain B (grey) and kinase domain (orange). (f) Location of the A243 residue (yellow) within the cloud structure of Zap70. Identified are the N-terminal SH2 domain (purple), interdomain A (blue), C-terminal SH2 domain (green), interdomain B (grey) and kinase domain (orange). (g) Modelling of SH2 domains (green) in contact with TCR-ζ peptide (blue). The A243 residue is shown in red at the binding interface. Structures from PyMOL. *P < 0·05.
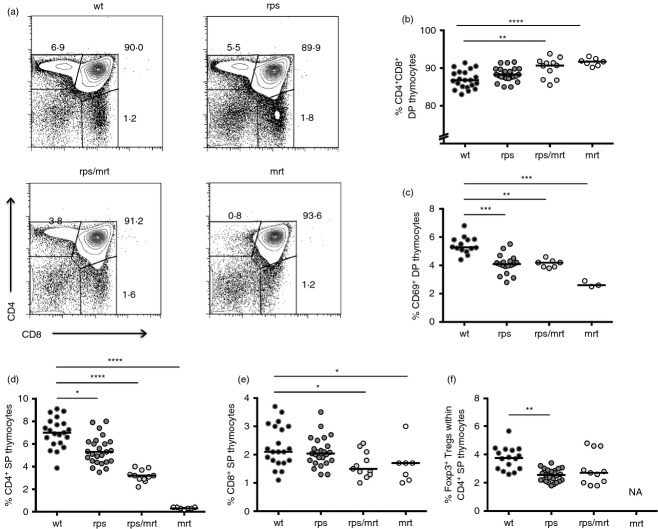
Due to the sensitivity of T-cell differentiation to defects in TCR signalling, we next investigated the impact of the Zap70A243V variant on thymic differentiation. Unlike mice bearing a null allele of Zap705, Zap70rps mice did not have a major defect in positive selection. The overall thymic cellularity was unchanged in Zap70rps mice (data not shown) and the percentage of CD4+ CD8+ double-positive thymocytes remained intact, while the frequency of post-selection CD69+ double-positive thymocytes was reduced by ˜ 20% (Fig. 2a–c). Similarly, a small impairment in lineage selection was observed, with a ˜ 25% decline in the number of CD4+ single-positive thymocytes (Fig. 2d and Supplementary material, Fig. S1a), whereas CD8+ single-positive thymocytes were unchanged (Fig. 2e and Supplementary material, Fig. S1b). In addition, positive selection into the Foxp3+ regulatory T-cell lineage was reduced by ˜ 30% in Zap70rps mice (Fig. 2f and Supplementary material, Fig. S1c). Non-complementation of the Zap70rps mutation was observed upon inter-crossing with the Zap70mrt strain (which is a severe hypomorph), as Zap70rps/mrt heterozygote mice bore defects in thymocyte differentiation ranging between that of the parental strains (Fig. 2b–f and Supplementary material, Fig. S1a–c). The mild defect in positive selection observed in the thymus was perpetuated in the periphery, with a ˜ 25% drop in both CD4+ and CD8+ T cells in the spleen of Zap70rps mice relative to wild-type mice (Fig. 3a–c and Supplementary material, Fig. S1d, e) but similar total splenocyte numbers (data not shown). Again, the Zap70rps mutation was not complemented by the Zap70mrt mutation (Fig. 3a–c and Supplementary material, Fig. S1d, e), reinforcing the conclusion that T-cell defects in the rps strain are caused by the Zap70A243V variant. Finally, consistent with other Zap70 hypomorphs9 and the robust niche-filling capacity of Treg cells20, splenic Treg cell numbers rebounded to normal levels despite the ˜ 30% reduction in thymic numbers, an effect which, when combined with the mild CD4+ lymphopenia, resulted in a relative enrichment of Treg cells within CD4+ T cells (Fig. 3d). In total numbers, the rebound was less obvious, but it was still apparent as the initial ˜ 70% decline in Zap70rps thymic Treg cell number output ended up in only a ˜ 30% decrease in peripheral numbers (Supplementary material, Fig. S1c,f). This relative enrichment was probably responsible for the phenotype of increased number of peripheral blood GFP+ Treg cells on which the rps strain was first identified and which remains constant with age (Supplementary material, Fig. S2).
Figure 2.
