Abstract
Background
Galectin-3 (Gal-3) is a marker of myocardial fibrosis, and elevated levels are associated with adverse outcomes. Mineralocorticoid receptor antagonists (MRA) modulate cardiac fibrosis in HF patients, and have been shown to improve long term outcomes. We examined whether treatment effects from MRA use differed by Gal-3 levels in ambulatory heart failure patients enrolled in the HF-ACTION study.
Methods and Results
HF-ACTION was a randomized controlled trial of exercise training versus usual care in patients with HF due to LV systolic dysfunction (NYHA Class II–IV, LVEF ≤ 0.35, median follow-up 2.5 years). Galectin-3 was assessed at baseline in 895 patients. The endpoint was all-cause mortality or all-cause hospitalization (ACM+ACH); all-cause mortality (ACM) was a key secondary endpoint. A differential association of MRA use by increasing Gal3 concentration was tested using interaction terms in Cox proportional hazards models, adjusted for covariates previously identified in this cohort, as well as age, sex, and race. Inverse Propensity Weighted (IPW) methods were also used to assess this association. Approximately half the patients were on an MRA (n=401). There was no significant interaction for the associations of Gal-3 levels and MRA use on either endpoint (adjusted interaction p-value=0.76 for ACM+ACH; p=0.26 for ACM). There was no evidence of improved outcomes for patients on an MRA compared to those not on MRA on either endpoint (HR=1.02, 95% CI [0.85–1.23], p=0.8; HR=1.15, 95% CI [0.82–1.61], p=0.4, respectively). IPW analysis was consistent with the results of the adjusted analysis.
Conclusion
Our study showed no evidence of interaction between Gal-3 and treatment effect of MRA. Whether biomarkers may be used to predict which patients may benefit from an mineralocorticoid receptor antagonist in HF requires further investigation.
Keywords: heart failure, biomarkers, galectin-3, mineralocorticoid receptor antagonists
Introduction
Galectin-3 (Gal-3) is a member of an evolutionarily conserved family of soluble β-galactoside-binding lectins that play a key role in several diverse biological processes and disease states.1 In the heart, galectin-3 is thought to augment fibrosis and modulate the immune response, both pivotal processes in maladaptive cardiac remodeling. Studies have also shown that elevated concentrations of galectin-3 provide important prognostic information, particularly in patients with chronic heart failure.2–4
Aldosterone is a mineralocorticoid hormone that has been shown to play a pathophysiologic role in cardiovascular remodeling through cardiac hypertrophy, fibrosis, and inflammation.5, 6 Mineralocorticoid receptor antagonists modulate cardiac fibrosis in heart failure patients, and have been shown to improve long term outcomes, reducing mortality by up to 30% and readmission for heart failure by nearly 40%.7 Despite this, their uptake has been slow in clinical practice, in part due to uncertainty about their effectiveness and safety outside clinical trials.8, 9 It has been hypothesized that patients with elevated plasma levels of Gal-3, indicating increased cardiac fibrosis, may be those who benefit most from mineralocorticoid receptor antagonists.10, 11 A study by Calvier et al in rats showed that cardiac fibrosis was mediated by Gal-3 and aldosterone activity, and that spironolactone reversed the inflammatory and fibrotic response to aldosterone in the setting of elevated Gal-3. Another study showed that in an experimental mouse model of cardiac hyperaldosteronism, the MRA eplerenone reduced Gal-3 levels.6, 12 However, there have been conflicting data. In a study by Weir et al, Gal-3 concentrations increased significantly, by approximately 14% in patients treated with eplerenone.13 This study also indicated there was no effect of eplerenone on remodeling, whether baseline galectin-3 levels were low or high. Additionally, in the Controlled Rosuvastatin Multinational Trial in Heart Failure (CORONA), higher Gal-3 levels were associated with current use of MRA’s.14 Thus, data substantiating the hypothesis that elevated Gal-3 levels may predict beneficial outcomes on MRA’s remain elusive.
To address this question, we examined whether the association between treatment effects from MRA use and clinical outcomes differed by Gal-3 levels in patients enrolled in the HF-ACTION study, a large, multicenter randomized trial of exercise training in ambulatory heart failure patients.
Methods
Study Population
The design, rationale, and primary results of the HF-ACTION study have been previously published.15 Briefly, HF-ACTION was a randomized clinical trial evaluating the effect of exercise training vs. usual care on long-term morbidity and mortality in patients with chronic heart failure due to left ventricular systolic dysfunction (NYHA class II–IV, left ventricular ejection fraction (LVEF) < 0.35). The primary endpoint of HF-ACTION was a composite of all-cause mortality and all-cause hospitalization over a median follow up of 2.5 years. Although not mandated, enrollment criteria included patients who were on optimal heart failure therapy according to American College of Cardiology/American Heart Association and Heart Failure Society of America guidelines.16, 17 An independent clinical events committee adjudicated deaths and first cardiovascular hospitalizations. HF-ACTION was approved by local Institutional Review Boards, and all patients provided written informed consent.
