Abstract
Purinergic control of vascular tone in the CNS has been largely unexplored. This study examines the contribution of endogenous extracellular ATP, acting on vascular smooth muscle cells, in controlling vascular tone in the in vivo rat retina. Retinal vessels were labelled by i.v. injection of a fluorescent dye and imaged with scanning laser confocal microscopy. The diameters of primary arterioles were monitored under control conditions and following intravitreal injection of pharmacological agents. Apyrase (500 units ml−1), an ATP hydrolysing enzyme, dilated retinal arterioles by 40.4 ± 2.8%, while AOPCP (12.5 mm), an ecto-5′-nucleotidase inhibitor that increases extracellular ATP levels, constricted arterioles by 58.0 ± 3.8% (P < 0.001 for both), demonstrating the importance of ATP in the control of basal vascular tone. Suramin (500 μm), a broad-spectrum P2 receptor antagonist, dilated retinal arterioles by 50.9 ± 3.7% (P < 0.001). IsoPPADS (300 μm) and TNP-ATP (50 μm), more selective P2X antagonists, dilated arterioles by 41.0 ± 5.3% and 55.2 ± 6.1% respectively (P < 0.001 for both). NF023 (50 μm), a potent antagonist of P2X1 receptors, dilated retinal arterioles by 32.1 ± 2.6% (P < 0.001). A438079 (500 μm) and AZ10606120 (50 μm), P2X7 antagonists, had no effect on basal vascular tone (P = 0.99 and P = 1.00 respectively). In the ex vivo retina, the P2X1 receptor agonist α,β-methylene ATP (300 nm) evoked sustained vasoconstrictions of 18.7 ± 3.2% (P < 0.05). In vivo vitreal injection of the gliotoxin fluorocitrate (150 μm) dilated retinal vessels by 52.3 ± 1.1% (P < 0.001) and inhibited the vasodilatory response to NF023 (50 μm, 7.9 ± 2.0%; P < 0.01). These findings suggest that vascular tone in rat retinal arterioles is maintained by tonic release of ATP from the retina. ATP acts on P2X1 receptors, although contributions from other P2X and P2Y receptors cannot be ruled out. Retinal glial cells are a possible source of the vasoconstricting ATP.
Introduction
Blood flow in the brain is regulated by the tone of cerebral arteries and arterioles. Vascular tone, defined as the degree of vessel constriction, is controlled by a number of mechanisms. These include extrinsic innervation from the autonomic nervous system, intrinsic innervation from distant subcortical neurons and cortical interneurons, and the local release of vasoactive agents from vascular cells and glial cells (Hamel, 2006). A number of vasoactive agents modulate cerebrovascular tone, including noradrenaline, released by sympathetic terminals and by locus coeruleus neurons (Bekar et al. 2012); serotonin, released by raphe nucleus neurons (Cohen et al. 1996); and neuropeptides, released from local interneurons (Cauli et al. 2004). The primary dilatory influences on vessels are nitric oxide, derived from endothelial cells, autonomic nitrergic nerves and brain neurons (Toda et al. 2009); acetylcholine, released by basal forebrain neurons (Hamel, 2004); and prostaglandins from vascular endothelial cells, neurons and glial cells (Attwell et al. 2010).
ATP, which is released from subcortical sympathetic neurons (Burnstock, 2007), local neurons (Piet & Jahr, 2007) and glial cells (Pascual et al. 2005) may also contribute to the vascular tone of cerebral vessels. ATP exerts both vasoconstricting and vasodilating influences acting on two types of P2 receptors, ligand-gated P2X cation channels and G-protein-coupled P2Y receptors (Lewis et al. 2000; Horiuchi et al. 2001). To date, there have been few reports addressing whether ATP contributes to the basal tone of cerebral vessels. However, it is known that ATP, when co-released with noradrenaline from perivascular sympathetic nerves, acts on vascular smooth muscle cell P2X1 receptors to transiently constrict vessels (Burnstock, 2007).
The retina offers unique advantages in studying the purinergic control of vascular tone. The intrinsic retinal and brain neurovascular units share many anatomical and physiological features (Kur et al. 2012) and the retinal vasculature can be imaged non-invasively through the optics of the eye. The intrinsic retinal vessels supply the inner two-thirds of the retina. A second vascular bed, the choroidal vessels, supplies the outer one-third, principally the photoreceptors (Kur et al. 2012). Owing to lack of extrinsic sympathetic innervation of the intrinsic retinal vasculature (Ye et al. 1990), vessel tone in the retina is controlled solely by autoregulatory mechanisms and by local release of vasoactive agents from neurons, glia and vascular cells.
Little is known about the factors and mechanisms that control the tone of retinal vessels, although nitric oxide and prostaglandin release has been shown to reverse vascular tone in animal and human studies (Brazitikos et al. 1993; Dorner et al. 2003). Müller cells, the principal glial cells of the retina (Newman & Reichenbach, 1996), release ATP (Newman, 2001) and retinal vessels constrict in response to ATP application (Kawamura et al. 2003; Scholfield et al. 2007), suggesting that ATP might contribute to the basal tone of retinal vessels.
We have now investigated whether purinergic signalling contributes to the maintenance of vascular tone in the intrinsic retinal vasculature. We find that reducing endogenous ATP levels by enzyme degradation and inhibiting purinergic signalling with P2X receptor antagonists decreases the tone of retinal arterioles. Conversely, raising ATP levels increases vessel tone. In addition, poisoning retinal glial cell metabolism results in the dilatation of retinal vessels. These results suggest that tonically released ATP acting on vascular smooth muscle cells generates tone in retinal arterioles. Retinal glial cells are a probable source of the ATP.
Methods
Studies were conducted in accordance with guidelines of the Institutional Animal Care and Use Committee of the University of Minnesota (approval no. 1004A80712). Male Long Evans rats (7–10 weeks old, 250–350 g) were purchased from Harlan Laboratories, Inc., Indianapolis, IN, USA. They were housed in rooms maintained on a 14:10 h light/dark cycle at 22°C. Animals were provided with Harlan Rodent Diet and water ad libitum. A maximum of three rats were housed in one cage.
In vivo rat preparation
The in vivo rat preparation has been described previously (Srienc et al. 2012). Briefly, the initial surgery was performed under isoflurane anaesthesia (2% in 30% O2/70% N2). Cannulas were placed in the left femoral vein and artery for drug administration and monitoring of blood pressure, respectively, and a tracheotomy was performed for artificial ventilation. Rats were secured firmly in a stereotaxic holder and a metal ring was sutured to the conjunctiva to stabilize the right eye. The pupil was dilated with 1% atropine sulphate (Alcon Laboratories, Fort Worth, TX, USA). A plano-concave contact lens (5.4 mm fundus lens; Ocular Instruments, Bellevue, WA, USA) was placed over the cornea to neutralize the refractive properties of the eye (Fig. 1A). Gonioscopic prism solution was introduced between the contact lens and the cornea to prevent the cornea from drying out (Wilson Ophthalmic, Mustang, OK, USA). Upon completion of the surgery, rats were gradually transitioned from isoflurane to α-chloralose anaesthesia and maintained under this regimen for the duration of the experiment. α-chloralose anaesthesia was induced and maintained by i.v. administration of α-chloralose–HBC complex (800 mg kg−1 bolus and 550 mg kg−1 h−1 continuous infusion).
Figure 1.
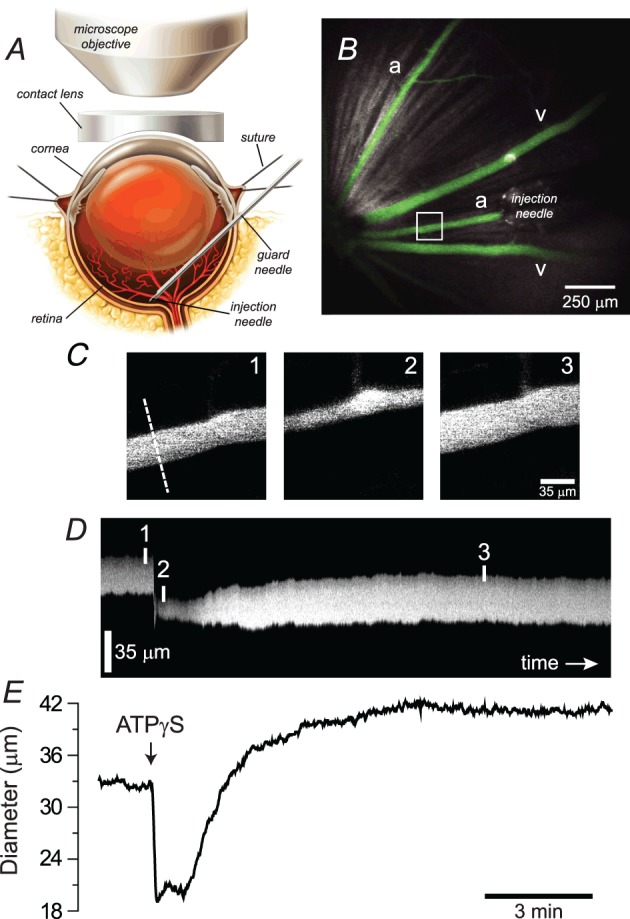
A, in vivo rat preparation. The retina is imaged through an upright microscope and a contact lens placed over the cornea. A hypodermic needle is advanced through the sclera into the vitreous humour and serves as a guard needle for the injection needle. B, low-magnification confocal image of the retinal surface showing arterioles (a) and venules (v) filled with a fluorescent dye and the injection needle positioned over an arteriole. C, high-magnification images of the boxed region in B showing the arteriole before, during and after injection of ATPγS. The dashed line indicates the position at which the vessel diameter was measured. D, line scan image showing the change in diameter of the arteriole as a function of time. The vertical lines indicate the times at which the images in (C) were captured. E, arteriole diameter as a function of time following intravitreal injection of ATPγS (10 μm). The time axes in D and E are aligned.
