Introduction
Approximately half of all patients with heart failure have an ejection fraction greater than 40–50% and may be diagnosed as having Heart Failure with preserved Ejection Fraction (HFpEF). Diastolic dysfunction is central to the pathophysiology of HFpEF (Borlaug & Paulus, 2011), and describes the slowing of ventricular relaxation and increased diastolic stiffness which ultimately impairs ventricular filling. The mechanistic basis of this impairment is complex and not yet well understood. Structural remodelling undoubtedly plays an important role in increasing left ventricular stiffness. However, the acute worsening of diastolic dysfunction during stress or exercise characteristic of HFpEF suggests an important contribution from dynamic changes in left ventricular (LV) functional properties. Frequency-dependent elevation of diastolic tension and intracellular Ca2+ ([Ca2+]i) has been observed in cardiac muscle strips from patients with left ventricular hypertrophy and diastolic dysfunction, or heart failure (Sossalla et al. 2008; Selby et al. 2011), implying that dysregulation of [Ca2+]i homeostasis of the cardiomyocyte contributes to diastolic dysfunction.
Intracellular Ca2+ regulation is closely linked to intracellular Na+ homeostasis, through the Na+–Ca2+ exchanger (NCX). Intracellular Na+ of cardiomyocytes from failing hearts is increased and associated with elevated diastolic tension (Pieske et al. 2002). An important mechanism underlying this observation may be an increase in the late sodium current (INa,L). The Na+ conductance responsible for rapid depolarization of cardiomyocytes does not completely inactivate during the action potential. (Noble & Noble, 2006; Maier, 2012) Some channels continue to conduct, or even reactivate at relatively positive membrane potentials during the plateau and repolarization phases. This is INa,L (Zaza et al. 2008). Consequently, about half of the myocyte Na+ entry occurs during the initial 2–3 ms, and about half during the remainder of the action potential (Makielski & Farley, 2006). At the molecular level, INa,L results from channel reopening during sustained depolarization by two different modes of gating: burst openings and late scattered openings (Maltsev & Undrovinas, 2008).
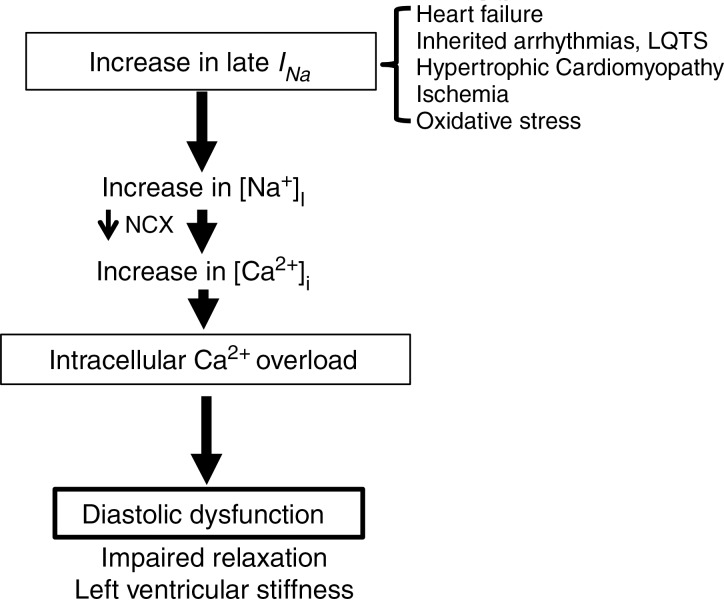
As outlined in Fig. 1, increased Na+ entry through INa,L increases intracellular Na+ ([Na+]i), which reduces the driving force for extrusion of Ca2+ and favours Ca2+ influx via the Na+–Ca2+ exchanger (NCX). This leads to increased [Ca2+]i. Elevated [Ca2+]i eventually increases actin–myosin filament interaction during diastole and thus increases diastolic tension. This mechanism of Ca2+ overload has been demonstrated in numerous animal studies, and in strips of ventricular muscle or myocytes isolated from patients with failing hearts (Valdivia et al. 2005; Makielski & Farley, 2006; Maltsev & Undrovinas, 2008; Sossalla et al. 2008; Selby et al. 2011; Coppini et al. 2013). Further, specific augmentation of INa,L with the sea anemone toxin ATXII in isolated myocytes and perfused hearts results in Na+ and Ca2+ overload (Fraser et al. 2006; Sossalla et al. 2008) and impaired diastolic function. Diastolic dysfunction with preserved systolic function has also been described in LQT syndrome type 3 patients, where INa,L is enhanced due to a Na+ channel mutation (Moss et al. 2008; Hummel et al. 2013).