Biomarker Assays
A sub-set of patients enrolled in the HF-ACTION study who agreed to participate in the Biomarker substudy. Baseline blood samples were obtained on the same day as baseline exercise testing but were obtained prior to exercise. Samples were collected via peripheral vein into EDTA containing tubes, and then centrifuged immediately and stored at −70 C for subsequent analysis. Galectin-3 levels were assessed on baseline samples using an enzyme-linked immunosorbent assay (ELISA) developed by BG Medicine, Inc. in Waltham, MA, USA, which quantitatively measures galectin-3 concentrations on plasma samples. The assays were run at an academic core laboratory that was blinded to clinical data. The core laboratory limit of quantification for Gal-3 measurement was unavailable, so values were truncated at the 99th percentile of the observed distribution.
Statistical Analysis
Baseline characteristics were described according to use of MRA treatment and high vs low Galectin-3 level at randomization. A Gal-3 cut-point of 17.8 ng/mL was used based on the FDA approved labeling for use of the assay. A sensitivity analysis was also conducted using the median split of Gal-3 levels in the HF ACTION cohort (14.01 ng/mL). Continuous characteristics are described using medians and interquartile ranges, and compared across the four combinations of MRA use and Gal-3 level using a Kruskal-Wallis test; categorical characteristics are described by proportions and compared using the Pearson chi-square test or exact test.
The primary endpoint was all-cause mortality or all-cause hospitalization; the key secondary endpoint was all-cause mortality. Other secondary endpoints included cardiovascular (CV) mortality or CV hospitalization, and CV mortality or HF hospitalization.
A differential association of MRA therapy by Gal-3 concentration was tested for each endpoint using interaction terms in Cox proportional hazards models. To maximize power and precision of our analyses, Gal-3 concentration was kept as a continuous variable in all statistical models. For each endpoint, the regression analysis was adjusted for clinical risk factors previously identified as HF-ACTION adjustment models. Adjustment models were built using the approach described by O’Connor et al but with a larger set of candidate variables.18 The clinical adjustment model for the primary endpoint included Weber class, Kansas City Cardiomyopathy Questionnaire (KCCQ) symptom stability, blood urea nitrogen, region (USA versus non-USA), LVEF, sex, beta blocker dose, mitral regurgitation (severe versus non-severe), and ventricular conduction. Adjustment covariates for the secondary endpoints are described in the footnote to Table 2. Further, all models included adjustment for age, sex, and race even when not present in the original HF-ACTION adjustment model. Linearity assumptions were assessed for Gal-3; results suggested truncation below 8 ng/mL and above the 25 ng/mL would provide an appropriate fit for each outcome. Proportional hazards assumptions were checked for Gal-3 and MRA use; no violation was suggested. The analysis plan specified that if the tested interaction was not significant, an additive model (i.e. without the interaction term) would assess the relationship between MRA use and outcome adjusted for Gal-3 level and the previously mentioned covariates. Direct adjusted survival curves were plotted for the primary and key secondary endpoint for MRA use and for combinations of low vs. high Gal3 and MRA use.
Table 2.
