Abstract
Hypoxia is a common challenge to the fetus, promoting a physiological defence to redistribute blood flow towards the brain and away from peripheral circulations. During acute hypoxia, reactive oxygen species (ROS) interact with nitric oxide (NO) to provide an oxidant tone. This contributes to the mechanisms redistributing the fetal cardiac output, although the source of ROS is unknown. Here, we investigated whether ROS derived from xanthine oxidase (XO) contribute to the fetal peripheral vasoconstrictor response to hypoxia via interaction with NO-dependent mechanisms. Pregnant ewes and their fetuses were surgically prepared for long-term recording at 118 days of gestation (term approximately 145 days). After 5 days of recovery, mothers were infused i.v. for 30 min with either vehicle (n = 11), low dose (30 mg kg−1, n = 5) or high dose (150 mg kg−1, n = 9) allopurinol, or high dose allopurinol with fetal NO blockade (n = 6). Following allopurinol treatment, fetal hypoxia was induced by reducing maternal inspired O2 such that fetal basal 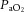 decreased approximately by 50% for 30 min. Allopurinol inhibited the increase in fetal plasma uric acid and suppressed the fetal femoral vasoconstrictor, glycaemic and lactate acidaemic responses during hypoxia (all P < 0.05), effects that were restored to control levels with fetal NO blockade. The data provide evidence for the activation of fetal XO in vivo during hypoxia and for XO-derived ROS in contributing to the fetal peripheral vasoconstriction, part of the fetal defence to hypoxia. The data are of significance to the understanding of the physiological control of the fetal cardiovascular system during hypoxic stress. The findings are also of clinical relevance in the context of obstetric trials in which allopurinol is being administered to pregnant women when the fetus shows signs of hypoxic distress.
decreased approximately by 50% for 30 min. Allopurinol inhibited the increase in fetal plasma uric acid and suppressed the fetal femoral vasoconstrictor, glycaemic and lactate acidaemic responses during hypoxia (all P < 0.05), effects that were restored to control levels with fetal NO blockade. The data provide evidence for the activation of fetal XO in vivo during hypoxia and for XO-derived ROS in contributing to the fetal peripheral vasoconstriction, part of the fetal defence to hypoxia. The data are of significance to the understanding of the physiological control of the fetal cardiovascular system during hypoxic stress. The findings are also of clinical relevance in the context of obstetric trials in which allopurinol is being administered to pregnant women when the fetus shows signs of hypoxic distress.
Introduction
Fetal hypoxia can result in marked fetal cardiovascular compromise with subsequent hypoxic–ischaemic encephalopathy (Primhak et al. 1985), which is associated with cerebral palsy and cognitive disability in later life (Cowan et al. 2003). Therefore, the prevention and management of fetal hypoxia remain major concerns in obstetric practice today.
The beneficial effects of the xanthine oxidase (XO) inhibitor allopurinol in reducing hypoxic damage in adult cardiology and in paediatric and adult cardiothoracic surgery have long been established (Johnson et al. 1991; Coghlan et al. 1994; Castelli et al. 1995; Clancy et al. 2001). In contrast, the effects of allopurinol in protecting the physiology of the fetus against hypoxia during the developmental period have been less well described. One study reported that treatment with allopurinol of the hypoxic neonate following complicated labour improved neonatal outcome (van Bel et al. 1998). However, if the time interval between the hypoxic challenge and treatment was too long, or the hypoxia too severe, no reductions in serious morbidity or mortality were reported (Benders et al. 2006). Consequently, there has been growing clinical interest in establishing whether perinatal outcome in complicated pregnancy may be improved if the window of treatment with allopurinol is advanced, for instance via maternal treatment to cover the actual period of fetal hypoxia in complicated labour. Allopurinol administered to the mother crosses the placenta, yielding therapeutic levels in the neonatal circulation (Boda et al. 1999), justifying this route of administration for preventative therapy in obstetric practice. However, virtually nothing is known about the effects of maternal treatment with allopurinol on the maternal or fetal physiology, or on maternal and fetal cardiovascular function.
Recently, we reported that antenatal maternal treatment with allopurinol reduces hippocampal brain damage after acute birth asphyxia in late gestation fetal sheep (Kaandorp et al. 2013). We have also discovered that the interaction between the superoxide anion (•O2−) and nitric oxide (NO) provides an oxidant tone in the fetal vasculature that controls blood flow in several circulations during basal and hypoxic conditions (Thakor et al. 2010a,b2010a; Herrera et al. 2012; Kane et al. 2012). Maternal treatment with allopurinol under basal conditions increased umbilical blood flow and the gain of the fetal cardiac baroreflex, but it impaired fetal α1 adrenergic-mediated pressor and femoral vasopressor responses via increasing NO bioavailability (Herrera et al. 2012). Therefore, it is possible that allopurinol-induced alterations in the fetal circulation, particularly in response to hypoxia, may offset some of its benefits in neuroprotection. However, the effect of maternal treatment with allopurinol on the fetal cardiovascular defence to acute hypoxia remains completely unknown. Therefore, in this study we tested the hypothesis that XO-derived reactive oxygen species (ROS) interact with NO in the fetal vasculature and have a role in the regulation of fetal cardiovascular function during acute hypoxic stress. If so, blocking XO activity may prevent an appropriate fetal circulatory defence to hypoxia. The hypothesis was tested by investigating the in vivo effects of maternal treatment with high and low doses of allopurinol on the fetal cardiovascular responses to hypoxia in the chronically catheterized ewe and fetus during late gestation. To determine whether enhanced NO bioavailability was involved in mediating the effects of allopurinol on fetal cardiovascular function, maternal treatment with allopurinol was repeated in the presence of fetal in vivo NO blockade with an NO clamp (Gardner & Giussani, 2003; Morrison et al. 2003; Thakor & Giussani, 2009).
Methods
Surgical preparation
Experiments were conducted on pregnant Welsh Mountain ewes using procedures approved by the Local Ethics Review Committee of the University of Cambridge and licensed by the Home Office under the UK Animals (Scientific Procedures) Act, 1986.
Eleven Welsh Mountain sheep fetuses and their mothers were surgically instrumented under general anaesthesia for long-term recording between 118 and 120 days of gestation (term approximately 145 days) using strict aseptic conditions, as described in detail (Fletcher et al. 2000; Herrera et al. 2012). Midline abdominal and uterine incisions were made, the fetal hind limbs were exteriorized and the right femoral artery and vein were isolated and catheterized with a polyvinylchloride catheter (i.d. 0.86 mm, o.d. 1.52 mm; Critchly Electrical Products, NSW, Australia). A further catheter was anchored onto the fetal hind limb for measurement of amniotic pressure and for administration of antibiotics into the amniotic cavity (600 mg in 2 ml benzylpenicillin, Crystapen; Schering-Plough, Animal Health Division, Welwyn Garden City, UK). A transonic flow probe was also implanted around the contralateral femoral artery (Type 2RSl Transonic Systems Inc., Ithaca, NY, USA). The dead space of the catheters was filled with heparinized saline (80 i.u. heparin ml−1 in 0.9% NaCl) and the catheter ends were plugged. A Teflon catheter (i.d. 1.0 mm, o.d. 1.6 mm; Altec, UK) was inserted in the maternal femoral artery and placed in the descending aorta, and a maternal venous catheter placed in the inferior vena cava (i.d. 0.86 mm, o.d. 1.52 mm; Critchly Electrical Products). The catheters and flow probe cable were then exteriorized via a keyhole incision in the maternal flank, and placed in a bag sutured to the skin of the ewe. Antibiotics were administered daily to the ewe (0.20–0.25 mg kg−1 i.m. Depocillin; Mycofarm, Cambridge, UK), to the fetus i.v. and into the amniotic cavity (300 mg Penbritin; SmithKline Beecham Animal Health, Welwyn Garden City, UK). Mean fetal arterial blood pressure (corrected for amniotic pressure), femoral blood flow, fetal heart rate (triggered from the arterial blood pressure or femoral blood flow pulse), maternal blood pressure and maternal heart rate (triggered from blood pressure) were continuously recorded using a custom made computerized data acquisition system (Department of Physiology, Development and Neuroscience, Cambridge University, UK).
Experimental protocol
Following 5 days of post-operative recovery, ewes and fetuses were subjected to the acute hypoxia protocol which consisted of 2 h normoxia, 0.5 h hypoxia and 1 h recovery (Fig. 1). Acute hypoxia in the fetus was induced by maternal inhalational hypoxia (Fletcher et al. 2000), changing the concentrations of gases breathed by the ewe to 6% O2 in N2 with small amounts of CO2 (15 l min−1 air, 35 l min−1 N2, 1.5–2.5 l min−1 CO2). This mixture was designed to reduce the fetal 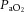 to about 10 mmHg whilst maintaining fetal
to about 10 mmHg whilst maintaining fetal 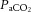 . Following the 0.5 h period of hypoxia, the ewe was returned to breathing air for the 1 h recovery period.
. Following the 0.5 h period of hypoxia, the ewe was returned to breathing air for the 1 h recovery period.
Figure 1.
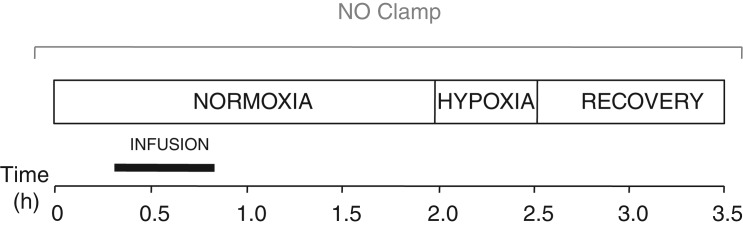
The experimental protocol consisted of 2h h of normoxia, 0.5 h of hypoxia (6% O2) and 1 h of recovery, with maternal infusion from 20 to 50 min of: saline vehicle (n = 11), the low dose of allopurinol (30 mg kg−1; n = 5), the high dose allopurinol (150 mg kg−1; n = 9) or high dose of allopurinol during fetal blockade of NO synthase with the NO clamp (n = 6).
