Abstract
One hundred years ago in this journal, Krogh and Lindhard published a seminal paper highlighting the importance of the brain in the control of breathing during exercise. This symposium report reviews the historical developments that have taken place since 1913, and attempts to place the detailed neurocircuitry thought to underpin exercise hyperpnoea into context by focusing on key structures that might form the command network. With the advent of enhanced neuroimaging and functional neurosurgical techniques, a unique window of opportunity has recently arisen to target potential circuits in humans. Animal studies have identified a priori sites of interest in mid‐brain structures, in particular the subthalamic locomotor region (subthalamic nucleus, STN) and the periaqueductal grey (PAG), which have now been recorded from in humans during exercise. When all data are viewed in an integrative manner, the PAG, in particular the lateral PAG, and aspects of the dorsal lateral PAG, appear to be key communicating circuitry for ‘central command’. Moreover, the PAG also fulfils many requirements of a command centre. It has functional connectivity to higher centres (dorsal lateral prefrontal cortex) and the basal ganglia (in particular, the STN), and receives a sensory input from contracting muscle, but, importantly, it sends efferent information to brainstem nuclei involved in cardiorespiratory control.
Abbreviations
- dPAG
dorsal periaqueductal grey
- dlPAG
dorsal lateral periaqueductal grey
- dmPAG
dorsal medial periaqueductal grey
- DLPFC
dorsal lateral prefrontal cortex
- lPAG
lateral periaqueductal grey
- MLR
mesencephalic locomotor region
- PAG
periaqueductal grey
- PMA
premotor area
- PPN
pedunculopontine nucleus
- SMA
supplementary motor area
- STN
subthalamic nucleus
- vlPAG
ventrolateral periaqueductal grey
One hundred years ago, The Journal of Physiology published a classic paper by the Nobel Prize winning physiologist August Krogh and his colleague Johannes Lindhard (Krogh & Lindhard, 1913) concerning the regulation of respiration and circulation during the initial stages of muscular work. This publication became a landmark study for those interested in understanding how ventilation and the cardiovascular system are controlled during exercise. Since then, many animal and human studies have built on their initial observation showing the importance of higher centre control. A wealth of information has been published, forgotten and latter re‐discovered (e.g. Mateika & Duffin, 1995; Forster et al. 2012). This symposium report reviews the historical developments that have taken place since 1913, and attempts to place the detailed neurocircuitry thought to underpin exercise hyperpnoea into context by focusing on key structures that might form the command network.
It has been known for some time that the stimulation of skeletal muscle can affect ventilation and cardiovascular function (Zuntz & Geppert, 1886; Geppert & Zuntz, 1888; Johansson, 1893). Given the difficulty of studying the central nervous system in humans, much of the effort in the last century focused on the peripheral neural and chemical factors targeting areas of known chemoreception (Heymans & Heymans, 1927) as putative drivers for exercise hyperpnoea (e.g. Haldane & Priestley, 1905; Douglas & Haldane, 1909; Paterson, 1928; Comroe & Schmidt, 1943; Grodins, 1950; Bannister & Cunningham, 1954; Dejours, 1959; Wasserman & Whipp, 1975; Dempsey & Forster, 1982). Several by‐products of muscle contraction received considerable attention in the 1970–80s, in particular H+ (e.g. Wasserman et al. 1973; Wasserman & Whipp, 1975) and arterial potassium (e.g. Linton & Band, 1985, Burger et al. 1988; Paterson & Nye, 1988; Paterson et al. 1989), were mooted as being factor x (Haggard & Henderson, 1920), later renamed the ‘hyperpnein’ (Henderson, 1938) or ‘anaerobic work substance’ (Asmussen & Nielsen, 1946). However, studies with McArdle's patients (myophosphorylase deficiency – hence no acid on exercise) still show a relatively normal respiratory and hyperkalaemic response to exercise (Paterson et al. 1990). Circumstantial evidence supports a role for potassium as the ‘work substance’, although there is no direct proof of whether it is critical in exercise hyperpnoea (Paterson, 1992).
It is clear that exercise rhythm can entrain breathing frequency to a subharmonic of that rhythm (e.g. Bechbache & Duffin, 1977; Paterson et al. 1986) and hypoxia can reduce this coupling (Paterson et al. 1987). However, the removal of peripheral signals in humans does not appreciably change the steady‐state isocapnic ventilatory response to exercise. This is seen following surgical denervation of the carotid bodies (Wasserman et al. 1975), in subjects with no functional chemoreception (congenital central hypoventilation syndrome) (Shea et al. 1993), following heart–lung transplantation (Banner et al. 1988), and in patients with spinal chord transection (Adams et al. 1984) or following epidural anaesthesia during exercise (Fernandes et al. 1990). However, recent work using selective afferent blockade during exercise suggests that muscle afferents play a significant role in the respiratory response (Amann et al. 2010). For some, these observations merely reinforce the notion that the essential nature of the control system resides in the brain (e.g. Rauch et al. 2013; ‘central governor’ hypothesis), whereas others would argue that these studies confirm that the control is multifactorial, and is based on a high degree of redundancy (Cunningham, 1987).