Reduced positive selection of regulatory T cells in mice bearing the Zap70A243V variant. Wild-type, Zap70rps, Zap70rps/mrt and Zap70mrt mice were assessed for thymic function at 5–13 weeks of age for T-cell subpopulations (n = 14–21, 18–25, 7–11, 3–7 mice/group). (a) Representative flow cytometry plots for CD4 versus CD8. (b) Percentage of CD4+ CD8+ double-positive (DP) thymocytes in mice of each genotype (median with individual values shown). (c) Percentage of DP thymocytes expressing CD69 in mice of each genotype (median with individual values shown). (d) Percentage of CD4+ single-positive (SP) thymocytes in mice of each genotype (median with individual values shown). (e) Percentage of CD8+ SP thymocytes in mice of each genotype (median with individual values shown). (f) Percentage of Foxp3+ regulatory T cells within the CD4+ SP population in mice of each genotype, with the exception of Zap70mrt mice where the parent population was absent (NA) (median with individual values shown). *P < 0·05; **P < 0·01; ***P < 0·001; ****P < 0·0001.
Figure 3.
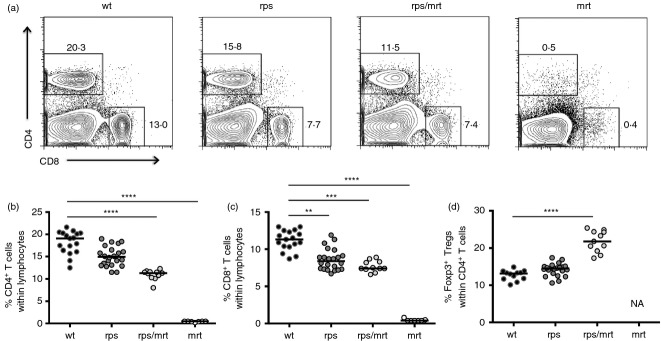
Mild lymphopenia and enrichment of regulatory T cells in Zap70rps mice. Wild-type, Zap70rps, Zap70rps/mrt and Zap70mrt mice were assessed at 5–13 weeks of age for T-cell subpopulations in the spleen (n = 12–17, 22, 11, 7 mice/group). (a) Representative flow cytometry plots for CD4 versus CD8. (b) Percentage of CD4+ T cells within the lymphocyte gate in mice of each genotype (median with individual values shown). (c) Percentage of CD8+ T cells within the lymphocyte gate in mice of each genotype (median with individual values shown). (d) Percentage of Foxp3+ Treg cells within the CD4+ T-cell population in mice of each genotype, with the exception of Zap70mrt mice where the parent population was absent (NA) (median with individual values shown). **P < 0·01; ***P < 0·001; ****P < 0·0001.
The Zap70A243V variant demonstrates reduced protein stability and impaired calcium signalling
To determine the mechanism of activity of the Zap70rps mutation the relative expression of the Zap70A243V variant was tested at the mRNA and protein levels. Complementary DNA was generated from sorted peripheral CD4+ and CD8+ T cells and the relative expression of Zap70 was assessed by quantitative PCR. No differences were observed between wild-type and Zap70rps T cells, either CD4+ or CD8+, indicating that the rps mutation does not affect mRNA expression or longevity (Fig. 4a). To determine the effect of the Zap70A243V variant on protein expression, Zap70 expression was measured by flow cytometry in CD4+ and CD8+ T cells of both the thymus and spleen. In the thymus, CD4+ and CD8+ single-positive T cells displayed a 50–70% reduction in Zap70 protein levels in Zap70rps mice (Fig. 4b,c). By contrast, Zap70 expression was almost undetectable in the same population from Zap70mrt mice, in which a major disruption to the catalytic site results in rapidly degraded protein16. Compound heterozygotes between the Zap70rps and Zap70mrt strains showed a further ˜ 50% reduction in Zap70 protein levels, to ˜ 30% of wild-type levels (Fig. 4c). A reduction of ˜ 55% in Zap70 protein level was also observed in the spleen of Zap70rps mice, indicating that peripheral compensation mechanisms were not effective (Fig. 4d,e). Together, these results demonstrate that the rps mutation results in a Zap70 variant with reduced protein stability, titrating down the available Zap70 protein levels to ˜ 45% across organs and lineages.
Figure 4.