Acute hypoxia was induced following maternal i.v. infusion with vehicle (n = 11). On a separate day, 5 of these 11 animals were also exposed to acute hypoxia following maternal i.v. infusion with low allopurinol (30 mg kg−1, n = 5). These 5 and another 4 of the 11 animals were exposed to acute hypoxia with high dose allopurinol treatment (150 mg kg−1, n = 9; Sigma, UK). Allopurinol was dissolved in the minimum volume of 4 m sodium hydroxide (NaOH) and made up with saline (van Dijk et al. 2008; Herrera et al. 2012). Vehicle was saline treated with 4 m NaOH to achieve the same pH of the allopurinol solution (van Dijk et al. 2008; Herrera et al. 2012). The 30 min infusion period started 20 min following the onset of recording during normoxia. The low dose of allopurinol was adapted from recent studies of allopurinol compatible with human treatment (van Bel et al. 1998; Benders et al. 2006). The high dose of allopurinol was five times the low dose. The timing of the acute hypoxic challenge coincided with peak concentrations of oxypurinol (the active metabolite of allopurinol) measured in fetal plasma following maternal treatment with a comparable dose of allopurinol of similar duration in the same breed of sheep (Masaoka et al. 2005; Derks et al. 2010).
In some animals, the experiment with the high dose of allopurinol was repeated following fetal treatment with the NO clamp (n = 6). The NO clamp is an established technique that combines fetal treatment with the NO synthase inhibitor NG-nitro-l-arginine methyl ester (l-NAME; 100 mg kg−1 bolus dissolved in 2 ml saline, fetal i.a.; Sigma) with the NO donor sodium nitroprusside (5.1 ± 2.0 μg kg−1 min−1, mean ± SD dissolved in saline, fetal i.v.; Sigma). The technique blocks de novo synthesis of NO while compensating for the tonic production of the gas and thereby maintaining basal cardiovascular function (Gardner & Giussani, 2003; Morrison et al. 2003; Thakor & Giussani, 2009).
At least 2 days were allowed between protocols in animals where two or more experiments were carried out in a single animal preparation. In animals that were exposed to high dose allopurinol with and without the NO clamp, at least 48 h was allowed for allopurinol to be cleared between experiments (Derks et al. 2010). The NO clamp experiment was always the final procedure in an animal preparation as we have previously found that cardiovascular function does not normalize for a long time following treatment with the NO clamp.
Blood sampling regimen and assays
During any acute hypoxia protocol, descending aortic blood samples (1.0 ml) were taken from the mother and fetus at set time intervals: 0, 50 and 120 min of normoxia, 5, 15 and 30 min of hypoxia, and 15 and 60 min of recovery. Arterial blood gas and acid base status (ABL5 Blood Gas Analyser; Radiometer, Copenhagen, Denmark; measurements corrected to 39.5°C for fetal blood and 38°C for maternal blood), percentage saturation of haemoglobin with oxygen (Sat Hb) and the blood haemoglobin concentration [Hb] (Haemoximeter OSM3; Radiometer), and blood glucose and lactate concentrations (Yellow Springs 2300 Stat Plus Glucose/Lactate Analyser; YSI Ltd, Farnborough, UK) were determined for each sample. Given that XO catalyses the conversion of hypoxanthine to uric acid, plasma concentrations of urate were also measured using high-performance liquid chromatography (HPLC) with electrochemical detection (Iriyama et al. 1984). In brief, aliquots of maternal and fetal plasma (acidified 1:1 with ice-cold 10% metaphosphoric acid, centrifuged and the supernatant stored at –80°C) were diluted 1:4 with ice-cold 5% metaphosphoric acid; final dilution of plasma 1:10. To this, 100 μl HPLC-grade heptane was added and following vortex mixing for 40 s, the samples were centrifuged (20,000g; 5 min) and the lower (aqueous) layer removed and treated with heptane again until the supernatant was clear. This clear supernatant was transferred to a 0.8 ml HPLC vial. An electrochemical detector (EG&G Instruments, Wokingham, UK) with a working electrode (set at 400 mV and sensitivity of 0.5 μA) was used for detection. Final concentrations for urate were calculated with external standards, which were run simultaneously. The limit of sensitivity for the assay was 0.1 μmol l−1 for urate, and the inter-assay coefficient of variation was less than 5%. An additional 1 ml of arterial blood was withdrawn at set intervals for fetal plasma catecholamine analyses during acute hypoxia following maternal i.v. infusion with vehicle (n = 6) or the high dose of allopurinol (n = 6) only to minimize fetal blood loss. These samples were collected under sterile conditions into chilled heparin tubes (2 ml Li+-heparin tubes; LIP Ltd, Shipley, UK) containing reduced glutathione (4 nmol per tube; G-4251; Sigma, UK) and EGTA (5 nmol per tube; E-4378; Sigma). Samples were then centrifuged at 4000 r.p.m. for 4 min at 4°C and stored at –80°C until analysis. Fetal plasma catecholamine concentrations were measured using a commercially available catecholamine radioimmunoassay previously validated for use with sheep plasma (2-CAT RIA; Diasourse, http://www.diasource-diagnostics.com) and as previously described in detail (Kane et al. 2012).
Data and statistical analyses
Values for blood gas, acid base and metabolic status are the mean ± SEM for normoxia before (Pre i), during (During i) or after (Post i,) infusion, hypoxia (H, the mean value of 5, 15 and 30 min of hypoxia) and recovery (R, the mean value of 15 and 60 min of recovery). Values for plasma uric acid are the mean ± SEM at 0, 50 and 120 min of normoxia, 30 min of hypoxia, and 60 min of recovery. Fetal and maternal cardiovascular variables were recorded continually, and were compiled into line graphs of the mean ± SEM for every minute for all animals. The serial cardiovascular variables were analysed using summary measures to focus the number of comparisons (Matthews et al. 1990). To determine the effects of allopurinol on basal maternal and fetal cardiovascular function, the mean of minute means were determined before (Pre i, 0–20 min), during (During i, 21–50 min) and after (Post i, 51–120 min) infusion. To determine the effects of allopurinol on maternal and fetal cardiovascular function during acute hypoxia, the means ± SEM for the absolute change in the area under the curve (AUC) from normoxic baseline in cardiovascular variables were calculated. These were determined as 30 min epochs during normoxia (N, 90–120 min), hypoxia (H, 121–150 min) and recovery (R, 151–180 min). For all data, comparisons within (effect of time) and between (effect of treatment) groups were assessed statistically using two-way analysis if variance (ANOVA) with repeated measures. Where a significant effect of time or treatment was indicated, the post hoc Tukey test was used to isolate the statistical differences. For all comparisons, statistical significance was accepted when P < 0.05.
Results
Maternal arterial blood gas, acid base and metabolic status
Pre-infusion values for maternal arterial blood gas, acid base and metabolic status were similar in all ewes and were within the normal range for pregnant Welsh Mountain sheep at this stage of gestation (Table 1). Infusion with saline vehicle or the low dose of allopurinol had no effect on arterial blood gas, acid base or metabolic status. In contrast, infusion with the high dose of allopurinol, with or without fetal treatment with the NO clamp, significantly increased maternal arterial pH (pHa) for the duration of the protocol. Further, high allopurinol infusion significantly increased maternal acid base excess (ABE) and decreased maternal 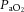 without affecting maternal haemoglobin saturation with oxygen (Sat Hb; Table 1).
without affecting maternal haemoglobin saturation with oxygen (Sat Hb; Table 1).
Table 1.