Evidence for ‘central command’
Krogh and Lindhard (1913) provided the first evidence for the concept of central command. They suggested that the rise in ventilation and heart rate (data provided by Miss Florence Buchanan in Haldane's laboratory at Oxford) was not produced reflexly, but by the ‘irradiation of impulses from the motor cortex’. The experiment was simple yet informative. In anticipation of exercise, subjects showed greater ventilation in the transition from rest to work. When one subject (JL) was told to expect a heavy load (when in fact it had not changed), the resulting ventilation during the first few seconds of exercise was essentially the same as if the extra load was present (see Fig. 4 from Krogh & Lindhard, 1913). The power of this observation in respiratory control is further illustrated when hypnotic suggestion is used to focus attention (Daly & Overly, 1966; Morgan et al. 1973). Figure 1 is an example that attests to the reproducibility of Krogh & Lindhard's original observation, when hypnotized subjects who are exercising on a cycle ergometer are told to imagine cycling ‘uphill’ at the same pedal frequency. Here, isocapnic buffering is lost as the subjects breathe disproportionately to the metabolic rate until they are told the imagined ‘hill’ has disappeared (Thornton, 1999). A similar response is seen at rest with no movement feedback when subjects are told to imagine cycling up a hill (Thornton et al. 2001).
Figure 1. Breathing response to altered perception of effort .
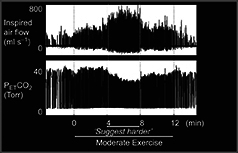
Respiratory responses during exercise under hypnotic suggestion. Note the increase in inspired air flow and loss of isocapnic buffering when subjects are told to imagine (‘Suggest harder’ line on x axis) the exercise has become hard whilst maintaining a constant pedal frequency and exercise intensity. From Thornton (1999) with permission.
The significance of the earlier observation by Krogh & Lindhard was recognized by Goodwin et al. 1972, who first used the term ‘central command’. In a clever experiment, they showed that ventilation, heart rate and arterial blood pressure would follow a change in central command brought about by tendon vibration on either the triceps or biceps muscle during isometric exercise at constant muscle tension. For example, when the triceps was contracting, tendon vibration of the biceps muscle would cause reflex inhibition, so that more central command would be required to maintain the same tension in the muscle during biceps contraction. This resulted in a greater cardiorespiratory response (Fig. 2). The converse was true for vibration of the biceps muscle. So, where are the command neurons for ‘central command’?
Figure 2. Altered central command using tendon vibration .
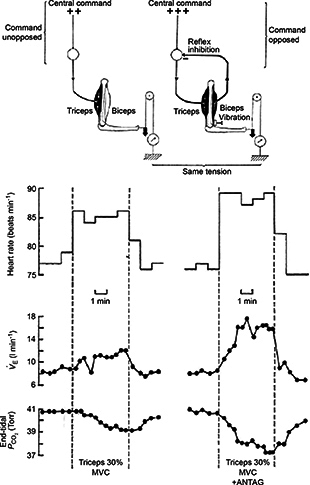
Top panel: principle of experiment to increase central command required to achieve the same muscle tension. On the left is the force exerted by the forearm through voluntary contraction of the triceps muscle: the magnitude of central command is given as ++. On the right is the vibration of the biceps; an antagonist of the contracting muscle is used to excite the primary afferents from the muscle spindles in the biceps. This contributes an element of reflex inhibition to the motor neurons innervating the contracting triceps. The same downward force can thus be exerted by a contraction of the triceps, only if a greater central command (here +++) is given. Bottom panel: increase in central command. On the right are the responses to a contraction of the same force in which the central command required was greater because of vibration applied to the antagonist, the biceps. Note the greater breathing and heart rate responses. ANTAG, antagonist; MVC, maximum voluntary contraction. Modified from Goodwin et al. (1972) with permission.
Higher central circuits
In humans, there have been several approaches to address this question employing a combination of neuroimaging techniques (e.g. Fink et al. 1995; Williamson et al. 2001), transcranial stimulation (e.g. Gandevia & Rothwell, 1987) and functional neurosurgical interventions (deep brain stimulation; Green et al. 2005). Penfield and Foerster, both leading neurosurgeons of their day, first showed that discrete electrical stimulation of the primary motor cortex excited the diaphragm (e.g. Foerster, 1936; Penfield & Boldrey, 1937). Others have refined this approach using percutaneous stimulation (Gandevia & Rothwell, 1987) or transcranial magnetic stimulation of the motor cortex (Corfield et al. 1998) to support the idea of a fast conducting oliosynaptic motor pathway that activates the diaphragm via a cortico‐spinal tract that presumably ‘by‐passes’ the brainstem respiratory centre. Fink et al. (1995) employed positron emission tomography and showed that the hyperpnoea observed during and immediately after exercise in humans was correlated with the activation of motor cortical sites, specifically, the left and right superomedial primary motor cortex and left and right superolateral primary cortex, areas associated with volitional breathing.
In an attempt to uncouple ‘central command’ from movement feedback, Thornton et al. (2001) used hypnotic suggestion of exercise during concurrent positron emission tomography scanning. Three cognitive conditions were used, involving the imagination of: (i) free‐wheeling downhill on a bicycle; (ii) cycling up a hill; and (iii) volitional hyperventilation with the CO2 clamped to match the breathing observed in (ii). Similar to Fink et al. (1995), significant activation was seen in the supplementary motor area (SMA) and premotor area (PMA). In addition, the thalamus, bilateral cerebellum and right dorsal lateral prefrontal cortex (DLPFC Brodmann area 9) were also activated (fig. 3). These areas are concerned with volitional/motor control that also includes respiratory muscle. However, when breathing was driven voluntarily, only the SMA and sensorimotor cortex were activated. The lack of activation of the dorsal lateral precortex is particularly interesting, because this area is important for working memory and executive function, which also includes the regulation of thinking and tasks (Frith et al. 1991). It projects to the SMA/PMA but not to the motor cortex itself. Moreover, it also has a projection to the lateral periaqueductal grey (lPAG) (An et al. 1998), which is an area that is widely believed to be important in cardiorespiratory control during stress and emotional states (Bandler & Shipley 1994; Dampney et al. 2013). Emerging evidence now suggests that the dorsal lateral periaqueductal grey (dlPAG)/lPAG, which is activated during exercise, could form a key part of the circuitry involved in ‘central command’ (Green et al. 2007; Basnayake et al. 2011 see below).