Mineralocorticoid receptor antagonist Use, Interaction with Gal-3, and Clinical Outcomes
| Model | Association between MRA use and Outcome without Interaction with Gal-3 | P-value for the Interaction between Gal-3 and MRA use | ||
|---|---|---|---|---|
| Covariate Adjusted HR (95% CI) | Inverse Propensity Weighted*,5 HR (95% CI) | Covariate Adjusted | IPW | |
| All-cause mortality or hospitalization [adjusted1 n=694] | 1.02 (0.85, 1.23) | 1.02 (0.84, 1.23) | 0.76 | 0.94 |
| All-cause mortality [adjusted2 n=771] | 1.15 (0.82, 1.61) | 1.05 (0.72, 1.53) | 0.26 | 0.45 |
| Cardiovascular (CV) mortality or CV hospitalization [adjusted3 n=699] | 0.97 (0.79, 1.18) | 1.01 (0.82, 1.25) | 0.52 | 0.56 |
| CV mortality or heart failure (HF) hospitalization [adjusted4 n=686] | 0.94 (0.72, 1.23) | 0.96 (0.73, 1.25) | 0.40 | 0.97 |
IPW n=674 for all outcomes
Adjusted for age, race (black vs white vs other), galectin-3, and the following HF-ACTION adjustment model covariates: sex, peak VO2 characterized by Weber class, KCCQ symptom stability score, blood urea nitrogen, county (US vs non-US), LVEF, beta blocker dosage, mitral regurgitation grade, and ventricular conduction on the baseline CPX test
Adjusted for age, race (black vs white vs other), galectin-3, and the following HF-ACTION adjustment model covariates: sex, exercise duration on the baseline CPX test, serum creatinine level, BMI, loop diuretic dosage, LVEF, CCS angina classification, and ventricular conduction on the baseline CPX test
Adjusted for age, galectin-3, and the following HF-ACTION adjustment model covariates: sex, race (black vs white vs other), LVEF, mitral regurgitation grade, ventricular conduction on the baseline CPX test, KCCQ symptom stability score, blood urea nitrogen, heart rate at peak exercise on the baseline CPX test, nitrate use, peak VO2 characterized by Weber class, and KCCQ total symptom score
Adjusted for galectin-3 and the following HF-ACTION adjustment model covariates: age, sex, race (black vs white vs other), loop diuretic dosage, LVEF, mitral regurgitation grade, ventricular conduction on the baseline CPX test, KCCQ symptom stability score, blood urea nitrogen, peak VO2 characterized by Weber class, and VE/VCO2 slope
IPW models used the following covariates in the propensity model: age, sex, race (black vs white vs other), galectin-3, peak VO2 characterized by Weber class, KCCQ symptom stability score, blood urea nitrogen, county (US vs non-US), LVEF, beta blocker dosage, mitral regurgitation grade, ventricular conduction on the baseline CPX test, CCS angina classification, exercise duration on the baseline CPX test, serum creatinine level, BMI, loop diuretic dosage, heart rate at peak exercise on the baseline CPX test, nitrate use, KCCQ total symptom score, and VE/VCO2 slope
Inverse Propensity Weighted (IPW) methods were also used to assess these interactions and associations. A propensity model for MRA use included all covariates identified in any of the HF-ACTION adjustment models previously described. Covariate balance was assessed following appropriate methods19 and demonstrated adequate balance. Cox proportional hazards models then assessed the association between MRA use and outcomes, weighted by the inverse of the estimated probability of MRA treatment received. Statistical analysis was performed by the Duke Clinical Research Institute using SAS software version 9.2 (SAS Institute, Cary, NC) and p<0.05 was considered statistically significant.
Results