Maternal arterial blood gas and acid base status
| N |
||||||
|---|---|---|---|---|---|---|
| Pre i | During i | Post i | H | R | ||
| pHa | Vehicle | 7.53 ± 0.01 | 7.51 ± 0.01 | 7.54 ± 0.01 | 7.52 ± 0.01 | 7.52 ± 0.01 |
| Low allopurinol | 7.52 ± 0.01 | 7.53 ± 0.01 | 7.52 ± 0.02 | 7.52 ± 0.03 | 7.53 ± 0.02 | |
| High allopurinol | 7.50 ± 0.02 | 7.61 ± 0.02*† | 7.57 ± 0.01* | 7.56 ± 0.01* | 7.56 ± 0.01* | |
| High allopurinol + NO clamp | 7.49 ± 0.02 | 7.62 ± 0.02*† | 7.57 ± 0.01* | 7.57 ± 0.02 | 7.57 ± 0.01* | |
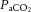 (mmHg) (mmHg) |
Vehicle | 34.5 ± 0.8 | 35.3 ± 1.3 | 33.5 ± 0.9 | 34.8 ± 0.8 | 34.2 ± 0.8 |
| Low allopurinol | 35.8 ± 1.6 | 35.8 ± 1.1 | 36.8 ± 0.4 | 35.7 ± 0.8 | 35.5 ± 1.3 | |
| High allopurinol | 36.1 ± 1.7 | 33.5 ± 1.1 | 33.8 ± 0.8 | 34.6 ± 0.8 | 34.2 ± 0.7 | |
| High allopurinol + NO clamp | 37.7 ± 0.8 | 34.7 ± 0.8 | 37.0 ± 1.0 | 34.9 ± 0.9 | 35.5 ± 1.0 | |
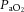 (mmHg) (mmHg) |
Vehicle | 106 ± 3 | 100 ± 3 | 109 ± 4 | 33 ± 1* | 101 ± 2 |
| Low allopurinol | 104 ± 3 | 101 ± 4 | 101 ± 3 | 35 ± 2* | 105 ± 4 | |
| High allopurinol | 105 ± 2 | 86 ± 5*† | 107 ± 2 | 37 ± 1* | 107 ± 3 | |
| High allopurinol + NO clamp | 104 ± 2 | 77 ± 3*† | 100 ± 2 | 37 ± 3* | 102 ± 3 | |
| Sat Hb (%) | Vehicle | 97.3 ± 0.7 | 96.5 ± 0.8 | 96.7 ± 0.7 | 54.1 ± 2.5* | 95.9 ± 0.6 |
| Low Allopurinol | 95.6 ± 0.4 | 95.5 ± 0.6 | 95.8 ± 0.5 | 60.4 ± 3.9* | 96.1 ± 0.4 | |
| High allopurinol | 98.5 ± 1.5 | 96.2 ± 1.3 | 99.0 ± 1.3 | 59.4 ± 2.8* | 99.1 ± 1.3 | |
| High allopurinol + NO clamp | 99.2 ± 0.6 | 97.2 ± 1.2 | 98.8 ± 1.0 | 57.8 ± 4.9* | 99.1 ± 0.5 | |
| ABE (meq l−1) | Vehicle | 5.8 ± 0.5 | 5.7 ± 0.4 | 5.8 ± 0.6 | 6.0 ± 0.5 | 5.4 ± 0.5 |
| Low allopurinol | 6.4 ± 1.3 | 7.2 ± 1.3 | 6.8 ± 1.4 | 7.2 ± 1.4 | 6.6 ± 1.3 | |
| High allopurinol | 5.9 ± 0.7 | 11.7 ± 1.3*† | 9.5 ± 1.0*† | 8.8 ± 1.2 | 8.6 ± 1.1 | |
| High allopurinol + NO clamp | 6.2 ± 1.4 | 12.7 ± 1.3*† | 8.8 ± 1.7 | 8.3 ± 1.6 | 8.7 ± 1.4 | |
| [Glucose] (mmol l−1) | Vehicle | 2.95 ± 0.24 | 3.08 ± 0.25 | 3.08 ± 0.24 | 3.10 ± 0.20 | 3.19 ± 0.29 |
| Low allopurinol | 3.38 ± 0.30 | 3.69 ± 0.35 | 3.73 ± 0.41 | 3.43 ± 0.36 | 3.54 ± 0.42 | |
| High allopurinol | 2.49 ± 0.18 | 2.56 ± 0.20 | 2.53 ± 0.23 | 2.63 ± 0.24 | 2.80 ± 0.23 | |
| High allopurinol + NO clamp | 2.89 ± 0.13 | 2.91 ± 0.15 | 2.89 ± 0.16 | 2.89 ± 0.17 | 3.31 ± 0.21 | |
| [Lactate] (mmol l−1) | Vehicle | 0.39 ± 0.07 | 0.45 ± 0.08 | 0.47 ± 0.10 | 0.56 ± 0.09 | 0.44 ± 0.05 |
| Low allopurinol | 0.50 ± 0.11 | 0.52 ± 0.11 | 0.54 ± 0.11 | 0.55 ± 0.11 | 0.52 ± 0.13 | |
| High allopurinol | 0.44 ± 0.04 | 0.69 ± 0.12 | 0.68 ± 0.16 | 0.95 ± 0.20 | 0.66 ± 0.15 | |
| High allopurinol + NO clamp | 0.40 ± 0.03 | 0.57 ± 0.03 | 0.56 ± 0.06 | 0.97 ± 0.23 | 0.75 ± 0.18 | |
Values represent the means ± SEM at 0 (Pre i), 50 (During i) and 115 (Post i) min of normoxia, at 30 min of hypoxia (H) and at 60 min of recovery (R) for mothers exposed to 0.5 h of hypoxia either during saline vehicle infusion (n = 11), treatment with the low dose of allopurinol (30 mg kg−1; n = 5), treatment with the high dose of allopurinol (150 mg kg−1; n = 9) or treatment with the high dose of allopurinol during fetal blockade of NO synthase with the NO clamp (n = 6). Significant differences (P < 0.05) are:
within group with respect to time period Pre i,
between groups with respect to saline vehicle infusion (two-way repeated measures ANOVA with post hoc Tukey test).
In all ewes, acute hypoxia induced significant falls of similar magnitude in maternal 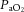 and Sat Hb without any alteration to
and Sat Hb without any alteration to 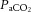 (Table 1). During recovery, infusion with the high dose of allopurinol, with or without fetal treatment with the NO clamp, maintained the increased maternal pHa. In contrast, all other variables across the groups returned to pre-infusion values.
(Table 1). During recovery, infusion with the high dose of allopurinol, with or without fetal treatment with the NO clamp, maintained the increased maternal pHa. In contrast, all other variables across the groups returned to pre-infusion values.
Fetal arterial blood gas, acid base and metabolic status
Pre-infusion values for fetal arterial blood gas, acid base and metabolic status were similar in all fetuses and were within the normal range for the Welsh Mountain sheep fetus at this stage of gestation (Table 2). Infusion with vehicle or allopurinol had no effect on basal arterial blood gas or acid base status. In all fetuses, acute hypoxia induced significant falls of similar magnitude in fetal 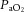 and Sat Hb without any alteration to
and Sat Hb without any alteration to 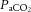 (Table 2). Acute hypoxia induced a significant decrease in pHa and ABE by the end of the hypoxic challenge in control fetuses only (Table 2). In all fetuses, acute hypoxia led to a significant increase in blood lactate. In contrast, a significant increase from baseline in blood glucose during hypoxia only reached significance in the control fetuses and fetuses from mothers treated with the low dose of allopurinol. When blood glucose and lactate were calculated as a change from normoxic baseline, the increments from baseline in blood glucose and lactate were significantly depressed in fetuses from mothers treated with the high dose of allopurinol relative to control (Δ[glucose]: 0.49 ± 0.15 vs. 0.12 ± 0.13 mmol l−1, Δ[lactate]: 2.59 ± 0.51 vs. 1.59 ± 0.48 mmol l−1, P < 0.05 for saline vs. high allopurinol). Fetal treatment with the NO clamp during maternal infusion with the high dose of allopurinol restored the increment in fetal blood glucose and lactate towards control levels (Δ[glucose]: 0.29 ± 0.11 mmol l−1, Δ[lactate]: 1.85 ± 0.61 mmol l−1).
(Table 2). Acute hypoxia induced a significant decrease in pHa and ABE by the end of the hypoxic challenge in control fetuses only (Table 2). In all fetuses, acute hypoxia led to a significant increase in blood lactate. In contrast, a significant increase from baseline in blood glucose during hypoxia only reached significance in the control fetuses and fetuses from mothers treated with the low dose of allopurinol. When blood glucose and lactate were calculated as a change from normoxic baseline, the increments from baseline in blood glucose and lactate were significantly depressed in fetuses from mothers treated with the high dose of allopurinol relative to control (Δ[glucose]: 0.49 ± 0.15 vs. 0.12 ± 0.13 mmol l−1, Δ[lactate]: 2.59 ± 0.51 vs. 1.59 ± 0.48 mmol l−1, P < 0.05 for saline vs. high allopurinol). Fetal treatment with the NO clamp during maternal infusion with the high dose of allopurinol restored the increment in fetal blood glucose and lactate towards control levels (Δ[glucose]: 0.29 ± 0.11 mmol l−1, Δ[lactate]: 1.85 ± 0.61 mmol l−1).
Table 2.