Figure 3. Activations during imagination of exercise and voluntary hyperventilation with associated respiratory responses .
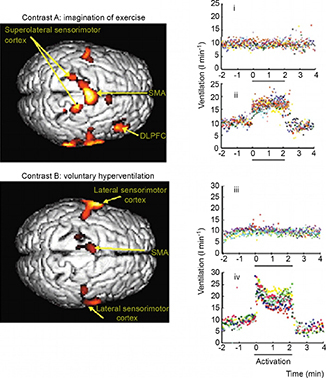
Left panel: main activations during contrasts A and B rendered on the dorsal aspect of a representative brain in a transverse view from above (right edge, anterior) shown at P < 0.01 (uncorrected for multiple comparisons), for clarity. Right panel: breath‐by‐breath ventilatory data in one subject for contrast A, imagining cycling uphill (ii), and for contrast B, breathing to an instructed frequency (iv) copying that in (ii). Graphs (i) (contrast A) and (iii) (contrast B) record the absence of effect when imagining freewheeling downhill. Each point represents a single breath and the eight colours represent each repeat of the protocol. Experimental periods of 2 min 10 s are marked by the full lines (x axis) when the scan was performed. DLPFC, dorsal lateral prefrontal cortex; SMA, supplementary motor area. Activations within 2 cm of the brain surface are represented. From Thornton et al. (2001) with permission.
Mid‐brain circuits
Subthalamic nucleus
The visualization of deep brain structures during exercise with neuroimaging techniques is technically challenging, given the blurring effects that can be detected as a result of the smallest of head movements caused by whole body exercise. Until relatively recently, direct electrophysiological evidence came from early animal investigations that focused on the subthalamic locomotor region of the posterior hypothalamus and the broader mesencephalic locomotor region (MLR) as key structures in cardiorespiratory neurocircuits. Pitts et al. (1941) provided some of the first evidence for increases in sympathetic discharge and arterial blood pressure following stimulation of the hypothalamic region in anaesthetized cats. Similarly, Smith et al. (1960) and Eldridge et al. (1981) refined this approach, and showed that the activation of the H2 fields of Forel, which include the dorsal aspect of the subthalamic nucleus (STN) in dogs, mimicked their arterial blood pressure response to exercise (Smith et al. 1960). Eldridge et al. (1981) then went on to elegantly demonstrate that the stimulation of the mesencephalic locomotor region (including the STN) in the decorticate cat caused a parallel activation of both cardiovascular and respiratory activity. Importantly, they observed that blocking all movement feedback during stimulation with curae (fictive locomotion) still caused an increase in arterial blood pressure, heart rate and phrenic nerve activity (Fig. 4). Other areas of the MLR, such as the pedunculopontine nucleus (PPN), can also cause a rise in cardiorespiratory variables following muscle contraction in the rat (Plowey & Waldrop 2004); moreover, c‐fos studies confirmed that the PPN is activated during treadmill running (Iwamoto et al. 1996). Of interest, the reflex diaphragmatic response caused by muscle contraction was attenuated by a cobalt injection into the rostral PPN, whereas the cardiovascular response remained intact, suggesting a differential neural integration (Plowey & Waldrop 2004).
Figure 4. Activation of mid‐brain nuclei to drive locomotion and cardiorespiration independent of peripheral feedback .
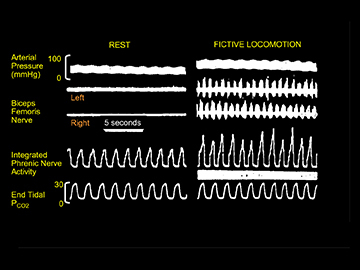
Left panel: example of locomotion induced by electrical stimulation of the subthalamic locomotor region. Right panel: example of cardiovascular and respiratory responses during fictive locomotion induced by stimulation of the subthalamic locomotor region. Here the decorticate animals were paralyzed and ventilated. Note the increase in phrenic nerve activity during stimulation. Modified from Eldridge et al. (1981) with permission.
As compelling as these studies might seem, they do not represent ‘central command’ per se, but rather subcortical control that can drive a cardiorespiratory response independent of movement feedback. Furthermore, the loss of descending cortical–thalamic inhibition in the exercising cat model also calls into question the exact quantitative role played by the MLR in exercise hyperpnoea. Nevertheless, these pioneering animal studies paved the way for deep mid‐brain neural sites to be targeted directly in humans with the advent of improved neuroimaging coupled with precise stereotaxic neurosurgical techniques (Fig. 5).
Figure 5. Targeting mid‐brain structures using functional neurosurgical techniques .
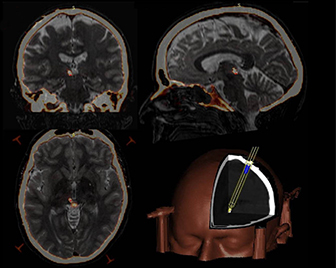
Fused pre‐operative magnetic resonance imaging and post‐operative computed tomography images highlighted using heat mapping (axial, coronal and sagittal clockwise from bottom left) showing left periaqueductal grey electrode placement. Three‐dimensional reconstruction of the electrode trajectory is shown bottom right. From Pereira et al. (2013) with permission.
