Abstract
Background
We evaluated the effect of marital status on risk of late-stage cutaneous melanoma diagnosis.
Methods
Information about melanoma patients was obtained from Surveillance Epidemiology and End Results (SEER), 1973-2006. A multivariable logistic regression model was used to estimate relative risks of late-stage disease at diagnosis.
Results
After exclusion criteria, 192,014 adult melanoma patients remained for analyses. After adjustment for age, race, year of diagnosis, tumor histology, anatomic site, socioeconomic status, and SEER site, the relationship between estimated risk of late-stage melanoma diagnosis and marital status was dependent on sex (P < .0001 for interaction). Although unmarried patients had a higher risk of being diagnosed at a late stage among men and women, the magnitude of the effect varied by sex. Moreover, among married, single, and divorced or separated patients, men had more than a 50% increase in risk of late-stage diagnosis when compared with women. Widowed men and widowed women, however, were not statistically different in their stage at diagnosis.
Conclusions
Results from this study are important and may be used by clinicians and public health practitioners interested in increasing the proportion of melanoma patients diagnosed at an early stage through screening, perhaps by specifically targeting unmarried individuals in addition to having broad-based skin cancer prevention programs.
Keywords: marital status, melanoma, SEER, stage at diagnosis, late stage, single individuals
The survival advantage of diagnosing melanoma at an early stage (in situ or localized) is evident in population-based studies. Although overall survival for melanoma is high, with more than 90% of patients surviving at least 5 years after diagnosis, survival decreases markedly with advancing stage. Specifically, 98%, 62%, and 15% of patients diagnosed at the localized, regional, and distant stages, respectively, survive 5 years.1 Thus, it is important not only to determine who is at high risk of developing melanoma, but also who is at high risk of late-stage diagnosis. Studies that have examined predictors of later stage at diagnosis or poor prognosis generally have reported that patients who are male,2-8 older age,2,3,5,6,9-13 low socioeconomic status (SES),2,3,8,13-16 nonwhite,2,4,17 cigarette smokers,8,18-22 living in areas with fewer dermatologists,23 or uninsured or on Medicaid24 are more likely to be diagnosed at late stage and to have correspondingly worse outcomes. In addition, physician discovery of melanoma (compared with self-discovery or discovery by a spouse or significant other) is more commonly associated with thinner lesions.16,25-28 Not surprisingly, these factors involve myriad patient-, provider-, and health care system-level dynamics.
Being married provides a survival advantage to those with cancer, including melanoma.29-33 Researchers have hypothesized that the advantage of marriage may result from an immunologic benefit of psychosocial support associated with marriage or from spousal support to follow-through with treatment. In the case of melanoma, advantage may also result from diagnosis at an earlier stage because spouses may identify suspicious or changing nevi or nevi on areas of the body not easily or routinely viewed by oneself.
Although research exists about survival advantage among married patients, few studies have examined the association between marital status and stage of melanoma at diagnosis. Swetter et al reported that tumor thickness at diagnosis was unrelated to marital status16; however, studies that examined stage at diagnosis have reported later stage at diagnosis among unmarried patients.8,34 To our knowledge, only 2 prior studies have specifically examined the association of stage at diagnosis with marital status; however, both lacked generalizability.8,34
Our primary objective was to examine the potential association between marital status and stage of melanoma at diagnosis, controlling for potentially confounding factors, in a large, highly generalizable population. We hypothesized that unmarried patients would be more likely to be diagnosed at a late stage. As a secondary aim, we examined additional variables previously thought to affect stage of melanoma at diagnosis.
Materials and Methods
Data Source
Public-use data from the Surveillance, Epidemiology and End Results (SEER) Program of the National Cancer Institute were obtained about patients diagnosed with melanoma reported to SEER registries from 1973 through 2006 using SEER*Stat software, version 6.5.1 (Silver Spring, MD).35 Today, the SEER program represents approximately 26% of the United States, and is relatively generalizable to the US population with few exceptions.36,37 The SEER program's methods of data collection, organizational structure, and mission have been described extensively elsewhere.38
Study Cohort
There were a total of 276,717 patients recorded in the SEER registry program diagnosed with melanoma (International Classification of Diseases for Oncology, Third Edition [ICD-O-3], codes C440-C449, types 8720-8790) from 1973 through 2006. Because of our interest in marital status and in stage at diagnosis, we excluded patients with unknown marital status or unstaged tumors. We additionally excluded patients younger than age 18 and those with unknown race, leaving a final study cohort of 192,014 (69.4%) melanoma patients (Fig. 1). Patients with unknown marital status, unknown race, and unstaged tumors appeared to be an amalgam of the other categories for each variable; however, a higher proportion of whites and patients diagnosed after 1990 had an unknown marital status. In addition, those with unknown marital status were more likely to be diagnosed at an early stage compared with other marital categories.
Figure 1.
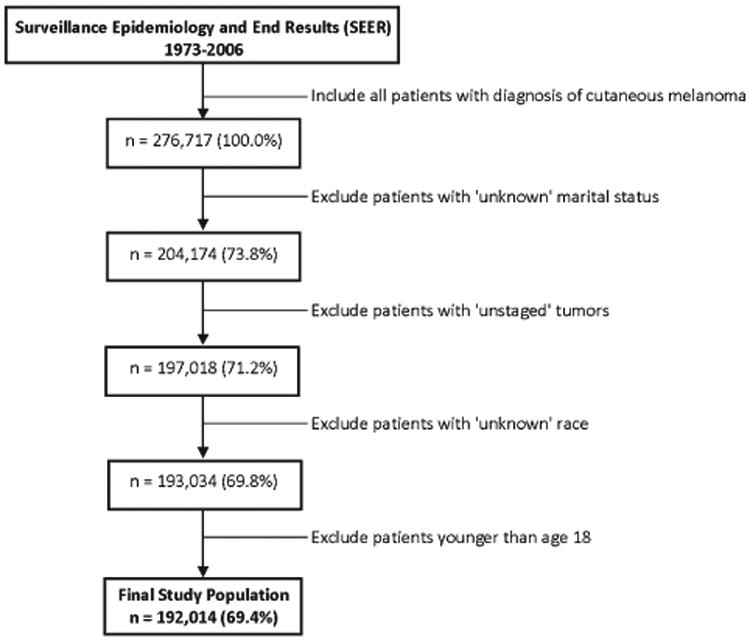
Inclusion and exclusion criteria with corresponding sample sizes and percentages of total cutaneous melanoma sample for the study cohort are shown.