During experiments, animals were artificially ventilated (35–50 breaths per min; CWE SAR-830-P, Ardmore PA, USA) with a mixture of O2 and N2 (30%/70%). Neuromuscular blockade was provided by gallamine triethiodide (20 mg kg−1 bolus, maintained at a rate of 20 mg kg−1 h−1, i.v.) to minimize eye movements. Mean arterial blood pressure (Pressure Monitor BP-1; World Precision Instruments, Sarasota, FL, USA), end-tidal CO2 (microCapStar; CWE, Ardmore, PA, USA) and blood O2 saturation levels (MouseOx; Starr Life Sciences Corp., Oakmount, PA, USA) were monitored continuously. These parameters were employed to ensure adequate depth of the anaesthesia throughout the experiment. Blood 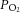 ,
, 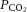 and pH were sampled periodically (Radiometer, ABL 800 Flex, WestLake, OH, USA), and maintained within physiological norms (Tables 1 and 2). Blood pressure, measured before intravitreal injection of pharmacological agents and after injection, when the steady-state diameter of arterioles was measured, did not vary significantly (Table 1). Core body temperature was monitored and maintained at 37°C (TC-1000 Temperature Controller; CWE, Ardmore, PA, USA). Following experiments, animals were killed by injection of 40 mm KCl (1 ml kg−1, i.v.).
and pH were sampled periodically (Radiometer, ABL 800 Flex, WestLake, OH, USA), and maintained within physiological norms (Tables 1 and 2). Blood pressure, measured before intravitreal injection of pharmacological agents and after injection, when the steady-state diameter of arterioles was measured, did not vary significantly (Table 1). Core body temperature was monitored and maintained at 37°C (TC-1000 Temperature Controller; CWE, Ardmore, PA, USA). Following experiments, animals were killed by injection of 40 mm KCl (1 ml kg−1, i.v.).
Table 1.
Blood pressure of rat preparation
| Baseline BP | BP after intravitreal injection(s) | |
|---|---|---|
| 3 μl saline vehicle (n = 5) | 127 ± 5 | 129 ± 6 |
| 10 μl saline vehicle (n = 5) | 117 ± 5 | 119 ± 5 |
| Fluorocitrate vehicle (n = 3) | 120 ± 5 | 122 ± 7 |
| Apyrase (n = 4) | 122 ± 8 | 123 ± 7 |
| AOPCP (n = 3) | 110 ± 8 | 108 ± 9 |
| ATPγS (n = 3) | 110 ± 8 | 113 ± 12 |
| Suramin (n = 3) | 128 ± 3 | 126 ± 3 |
| isoPPADS (n = 3) | 115 ± 8 | 114 ± 5 |
| TNP-ATP (n = 4) | 102 ± 6 | 105 ± 6 |
| NF023 (n = 4) | 125 ± 4 | 120 ± 4 |
| A438079 (n = 3) | 129 ± 5 | 127 ± 5 |
| AZ10606120 (n = 3) | 124 ± 4 | 122 ± 6 |
| Fluorocitrate (n = 4) | 119 ± 4 | 121 ± 3 |
| Fluorocitrate, endothelin-1 and NF023 (n = 4) | 126 ± 3 | 124 ± 3 |
A430879, 3-[[5-(2,3-dichlorophenyl)-1H-tetrazol-1-yl]methyl]pyridine; AOPCP, α,β-methylene ADP; AZ10606120, N-[2-[[2-[(2-hydroxyethyl)amino]ethyl]amino]-5-quinolinyl]-2-tricyclo[3.3.1.13,7]dec-1-ylacetamide dihydrochloride; BP, blood pressure; isoPPADS, pyridoxalphosphate-6-azophenyl-2′,5′disulphonic acid; NF023, 8,8′-[carbonylbis(imino-3,1-phenylenecarbonylimino)]bis-1,3,5-naphthalene-trisulphonic; TNP-ATP, 2′,3′-O-(2,4,6-trinitrophenyl)-ATP. Mean arterial BP in mmHg. BP was measured before intravitreal injection and after injection, when steady-state arteriole diameter measurements were made. For all experiments, there were no significant differences in BP before and after injection(s).
Table 2.
Physiological parameters of rat preparation
| Physiological parameter | Mean ± s.e.m. |
|---|---|
| BP | 120 ± 2 mmHg |
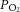 |
110 ± 2 mmHg |
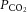 |
29 ± 1 mmHg |
| pH | 7.40 ± 0.01 |
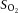 |
95.1 ± 0.3% |
BP, mean arteriole blood pressure; 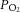 , O2 partial pressure;
, O2 partial pressure; 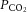 , CO2 partial pressure;
, CO2 partial pressure; 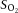 , arteriole O2 saturation. n = 51 animals.
, arteriole O2 saturation. n = 51 animals.
Blood vessel labelling and diameter measurements
Retinal vessels were labelled by injection of a fluorescent dye (Fig. 1B), either fluorescein- or rhodamine B-isothiocyanate, conjugated to high-molecular-weight dextran (2000 kDa and 70 kDa respectively; Sigma, St. Louis, MO, USA) dissolved in saline (1–2 ml of 1.5% solution, infused over 5 min, i.v.). Cromolyn (20 mg kg−1) was administered i.v. to prevent dextran-evoked mast cell activation (Berstad, 1982; Blom et al. 2004). The retina was imaged through the cornea and lens with an Olympus FluoView 1000 confocal scanning laser microscope equipped with a UPlanSApo 4× dry objective, 0.16 NA (Fig. 1A and B). Fluorescein and rhodamine B dyes were excited at 488 and 559 nm respectively. Arterioles were distinguished from venules based on their morphology, i.e. caliber and branching pattern, and based on their filling pattern following i.v. injection of dye – retinal arterioles filled before venules. The luminal diameters of first-order arterioles were measured from line scans of sequences of high-power (0.81 μm per pixel) retinal images (Fig. 1C–E) using a custom MatLab algorithm. Image acquisition was limited to small regions of the retina containing a single arteriole, and venular responses were not assessed.
Intravitreal injections
Pharmacological agents targeting extracellular ATP levels and P2 receptors on the abluminal face of vessels were injected intravitreally using a micro-advancer device (Hultman & Newman, 2011). This instrument positions a 25-gauge guard needle within the vitreous humour with a standard micromanipulator. A 31-gauge injection needle attached to a Hamilton syringe is then advanced through the guard needle with a manually controlled lead screw (Fig. 1A and B). The position of the injection needle and guard needle can be adjusted independently and the injection needle can be withdrawn, refilled and reinserted while the guard needle remains in place. This permits different solutions to be injected sequentially. The injection site was within 200 μm of the retinal surface and within 250 μm of the arteriole being analysed. All drugs were introduced into the vitreous in a small volume of vehicle (3 μl or 10 μl) to limit elevation of intraocular pressure and reflux of the injection solution. Reported drug concentrations represent the estimated end vitreal concentrations, which were calculated assuming a 60 μl vitreal volume, as previously estimated (Berkowitz et al. 1998), and a homogeneous distribution of the drug throughout the vitreous humour.
In preliminary studies, we tested intravitreal injections of suramin, 8,8′-[carbonylbis(imino-3,1-phenylenecarbonylimino)]bis-1,3,5-naphthalene-trisulphonic (NF023), and 2′,3′-O-(2,4,6-trinitrophenyl)-ATP (TNP-ATP), at doses of 5, 50 and 500 μm, and found 50 μm to be the minimal dose that produced a change in vessel tone. Pyridoxalphosphate-6-azophenyl-2′,5′disulphonic acid (isoPPADS) was effective at 300 μm, but not 30 μm. These values are within the range of concentrations reported previously for in situ vascular preparations (Lewis et al. 2000; Horiuchi et al. 2001; Koltsova et al. 2009).
Ex vivo rat retina preparation
The ex vivo whole mount retina preparation has been described previously (Newman, 2001). Animals were killed by an overdose of isoflurane and bilateral pneumothorax, and eyes were enucleated. Following removal of the vitreous, retinal pieces were placed in a chamber and superfused at 2–3 ml min−1 with HEPES-buffered saline (22–24°C) bubbled with air. Retinas were imaged with a 40× water immersion objective (Olympus LUMPlan Fl, 0.80 NA), infrared differential interference contrast optics and a CCD camera (CoolSnap ES; Roper Scientific, Duluth, GA, USA). Images of first- and second-order retinal arterioles were captured using MetaMorph image processing software (Molecular Devices, Downingtown, PA, USA) and luminal diameters measured manually. Arterioles in the ex vivo preparation lacked tone and experiments were conducted without the addition of drugs to pre-constrict vessels. Hence, only vasoconstrictive responses were observed.
Drugs and solutions
Stock solutions of drugs for intravitreal injections were dissolved in saline containing (in mm): 132.5 NaCl, 3 KCl, 2 CaCl2, 1 MgSO4, 1 NaH2PO4 and 10 HEPES, pH 7.4. The HEPES-buffered saline solution used in ex vivo experiments contained (in mm): 128 NaCl, 3 KCl, 2 CaCl2, 1 MgSO4, 0.5 NaH2PO4, 15 dextrose and 20 HEPES, pH 7.4. Apyrase, α,β-methylene ADP (AOPCP), N-[2-[[2-[(2-hydroxyethyl)amino]ethyl]amino]-5-quinolinyl]-2-tricyclo[3.3.1.13,7]dec-1-ylacetamide dihydrochloride (AZ10606120), suramin, ATP, fluorocitrate, endothelin-1 and cromolyn were purchased from Sigma (St. Louis, MO, USA) and ATPγS, isoPPADS, TNP-ATP, NF023, 3-[[5-(2,3-dichlorophenyl)-1H-tetrazol-1-yl]methyl]pyridine (A430879), and α,β-methylene ATP from Tocris (Bristol, UK).
The fluorocitrate solution was prepared as described previously (Paulsen et al. 1987). Briefly, 8 mg of dl-fluorocitric acid, barium salt was dissolved in 1 ml of 0.1 m HCl and three drops of 0.1 m Na2SO4 added to precipitate the barium. Two ml of 0.1 m Na2HPO4 was added and the suspension centrifuged at 1000 g for 5 min. The supernatant containing the fluorocitrate was adjusted to pH 7.4. The concentration of fluorocitrate used in our experiments (150 μm in the vitreous humour) was similar to that of a previous in vivo study showing selective inhibition of glial cell metabolism resulting in decreased ATP levels (Lian & Stringer, 2004).