Figure 1.
A pathological enhanced INa,L contributes to Na+-dependent Ca2+ overload, diastolic dysfunction
We propose that a pathological increase in Na+ influx through cardiac Na+ channels, specifically due to enhanced INa,L is a major contributor to Ca2+ overload and diastolic dysfunction in HFpEF. Key evidence to support this hypothesis is outlined below.
In pathological conditions with diastolic dysfunction, cardiomyocyte INa,L is enhanced up to 5-fold
This has been characterized in cardiomyocytes isolated from patients with hypertrophic cardiomyopathy (Coppini et al. 2013), from human (Maltsev et al. 2007; Sossalla et al. 2008) and dog failing hearts (Maltsev et al. 2007), in rat (Xi et al. 2009; Aistrup et al. 2013) and mouse (Toischer et al. 2013) models of pressure overload and in numerous species following hypoxia, ischaemia or metabolic stress (Shryock et al. 2013); all factors of relevance to the genesis of diastolic dysfunction in heart failure. Elucidation of the underlying mechanisms whereby INa,L is enhanced is incomplete. Single channel studies on myocytes isolated from failing human hearts suggest that functional changes such as slowing of the two modes of gating comprising INa,L (late scattered and bursting modes) contribute to enhanced INa,L (Maltsev & Undrovinas, 2008). Evidence has also been gathered that Na+ channel isoform expression (Xi et al. 2009) and functional regulation (Zaza et al. 2008) differs in diastolic dysfunction. There is considerable evidence that under pathological conditions INa,L can, in a rate-dependent manner, induce Ca2+ overload and consequently ventricular dysfunction and arrhythmogenesis (Valdivia et al. 2005; Maltsev & Undrovinas, 2008; Zaza et al. 2008; Shryock et al. 2013).
Inhibiting INa,L in isolated cardiac tissue improves relaxation and diminishes Ca2+ accumulation
One pivotal early study demonstrated that the INa,L inhibitor ranolazine effectively prevented the frequency-dependent increase in diastolic tension in tissue strips from failing human hearts (Sossalla et al. 2008). This observation is key in understanding the role of INa,L in HFpEF, characterized by exercise intolerance in part due to the worsening of diastolic function at elevated heart rates. Similar observations were made in myocytes isolated from dog failing hearts and from patients with hypertrophic cardiomyopathy. Diastolic Ca2+ became elevated at high pacing rates compared to healthy cells, and this effect could be diminished by ranolazine (Undrovinas et al. 2010; Coppini et al. 2013). Although selective for INa,L over peak INa, ranolazine has multiple pharmacological targets of potential relevance to diastolic dysfunction. However, similar to ranolazine, other INa,L inhibitors including the specific Na+ channel inhibitor tetrodotoxin (TTX) attenuate INa,L induced- Na+-dependent Ca2+ overload in failing ventricular myocytes, and in myocytes exposed to H2O2 or ATXII (Undrovinas et al. 2010; Qian et al. 2012; Belardinelli et al. 2013). Although Na+ channel knockout mice studies have shed light on mechanisms of arrhythmia, they did not specifically investigate the role of INa,L on relaxation and diastolic calcium (Derangeon et al. 2012; Yang et al. 2012).
Inhibiting INa,L improves diastolic function in experimental heart failure
Early insights into the potential contribution of INa,L to diastolic dysfunction were gained from studies that examined the effects of INa,L inhibition in heart failure. In 2002, the effect of ranolazine to acutely improve heart function was examined in dogs with chronic heart failure. Whereas ranolazine had no significant effects in normal dogs, ranolazine both decreased end-diastolic pressure and improved systolic functional parameters in dogs with heart failure (Sabbah et al. 2002). In a follow-up experiment, 3 months’ treatment with ranolazine decreased end-diastolic pressure and circumferential wall stress whether alone, or combined with beta blockade or ACE inhibition in dogs with heart failure (Rastogi et al. 2008). Interestingly, all treatment regimens also diminished the pathological LV remodelling that occurred relative to placebo.
