Abstract
Calcium cycling is integral to muscle performance during the rapid muscle contraction and relaxation of high-intensity exercise. Ca2+ handling is altered by diabetes mellitus, but has not previously been investigated in human skeletal muscle. We investigated effects of high-intensity exercise and sprint training on skeletal muscle Ca2+ regulation among men and women with type 1 diabetes (T1D, n = 8, 3F, 5M) and matched non-diabetic controls (CON, n = 8, 3F, 5M). Secondarily, we examined sex differences in Ca2+ regulation. Subjects undertook 7 weeks of three times-weekly cycle sprint training. Before and after training, performance was measured, and blood and muscle were sampled at rest and after high-intensity exercise. In T1D, higher Ca2+-ATPase activity (+28%) and Ca2+ uptake (+21%) than in CON were evident across both times and days (P < 0.05), but performance was similar. In T1D, resting Ca2+-ATPase activity correlated with work performed until exhaustion (r = 0.7, P < 0.01). Ca2+-ATPase activity, but not Ca2+ uptake, was lower (−24%, P < 0.05) among the women across both times and days. Intense exercise did not alter Ca2+-ATPase activity in T1D or CON. However, sex differences were evident: Ca2+-ATPase was reduced with exercise among men but increased among women across both days (time × sex interaction, P < 0.05). Sprint training reduced Ca2+-ATPase (−8%, P < 0.05), but not Ca2+ uptake, in T1D and CON. In summary, skeletal muscle Ca2+ resequestration capacity was increased in T1D, but performance was not greater than CON. Sprint training reduced Ca2+-ATPase in T1D and CON. Sex differences in Ca2+-ATPase activity were evident and may be linked with fibre type proportion differences.
Introduction
High-intensity exercise challenges metabolic regulation in skeletal muscle. During intense exercise most cellular ATP is used by the ATPases (sodium–potassium pumps, actomyosin crossbridge interactions and calcium pumps) (Green, 1997). Muscle contraction and relaxation depends on the rates of sarcoplasmic reticulum (SR) Ca2+ release and resequestration and on other Ca2+ buffers that influence cytosolic and intra-SR [Ca2+]; hence, SR Ca2+ cycling is integral to muscle performance. It has been suggested that there may be a causal link between impairment of muscle Ca2+ regulation and fatigue during exercise in human muscle (Li et al. 2002). During muscular activity propagation of an action potential along the sarcolemma and into the t-tubules causes conformational change of the dihydropyridine receptors, which then activate the ryanodine receptors resulting in release of Ca2+ from the SR and hence a rise in cytosolic [Ca2+], thereby allowing muscle contraction. Calcium removal is accomplished by the sarcoplasmic reticulum calcium (SERCA) pumps (or Ca2+-ATPase) that transport Ca2+ ions against the concentration gradient into the SR (Allen et al. 2008). Rapid removal of intracellular Ca2+ is essential for muscle relaxation and to prevent proteolytic muscle cell damage (Lamb, 2009). During high-frequency electrical stimulation (a model for intense exercise) the sodium–calcium exchangers (NCXs), which can extrude or allow influx of Ca2+ depending on the cellular sodium gradient generated by the sodium–potassium ATPase (Na+–K+-ATPase), also contribute to intracellular Ca2+ handling (Germinario et al. 2008).
Altered cellular Ca2+ handling is evident in diabetes, and indeed is a fundamental disorder of the diabetic state (Levy et al. 1994), and hence affects cellular function. In skeletal muscle of male rats with streptozotocin (STZ)-induced diabetes, Ca2+-ATPase activity, Ca2+ uptake and NCX activity were increased and muscle function was enhanced (Ganguly et al. 1986; Taira et al. 1991). In human diabetes, Ca2+ handling is also altered in various tissues including red cells, platelets, arteries and adipocytes (Levy et al. 1994). In platelets drawn from patients with diabetes, intracellular Ca2+ and Ca2+-ATPase activity were higher than in non-diabetic controls and these changes have been linked to enhanced platelet aggregability (Mazzanti et al. 1990). However, despite the integral role of SR Ca2+ handling in regulating skeletal muscle function, no previous study has examined Ca2+ handling in skeletal muscle of patients with diabetes, and this is the first aim of the study.
Exercise training is a key component of diabetes management, especially given that people with diabetes report greater daily fatigue than people without diabetes (Fritschi & Quinn, 2010). High-intensity (sprint) exercise training improves objectively measured fatigue resistance during intense exercise (McKenna et al. 1997; Harmer et al. 2000). It is not known whether altered Ca2+ handling contributes to fatigue resistance during intense exercise after high-intensity exercise training or whether any effects of exercise training differ between people with or without diabetes. This is the second aim of the study.
Based on rodent muscle studies (Ganguly et al. 1986; Taira et al. 1991) and the limited data on human tissue other than muscle, we hypothesised that skeletal muscle SR Ca2+ uptake and ATPase activity would be higher in young patients with type 1 diabetes (T1D) compared with age-, body mass index (BMI)- and fitness-matched young non-diabetic controls. We also hypothesised that objectively measured fatigue resistance would be greater before and after high-intensity sprint training in the patients with diabetes compared with the controls. Although not part of our original hypotheses, after examination of our results from this study, we also compared responses to acute high-intensity exercise and sprint training between men and women to determine if sex differences in Ca2+ handling were significant.
Methods
Ethical approval
This study was approved by the Human Research Ethics Committees of The University of Sydney and Sydney South West Area Health Service, was conducted in accordance with the Declaration of Helsinki, and is registered with the Australia–New Zealand Clinical Trial Registry (ACTRN012606000368538-ANZCTR.org). Eight subjects with type 1 diabetes mellitus (T1D group; 3F, 5M) and eight healthy controls (CON group; 3F, 5M) were recruited. Each subject provided written informed consent after being provided with verbal and written information regarding the nature and risks of the experimental procedures. We have previously reported data for all but one of these subjects on muscle and blood potassium regulation (Harmer et al. 2006) and respiratory gas exchange and muscle metabolism (Harmer et al. 2008) during a single bout of high-intensity matched-work exercise, and on blood ion regulation during repeated 30 s sprint bouts (Harmer et al. 2007) before and after sprint training. Here we also include data from an extra male control subject from whom we obtained muscle for Ca2+ assays.
In subjects with T1D, duration of diabetes mellitus was 7.1 ± 4.0 years (mean ± SD), average daily insulin dose was 52 ± 11 U day−1 and glycated haemoglobin (HbA1c) was 8.6 ± 0.8%. Control subjects (HbA1c, 5.3 ± 0.3%) were closely matched with those with diabetes for age (CON, 23 ± 5; T1D, 25 ± 4 years), BMI (CON, 23.9 ± 4.6; T1D, 25.4 ± 3.2 kg m−2) and peak oxygen uptake (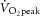 : CON, 3.27 ± 0.71; T1D, 3.30 ± 0.97 l min−1). Potential subjects with T1D were included if diabetes duration was ≥1.5 years and HbA1c was <10%, and were excluded from the study if overt complications of diabetes were present. Subjects without diabetes had no family history of metabolic disorders. No subject smoked, took regular medication (other than insulin in the T1D group) or had previously engaged in high-intensity cycle training.
: CON, 3.27 ± 0.71; T1D, 3.30 ± 0.97 l min−1). Potential subjects with T1D were included if diabetes duration was ≥1.5 years and HbA1c was <10%, and were excluded from the study if overt complications of diabetes were present. Subjects without diabetes had no family history of metabolic disorders. No subject smoked, took regular medication (other than insulin in the T1D group) or had previously engaged in high-intensity cycle training.
Experimental procedures
Testing was conducted in the fasted state. Subjects in the T1D group reduced their night-time dose of insulin by 1–2 U to prevent a hypoglycaemic episode on the morning of the tests, and delayed their morning insulin dose until after testing had been completed. Subjects abstained from alcohol consumption and vigorous exercise for 48 h prior to each test. Female subjects were tested in the follicular phase of the menstrual cycle (determined by subject feedback and subsequent plasma progesterone concentration) when possible. However, two women, one in each of the T1D and CON groups, had irregular menstrual cycles, and testing could not be accurately scheduled.