Fetal arterial blood gas and acid base status
| N |
||||||
|---|---|---|---|---|---|---|
| Pre i | During i | Post i | H | R | ||
| pHa | Vehicle | 7.36 ± 0.01 | 7.37 ± 0.01 | 7.37 ± 0.01 | 7.30 ± 0.02 | 7.26 ± 0.02 |
| Low allopurinol | 7.36 ± 0.00 | 7.36 ± 0.00 | 7.36 ± 0.01 | 7.32 ± 0.01 | 7.30 ± 0.01* | |
| High allopurinol | 7.36 ± 0.01 | 7.37 ± 0.01 | 7.36 ± 0.01 | 7.32 ± 0.02 | 7.30 ± 0.02 | |
| High allopurinol + NO Clamp | 7.33 ± 0.02 | 7.34 ± 0.01 | 7.35 ± 0.01 | 7.35 ± 0.01 | 7.27 ± 0.03 | |
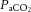 (mmHg) (mmHg) |
Vehicle | 53.1 ± 1.5 | 51.5 ± 1.2 | 51.5 ± 1.0 | 53.8 ± 1.2 | 52.9 ± 1.3 |
| Low allopurinol | 57.0 ± 1.2 | 54.3 ± 1.8 | 55.8 ± 0.9 | 55.0 ± 1.0 | 53.5 ± 1.4 | |
| High allopurinol | 53.1 ± 0.8 | 51.3 ± 1.2 | 52.8 ± 1.2 | 52.9 ± 1.0 | 52.7 ± 0.8 | |
| High allopurinol + NO clamp | 55.8 ± 0.7 | 53.0 ± 0.8 | 53.5 ± 0.9 | 52.6 ± 1.2 | 52.6 ± 0.9 | |
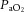 (mmHg) (mmHg) |
Vehicle | 20 ± 1 | 20 ± 1 | 20 ± 1 | 9 ± 1* | 20 ± 1 |
| Low allopurinol | 19 ± 1 | 19 ± 1 | 19 ± 1 | 9 ± 0* | 18 ± 1 | |
| High allopurinol | 21 ± 1 | 19 ± 1 | 20 ± 1 | 10 ± 1* | 20 ± 1 | |
| High allopurinol + NO clamp | 22 ± 1 | 20 ± 1 | 21 ± 1 | 11 ± 1* | 22 ± 1 | |
| Sat Hb (%) | Vehicle | 56.4 ± 3.0 | 52.9 ± 3.3 | 51.5 ± 2.3 | 17.3 ± 1.5* | 50.8 ± 3.3 |
| Low allopurinol | 51.3 ± 1.4 | 50.7 ± 2.5 | 52.9 ± 2.1 | 19.1 ± 1.4* | 49.4 ± 2.1 | |
| High allopurinol | 55.0 ± 3.2 | 49.5 ± 3.5 | 52.5 ± 3.1 | 20.3 ± 2.0* | 52.9 ± 3.1 | |
| High Allopurinol + NO Clamp | 58.8 ± 2.9 | 52.5 ± 3.1 | 54.0 ± 3.7 | 21.5 ± 3.6* | 54.3 ± 4.4 | |
| ABE (meq l−1) | Vehicle | 3.6 ± 0.5 | 3.4 ± 0.4 | 3.6 ± 0.5 | –1.4 ± 1.2* | –3.8 ± 1.3* |
| Low allopurinol | 4.6 ± 0.7 | 4.2 ± 0.6 | 4.2 ± 0.6 | 0.7 ± 1.2 | –1.5 ± 1.1* | |
| High allopurinol | 3.0 ± 0.6 | 0.9 ± 2.6 | 3.4 ± 0.5 | 0.1 ± 1.3 | –0.9 ± 1.4 | |
| High allopurinol + NO clamp | 1.7 ± 1.0 | 1.3 ± 0.8 | 1.7 ± 0.6 | –1.3 ± 1.3 | –3.0 ± 1.8* | |
| [Glucose] (mmol l−1) | Vehicle | 0.91 ± 0.10 | 0.97 ± 0.10 | 0.96 ± 0.09 | 1.40 ± 0.18 | 1.38 ± 0.18 |
| Low allopurinol | 1.15 ± 0.12 | 1.17 ± 0.12 | 1.16 ± 0.14 | 1.52 ± 0.23 | 1.40 ± 0.24 | |
| High allopurinol | 0.97 ± 0.09 | 0.84 ± 0.08 | 0.82 ± 0.08 | 1.09 ± 0.12 | 1.08 ± 0.16 | |
| High allopurinol + NO clamp | 0.91 ± 0.08 | 0.89 ± 0.07 | 0.88 ± 0.04 | 1.09 ± 0.12 | 1.21 ± 0.07 | |
| [Lactate] (mmol l−1) | Vehicle | 0.87 ± 0.06 | 0.87 ± 0.08 | 1.10 ± 0.06 | 3.46 ± 0.51 | 4.97 ± 0.49* |
| Low allopurinol | 1.02 ± 0.11 | 1.02 ± 0.12 | 1.07 ± 0.17 | 2.88 ± 0.59 | 3.80 ± 0.51*† | |
| High allopurinol | 0.85 ± 0.06 | 0.98 ± 0.09 | 1.00 ± 0.06 | 2.63 ± 0.50 | 3.30 ± 0.51*† | |
| High allopurinol + NO clamp | 0.95 ± 0.10 | 1.16 ± 0.11 | 1.36 ± 0.10 | 2.79 ± 0.62 | 3.95 ± 0.72 | |
Values represent the means ± SEM at 0 (Pre i), 50 (During i) and 115 (Post i) min of normoxia, at 30 min of hypoxia (H) and at 60 min of recovery (R) for fetuses exposed to 0.5 h of hypoxia either during maternal saline vehicle infusion (n = 11), maternal treatment with the low dose of allopurinol (30 mg kg−1; n = 5), maternal treatment with the high dose of allopurinol (150 mg kg−1; n = 9) or maternal treatment with the high dose of allopurinol during fetal blockade of NO synthase with the NO clamp (n = 6). Significant differences (P < 0.05) are:
within group with respect to time period Pre i,
between groups with respect to saline vehicle infusion (two-way repeated measures ANOVA with post hoc Tukey test).
During recovery, 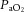 and Sat Hb returned to pre-hypoxic levels in all fetuses whilst
and Sat Hb returned to pre-hypoxic levels in all fetuses whilst 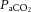 remained unaltered (Table 2). There was a significant decrease in pHa and ABE in all fetuses (Table 2). All fetuses continued to show a significant increase in blood lactate during recovery and blood glucose remained significantly elevated from normoxic baseline only in control fetuses (Table 2). The increments from baseline in blood glucose and lactate during recovery were again significantly depressed in fetuses from mothers treated with the high dose of allopurinol relative to control (Δ[glucose]: 0.47 ± 0.15 vs. 0.10 ± 0.16 mmol l−1, Δ[lactate]: 4.10 ± 0.50 vs. 2.45 ± 0.48 mmol l−1, P < 0.05 for saline vs. high allopurinol). Fetal treatment with the NO clamp during maternal infusion with the high dose of allopurinol restored the increment in fetal blood glucose but not lactate towards control levels during recovery (Δ[glucose]: 0.31 ± 0.10 mmol l−1, Δ[lactate]: 3.01 ± 0.71 mmol l−1, P < 0.05 for saline vs. high allopurinol + NO clamp).
remained unaltered (Table 2). There was a significant decrease in pHa and ABE in all fetuses (Table 2). All fetuses continued to show a significant increase in blood lactate during recovery and blood glucose remained significantly elevated from normoxic baseline only in control fetuses (Table 2). The increments from baseline in blood glucose and lactate during recovery were again significantly depressed in fetuses from mothers treated with the high dose of allopurinol relative to control (Δ[glucose]: 0.47 ± 0.15 vs. 0.10 ± 0.16 mmol l−1, Δ[lactate]: 4.10 ± 0.50 vs. 2.45 ± 0.48 mmol l−1, P < 0.05 for saline vs. high allopurinol). Fetal treatment with the NO clamp during maternal infusion with the high dose of allopurinol restored the increment in fetal blood glucose but not lactate towards control levels during recovery (Δ[glucose]: 0.31 ± 0.10 mmol l−1, Δ[lactate]: 3.01 ± 0.71 mmol l−1, P < 0.05 for saline vs. high allopurinol + NO clamp).
Effects of allopurinol on maternal and fetal basal cardiovascular function
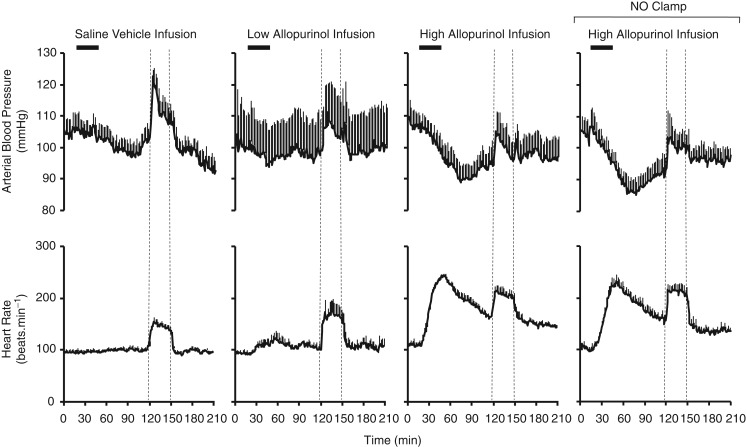
Pre-infusion values for maternal arterial blood pressure and heart rate were similar in all ewes (Fig. 2). Infusion with saline or the low dose of allopurinol had no effect on basal maternal cardiovascular function. In contrast, infusion with the high dose of allopurinol led to a significant decrease in maternal basal arterial blood pressure and a significant increase in maternal basal heart rate after the cessation of the infusion (Figs 2 and 4A).
Figure 2.
Values represent the means ± SEM calculated every minute for arterial blood pressure and heart rate during 2 h of normoxia, 0.5 h of hypoxia (dashed lines) and 1 h of recovery for mothers either during saline vehicle infusion (n = 11), treatment with the low dose of allopurinol (30 mg kg−1; n = 5), treatment with the high dose of allopurinol (150 mg kg−1; n = 9) or treatment with the high dose of allopurinol during fetal blockade of NO synthase with the NO clamp (n = 6).
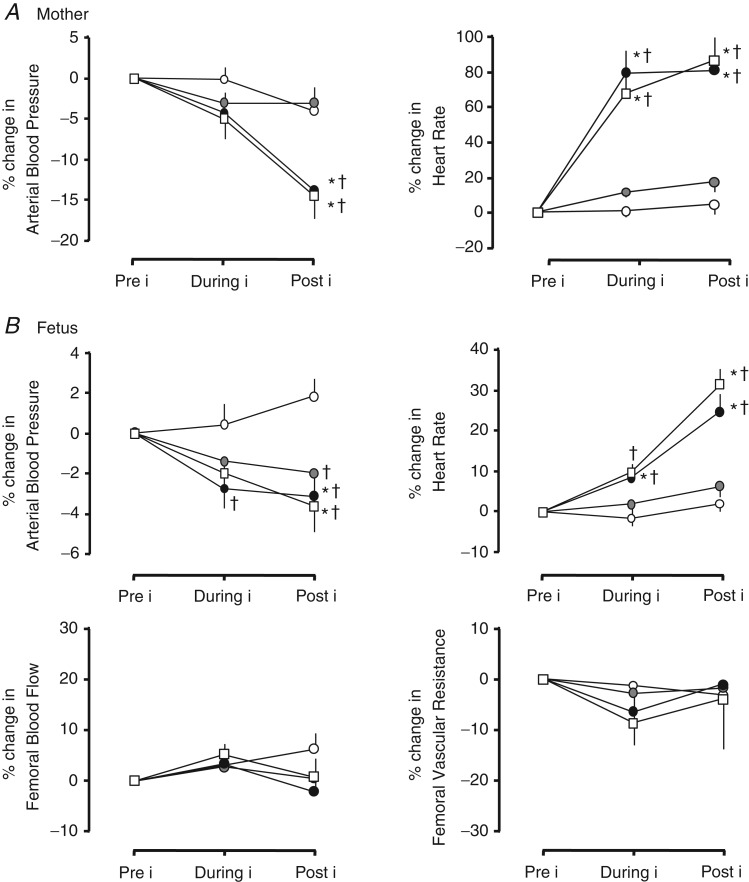
Figure 4.