Measures
Marital status at diagnosis was categorized as married (including common-law marriages), single (ie, never married), divorced or separated, or widowed. Stage at diagnosis was dichotomized using SEER summary staging as either early stage (in situ or localized) or late stage (regional or distant). SEER summary stage reflects several tumor characteristics, including the depth of melanoma invasion (ie, Breslow's depth) and elements of tumornode-metastases (TNM) staging. Race was classified as white, black, or other. Consistent with previous research, tumor histology was classified according to the International Classification of Diseases for Oncology (Third Edition) into categories of lentigo maligna melanoma, nodular, acral lentiginous, superficial spreading, or other or unspecified.34,39-41 Other or unspecified includes, in order of most to least frequent, spindle cell melanoma, no otherwise specified (NOS); malignant desmoplastic melanoma; malignant melanoma, regression; amelanotic melanoma; malignant melanoma in junctional nevus; malignant melanoma in giant pigmented nevus; epithelioid cell melanoma; mixed epithelioid and spindle cell melanoma; balloon cell melanoma; malignant blue nevus; malignant melanoma in precancerous melanosis; and spindle cell melanoma, type A. Anatomic site was categorized as skin of the trunk, lower limb and hip, upper limb and shoulder, head and neck, overlapping lesion of the skin (a single neoplasm that overlaps 2 or more contiguous sites whose point of origin cannot be determined), or skin, NOS. SES-proxy variables were obtained from the US Census Bureau data and included county-level information about median household income and percentages of individuals who were below the poverty level, unemployed, “white collar” employees, living in urban areas, and who had less than a high school education by age 25 years. For individuals diagnosed before 1995, 1990 US Census data were used (because of lack of availability of US Census data prior to 1990), and data from the 2000 US Census were used for patients diagnosed in 1995 or later.
Statistical Analyses
Descriptive statistics were used to provide overall characteristics by marital status and stage at diagnosis. Univariate differences in late-stage melanoma according to marital status and other independent variables were tested using likelihood ratio chi-square tests. A multivariable logistic regression model was built to estimate the risk of diagnosis at late stage (versus early stage) with simultaneous adjustment of confounding variables. SEER site and county-level SES variables were modeled as fixed effects. Because the number of observations was very large, small effects could have been statistically significant at the usual nominal alpha level of .05. Consequently, a priori, we decided to include only covariates that 1) changed the odds ratios (ORs) for marital status by at least 10%-15% (ie, confounded),42 2) improved the precision of the estimated marital status parameters, or 3) were statistically significant at a very stringent alpha level of .0001. All P values were 2-sided. For logistic regression analyses, likelihood ratio chi-square tests were used to determine improved statistical fit. Any meaningful, statistically significant interaction terms or appreciable confounders remained in the final model. All statistical analyses were conducted using SAS version 9.2.
Results
Of the 192,014 patients who met study criteria, the majority (87.6%) were diagnosed at an early stage. Most patients (70.8%) reported they were married at the time of cancer diagnosis, and nearly all (98.7%) were white. Few patients were younger than age 25 (1.9%). In addition, the majority of patients were diagnosed from 2000 through 2006 (58.4%) and had a tumor histology type of “other or unspecified” (52.0%). Table 1 shows descriptive statistics for independent variables (potential confounders) by categories of marital status. A higher proportion of married patients were male (61.1%) compared with other marital categories, and a lower proportion of widows were male (30.7%). Not surprisingly, most widows were older, and a higher proportion of single patients were younger. Widowed patients had a higher proportion of lentigo maligna melanoma, a lower proportion of superficial spreading melanoma, and a higher proportion of tumors of the head or neck. Race, year of diagnosis, and proxy indicators of SES were relatively similar across marital status categories.
Table 1. Patient Demographic and Tumor Characteristics by Marital Status at Diagnosis of Cutaneous Melanoma, SEER 1973-2006 (n = 192,014).