Thornton et al. (2002) targeted the STN in patients with a Parkinsonian phenotype and reported that high‐frequency stimulation (depolarization block) facilitated movement, but also increased the heart rate and systolic and diastolic blood pressure by removing an inhibitory ‘break’ on the system (Thornton et al. 2002). Advancing this study further, Basnayake et al. (2012) recently re‐visited the ‘Goodwin et al.’ paradigm using muscle vibration during constant tension‐based isometric contraction, whilst recording field potentials in the STN. Here, a decrease in the inhibitory action of the STN during biceps muscle contraction was further potentiated when the triceps tendon was vibrated, thereby activating the muscle spindle afferents to cause reflex inhibition. This resulted in more ‘central command’ in order to maintain the same tension (Basnayake et al. 2012). It is clear that the basal ganglia is a complex circuit and may form only a part of the circuitry involved in central command itself (e.g. Williamson et al. 2001), especially for cardiovascular control during exercise (Williamson, 2010). In particular, the posterior hypothalamus receives a heavy projection from the periaqueductal grey (PAG) (Mantyh, 1983), which appears to be diffuse in nature, rather than from distinct regions of the PAG, although Sakai et al. (1990) reported notable connectivity from the lPAG and dlPAG.
Periaqueductal grey
This structure provides a functional interface between the forebrain and lower brainstem, and has been referred to as a visceral, nociceptor and ‘cognitive integrator’ (Mantyh, 1983). Its well‐established role in the modulation of pain (internal stress) and defensive action (fight/flight or coping: external stress) (e.g. Benarroch, 2012) is now being extended to our understanding of cardiorespiratory control during exercise (Green et al. 2007; Basnayake et al. 2011). Interestingly, removal of the PAG through lesioning results in immediate loss of consciousness (Watt et al. 2000), mutism (Esposito et al. 1999), attenuation of carbon dioxide sensitivity (Lopes et al. 2012), loss of the muscle pressure reflex (Williams et al. 1990) and instances of respiratory failure in the case of subacute necrotizing encephalomyelopathy (Leigh's syndrome) (Arii & Tanabe, 2000). The neural architectural of the PAG is very heterogeneous where it is divided into four distinct longitudinal columns comprising the dorsal medial periaqueductal grey (dmPAG), dlPAG, lPAG and ventrolateral periaqueductal grey (vlPAG) (Dampney et al. 2013). This structure is closely related to function. Each column has its own cytoarchitecture comprising neurons that are heterogeneous in size and shape with overlapping connections (Mantyh, 1983). The PAG circuits associated with cardiovascular responses are now well established in animals (Kabat et al. 1935; Lovick, 1985; Carrive et al. 1988) and human studies (Green et al. 2005), whereas we are only now obtaining a fuller understanding of the circuitry to and from the PAG that can control breathing (Horn & Waldrop 1998; Horiuchi et al. 2009; Subramanian, 2013).
An et al. (1998), Benarroch (2012) and Dampney et al. (2013) have provided a comprehensive review of the inputs and outputs of the PAG. In brief, the rat PAG receives selective inputs from the medial prefrontal cortex, amygdala, hypothalamus and nociceptive pathways, and sends outputs via the dorsal medial and lateral columns to thalamic and brainstem areas, especially those involved in cardiovascular regulation (e.g. rostral and caudal ventrolateral medulla) associated with ‘fight and flight’ responses (Bandler & Carrive, 1988). Conversely, coping responses, such as hypotension, bradycardia and hypopnoea, are observed when the ventrolateral column of the PAG is activated (Benarroch, 2012). Indeed, there is an especially close relationship between the PAG and the hypothalamus, given the functional connectivity reported by Horiuchi et al. (2009). In particular, there is a prominent synaptic connection from the dlPAG to the dorsal medial hypothalamus, which has been shown to increase respiratory activity when stimulated (e.g. Dampney et al. 2013).
Subramanian et al. (2008) and Subramanian (2013) have undertaken a detailed electrophysiological profile of various aspects of the rat PAG, and have shown different respiratory patterns depending upon the site of stimulation. Activation of the dlPAG causes active breathing and tachypnoea, consistent with a defence response, whereas stimulation of the medial part of the lPAG causes apneusis (Subramanian et al. 2008). However, stimulation of the dorsal aspect of the lPAG increases diaphragm electromyogram activity twofold as a consequence of increases in inspiratory time and decreases in expiratory time (Subramanian, 2013). Taken together, these data provide a framework to suggest that the PAG can serve as a strong modulator of breathing. Of interest, the DLPFC that is activated during ‘exercise’ (see Thornton et al. 2001) projects to the lateral PAG (An et al. 1998). Two big questions then arise. First, are all the PAG‐activated cardiorespiratory responses only related to internal or external stress? Second, is it possible that neurons in the lPAG are activated in a reciprocal fashion to increase and decrease respiratory activity associated with exercise and recovery?
Treadmill running and static muscle contraction in rats increase c‐fos activation of the PAG (Iwamoto et al. 1996; Li & Mitchell, 2003). When Green et al. (2007) re‐visited the ‘Krogh and Lindhard’ paradigm of anticipation of exercise, they observed marked increases in PAG activity when their patients were alerted to impending exercise. Neural activity increased further during actual exercise and then subsided during recovery as cardiorespiratory variables returned to baseline values (Figs 6 and 7). This study provided direct neurophysiological support in an exercising human for the idea that the dorsal periaqueductal grey (dPAG) may be a key part of the subcortical neurocircuitry of central command. What was not established was the precise location of the electrodes in the dPAG. If the PAG is a ‘cognitive integrator’, what is its relationship with the peripheral nervous system given the abundance of information travelling into the brain during exercise? There is convincing evidence that muscle contraction brought about by ventral root stimulation increases activity in both the posterior hypothalamus (Waldrop & Stremel 1989) and the PAG in the cat (Kramer et al. 1996). Group III and IV afferents connect to the PAG and carry important sensory information from muscle regarding nociception, but they also transmit the signal underpinning the exercise pressor reflex (Coote et al. 1971; McCloskey & Mitchell 1972). Basnayake et al. (2011) provided direct evidence for excitation of the dPAG in patients during activation of the exercise pressor reflex, confirming the functional significance of the PAG as an integrator of this reflex (Fig. 8).