Exercise testing
Constant load sprint tests
(i) Prior to training, following an identical test (used for familiarisation) on a separate day, a sprint test (Pre) was conducted to exhaustion on an electronically braked cycle ergometer. After a 3-min warm up at 20 W, subjects pedalled at a power output equivalent to 130% 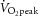 at 110 rev min−1 until exhaustion – defined as the inability to maintain a cadence ≥ 80 rev min−1, despite strong verbal encouragement. Muscle and blood were sampled in this sprint test. This test has been described in more detail in our previous reports of data sampled from the subjects in the present study (Harmer et al. 2006, 2008). (ii) After completion of training a test was conducted in which the pre-training power output and exercise duration were intentionally replicated for each individual, i.e. the post-training work (PostM) was matched with Pre. Blood and muscle were sampled in this test. (iii) A second post-training test (conducted on a separate day) was performed at the same power output, but continued until exhaustion (PostExh). This test was conducted to assess performance (time to fatigue and work) after training and there was no blood or muscle sampled.
at 110 rev min−1 until exhaustion – defined as the inability to maintain a cadence ≥ 80 rev min−1, despite strong verbal encouragement. Muscle and blood were sampled in this sprint test. This test has been described in more detail in our previous reports of data sampled from the subjects in the present study (Harmer et al. 2006, 2008). (ii) After completion of training a test was conducted in which the pre-training power output and exercise duration were intentionally replicated for each individual, i.e. the post-training work (PostM) was matched with Pre. Blood and muscle were sampled in this test. (iii) A second post-training test (conducted on a separate day) was performed at the same power output, but continued until exhaustion (PostExh). This test was conducted to assess performance (time to fatigue and work) after training and there was no blood or muscle sampled.
Exercise training
Subjects undertook seven weeks of three times-weekly supervised cycle sprint training. The training load was progressed from four to ten 30-s ‘all out’ sprints, separated by a 3- to 4-min rest period, as previously described (Harmer et al. 2006).
Muscle sampling and analyses
The skin overlying the vastus lateralis muscle was anaesthetised using 2% xylocaine (without adrenaline). Two percutaneous muscle biopsies (n = 7, CON; n = 8, T1D), obtained using an aseptic technique (Bergström 1962) with 6 mm biopsy needles (AB Stille-Werner, Stockholm, Sweden) with suction applied via a 50 ml syringe, were performed at rest and immediately after exercise (whilst still on the cycle ergometer) before (Pre test) and after training (PostM test). One female subject in the CON group declined muscle biopsy. The first muscle biopsy sample at each time was immediately immersed in liquid nitrogen for determination of muscle metabolites, high-energy phosphates and enzyme activities (Harmer et al. 2008). The second sample was used to determine Na+–K+-ATPase content (Harmer et al. 2006), Ca2+ uptake, Ca2+-ATPase activity and protein content. The average muscle tissue wet weight used for the calcium assays was 51.8 ± 2.7 mg (range 20–108 mg). Muscle for Ca2+ assays was rapidly weighed and then homogenised in 10 volumes of a pre-cooled homogenizing buffer (40 mm Tris, 0.3 m sucrose, 5 mm dithiothreitol, pH 7.9). Homogenisation was performed in a 10 mm Kimble tube on ice using an Omni 2000 hand-held electric homogeniser for three bursts of 15 s (with 15-s rests between each burst) at 60% maximum power.
Muscle homogenate protein content
Homogenate protein content was determined in triplicate, using serum albumin as the standard (Precimat, Boehringer Mannheim, Mannheim, Germany), according to a published method (Markwell et al. 1978). SR Ca2+-ATPase activity and Ca2+ uptake were corrected for muscle homogenate protein content and expressed as nmol min−1 (mg muscle protein)−1.
SR Ca2+-ATPase activity
The maximal in vitro rate of SR Ca2+-ATPase activity was measured in triplicate as previously described by our laboratory (Ruell et al. 1995; Hunter et al. 1999; Thom et al. 2001). Assays were performed at 37°C using a spectrophotometer (UV-1601PC; Shimadzu, Tokyo, Japan) at 340 nm, which allowed the quantification of the progressive decrease in absorbance of NADH as it was converted to NAD. The reaction medium consisted of 18 mm HEPES buffer, pH 7.5, 180 mm KCl, 13 mm MgCl2, 9 mm NaN3, 1 mm EGTA, 0.3 mm NADH, 9 mm phosphoenolpyruvate, 22 U ml−1 lactate dehydrogenase, 16 U ml−1 pyruvate kinase and 4 mm ATP. The calcium ionophore A-23187 (2.5 μm) was added to the reaction medium to permeabilise the SR membrane and prevent feedback inhibition of SR Ca2+-ATPase activity. One millilitre of assay buffer was mixed with 1.4 mg tissue wet weight of muscle homogenate and the reaction was initiated with the addition of 1.1 mm CaCl2, corresponding to a free Ca2+ concentration ([Ca2+]f) of ∼12.1 μm (Green et al. 1996). After 90 s, 36 mm CaCl2 was added to completely inhibit the Ca2+-ATPase activity, thus allowing the measurement of basal ATPase activity, and the reaction was followed for a further 90 s. The maximal SR Ca2+-ATPase activity was calculated by subtracting the basal ATPase (Ca2+-independent) from the total (Ca2+-dependent + Ca2+-independent) ATPase activities.
Ca2+ uptake
The peak rate of oxalate-supported muscle homogenate SR Ca2+ uptake was measured at 37°C using ratiometric dual-emission spectrofluorometry, with continuous stirring, using the fluorescent Ca2+-binding dye indo-1 (Ruell et al. 1995). The luminescence spectrophotometer (Aminco Bowman Series 2; SLM Instruments, Urbana, IL, USA) was set with an excitation wavelength of 349 nm, while the emission wavelength cycled between 410 and 485 nm (emission maxima for Ca2+-bound and Ca2+-free indo-1, respectively). Excitation bandpass width was set to 1 nm and emission bandpass width at 8 nm, with ratiometric data being collected every second. The reaction medium consisted of 20 mm HEPES, 150 mm KCl, 10 mm NaN3, 6.8 mm oxalate, 5 μm N,N,N8,N8-tetrakis(2-pyridylmethyl)-ethane diamine, 4.5 mm MgATP and 1 μm indo 1, pH 7.0. Addition of extra CaCl2 was not necessary because the Ca2+ in the assay buffers gave a starting [Ca2+]f of ∼1 μm. Calcium uptake was measured in triplicate after the addition of 50 μl of muscle homogenate to 2.2 ml of reaction medium. The reaction was allowed to proceed for ∼100 s before the addition of 40 μl of 200 mm EGTA and 120 μl of 100 mm CaCl2 for calibration purposes to give final concentrations of 3.5 and 5.0 mm, respectively. The dissociation constant for the Ca2+–indo 1 complex in 150 mm KCl buffer and 20 mm HEPES, pH 7.0, was determined to be 170 nm by using precise mixtures of Ca2+/EGTA. Alteration in the ratio of the emission signal at 410–485 nm reflects a change in the [Ca2+], which was calculated using a standard equation (Grynkiewicz et al. 1985). The maximal rate of Ca2+ uptake was determined by dividing the smoothed first derivative of [Ca2+] by the [Ca2+] versus time graph, such that Ca2+ uptake values were corrected to the same [Ca2+] of 1 μm (Ruell et al. 1995).
Blood sampling and analyses
A 22G flexible catheter (Optiva 225; Johnson & Johnson, Sydney, Australia) was inserted into a dorsal hand vein and the venous blood sampled at rest and during and after exercise (Pre and PostM) was arterialised and analysed as we previously described for these subjects (Harmer et al. 2006). Previously we reported the change in plasma glucose from the rest value (Harmer et al. 2006), whereas here we report absolute values. Plasma glucose was analysed using a commercial kit (Thermo Electron, Melbourne, Australia). Free (ionised) plasma calcium concentration (iCa) was determined using an automated blood gas analyser (Corning 865; Chiron Diagnostics, Medfield, MA, USA) and was analysed at the same time as our previously reported blood gas and plasma ion values (Harmer et al. 2006, 2008). A 5 ml blood sample, collected at rest before and after training from the six female subjects, was allowed to clot, then serum was drawn off and analysed for progesterone concentration with an automated immunoassay analyser (Abbott IMX, Abbott Diagnostics, Abbot Park, IL, USA) at Lidcombe-Bankstown Hospital.
Statistics
Blood and muscle data from Pre and PostM tests were analysed using repeated measures analysis of variance (ANOVA) with within-subjects factors: training status (‘day’; before training, after training), time (rest, exercise); and between-subjects factor: group (diabetes, no diabetes). Performance data from the Pre and PostExh tests were analysed using repeated-measures ANOVA with within-subjects factor: training status, and between-subjects factor: group (diabetes, no diabetes). Based on post hoc observation of possible differences between men and women, secondary analyses that used the same ANOVA procedures, but with a between-subjects factor of sex, were conducted. Main effects and interaction effects are reported. Significant F ratios were further examined using an ANOVA contrast technique. Data are expressed as mean ± SEM. Correlations were determined using linear regression; and Pearson's r is reported. All statistical procedures were performed using IBM SPSS Statistics v 21 (IBM Corp).
Results
Performance – Pre and PostExh tests
Exercise to fatigue
While there was a tendency for those in the T1D group to have a longer time to fatigue before (Pre) and after (PostExh) training compared with the control group (CON, 74 ± 8; T1D 95 ± 8 s; P = 0.08), work performed until fatigue did not differ between groups (CON, 26 ± 3; T1D, 31 ± 3 kJ; P = 0.33). High-intensity training increased the work performed until fatigue by 43 ± 6% (PostExh; P < 0.0001) compared with Pre, with no difference between CON (+41 ± 8%) and T1D (+45 ± 9%; training status × group interaction, P = 0.78; Table 1).