| Marital Status at Diagnosis | Married | Singlea | Divorcedb | Widowed |
|---|---|---|---|---|
| Demographic Variable | n (%)c | |||
| Sex | ||||
| Male | 83,106 (61.1) | 13,496 (51.9) | 5702 (45.6) | 5376 (30.7) |
| Female | 52,885 (38.9) | 12,506 (48.1) | 6801 (54.4) | 12,142 (69.3) |
| Age, y | ||||
| 18-24 | 813 (0.6) | 2818 (10.8) | 70 (0.6) | 6 (0.0) |
| 25-39 | 16,697 (12.3) | 6643 (25.5) | 1360 (10.9) | 68 (0.4) |
| 40-54 | 36,558 (26.9) | 7166 (27.6) | 4131 (33.0) | 532 (3.0) |
| 55-69 | 43,290 (31.8) | 5199 (20.0) | 4264 (34.1) | 2991 (17.1) |
| 70 or older | 38,633 (28.4) | 4176 (16.1) | 2678 (21.4) | 13,921 (79.5) |
| Race | ||||
| White | 134,449 (98.9) | 25,495 (98.1) | 12,334 (98.7) | 17,185 (98.1) |
| Black | 444 (0.3) | 224 (0.9) | 89 (0.7) | 162 (0.9) |
| Otherd | 1098 (0.8) | 283 (1.1) | 80 (0.6) | 171 (1.0) |
| Year of diagnosis | ||||
| 1973-1979 | 6189 (4.6) | 890 (3.4) | 537 (4.3) | 703 (4.0) |
| 1980-1989 | 15,845 (11.7) | 2682 (10.3) | 1333 (10.7) | 2057 (11.7) |
| 1990-1999 | 34,737 (25.5) | 7028 (27.0) | 3226 (25.8) | 4627 (26.4) |
| 2000-2006 | 79,220 (58.3) | 15,402 (59.2) | 7407 (59.2) | 10,131 (57.8) |
| Tumor histology | ||||
| Superficial spreading (SS) | 36,047 (26.5) | 7621 (29.3) | 3403 (27.2) | 3270 (18.7) |
| Nodular | 7678 (5.7) | 1780 (6.9) | 965 (7.7) | 1519 (8.7) |
| Lentigo maligna melanoma | 20,352 (15.0) | 2408 (9.3) | 1534 (12.3) | 3816 (21.8) |
| Acral lentiginous (AL) | 1071 (0.8) | 230 (0.9) | 128 (1.0) | 257 (1.5) |
| Other or unspecified | 70,843 (52.1) | 13,963 (53.7) | 6473 (51.8) | 8656 (49.4) |
| Anatomical site | ||||
| Upper limb and shoulder | 31,441 (23.1) | 5760 (22.2) | 2971 (23.8) | 4166 (23.8) |
| Lower limb and hip | 23,587 (17.3) | 5449 (21.0) | 2609 (20.9) | 3240 (18.5) |
| Trunk | 41,885 (30.8) | 8794 (33.8) | 3896 (31.2) | 3165 (18.1) |
| Head and neck | 35,353 (26.0) | 5176 (19.9) | 2572 (20.6) | 6313 (36.0) |
| Overlapping lesion of the skin | 126 (0.1) | 27 (0.1) | 13 (0.1) | 29 (0.2) |
| Skin, not otherwise specified | 3599 (2.7) | 796 (3.1) | 442 (3.5) | 605 (3.5) |
| Socioeconomic statuse,f | mean (SD) | |||
| Median household incomeg | 45.7 (12.1) | 46.2 (11.5) | 45.5 (11.3) | 44.9 (12.4) |
| Below poverty (%) | 8.3 (4.3) | 8.6 (4.2) | 8.6 (4.3) | 8.6 (4.4) |
| Unemployed (%) | 6.0 (2.1) | 6.0 (2.0) | 6.1 (2.0) | 6.1 (2.1) |
| Less than high school education (%) | 18.8 (6.8) | 19.4 (6.8) | 19.5 (6.9) | 19.5 (6.9) |
| Urban (%) | 87.7 (19.1) | 90.7 (16.0) | 88.9 (17.8) | 86.6 (20.9) |
| White collar (%) | 36.8 (5.8) | 37.5 (5.7) | 36.8 (5.7) | 36.5 (5.9) |
Single includes individuals who have never been married.
Divorced includes individuals who are separated.
Percentages may not add to 100.0% due to rounding.
Other includes American Indian, Alaska Native, Asian/Pacific Islander, oand other race, unspecified.
Socioeconomic variables are proxy measures for each individual and represent the county-level median or percentage for the county of residence.
Fourteen patients were missing data about socioeconomic variables; thus, the sample size was 192,000 for those variables.
In thousands of 2000 US dollars.
Table 2 presents descriptive statistics for independent variables by stage at diagnosis, with unadjusted (crude) ORs and 95% confidence intervals (CIs) estimating risk of late-stage diagnosis of melanoma. Final adjusted logistic modeling results indicated that sex modified the relationship between marital status and stage at diagnosis (P < .0001 for interaction). Namely, although unmarried patients had a higher risk of being diagnosed at a late stage among both men and women, when compared with married patients, widows were most likely to be diagnosed at a late stage among women and least likely among men. Table 3 shows this interaction and the additional factors remaining in the final logistic model. Alternatively (also shown in Table 3), the interaction revealed that among married, single, and divorced or separated patients, men had 1.51 (95% CI, 1.45-1.58), 1.89 (95% CI, 1.74-2.05), and 1.53 (95% CI, 1.37-1.71) times the risk, respectively, of being diagnosed at a late stage when compared with women. Widowed men and widowed women, however, were not statistically different in their stage at diagnosis (P = .60).
Table 2. Patient Demographic and Tumor Characteristics by Stage at Diagnosis of Cutaneous Melanoma With Corresponding Univariate Odds Ratios (ORs) and 95% Confidence Intervals (CIs), SEER 1973-2006 (n = 192,014).