Evaluable baseline plasma samples were available for 895 patients, and baseline characteristics for this study cohort stratified by MRA use (yes/no) and Gal-3 (high/low) are shown in Table 1. Of the 895 patients, approximately half were on an MRA (n=401). The median age of the study cohort was 59 years. There was no evidence to suggest a significant difference in the median Gal-3 level in patients on MRA (13.8 [11.0 – 18.1] verses not on MRA (14.2 [10.8 – 18.7].
Table 1.
Baseline Patient Characteristics by Galectin-3 and Mineralocorticoid receptor antagonist Use
| Category of Galectin-3 and Mineralocorticoid receptor antagonist Use | |||||
|---|---|---|---|---|---|
|
| |||||
| Characteristic# | Gal-3 <17.8 ng/mL Not on MRA N=351 | Gal-3 <17.8 ng/mL On MRA N=295 | Gal-3 >17.8 ng/mL Not on MRA N=143 | Gal-3 >17.8 ng/mL On MRA N=106 | P-Value |
| * Age, yrs | 60 | 55 | 66 | 60 | <.001 |
| Female Sex | 90 (26%) | 95 (32%) | 38 (27%) | 36 (34%) | 0.17 |
| Race | 0.02 | ||||
| Black | 99 (28.5%) | 110/290 (37.9%) | 30/142 (21.1%) | 32/104 (30.8%) | |
| White | 233 (67%) | 164 (57%) | 103 (73%) | 67 (64%) | |
| Other | 15 (4%) | 16 (6%) | 9 (6%) | 5 (5%) | |
| Ischemic Etiology | 183 (52%) | 115 (39%) | 93 (65%) | 63 (59%) | <.001 |
| Hx of Myocardial Infarction | 149 (43%) | 100 (34%) | 77 (54%) | 46 (43%) | <.001 |
| Hx of Hypertension | 232 (66%) | 164 (56%) | 100 (71%) | 74 (70%) | 0.003 |
| Hx of Diabetes | 100 (29%) | 79 (27%) | 67 (47%) | 45 (43%) | <.001 |
| NYHA III–IV vs II | 101 (29%) | 104 (36%) | 73 (51%) | 57 (54%) | <.001 |
| * LVEF, % | 26 | 23 | 25 | 22 | <.001 |
| Atrial Fibrillation or Flutter | 62 (18%) | 58 (20%) | 38 (27%) | 38 (36%) | <.001 |
| * BMI, kg/m^2 | 30 | 31 | 30 | 31 | 0.07 |
| * Systolic BP, mmHg | 118 | 110 | 114 | 108 | <.001 |
| * Diastolic BP, mmHg | 73 | 68 | 70 | 66 | <.001 |
| * Heart Rate, bpm | 70 | 72 | 70 | 72 | 0.08 |
| * Sodium, mmol/L | 140 | 139 | 139 | 139 | 0.001 |
| * Creatinine, mg/dL | 1.1 | 1.1 | 1.5 | 1.4 | <.001 |
| * BUN, mg/dL | 17 | 19 | 27 | 28 | <.001 |
| ACE-i or ARB | 338 (96%) | 291 (99%) | 129 (90%) | 94 (89%) | <.001 |
| Beta Blocker | 331 (94%) | 283 (96%) | 132 (92%) | 102 (96%) | 0.37 |
| Loop Diuretic | 243 (69%) | 243 (82%) | 120 (84%) | 100 (94%) | <.001 |
| Digoxin | 136 (39%) | 175 (59%) | 55 (39%) | 63 (59%) | <.001 |
| ICD | 122 (35%) | 143 (49%) | 62 (43%) | 60 (57%) | <.001 |
| Biventricular Pacemaker | 53 (15%) | 58 (20%) | 23 (16%) | 42 (40%) | <.001 |
| * Peak VO2 mL/kg/min | 15.4 | 15.0 | 12.9 | 12.1 | <.001 |
| *6MW Distance, m | 379 | 381 | 330 | 321 | <.001 |
| * NT-proBNP, pg/mL | 643 | 654 | 1562 | 1446 | <.001 |
n, % except as specified
Median values
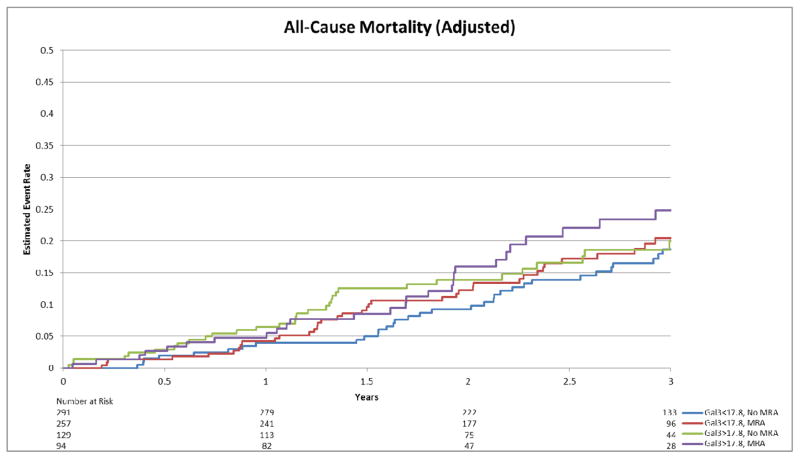
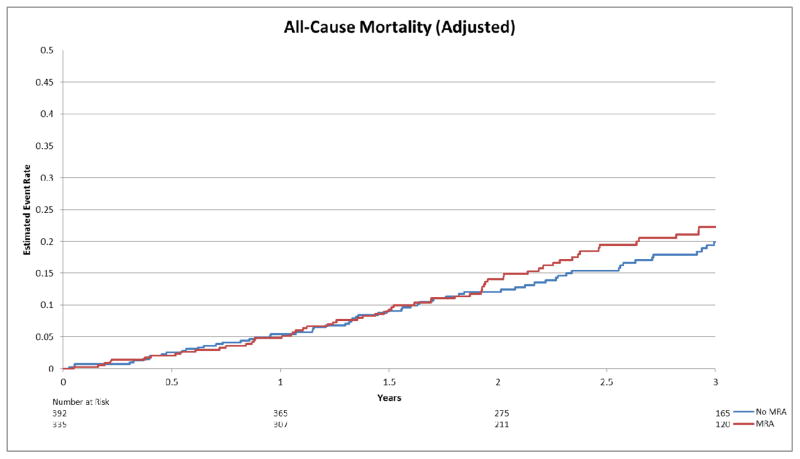
There was no significant interaction for the associations of Gal-3 levels and MRA use on either endpoint after adjusting for predictors of adverse outcomes in this cohort (adjusted interaction p-value=0.76 for all-cause mortality + all-cause hospitalization; p=0.26 for all-cause mortality). The association of MRA use with outcome was approximately the same over the range of Gal-3 values (Figure 1-ACM+ACH; Figure 2-ACM). In adjusted analysis, there was no evidence of improved outcomes for patients on an MRA compared to those not on an MRA, on the primary endpoint (Figure 3) or mortality alone (Figure 4) (HR=1.02, 95% CI [0.85–1.23], p=0.80; HR=1.15, 95% CI [0.82–1.61], p=0.43, respectively). Inverse Propensity Weighted analysis was consistent with the results of the adjusted analysis (Table 2). Results of the secondary endpoints of CV mortality/CV hospitalization and CV mortality/HF hospitalization also showed no evidence of an interaction (Table 2). Baseline characteristics and survival plots were also evaluated as a sensitivity analysis using median Gal-3 levels (14.01 ng/mL), which yieldeding similar results.