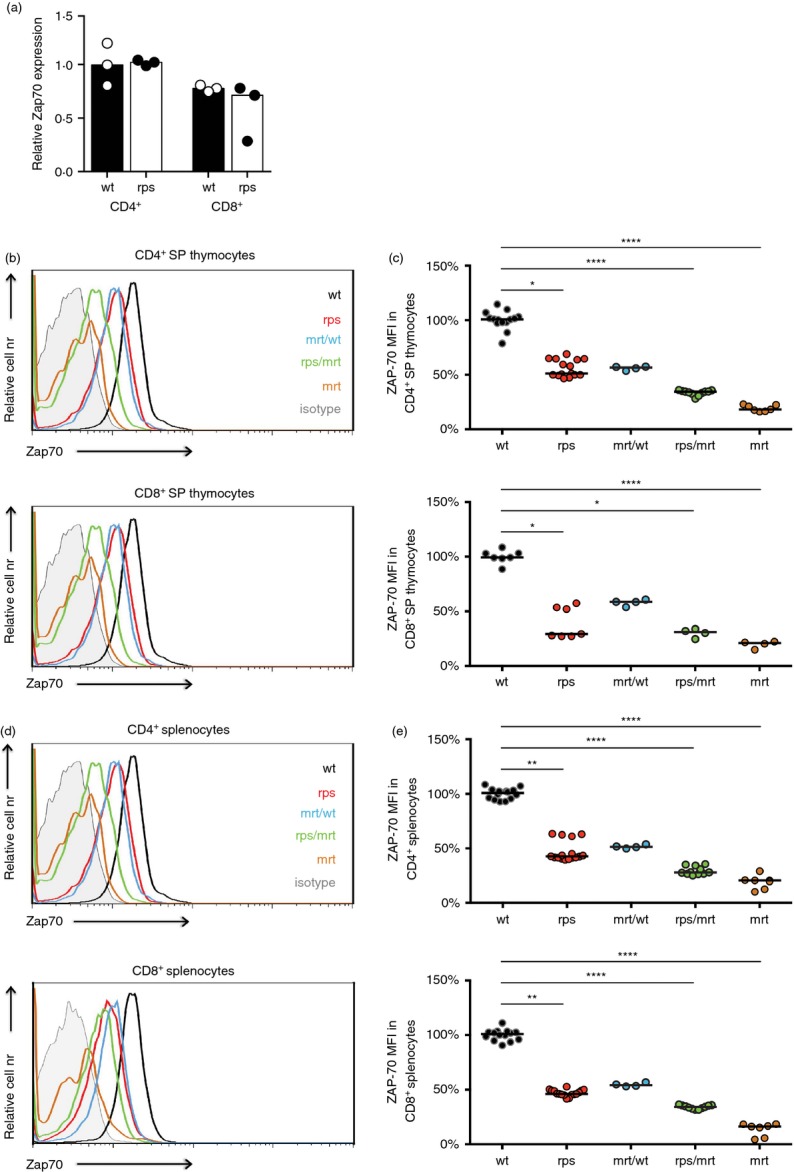
Decreased Zap70 protein levels in Zap70rps mice. (a) CD4+ and CD8+ T cells were purified from the spleen of wild-type and Zap70rps mice. Relative Zap70 expression was assayed through quantitative PCR measurement, relative to β-actin (n = 3/group). (b–e) The thymus and spleen of wild-type, Zap70rps, Zap70rps/mrt and Zap70mrt mice was stained with antibodies against CD4, CD8 and Zap70 for flow cytometric analysis (n = 7–14, 7–16, 4, 4–11, 4–7/group). (b) Representative flow cytometry plots and (c) mean fluorescence intensity (MFI) for CD4+ and CD8+ single-positive thymocytes (median with individual values shown). (d) Representative flow cytometry plots and (e) mean fluorescence intensity for CD4+ and CD8+ splenocytes (median with individual values shown). *P < 0·05; **P < 0·01; ****P < 0·0001.