Drugs were delivered either by intravitreal injection (in vivo) or by superfusion (ex vivo). Hence, observed effects on arterioles most probably reflect actions of the drugs at the abluminal face of the vessels.
Data analysis
Experimental values are reported as mean ± s.e.m., with n representing the number of rats (in vivo experiments) or the number of retinal preparations (ex vivo experiments). Changes in vessel diameter are expressed as a percentage change from baseline diameter in the text and in absolute units in Tables 3 and 4. The recovery from arteriolar dilatation following intravitreal injection of vehicle (control experiments) was fit by a first-order exponential. Vessel diameter data followed a Gaussian distribution, as shown by the normality test (Shapiro–Wilk W test). Statistical analysis of within-group variation (i.e. response to intravitreal injection) was performed with two-tailed paired t test or repeated measures one-way ANOVA followed by Tukey's multiple comparison test, as appropriate. Multigroup comparisons, i.e. between responses to intravitreal injections of pharmacological agents and vehicle controls, were tested for significance with the standard least squares method, unless otherwise indicated. When significant results were found, a post hoc Tukey's test was used. Significance was defined as P < 0.05. Descriptive statistics, normality test and analysis of variance were performed using JMP software (SAS Institute Inc., Cary, NC, USA).
Table 3.
The effects of altered ATP levels and P2X receptor antagonists on arteriole diameter in vivo
| Diameter following | |||
|---|---|---|---|
| Compound | Baseline diameter | intravitreal injection | |
| Vehicles | 3 μl saline vehicle (n = 5) | 29.3 ± 2.2 | 31.0 ± 2.6 |
| 10 μl saline vehicle (n = 5) | 31.9 ± 1.4 | 33.7 ± 1.4 | |
| Fluorocitrate vehicle (n = 3) | 37.7 ± 5.1 | 37.9 ± 4.4 | |
| Compounds changing ATP levels | Apyrase (n = 4) | 35.8 ± 3.1 | 50.4 ± 4.4*** |
| AOPCP (n = 3) | 37.8 ± 1.0 | 16.0 ± 1.9*** | |
| peak ATPγS (n = 3) | 35.9 ± 3.4 | 20.0 ± 1.7*** | |
| P2X antagonists | Suramin (n = 3) | 33.2 ±1.0 | 50.2 ± 2.8*** |
| isoPPADS (n = 3) | 33.8 ± 2.7 | 47.8 ± 4.8*** | |
| TNP-ATP (n = 4) | 33.3 ± 2.2 | 51.5 ± 2.7*** | |
| NF023 (n = 4) | 32.5 ± 2.1 | 43.0 ± 3.3*** | |
| A438079 (n = 3) | 38.2 ± 3.1 | 37.8 ± 2.8 | |
| AZ10606120 (n = 3) | 34.8 ± 1.0 | 37.7 ± 0.9 | |
| Gliotoxin | Fluorocitrate (n = 4) | 38.0 ± 0.6 | 53.6 ± 5.4*** |
| Gliotoxin – P2X1 antagonist (n = 4) | Fluorocitrate | 39.9 ± 1.4 | 57.8 ± 3.4*** |
| Endothelin-1 | 53.4 ± 1.7 | 35.3 ± 3.3*** | |
| NF023 | 40.7 ± 3.1 | 43.8 ± 3.0 |
A430879, 3-[[5-(2,3-dichlorophenyl)-1H-tetrazol-1-yl]methyl]pyridine; AOPCP, α,β-methylene ADP; AZ10606120, N-[2-[[2-[(2-hydroxyethyl)amino]ethyl]amino]-5-quinolinyl]-2-tricyclo[3.3.1.13,7]dec-1-ylacetamide dihydrochloride; isoPPADS, pyridoxalphosphate-6-azophenyl-2′,5′disulphonic acid; NF023, 8,8′-[carbonylbis(imino-3,1-phenylenecarbonylimino)]bis-1,3,5-naphthalene-trisulphonic; TNP-ATP, 2′,3′-O-(2,4,6-trinitrophenyl)-ATP. Arteriole diameter in μm, means ± s.e.m. ***P < 0.001 vs. matched vehicle control. Numbers in parentheses indicate number of rats.
Table 4.
The effects of P2 receptor agonists on arteriole diameter ex vivo
| Agonist alone | After pretreatment with fluorocitrate | After pretreatment3 with NF023 | ||||
|---|---|---|---|---|---|---|
| Baseline | Sustained | Baseline | Sustained | Baseline | Sustained | |
| 300 nm α,β-methylene ATP | 25.5 ± 1.2 | 17.4 ± 1.3*** | NA | NA | 27.6 ± 0.8 | 27.9 ± 0.8 |
| 0.1 μm ATP | 27.3 ± 1.2 | 26.7 ± 1.5 | 25.7 ± 1.5 | 25.1 ± 1.2 | NA | NA |
| 1 μm ATP | 26.6 ± 1.6 | 26.8 ± 1.5 | 25.4 ± 1.3 | 25.1 ± 1.2 | NA | NA |
| 10 μm ATP | 26.0 ± 1.1 | 23.5 ± 1.4* | 25.4 ± 1.3 | 23.4 ± 1.4* | NA | NA |
| 100 μm ATP | 25.4 ± 1.1 | 19.1 ± 1.1*** | 24.2 ± 1.4 | 20.0 ± 1.4*** | NA | NA |
| 1 mm ATP | 23.0 ± 0.9 | 13.9 ± 1.5*** | 23.5 ± 1.2 | 13.1 ± 0.8*** | NA | NA |
Arteriole diameter in μm, means ± s.e.m. *P < 0.05 and ***P < 0.001 vs. baseline. Data for P2 agonists alone and after pretreatment with either fluorocitrate (150 μm, 45 min) or NF023 (50 μm, 5 min) represent independent observations from a minimum of six preparations and three animals each. Note that baseline diameters from in vivo and ex vivo experiments cannot be compared directly as values were obtained using different measurement techniques. In addition, ex vivo results include data from both first- and second-order arterioles, whereas in vivo results are from first-order arterioles only.
Results
The tone of retinal vessels in vivo was assessed by monitoring the diameter of first-order arterioles visualized by i.v. injection of a fluorescent dye. Vessels were imaged with confocal microscopy. Vessel diameter was measured at an eccentricity of 250–750 μm from the edge of the optic disk in the superior nasal quadrant of the retina (Fig. 1B). The role of purinergic signalling in controlling vessel tone was studied by injecting purinergic agonists and antagonists into the vitreous humour, which would interact with receptors on the abluminal face of blood vessels.
Control experiments
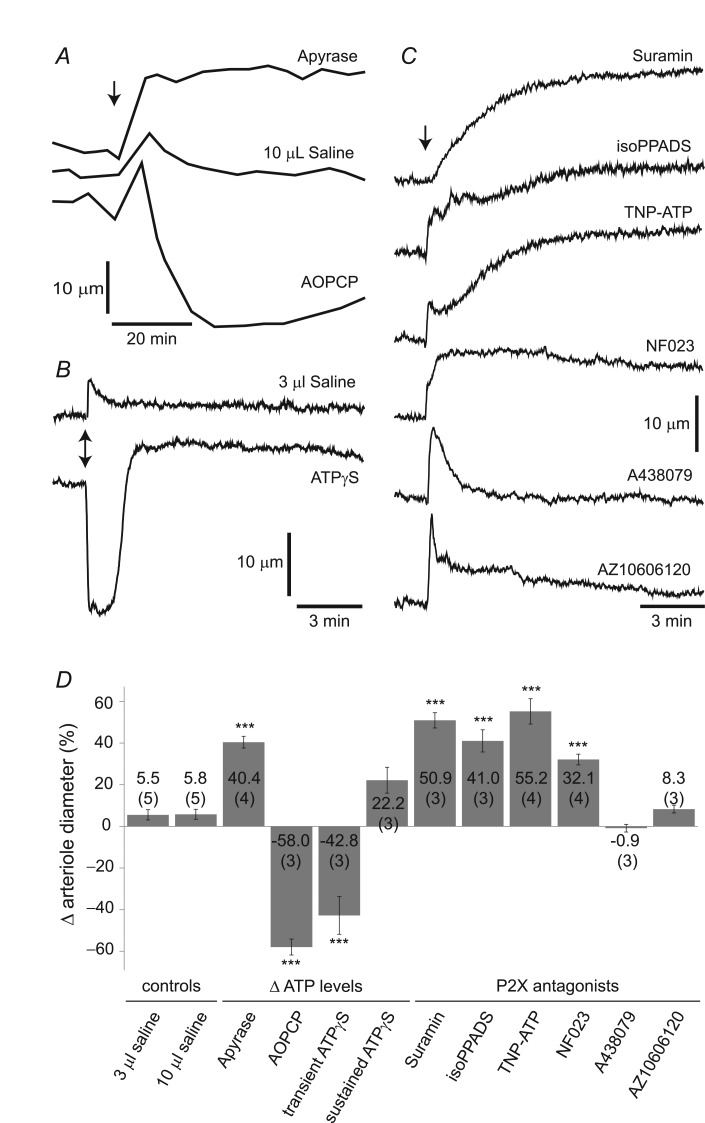
Control experiments were performed to determine the effect of vehicle injections alone. Intravitreal injections of vehicles induced transient dilatations that rapidly declined to a plateau at or just above baseline diameter (Fig. 2A and B; Table 3). Following injections of 3 μl saline, 10 μl saline and 3 μl fluorocitrate vehicle, vessel diameter transiently increased and then decayed to 5.5 ± 2.5% (n = 5, not significant, NS, P = 0.07 vs. baseline), 5.8 ± 2.4% (n = 5, NS P = 0.06 vs. baseline), and 1.1 ± 2.4% (n = 3, NS P = 0.85 vs. baseline) greater than basal diameter, respectively. The recovery of arteriole diameter after transient dilatation followed an exponential decay, with time constants of 1.4 ± 0.4 min, 8.9 ± 1.6 min, and 6.1 ± 2.0 min for 3 μl and 10 μl saline and 3 μl fluorocitrate vehicle, respectively. The origin of the transient dilatation in response to intravitreal vehicle injection is not clear. Based on previous observations in the human eye (Morlet & Young, 1993), this phenomenon may be related to a rapid, transient elevation of intraocular pressure resulting in a vascular autoregulatory response. In the experiments described below, vascular responses to pharmacological agents were measured at 13.5 min or 60 min (for 3 μl and 10 μl injection volume, respectively), after the response to vehicle injection alone had decayed.