Table 1.
Work (kJ) performed to fatigue before and after training
| n | Pre | PostExh*† | |
|---|---|---|---|
| CON | 8 | 21 ± 2 | 30 ± 4 |
| T1D | 8 | 25 ± 3 | 37 ± 4 |
| Men | 10 | 27 ± 2 | 41 ± 3 |
| Women | 6 | 17 ± 2 | 22 ± 3 |
Data are mean ± SEM. CON, non-diabetic control group; T1D, group with type 1 diabetes. Pre, pre-training test to fatigue; PostExh, post-training test to fatigue. *P < 0.001, main effect of training status, PostExh>Pre in CON and T1D; †P = 0.001, main effect of sex, Men>Women.
Work performed to fatigue was less before and after training (P = 0.001) among the women (20 ± 3 kJ) than the men (34 ± 2 kJ) with a mean difference of 14 ± 3 kJ (95% confidence interval (CI) 7-21). Improvement in work to fatigue with training was also less among the women than the men (Women, +29 ± 5%; Men, +51 ± 8%; training status × sex interaction, P < 0.01; Table 1).
Muscle – Pre and PostM tests
Total protein content
Vastus lateralis muscle homogenate total protein content (wet weight) did not differ between T1D and CON groups (across both times and days, P = 0.83; Table 2), with exercise (main effect of time, P = 0.86), with exercise training (main effect of training status, P = 0.36), or between the sexes across both times and days (P = 0.82; Table 2).
Table 2.
Muscle total protein content (mg g−1 ww)
| Before training (Pre) |
After training (PostM) |
|||
|---|---|---|---|---|
| Group | Rest | Exercise | Rest | Exercise |
| CON | 144 ± 6 | 143 ± 4 | 143 ± 7 | 142 ± 4 |
| T1D | 142 ± 5 | 140 ± 4 | 147 ± 7 | 140 ± 4 |
| Men | 142 ± 5 | 144 ± 3 | 142 ± 6 | 141 ± 3 |
| Women | 145 ± 7 | 137 ± 4 | 151 ± 8 | 141 ± 5 |
Data are mean ± SEM. CON, n = 7, except Pre Exercise where n = 6; T1D, n = 8; Men, n = 10, except Pre Exercise where n = 9; Women, n = 5; ww, wet weight.
Basal, total and Ca2+-ATPase activity
Basal ATPase activity did not differ with time (P = 0.55), training status (P = 0.79), or between T1D and CON groups (P = 0.40). There was a tendency for basal ATPase to be higher in the women than the men across both times and days (Women, 14.4 ± 0.9; Men, 12.2 ± 0.7 nmol min−1 (mg Pr)−1; P = 0.08).
Total ATPase activity was higher in the T1D group across both times and days (CON, 91.0 ± 6.3; T1D, 111.7 ± 5.9 nmol min−1 (mg Pr)−1; P < 0.05) with a mean difference of 20.7 ± 8.7 nmol min−1 (mg Pr)−1 (95% CI 2.0–39.4). Total ATPase activity was not altered by acute high-intensity dynamic exercise (Rest, 102.5 ± 5.5; Exercise, 100.2 ± 3.9 nmol min−1 (mg Pr)−1; main effect of time, P = 0.57) but tended to be lower after sprint training (Before training, 104.9 ± 4.8; After training, 97.8 ± 4.5 nmol min−1 (mg Pr)−1; main effect of training status, P = 0.05). Total ATPase activity was lower (P < 0.05) in the women (88.0 ± 7.6 nmol min−1 (mg Pr)−1) than the men (109.0 ± 5.4 nmol min−1 (mg Pr)−1) across both times and days. In response to acute high-intensity exercise, total ATPase activity was increased in women but reduced in men (Women, Rest 82.9 ± 8.0; Exercise 93.2 ± 8.1; Men, Rest 113.3 ± 5.6, Exercise 104.8 ± 5.7 nmol min−1 (mg Pr)−1; time × sex interaction, P < 0.05).
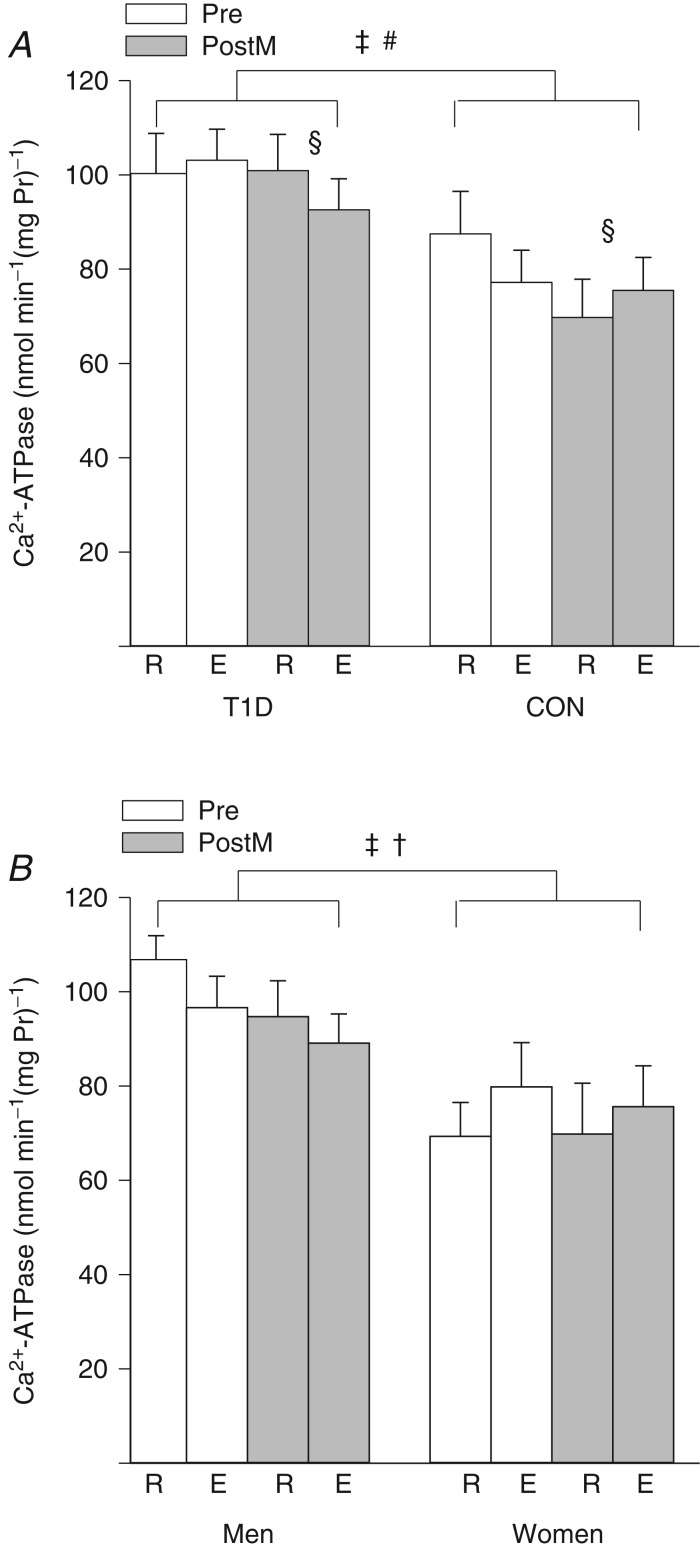
Maximal in vitro Ca2+-ATPase activity was 28% higher across both times and days in the T1D group (CON, 77.5 ± 6.7; T1D, 99.2 ± 6.3 nmol min−1 (mg Pr)−1; P < 0.05; Fig. 1A) with a mean difference of 21.7 ± 9.2 nmol min−1 (mg Pr)−1 (95% CI 1.9–41.6). Ca2+-ATPase activity was not altered by acute high-intensity dynamic exercise (Rest, 89.6 ± 5.6; Exercise, 87.1 ± 4.1 nmol min−1 (mg Pr)−1; main effect of time, P = 0.47). Although Ca2+-ATPase activity was 8% lower across both times after sprint training (Before training, 92.0 ± 5.1; After training, 84.7 ± 4.7 nmol min−1 (mg Pr)−1; P < 0.05; Fig. 1A), Ca2+-ATPase activity at rest was lower in CON, but preserved in T1D after training (training status × time × group interaction, P < 0.05). Ca2+-ATPase activity was 24% lower (P < 0.05) among the women (n = 5; 73.6 ± 7.9 nmol min−1 (mg Pr−1)) than the men (n = 10; 96.8 ± 5.6 nmol min−1 (mg Pr)−1; Fig. 1B) across both times and days. Ca2+-ATPase activity increased in women after acute high-intensity exercise but decreased in men (time × sex interaction, P < 0.05; Fig. 1B).
Figure 1.