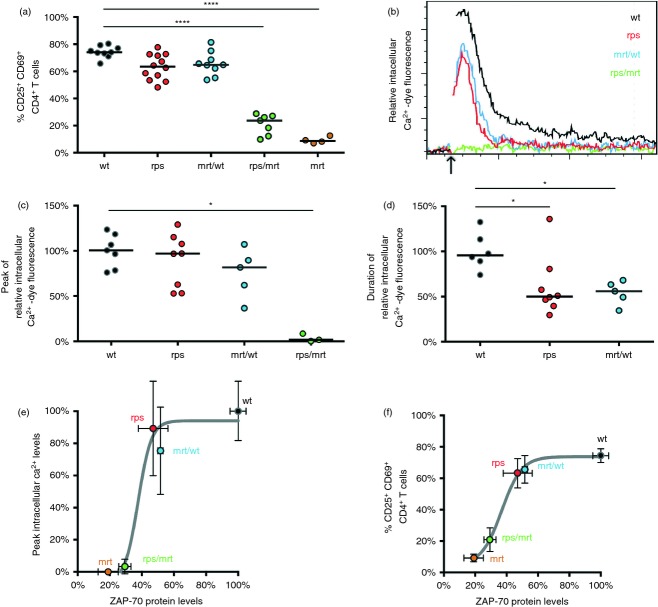
To determine the impact of reduced Zap70 levels on downstream signalling, CD4+ and CD8+ T cells from wild-type and Zap70rps mice were stimulated in vitro for 24 hr. For both Zap70rps CD4+ and CD8+ T cells, the up-regulation of early activation markers (CD25+ CD69+ T cells) was slightly reduced from that of wild-type cells (Fig. 5a), similar to that observed in Zap70mrt/wt mice, in which functional Zap70 is reduced by ˜ 45%. Notably, reliance on a single copy of Zap70rps in combination with the severly hypomorphic Zap70mrt allele (in Zap70rps/mrt T cells) resulted in a more significant drop in T-cell activation, although still less than that observed in Zap70mrt mice (Fig. 5a). To determine the quantity and quality of the immediate TCR signalling response, wild-type and Zap70rps T cells were challenged in a Ca2+ flux assay. Following loading with Ca2+-sensitive dye and recording of baseline fluorescence, wild-type and Zap70rps CD4+ T cells were stimulated via cross-linking of CD3 and CD4, respectively. No differences in initial activation status between Zap70rps, Zap70wt/mrt and wild-type CD4+ T cells were observed, whereas Zap70rps/mrt CD4+ T cells showed significantly increased proportions of activated cells (Supplementary material, Fig. S3a). Individual Ca2+ signalling results showed a uniform response, excluding the possibility that the observed difference was due to population heterogeneity (Supplementary material, Fig. S3b). Relative to wild-type CD4+ T cells, Zap70rps CD4+ T cells showed a mild impairment of TCR signalling (Fig. 5b), with no significant reduction in peak intracellular Ca2+ (Fig. 5c), but a reduced duration of elevated intracellular Ca2+ (Fig. 5d). Hence, despite a reduction to ˜ 55% of wild-type Zap70 protein levels, TCR signalling remained relatively unchanged in Zap70rps T cells. These results were very similar to Zap70wt/mrt T cells, with ˜ 45% of functional Zap70 levels. Strikingly, however, further reduction of functional Zap70 protein levels to ˜ 30% of wild-type levels (observed in Zap70rps/mrt T cells) led to a complete blockade of effective Ca2+ flux in response to TCR stimulation (Fig. 5b–d). This discrepancy between Zap70rps T cells and Zap70rps/mrt T cells demonstrates a non-linear relationship between Zap70 protein availability and TCR signalling events, as exemplified by the sigmoidal curve of Zap70 protein levels versus Ca2+ flux peaks (Fig. 5e) or versus general T-cell activation (Fig. 5f). Analysis of this relationship suggests a relatively narrow protein concentration range in which near-normal TCR signalling switches into an effectively non-functional state.
Figure 5.
Reduced Zap70 protein levels in Zap70rps CD4+ and CD8+ T cells leave T-cell receptor (TCR) signalling above the critical threshold for activity. (a) Splenocytes from wild-type, Zap70rps, Zap70mrt/wt, Zap70rps/mrt and Zap70mrt mice were stimulated for 24 hr with anti-CD3 and anti-CD28 before measurement of activation via CD25 and CD69 up-regulation in CD4+ T cells (n = 9, 12, 9, 7, 4 mice/group, median and individual values shown). (b) CD4+ splenocytes from wild-type, Zap70rps, Zap70mrt/wt,and Zap70rps/mrt mice were loaded with Ca2+-dependent dye and assessed by flow cytometry for intracellular Ca2+. The arrow indicates the addition of streptavidin to cause the cross-linking of CD3 and CD4. Representative histograms are shown gated on CD4+ T cells. n = 7, 8, 5, 3 mice/group (c) Relative peak intracellular Ca2+-dependent dye fluorescence (median and individual values shown) and (d) duration of elevated intracellular Ca2+-dependent dye fluorescence in the cells described in (b) (median and individual values shown). (e, f) Relationship between Zap70 protein levels (relative to wild-type T cells based on data in Fig. 4e) and (e) peak intracellular Ca2+ levels following TCR stimulation (relative to wild-type T cells based on data in part c of this figure) or (f) activation (as depicted in panel a of this figure) in wild-type, Zap70rps, Zap70mrt/wt, Zap70rps/mrt and Zap70mrt mice for CD4+ splenocytes. Mean and standard deviation for each genotype is shown, with the Boltzmann sigmoidal function of best fit. *P < 0·05; ****P < 0·0001.