Figure 2.
A, vitreal injection of apyrase (500 units ml−1), an ATP hydrolysing enzyme that reduces endogenous ATP levels, dilated vessels while AOPCP (12.5 mm), an ecto-5′-nucleotidase inhibitor which increases ATP levels, constricted vessels. B, injection of ATPγS (10 μm), a slowly hydrolysable ATP analogue, evoked a large transient vasoconstriction followed by a small, sustained vasodilatation. C, suramin (500 μm), a non-selective P2 receptor antagonist; isoPPADS (300 μm), a P2X receptor antagonist; TNP-ATP (50 μm), a P2X1, P2X3 and P2X2/3 receptor antagonist; and NF023 (50 μm), a P2X1 receptor antagonist, all evoked dilatations of retinal arterioles. A438079 (500 μm) and AZ10606120 (50 μm), P2X7 receptor antagonists, had no effect on arteriole diameter. Vehicle injections (3 μl and 10 μl saline) evoked small, transient vasodilatations. Saline injection volumes in A and B matched the volumes of injected drugs in the respective trials. Traces in A differ in appearance from B and C due to different rates of data collection. Arrows indicate time of vitreal injections. D, summary of in vivo data showing arteriole diameter changes evoked by altered ATP levels and by vitreal injection of P2X receptor antagonists. Vessel diameters were measured after they reached plateau values. Numbers in parentheses indicate number of rats; error bars denote ± s.e.m.; ***P < 0.001 relative to vehicle control. A430879, 3-[[5-(2,3-dichlorophenyl)-1H-tetrazol-1-yl]methyl]pyridine; AOPCP, α,β-methylene ADP; AZ10606120, N-[2-[[2-[(2-hydroxyethyl)amino]ethyl]amino]-5-quinolinyl]-2-tricyclo[3.3.1.13,7]dec-1-ylacetamide dihydrochloride; isoPPADS, pyridoxalphosphate-6-azophenyl-2′,5′disulphonic acid; NF023, 8,8′-[carbonylbis(imino-3,1-phenylenecarbonylimino)]bis-1,3,5-naphthalene-trisulphonic; TNP-ATP, 2′,3′-O-(2,4,6-trinitrophenyl)-ATP.
Altered endogenous ATP levels
We initially investigated the importance of purinergic signalling for the regulation of basal vascular tone in the retina by changing endogenous ATP levels. ATP levels were lowered with apyrase (500 units ml−1), an enzyme that hydrolyses extracellular ATP and ADP (Zimmermann & Braun, 1996; Resta et al. 2005). Apyrase dilated retinal arterioles by 40.4 ± 2.8% (n = 4, P < 0.001 vs. vehicle control; Fig. 2A, Table 3), indicating that endogenous ATP or ADP constricted vessels. We then raised endogenous ATP and ADP levels with the ecto-5′-nucleotidase inhibitor AOPCP (12.5 mm), which blocks the conversion of AMP to adenosine, thus increasing extracellular ATP and ADP levels (Melani et al. 2005). AOPCP constricted arterioles by 58.0 ± 3.8% (n = 3, P < 0.001 vs. vehicle control; Fig. 2A, Table 3).
As AOPCP application reduces adenosine levels as well as increases ATP and ADP levels, it is possible that the vasoconstriction produced by AOPCP was due to a decrease in adenosine-mediated vasodilatation rather than to an ATP- or ADP-mediated vasoconstriction. However, we found that when the P2 receptor antagonist suramin (500 μm) was applied following AOPCP-induced vasoconstriction, vessels dilated to a diameter greater than control (data not shown), demonstrating that the AOPCP-induced constriction was mediated by ATP or ADP activation of P2 receptors rather than by a reduction of P1 adenosine receptor activation.
Exogenous ATPγS
Previous studies in the retina have demonstrated that arteriole constrictions can be induced by exogenous ATP (Scholfield et al. 2007). As extracellular ATP is rapidly metabolized to adenosine, we assessed vascular responses to the slowly hydrolysable ATP analogue ATPγS. Vitreal injection of 10 μm ATPγS caused a biphasic response (Figs 1E and 2B). The diameter initially decreased by 42.8 ± 9.1% (n = 3, P < 0.001 vs. vehicle control) and then increased to a dilated steady-state level of 22.2 ± 6.2% greater than baseline (Fig. 2D, Table 3). The dilatory component of the response was not significantly different from baseline (n = 3, NS, P = 0.26).
P2X receptor antagonists
We further assessed the contribution of endogenous ATP to basal tone by testing vascular responses to a wide range of purinergic receptors antagonists. If tonic ATP-evoked vasoconstriction is present in the retinal vasculature, ATP receptor antagonists should reduce vascular tone. The broad-spectrum P2 receptor antagonist suramin (500 μm) induced a 50.9 ± 3.7% dilatation of retinal arterioles (n = 3, P < 0.001 vs. vehicle control; Fig. 2C, Table 3), supporting the role of purinergic signalling in controlling basal vessel tone.
Suramin, in addition to blocking purinergic receptors, has been shown to disrupt G-protein-coupled signalling by blocking association of G protein α and β/γ subunits (Chung & Kermode, 2005). We therefore examined whether suramin treatment compromised the efficacy of a vasoconstricting agent acting via G-protein-coupled receptors. Endothelin-1 (750 nm), a well-characterized vasoactive peptide secreted by stimulated endothelial cells (Stewart, 2012; Hinds, 2013), was injected into the vitreous following pretreatment with suramin (500 μm). The vasoconstricting action of endothelin-1 was unaffected by suramin pretreatment (data not shown) suggesting that suramin did not block G-protein signalling in vascular smooth muscle cells.
We used more selective purinergic antagonists to identify the purinergic receptor(s) responsible for generating vessel tone. isoPPADS (300 μm), which blocks P2X but not P2Y receptors (Connolly, 1995), dilated arterioles by 41.0 ± 5.3% (n = 3, P < 0.001 vs. vehicle control; Fig. 2C, Table 3). TNP-ATP (50 μm), which blocks P2X1, P2X3 and P2X2/3 receptors (Lewis et al. 1998), induced a 55.2 ± 6.1% dilatation (n = 4, P < 0.001 vs. vehicle control). NF023 (50 μm), a selective antagonist of P2X1 receptors (Soto et al. 1999), evoked a 32.1 ± 2.6% dilatation (n = 4, P < 0.001 vs. vehicle control). Together, these results indicate that purinergic vasoconstriction is mediated, at least in part, by P2X1 receptors.
ATP has been shown to contract pericytes from rat microvessels by activating P2X7 receptors (Kawamura et al. 2003). However, we found that two structurally distinct antagonists of P2X7 receptors, A438079 (500 μm) and AZ10606120 (50 μm) had no effect on basal vascular tone (n = 3, P = 0.99 and n = 3, P = 1.00 respectively; Fig. 2C, Table 3). Therefore, it is unlikely that P2X7 receptors contribute to tonic vasoconstriction of retinal arterioles.
Ex vivo agonist studies
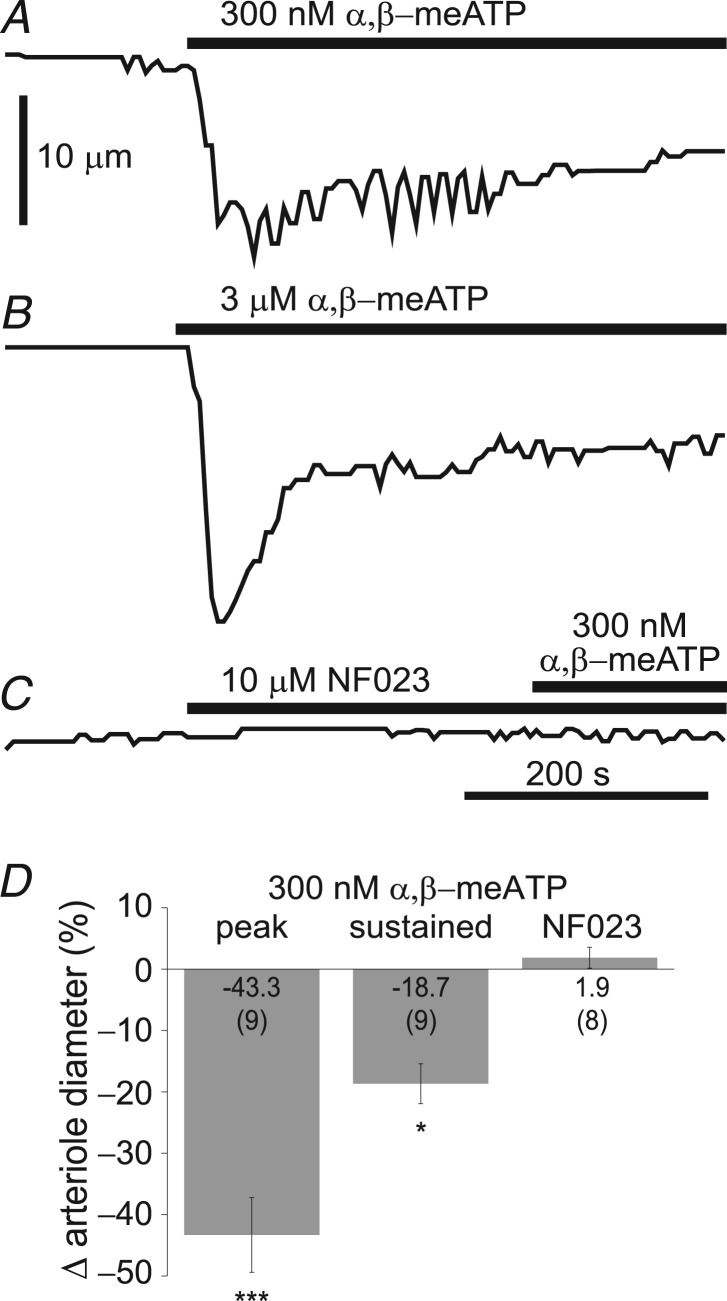
Our results indicate that P2X1 receptors, at least in part, control the basal tone of retinal arterioles. This presents a puzzle as P2X1 receptors are generally believed to mediate transient, but not sustained constriction of vascular smooth muscle cells (Lamont et al. 2006). We therefore tested the effect of the selective P2X1 receptor agonist α,β-methylene ATP (Burnstock, 2007). We used the ex vivo retinal whole mount preparation, permitting us to rapidly change superfusion solutions. We found that during bath application of α,β-methylene ATP (300 nm and 3 μm) a transient constriction of primary retinal arterioles followed by a smaller sustained constriction was generated (Fig. 3A and B, Table 4). Peak and sustained constriction in response to 300 nm α,β-methylene ATP was 43.3 ± 6.1% and 18.7 ± 3.2% respectively (n = 9, P < 0.001 and P < 0.05 vs. baseline; Fig. 3D, Table 4). Next, we investigated the effect of the P2X1 receptor antagonist NF023 on the α,β-methylene ATP-induced vasoconstriction. When vessels were pretreated with NF023 (10 μm), addition of α,β-methylene ATP (300 nm) had no effect on vessel diameter (n = 8, P = 0.97 vs. baseline; Fig. 3C and D, Table 4), demonstrating that the agonist was acting on P2X1 receptors. NF023 alone had no effect on arteriole diameter (n = 8, P = 0.96 vs. baseline). (NF023 was not capable of producing dilatation in the ex vivo preparation because ex vivo retinal vessels lack tone.) Together, these results indicate that activation of P2X1 receptors evokes both a transient and a sustained vasoconstriction of retinal arterioles.