Maximal in vitro calcium ATPase activity (mean ± SEM) in vastus lateralis at rest (R) and immediately after high-intensity exercise (E) before (Pre; open bars) and after sprint training (PostM; grey bars). A, among the group with type 1 diabetes (T1D; n = 8) and the non-diabetic control group (CON; n = 7, except in Pre rest where n = 6 and Pre exercise where n = 5). ‡Main effect for group, T1D > CON, P < 0.05; §main effect of sprint training, Pre > PostM, P < 0.05; and #training status × time × group interaction, T1D rest unchanged and CON rest lower after training, P < 0.05. B, among the men (n = 10, except Pre rest where n = 9 and Pre exercise where n = 8) and the women (n = 5). ‡Main effect for sex, Men>Women, P < 0.05; and †time × sex interaction, E < R in men, but not women, P < 0.05.
Ca2+ uptake
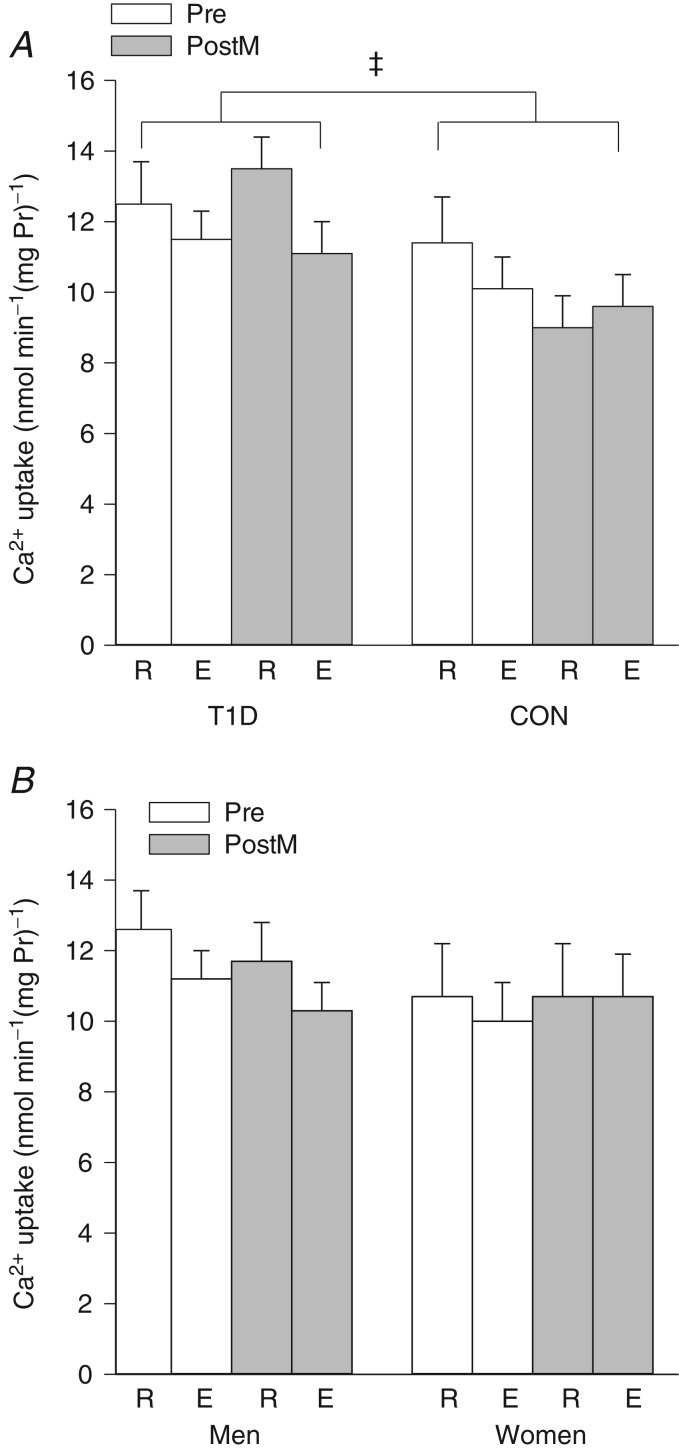
In vitro Ca2+ uptake was 21% higher in the T1D group across both times and days (CON, 10.0 ± 0.6; T1D, 12.1 ± 0.5 nmol min−1 (mg Pr)−1; P < 0.05; Fig. 2A) with a mean difference of 2.1 ± 0.8 nmol min−1 (mg Pr)−1 (95% CI 0.5–3.8). Ca2+ uptake was not significantly altered by acute high-intensity dynamic exercise (main effect of time, P = 0.10) or by sprint training (main effect of training status, P = 0.47; Fig. 2A). There was no significant effect of sex on Ca2+ uptake (P = 0.35; Fig. 2B); however, when the men were analysed separately there was a tendency for lower Ca2+ uptake after intense exercise (main effect of time, P = 0.06).
Figure 2.
Maximal in vitro calcium uptake (mean ± SEM) in vastus lateralis at rest (R) and immediately after high-intensity exercise (E) before (Pre; open bars) and after sprint training (PostM; grey bars). A, among the group with type 1 diabetes (T1D; n = 8) and the non-diabetic control group (CON; n = 7, except in Pre rest where n = 6 and Pre exercise where n = 5). ‡Main effect for group, T1D>CON, P < 0.05. B, among the men (n = 10, except Pre rest where n = 9 and Pre exercise where n = 8) and the women (n = 5).
Ratio of Ca2+ uptake to Ca2+-ATPase activity
The ratio of in vitro Ca2+ uptake to Ca2+-ATPase activity did not differ between T1D and CON groups across both times and days (CON, 0.13 ± 0.01; T1D, 0.13 ± 0.01; P = 0.75) and was not significantly changed by acute high-intensity exercise across both days (Rest, 0.13 ± 0.01, Exercise, 0.12 ± 0.01; P = 0.16) or by sprint training (Before training, 0.13 ± 0.01, After training, 0.13 ± 0.01; P = 0.47). There was a higher ratio of Ca2+ uptake to Ca2+-ATPase activity among the women than the men across both times and days (Women, 0.14 ± 0.01; Men, 0.12 ± 0.01; P < 0.05).
Correlations
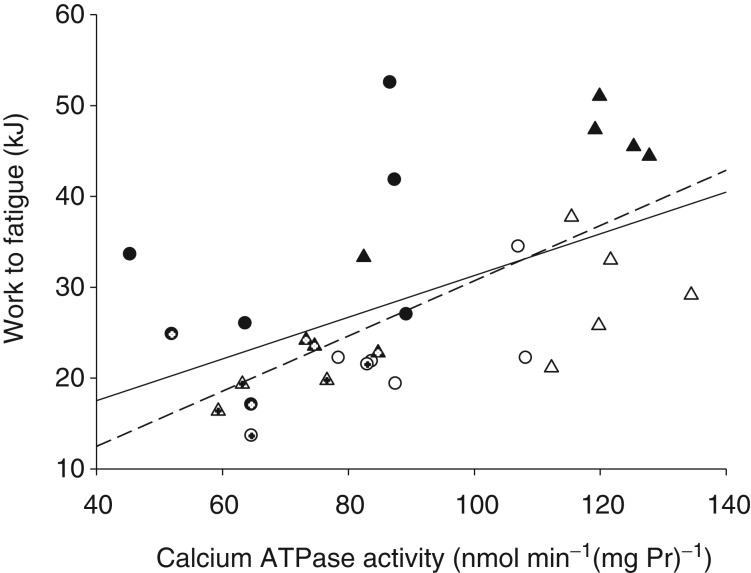
Ca2+-ATPase activity before exercise (at rest) was highly correlated with Ca2+-ATPase activity at the end of exercise (r = 0.73; P < 0.001), and with Ca2+uptake at rest (r = 0.53; P = 0.01) and at the end of exercise (r = 0.44; P < 0.05) across both days for all subjects. Ca2+-ATPase activity at rest was correlated with work performed until exhaustion across both days for all subjects (r = 0.53, r2 = 0.28, P < 0.01; Fig. 3, continuous line).
Figure 3.
Maximal in vitro calcium ATPase activity at rest and the amount of work performed to fatigue among the group with type 1 diabetes (T1D) before (open triangles) and after sprint training (filled triangles) and the non-diabetic control group (CON) before (open circles) and after sprint training (filled circles). The women in each group are indicated by cross-hairs within the symbols. Solid line, regression for all subjects, pooled data: r = 0.53, r2 = 0.28, P < 0.01. Dashed line, regression for the T1D group only: r = 0.70, r2 = 0.49, P = 0.002.
Interestingly, when considered as separate groups, there was no correlation between Ca2+-ATPase activity at rest and work performed to exhaustion for CON, women or men. However, for T1D only, Ca2+-ATPase activity at rest was highly correlated with work performed to exhaustion (r = 0.70, r2 = 0.49, P = 0.002; Fig. 3, dashed line).