Discussion
In the present study, we characterized a novel Zap70 hypomorphic mutant mouse strain, Zap70rps mice, in which an A243V change resulted in a 55% reduction in the Zap70 protein concentration. The phenotypic changes induced by ˜ 50% reduction in Zap70 protein, either in Zap70rps mice or mice heterozygous for the mrt mutation, proved relatively subtle, with a few notable exceptions. In both genetic contexts, TCR signalling remained largely intact, with normal peak intracellular Ca2+ achieved following TCR stimulation but a reduction in the duration of elevated intracellular Ca2+. This defect can be explained by the ˜ 50% reduction in Zap70 protein levels in Zap70rps T cells, as Zap70mrt/wt T cells, which have one normal fully-function copy of Zap70 and one severely hypomorphic and rapidly degraded copy of Zap70, have an indistinguishable phenotype. Nevertheless, it is conceivable that the A243V change also results in impaired function of the remaining protein. The two SH2 domains of Zap70 are involved in the binding of Zap70 to the TCR-ζ chain, following the phosphorylation of the immunoreceptor tyrosine-based activation motif by Lck. This binding releases Zap70 from its auto-inhibited conformation, allowing subsequent signal transduction.4 Crystal structure studies indicate that Y238 and K242 are closely associated with the phosphotyrosine of the immunoreceptor tyrosine-based activation motif peptide,21 suggesting that changes to the neighbouring 243 residue could impair docking of Zap70 to CD3ζ. Such a function would of necessity be minor, because otherwise the additive effects of reduced protein level and impaired protein function would be predicted to be greater than that of a similar reduction in protein level alone.
Regardless of the molecular mechanism by which Zap70 signalling is affected, the mild alteration in signalling capacity of Zap70rps and Zap70mrt/wt T cells produced only small decreases in the TCR-dependent processes of positive selection and peripheral T-cell activation. The TCR-dependent process, which demonstrated the most substantial defect due to the 50% reduction in Zap70 protein levels, was the positive selection of Foxp3+ Treg cells. There are several reasonable hypotheses for this. First, thymic Foxp3 induction in Treg cell precursors is a positive selection event requiring an elevated level of TCR signalling22 and qualitatively different downstream mediators of signal transduction,23 such that reduction of the kinase function may differentially impact Treg cells. Alternatively, Treg cells have been demonstrated to be dependent on a non-kinase function of Zap70 for activation of Rap1.24 As Rap1 is involved in thymic Treg cell induction,25,26 it is plausible that partial loss of Zap70 protein has a stronger effect on Treg cell induction via reduction of both the kinase-dependent and Rap1-dependent Zap70 signalling pathways.