Figure 3.
A and B, superfusate application of 300 nm and 3 μm α,β-meATP, a selective P2X1 receptor agonist, evoked both transient and sustained vasoconstriction. C, pretreatment with the P2X1 antagonist NF023 (10 μm) abolished vascular responses to α,β-meATP (300 nm). Bars represent application intervals. D, summary data for 300 nm α,β-meATP applied alone (peak and sustained) and after pretreatment with NF023 (10 μm). Note that in contrast to in vivo experiments, NF023 applied ex vivo did not produce dilatation because vessels in the isolated retina lack tone. Numbers in parentheses represent number of retinal preparations (minimum of three animals). *P < 0.05, ***P < 0.001, relative to baseline. α,β-meATP, α,β-methylene ATP; NF023, 8,8′-[carbonylbis(imino-3,1-phenylenecarbonylimino)]bis-1,3,5-naphthalene-trisulphonic.
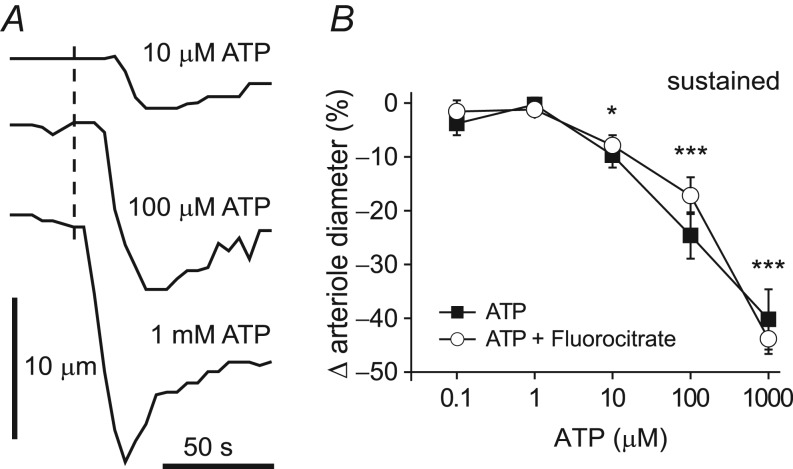
Finally, we used the ex vivo preparation to determine the minimal concentration of ATP needed to produce sustained constrictions in retinal arterioles. We found that bath application of 10 μm, 100 μm and 1 mm ATP produced constrictions of 9.7 ± 2.3% (P < 0.05 vs. baseline), 24.3 ± 4.3% (P < 0.001 vs. baseline) and 40.2 ± 5.6% (P < 0.001 vs. baseline) respectively (n = 6; Fig. 4A and B, Table 4). There were no significant changes in response to 0.1 μm and 1 μm ATP (−3.8 ± 2.2% and −0.3 ± 1.3%, respectively; n = 6, NS vs. baseline, Table 4).
Figure 4.
A, superfusate application of 10 μm, 100 μm and 1 mm ATP evoked transient and sustained vasoconstriction. Dashed line represents onset of ATP application. B, filled squares: summary data for six retinal preparations from four animals showing the arteriole response to increasing concentrations of ATP (measured at the end of agonist application, 100 s; *P < 0.05, ***P < 0.001, relative to baseline). Open circles: pretreatment with the gliotoxin fluorocitrate (150 μm, 45 min) did not affect the ATP-evoked constrictions of retinal arterioles (n = 6 retinal preparations from three animals, not significant for comparison between ATP alone and after pretreatment with fluorocitrate).
Cellular source of ATP
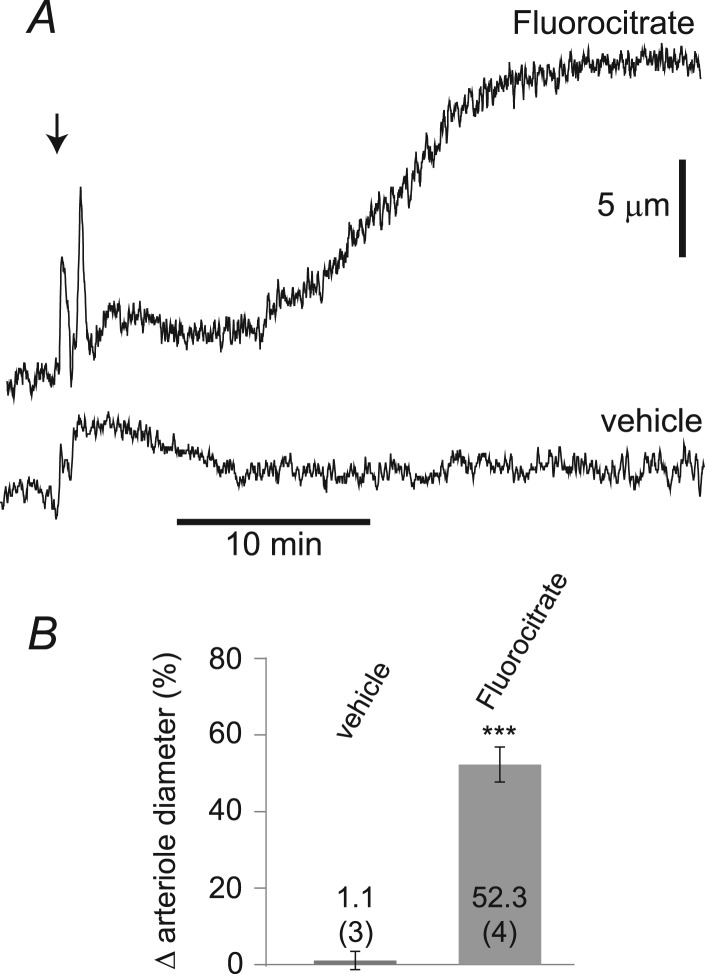
Glial cells are a probable source of the ATP that tonically constricts retinal vessels (Pascual et al. 2005). We assessed the contribution of glial cells to the control of vascular tone with intravitreal injections of fluorocitrate, a metabolic toxin that inhibits the TCA cycle in glial cells by blocking aconitase (Paulsen et al. 1987), leading to a reduction in glial ATP levels (Lian & Stringer, 2004). Following a delay of 10.0 ± 1.3 min, intravitreal injection of fluorocitrate (150 μm) induced vessel dilatation that plateaued after 28.3 ± 1.4 min. At 45 min after injection, fluorocitrate produced a dilatation of 52.3 ± 1.1% (n = 4, P < 0.001 vs. vehicle control; Fig. 5 and Table 3), similar to the dilatation produced by purinergic antagonists. This result suggests that the ATP responsible for producing vessel tone in the retina may originate, at least in part, from glial cells.
Figure 5.
A, intravitreal injection of the gliotoxin fluorocitrate (150 μm) dilated retinal arterioles. Vehicle alone had no effect on arteriole diameter. B, summary data showing the change in arteriole diameter in response to vehicle and fluorocitrate measured 45 min after injection. Numbers in parentheses indicate number of rats. ***P < 0.001, relative to vehicle control.
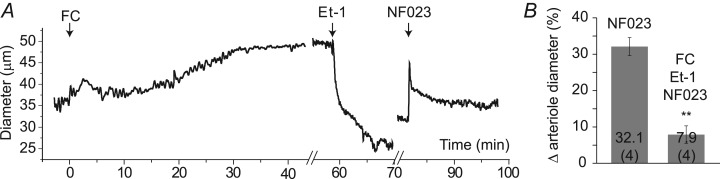
If the vasodilatory action of fluorocitrate is due to a reduction in the release of ATP from glial cells, then arteriole dilatation evoked by purinergic antagonists should be reduced in the presence of fluorocitrate. We tested this by recording changes in arteriole diameter in vivo in response to sequential intravitreal injections of fluorocitrate (150 μm), endothelin-1 (7–12 nm) and NF023 (50 μm). It was necessary to inject endothelin-1, a vasoconstrictive peptide (Stewart, 2012; Hinds, 2013), to restore arteriole tone following fluorocitrate injection so that NF023-evoked vasodilatations, if present, could be observed. As demonstrated above, fluorocitrate injection dilated retinal arterioles (Fig. 6A). Endothelin-1 was then injected to constrict vessels to a pre-fluorocitrate level; there were no significant differences between baseline diameters before fluorocitrate injection and after endothelin-1 treatment (P = 0.67; Table 3). The P2X1 antagonist NF023 was then injected. We found that pretreatment with fluorocitrate inhibited NF023-mediated vasodilatations (n = 4, P < 0.01). In the presence of fluorocitrate, NF023 dilated arterioles by 7.9 ± 2.0%, compared to 32.1 ± 2.6% in controls (Fig. 6 and Table 3). The result suggests that fluorocitrate, presumably by poisoning glial cell metabolism and lowering extracellular ATP levels, reduces activation of P2X1 receptors on vascular smooth muscle cells.