Figure 6. Changes in cardiorespiratory parameters with exercise and anticipation of exercise .
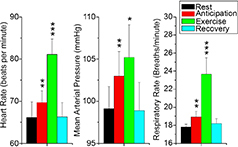
Note that all three parameters, i.e. heart rate, mean arterial pressure and respiratory rate, increased with anticipation, and further with exercise. Similarly, all parameters recovered after exercise was stopped. Error bars show one standard error of the mean; **P < 0.01, ***P < 0.001, *P < 0.05. From Green et al. (2007) with permission.
Figure 7. Local field potential (LFP) changes in the periaqueductal grey (PAG) during rest, anticipation, exercise and recovery .
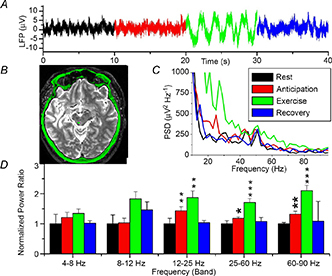
A, raw data trace. B, post‐operative magnetic resonance image showing a unilateral electrode in the left PAG (green dot). C, mean power spectral density (PSD) for all three patients (recovery not shown for clarity). D, normalized spectral changes (rest = 1.0) divided into frequency bands. *P < 0.05, **P < 0.01, ***P < 0.001. Note increase in neural activity during both anticipation of exercise and actual exercise. From Green et al. (2007) with permission.
Figure 8. Local field potential (LFP) changes in the periaqueductal grey (PAG) during activation of muscle pressor reflex .
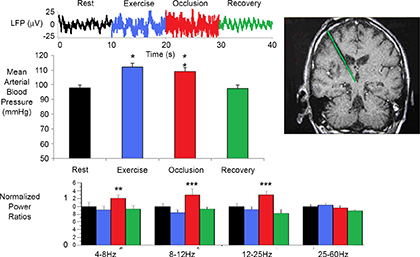
Top panel: raw data trace. Middle left panel: activation of muscle pressor reflex during light muscle contraction. Middle right panel: post‐operative image (post‐operative computed tomography fused to pre‐operative magnetic resonance) showing a unilateral electrode in the right PAG. Bottom panel: normalized spectral changes (rest = 1.0) divided into frequency bands. n = 6, **P < 0.01, ***P < 0.001. Note that PAG remains elevated when the pressor reflex is active. Modified from Basnayake et al. (2011) with permission.
Conclusion
When all data are viewed in an integrative manner, the PAG, in particular the lPAG and aspects of the dlPAG, appears to be a key communicating circuit for ‘central command’. Moreover, the PAG also fulfils many requirements of a command centre. It has functional connectivity to higher centres (DLPFC) and the basal ganglia (in particular, the STN), and receives a sensory input from contracting muscle, but, importantly, it sends efferent information to brainstem nuclei involved in cardiorespiratory control (see Fig. 9).
Figure 9. Proposed circuitry for exercise hyperpnoea .
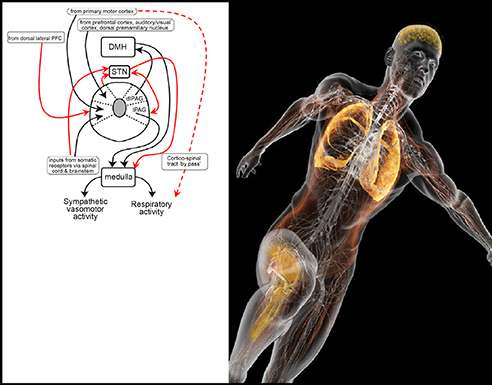
Left panel illustrates, in schematic form, the key circuits that may underpin the networks and structures involved in exercise hyperpnoea. dlPAG, dorsal lateral periaqueductal grey; DMH, dorsal medial hypothalamus; lPAG, lateral periaqueductal grey; PFC, prefrontal cortex; STN, subthalamic nucleus. Modified from Dampney et al. (2013) with permission. New proposed circuits in red. In particular, this network may underpin the ‘central governor hypothesis’ proposed by Noakes (Rauch et al. 2013). Running man image with permission from Bryan Christie Design.
The future challenge will be to build on these observations to test the robustness of the PAG as an essential structure in the circuitry underpinning exercise hyperpnoea, but to also establish the connections to other structures that are coupled to the exercise response. We have come a long way since Krogh and Lindhard (1913), although, in many ways, we are also now at the beginning of a new effort to re‐assemble the neural circuits responsible for exercise hyperpnoea.
Additional information
Competing interests
None.
Acknowledgments
I would like to thank Professor Roger Dampney for helpful feedback and thoughtful discussions on this article.
Biography
David Paterson is Professor of Physiology in the Department of Physiology, Anatomy & Genetics at the University of Oxford. He graduated from the Universities of Otago (NZ), Western Australia and Oxford, gaining his DPhil from Oxford and DSc from the University of Western Australia. He is a group leader in the British Heart Foundation Centre of Research Excellence at Oxford, and is Joint Director of the Burdon Sanderson Cardiac Science Centre. As a cardiac neurobiologist, his research focuses on the neural control of the cardiorespiratory system in normal and diseased states.

This review was presented at the symposium Recent advances in understanding mechanisms regulating breathing during exercise, which took place at Experimental Biology 2013, Boston, MA, USA on 24 April 2013.