Values are the means ± SEM for the percentage change in mean cardiovascular variables in the mother (A) and in the fetus (B). These were calculated before (Pre i, 0–20 min), during (During i, 21–50 min) and after (Post i, 51–120 min) maternal infusion with saline vehicle (open circles; n = 11), maternal treatment with the low dose of allopurinol (30 mg kg−1; grey circles; n = 5), maternal treatment with the high dose of allopurinol (150 mg kg−1; filled circles; n = 9) or maternal treatment with the high dose of allopurinol during fetal blockade of NO synthase with the NO clamp (open squares; n = 6). Significant differences (P < 0.05) are: *within group with respect to time period Pre i; † between groups with respect to saline vehicle infusion (two-way repeated measures ANOVA with post hoc Tukey test).
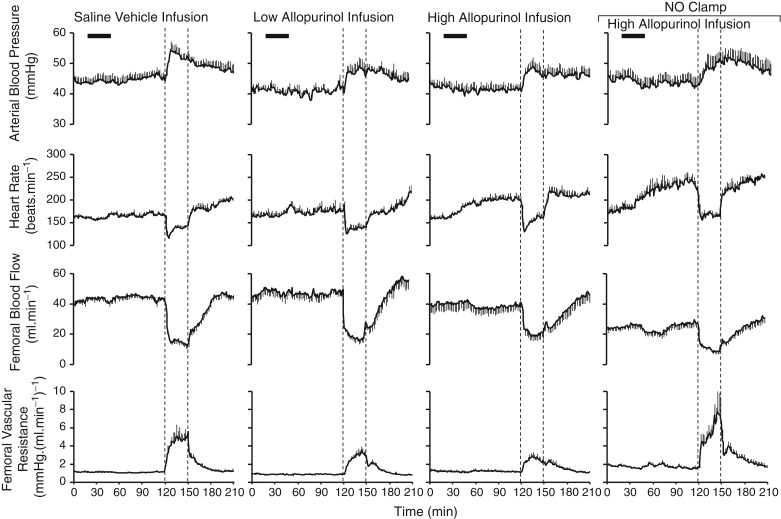
Pre-infusion values for fetal arterial blood pressure, heart rate and femoral vascular resistance were similar in all fetuses (Fig. 3). Maternal infusion with the low or high dose of allopurinol, with or without the NO clamp, significantly decreased basal fetal arterial blood pressure but only infusion with the high dose of allopurinol, with or without fetal treatment with the NO clamp, significantly increased basal fetal heart rate. Allopurinol treatment at either dose did not affect basal fetal femoral blood flow or fetal femoral vascular resistance (Figs 3 and 4B).
Figure 3.
Values represent the means ± SEM calculated every minute for fetal arterial blood pressure, fetal heart rate, fetal femoral blood flow and fetal femoral vascular resistance during 2 h of normoxia, 0.5 h of hypoxia (dashed lines) and 1 h of recovery for fetuses either during maternal saline vehicle infusion (n = 11), maternal treatment with the low dose of allopurinol (30 mg kg−1; n = 5), maternal treatment with the high dose of allopurinol (150 mg kg−1; n = 9) or maternal treatment with the high dose of allopurinol during fetal blockade of NO synthase with the NO clamp (n = 6).
Effects of allopurinol on maternal and fetal cardiovascular function during hypoxia
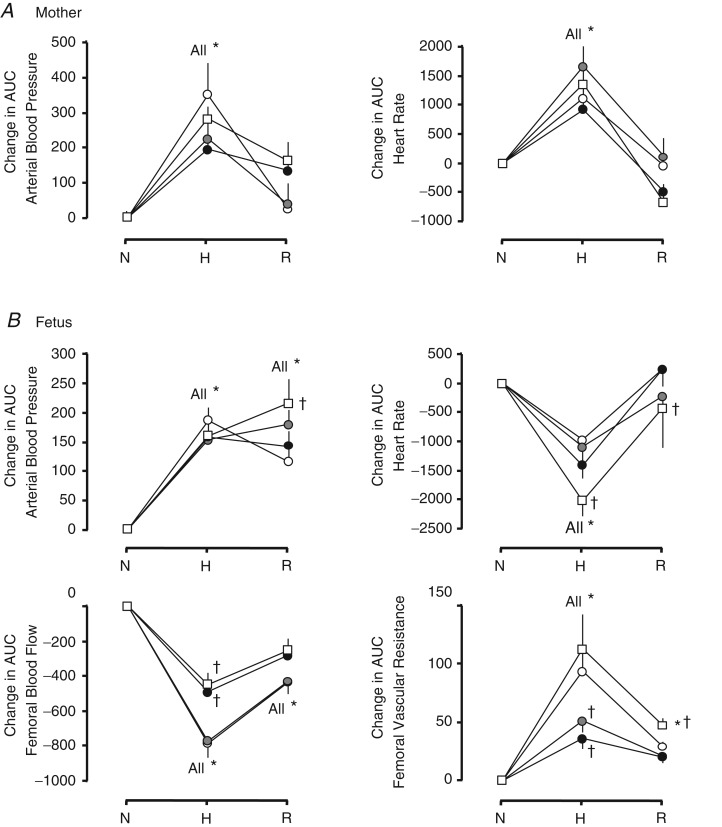
Maternal arterial blood pressure and heart rate increased significantly during acute hypoxia following maternal infusion with vehicle (Figs 2 and 5A). Although treatment with either dose of allopurinol tended to diminish the increment in maternal arterial blood pressure during hypoxia, this failed to reach significance (Fig. 5A). Both maternal arterial blood pressure and heart rate returned to pre-hypoxic levels during recovery across all groups.
Figure 5.
Values are the means ± SEM for the absolute change in the area under the curve (AUC) from normoxic baseline in cardiovascular variables in the mother (A) and in the fetus (B). These were calculated as 30 min epochs during normoxia (N, 90–120 min), hypoxia (H, 121–150 min) and recovery (R, 151–180 min) after maternal infusion with saline vehicle (open circles; n = 11), maternal treatment with the low dose of allopurinol (30 mg kg−1; grey circles; n = 5), maternal treatment with the high dose of allopurinol (150 mg kg−1; filled circles; n = 9) or maternal treatment with the high dose of allopurinol during fetal blockade of NO synthase with the NO clamp (open squares; n = 6). Significant differences (P < 0.05) are: *within group with respect to time period Pre i; †between groups with respect to saline vehicle infusion (two-way repeated measures ANOVA with post hoc Tukey test).
In all groups, acute hypoxia led to a significant increase in fetal arterial blood pressure and femoral vascular resistance and a significant decrease in fetal heart rate and fetal femoral blood flow (Figs 3 and 5B). The increase in arterial blood pressure and the decrease in heart rate were similar across all groups whilst the increase in femoral vascular resistance was markedly diminished in fetuses treated with maternal infusion with allopurinol (Figs 3 and 5B). Fetal treatment with the NO clamp returned the femoral vascular resistance response to control levels in fetuses from mothers infused with the high dose of allopurinol. The increase in fetal arterial blood pressure was sustained in all groups during the recovery period (Figs 3 and 5B). In contrast, fetal heart rate, femoral blood flow and femoral vascular resistance returned to pre-hypoxic values.
Fetal plasma catecholamines
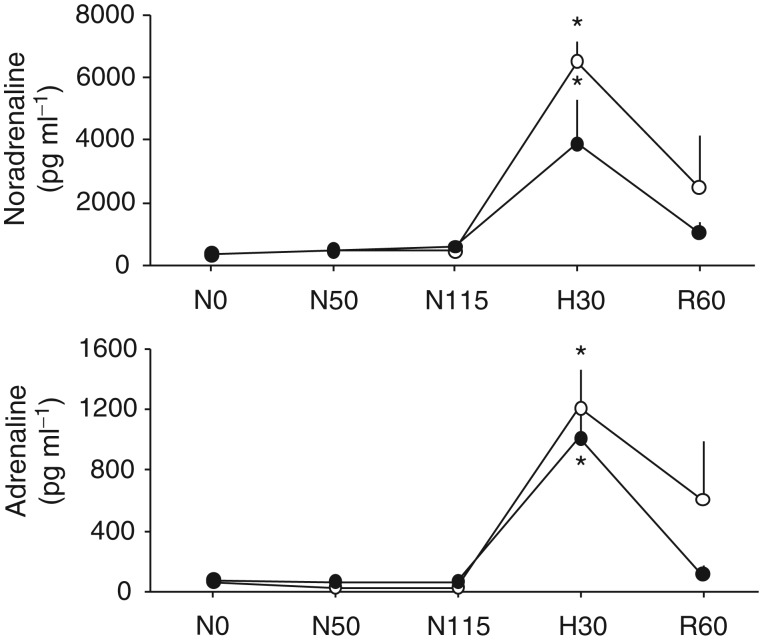
As increases in fetal plasma catecholamines contribute to the femoral vasoconstrictor response to acute hypoxia (Fletcher et al. 2000), it was of interest to determine if maternal allopurinol affected this response in the fetal circulation. Plasma noradrenaline and adrenaline showed a significant increase from baseline concentrations during acute hypoxia. These elevations in fetal plasma catecholamines were not affected by maternal treatment with the high dose of allopurinol (Fig. 6).
Figure 6.
Values represent the means ± SEM for fetal plasma adrenaline and noradrenaline at 0 (Pre i), 50 (During i) and 115 (Post i) min of normoxia, at 30 min of hypoxia (H) and at 60 min of recovery (R) for fetuses exposed to 0.5 h of hypoxia either during maternal saline vehicle infusion (n = 5) or maternal treatment with the high dose of allopurinol (150 mg kg−1; n = 5).