Blood – Pre and PostM tests
Ionised (free) plasma calcium (iCa)
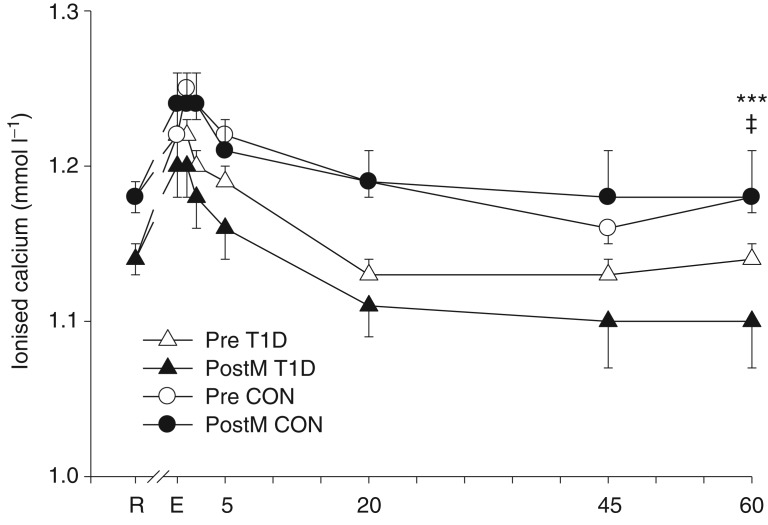
Plasma iCa was lower across both days and all times in T1D compared with CON (CON 1.21 ± 0.01, T1D 1.16 ± 0.01 mmol l−1; P < 0.05; Fig. 4). In both T1D and CON, iCa rose with high-intensity exercise (effect of time, P < 0.001) and remained above the resting value until 20 min of recovery; it was not affected by sprint training (P = 0.40). Sex did not affect iCa (Women, 1.19 ± 0.02; Men, 1.19 ± 0.01 mmol l−1; P = 0.90).
Figure 4.
Plasma ionised calcium concentration (mean ± SEM) among the T1D (triangles; n = 8) and CON (circles; n = 8, except where n = 7 for CON post training at 5–60 min recovery) groups before (open symbols) and after (filled symbols) sprint training at rest (R), immediately after intense exercise (E), and at 1, 2, 5, 20, 45 and 60 min of recovery. ‡Main effect of group, T1D < CON, P < 0.05; and ***main effect of time, E, 1, 2 and 5>R, P < 0.001.
Plasma glucose
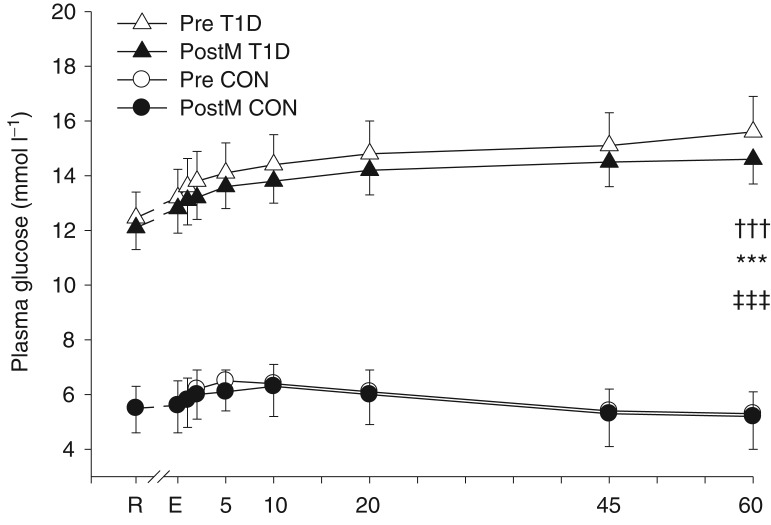
Plasma glucose concentration was higher across both days and all times in T1D than CON (CON, 5.8 ± 0.8; T1D, 13.89 ± 0.8 mmol l−1; P < 0.001; Fig. 5). In CON, across both days, plasma glucose rose with exercise, peaked at 6.4 ± 0.8 mmol l−1 at 10 min of recovery and returned to resting values by 45 min recovery. In T1D, across both days, plasma glucose also rose with exercise, but continued to rise unabated throughout recovery (time × group interaction, P < 0.001). There was no effect of sprint training (P = 0.70) or sex on plasma glucose concentration (Women, 10.7 ± 2.0; Men, 9.3 ± 1.5 mmol l−1; P = 0.59).
Figure 5.
Plasma glucose concentration (mean ± SEM) among the T1D (triangles; n = 8) and CON (circles; n = 8) groups before (open symbols) and after (filled symbols) sprint training at rest (R), immediately after intense exercise (E), and at 1, 2, 5, 10, 20, 45 and 60 min of recovery. ‡‡‡Main effect of group, T1D < CON, P < 0.0001; †††time × group interaction, T1D>CON at every time, P < 0.001; and ***main effect of time, P < 0.001.
Serum progesterone
Indicative of successfully testing four of the six females in the follicular phase of the menstrual cycle, progesterone concentrations were low before (1.8 ± 0.2 nmol l−1) and after (2.6 ± 0.6 nmol l−1) training. In the remaining two females (one each from CON and T1D groups), due to irregular menstrual cycles, one test was successfully conducted in the follicular phase (2.0 and 4.8 nmol l−1) whilst the other was during the luteal phase (18.7 and 33.4 nmol l−1).
Discussion
The effects of acute high-intensity exercise and high-intensity (sprint) cycle exercise training on SR Ca2+-ATPase activity, Ca2+ uptake and performance were investigated for the first time among young patients with T1D and age-, BMI- and fitness-matched control subjects without diabetes. The major findings were that (1) Ca2+-ATPase activity and Ca2+ uptake were higher across both times and days in T1D than CON, (2) Ca2+-ATPase activity was lower across both times and days in women than men, (3) Ca2+-ATPase activity and Ca2+ uptake were preserved after acute high-intensity exercise in T1D and CON, although sex differences were evident: Ca2+-ATPase activity was reduced in men but increased in women after high-intensity exercise across both days, and (4) sprint training reduced Ca2+-ATPase activity but not Ca2+ uptake.
Higher Ca2+-ATPase and Ca2+ uptake in patients with T1D
The present study demonstrates for the first time that Ca2+-ATPase activity and Ca2+ uptake are higher in skeletal muscle of young patients with T1D than in non-diabetic controls. Consistent with our results, Ca2+ uptake and Ca2+-ATPase activity (Ganguly et al. 1986; Taira et al. 1991) were higher in skeletal muscle of male rats with STZ-induced diabetes than in male non-diabetic control rats. Moreover, 2–4 weeks of insulin treatment returned Ca2+ uptake and ATPase activity to normal levels. Briefer periods of insulin treatment were not examined, and thus the time course of the response is unknown. Insulin regulates skeletal muscle Ca2+ pumps (SERCA1 and 2) via the insulin receptor substrates (IRS)-1 and -2 and the subsequent tyrosine phosphorylation cascade; and in STZ diabetes, the association of IRS-1 and IRS-2 with SERCA1 and SERCA2 was reduced (Algenstaedt et al. 1997).
Patients with diabetes in the present study reduced their usual evening insulin dose by 1–2 U to prevent hypoglycaemia on the morning of testing and delayed their morning insulin dosage until laboratory testing was completed. We previously reported (Harmer et al. 2006) immunoreactive insulin concentrations in the T1D (free insulin) and CON groups and there was no difference between groups prior to exercise. However, as evidenced by the elevated mean blood glucose level before exercise (Fig. 5), the subjects in the T1D group were mildly relatively hypoinsulinaemic. In contrast, in the non-fasted state and when insulin has been administered, patients with T1D will probably have moderate basal hyperinsulinaemia because of the need to supply an insulin dose sufficient to normalize portal vein insulin levels and control hepatic glucose output. Whether Ca2+-ATPase activity and Ca2+ uptake were chronically elevated in our patients or transiently elevated due to direct or indirect effects of mild relative hypoinsulinaemia cannot be determined from the present study. Interestingly, and probably relevant to the present study, red blood cell sodium–potassium ATPase activity was lower and magnesium ATPase activity higher in five patients with uncontrolled T1D than in non-diabetic controls, and activities were restored to control levels after a 24 h insulin infusion titrated to achieve euglycaemia (Rahmani-Jourdheuil et al. 1987). These data imply that cellular ATPases are rapidly responsive to changing concentrations of insulin (and/or glycaemia) in humans.
Other Ca2+-regulating systems are also affected in diabetes and probably contribute to Ca2+ cycling to a lesser extent than Ca2+-ATPase activity. NCXs in the plasma membrane can extrude or allow influx of Ca2+ to the muscle cell according to the sodium gradient established by the Na+–K+-ATPase. There is a paucity of data regarding the effects of insulin, hyperglycaemia or diabetes on skeletal muscle sodium–calcium exchange. However, NCX activity and Ca2+ influx were elevated in sarcolemmal preparations from diabetic rat muscle (Taira et al. 1991). Mild relative hypoinsulinaemia and consequent hyperglycaemia, as our patients with diabetes experienced during testing, may have affected Ca2+ exchange via the NCX and contributed to differences in Ca2+ regulation via alteration in muscle [Ca2+], but this remains to be tested.