With increased recognition of the unique pathology of HFpEF relative to heart failure with a reduced ejection fraction, studies are being performed in models that more specifically reproduce the phenotype of diastolic dysfunction (Doi et al. 2000; LeGrice et al. 2012). In a recent study Aistrup et al. (2013) demonstrated that INa,L is elevated in the spontaneously hypertensive rat and that 3 months’ treatment with ranolazine prevented progression of LV hypertrophy, disruption of t-tubule architecture, and improved intracellular Ca2+ cycling. Combined, these data suggest that sustained inhibition of enhanced INa,L and subsequent Ca2+ overload may improve diastolic function not only by improving dynamic Ca2+ regulation, but also interrupting the aberrant structural remodelling characteristic of diastolic heart failure.
The INa,L inhibitor ranolazine improves diastolic function in patients
When ranolazine was administered acutely to 15 patients with ischaemic heart disease the regional peak filling rate and wall lengthening increased in ischaemic regions and the diastolic pressure volume relation was shifted downward, suggesting improvement of diastolic function (Hayashida et al. 1994). In a cohort of 22 patients with angina, ranolazine treatment for a mean of 65 days improved both systolic and diastolic parameters assessed by echocardiography (Figueredo et al. 2011). Systolic and diastolic left ventricular wall synchrony was also increased by 4 weeks’ ranolazine treatment in patients with coronary artery disease (Venkataraman et al. 2012). While ranolazine has multiple mechanisms of action, in long QT syndrome type 3 patients with a specific enhancement of INa,L, acute infusion of ranolazine improved parameters of relaxation (Moss et al. 2008). In the recent RALI-DHF trial studying HFpEF patients, acute intravenous ranolazine was found to improve measures of haemodynamics including LV end-diastolic pressure and pulmonary artery pressure, but did not alter the LV relaxation rate. Oral treatment was extended for a further 13 days. Although no improvements in diastolic function were apparent by echocardiography at this time, this clinical study was underpowered to address this hypothesis (Maier et al. 2013).
Conclusion
Taken together, experimental data suggest that INa,L is enhanced in many conditions and is an important contributor to Ca2+ overload and diastolic dysfunction.
Call for comments
Readers are invited to give their views on this and the accompanying CrossTalk articles in this issue by submitting a brief comment. Comments may be posted up to 6 weeks after publication of the article, at which point the discussion will close and authors will be invited to submit a ‘final word'. To submit a comment, go to http://jp.physoc.org/letters/submit/jphysiol;592/3/411
Competing interests
LB is an employee of Gilead Sciences, Inc. (owner of ranolazine).
References
- Aistrup GL, Gupta DK, Kelly JE, O'Toole MJ, Nahhas AF, Chirayil N, Misener S, Beussink L, Singh N, Ng J, Reddy M, Mongkolrattanothai T, El Bizri N, Rajamani S, Shryock JC, Belardinelli L, Shah SJ, Wasserstrom JA. Inhibition of the late sodium current slows t-tubule disruption during the progression of hypertensive heart disease in the rat. Am J Physiol Heart Circ Physiol. 2013;305:H1068–H1079. doi: 10.1152/ajpheart.00401.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belardinelli L, Liu G, Smith-Maxwell C, Wang WQ, El Bizri N, Hirakawa R, Karpinski S, Li CH, Hu L, Li XJ, Crumb W, Wu L, Koltun D, Zablocki J, Yao L, Dhalla AK, Rajamani S, Shryock JC. A novel, potent, and selective inhibitor of cardiac late sodium current suppresses experimental arrhythmias. J Pharmacol Exp Ther. 2013;344:23–32. doi: 10.1124/jpet.112.198887. [DOI] [PubMed] [Google Scholar]
- Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur Heart J. 2011;32:670–679. doi: 10.1093/eurheartj/ehq426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppini R, Ferrantini C, Yao L, Fan P, Del Lungo M, Stillitano F, Sartiani L, Tosi B, Suffredini S, Tesi C, Yacoub M, Olivotto I, Belardinelli L, Poggesi C, Cerbai E, Mugelli A. Late sodium current inhibition reverses electromechanical dysfunction in human hypertrophic cardiomyopathy. Circulation. 2013;127:575–584. doi: 10.1161/CIRCULATIONAHA.112.134932. [DOI] [PubMed] [Google Scholar]