Maternal and fetal plasma uric acid
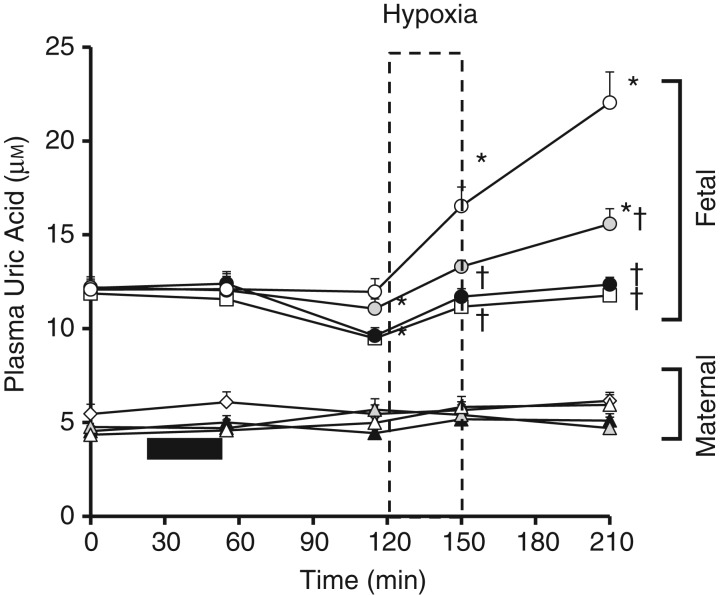
Pre-infusion maternal uric acid levels were similar in all groups and were not significantly altered by allopurinol infusion or hypoxia (Fig. 7). Fetal plasma uric acid levels were higher than maternal levels during the pre-infusion period. Hypoxia increased fetal plasma uric acid levels and there was a continued increase during the recovery period (Fig. 7). In contrast, maternal plasma uric acid remained unaltered from baseline during acute hypoxia. Maternal allopurinol treatment led to a decrease in fetal plasma basal uric acid levels when given at 150 mg kg−1, with and without the fetal NO clamp (Fig. 7). Both low and high doses of maternal allopurinol led to a dose-dependent inhibition of the increase in fetal plasma uric acid measured during acute hypoxia and recovery.
Figure 7.
Values are the means ± SEM for maternal (triangles/diamonds) and fetal (circles/squares) plasma uric acid during the hypoxia protocol after maternal infusion with saline vehicle (open triangles and circles; n = 5), maternal treatment with the low dose of allopurinol (30 mg kg−1; grey triangles and circles; n = 5), maternal treatment with the high dose of allopurinol (150 mg kg−1; filled triangles and circles; n = 5) or maternal treatment with the high dose of allopurinol during fetal blockade of NO synthase with the NO clamp (open diamonds and squares; n = 5). Significant differences (P < 0.05) are: *within group and †between groups with respect to saline vehicle infusion (two-way repeated measures ANOVA with post hoc Tukey test).
Discussion
This study tested the hypothesis that XO has a role in the regulation of fetal cardiovascular function during acute hypoxia. The principal findings of the study show that maternal treatment with allopurinol significantly diminished the rise in fetal plasma uric acid and the fetal femoral vasoconstrictor, hyperglycaemic and lactic acidaemic responses to acute hypoxia. The effects of maternal allopurinol on fetal femoral vascular resistance, glucose and lactate concentrations in fetal blood were prevented by fetal in vivo NO blockade. Therefore, the data support the hypothesis that enhanced NO bioavailability is involved in mediating the effects of maternal allopurinol treatment on fetal cardiovascular function during acute hypoxia.
The fetal defence to acute hypoxia is largely contingent on fetal cardiovascular responses, which have been well characterized. This fetal cardiovascular defence includes bradycardia and peripheral vasoconstriction (Cohn et al. 1974; Giussani et al. 1993). The latter aids the redistribution of the fetal combined ventricular output away from less essential vascular beds to maintain oxygen and nutrient delivery to the brain: the so-called brain sparing effect (Rudolph, 1985; Giussani & Davidge, 2013). The physiology underlying this response is also well delineated. The fetal bradycardia and peripheral vasoconstriction are triggered exclusively by a carotid body chemoreflex (Bartelds et al. 1993; Giussani et al. 1993). Release of hormones, such as catecholamines, into the fetal circulation maintain the neurally triggered peripheral vasoconstriction (Fletcher et al. 2000) and return fetal heart rate back to basal levels, opposing the enhanced vagal tone (Giussani et al. 1993). The neural and endocrine peripheral vasoconstriction is further fine-tuned by an oxidant tone, created by the interaction between •O2− and NO during acute hypoxia, whereby a fall in the ratio favours dilatation and an increase enhances constriction (Morrison et al. 2003; Thakor et al. 2010a,b; Herrera et al. 2012; Kane et al. 2012). Data in the present study show that maternal treatment with low and high doses of allopurinol markedly diminished the fetal peripheral vasoconstrictor response to acute hypoxia without affecting fetal bradycardia and that this haemodynamic effect was prevented by fetal in vivo NO blockade. Therefore, the data in this study support that activation of XO contributes to the femoral vasoconstrictor response during acute hypoxia by altering the peripheral vascular oxidant tone. Hence, maternal treatment with allopurinol shifts the ratio between •O2− and NO towards dilatation, opposing chemoreflex and endocrine vasoconstrictor influences on the fetal femoral vascular bed. The normalization of the magnitude of the femoral vasoconstrictor response to acute hypoxia in the presence of fetal in vivo NO blockade during maternal treatment with allopurinol confirms this mechanism as being involved.
The activation of XO in the fetus during hypoxia is, in itself, intriguing. Clearly, the production of uric acid is dependent upon XO being in the active state and the supply of both hypoxanthine and molecular oxygen as reactants (Berry & Hare, 2004). Whilst enzymes dependent upon oxygen may decrease their activity during hypoxia (e.g. prolyl hydroxylase; Majmundar et al. 2010), the fact that hypoxic conditions increase the availability of hypoxanthine as a key substrate to XO may explain the increase in uric acid levels, and XO activity during hypoxic conditions (Berry & Hare, 2004). The further rise in uric acid during the recovery period may be explained by re-oxygenation providing further reactants for XO. Furthermore, the prevention of an increase in uric acid levels in the allopurinol treatment group supports an increase in the activity of XO.
The increase in fetal blood glucose concentrations during acute hypoxia results from an inhibition in glucose uptake and utilization by peripheral tissues coupled with stimulation of hepatic glucose production (Jones, 1977; Jones & Ritchie, 1983). The fetal lactic acidaemia response to acute hypoxia principally arises from the anaerobic metabolism of glucose in hypoxic fetal tissues, in particular the hind limbs in which blood flow is markedly reduced (Boyle et al. 1990). As well as helping in the redistribution of blood flow away from less essential vascular beds towards the fetal brain, the peripheral vasoconstriction also markedly decreases oxygen consumption in the fetus, as the latter is exquisitely coupled to oxygen delivery (Boyle et al. 1990). As fetal treatment with phentolamine prevented the glycaemic response but enhanced insulin secretion during acute hypoxia (Jones et al. 1983), and because infusion of catecholamines increased glucose output in the sheep fetus (Apatu & Barnes, 1991), both the reduction in insulin-dependent glucose uptake and the increase in glucose production by the fetal tissues may be mediated via neural and endocrine adrenergic pathways. Depression of the glycaemic response to acute hypoxia following fetal exposure to allopurinol in this study may therefore represent an effect on insulin release and/or on the glucogenic pathways mediated either via the neural sympathetic or plasma amine activities. Indeed, allopurinol has been reported to depress the hyperglycaemia of haemorrhagic shock via similar mechanisms (Salles et al. 1972). The depressed circulating lactate concentrations during acute hypoxia in the fetus following exposure to allopurinol in the present study may have resulted from the diminished increased in blood glucose availability (Lawrence et al. 1982) and/or from the decreased production of lactate by the fetal hind limbs (Boyle et al. 1990). Reversal of the depressive effects of allopurinol on the fetal lactic acidaemic response to acute hypoxia following fetal NO blockade may thus result from the restoration towards control levels of the hyperglycaemic response and/or the femoral vasoconstrictor response during acute hypoxia in the fetus.
One could argue that inhibition of the fetal peripheral vasoconstrictor response to acute hypoxia following exposure to XO inhibition may be mediated via depressed chemo-transduction mechanisms within the carotid body and/or due to reduced activation of endocrine constrictor responses, such as the increase in plasma catecholamine levels in the fetus during acute hypoxia. However, in the present study, we further show that maternal treatment with even very high doses of allopurinol did not affect the magnitude of the increase in fetal plasma catecholamine concentrations during acute hypoxia. Inhibition of the fetal femoral vasoconstriction and depression of the fetal glycaemic response during acute hypoxia as a consequence of an effect of allopurinol on plasma amine activities is therefore not supported. Similarly, dissociation between a lack of an effect of allopurinol on the fetal bradycardia, which persists during acute hypoxia, and inhibition of the fetal femoral vasoconstriction during the same time period does not support an effect of allopurinol at the level of the carotid chemoreflex, as both fetal bradycardia and fetal femoral vasoconstriction are triggered by the same carotid body chemoreflex (Bartelds et al. 1993; Giussani et al. 1993). Rather, these additional findings further support an affect of allopurinol acting to alter the local oxidant tone at the level of the fetal peripheral vasculature. Accordingly, we have reported that fetal treatment with other antioxidants, such as vitamin C, or other agents that increase NO bioavailability, such as statins, has a similar effect on the fetal peripheral vascular oxidant tone, shifting the ratio towards dilatation via NO-dependent pathways, and impairing the redistribution of blood flow away from peripheral circulations during acute hypoxia in the fetus (Thakor et al. 2010b; Kane et al. 2012).