STZ diabetes can also upregulate muscle calmodulin concentration and increase phospholipid membrane permeability to Ca2+, thus potentially increasing Ca2+-ATPase activity (Taira et al. 1988); however, this has not been examined in humans. Skeletal muscle SR Ca2+-ATPase activity may also be altered via elevated or depressed levels of thyroid hormone (triiodothyronine, T3; Simonides et al. 2001). In animals with STZ diabetes, insulin administration restored or elevated T3 levels above normal (Sundaresan et al. 1984). However, among human patients with T1D, thyroid hormone levels are normal unless there is coincident thyroid disease (which, being autoimmune, is more common in T1D than in the general population – but is still uncommon). To our knowledge there is no evidence in humans that intermittent hyperinsulinaemia (due to insulin therapy) influences thyroid hormone levels. Conversely, the low levels of T3 (and thyroxine (T4) and thyrotropin (TSH)) evident in human diabetic ketoacidosis (Schmitz et al. 1981), one among many acute or chronic disease states that induce ‘sick euthyroid syndrome’ (or non-thyroidal illness syndrome) (Economidou et al. 2011), would be anticipated to depress SR Ca2+-ATPase activity (Simonides et al. 2001); however, this has not been investigated. Although our diabetic patients reduced their evening insulin dose by one or two units and delayed their morning insulin administration until after testing was completed, none was in a state of ketoacidosis.
An interesting finding in the present study was lower iCa across all times and both days in T1D compared with CON. Lower iCa has previously been reported in T1D (Fogh-Andersen et al. 1982) and is positively related to lower bone mineral density (Hamilton et al. 2009); however, the relationship between iCa and muscle Ca2+ regulation is unknown.
Ca2+-ATPase activity is lower in women than men
Ca2+-ATPase activity was lower among the women than the men in the present study when data from the CON and T1D groups were combined. Only one previous study (Fano et al. 2001) has examined sex differences in skeletal muscle Ca2+ regulation. Consistent with results of the present study, albeit not statistically significant, Ca2+-ATPase activity tended to be lower (∼20% lower) in young women (n = 5) than young men (n = 5) (Fano et al. 2001). Two previous studies exclusively recruited women, and investigated effects of strength training in young compared with older women (Hunter et al. 1999) and effects of leg cast immobilisation (Thom et al. 2001). Maximal in vitro Ca2+-ATPase activity among the women in the present study was 73.6 ± 7.9 nmol min−1 (mg Pr)−1, which compares very closely to ∼80 nmol min−1 (mg Pr)−1 for the young women in Hunter et al. (1999) and 75.7 ± 4.5 nmol min−1 (mg Pr)−1 in Thom et al. (2001). In contrast, in a number of studies that have exclusively recruited men, Ca2+-ATPase activity varied between ∼89 and 96 nmol min−1 (mg Pr)−1 (Booth et al. 1997; Ørtenblad et al. 2000; Li et al. 2002) and these data compare very closely with our finding of 96.8 ± 5.6 nmol min−1 (mg Pr)−1 among the men in the present study.
A probable contributor to the 24% lower Ca2+-ATPase activity in the women in the present study is the sex difference in muscle fibre type. Women have a higher type I proportional area (+6 to 19%) of the muscle than men (Simoneau & Bouchard, 1989; Roepstorff et al. 2006) and lower type II subgroup proportional areas (11% IIA Roepstorff et al. 2006; 3% IIB Simoneau & Bouchard, 1989). Human type II fibres have been estimated to have approximately 3- to 4-fold higher Ca2+-ATPase activity (Li et al. 2002; Green et al. 2009) and 2-fold higher Ca2+ uptake (Li et al. 2002) than type I fibres; and a strong linear relationship (r = 0.72) exists between the proportion of type II fibres and Ca2+-ATPase content (Madsen et al. 1994). Thus, it may be anticipated that women will have lower Ca2+-ATPase activity – which we confirmed in the present study. Similarly, endurance-trained athletes had a higher percentage of type I fibres than either untrained or resistance-trained subjects and had correspondingly lower Ca2+-ATPase activity (Li et al. 2002). Ca2+ uptake was not different amongst women and men in the present study, although this may be a type II error given our small number of women; or perhaps sensitivity of Ca2+-ATPase to activation is higher in women than men given the higher ratio of Ca2+ uptake to Ca2+-ATPase activity in women in the present study. The tendency for higher basal ATPase activity in the women in the present study is consistent with the higher basal ATPase activity in soleus (predominantly slow twitch) compared with white gastrocnemius (predominantly fast twitch) in rodent muscle (Dossett-Mercer et al. 1994); however, the physiological significance of this finding is not known.
To our knowledge, only one study (Liu et al. 2008) has examined the effects of sex hormones on skeletal muscle Ca2+ handling. In aged male rats, oestrogen injections had little effect on genioglossal muscle SR Ca2+-ATPase activity (Liu et al. 2008). However, in whole heart preparations from ovariectomised female rats, Ca2+ uptake and SR Ca2+-ATPase activity were suppressed; and with oestrogen and/or progesterone supplementation, Ca2+ sequestration capacity was restored (Bupha-Intr & Wattanapermpool, 2006). We attempted to test our female subjects, both before and after training, in the follicular phase of the menstrual cycle, and were successful in four of the five women from whom we obtained a muscle biopsy. Whether low progesterone levels contributed to lower skeletal muscle Ca2+-ATPase activity in our female subjects is unknown; however, it may be plausible given the effects of progesterone in rodent heart muscle.
Ca2+-ATPase activity and uptake were preserved with intense exercise, but the response differs by sex
Ca2+-ATPase activity and Ca2+ uptake were preserved, in both T1D and CON across both days, immediately after acute high-intensity exercise that continued for a period of 70 ± 5 s. Hill et al. (2001), who induced fatigue during 7 min of maximal one-legged kicking, also found no reduction in Ca2+-ATPase activity at fatigue; however, Ca2+ uptake was reduced. Li et al. (2002) reported marked reductions in both Ca2+-ATPase activity and Ca2+ uptake after 50 maximal one-legged knee extensions. The differences between these studies and the present study may be attributable to different modes of exercise; however, they cannot be attributed to a lesser degree of muscle metabolic perturbation in the present study. Intracellular perturbation, as evidenced by glycogen depletion, lactate accumulation and ATP and phosphocreatine (PCr) depletion, was at least as great prior to training in subjects in the present study (Harmer et al. 2008) as in Hill et al. (2001) and Li et al. (2002).
However, it appears that the main difference between studies, with regard to maximal in vitro Ca2+-ATPase activity, is explained by the greater inclusion of women in the present study, as demonstrated by the differing responses to acute exercise: preservation of activity in women and reduction of activity in men (Fig. 2). All eight untrained subjects and all but one of the 16 athletes in Li et al. (2002) and all but two subjects in Hill et al. (2001) (n = 9) were healthy young men. Prolonged moderate-intensity exercise consistently resulted in reduction of Ca2+ uptake (Booth et al. 1997; Leppik et al. 2004) and Ca2+-ATPase activity (Booth et al. 1997; Green et al. 1998); and all but one of the subjects in these studies were men. Reduction in maximal Ca2+-ATPase activity and Ca2+ uptake with acute intense exercise was related to proportion of type II fibres (Li et al. 2002), and given that women generally have a higher proportion of type I fibres than men (Simoneau & Bouchard, 1989; Roepstorff et al. 2006), this may partially explain the preservation of Ca2+-ATPase activity with intense exercise in women in the present study. With regard to Ca2+ uptake, when data only from the men were analysed, there was a tendency for lower uptake after intense exercise (P = 0.06), suggesting a type II error.
Reduced Ca2+-ATPase activity but preserved Ca2+ uptake with sprint training
The present study demonstrated lower Ca2+-ATPase activity across both times, but no significant change in Ca2+ uptake, after sprint training. Interestingly, Ca2+-ATPase activity at rest did not change after training in the T1D group, but was lower in CON. No previous study has examined Ca2+ handling in skeletal muscle of patients with diabetes, so these data are novel. The only previous study to investigate sprint training effects on Ca2+ handling in human skeletal muscle did not include post-exercise data, but demonstrated no change in Ca2+ uptake or Ca2+-ATPase activity at rest, but increased density of SERCA1 and SERCA2 isoforms after 5 weeks of training (Ørtenblad et al. 2000).
With regard to other forms of exercise training and muscle Ca2+ regulation, 3 months of strength training in young men (Green et al. 1998) or women (Hunter et al. 1999) had no effect on resting Ca2+ uptake and/or ATPase activity; however, it did attenuate the reduction in Ca2+-ATPase activity with moderate-intensity exercise in men (Green et al. 1998) and increased both resting Ca2+ uptake and Ca2+-ATPase activity in elderly women (Hunter et al. 1999). Intensified training in moderately endurance-trained subjects did not alter Ca2+-ATPase activity (Madsen et al. 1994), but values may have already been depressed by exercise training. Cross-sectional data demonstrate lower Ca2+ uptake, Ca2+-ATPase and Ca2+ release in endurance-trained men compared with resistance-trained or untrained subjects, and lower Ca2+ uptake in resistance-trained subjects compared with untrained subjects (Li et al. 2002). Exercise training thus appears capable of inducing alterations in muscle Ca2+ handling at rest and during exercise, as confirmed by the present study; however, effects may be dose-related and affected by sex and/or age.