- Derangeon M, Montnach J, Baró I, Charpentier F. Mouse models of SCN5A-related cardiac arrhythmias. Front Physiol. 2012;3:210. doi: 10.3389/fphys.2012.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi R, Masuyama T, Yamamoto K, Doi Y, Mano T, Sakata Y, Ono K, Kuzuya T, Hirota S, Koyama T, Miwa T, Hori M. Development of different phenotypes of hypertensive heart failure: systolic versus diastolic failure in Dahl salt-sensitive rats. J Hypertens. 2000;18:111–120. doi: 10.1097/00004872-200018010-00016. [DOI] [PubMed] [Google Scholar]
- Figueredo VM, Pressman GS, Romero-Corral A, Murdock E, Holderbach P, Morris DL. Improvement in left ventricular systolic and diastolic performance during ranolazine treatment in patients with stable angina. J Cardiovasc Pharmacol Ther. 2011;16:168–172. doi: 10.1177/1074248410382105. [DOI] [PubMed] [Google Scholar]
- Fraser H, Belardinelli L, Wang L, Light PE, McVeigh JJ, Clanachan AS. Ranolazine decreases diastolic calcium accumulation caused by ATX-II or ischemia in rat hearts. J Mol Cell Cardiol. 2006;41:1031–1038. doi: 10.1016/j.yjmcc.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Hayashida W, van Eyll C, Rousseau MF, Pouleur H. Effects of ranolazine on left ventricular regional diastolic function in patients with ischemic heart disease. Cardiovasc Drugs Ther. 1994;8:741–747. doi: 10.1007/BF00877121. [DOI] [PubMed] [Google Scholar]
- Hummel YM, Wilde AA, Voors AA, Bugatti S, Hillege HL, Van den Berg MP. Ventricular dysfunction in a family with long QT syndrome type 3. Europace. 2013;15:1516–1521. doi: 10.1093/europace/eut101. [DOI] [PubMed] [Google Scholar]
- LeGrice IJ, Pope AJ, Sands GB, Whalley G, Doughty RN, Smaill BH. Progression of myocardial remodeling and mechanical dysfunction in the spontaneously hypertensive rat. Am J Physiol Heart Circ Physiol. 2012;303:H1353–H1365. doi: 10.1152/ajpheart.00748.2011. [DOI] [PubMed] [Google Scholar]
- Maier LS. New treatment options for late Na current, arrhythmias, and diastolic dysfunction. Curr Heart Fail Rep. 2012;9:183–191. doi: 10.1007/s11897-012-0099-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier LS, Layug B, Karwatowska-Prokopczuk E, Belardinelli L, Lee S, Sander J, Lang C, Wachter R, Edelmann F, Hasenfuss G, Jacobshagen C. RAnoLazIne for the treatment of diastolic heart failure in patients with preserved ejection fraction: The RALI-DHF Proof-of-Concept Study. J Am Coll Cardiol. 2013;1:115–122. doi: 10.1016/j.jchf.2012.12.002. [DOI] [PubMed] [Google Scholar]
- Makielski JC, Farley AL. Na+ current in human ventricle: implications for sodium loading and homeostasis. J Cardiovasc Electrophysiol. 2006;17(Suppl. 1):S15–S20. doi: 10.1111/j.1540-8167.2006.00380.x. [DOI] [PubMed] [Google Scholar]
- Maltsev VA, Silverman N, Sabbah HN, Undrovinas AI. Chronic heart failure slows late sodium current in human and canine ventricular myocytes: implications for repolarization variability. Eur J Heart Fail. 2007;9:219–227. doi: 10.1016/j.ejheart.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltsev VA, Undrovinas A. Late sodium current in failing heart: friend or foe? Prog Biophys Mol Biol. 2008;96:421–451. doi: 10.1016/j.pbiomolbio.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss AJ, Zareba W, Schwarz KQ, Rosero S, McNitt S, Robinson JL. Ranolazine shortens repolarization in patients with sustained inward sodium current due to type-3 long-QT syndrome. J Cardiovasc Electrophysiol. 2008;19:1289–1293. doi: 10.1111/j.1540-8167.2008.01246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble D, Noble PJ. Late sodium current in the pathophysiology of cardiovascular disease: consequences of sodium-calcium overload. Heart. 2006;92(Suppl. 4):iv1–iv5. doi: 10.1136/hrt.2005.078782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieske B, Maier LS, Schmidt-Schweda S. Sarcoplasmic reticulum Ca2+ load in human heart failure. Basic Res Cardiol. 2002;97(Suppl. 1):I63–I71. doi: 10.1007/s003950200032. [DOI] [PubMed] [Google Scholar]