A hypotensive effect of allopurinol during basal conditions supports the idea that the cellular oxidant milieu also plays a tonic contribution to peripheral vascular resistance and that, under basal conditions, XO-derived •O2− is involved in arterial blood pressure maintenance. The mechanisms mediating the tachycardic responses to allopurinol in either the mother or the fetus are less clear. The dissociation between the magnitude and timing of the depressor and cardiac responses both suggest that baroreflex activation is an unlikely contributing mechanism increasing heart rate. A more likely explanation is that allopurinol has direct chronotropic effects. Studies have reported that allopurinol increases myocardial contractility (Ekelund et al. 1999; Cappola et al. 2001; Kögler et al. 2003). We have also reported that the tachycardic response to allopurinol during basal conditions in late gestation fetal sheep can be prevented by fetal treatment with the β1-adrenergic antagonist atenolol, suggesting that allopurinol may enhance sympathetic influences on the heart (Herrera et al. 2012).
In summary, data in the present study show that maternal treatment with low and high doses of allopurinol induces significant effects on maternal and fetal cardiovascular function not only during basal conditions but also in response to acute hypoxia. The data are not only of significance to the understanding of the physiological control of the fetal cardiovascular system during acute hypoxic stress, but they are also of particular clinical relevance in the context of ongoing trials in which allopurinol is being administered to pregnant women when their unborn child shows signs of hypoxic distress (Kaandorp et al. 2010, 2012).
Key points
Periods of impaired oxygenation or acute hypoxia in the fetus can be common during labour and how the fetus withstands these challenges is of interest.
During hypoxia, the fetus shunts blood flow away from peripheral and towards essential vascular beds: the so called brain-sparing effect.
Part of the peripheral vasoconstriction is driven by reactive oxygen species (ROS) that inactivate nitric oxide (NO), thereby limiting its vasodilator action.
Here, we investigate the source of ROS generation contributing to fetal peripheral vasoconstriction during hypoxia, and show that xanthine oxidase (XO) is fundamentally involved. Fetal exposure to the XO inhibitor allopurinol markedly diminished the peripheral vasoconstriction during hypoxia via NO-dependent mechanisms.
The data increase our understanding of the physiological control of fetal cardiovascular function during stress. The findings are also of significant clinical relevance as allopurinol is being administered to pregnant women in clinical obstetric trials.
Acknowledgments
We are thankful to Mr Scott Gentle and Mrs Sue Nicholls for their invaluable help with the maintenance of the animals. D.A.G. is Professor of Cardiovascular Developmental Physiology & Medicine at the Department of Physiology, Development & Neuroscience at the University of Cambridge, Professorial Fellow and Director of Studies in Medicine at Gonville & Caius College, a Lister Institute Fellow and a Royal Society Wolfson Research Merit Award Holder.
Glossary
- ABE
acid base excess
- AUC
area under the curve
- HPLC
high-performance liquid chromatography
- pHa
arterial pH
- ROS
reactive oxygen species
- XO
xanthine oxidase.
Translational perspective.
Fetal hypoxia is common in high risk pregnancy and complicated birth and can have serious consequences for the development of the neonate, in particular its brain. Recent basic science studies have hypothesized that antioxidants such as the xanthine oxidase inhibitor allopurinol may be neuroprotective in labour complicated by fetal hypoxic distress. In support of this hypothesis, maternal antenatal treatment with allopurinol reduced hippocampal brain damage after repeated episodes of acute birth asphyxia in late gestation fetal sheep (Kaandorp et al. 2013). Recent clinical studies have expanded on this and other studies to raise the hypothesis that maternal treatment with allopurinol may therefore be neuroprotective in human labour complicated by clinical signs of fetal hypoxic distress (van Bel et al. 1998; Benders et al. 2006; Kaandorp et al. 2010; Kaandorp et al. 2012). However, in a separate line of investigation, we have made the discovery that antioxidants, by quenching free radicals and increasing NO bioavailability, can also markedly impair the fetal peripheral vasoconstrictor response to acute episodes of hypoxia: part of the fetal brain sparing effect during hypoxia (Thakor et al. 2010b; Kane et al. 2012). Therefore, any fetal neuroprotective benefit of maternal treatment with allopurinol in the clinical setting may be offset by a compromised fetal circulatory response. However, the effect of maternal treatment with allopurinol on the fetal cardiovascular defence to hypoxia had not been investigated. In the present basic science study we provide this evidence to give insight into the cost–benefit interaction of maternal treatment with antioxidants during fetal hypoxic stress. The data show that maternal treatment with allopurinol in low doses that can be extrapolated to the human clinical situation does indeed impair the fetal circulatory defence response to hypoxia. Maternal treatment with allopurinol in much higher doses makes this effect worse, almost abolishing the fetal peripheral vasoconstrictor response to hypoxia. More is not better. Additional data show that the mechanism of suppression of the fetal circulatory response to hypoxia is via increasing NO bioavailability as fetal in vivo NO blockade reversed the effect of maternal treatment with allopurinol. Collectively, past and present evidence on the effects of allopurinol on the fetus are of mixed clinical implications. Beneficial effects of maternal treatment with allopurinol are supported by its protective effects on umbilical blood flow (Derks et al. 2010; Herrera et al. 2012), and on the fetal heart (Derks et al. 2010) and fetal brain (Kaandorp et al. 2013) during and following periods of ischaemia and reperfusion. Detrimental consequences on the fetus of maternal treatment with allopurinol are supported by its effects in impairing fetal peripheral vascular reactivity to constrictor agonists (Herrera et al. 2012) and on the fetal cardiovascular defence to acute hypoxia (present work). As highlighted in a recent editorial (Peebles, 2012), studies that include parallel in vivo measurement of peripheral and cerebral blood flow in the fetus during acute hypoxia or asphyxia in the presence of antioxidant treatment in doses that are administered to humans with subsequent analysis of indices of brain damage have now become indispensable before any potential cost–benefit assessment of fetal antioxidant therapy can be determined. Until then, the therapeutic use of allopurinol or of any other antioxidant in clinical obstetric practice should be approached with caution.
Author contributions
Competing interests
The authors confirm that there are no conflicts of interest.
Author contributions
The experiments in this study were performed in the Department of Physiology, Development and Neuroscience, University of Cambridge. J.A.H., A.D.K., E.A.H. and D.A.G. conceived and designed the experiments. J.A.H., A.D.K., E.A.H., B.J.A., Y.N., K.L.B., J.J.K., J.B.D. and D.A.G. collected, analysed and interpreted the experimental data. J.A.H., A.D.K., E.A.H., J.B.D. and D.A.G. drafted the article and revised it critically for important intellectual content. All authors have read and approved the manuscript.
Funding
This work was supported by the British Heart Foundation, the Biotechnology and Biological Sciences Research Council, UK.
References
- Apatu RS, Barnes RJ. Release of glucose from the liver of fetal and postnatal sheep by portal vein infusion of catecholamines or glucagon. J Physiol. 1991;436:449–468. doi: 10.1113/jphysiol.1991.sp018560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartelds B, van Bel F, Teitel D, Rudolph A. Carotid, not aortic, chemoreceptors mediate the fetal cardiovascular response to acute hypoxemia in lambs. Pediatr Res. 1993;34:51–55. doi: 10.1203/00006450-199307000-00013. [DOI] [PubMed] [Google Scholar]
- Benders M, Bos A, Rademaker C, Rijken M, Torrance H, Groenendaal F, van Bel F. Early postnatal allopurinol does not improve short term outcome after severe birth asphyxia. Arch Dis Child Fetal Neonatal Ed. 2006;91:163–165. doi: 10.1136/adc.2005.086652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry C, Hare M. Xanthine oxidoreductase and cardiovascular disease: molecular mechanisms and pathophysiological implications. J Physiol. 2004;555:589–606. doi: 10.1113/jphysiol.2003.055913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boda D, Nemeth P, Kiss P, Orvos H. Treatment of mothers with allopurinol to produce therapeutic blood levels in newborns. Prenat Neonat Med. 1999;4:130–134. [Google Scholar]
- Boyle D, Hirst K, Zerbe G, Meschia G, Wilkening R. Fetal hind limb oxygen consumption and blood flow during acute graded hypoxia. Pediatr Res. 1990;28:94–100. doi: 10.1203/00006450-199008000-00004. [DOI] [PubMed] [Google Scholar]
- Cappola TP, Kass DA, Nelson GS, Berger RD, Rosas GO, Kobeissi ZA, Marbán E, Hare JM. Allopurinol improves myocardial efficiency in patients with idiopathic dilated cardiomyopathy. Circulation. 2001;104:2407–2411. doi: 10.1161/hc4501.098928. [DOI] [PubMed] [Google Scholar]