Performance during high-intensity exercise was similarly enhanced in both groups after sprint training in the present study. Work performed to fatigue was correlated with Ca2+-ATPase activity at rest in the whole cohort, but this was due to the strong correlation among the T1D group (r = 0.70). This finding demonstrates that calcium handling is important in diabetes, but that other mechanisms are also of importance in fatigue given that performance did not differ among the groups with and without diabetes. Li et al. (2002) demonstrated an association between the fatigue index and the change in Ca2+ uptake and ATPase activity with intense fatiguing exercise among a cohort that included exercise-trained and untrained subjects.
In conclusion, this study demonstrated that young patients with T1D had similar performance, but higher Ca2+ uptake and ATPase activity than age-, BMI-, and fitness-matched non-diabetic controls. The mechanisms of these differences remain to be investigated, but may be related to (1) prior relative hyperinsulinaemia (from therapy) or (2) relative hypoinsulinaemia and/or hyperglycaemia during testing. The present study also demonstrated that young patients with T1D adapted equally well, compared with non-diabetic controls, to the marked stresses imposed by sprint training. Sex differences in Ca2+ resequestration capacity at rest and during exercise were notable, with the women exhibiting lower Ca2+-ATPase activity and performing less work before fatigue, but showing preservation of Ca2+-ATPase activity with high-intensity exercise. The mechanisms for the sex differences may include differences in muscle fibre type proportions and/or sex hormones. Sprint training improved performance during intense exercise, and although we cannot speculate regarding the mechanisms involved, training also reduced Ca2+-ATPase activity.
Key points
Skeletal muscle calcium resequestration and performance is increased in male rats with induced diabetes; and whilst muscle calcium resequestration is important during exercise, it has not been investigated in human diabetes or compared between sexes.
We show that Ca2+-ATPase activity and Ca2+ uptake are higher among people with type 1 diabetes (T1D) compared with matched non-diabetic controls (CON), but that performance during intense exercise was similar; Ca2+-ATPase activity and Ca2+ uptake are also higher among men than women.
We show that Ca2+-ATPase activity is reduced during intense exercise in men but not women, and is reduced by high-intensity exercise training (HIET) in T1D and CON.
Fatigue is commonly reported by people with diabetes, but our data show that muscle calcium resequestration and performance during intense exercise and after HIET is not impaired in T1D, and hence other physiological or psychological mechanisms for fatigue in diabetes must be sought.
Sex influences muscle calcium regulation.
Acknowledgments
We are very grateful to Drs Grace Bryant and James Harrison who skilfully performed the muscle biopsies; and to our wonderful subjects for their dedication to exercise testing and training.
Glossary
- BMI
body mass index
- CI
confidence interval
- CON
control group
- HbA1c
glycated haemoglobin
- iCa
ionised (free) calcium
- NCX
sodium–calcium exchanger
- PostExh
post-training performance test to fatigue
- PostM
post-training matched work blood and biopsy test
- Pre
pre-training blood and biopsy test to fatigue
- SERCA
sarcoplasmic reticulum calcium ATPase
- SR
sarcoplasmic reticulum
- STZ
streptozotocin
- T3
triiodothyronine
- T1D
type 1 diabetes
Competing interests
None.
Author contributions
The work presented in this paper was undertaken at The University of Sydney in laboratories located in the Discipline of Exercise Science, Lidcombe. Analyses were performed in the Biochemistry laboratories at the University of Sydney, Lidcombe, and in the biochemistry laboratory at Victoria University, Melbourne, Australia. A.R.H.: conception and design of the experiments; collection, analysis and interpretation of data; drafting and revising the article. P.A.R.: collection, analysis and interpretation of data; revising the article. S.K.H.: collection, analysis and interpretation of data; revising the article. M.J.M.: conception and design of the experiments; collection, analysis and interpretation of data; revising the article. J.M.T.: collection, analysis and interpretation of data; revising the article. D.J.C.: conception and design of the experiments; interpretation of data; revising the article. J.R.F.: interpretation of data; revising the article. We confirm that all authors approved the final version of the manuscript and all persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
Funds for the study were provided by the University of Sydney and Victoria University, Australia.
References
- Algenstaedt P, Antonetti DA, Yaffe MB, Kahn CR. Insulin receptor substrate proteins create a link between the tyrosine phosphorylation cascade and the Ca2+-ATPases in muscle and heart. J Biol Chem. 1997;272:23696–23702. doi: 10.1074/jbc.272.38.23696. [DOI] [PubMed] [Google Scholar]
- Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev. 2008;88:287–332. doi: 10.1152/physrev.00015.2007. [DOI] [PubMed] [Google Scholar]
- Bergström J. Muscle electrolytes in man. Determined by neutron activation analysis on needle biopsy specimens. A study on normal subjects, kidney patients, and patients with chronic diarrhaea. Scand J Clin Lab Invest. 1962;14((Suppl 68)):1–110. [Google Scholar]
- Booth J, McKenna MJ, Ruell PA, Gwinn TH, Davis GM, Thompson MW, Harmer AR, Hunter SK, Sutton JR. Impaired calcium pump function does not slow relaxation in human skeletal muscle after prolonged exercise. J Appl Physiol. 1997;83:511–521. doi: 10.1152/jappl.1997.83.2.511. [DOI] [PubMed] [Google Scholar]
- Bupha-Intr T, Wattanapermpool J. Regulatory role of ovarian sex hormones in calcium uptake activity of cardiac sarcoplasmic reticulum. Am J Physiol Heart Circ Physiol. 2006;291:H1101–1108. doi: 10.1152/ajpheart.00660.2005. [DOI] [PubMed] [Google Scholar]
- Dossett-Mercer J, Green HJ, Chin E, Grange F. Preservation of sarcoplasmic reticulum Ca2+-sequestering function in homogenates of different type composition following sprint activity. Can J Physiol Pharmacol. 1994;72:1231–1237. doi: 10.1139/y94-175. [DOI] [PubMed] [Google Scholar]
- Economidou F, Douka E, Tzanela M, Nanas S, Kotanidou A. Thyroid function during critical illness. Hormones. 2011;10:117–124. doi: 10.14310/horm.2002.1301. [DOI] [PubMed] [Google Scholar]
- Fano G, Mecocci P, Vecchiet J, Belia S, Fulle S, Polidori MC, Felzani G, Senin U, Vecchiet L, Beal MF. Age and sex influence on oxidative damage and functional status in human skeletal muscle. J Muscle Res Cell Motil. 2001;22:345–351. doi: 10.1023/a:1013122805060. [DOI] [PubMed] [Google Scholar]
- Fogh-Andersen N, McNair P, Moller-Petersen J, Madsbad S. Serum calcium fractions in diabetes mellitus. Clin Chem. 1982;28:2073–2076. [PubMed] [Google Scholar]
- Fritschi C, Quinn L. Fatigue in patients with diabetes: a review. J Psychosom Res. 2010;69:33–41. doi: 10.1016/j.jpsychores.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly PK, Mathur S, Gupta MP, Beamish RE, Dhalla NS. Calcium pump activity of sarcoplasmic reticulum in diabetic rat skeletal muscle. Am J Physiol. 1986;251:E515–523. doi: 10.1152/ajpendo.1986.251.5.E515. [DOI] [PubMed] [Google Scholar]
- Germinario E, Esposito A, Midrio M, Peron S, Palade PT, Betto R, Danieli-Betto D. High-frequency fatigue of skeletal muscle: role of extracellular Ca2+ Eur J Appl Physiol. 2008;104:445–453. doi: 10.1007/s00421-008-0796-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green HJ. Mechanisms of muscle fatigue in intense exercise. J Sports Sci. 1997;15:247–256. doi: 10.1080/026404197367254. [DOI] [PubMed] [Google Scholar]