References
- Adams L, Frankel H, Garlick J, Guz A, Murphy K & Semple SJ (1984). The role of spinal cord transmission in the ventilatory response to exercise in man. J Physiol 355, 85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF & Dempsey JA (2010). Group III and IV muscle afferents contribute to ventilatory and cardiovascular response to rhythmic exercise in humans. J Appl Physiol 109, 966–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An X, Bandler R, Ongur D & Price JL (1998). Prefrontal cortical projections to longitudinal columns in the midbrain periaqueductal grey in macaque monkeys. J Comp Neurol 401, 455–479. [PubMed] [Google Scholar]
- Arii J & Tanabe Y (2000). Leigh syndrome: serial MR imaging and clinical follow‐up. Am J Neuroradiol 21, 1502–1509. [PMC free article] [PubMed] [Google Scholar]
- Asmussen E & Nielsen M (1946). Studies on the regulation of respiration in heavy work. Acta Physiol Scand 12, 171–188. [Google Scholar]
- Bandler R & Carrive P (1988). Integrated defence reaction elicited by excitatory amino acid microinjection in the midbrain periaqueductal grey region of the unrestrained cat. Brain Res 439, 95–106. [DOI] [PubMed] [Google Scholar]
- Bandler R & Shipley MT (1994). Columnar organization in the midbrain periaqueductal grey: modules for emotional expression? Trends Neurosci 17, 379–389. [DOI] [PubMed] [Google Scholar]
- Banner N, Guz A, Heaton R, Innes JA, Murphy K & Yacoub M (1988). Ventilatory and circulatory responses at the onset of exercise in man following heart or heart–lung transplantation. J Physiol 399, 437–449, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannister RG & Cunningham DJ (1954). The effects on the respiration and performance during exercise of adding oxygen to the inspired air. J Physiol 125, 118–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basnayake SD, Green AL & Paterson DJ (2012). Mapping the central neurocircuitry that integrates the cardiovascular response to exercise in humans. Exp Physiol 97, 29–38. [DOI] [PubMed] [Google Scholar]
- Basnayake SD, Hyam JA, Pereira EA, Schweder PM, Brittain JS, Aziz TZ, Green AL & Paterson DJ (2011). Identifying cardiovascular neurocircuitry involved in the exercise pressor reflex in humans using functional neurosurgery. J Appl Physiol 110, 881–891. [DOI] [PubMed] [Google Scholar]
- Bechbache R & Duffin J (1977). The entrainment of breathing frequency by exercise rhythm. J Physiol 272, 553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch EE (2012). Periaqueductal grey: an interface for behavioral control. Neurology 78, 210–217. [DOI] [PubMed] [Google Scholar]
- Burger RE, Estavillo JA, Kumar P, Nye PC & Paterson DJ (1988). Effects of potassium, oxygen and carbon dioxide on the steady‐state discharge of cat carotid body chemoreceptors. J Physiol 401, 519–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrive P, Bandler R & Dampney R (1988). Anatomical evidence that hypertension associated with the defence reaction in the cat is mediated by a direct projection from a restricted portion of the midbrain periaqueductal grey to the subretrofacial nucleus of the medulla. Brain Res 460, 339–345. [DOI] [PubMed] [Google Scholar]
- Comroe JH & Schmidt CF (1943). Reflexes from the limbs as a factor in the hyperpnea of muscular exercise. Am J Physiol 138, 536–547. [Google Scholar]
- Coote JH, Hilton SM & Perez‐Gonzalez JF (1971). The reflex nature of the pressor response to muscular exercise. J Physiol 215, 789–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corfield DR, Murphy K & Guz A (1998). Does the motor cortical control of the diaphragm ‘bypass’ the brain stem respiratory centres in man? Respir Physiol 114, 109–117. [DOI] [PubMed] [Google Scholar]
- Cunningham DJ (1987). Studies on arterial chemoreceptors in man. J Physiol 384, 1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly WJ & Overley T (1966). Modification of ventilatory regulation by hypnosis. J Lab Clin Med 68, 279–285. [PubMed] [Google Scholar]
- Dampney RAL, Furlong TM, Horiuchi J & Iigaya K (2013). Role of dorsolateral periaqueductal grey in the coordinated regulation of cardiovascular and respiratory function. Aut Neurosci 175, 17–25. [DOI] [PubMed] [Google Scholar]
- Dejours P (1959). La regulation de la ventilation au cours de l'exercice musculaire chez l'homme. J Physiol (Paris) 51, 163–261. [PubMed] [Google Scholar]
- Dempsey JA & Forster HV (1982). Mediation of ventilatory adaptations. Physiol Rev 62, 262–346. [DOI] [PubMed] [Google Scholar]
- Douglas CG & Haldane JS (1909). The regulation of normal breathing. J Physiol 38, 420–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge FL, Millhorn DE & Waldrop TG (1981). Exercise hyperpnea and locomotion: parallel activation from the hypothalamus. Science 211, 844–846. [DOI] [PubMed] [Google Scholar]
- Esposito A, Demeurisse G, Alberti B & Fabbro F (1999). Complete mutism after midbrain periaqueductal grey lesion. Neuroreport 10, 681–685. [DOI] [PubMed] [Google Scholar]
- Fernandes A, Galbo H, Kjaer M, Mitchell JH, Secher NH & Thomas SN (1990). Cardiovascular and ventilatory responses to dynamic exercise during epidural anaesthesia in man. J Physiol 420, 281–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink GR, Adams L, Watson JD, Innes JA, Wuyam B, Kobayashi I, Corfield DR, Murphy K, Jones T, Frackowiak RS & et al. (1995). Hyperpnoea during and immediately after exercise in man: evidence of motor cortical involvement. J Physiol 489, 663–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foerster O (1936). Motorische Felder und Bahen. In: Bumke O. Handbuch der Neurologie. Springer. 50–51. [Google Scholar]