| Stage at Diagnosis | Earlya | Lateb | Crude OR (95% CI) | Pc |
|---|---|---|---|---|
| Demographic Variable | n (%)d | |||
| Marital status at diagnosis | <.0001 | |||
| Married | 121,008 (71.9) | 14,983 (63.0) | 1.00 (reference) | |
| Single (never married) | 22,318 (13.3) | 3684 (15.5) | 1.33 (1.28, 1.39) | |
| Divorced or separated | 10,500 (6.2) | 2003 (8.4) | 1.54 (1.46, 1.62) | |
| Widowed | 14,411 (8.6) | 3107 (13.1) | 1.74 (1.67, 1.82) | |
| Sex | <.0001 | |||
| Female | 75,518 (44.9) | 8816 (37.1) | 1.00 (reference) | |
| Male | 92,719 (55.1) | 14,961 (62.9) | 1.38 (1.34, 1.42) | |
| Age, y | <.0001 | |||
| 18-24 | 3269 (1.9) | 438 (1.8) | 1.00 (referent) | |
| 25-39 | 22,263 (13.2) | 2505 (10.5) | 0.84 (0.75, 0.94) | |
| 40-54 | 42,827 (25.5) | 5560 (23.4) | 0.97 (0.87, 1.08) | |
| 55-69 | 49,001 (29.1) | 6743 (28.4) | 1.03 (0.93, 1.14) | |
| 70 or older | 50,877 (30.2) | 8531 (35.9) | 1.25 (1.13, 1.39) | |
| Race | <.0001 | |||
| White | 166,383 (98.9) | 23,080 (97.1) | 1.00 (reference) | |
| Black | 616 (0.4) | 303 (1.3) | 3.55 (3.09, 4.07) | |
| Othere | 1238 (0.7) | 394 (1.7) | 2.29 (2.05, 2.57) | |
| Year of diagnosis | <.0001 | |||
| 1973-1979 | 6879 (4.1) | 1440 (6.1) | 1.00 (reference) | |
| 1980-1989 | 19,007 (11.3) | 2910 (12.2) | 0.73 (0.68, 0.78) | |
| 1990-1999 | 43,525 (25.9) | 6093 (25.6) | 0.67 (0.63, 0.71) | |
| 2000-2006 | 98,826 (58.7) | 13,334 (56.1) | 0.65 (0.61, 0.68) | |
| Tumor histology | <.0001 | |||
| Lentigo maligna melanoma | 27,681 (16.5) | 429 (1.8) | 1.00 (reference) | |
| Superficial spreading (SS) | 47,018 (28.0) | 3323 (14.0) | 4.56 (4.12, 5.05) | |
| Nodular | 7250 (4.3) | 4692 (19.7) | 41.8 (37.7, 46.3) | |
| Acral lentiginous (AL) | 1179 (0.7) | 507 (2.1) | 27.7 (24.1, 32.0) | |
| Other or unspecified | 85,109 (50.6) | 14,826 (62.4) | 11.2 (10.2, 12.4) | |
| Anatomical site | <.0001 | |||
| Upper limb and shoulder | 40,411 (24.0) | 3927 (16.5) | 1.00 (reference) | |
| Lower limb and hip | 30,455 (18.1) | 4430 (18.6) | 1.50 (1.43, 1.57) | |
| Trunk | 51,831 (30.8) | 5909 (24.9) | 1.17 (1.12, 1.22) | |
| Head and neck | 44,670 (26.6) | 4744 (20.0) | 1.09 (1.05, 1.14) | |
| Overlapping lesion of the skin | 166 (0.1) | 29 (0.1) | 1.80 (1.21, 2.67) | |
| Skin, not otherwise specified | 704 (0.4) | 4738 (19.9) | 69.3 (63.6, 75.5) | |
| Socioeconomic statusf,g | Mean (SD) | |||
| Median household incomeh | 45.9 (12.0) | 44.4 (11.7) | 0.95 (0.94, 0.95)i | <.0001 |
| Below poverty (%) | 8.3 (4.2) | 8.7 (4.5) | 1.11 (1.09, 1.13)j | <.0001 |
| Unemployed (%) | 6.0 (2.1) | 6.1 (2.2) | 1.20 (1.16, 1.24)j | <.0001 |
| Urban (%) | 88.3 (18.6) | 86.6 (20.5) | 0.96 (0.95, 0.96)k | <.0001 |
| Less than high school education (%) | 18.9 (6.8) | 19.6 (7.1) | 1.07 (1.06, 1.08)j | <.0001 |
| White collar (%) | 37.0 (5.8) | 36.4 (5.8) | 0.84 (0.82, 0.86)k | <.0001 |
Early stage is in situ or localized stage using SEER summary staging.
Late stage is regional or distant stage using SEER summary staging.
P values are from a global univariate likelihood ratio test.
Percentages may not add to 100.0% due to rounding.
Other includes American Indian, Alaska Native, Asian/Pacific Islander, and other race, unspecified.
Socioeconomic variables are proxy measures for each individual and represent the county-level median or percentage for the county of residence.
Fourteen patients were missing data about socioeconomic variables; thus, the sample size was 192,000 for those variables.
In thousands of 2000 US dollars.
OR represents an increase in median household income of $5000.
OR represents an absolute 5% increase.
OR represents an absolute 10% increase.
Table 3. Multivariate Logistic Regression Model of Late Versus Early Stage at Cutaneous Melanoma Diagnosis With Corresponding Odds Ratios (ORs) and 95% Confidence Intervals (CIs), SEER 1973-2006 (n = 192,014).