One interpretation of the resilience of TCR-dependent signalling to substantial losses in Zap70 protein could be that the Zap70 signalling pathway requires very little functional protein to signal. In this scenario it could be predicted that further reduction in Zap70 protein levels would lead to a progressive decline in total Zap70-dependent signalling. In contrast to this supposition, however, Zap70rps/mrt T cells demonstrated a profound defect in TCR signalling. In Zap70rps/mrt T cells the functional level of Zap70 protein was reduced to ˜ 30% of normal levels, as a result of the compound effect of one allele of reduced protein stability producing half the normal Zap70 quotient and one allele (mrt) largely unable to produce functional levels of Zap70. The phenotype of these Zap70rps/mrt T cells was striking. Positive selection was essentially intact, however peripheral T-cell signalling was essentially absent. No intracellular Ca2+ flux was observed upon TCR-stimulation and even 24 hr of stimulation only resulted in very minor up-regulation of CD69 and CD25, consistent with just a ‘trickle’ of signalling capacity being left intact. It is tempting to speculate that the largely intact nature of positive selection in Zap70rps/mrt mice, in contrast to the defective peripheral signalling, is due to cooperation between remnant Zap70 with Syk. In human thymocytes, SYK is expressed at a higher level than in mouse thymocytes, and can substitute for ZAP70 until SYK expression is lost in the periphery.7 It is plausible that murine Syk, expressed at lower levels than SYK, cannot substitute for complete Zap70-deficiency but can substitute for the ˜ 70% Zap70-deficiency observed in Zap70rps/mrt mice,27 until Syk is lost in the periphery. Alternatively, this feature may simply emerge as a result of the lower signalling requirement of positive selection versus peripheral T-cell activation.9,22 Regardless, it is striking that the reduction of Zap70 protein from 100% to 50% has almost no impact on TCR signalling, whereas further reduction from 50% to 30% results in an essentially complete blockade. This sigmoidal relationship between protein concentration and protein function constitutes a switch-like function, analogous to the dose–response function of TCR ligand affinity, which oscillates from ‘all on’ to ‘all off’ over a relatively small affinity range.28,29
Finally, these observations serve as a case study for the emergent phenotypes that can develop in complex genetic contexts. A recent study of publicly available exome sequencing data concluded that about 2% of the human population carries a rare, non-synonymous (protein sequence-altering) variant in any given gene, most of which are rare variants, i.e. found in < 1% of the population.1 Assuming ZAP70 is not exceptional, about four individuals per 10 000 will inherit compound heterozygous mutations in ZAP70. Our results here suggest that most of these combinations will have no observable phenotype, as the change induced by ˜ 50% reduction in functional Zap70 in mice is well within the normal variation observed in humans. At the other end of the spectrum are ZAP70 deficiencies sufficient to cause SCID.6 Genetic defects within these extremes have been observed in humans, such as a case of hypomorphic ZAP70, which resulted in late onset immunodeficiency.30 Notably, most of these mutations affect protein stability of ZAP70,6,31–33 functioning in a similar manner to the rps mutation described here. Since we know from mouse studies that Zap70 mutations in the intermediate range produce an admixture of immunodeficiency and autoimmunity,9–12 it is surprising that such cases have not been observed in humans, as genetic combinations that fall within the midrange of the mutational spectrum should be more common than those at the extremes. To some degree this may be due to ascertainment bias in genetic diagnosis; however, the recent growth in all-exome sequencing approaches1,2 should negate such effects. Our results here provide an alternative explanation for the absence of clinical phenotypes representative of intermediate defects: namely that the window for intermediate defects is narrow, such that most defects in ZAP70 will fall either into the phenotypically normal or phenotypically null range. The extrapolation of these results on Zap70 to other genes would likewise provide an explanation for the vast majority of individuals having normal healthy phenotypes despite a surprisingly high genome-wide inherited mutational burden.
Acknowledgments
AL designed and supervised the study. BC, LT, DF, WP, KAS and SMS performed the experiments. BC and AL wrote the paper. We thank the staff of the Australian Phenomics Facility for ENU mutagenesis screening and S. Schönefeldt for maintenance of the mouse colonies. Foxp3GFP mice were provided by A. Rudensky (Sloan-Kettering, New York, NY) and Zap70mrt mice were provided by C. Goodnow (Australian National University, Canberra, ACT, Australia). This work was supported by the Fonds Wetenschappelijk Onderzoek (FWO), University of Leuven and the VIB. BC and SMS are postdoctoral fellows of the Fonds Wetenschappelijk Onderzoek (FWO) Vlaanderen. WP is a PhD fellow of the Agentschap voor Innovatie door Wetenschap en Technologie (IWT).
Glossary
- APC
allophycocyanin
- ENU
N-ethyl-N-nitrosourea
- PE
phycoerythrin
- PerCP
peridinin chlorophyll protein
- Rps
reduced protein stability
- SP
single positive
- TCR
T-cell receptor
- Treg
regulatory T
Disclosures
The authors declare no financial or commercial conflict of interest.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Figure S1. Total numbers of thymic and splenic T-cell subpopulations.
Figure S2. Increased peripheral regulatory T (Treg) cell frequencies in Zap70rps mice remain constant over age.
Figure S3. Individual cellular Ca2+ flux responses are uniform and unaffected by activation status.