- Forster HV, Haouzi P & Dempsey JA (2012). Control of breathing during exercise. Comp Physiol 2, 743–777. [DOI] [PubMed] [Google Scholar]
- Frith C, Friston K, Liddle P & Frackowiak R (1991). Willed action and the prefrontal cortex in man: a study with PET. Proc Biol Sci 244, 241–246. [DOI] [PubMed] [Google Scholar]
- Gandevia SC & Rothwell JC (1987). Activation of the human diaphragm from the motor cortex. J Physiol 384, 109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geppert J & Zuntz N (1888). Ueber die Regulation der Atmung. Arch Ges Physiol 42, 189–244. [Google Scholar]
- Goodwin GM, McCloskey DI & Mitchell, JH (1972). Cardiovascular and respiratory responses to changes in central command during isometric exercise at constant muscle tension. J Physiol 226, 173–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AL, Wang S, Owen SL, Xie K, Liu X, Paterson DJ, Stein JF, Bain PG & Aziz TZ (2005). Deep brain stimulation can regulate arterial blood pressure in awake humans. Neuroreport 16, 1741–1745. [DOI] [PubMed] [Google Scholar]
- Green AL, Wang S, Purvis S, Owen SL, Bain PG, Stein JF, Guz A, Aziz TZ & Paterson DJ (2007). Identifying cardiorespiratory neurocircuitry involved in central command during exercise in humans. J Physiol 578, 605–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodins FS (1950). Analysis of factors concerned in regulation of breathing in exercise. Physiol Rev 30, 220–239. [DOI] [PubMed] [Google Scholar]
- Haggard HW & Henderson YB (1920). The fallacy of asphyxial acidosis. J Biol Chem 43, 3–13. [Google Scholar]
- Haldane JS & Priestley JG (1905). The regulation of the lung‐ventilation. J Physiol 32, 225–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson YB (1938). Adventures in Respiration Baltimore, MD: Williams & Wilkins. 316. [Google Scholar]
- Heymans JF & Heymans C (1927). Sur les modifications directes et sur la régulation réflexe de l'activité du centre respiratoire de la tête isolée du chien. Arch Int Pharmacodyn 33, 273–370. [Google Scholar]
- Horiuchi J, McDowall LM & Dampney RAL (2009). Vasomotor and respiratory responses evoked from the dorsolateral periaqueductal grey are mediated by the dorsomedial hypothalamus. J Physiol 587, 5149–5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn EM & Waldrop TG (1998). Suprapontine control of respiration. Respir Physiol 114, 201–211. [DOI] [PubMed] [Google Scholar]
- Iwamoto G, Wappel S, Fox G, Buetow K & Waldrop T (1996). Identification of diencephalic and brainstem cardiorespiratory areas activated during exercise. Brain Res 726, 109–122. [PubMed] [Google Scholar]
- Johansson JE (1893). Ueber die Einwirkung der Muskelthatigkeit auf die Athmung und die Hertzhiitigkeit. Skand Arch Physiol 5, 20–66. [Google Scholar]
- Kabat H, Magoum HW & Ranson SW (1935). Electrical stimulation of points in the forebrain and midbrain. The resultant alteration in blood pressure. Arch Neurol Psychiatr 34, 931–940. [Google Scholar]
- Kramer J, Jarboe M & Waldrop T (1996). Periaqueductal grey neuronal responses to hindlimb muscle contraction in the cat. Soc Neurosci Abstr 22, 89. [Google Scholar]
- Krogh A & Lindhard J (1913). The regulation of respiration and circulation during the initial stages of muscular work. J Physiol 47, 112–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J & Mitchell JH (2003). Glutamate release in midbrain periaqueductal grey by activation of skeletal muscle receptors and arterial baroreceptors. Am J Physiol 285, H137–144. [DOI] [PubMed] [Google Scholar]
- Linton RA & Band DM (1985). The effect of potassium on carotid chemoreceptor activity and ventilation in the cat. Respir Physiol 59, 65–70. [DOI] [PubMed] [Google Scholar]
- Lopes LT, Patrone LG, Bicego KC, Coimbra NC & Gargaglioni LH (2012). Periaqueductal grey matter modulates the hypercapnic ventilatory response. Pflugers Arch 464, 155–166. [DOI] [PubMed] [Google Scholar]
- Lovick T (1985). Ventrolateral medullary lesions block the antinociceptive and cardiovascular responses elicited by stimulating the dorsal periaqueductal grey matter in rats. Pain 3, 241–252. [DOI] [PubMed] [Google Scholar]
- Mantyh PW (1983). Connections of midbrain periaqueductal grey in the monkey. II. Descending efferent projections. J Neurophysiol 49, 582–594. [DOI] [PubMed] [Google Scholar]
- Mateika JH & Duffin J (1995). A review of the control of breathing during exercise. Eur J Appl Physiol 71, 1–27. [DOI] [PubMed] [Google Scholar]
- McCloskey DI & Mitchell JH (1972). Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol 224, 173–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan WP, Raven PB, Drinkwater BL & Horvath SM (1973). Perceptual and metabolic responsivity to standard bicycle ergometry following various hypnotic suggestions. Int J Clin Exp Hypnosis 21, 86–101. [Google Scholar]
- Paterson DJ (1992). Potassium and ventilation in exercise. J Appl Physiol 72, 811–820. [DOI] [PubMed] [Google Scholar]