Figure 6.
A, changes in arteriole diameter following sequential intravitreal injections of the gliotoxin FC (150 μm), Et-1 (7–12 nm), a vasoconstricting peptide, and NF023 (50 μm), a P2X1 receptor antagonist. Et-1 restored the tone of the vessel, which was decreased following FC treatment. NF023 injection evoked a small dilatation. B, summary data showing arteriole diameter changes evoked by application of NF023 without pretreatment (data from Fig. 2) and following pretreatment with FC and Et-1. Numbers in parentheses indicate number of rats. **P < 0.01 relative to NF023 alone. Et-1, endothelin-1; FC, fluorocitrate; NF023, 8,8′-[carbonylbis(imino-3,1-phenylenecarbonylimino)]bis-1,3,5-naphthalene-trisulphonic.
It is conceivable that fluorocitrate acts on vascular cells directly rather than on glial cells to produce vessel dilatation. To control for this possible effect of fluorocitrate, we tested whether pre-incubation with fluorocitrate affects ATP-induced constrictions in the ex vivo preparation. We found that there was no difference in the magnitude of ATP-evoked constrictions following fluorocitrate treatment (150 μm, 45 min) as compared to responses to ATP alone (n = 6, NS, two-way ANOVA; Fig. 4B). This result demonstrates that fluorocitrate has no direct effect on vascular responsiveness to ATP. (Ex vivo arterioles lack vessel tone. Thus, in contrast to in vivo experiments, fluorocitrate did not dilate vessels in the ex vivo preparation; Table 4.)
Discussion
Our results support the hypothesis that ATP provides a tonic level of constriction in retinal vessels. When endogenous ATP levels are lowered, retinal arterioles dilate and when endogenous levels are increased, vessels constrict. The purinergic antagonist experiments we conducted indicate that vasoconstriction is mediated, at least in part, by P2X1 receptors. To our knowledge, these results constitute the first in vivo demonstration of local control of vascular tone through P2X1 activation. Retinal arterioles dilated to over 150% of their resting diameter when ATP levels were lowered and when P2X receptors were blocked. This indicates that purinergic constriction of the arterioles is responsible for generating a substantial fraction of vascular tone in these vessels. As our in vivo studies were performed in anaesthetized animals, the direct actions of anaesthetics on cellular mechanisms regulating vascular tone should be taken into consideration (Akata, 2007).
Previous electrophysiological studies have indicated that P2X1 receptors inactivate within several hundreds of milliseconds (Burnstock, 2007), raising the question of how these receptors could mediate tonic vasoconstriction. However, exogenous application of the P2X1 agonist α,β-methylene ATP in both rat pial arterioles (Lewis et al. 2000) and afferent renal arterioles (Zhao et al. 2001; Inscho & Cook, 2002) evokes vasoconstrictions with sustained as well as transient components. This is consistent with the results of our ex vivo experiments demonstrating that P2X1 receptors can mediate sustained constriction of rat retinal arterioles.
There are several possible explanations why the activation of P2X1 receptors might mediate tonic vasoconstriction. P2X1 receptor activation results in the depolarization of smooth muscle cells and can elicit Ca2+ influx through voltage-dependent L-type Ca2+ channels. This influx results in sustained vasoconstriction (Inscho & Cook, 2002). Ca2+ influx through P2X1 receptors can also trigger the release of Ca2+ from intracellular stores, leading to an amplified Ca2+ increase (Mironneau et al. 2001; Povstyan et al. 2011). In addition, other P2X receptor isoforms, with lower rates of desensitization, may form functional heteromeric channels with P2X1 receptors and contribute to a sustained vascular response (Harhun et al. 2010). Genetic knockout and pharmacological blockade studies of pressure-dependent autoregulation of renal arterioles demonstrate that P2X1 receptors can mediate tonic vasoconstriction (Inscho et al. 2003).
There is growing evidence that endothelium-derived polyphosphates, including uridine adenosine tetraphosphate (UpA4) and adenosine 5′-tetraphosphate (Ap4), can mediate vasoconstriction, at least in part, by activation of P2X1 receptors (Tolle et al. 2008). Whether these nucleotides contribute to the control of vascular tone in the retina remains to be examined.
The purinergic antagonists we employed were only effective in reducing vascular tone when used at relatively high concentrations. It could be argued that at such concentrations the antagonists were not selective. However, the EC50s of purinergic antagonists have been shown to be ∼15,000-fold higher in intact vascular preparations compared to isolated smooth muscle cells due to the metabolic breakdown of antagonists in intact tissue (Lewis et al. 1998). In addition, we found that the selective P2X7 antagonist A438079 was ineffective in reducing vascular tone, indicating that even at a concentration of 500 μm, this drug retained its selectivity and did not block the purinergic receptors responsible for generating tone.
It is conceivable that our purinergic antagonists could be acting upstream from vascular P2X receptors by altering neuronal activity. We believe this to be unlikely, as suramin does not reduce neuronal activity in the retina, at least in retinal ganglion cells (Newman, 2005).
While the effects of P2 receptor antagonists are consistent with the hypothesis that ATP provides a tonic level of constriction in retinal arterioles, the biphasic response to ATPγS, a slowly hydrolysable analogue of ATP, is somewhat surprising. The biphasic response may be due to ATPγS activation of P2Y receptors on vascular endothelial cells as well as P2X receptors on vascular smooth muscle cells, resulting in vasoconstriction followed by delayed dilatation. In agreement with this proposal and our results, Horiuchi et al. (2003) showed that in rat intracerebral arterioles, an initial ATPγS-evoked constriction was followed by a dilatation resulting from P2Y1 and/or P2Y2 receptor stimulation.
Where is ATP acting?
It is probable that the endogenous ATP contributing to the tone of retinal vessels is acting directly on P2X receptors on vascular smooth muscle cells. P2X1 receptors are expressed on vascular smooth muscle cells in peripheral and cerebral vessels (Nori et al. 1998; Lewis & Evans, 2001). P2X1 immunoreactivity has been observed in the retina but has not been localized to blood vessels (Yazulla & Studholme, 2004).
ATP could also be acting on vascular endothelial cells or on retinal neurons or glial cells, which express P2X receptors (Burnstock, 2007). ATP action on endothelial cells is unlikely, as activation of endothelial purinergic receptors normally produces vasodilatation rather than vasoconstriction (Horiuchi et al. 2001). Action on retinal glial cells is also unlikely, as activation of purinergic receptors on glial cells evokes glial Ca2+ increases, leading to vasodilatation rather than vasoconstriction (Mishra et al. 2011; Srienc et al. 2012). ATP could be stimulating or inhibiting retinal neurons, although this is unlikely as purinergic antagonists do not substantially alter neuronal activity (Newman, 2005).
Cellular origin of ATP
Glial cells are the probable source of the ATP that tonically constricts retinal vessels. We have shown previously that retinal glial cells release ATP when stimulated (Newman, 2001). In addition, astrocytes in the brain release ATP tonically (Pascual et al. 2005). Our fluorocitrate experiments also support the view that ATP release from glial cells is responsible for controlling vessel tone. When glial cell metabolism is poisoned with fluorocitrate, retinal arterioles dilate. This dilatation is due to reduced activation of P2 receptors, presumably due to lowered extracellular ATP levels at the abluminal face of vessels.
Fluorocitrate-induced inhibition of glial metabolism has previously been shown to decrease glial ATP content (Lian & Stringer, 2004), which probably impairs ATP release and lowers interstitial ATP levels. In addition, genetic ablation of Müller cells, the principal glial cells of the retina (Newman & Reichenbach, 1996) results in the dilatation of retinal vessels (Shen et al. 2012). It is worth noting that in our ex vivo experiments, ATP levels of 10 μm were sufficient to produce tonic constriction of retinal vessels. Tonic release of ATP from brain astrocytes produces extracellular ATP levels of approximately 10 μm as well (Pascual et al. 2005). This observation further supports a glial-mediated mechanism of the generation of vascular tone.
ATP is rapidly hydrolysed to adenosine once it is released into the extracellular space. Thus, the cells releasing ATP must be in close proximity to blood vessels to evoke vasoconstriction. In this respect, retinal glial cells, whose endfeet directly contact vessels, are ideally situated to mediate purinergic constriction. It is probable that ATP is released directly from glial cell endfeet on to vascular smooth muscle cells. Retinal neurons, which lie farther from vessels, are less likely to mediate vasoconstriction.
For the same reason, glial-mediated generation of vascular tone would break down under pathological conditions if glial endfeet were separated from blood vessels. If the distance between glial cells and vessels were increased substantially, released ATP would be converted to adenosine before reaching the vessel wall, resulting in dilatation rather than constriction. A separation of glial endfeet from blood vessels occurs in many pathologies, including ischaemia (Zhang et al. 2011) and an animal model of cerebral amyloid angiopathy (Merlini et al. 2011).
In this and in many other studies, fluorocitrate has been used as a selective inhibitor of glial cell metabolism (Virgili et al. 1991; Lian & Stringer, 2004; Zielke et al. 2007). However, fluorocitrate may directly affect other cells as well. For instance, fluorocitrate-induced energy deprivation causes changes in the F-actin cytoskeleton of brain vascular endothelial cells in vitro (Rist et al. 1996). Indeed, vascular endothelial cells may represent a possible source of ATP, as endothelial cells release ATP in response to shear stress (Yamamoto et al. 2011). However, it is unlikely that endothelial cells represent a major source of vasoconstricting ATP as ATP released from these cells into the vessel lumen functions as a potent autocrine and/or paracrine signal that promotes flow-induced vasodilatation rather than constriction (Winter & Dora, 2007).