References
- 1.Andrews TD, Sjollema G, Goodnow CC. Understanding the immunological impact of the human mutation explosion. Trends Immunol. 2013;34:99–106. doi: 10.1016/j.it.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Tennessen JA, Bigham AW, O'Connor TD, et al. Evolution and functional impact of rare coding variation from deep sequencing of human exomes. Science. 2012;337:64–9. doi: 10.1126/science.1219240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan AC, Iwashima M, Turck CW, Weiss A. ZAP-70: a 70 kd protein-tyrosine kinase that associates with the TCR ζ chain. Cell. 1992;71:649–62. doi: 10.1016/0092-8674(92)90598-7. [DOI] [PubMed] [Google Scholar]
- 4.Wang H, Kadlecek TA, Au-Yeung BB, Goodfellow HE, Hsu LY, Freedman TS, Weiss A. ZAP-70: an essential kinase in T-cell signaling. Cold Spring Harb Perspect Biol. 2010;2:a002279. doi: 10.1101/cshperspect.a002279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Negishi I, Motoyama N, Nakayama K, et al. Essential role for ZAP-70 in both positive and negative selection of thymocytes. Nature. 1995;376:435–8. doi: 10.1038/376435a0. [DOI] [PubMed] [Google Scholar]
- 6.Arpaia E, Shahar M, Dadi H, Cohen A, Roifman CM. Defective T cell receptor signaling and CD8+ thymic selection in humans lacking zap-70 kinase. Cell. 1994;76:947–58. doi: 10.1016/0092-8674(94)90368-9. [DOI] [PubMed] [Google Scholar]
- 7.Chu DH, Oers van NS, Malissen M, Harris J, Elder M, Weiss A. Pre-T cell receptor signals are responsible for the down-regulation of Syk protein tyrosine kinase expression. J Immunol. 1999;163:2610–20. [PubMed] [Google Scholar]
- 8.Wiest DL, Yuan L, Jefferson J, et al. Regulation of T cell receptor expression in immature CD4+ CD8+ thymocytes by p56lck tyrosine kinase: basis for differential signaling by CD4 and CD8 in immature thymocytes expressing both coreceptor molecules. J Exp Med. 1993;178:1701–12. doi: 10.1084/jem.178.5.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siggs OM, Miosge LA, Yates AL, et al. Opposing functions of the T cell receptor kinase ZAP-70 in immunity and tolerance differentially titrate in response to nucleotide substitutions. Immunity. 2007;27:912–26. doi: 10.1016/j.immuni.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jakob T, Kollisch GV, Howaldt M, et al. Novel mouse mutants with primary cellular immunodeficiencies generated by genome-wide mutagenesis. J Allergy Clin Immunol. 2008;121:e7. doi: 10.1016/j.jaci.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 11.Hsu LY, Tan YX, Xiao Z, Malissen M, Weiss A. A hypomorphic allele of ZAP-70 reveals a distinct thymic threshold for autoimmune disease versus autoimmune reactivity. J Exp Med. 2009;206:2527–41. doi: 10.1084/jem.20082902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakaguchi N, Takahashi T, Hata H, et al. Altered thymic T-cell selection due to a mutation of the ZAP-70 gene causes autoimmune arthritis in mice. Nature. 2003;426:454–60. doi: 10.1038/nature02119. [DOI] [PubMed] [Google Scholar]
- 13.Liston A, Enders A, Siggs OM. Unravelling the association of partial T-cell immunodeficiency and immune dysregulation. Nat Rev Immunol. 2008;8:545–58. doi: 10.1038/nri2336. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka S, Maeda S, Hashimoto M, et al. Graded attenuation of TCR signaling elicits distinct autoimmune diseases by altering thymic T cell selection and regulatory T cell function. J Immunol. 2010;185:2295–305. doi: 10.4049/jimmunol.1000848. [DOI] [PubMed] [Google Scholar]
- 15.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–41. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 16.Siggs OM, Yates AL, Schlenner S, Liston A, Lesage S, Goodnow CC. A ZAP-70 kinase domain variant prevents thymocyte positive selection despite signalling CD69 induction. Immunology. 2013 doi: 10.1111/imm.12220. doi: 10.1111/imm.12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
-
17.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the