| Demographic Variable | Adjusted OR (95% CI)a | Pb |
|---|---|---|
| Interaction: marital status by sexg | ||
| Among men | ||
| Married | 1.00 (reference) | |
| Single (never married) | 1.56 (1.47, 1.66) | <.0001 |
| Divorced or Separated | 1.60 (1.47, 1.73) | <.0001 |
| Widowed | 1.31 (1.20, 1.42) | <.0001 |
| Among women | ||
| Married | 1.00 (reference) | |
| Single (never married) | 1.25 (1.16, 1.35) | <.0001 |
| Divorced or separated | 1.57 (1.44, 1.71) | <.0001 |
| Widowed | 1.92 (1.80, 2.05) | <.0001 |
| Alternatively (sex by marital status)g | ||
| Among those married | ||
| Women | 1.00 (reference) | |
| Men | 1.51 (1.45, 1.58) | <.0001 |
| Among those single (never married) | ||
| Women | 1.00 (reference) | |
| Men | 1.89 (1.74, 2.05) | <.0001 |
| Among those divorced or separated | ||
| Women | 1.00 (reference) | |
| Men | 1.53 (1.37, 1.71) | <.0001 |
| Among those widowed | ||
| Women | 1.00 (reference) | |
| Men | 1.03 (0.93, 1.13) | .5966 |
| Age, y | ||
| 18-24 | 1.00 (reference) | |
| 25-39 | 0.99 (0.88, 1.12) | .8907 |
| 40-54 | 1.18 (1.04, 1.33) | .0111 |
| 55-69 | 1.28 (1.13, 1.44) | .0001 |
| 70 or older | 1.60 (1.41, 1.80) | <.0001 |
| Race | ||
| White | 1.00 (reference) | |
| Black | 2.09 (1.77, 2.46) | <.0001 |
| Otherc | 2.12 (1.84, 2.43) | <.0001 |
| Year of diagnosis | ||
| 1973-1979 | 1.00 (reference) | |
| 1980-1989 | 0.67 (0.62, 0.73) | <.0001 |
| 1990-1999 | 0.68 (0.63, 0.73) | <.0001 |
| 2000-2006 | 0.69 (0.64, 0.74) | <.0001 |
| Tumor histology | ||
| Superficial spreading (SS) | 1.00 (reference) | |
| Nodular | 53.6 (48.1, 59.6) | <.0001 |
| Lentigo maligna melanoma | 28.5 (24.5, 33.1) | <.0001 |
| Acral lentiginous (AL) | 6.25 (5.62, 6.96) | <.0001 |
| Other or unspecified | 11.1 (10.0, 12.3) | <.0001 |
| Anatomical site | ||
| Upper limb and shoulder | 1.00 (reference) | |
| Lower limb and hip | 1.56 (1.48, 1.64) | <.0001 |
| Trunk | 1.16 (1.11, 1.21) | <.0001 |
| Head and neck | 1.38 (1.31, 1.45) | <.0001 |
| Overlapping lesion of the skin | 1.96 (1.27, 3.00) | <.0001 |
| Skin, not otherwise specified | 73.8 (67.4, 80.7) | <.0001 |
| Socioeconomic statusd,e | ||
| Less than high school education | 1.09 (1.07, 1.11)f | <.0001 |
aOdds ratios also adjusted for the 17 SEER sites as a fixed effect.
P values are from the Wald test. All variables, including the interaction, were globally significant at P < .0001 using the likelihood ratio test.
Other includes American Indian, Alaska Native, Asian/Pacific Islander, and other race, unspecified.
Socioeconomic variables are proxy measures for each individual and represent the county-level median or percentage for the county of residence.
Fourteen patients were missing data about socioeconomic variables; thus, the sample size was 192,000 for the final model.
OR represents an absolute 5% increase in the percentage of the county's residents with less than a high school education.
Results from the adjusted single model term marital status × sex.
Older patients were progressively (P < .0001 for trend) more likely to be diagnosed at a late stage. Compared with whites, blacks and members of other races had more than twice the risk of being diagnosed at a late stage (P < .0001). Tumor histology and anatomical site of tumor influenced stage at diagnosis as well. In addition, patients diagnosed after 1979 had more than a 30% reduction in risk of being diagnosed at a late stage, and for every 5% absolute increase in the percentage of individuals in each county without a high school education, there was a corresponding 1.09-fold increase (95% CI, 1.07- to 1.11-increase) in the risk of late-stage diagnosis. None of the other measures of SES had an effect on marital status parameters, nor were they significant in the final model.
Discussion
The majority of early-stage melanomas are curable; however, late-stage disease is much more difficult to treat and oftentimes fatal. In this population-based study of patients diagnosed with melanoma in the SEER registries from 1973 through 2006, unmarried patients were considerably more likely to be diagnosed with late-stage disease, even after adjusting for biologically and socially meaningful confounding influences, and the magnitude of the protective effect of marriage depended on the sex of the patient. This finding suggests that being married confers a substantial protective effect in preventing late-stage melanoma diagnosis. The magnitude of this effect was considerable for both men and women, and it could be the result of an immunologically protective response resulting from the social support associated with being married, differences in insurance status between married and unmarried patients, and/or the benefit of married patients being prompted to earlier screening and dermatological consultation than are their unmarried counterparts. The latter possibility could be the result of many influences, including benefits from spouses spotting suspicious lesions earlier (lesions that would have otherwise remained unseen by the patient), spousal peer pressure to seek regular or early screening for melanoma, or spousal facilitation of early screening by helping to remove barriers, including providing transportation, childcare, or support of daily activities or responsibilities in the other's absence.
These findings have significant implications for screening recommendations, providers, and programs, suggesting that extra emphasis should be placed on prompting unmarried individuals to seek early routine screening for skin cancer. Although it is certainly important to maintain proper screening for all individuals, health behavior experts may want to specifically target the high-risk groups identified in this study to achieve a meaningful decrease in melanoma mortality. Screening programs designed for different groups of unmarried individuals, with particular attention paid to the nuances of the effects of an individual's sex, may help to reduce or eliminate the disparity in stage at diagnosis between those who are married and those who are not. How to effectively target and tailor messages to unmarried individuals requires future investigation, but could include increasing physician awareness of marital status as a risk factor for late-stage melanoma diagnosis through training and/or academic detailing or cleverly targeting unmarried individuals via ad campaigns directed at singles Web sites or consumer-specific marketing that utilizes online social media Web sites where “relationship status” is known (eg, Facebook).