- Green HJ, Burnett ME, D'Arsigny C, Iqbal S, Ouyang J, Webb KA, O'Donnell DE. Muscle fibre type characteristics in females with chronic obstructive pulmonary disease. A preliminary study. J Mol Histol. 2009;40:41–51. doi: 10.1007/s10735-009-9211-8. [DOI] [PubMed] [Google Scholar]
- Green HJ, Grange F, Chin C, Goreham C, Ranney D. Exercise-induced decreases in sarcoplasmic reticulum Ca2+-ATPase activity attenuated by high-resistance training. Acta Physiol Scand. 1998;164:141–146. doi: 10.1046/j.1365-201X.1998.00425.x. [DOI] [PubMed] [Google Scholar]
- Green HJ, McKee NH, Carvalho AJ, Dossett-Mercer JC. Ischemia-induced alterations in sarcoplasmic reticulum Ca2+-ATPase activity in rat soleus and EDL muscles. Am J Physiol. 1996;271:C1942–1948. doi: 10.1152/ajpcell.1996.271.6.C1942. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Hamilton EJ, Rakic V, Davis WA, Chubb SAP, Kamber N, Prince RL, Davis TME. Prevalence and predictors of osteopenia and osteoporosis in adults with Type 1 diabetes. Diabet Med. 2009;26:45–52. doi: 10.1111/j.1464-5491.2008.02608.x. [DOI] [PubMed] [Google Scholar]
- Harmer AR, Chisholm DJ, McKenna MJ, Hunter SK, Ruell PA, Naylor JM, Maxwell LJ, Flack JR. Sprint training increases muscle oxidative metabolism during high-intensity exercise in patients with type 1 diabetes. Diabetes Care. 2008;31:2097–2102. doi: 10.2337/dc08-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmer AR, Chisholm DJ, McKenna MJ, Morris NR, Thom JM, Bennett G, Flack JR. High-intensity training improves plasma glucose and acid-base regulation during intermittent maximal exercise in type 1 diabetes. Diabetes Care. 2007;30:1269–1271. doi: 10.2337/dc06-1790. [DOI] [PubMed] [Google Scholar]
- Harmer AR, McKenna MJ, Sutton JR, Snow RJ, Ruell PA, Booth J, Thompson MW, Mackay NA, Stathis CG, Crameri RM, Carey MF, Eager DM. Skeletal muscle metabolic and ionic adaptations during intense exercise following sprint training in humans. J Appl Physiol. 2000;89:1793–1803. doi: 10.1152/jappl.2000.89.5.1793. [DOI] [PubMed] [Google Scholar]
- Harmer AR, Ruell PA, McKenna MJ, Chisholm DJ, Hunter SK, Thom JM, Morris NR, Flack JR. Effects of sprint training on extrarenal potassium regulation with intense exercise in type 1 diabetes. J Appl Physiol. 2006;100:26–34. doi: 10.1152/japplphysiol.00240.2005. [DOI] [PubMed] [Google Scholar]
- Hill CA, Thompson MW, Ruell PA, Thom JM, White MJ. Sarcoplasmic reticulum function and muscle contractile character following fatiguing exercise in humans. Journal of Physiology. 2001;531:871–878. doi: 10.1111/j.1469-7793.2001.0871h.x. (Pt 3), [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter SK, Thompson MW, Ruell PA, Harmer AR, Thom JM, Gwinn TH, Adams RD. Human skeletal sarcoplasmic reticulum Ca2+ uptake and muscle function with aging and strength training. J Appl Physiol. 1999;86:1858–1865. doi: 10.1152/jappl.1999.86.6.1858. [DOI] [PubMed] [Google Scholar]
- Lamb GD. Mechanisms of excitation-contraction uncoupling relevant to activity-induced muscle fatigue. Appl Physiol Nutr Metab. 2009;34:368–372. doi: 10.1139/H09-032. [DOI] [PubMed] [Google Scholar]
- Leppik JA, Aughey RJ, Medved I, Fairweather I, Carey MF, McKenna MJ. Prolonged exercise to fatigue in humans impairs skeletal muscle Na+-K+-ATPase activity, sarcoplasmic reticulum Ca2+ release, and Ca2+ uptake. J Appl Physiol. 2004;97:1414–1423. doi: 10.1152/japplphysiol.00964.2003. [DOI] [PubMed] [Google Scholar]
- Levy J, Gavin JR, 3rd, Sowers JR. Diabetes mellitus: a disease of abnormal cellular calcium metabolism? Am J Med. 1994;96:260–273. doi: 10.1016/0002-9343(94)90152-x. [DOI] [PubMed] [Google Scholar]
- Li JL, Wang XN, Fraser SF, Carey MF, Wrigley TV, McKenna MJ. Effects of fatigue and training on sarcoplasmic reticulum Ca2+ regulation in human skeletal muscle. J Appl Physiol. 2002;92:912–922. doi: 10.1152/japplphysiol.00643.2000. [DOI] [PubMed] [Google Scholar]
- Liu Y-H, Qi J, Hou Y-X, Wang F. Effects of sex hormones on genioglossal muscle contractility and SR Ca2+-ATPase activity in aged rat. Arch Oral Biol. 2008;53:353–360. doi: 10.1016/j.archoralbio.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Madsen K, Franch J, Clausen T. Effects of intensified endurance training on the concentration of Na,K-ATPase and Ca-ATPase in human skeletal muscle. Acta Physiol Scand. 1994;150:251–258. doi: 10.1111/j.1748-1716.1994.tb09684.x. [DOI] [PubMed] [Google Scholar]
- Markwell MAK, Haas SM, Bieber LL, Tolbert NE. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978;87:206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Mazzanti L, Rabini RA, Faloia E, Fumelli P, Bertoli E, De Pirro R. Altered cellular Ca2+ and Na+ transport in diabetes mellitus. Diabetes. 1990;39:850–854. doi: 10.2337/diab.39.7.850. [DOI] [PubMed] [Google Scholar]
- McKenna MJ, Heigenhauser GJF, McKelvie RS, MacDougall JD, Jones NL. Sprint training enhances ionic regulation during intense exercise in men. J Physiol. 1997;501:687–702. doi: 10.1111/j.1469-7793.1997.687bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ørtenblad N, Lunde PK, Levin K, Andersen JL, Pedersen PK. Enhanced sarcoplasmic reticulum Ca2+ release following intermittent sprint training. Am J Physiol Regul Integr Comp Physiol. 2000;279:R152–160. doi: 10.1152/ajpregu.2000.279.1.R152. [DOI] [PubMed] [Google Scholar]
- Rahmani-Jourdheuil D, Mourayre Y, Vague P, Boyer J, Juhan-Vague I. In vivo insulin effect on ATPase activities in erythrocyte membrane from insulin-dependent diabetics. Diabetes. 1987;36:991–995. doi: 10.2337/diab.36.9.991. [DOI] [PubMed] [Google Scholar]
- Roepstorff C, Thiele M, Hillig T, Pilegaard H, Richter EA, Wojtaszewski JFP, Kiens B. Higher skeletal muscle α2AMPK activation and lower energy charge and fat oxidation in men than in women during submaximal exercise. J Physiol. 2006;574:125–138. doi: 10.1113/jphysiol.2006.108720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruell PA, Booth J, McKenna MJ, Sutton JR. Measurement of sarcoplasmic reticulum function in mammalian skeletal muscle: technical aspects. Anal Biochem. 1995;228:194–201. doi: 10.1006/abio.1995.1339. [DOI] [PubMed] [Google Scholar]
- Schmitz O, Alberti KG, Hreidarsson AB, Laurberg P, Weeke J, Orskov H. Suppression of the night increase in serum TSH during development of ketosis in diabetic patients. J Endocrinol Invest. 1981;4:403–407. doi: 10.1007/BF03348303. [DOI] [PubMed] [Google Scholar]
- Simoneau JA, Bouchard C. Human variation in skeletal muscle fibre-type proportion and enzyme activities. Am J Physiol. 1989;257:E567–572. doi: 10.1152/ajpendo.1989.257.4.E567. [DOI] [PubMed] [Google Scholar]
- Simonides WS, Thelen MH, van der Linden CG, Muller A, van Hardeveld C. Mechanism of thyroid-hormone regulated expression of the SERCA genes in skeletal muscle: implications for thermogenesis. Biosci Rep. 2001;21:139–154. doi: 10.1023/a:1013692023449. [DOI] [PubMed] [Google Scholar]
- Sundaresan PR, Sharma VK, Gingold SI, Banerjee SP. Decreased β-adrenergic receptors in rat heart in streptozotocin-induced diabetes: role of thyroid hormones. Endocrinology. 1984;114:1358–1363. doi: 10.1210/endo-114-4-1358. [DOI] [PubMed] [Google Scholar]
- Taira Y, Ganguly PK, Panagia V, Dhalla NS. Increased SR phospholipid N-methylation in skeletal muscle of diabetic rats. Am J Physiol. 1988;255:E347–E352. doi: 10.1152/ajpendo.1988.255.3.E347. [DOI] [PubMed] [Google Scholar]
- Taira Y, Hata T, Ganguly PK, Elimban V, Dhalla NS. Increased sarcolemmal Ca2+ transport activity in skeletal muscle of diabetic rats. Am J Physiol. 1991;260:E626–E632. doi: 10.1152/ajpendo.1991.260.4.E626. [DOI] [PubMed] [Google Scholar]
- Thom JM, Thompson MW, Ruell PA, Bryant GJ, Fonda JS, Harmer AR, Janse de Jonge XA, Hunter SK. Effect of 10-day cast immobilization on sarcoplasmic reticulum calcium regulation in humans. Acta Physiol Scand. 2001;172:141–147. doi: 10.1046/j.1365-201X.2001.00853.x. [DOI] [PubMed] [Google Scholar]