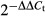 Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar] - 18.Waterhouse AM, Procter JB, Martin DM, Clamp M, Barton GJ. Jalview Version 2–a multiple sequence alignment editor and analysis workbench. Bioinformatics. 2009;25:1189–91. doi: 10.1093/bioinformatics/btp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nelms KA, Goodnow CC. Genome-wide ENU mutagenesis to reveal immune regulators. Immunity. 2001;15:409–18. doi: 10.1016/s1074-7613(01)00199-6. [DOI] [PubMed] [Google Scholar]
- 20.Pierson W, Cauwe B, Policheni A, et al. Antiapoptotic Mcl-1 is critical for the survival and niche-filling capacity of Foxp3 regulatory T cells. Nat Immunol. 2013;14:959–65. doi: 10.1038/ni.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hatada MH, Lu X, Laird ER, et al. Molecular basis for interaction of the protein tyrosine kinase ZAP-70 with the T-cell receptor. Nature. 1995;377:32–8. doi: 10.1038/377032a0. [DOI] [PubMed] [Google Scholar]
- 22.Liston A, Rudensky AY. Thymic development and peripheral homeostasis of regulatory T cells. Curr Opin Immunol. 2007;19:176–85. doi: 10.1016/j.coi.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 23.Koonpaew S, Shen S, Flowers L, Zhang W. LAT-mediated signaling in CD4+ CD25+ regulatory T cell development. J Exp Med. 2006;203:119–29. doi: 10.1084/jem.20050903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Au-Yeung BB, Levin SE, Zhang C, Hsu LY, Cheng DA, Killeen N, Shokat KM, Weiss A. A genetically selective inhibitor demonstrates a function for the kinase Zap70 in regulatory T cells independent of its catalytic activity. Nat Immunol. 2010;11:1085–92. doi: 10.1038/ni.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li L, Greenwald RJ, Lafuente EM, Tzachanis D, Berezovskaya A, Freeman GJ, Sharpe AH, Boussiotis VA. Rap1-GTP is a negative regulator of Th cell function and promotes the generation of CD4+ CD103+ regulatory T cells in vivo. J Immunol. 2005;175:3133–9. doi: 10.4049/jimmunol.175.5.3133. [DOI] [PubMed] [Google Scholar]
- 26.Li L, Kim J, Boussiotis VA. Rap1A regulates generation of T regulatory cells via LFA-1-dependent and LFA-1-independent mechanisms. Cell Immunol. 2010;266:7–13. doi: 10.1016/j.cellimm.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gong Q, White L, Johnson R, White M, Negishi I, Thomas M, Chan AC. Restoration of thymocyte development and function in zap-70–/– mice by the Syk protein tyrosine kinase. Immunity. 1997;7:369–77. doi: 10.1016/s1074-7613(00)80358-1. [DOI] [PubMed] [Google Scholar]
- 28.Palmer E, Naeher D. Affinity threshold for thymic selection through a T-cell receptor-co-receptor zipper. Nat Rev Immunol. 2009;9:207–13. doi: 10.1038/nri2469. [DOI] [PubMed] [Google Scholar]
- 29.Mallaun M, Zenke G, Palmer E. A discrete affinity-driven elevation of ZAP-70 kinase activity initiates negative selection. J Recept Signal Transduct Res. 2010;30:430–43. doi: 10.3109/10799893.2010.518151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Picard C, Dogniaux S, Chemin K, et al. Hypomorphic mutation of ZAP70 in human results in a late onset immunodeficiency and no autoimmunity. Eur J Immunol. 2009;39:1966–76. doi: 10.1002/eji.200939385. [DOI] [PubMed] [Google Scholar]
- 31.Monafo WJ, Polmar SH, Neudorf S, Mather A, Filipovich AH. A hereditary immunodeficiency characterized by CD8+ T lymphocyte deficiency and impaired lymphocyte activation. Clin Exp Immunol. 1992;90:390–3. doi: 10.1111/j.1365-2249.1992.tb05856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elder ME, Lin D, Clever J, Chan AC, Hope TJ, Weiss A, Parslow TG. Human severe combined immunodeficiency due to a defect in ZAP-70, a T cell tyrosine kinase. Science. 1994;264:1596–9. doi: 10.1126/science.8202712. [DOI] [PubMed] [Google Scholar]
- 33.Matsuda S, Suzuki-Fujimoto T, Minowa A, Ueno H, Katamura K, Koyasu S. Temperature-sensitive ZAP70 mutants degrading through a proteasome-independent pathway. Restoration of a kinase domain mutant by Cdc37. J Biol Chem. 1999;274:34515–8. doi: 10.1074/jbc.274.49.34515. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Total numbers of thymic and splenic T-cell subpopulations.
Figure S2. Increased peripheral regulatory T (Treg) cell frequencies in Zap70rps mice remain constant over age.
Figure S3. Individual cellular Ca2+ flux responses are uniform and unaffected by activation status.