- Qian C, Ma J, Zhang P, Luo A, Wang C, Ren Z, Kong L, Zhang S, Wang X, Wu Y. Resveratrol attenuates the Na+-dependent intracellular Ca2+ overload by inhibiting H2O2-induced increase in late sodium current in ventricular myocytes. PLoS One. 2012;7:e51358. doi: 10.1371/journal.pone.0051358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi S, Sharov VG, Mishra S, Gupta RC, Blackburn B, Belardinelli L, Stanley WC, Sabbah HN. Ranolazine combined with enalapril or metoprolol prevents progressive LV dysfunction and remodeling in dogs with moderate heart failure. Am J Physiol Heart Circ Physiol. 2008;295:H2149–H2155. doi: 10.1152/ajpheart.00728.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbah HN, Chandler MP, Mishima T, Suzuki G, Chaudhry P, Nass O, Biesiadecki BJ, Blackburn B, Wolff A, Stanley WC. Ranolazine, a partial fatty acid oxidation (pFOX) inhibitor, improves left ventricular function in dogs with chronic heart failure. J Card Fail. 2002;8:416–422. doi: 10.1054/jcaf.2002.129232. [DOI] [PubMed] [Google Scholar]
- Selby DE, Palmer BM, LeWinter MM, Meyer M. Tachycardia-induced diastolic dysfunction and resting tone in myocardium from patients with a normal ejection fraction. J Am Coll Cardiol. 2011;58:147–154. doi: 10.1016/j.jacc.2010.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shryock JC, Song Y, Rajamani S, Antzelevitch C, Belardinelli L. The arrhythmogenic consequences of increasing late INa in the cardiomyocyte. Cardiovasc Res. 2013;99:600–611. doi: 10.1093/cvr/cvt145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sossalla S, Wagner S, Rasenack EC, Ruff H, Weber SL, Schondube FA, Tirilomis T, Tenderich G, Hasenfuss G, Belardinelli L, Maier LS. Ranolazine improves diastolic dysfunction in isolated myocardium from failing human hearts – role of late sodium current and intracellular ion accumulation. J Mol Cell Cardiol. 2008;45:32–43. doi: 10.1016/j.yjmcc.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Toischer K, Hartmann N, Wagner S, Fischer TH, Herting J, Danner BC, Sag CM, Hund TJ, Mohler PJ, Belardinelli L, Hasenfuss G, Maier LS, Sossalla S. Role of late sodium current as a potential arrhythmogenic mechanism in the progression of pressure-induced heart disease. J Mol Cell Cardiol. 2013;61:111–122. doi: 10.1016/j.yjmcc.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Undrovinas NA, Maltsev VA, Belardinelli L, Sabbah HN, Undrovinas A. Late sodium current contributes to diastolic cell Ca2+ accumulation in chronic heart failure. J Physiol Sci. 2010;60:245–257. doi: 10.1007/s12576-010-0092-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdivia CR, Chu WW, Pu J, Foell JD, Haworth RA, Wolff MR, Kamp TJ, Makielski JC. Increased late sodium current in myocytes from a canine heart failure model and from failing human heart. J Mol Cell Cardiol. 2005;38:475–483. doi: 10.1016/j.yjmcc.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Venkataraman R, Chen J, Garcia EV, Belardinelli L, Hage FG, Heo J, Iskandrian AE. Effect of ranolazine on left ventricular dyssynchrony in patients with coronary artery disease. Am J Cardiol. 2012;110:1440–1445. doi: 10.1016/j.amjcard.2012.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Y, Wu G, Yang L, Han K, Du Y, Wang T, Lei X, Bai X, Ma A. Increased late sodium currents are related to transcription of neuronal isoforms in a pressure-overload model. Eur J Heart Fail. 2009;11:749–757. doi: 10.1093/eurjhf/hfp092. [DOI] [PubMed] [Google Scholar]
- Yang T, Atack TC, Stroud DM, Zhang W, Hall L, Roden DM. Blocking Scn10a channels in heart reduces late sodium current and is antiarrhythmic. Circ Res. 2012;111:322–332. doi: 10.1161/CIRCRESAHA.112.265173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaza A, Belardinelli L, Shryock JC. Pathophysiology and pharmacology of the cardiac "late sodium current.". Pharmacol Ther. 2008;119:326–339. doi: 10.1016/j.pharmthera.2008.06.001. [DOI] [PubMed] [Google Scholar]