ATP contribution to vascular tone in the brain
It is probable that purinergic signalling contributes to the maintenance of vascular tone in the brain as well as the retina. Brain arteries and arterioles express both P2X and P2Y receptors and application of purinergic agonists on to the abluminal face of isolated cerebral vessels produces constriction (Lewis et al. 2000; Horiuchi et al. 2001). ATP is present in the brain parenchyma, where it is co-released from neurons along with many classical transmitters (Burnstock, 2007) and is tonically released from brain astrocytes (Pascual et al. 2005). However, definitive evidence demonstrating that purinergic signalling contributes to vascular tone in the brain is lacking.
In conclusion, our results demonstrate that ATP, acting at least in part on P2X1 receptors, is responsible for generating tone in retinal arterioles. Experiments with the gliotoxin fluorocitrate provide evidence that glial cells control arteriole tone by releasing vasoconstricting ATP.
Key points
The blood supply to the CNS is controlled by the tone of arteries and arterioles. However, little is known about purinergic regulation of vascular tone in the CNS.
We have investigated purinergic control of vascular tone in the in vivo rat retina.
Reducing endogenous ATP levels by enzyme degradation and inhibiting purinergic signalling with P2X receptor antagonists decreases the tone of retinal arterioles. Raising ATP levels increases vessel tone.
Experiments using fluorocitrate to poison the metabolism of glial cells suggest glia are a source of the ATP that tonically constricts retinal vessels.
The results suggest a novel mechanism of local control of vascular tone through activation of vascular smooth muscle cell P2X receptors.
Acknowledgments
The authors thank Michael Burian for his excellent technical assistance and Edith Hamel for comments on an early version of the manuscript.
Glossary
- A430879
3-[[5-(2,3-dichlorophenyl)-1H-tetrazol-1-yl]methyl]pyridine
- AOPCP
α,β-methylene ADP
- AZ10606120
N-[2-[[2-[(2-hydroxyethyl)amino]ethyl]amino]-5-quinolinyl]-2-tricyclo[3.3.1.13,7]dec-1-ylacetamide dihydrochloride
- isoPPADS
pyridoxalphosphate-6-azophenyl-2′,5′disulphonic acid
- NF023
8,8′-[carbonylbis(imino-3,1-phenylenecarbonylimino)]bis-1,3,5-naphthalene-trisulphonic
- TNP-ATP
2′,3′-O-(2,4,6-trinitrophenyl)-ATP.
Competing interests
The authors declare no conflict of interest.
Author contributions
J.K. and E.A.N contributed equally to the design of the experiments, interpretation of the data, and writing of the article. J.K. performed and analysed the experiments. Both authors approved the final version of the manuscript.
Funding
This work was supported by Fondation Leducq and NIH grant EY004077.
References
- Akata T. General anaesthetics and vascular smooth muscle: direct actions of general anaesthetics on cellular mechanisms regulating vascular tone. Anesthesiology. 2007;106:365–391. doi: 10.1097/00000542-200702000-00026. [DOI] [PubMed] [Google Scholar]
- Attwell D, Buchan AM, Charpak S, Lauritzen M, MacVicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature. 2010;468:232–243. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekar LK, Wei HS, Nedergaard M. The locus coeruleus-norepinephrine network optimizes coupling of cerebral blood volume with oxygen demand. J Cereb Blood Flow Metab. 2012;32:2135–2145. doi: 10.1038/jcbfm.2012.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz BA, Lukaszew RA, Mullins CM, Penn JS. Impaired hyaloidal circulation function and uncoordinated ocular growth patterns in experimental retinopathy of prematurity. Invest Ophthalmol Vis Sci. 1998;39:391–396. [PubMed] [Google Scholar]
- Berstad J. The initial phase of the dextran-induced anaphylactoid reaction in the rat: a comparison of inhibitors of the blood pressure fall. Acta Pharmacol Toxicol (Copenh) 1982;51:141–146. doi: 10.1111/j.1600-0773.1982.tb01005.x. [DOI] [PubMed] [Google Scholar]
- Blom JD, Yang PC, Nicholson NS, Case BL, Parlow JJ, South MS, Wegner CD. A method for determining whether hypotension caused by novel compounds in preclinical development results from histamine release. J Pharmacol Toxicol Methods. 2004;49:31–37. doi: 10.1016/j.vascn.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Brazitikos PD, Pournaras CJ, Munoz JL, Tsacopoulos M. Microinjection of l-lactate in the preretinal vitreous induces segmental vasodilatation in the inner retina of miniature pigs. Invest Ophthalmol Vis Sci. 1993;34:1744–1752. [PubMed] [Google Scholar]
- Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- Cauli B, Tong XK, Rancillac A, Serluca N, Lambolez B, Rossier J, Hamel E. Cortical GABA interneurons in neurovascular coupling: relays for subcortical vasoactive pathways. J Neurosci. 2004;24:8940–8949. doi: 10.1523/JNEUROSCI.3065-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung WC, Kermode JC. Suramin disrupts receptor-G protein coupling by blocking association of G protein alpha and betagamma subunits. J Pharmacol Exp Ther. 2005;313:191–198. doi: 10.1124/jpet.104.078311. [DOI] [PubMed] [Google Scholar]
- Cohen Z, Bonvento G, Lacombe P, Hamel E. Serotonin in the regulation of brain microcirculation. Prog Neurobiol. 1996;50:335–362. doi: 10.1016/s0301-0082(96)00033-0. [DOI] [PubMed] [Google Scholar]
- Connolly GP. Differentiation by pyridoxal 5-phosphate, PPADS and IsoPPADS between responses mediated by UTP and those evoked by a,b-methylene-ATP on rat sympathetic ganglia. Br J Pharmacol. 1995;114:727–731. doi: 10.1111/j.1476-5381.1995.tb17199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorner GT, Garhofer G, Kiss B, Polska E, Polak K, Riva CE, Schmetterer L. Nitric oxide regulates retinal vascular tone in humans. Am J Physiol Heart Circ Physiol. 2003;285:H631–H636. doi: 10.1152/ajpheart.00111.2003. [DOI] [PubMed] [Google Scholar]
- Hamel E. Cholinergic modulation of the cortical microvascular bed. Prog Brain Res. 2004;145:171–178. doi: 10.1016/S0079-6123(03)45012-7. [DOI] [PubMed] [Google Scholar]
- Hamel E. Perivascular nerves and the regulation of cerebrovascular tone. J Appl Physiol. 2006;100:1059–1064. doi: 10.1152/japplphysiol.00954.2005. [DOI] [PubMed] [Google Scholar]
- Harhun MI, Povstyan OV, Gordienko DV. Purinoreceptor-mediated current in myocytes from renal resistance arteries. Br J Pharmacol. 2010;160:987–997. doi: 10.1111/j.1476-5381.2010.00714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinds K, Monaghan KP, Frolund B, McGeown JG, Curtis T. GABAergic control of arteriolar diameter in the rat retina. Invest Ophthalmol Vis Sci. 2013;54:6798–6805. doi: 10.1167/iovs.13-12362. [DOI] [PubMed] [Google Scholar]
- Horiuchi T, Dietrich HH, Tsugane S, Dacey RG., Jr Analysis of purine- and pyrimidine-induced vascular responses in the isolated rat cerebral arteriole. Am J Physiol Heart Circ Physiol. 2001;280:H767–H776. doi: 10.1152/ajpheart.2001.280.2.H767. [DOI] [PubMed] [Google Scholar]
- Horiuchi T, Dietrich HH, Hongo K, Dacey RG., Jr Comparison of P2 receptor subtypes producing dilatation in rat intracerebral arterioles. Stroke. 2003;34:1473–1478. doi: 10.1161/01.STR.0000071527.10129.65. [DOI] [PubMed] [Google Scholar]
- Hultman D, Newman EA. A micro-advancer device for vitreal injection and retinal recording and stimulation. Exp Eye Res. 2011;93:767–770. doi: 10.1016/j.exer.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inscho EW, Cook AK. P2 receptor-mediated afferent arteriolar vasoconstriction during calcium blockade. Am J Physiol Renal Physiol. 2002;282:F245–F255. doi: 10.1152/ajprenal.0038.2001. [DOI] [PubMed] [Google Scholar]
- Inscho EW, Cook AK, Imig JD, Vial C, Evans RJ. Physiological role for P2X1 receptors in renal microvascular autoregulatory behaviour. J Clin Invest. 2003;112:1895–1905. doi: 10.1172/JCI18499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura H, Sugiyama T, Wu DM, Kobayashi M, Yamanishi S, Katsumura K, Puro DG. ATP: a vasoactive signal in the pericyte-containing microvasculature of the rat retina. J Physiol. 2003;551.3:787–799. doi: 10.1113/jphysiol.2003.047977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koltsova SV, Maximov GV, Kotelevtsev SV, Lavoie JL, Tremblay J, Grygorczyk R, Hamet P, Orlov SN. Myogenic tone in mouse mesenteric arteries: evidence for P2Y receptor-mediated, Na+, K+, 2Cl– cotransport-dependent signalling. Purinergic Signal. 2009;5:343–349. doi: 10.1007/s11302-009-9160-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kur J, Newman EA, Chan-Ling T. Cellular and physiological mechanisms underlying blood flow regulation in the retina and choroid in health and disease. Prog Retin Eye Res. 2012;31:377–406. doi: 10.1016/j.preteyeres.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamont C, Vial C, Evans RJ, Wier WG. P2X1 receptors mediate sympathetic postjunctional Ca2+ transients in mesenteric small arteries. Am J Physiol Heart Circ Physiol. 2006;291:H3106–H3113. doi: 10.1152/ajpheart.00466.2006. [DOI] [PubMed] [Google Scholar]