- Paterson DJ, Friedland JS, Bascom DA, Clement ID, Cunningham DA, Painter R & Robbins PA (1990). Changes in arterial K+ and ventilation during exercise in normal subjects and subjects with McArdle's syndrome. J Physiol 429, 339–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson DJ & Nye PC (1988). The effect of beta adrenergic blockade on the carotid body response to hyperkalaemia in the cat. Respir Physiol 74, 229–237. [DOI] [PubMed] [Google Scholar]
- Paterson DJ, Robbins PA & Conway J (1989). Changes in arterial plasma potassium and ventilation during exercise in man. Respir Physiol 78, 323–330. [DOI] [PubMed] [Google Scholar]
- Paterson DJ, Wood GA, Marshall RN, Morton AR & Harrison AB (1987). Entrainment of respiratory frequency to exercise rhythm during hypoxia. J Appl Physiol 62, 1767–1771. [DOI] [PubMed] [Google Scholar]
- Paterson DJ, Wood GA, Morton AR & Henstridge JD (1986). The entrainment of ventilation frequency to exercise rhythm. Eur J Appl Physiol 55, 530–537. [DOI] [PubMed] [Google Scholar]
- Paterson WD (1928). Circulatory and respiratory changes in response to muscular exercise in man. J Physiol 66, 323–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfield W & Boldrey E (1937). Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain 60, 389–443. [Google Scholar]
- Pereira EAC, Wang Y, Peachey T, Lu G, Shlugman D, Stein JF, Aziz T & Green AL (2013). Elevated gamma band power in humans receiving naloxone suggests dorsal periaqueductal and periventricular grey deep brain stimulation produced analgesia is opioid mediated. Exp Neurol. 239, 248–255. [DOI] [PubMed] [Google Scholar]
- Pitts RF, Larrabee MG & DW B (1941). An analysis of hypothalamic cardiovascular control. Am J Physiol 134, 359–383. [Google Scholar]
- Plowey E & Waldrop T (2004). Cobalt injections into the pedunculopontine nuclei attenuate the reflex diaphragmatic responses to muscle contraction in rats. J Appl Physiol 96, 301–307. [DOI] [PubMed] [Google Scholar]
- Rauch HG, Schonbachler G & Noakes TD (2013). Neural correlates of motor vigour and motor urgency during exercise. Sports Med 43, 227–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K, Yoshimoto Y, Luppi PH, Fort P, el Mansari M, Salvert D & Jouvet M (1990). Lower brainstem afferents to the cat posterior hypothalamus: a double‐labelling study. Brain Res Bull 24, 437–455. [DOI] [PubMed] [Google Scholar]
- Shea SA, Andres LP, Shannon DC, Guz A & Banzett RB (1993). Respiratory sensations in subjects who lack a ventilatory response to CO2 . Respir Physiol 93, 203–219. [DOI] [PubMed] [Google Scholar]
- Smith OA, Jr. , Rushmer RF & Lasher EP (1960). Similarity of cardiovascular responses to exercise and to diencephalic stimulation. Am J Physiol 198, 1139–1142. [DOI] [PubMed] [Google Scholar]
- Subramanian HH (2013). Descending control of the respiratory neuronal network by the midbrain periaqueductal grey in the rat in vivo. J Physiol 591, 109–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian HH, Balnave RJ & Holstege G (2008). The midbrain periaqueductal grey control of respiration. J Neurosci 28, 12274–12283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton JM (1999). Higher centre and autonomic control of cardiorespiratory function. Phil Thesis. University of Oxford. [Google Scholar]
- Thornton JM, Aziz T, Schlugman D & Paterson DJ (2002). Electrical stimulation of the midbrain increases heart rate and arterial blood pressure in awake humans. J Physiol 539, 615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton JM, Guz A, Murphy K, Griffith AR, Pedersen DL, Kardos A, Leff A, Adams L, Casadei B & Paterson DJ (2001). Identification of higher brain centres that may encode the cardiorespiratory response to exercise in humans. J Physiol 533, 823–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldrop T & Stremel R (1989). Muscular contraction stimulates posterior hypothalamic neurons. Am J Physiol 256, R348–356. [DOI] [PubMed] [Google Scholar]
- Wasserman K & Whipp BJ (1975). Exercise physiology in health and disease. Am Rev Respir Dis 112, 219–249. [DOI] [PubMed] [Google Scholar]
- Wasserman K, Whipp BJ, Koyal SN & Beaver WL (1973). Anaerobic threshold and respiratory gas exchange during exercise. J Appl Physiol 35, 236–243. [DOI] [PubMed] [Google Scholar]
- Wasserman K, Whipp BJ, Koyal SN & Cleary MG (1975). Effect of carotid body resection on ventilatory and acid–base control during exercise. J Appl Physiol 39, 354–358. [DOI] [PubMed] [Google Scholar]
- Watt DF (2000). The centrecephalon and thalamocortical integration: neglected contributions of periaqueductal grey. Consciousness & Emotion 1, 91–114. [Google Scholar]
- Williams CA, Roberts JR & Freels DB (1990). Changes in blood pressure during isometric contractions to fatigue in the cat after brain stem lesions: effects of clonidine. Cardiovasc Res 24, 821–833. [DOI] [PubMed] [Google Scholar]
- Williamson JW (2010). The relevance of central command for the neural cardiovascular control of exercise. Exp Physiol 95 (11), 1043–1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson JW, McColl R, Matthews D, Mitchell JH, Raven PB & Morgan WP (2001). Hypnotic manipulation of effort sense during dynamic exercise: cardiovascular responses and brain activation. J Appl Physiol 90, 1392–1399. [DOI] [PubMed] [Google Scholar]
- Zuntz N & Geppert J (1886). Über die Natur der normalen Atemreize und den ort ihrer Wirkung. Arch Gen Physiol 38, 337–338. [Google Scholar]