Figure 1.
Adjusted estimated event rate for all-cause mortality or all-cause hospitalization by Gal-3 and MRA use.
Figure 2.
Adjusted estimated event rate for all-cause mortality by Gal-3 and MRA use.
Figure 3.
Adjusted estimated event rate for all-cause mortality or all-cause hospitalization by MRA use.
Figure 4.
Adjusted estimated event rate for all-cause mortality by MRA use.
Discussion
Our study showed that there was no evidence of a differential association of MRA use with outcomes by Gal3 level. Further, in this cohort, there was not a significant difference in outcomes of patients on MRA after adjustment for important clinical variables.
Galectin-3 is a novel biomarker that has been shown to mediate fibrosis in the failing heart.20 Studies have shown that Gal-3 can be used to predict adverse outcomes in patients with chronic heart failure, with those with elevated levels having a higher risk of adverse outcomes, including mortality or hospitalization.18, 21–23 Data from the CORONA trial suggested that lower Gal-3 levels may confer a benefit with rosuvastatin therapy.14 Additionally, this study showed that MRA use was associated with higher levels of Gal-3. It has been suggested, however, that this may be related to the antifibrotic effects of MRA’s which may trigger feedback upregulation of Gal-3. It is also unclear whether galectin-3 may be rising in response to MRA induced renal dysfunction.24
Another possible explanation for our findings may be related to the timing of Gal-3 measurements and use of MRA’s. Galectin-3 levels are not affected by decompensation, and generally remain stable over time.25 Therefore, it may be plausible that by the time the biomarker is elevated, it is too late to observe the benefit in patients who receive MRA’s.
We previously demonstrated that in HF-ACTION, after multivariable adjustment for a large number of clinical variables including NT-proBNP, galectin-3 was no longer a significant predictor of any cardiovascular outcomes examined in our study.2 In the context of this finding, whether treatment decisions could be determined with Gal-3 levels would be valuable. Mineralocorticoid receptor antagonists have been shown to significantly improve long term outcomes in patients with systolic heart failure.26–28 Their mechanism of action includes modulating cardiac fibrosis in these patients.29 However, findings from large registries have demonstrated conflicting results. While some have shown a benefit, others have failed to note this in clinical practice.8, 30, 31 For example, a recent study using clinical registry data linked to Medicare claims from 2005 – 2010, there was no benefit observed in treated versus untreated patients on the endpoints of mortality (p=0.62), or for cardiovascular readmissions (p=0.65). However, there was a significant reduction in hospitalizations for heart failure in patients treated with an MRA (p<0.001).8 Similarly, in our cohort, there was no evidence of improved outcomes for patients treated with MRA’s compared to those not on an MRA on the endpoint of all-cause mortality + all-cause hospitalization, or the endpoint of all-cause mortality alone.