Furthermore, results of our final model revealed that, with the exception of widowed patients for whom there was no difference in stage at diagnosis between men and women, men had at least a 50% increase in risk of being diagnosed at a late stage, when compared with women in the same marital category. This finding likely reflects that women have a higher melanoma awareness43 and are more likely to participate in routine screening and skin health maintenance practices—practices that ultimately lead to women having cancerous lesions that are smaller, more localized, and easier to treat than comparable lesions found in men.8 Indeed, it is true that melanoma has a worse prognosis in men than in women, and, in part, this may reflect differences in cancer stage at diagnosis.44 The finding that there was no difference in stage at diagnosis between widowed men and widowed women may be because among widows, older age is more important than marital status as a predictor of melanoma stage at diagnosis. Previous reports have suggested that middle-aged and older men may benefit most from targeted interventions to promote the uptake of skin-screening practices.16 Our results support this conclusion but also suggest that targeting unmarried individuals may be equally as beneficial.
We also investigated a potential 3-way interaction between marital status, sex, and age at diagnosis (data not shown). Although the effect of marital status and sex on stage at diagnosis did not heavily depend on age at diagnosis, it is worth noting that among men, the protective effect of being married was diminished somewhat with increasing age. In addition, the risk of being diagnosed at a late stage for men compared with women was somewhat dampened as age increased.
Results also revealed that patients who were older and who were nonwhite had a higher risk of being diagnosed at a late stage. These findings are similar to those reported previously.2-6,9-13,17 Older patients are less likely to participate in melanoma screening and are more likely to be diagnosed with nodular melanoma6—a histologic type that, in our study, was more than 50 times more likely to be diagnosed at a late stage. Because older patients are also more likely to be widowed, our primary results (shown in Table 3) were adjusted for histology to control for confounding.
Although all SES-proxy variables were significant at the univariate level, only the percentage of individuals with less than a high school education remained in the final model. Low SES has previously been associated with a later stage of melanoma at diagnosis.2,3,8,13-15 Interestingly, however, this finding does suggest that education may play a larger role than income in adherence to screening and diagnosing melanoma at an early stage. Other characteristics of SES neither confounded the effect of marital status on stage at diagnosis nor were significant at the predetermined alpha level. The effect of year of diagnosis, namely, that those diagnosed after 1979 had more than a 60% reduction in risk of being diagnosed at a late stage, likely reflects a combination of increased focus on the importance of screening and the recognition of suspicious lesions (eg, the American Cancer Society's ABCD campaign) from the 1980s through the present,45 better medical training, an increase in the number of dermatologists, and improved technology used to recognize malignant nevi.
Results also showed that the histologic types nodular, lentigo maligna melanoma, and acral lentiginous were much more likely to be diagnosed at a late stage than were superficial spreading tumors. These results are nearly identical to those from another study using SEER data to examine stage of melanoma at diagnosis among older patients34 and reflect the pathologic nature of the histologic types. Analysis of anatomic site revealed that patients with melanoma lesions found on the trunk, head or neck, and lower limb or hip had nearly a 20%, 40%, and 60% increase in risk, respectively, of being diagnosed at a late stage when compared with those with cancerous lesions on the upper limb or shoulder. This suggests that lesions on areas other than the arms or shoulders may be spotted later. Patients with an anatomic site coded as “overlapping lesion of the skin” had nearly double the risk of being diagnosed at a late stage, and those with unspecified anatomic location had 73.8 times the risk (95% CI, 67.4-80.7) of late-stage diagnosis.
This study is not without limitations. Marital status categories were self-reported and reflected marital status only at the time of diagnosis; therefore, our marital status categories do not present a life course view of marital history. For example, patients who reported being married may have been married for the first time or for the third. Likewise, for patients who reported being widowed, no data were available describing the amount of time since their loss, which is likely to be highly variable and possibly important in relation to stage of melanoma at diagnosis. Moreover, data about marital status was missing for 26.2% of patients who were ultimately excluded. Furthermore, detailed information about individual-level SES and factors related to cancer screening behaviors, cancer-related health behaviors (eg, diet, exercise, and smoking status), occupational sun exposure, psychosocial health, and comorbidities is also not available in the SEER database. As a result, these factors were not accounted for in our analyses. However, proxy SES information from the US census has been effectively used in epidemiologic cancer research.46-48 SEER data are also limited by the fact that anatomic classification restricted our analyses to the broad groupings of upper limb and shoulder, lower limb and hip, trunk, head and neck, overlapping lesion of the skin, and skin, NOS. This was especially limiting for the category of trunk (which includes chest and back) because melanomas found on the back are more common in men, more difficult to see, and more likely to be found at a later stage than are more visible and earlier-detected chest lesions. Future studies using population-based data should consider evaluating more specific anatomic sites. In addition, patients' insurance status was unavailable, and the finding that a higher proportion of unmarried individuals may be without health insurance may partially explain an increased risk of late-stage diagnosis among unmarried patients. However, the protective effect of marital status remained among older patients, who all had similar medical coverage (Medicare) in a previous study.34 It is also important to note that we reported findings from all SEER data and that during the initial 15-20 years of the SEER program (1970s and 1980s), SEER covered only around 10% of the US population and may have been less generalizable to the United States as a whole. Last, we were unable to evaluate the impact of cohabitation in the absence of marriage. Specifically, the impact of gay and lesbian, or homosexual, relationships could not be examined, and straight, or heterosexual, relationships were only examined in the presence of marriage. It would be interesting to determine whether the apparent protection afforded to married individuals in terms of risk of late-stage melanoma diagnosis is extended to those cohabiting but not married. According to the US census, there were more than 12 million individuals living as unmarried partners in the United States in 2005-2007. Our study is limited by the fact that we could not examine the impact of these relationships, which represent approximately 4% of US residents, on stage of melanoma at diagnosis.