- Lewis CJ, Evans RJ. P2X receptor immunoreactivity in different arteries from the femoral, pulmonary, cerebral, coronary and renal circulations. J Vasc Res. 2001;38:332–340. doi: 10.1159/000051064. [DOI] [PubMed] [Google Scholar]
- Lewis CJ, Surprenant A, Evans RJ. 2′,3′-O-(2,4,6-trinitrophenyl) adenosine 5′-triphosphate (TNP-ATP) – a nanomolar affinity antagonist at rat mesenteric artery P2X receptor ion channels. Br J Pharmacol. 1998;124:1463–1466. doi: 10.1038/sj.bjp.0702001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis CJ, Ennion SJ, Evans RJ. P2 purinoceptor-mediated control of rat cerebral (pial) microvasculature; contribution of P2X and P2Y receptors. J Physiol. 2000;527(Pt 2):315–324. doi: 10.1111/j.1469-7793.2000.00315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian XY, Stringer JL. Energy failure in astrocytes increases the vulnerability of neurons to spreading depression. Eur J Neurosci. 2004;19:2446–2454. doi: 10.1111/j.0953-816X.2004.03289.x. [DOI] [PubMed] [Google Scholar]
- Melani A, Turchi D, Vannucchi MG, Cipriani S, Gianfriddo M, Pedata F. ATP extracellular concentrations are increased in the rat striatum during in vivo ischemia. Neurochem Int. 2005;47:442–448. doi: 10.1016/j.neuint.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Merlini M, Meyer EP, Ulmann-Schuler A, Nitsch RM. Vascular β-amyloid and early astrocyte alterations impair cerebrovascular function and cerebral metabolism in transgenic arcAb mice. Acta Neuropathol. 2011;122:293–311. doi: 10.1007/s00401-011-0834-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mironneau J, Coussin F, Morel JL, Barbot C, Jeyakumar LH, Fleischer S, Mironneau C. Calcium signalling through nucleotide receptor P2X1 in rat portal vein myocytes. J Physiol. 2001;536:339–350. doi: 10.1111/j.1469-7793.2001.0339c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra A, Hamid A, Newman EA. Oxygen modulation of neurovascular coupling in the retina. Proc Natl Acad Sci U S A. 2011;108:17827–17831. doi: 10.1073/pnas.1110533108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morlet N, Young SH. Prevention of intraocular pressure rise following intravitreal injection. Br J Ophthalmol. 1993;77:572–573. doi: 10.1136/bjo.77.9.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman EA. Propagation of intercellular calcium waves in retinal astrocytes and Müller cells. J Neurosci. 2001;21:2215–2223. doi: 10.1523/JNEUROSCI.21-07-02215.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman EA. Calcium increases in retinal glial cells evoked by light-induced neuronal activity. J Neurosci. 2005;25:5502–5510. doi: 10.1523/JNEUROSCI.1354-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman EA, Reichenbach A. The Müller cell: a functional element of the retina. Trends Neurosci. 1996;19:307–312. doi: 10.1016/0166-2236(96)10040-0. [DOI] [PubMed] [Google Scholar]
- Nori S, Fumagalli L, Bo X, Bogdanov Y, Burnstock G. Coexpression of mRNAs for P2X1, P2X2 and P2X4 receptors in rat vascular smooth muscle: an in situ hybridization and RT-PCR study. J Vasc Res. 1998;35:179–185. doi: 10.1159/000025582. [DOI] [PubMed] [Google Scholar]
- Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, Takano H, Moss SJ, McCarthy K, Haydon PG. Astrocytic purinergic signalling coordinates synaptic networks. Science. 2005;310:113–116. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- Paulsen RE, Contestabile A, Villani L, Fonnum F. An in vivo model for studying function of brain tissue temporarily devoid of glial cell metabolism: the use of fluorocitrate. J Neurochem. 1987;48:1377–1385. doi: 10.1111/j.1471-4159.1987.tb05674.x. [DOI] [PubMed] [Google Scholar]
- Piet R, Jahr CE. Glutamatergic and purinergic receptor-mediated calcium transients in Bergmann glial cells. J Neurosci. 2007;27:4027–4035. doi: 10.1523/JNEUROSCI.0462-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povstyan OV, Harhun MI, Gordienko DV. Ca2+ entry following P2X receptor activation induces IP3 receptor-mediated Ca2+ release in myocytes from small renal arteries. Br J Pharmacol. 2011;162:1618–1638. doi: 10.1111/j.1476-5381.2010.01169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resta V, Novelli E, Di VF, Galli-Resta L. Neuronal death induced by endogenous extracellular ATP in retinal cholinergic neuron density control. Development. 2005;132:2873–2882. doi: 10.1242/dev.01855. [DOI] [PubMed] [Google Scholar]
- Rist RJ, Romero IA, Chan MW, Abbott NJ. Effects of energy deprivation induced by fluorocitrate in immortalised rat brain microvessel endothelial cells. Brain Res. 1996;730:87–94. doi: 10.1016/0006-8993(96)00438-6. [DOI] [PubMed] [Google Scholar]
- Scholfield CN, McGeown JG, Curtis TM. Cellular physiology of retinal and choroidal arteriolar smooth muscle cells. Microcirculation. 2007;14:11–24. doi: 10.1080/10739680601072115. [DOI] [PubMed] [Google Scholar]
- Shen W, Fruttiger M, Zhu L, Chung SH, Barnett NL, Kirk JK, Lee S, Coorey NJ, Killingsworth M, Sherman LS, Gillies MC. Conditional Müller cell ablation causes independent neuronal and vascular pathologies in a novel transgenic model. J Neurosci. 2012;32:15715–15727. doi: 10.1523/JNEUROSCI.2841-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto F, Lambrecht G, Nickel P, Stuhmer W, Busch AE. Antagonistic properties of the suramin analogue NF023 at heterologously expressed P2X receptors. Neuropharmacology. 1999;38:141–149. doi: 10.1016/s0028-3908(98)00158-0. [DOI] [PubMed] [Google Scholar]
- Srienc AI, Kornfield TE, Mishra A, Burian MA, Newman EA. Assessment of glial function in the in vivo retina. In: Milner R, editor. Astrocytes: Methods and Protocols. Springer, New York; 2012. pp. 499–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart M, Needham M, Bankhead P, Gardiner TA, Scholfield CN, Curtis TM, McGeown JG. Feedback via Ca2+-activated ion channels modulates endothelin-1 signalling in retinal arteriolar smooth muscle. Invest Ophthalmol Vis Sci. 2012;53:3059–3066. doi: 10.1167/iovs.11-9192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda N, Ayajiki K, Okamura T. Cerebral blood flow regulation by nitric oxide: recent advances. Pharmacol Rev. 2009;61:62–97. doi: 10.1124/pr.108.000547. [DOI] [PubMed] [Google Scholar]
- Tolle M, Jankowski V, Schuchardt M, Wiedon A, Huang T, Hub F, Kowalska J, Jemielity J, Guranowski A, Loddenkemper C, Zidek W, Jankowski J, van der Giet M. Adenosine 5′-tetraphosphate is a highly potent purinergic endothelium-derived vasoconstrictor. Circ Res. 2008;103:1100–1108. doi: 10.1161/CIRCRESAHA.108.177865. [DOI] [PubMed] [Google Scholar]
- Virgili M, Paulsen R, Villani L, Contestabile A, Fonnum F. Temporary impairment of Müller cell metabolism in the rat retina by intravitreal injection of fluorocitrate. Exp Eye Res. 1991;53:115–122. doi: 10.1016/0014-4835(91)90153-6. [DOI] [PubMed] [Google Scholar]
- Winter P, Dora KA. Spreading dilatation to luminal perfusion of ATP and UTP in rat isolated small mesenteric arteries. J Physiol. 2007;582:335–347. doi: 10.1113/jphysiol.2007.135202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K, Furuya K, Nakamura M, Kobatake E, Sokabe M, Ando J. Visualization of flow-induced ATP release and triggering of Ca2+ waves at caveolae in vascular endothelial cells. J Cell Sci. 2011;124:3477–3483. doi: 10.1242/jcs.087221. [DOI] [PubMed] [Google Scholar]
- Yazulla S, Studholme KM. Vanilloid receptor like 1 (VRL1) immunoreactivity in mammalian retina: colocalization with somatostatin and purinergic P2X1 receptors. J Comp Neurol. 2004;474:407–418. doi: 10.1002/cne.20144. [DOI] [PubMed] [Google Scholar]
- Ye XD, Laties AM, Stone RA. Peptidergic innervation of the retinal vasculature and optic nerve head. Invest Ophthalmol Vis Sci. 1990;31:1731–1737. [PubMed] [Google Scholar]
- Zhang J, Takahashi HK, Liu K, Wake H, Liu R, Maruo T, Date I, Yoshino T, Ohtsuka A, Mori S, Nishibori M. Anti-high mobility group box-1 monoclonal antibody protects the blood-brain barrier from ischemia-induced disruption in rats. Stroke. 2011;42:1420–1428. doi: 10.1161/STROKEAHA.110.598334. [DOI] [PubMed] [Google Scholar]
- Zhao X, Inscho EW, Bondlela M, Falck JR, Imig JD. The CYP450 hydroxylase pathway contributes to P2X receptor-mediated afferent arteriolar vasoconstriction. Am J Physiol Heart Circ Physiol. 2001;281:H2089–H2096. doi: 10.1152/ajpheart.2001.281.5.H2089. [DOI] [PubMed] [Google Scholar]
- Zielke HR, Zielke CL, Baab PJ, Tildon JT. Effect of fluorocitrate on cerebral oxidation of lactate and glucose in freely moving rats. J Neurochem. 2007;101:9–16. doi: 10.1111/j.1471-4159.2006.04335.x. [DOI] [PubMed] [Google Scholar]
- Zimmermann H, Braun N. Extracellular metabolism of nucleotides in the nervous system. J Auton Pharmacol. 1996;16:397–400. doi: 10.1111/j.1474-8673.1996.tb00062.x. [DOI] [PubMed] [Google Scholar]