A number of possibilities may explain differences in our findings from those of previous randomized, controlled trials. Prospective, randomized trials testing the benefit of MRA’s have a pre-specified patient population, rigorous follow-up, and measures to enforce adherence. Because HF-ACTION was not designed to test the effects of MRA’s, the setting of MRA use was more similar to the registries, where patients are treated based on clinical use. In addition, important differences exist between the study populations. Patients in HF ACTION were slightly younger than those in the EMPHASIS trial, with a lower SBP and slightly lower LVEF. Patients in HF ACTION also had a higher use of ICD’s and biventricular pacemakers than the cohorts of the mineralocorticoid receptor antagonist trials, which may further complicate the benefit that may be achieved by mineralocorticoid receptor antagonists. In addition, only 3% of the EMPHASIS cohort was made up of black patients, yet they comprised 31% of the HF ACTION cohort. A small number of studies have indicated that there may be racial differences in aldosterone concentrations and K+ response to aldosterone blockade with spironolactone. However the mechanisms for these findings have not been clearly identified and a racial difference in response to these agents has not been confirmed.32, 33
Our findings could be confounded by several important limitations. This was a retrospective analysis, and although we adjusted for known predictors of adverse outcome, the possibility of important unidentified prognostic indicators must be considered. In addition, the timing, dose, and type of mineralocorticoid receptor antagonists in each group were not known, which may play a role in the benefits observed in prospective trials.28 Further, the trial inclusion/exclusion criteria may limit generalizability of these findings. Gal-3 was only collected on a subsample of the original trial leaving a limited available sample size; a power calculation for this biomarker analysis was not done a priori. In our study, the overall effect of MRA therapy was neutral. Whether Gal-3 may be useful in trials where MRA therapy is successful cannot be determined by this study. However, using a biomarker to determine which patients may benefit in the context of a neutral effect of therapy in a general cohort is perhaps the most valuable potential of these markers.
Conclusions
In a retrospective analysis of a large well-treated cohort of ambulatory patients with systolic heart failure, there was no evidence of a differential association between Galectin-3 levels and mineralocorticoid receptor antagonist and clinical outcomes. Whether biomarkers may be used to predict which patients may benefit from an MRA in HF requires further investigation in a prospective, randomized clinical trial.
Acknowledgments
Funding Source
The HF-ACTION study was funded by the National Heart Lung and Blood Institute. The HF-ACTION Biobank was funded independently by the Duke Clinical Research Institute. This analysis was funded by a grant from BG Medicine.
Footnotes
None of the other authors report any conflicts.
Disclosures
Drs. Felker, Fiuzat, and O’Connor have received research funding from BG Medicine, Critical Diagnostics, and Roche Diagnostics. Drs. Felker and O’Connor have served as consultants for BG Medicine, Critical Diagnostics, and Roche Diagnostics. Dr. Adams has received research funding from Roche Diagnostics.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.de Boer RA, Voors AA, Muntendam P, van Gilst WH, van Veldhuisen DJ. Galectin-3: A novel mediator of heart failure development and progression. European journal of heart failure. 2009;11:811–817. doi: 10.1093/eurjhf/hfp097. [DOI] [PubMed] [Google Scholar]
- 2.Felker GM, Fiuzat M, Shaw LK, Clare R, Whellan DJ, Bettari L, Shirolkar SC, Donahue M, Kitzman DW, Zannad F, Pina IL, O’Connor CM. Galectin-3 in ambulatory patients with heart failure: Results from the hf-action study. Circulation. Heart failure. 2012;5:72–78. doi: 10.1161/CIRCHEARTFAILURE.111.963637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gullestad L, Ueland T, Kjekshus J, Nymo SH, Hulthe J, Muntendam P, McMurray JJ, Wikstrand J, Aukrust P. The predictive value of galectin-3 for mortality and cardiovascular events in the controlled rosuvastatin multinational trial in heart failure (corona) American heart journal. 2012;164:878–883. doi: 10.1016/j.ahj.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 4.Ho JE, Liu C, Lyass A, Courchesne P, Pencina MJ, Vasan RS, Larson MG, Levy D. Galectin-3, a marker of cardiac fibrosis, predicts incident heart failure in the community. Journal of the American College of Cardiology. 2012;60:1249–1256. doi: 10.1016/j.jacc.2012.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayashi M, Tsutamoto T, Wada A, Maeda K, Mabuchi N, Tsutsui T, Matsui T, Fujii M, Matsumoto T, Yamamoto T, Horie H, Ohnishi M, Kinoshita M. Relationship between transcardiac extraction of aldosterone and left ventricular remodeling in patients with first acute myocardial infarction: Extracting aldosterone through the heart promotes ventricular remodeling after acute myocardial infarction. Journal of the American College of Cardiology. 2001;38:1375–1382. doi: 10.1016/s0735-1097(01)01539-x. [DOI] [PubMed] [Google Scholar]