These limitations were unavoidable using the SEER database; however, SEER data represent a large and heterogeneous group of melanoma patients, are highly generalizable, and are extensive in describing tumor characteristics and known confounders of the association between marital status and stage at diagnosis.36,37 This study presents a complete picture of melanoma staging, including the factors that predict it, from 1973 through 2006. Our study confirms the findings of 2 previous studies that showed unmarried individuals were more likely to be diagnosed at a late stage8,34; however, ours is unique in that it utilized a large, heterogeneous, and generalizable population.
Of the 2 studies preceding ours that examined the influence of marital status on stage of melanoma at diagnosis, the earlier study included data about fewer than 2000 patients from only 1 US state (Florida, whose melanoma population is likely not representative) and for only 1 year of data.8 In the more recent study, SEER-Medicare linked data were used, thereby restricting the analyses and generalizability, admittedly so, only to patients eligible for and enrolled in Medicare.34 The median age of melanoma diagnosis from 2002 through 2006 was 59,1 and only including patients ages 65 years and older prevents generalization of results to younger patients.
Results of this study have demonstrated that marital status is an important factor for predicting stage of melanoma at diagnosis, not only in older patients, but among all patients. In addition, our results revealed that sex may modify this relationship, namely, that the magnitude of the effect of marital status is largest when comparing widowed women with married women. The interaction also revealed that men are more likely than women to be diagnosed at a late stage in every category of marital status, exception for widows. These results have important implications for researchers studying melanoma screening practices, health behavior experts interested in increasing melanoma awareness and the utilization of skin cancer screening, and general practitioners and dermatologists who perform screening tests and provide recommendations about risks and screening intervals. Aside from oneself or a health provider, an individual's spouse is probably the most likely person to recognize suspicious, large, or unusual skin lesions,49 and results from our study suggest that this additional mechanism of spousal detection may facilitate fewer cases of late-stage disease, ultimately increasing the probability of surviving melanoma. The effect of spousal detection, however, cannot be teased apart from other potential protective factors of being married in our study. Thus, future studies are warranted.
Footnotes
Conflict of Interest Disclosures: The authors made no disclosures.
References
- 1.Cancer Statistics Review. Bethesda, MD: National Cancer Institute; 2009. Surveillance Epidemiology End Results (SEER) Program. [Google Scholar]
- 2.Chang AE, Karnell LH, Menck HR. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: a summary of 84,836 cases from the past decade. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer. 1998;83:1664–1678. doi: 10.1002/(sici)1097-0142(19981015)83:8<1664::aid-cncr23>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 3.Bonett A, Roder D. Epidemiological features of melanoma in South Australia: implications for cancer control. Med J Aust. 1989;151:502–504. 506–509. [PubMed] [Google Scholar]
- 4.Cress RD. Incidence of cutaneous melanoma among non-Hispanic whites, Hispanics, Asians, and blacks: an analysis of california cancer registry data, 1988-93. Cancer Causes Control. 1997;8:246–252. doi: 10.1023/a:1018432632528. [DOI] [PubMed] [Google Scholar]
- 5.Hersey P, Sillar RW, Howe CG, et al. Factors related to the presentation of patients with thick primary melanomas. Med J Aust. 1991;154:583–587. doi: 10.5694/j.1326-5377.1991.tb121217.x. [DOI] [PubMed] [Google Scholar]
- 6.Hanrahan PF, Hersey P, D'Este CA. Factors involved in presentation of older people with thick melanoma. Med J Aust. 1998;169:410–414. doi: 10.5694/j.1326-5377.1998.tb126830.x. [DOI] [PubMed] [Google Scholar]
- 7.Thorn M, Ponten F, Bergstrom R, Sparen P, Adami HO. Clinical and histopathologic predictors of survival in patients with malignant melanoma: a population-based study in Sweden. J Natl Cancer Inst. 1994;86:761–769. doi: 10.1093/jnci/86.10.761. [DOI] [PubMed] [Google Scholar]
- 8.Van Durme DJ, Ferrante JM, Pal N, Wathington D, Roetz-heim RG, Gonzalez EC. Demographic predictors of melanoma stage at diagnosis. Arch Fam Med. 2000;9:606–611. doi: 10.1001/archfami.9.7.606. [DOI] [PubMed] [Google Scholar]
- 9.Austin PF, Cruse CW, Lyman G, Schroer K, Glass F, Reintgen DS. Age as a prognostic factor in the malignant melanoma population. Ann Surg Oncol. 1994;1:487–494. doi: 10.1007/BF02303614. [DOI] [PubMed] [Google Scholar]
- 10.Goodwin JS, Samet JM, Key CR, Humble C, Kutvirt D, Hunt C. Stage at diagnosis of cancer varies with the age of the patient. J Am Geriatr Soc. 1986;34:20–26. doi: 10.1111/j.1532-5415.1986.tb06335.x. [DOI] [PubMed] [Google Scholar]
- 11.Hanrahan PF, Hersey P, Watson AB, Callaghan TM. The effect of an educational brochure on knowledge and early detection of melanoma. Aust J Public Health. 1995;19:270–274. doi: 10.1111/j.1753-6405.1995.tb00442.x. [DOI] [PubMed] [Google Scholar]