- Castelli P, Condemi A, Brambillasca C, Fundarò P, Botta M, Lemma M, Vanelli P, Santoli C, Gatti S, Riva E. Improvement of cardiac function by allopurinol in patients undergoing cardiac surgery. J Cardiovasc Pharmacol. 1995;25:119–125. doi: 10.1097/00005344-199501000-00019. [DOI] [PubMed] [Google Scholar]
- Clancy RR, McGaurn SA, Goin JE, Hirtz DG, Norwood WI, Gaynor JW, Jacobs ML, Wernovsky G, Mahle WT, Murphy JD, Nicolson SC, Steven JM, Spray TL. Allopurinol neurocardiac protection trial in infants undergoing heart surgery using deep hypothermic circulatory arrest. Pediatrics. 2001;108:61–70. doi: 10.1542/peds.108.1.61. [DOI] [PubMed] [Google Scholar]
- Coghlan J, Flitter W, Clutton S, Panda R, Daly R, Wright G, Ilsley C, Slater T. Allopurinol pretreatment improves postoperative recovery and reduces lipid peroxidation in patients undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg. 1994;107:248–256. [PubMed] [Google Scholar]
- Cohn HE, Sacks EJ, Heymann MA, Rudolph AM. Cardiovascular responses to hypoxemia and acidemia in fetal lambs. Am J Obstet Gynecol. 1974;120:817–824. doi: 10.1016/0002-9378(74)90587-0. [DOI] [PubMed] [Google Scholar]
- Cowan F, Rutherford M, Groenendaal F, Eken P, Mercuri E, Bydder GM, Meiners LC, Dubowitz LM, de Vries LS. Origin and timing of brain lesions in term infants with neonatal encephalopathy. Lancet. 2003;361:736–742. doi: 10.1016/S0140-6736(03)12658-X. [DOI] [PubMed] [Google Scholar]
- Derks J, Oudijk M, Torrance H, Rademaker C, Benders M, Rosen K, Cindrova-Davies T, Thakor A, Visser G, Burton G, van Bel F, Giussani D. Allopurinol reduces oxidative stress in the ovine fetal cardiovascular system following repeated episodes of ischemia-reperfusion. Pediatr Res. 2010;68:374–380. doi: 10.1203/PDR.0b013e3181ef7780. [DOI] [PubMed] [Google Scholar]
- Ekelund UEG, Harrison RW, Shokek O, Thakkar RN, Tunin RS, Senzaki H, Kass DA, Marbán E, Hare JM. Intravenous allopurinol decreases myocardial oxygen consumption and increases mechanical efficiency in dogs with pacing-induced heart failure. Circ Res. 1999;85:437–445. doi: 10.1161/01.res.85.5.437. [DOI] [PubMed] [Google Scholar]
- Fletcher AJ, Edwards CM, Gardner DS, Fowden AL, Giussani DA. Neuropeptide Y in the sheep fetus: effects of acute hypoxemia and dexamethasone during late gestation. Endocrinology. 2000;141:3976–3982. doi: 10.1210/endo.141.11.7770. [DOI] [PubMed] [Google Scholar]
- Gardner DS, Giussani DA. Enhanced umbilical blood flow during acute hypoxemia after chronic umbilical cord compression: a role for nitric oxide. Circulation. 2003;108:331–335. doi: 10.1161/01.CIR.0000080323.40820.A1. [DOI] [PubMed] [Google Scholar]
- Giussani DA, Davidge ST. Developmental programming of cardiovascular disease by prenatal hypoxia. J Dev Orig Health Dis. 2013 doi: 10.1017/S204017441300010X. doi: 10.1017/S204017441300010X http://journals.cambridge.org/action/displayAbstract?fromPage=online&aid=8993851. [DOI] [PubMed] [Google Scholar]
- Giussani DA, Spencer JA, Moore PJ, Bennet L, Hanson MA. Afferent and efferent components of the cardiovascular reflex responses to acute hypoxia in term fetal sheep. J Physiol. 1993;461:431–449. doi: 10.1113/jphysiol.1993.sp019521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera EA, Kane AD, Hansell JA, Thakor AS, Allison BJ, Niu Y, Giussani DA. A role for xanthine oxidase in the control of fetal cardiovascular function in late gestation sheep. J Physiol. 2012;590:1825–1837. doi: 10.1113/jphysiol.2011.224576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iriyama K, Yoshiura M, Iwamoto T, Ozaki Y. Simultaneous determination of uric and ascorbic acids in human serum by reversed-phase high-performance liquid chromatography with electrochemical detection. Anal Biochem. 1984;141:238–243. doi: 10.1016/0003-2697(84)90451-2. [DOI] [PubMed] [Google Scholar]
- Johnson W, Kayser K, Brenowitz J, Saedi S. A randomized controlled trial of allopurinol in coronary bypass surgery. Am Heart J. 1991;121:20–24. doi: 10.1016/0002-8703(91)90950-m. [DOI] [PubMed] [Google Scholar]
- Jones C, Ritchie J, Walker D. The effects of hypoxia on glucose turnover in the fetal sheep. J Dev Physiol. 1983;5:223–235. [PubMed] [Google Scholar]
- Jones CT. The development of some metabolic responses to hypoxia in the foetal sheep. J Physiol. 1977;265:743–762. doi: 10.1113/jphysiol.1977.sp011741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CT, Ritchie J. The effects of adrenergic blockade on fetal response to hypoxia. J Dev Physiol. 1983;5:211–222. [PubMed] [Google Scholar]
- Kaandorp J, Benders M, Rademaker C, Torrance H, Oudijk M, de Haan T, Bloemenkamp K, Rijken M, van Pampus M, Bos A, Porath M, Oetomo S, Willekes C, Gavilanes AD, Wouters M, van Elburg R, Huisjes A, Bakker S, van Meir C, von Lindern J, Boon J, de Boer I, Rijnders R, Jacobs C, Uiterwaal C, Mol BW, Visser G, van Bel F, Derks J. Antenatal allopurinol for reduction of birth asphyxia induced brain damage (ALLO-Trial); a randomized double blind placebo controlled multicenter study. BMC Pregnancy Childbirth. 2010;10:8. doi: 10.1186/1471-2393-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaandorp JJ, Derks JB, Oudijk MA, Torrance HL, Harmsen MG, Nikkels PGJ, van Bel F, Visser GHA, Giussani DA. Antenatal allopurinol reduces hippocampal brain damage after acute birth asphyxia in late gestation fetal sheep. Reprod Sci. 2013 doi: 10.1177/1933719113493516. doi: 10.1177/1933719113493516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaandorp JJ, van Bel F, Veen S, Derks JB, Groenendaal F, Rijken M, Roze E, Venema MMU, Rademaker CM, Bos AF, Benders MJ. Long-term neuroprotective effects of allopurinol after moderate perinatal asphyxia: follow-up of two randomised controlled trials. Arch Dis Child Fetal Neonatal Ed. 2012;97:F162–F166. doi: 10.1136/archdischild-2011-300356. [DOI] [PubMed] [Google Scholar]
- Kane AD, Herrera EA, Hansell JA, Giussani DA. Statin treatment depresses the fetal defence to acute hypoxia via increasing nitric oxide bioavailability. J Physiol. 2012;590:323–334. doi: 10.1113/jphysiol.2011.217968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kögler H, Fraser H, McCune S, Altschuld R, Marbán E. Disproportionate enhancement of myocardial contractility by the xanthine oxidase inhibitor oxypurinol in failing rat myocardium. Cardiovasc Res. 2003;59:582–592. doi: 10.1016/s0008-6363(03)00512-1. [DOI] [PubMed] [Google Scholar]
- Lawrence GF, Brown VA, Parsons RJ, Cooke ID. Feto-maternal consequences of high-dose glucose infusion during labour. Br J Obstet Gynaecol. 1982;89:27–32. doi: 10.1111/j.1471-0528.1982.tb04630.x. [DOI] [PubMed] [Google Scholar]
- Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell. 2010;40:294–309. doi: 10.1016/j.molcel.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masaoka N, Nakajima Y, Hayakawa Y, Ohgame S, Hamano S, Nagaishi M, Yamamoto T. Transplacental effects of allopurinol on suppression of oxygen free radical production in chronically instrumented fetal lamb brains during intermittent umbilical cord occlusion. J Matern Fetal Neonatal Med. 2005;18:1–7. doi: 10.1080/14767050500127716. [DOI] [PubMed] [Google Scholar]
- Matthews J, Altman D, Campbell M, Royston P. Analysis of serial measurements in medical research. BMJ. 1990;300:230–235. doi: 10.1136/bmj.300.6719.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison S, Gardner DS, Fletcher AJ, Bloomfield MR, Giussani DA. Enhanced nitric oxide activity offsets peripheral vasoconstriction during acute hypoxaemia via chemoreflex and adrenomedullary actions in the sheep fetus. J Physiol. 2003;547:283–291. doi: 10.1113/jphysiol.2002.032615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peebles D. Radical change for the fetus. J Physiol. 2012;590:1773. doi: 10.1113/jphysiol.2012.231217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primhak R, Jedeikin R, Ellis G, Makela S, Gillan J, Swyer P, Rowe R. Myocardial ischaemia in asphyxia neonatorum. Electrocardiographic, enzymatic and histological correlations. Acta Paediatr Scand. 1985;74:595–600. doi: 10.1111/j.1651-2227.1985.tb11036.x. [DOI] [PubMed] [Google Scholar]
- Rudolph A. Distribution and regulation of blood flow in the fetal and neonatal lamb. Circ Res. 1985;57:811–821. doi: 10.1161/01.res.57.6.811. [DOI] [PubMed] [Google Scholar]
- Salles M, Tabatabai M, Shahidi H. Blood glucose levels in dogs pretreated with allopurinol during hemorrhagic shock. Am J Physiol. 1972;223:679–681. doi: 10.1152/ajplegacy.1972.223.3.679. [DOI] [PubMed] [Google Scholar]
- Thakor A, Giussani D. Nitric oxide reduces vagal baroreflex sensitivity in the late gestation fetus. Pediatr Res. 2009;65:269–273. doi: 10.1203/PDR.0b013e318193f134. [DOI] [PubMed] [Google Scholar]
- Thakor AS, Herrera EA, Serón-Ferré M, Giussani DA. Melatonin and vitamin C increase umbilical blood flow via nitric oxide-dependent mechanisms. J Pineal Res. 2010a;49:399–406. doi: 10.1111/j.1600-079X.2010.00813.x. [DOI] [PubMed] [Google Scholar]
- Thakor AS, Richter HG, Kane AD, Dunster C, Kelly FJ, Poston L, Giussani DA. Redox modulation of the fetal cardiovascular defence to hypoxaemia. J Physiol. 2010b;588:4235–4247. doi: 10.1113/jphysiol.2010.196402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bel F, Shadid M, Moison R, Dorrepaal C, Fontijn J, Monteiro L, van de Bor, Berger H. Effect of allopurinol on postasphyxial free radical formation, cerebral hemodynamics, and electrical brain activity. Pediatrics. 1998;101:185–193. doi: 10.1542/peds.101.2.185. [DOI] [PubMed] [Google Scholar]
- van Dijk A, ParviziI N, Taverne M, Fink-Gremmels J. Placental transfer and pharmacokinetics of allopurinol in late pregnant sows and their fetuses. J Vet Pharmacol Ther. 2008;31:489–495. doi: 10.1111/j.1365-2885.2008.00976.x. [DOI] [PubMed] [Google Scholar]