- 6.Calvier L, Miana M, Reboul P, Cachofeiro V, Martinez-Martinez E, de Boer RA, Poirier F, Lacolley P, Zannad F, Rossignol P, Lopez-Andres N. Galectin-3 mediates aldosterone-induced vascular fibrosis. Arteriosclerosis, thrombosis, and vascular biology. 2013;33:67–75. doi: 10.1161/ATVBAHA.112.300569. [DOI] [PubMed] [Google Scholar]
- 7.Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M Eplerenone Post-Acute Myocardial Infarction Heart Failure E Survival Study I. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. The New England journal of medicine. 2003;348:1309–1321. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- 8.Hernandez AF, Mi X, Hammill BG, Hammill SC, Heidenreich PA, Masoudi FA, Qualls LG, Peterson ED, Fonarow GC, Curtis LH. Associations between aldosterone antagonist therapy and risks of mortality and readmission among patients with heart failure and reduced ejection fraction. JAMA: the journal of the American Medical Association. 2012;308:2097–2107. doi: 10.1001/jama.2012.14795. [DOI] [PubMed] [Google Scholar]
- 9.Ahmad T, Felker GM. Galectin-3 in heart failure: More answers or more questions? Journal of the American Heart Association. 2012;1:e004374. doi: 10.1161/JAHA.112.004374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shah RV, Chen-Tournoux AA, Picard MH, van Kimmenade RR, Januzzi JL. Galectin-3, cardiac structure and function, and long-term mortality in patients with acutely decompensated heart failure. European journal of heart failure. 2010;12:826–832. doi: 10.1093/eurjhf/hfq091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahmad T, O’Connor CM. Therapeutic implications of biomarkers in chronic heart failure. Clinical pharmacology and therapeutics. 2013;94:468–479. doi: 10.1038/clpt.2013.139. [DOI] [PubMed] [Google Scholar]
- 12.Azibani F, Benard L, Schlossarek S, Merval R, Tournoux F, Fazal L, Polidano E, Launay JM, Carrier L, Chatziantoniou C, Samuel JL, Delcayre C. Aldosterone inhibits antifibrotic factors in mouse hypertensive heart. Hypertension. 2012;59:1179–1187. doi: 10.1161/HYPERTENSIONAHA.111.190512. [DOI] [PubMed] [Google Scholar]
- 13.Weir RA, Petrie CJ, Murphy CA, Clements S, Steedman T, Miller AM, McInnes IB, Squire IB, Ng LL, Dargie HJ, McMurray JJ. Galectin-3 and cardiac function in survivors of acute myocardial infarction. Circulation. Heart failure. 2013;6:492–498. doi: 10.1161/CIRCHEARTFAILURE.112.000146. [DOI] [PubMed] [Google Scholar]
- 14.Gullestad L, Ueland T, Kjekshus J, Nymo SH, Hulthe J, Muntendam P, Adourian A, Bohm M, van Veldhuisen DJ, Komajda M, Cleland JG, Wikstrand J, McMurray JJ, Aukrust P. Galectin-3 predicts response to statin therapy in the controlled rosuvastatin multinational trial in heart failure (corona) European heart journal. 2012;33:2290–2296. doi: 10.1093/eurheartj/ehs077. [DOI] [PubMed] [Google Scholar]
- 15.O’Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, Leifer ES, Kraus WE, Kitzman DW, Blumenthal JA, Rendall DS, Miller NH, Fleg JL, Schulman KA, McKelvie RS, Zannad F, Pina IL. Efficacy and safety of exercise training in patients with chronic heart failure: Hf-action randomized controlled trial. JAMA: the journal of the American Medical Association. 2009;301:1439–1450. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW. 2009 focused update incorporated into the acc/aha 2005 guidelines for the diagnosis and management of heart failure in adults: A report of the american college of cardiology foundation/american heart association task force on practice guidelines: Developed in collaboration with the international society for heart and lung transplantation. Circulation. 2009;119:e391–479. doi: 10.1161/CIRCULATIONAHA.109.192065. [DOI] [PubMed] [Google Scholar]
- 17.Jessup M, Abraham WT, Casey DE, Feldman AM, Francis GS, Ganiats TG, Konstam MA, Mancini DM, Rahko PS, Silver MA, Stevenson LW, Yancy CW. 2009 focused update: Accf/aha guidelines for the diagnosis and management of heart failure in adults: A report of the american college of cardiology foundation/american heart association task force on practice guidelines: Developed in collaboration with the international society for heart and lung transplantation. Circulation. 2009;119:1977–2016. doi: 10.1161/CIRCULATIONAHA.109.192064. [DOI] [PubMed] [Google Scholar]
- 18.O’Connor CM, Whellan DJ, Wojdyla D, Leifer E, Clare RM, Ellis SJ, Fine LJ, Fleg JL, Zannad F, Keteyian SJ, Kitzman DW, Kraus WE, Rendall D, Pina IL, Cooper LS, Fiuzat M, Lee KL. Factors related to morbidity and mortality in patients with chronic heart failure with systolic dysfunction: The hf-action predictive risk score model. Circulation. Heart failure. 2012;5:63–71. doi: 10.1161/CIRCHEARTFAILURE.111.963462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Statistics in medicine. 2009;28:3083–3107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharma UC, Pokharel S, van Brakel TJ, van Berlo JH, Cleutjens JP, Schroen B, Andre S, Crijns HJ, Gabius HJ, Maessen J, Pinto YM. Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation. 2004;110:3121–3128. doi: 10.1161/01.CIR.0000147181.65298.4D. [DOI] [PubMed] [Google Scholar]
- 21.Ueland T, Aukrust P, Broch K, Aakhus S, Skardal R, Muntendam P, Gullestad L. Galectin-3 in heart failure: High levels are associated with all-cause mortality. International journal of cardiology. 150:361–364. doi: 10.1016/j.ijcard.2011.05.081. [DOI] [PubMed] [Google Scholar]
- 22.de Boer RA, Lok DJ, Jaarsma T, van der Meer P, Voors AA, Hillege HL, van Veldhuisen DJ. Predictive value of plasma galectin-3 levels in heart failure with reduced and preserved ejection fraction. Annals of medicine. 2011;43:60–68. doi: 10.3109/07853890.2010.538080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lok DJ, Van Der Meer P, de la Porte PW, Lipsic E, Van Wijngaarden J, Hillege HL, van Veldhuisen DJ. Prognostic value of galectin-3, a novel marker of fibrosis, in patients with chronic heart failure: Data from the deal-hf study. Clinical research in cardiology: official journal of the German Cardiac Society. 2010;99:323–328. doi: 10.1007/s00392-010-0125-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weir RA, Petrie CJ, Murphy CA, Clements S, Steedman T, Miller AM, McInnes IB, Squire IB, Ng LL, Dargie HJ, McMurray JJ. Response to letter regarding article, “galectin-3 and cardiac function in survivors of acute myocardial infarction”. Circulation. Heart failure. 2013;6:e58. doi: 10.1161/CIRCHEARTFAILURE.112.000146. [DOI] [PubMed] [Google Scholar]
- 25.Anand IS, Rector TS, Kuskowski M, Adourian A, Muntendam P, Cohn JN. Baseline and serial measurements of galectin-3 in patients with heart failure: Relationship to prognosis and effect of treatment with valsartan in the val-heft. European journal of heart failure. 2013;15:511–518. doi: 10.1093/eurjhf/hfs205. [DOI] [PubMed] [Google Scholar]
- 26.Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B, Group E-HS. Eplerenone in patients with systolic heart failure and mild symptoms. The New England journal of medicine. 2011;364:11–21. [Google Scholar]
- 27.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized aldactone evaluation study investigators. The New England journal of medicine. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 28.Albert NM, Yancy CW, Liang L, Zhao X, Hernandez AF, Peterson ED, Cannon CP, Fonarow GC. Use of aldosterone antagonists in heart failure. JAMA: the journal of the American Medical Association. 2009;302:1658–1665. doi: 10.1001/jama.2009.1493. [DOI] [PubMed] [Google Scholar]
- 29.Brilla CG, Matsubara LS, Weber KT. Antifibrotic effects of spironolactone in preventing myocardial fibrosis in systemic arterial hypertension. The American journal of cardiology. 1993;71:12A–16A. doi: 10.1016/0002-9149(93)90239-9. [DOI] [PubMed] [Google Scholar]
- 30.Fonarow GC, Albert NM, Curtis AB, Gheorghiade M, Liu Y, Mehra MR, O’Connor CM, Reynolds D, Walsh MN, Yancy CW. Incremental reduction in risk of death associated with use of guideline-recommended therapies in patients with heart failure: A nested case-control analysis of improve hf. Journal of the American Heart Association. 2012;1:16–26. doi: 10.1161/JAHA.111.000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamaguchi S, Kinugawa S, Tsuchihashi-Makaya M, Goto K, Goto D, Yokota T, Yamada S, Yokoshiki H, Takeshita A, Tsutsui H. Spironolactone use at discharge was associated with improved survival in hospitalized patients with systolic heart failure. American heart journal. 2010;160:1156–1162. doi: 10.1016/j.ahj.2010.08.036. [DOI] [PubMed] [Google Scholar]
- 32.Cavallari LH, Groo VL, Momary KM, Fontana D, Viana MA, Vaitkus P. Racial differences in potassium response to spironolactone in heart failure. Congestive heart failure. 2006;12:200–205. doi: 10.1111/j.1527-5299.2006.05502.x. [DOI] [PubMed] [Google Scholar]
- 33.Fisher ND, Gleason RE, Moore TJ, Williams GH, Hollenberg NK. Regulation of aldosterone secretion in hypertensive blacks. Hypertension. 1994;23:179–184. doi: 10.1161/01.hyp.23.2.179. [DOI] [PubMed] [Google Scholar]