- 12.Holmes FF, Hearne E., 3rd Cancer stage-to-age relationship: implications for cancer screening in the elderly. J Am Geriatr Soc. 1981;29:55–57. doi: 10.1111/j.1532-5415.1981.tb01227.x. [DOI] [PubMed] [Google Scholar]
- 13.Roder DM, Luke CG, McCaul KA, Esterman AJ. Trends in prognostic factors of melanoma in South Australia, 1981-1992: implications for health promotion. Med J Aust. 1995;162:25–29. doi: 10.5694/j.1326-5377.1995.tb138407.x. [DOI] [PubMed] [Google Scholar]
- 14.Geller AC, Miller DR, Lew RA, Clapp RW, Wenneker MB, Koh HK. Cutaneous melanoma mortality among the socioeconomically disadvantaged in Massachusetts. Am J Public Health. 1996;86:538–543. doi: 10.2105/ajph.86.4.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vagero D, Persson G. Risks, survival and trends of malignant melanoma among white and blue collar workers in Sweden. Soc Sci Med. 1984;19:475–478. doi: 10.1016/0277-9536(84)90207-7. [DOI] [PubMed] [Google Scholar]
- 16.Swetter SM, Johnson TM, Miller DR, Layton CJ, Brooks KR, Geller AC. Melanoma in middle-aged and older men: a multi-institutional survey study of factors related to tumor thickness. Arch Dermatol. 2009;145:397–404. doi: 10.1001/archdermatol.2008.603. [DOI] [PubMed] [Google Scholar]
- 17.Vayer A, Lefor AT. Cutaneous melanoma in African-Americans. South Med J. 1993;86:181–182. doi: 10.1097/00007611-199302000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Koh HK, Sober AJ, Day CL, Jr, Lew RA, Fitzpatrick TB. Cigarette smoking and malignant melanoma. Prognostic implications. Cancer. 1984;53:2570–2573. doi: 10.1002/1097-0142(19840601)53:11<2570::aid-cncr2820531135>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 19.Rigel DS, Friedman RJ, Levine J, Kopf AW, Levenstein M. Cigarette smoking and malignant melanoma. Prognostic implications. J Dermatol Surg Oncol. 1981;7:889–891. doi: 10.1111/j.1524-4725.1981.tb00184.x. [DOI] [PubMed] [Google Scholar]
- 20.Dintenfass L. Effect of smoking on the recurrence of malignant melanoma. Med J Aust. 1979;1:620. [PubMed] [Google Scholar]
- 21.Shaw HM, Milton GW, McCarthy WH, Farago GA, Dil-worth P. Effect of smoking on the recurrence of malignant melanoma. Med J Aust. 1979;1:208–209. [PubMed] [Google Scholar]
- 22.Shaw HM, Milton GW. Smoking and the development of metastases from malignant melanoma. Int J Cancer. 1981;28:153–156. doi: 10.1002/ijc.2910280207. [DOI] [PubMed] [Google Scholar]
- 23.Roetzheim RG, Pal N, van Durme DJ, et al. Increasing supplies of dermatologists and family physicians are associated with earlier stage of melanoma detection. J Am Acad Dermatol. 2000;43:211–218. doi: 10.1067/mjd.2000.106242. [DOI] [PubMed] [Google Scholar]
- 24.Roetzheim RG, Pal N, Tennant C, et al. Effects of health insurance and race on early detection of cancer. J Natl Cancer Inst. 1999;91:1409–1415. doi: 10.1093/jnci/91.16.1409. [DOI] [PubMed] [Google Scholar]
- 25.Geller AC, Johnson TM, Miller DR, Brooks KR, Layton CJ, Swetter SM. Factors associated with physician discovery of early melanoma in middle-aged and older men. Arch Dermatol. 2009;145:409–414. doi: 10.1001/archdermatol.2009.8. [DOI] [PubMed] [Google Scholar]
- 26.Fisher NM, Schaffer JV, Berwick M, Bolognia JL. Breslow depth of cutaneous melanoma: impact of factors related to surveillance of the skin, including prior skin biopsies and family history of melanoma. J Am Acad Dermatol. 2005;53:393–406. doi: 10.1016/j.jaad.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 27.McPherson M, Elwood M, English DR, Baade PD, Youl PH, Aitken JF. Presentation and detection of invasive melanoma in a high-risk population. J Am Acad Dermatol. 2006;54:783–792. doi: 10.1016/j.jaad.2005.08.065. [DOI] [PubMed] [Google Scholar]
- 28.Brady MS, Oliveria SA, Christos PJ, et al. Patterns of detection in patients with cutaneous melanoma. Cancer. 2000;89:342–347. doi: 10.1002/1097-0142(20000715)89:2<342::aid-cncr19>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 29.Vercelli M, Lillini R, Capocaccia R, et al. Cancer survival in the elderly: effects of socio-economic factors and health care system features (ELDCARE project) Eur J Cancer. 2006;42:234–242. doi: 10.1016/j.ejca.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 30.De Boer MF, Ryckman RM, Pruyn JF, Van den Borne HW. Psychosocial correlates of cancer relapse and survival: a literature review. Patient Educ Couns. 1999;37:215–30. doi: 10.1016/s0738-3991(99)00029-4. [DOI] [PubMed] [Google Scholar]
- 31.de Graeff A, de Leeuw JR, Ros WJ, Hordijk GJ, Blijham GH, Winnubst JA. Sociodemographic factors and quality of life as prognostic indicators in head and neck cancer. Eur J Cancer. 2001;37:332–339. doi: 10.1016/s0959-8049(00)00385-3. [DOI] [PubMed] [Google Scholar]
- 32.Hansen RP, Olesen F, Sorensen HT, Sokolowski I, Sonder-gaard J. Socioeconomic patient characteristics predict delay in cancer diagnosis: a Danish cohort study. BMC Health Serv Res. 2008;8:49. doi: 10.1186/1472-6963-8-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lai H, Lai S, Krongrad A, Trapido E, Page JB, McCoy CB. The effect of marital status on survival in late-stage cancer patients: an analysis based on surveillance, epidemiology, and end results (SEER) data, in the United States. Int J Behav Med. 1999;6:150–176. doi: 10.1207/s15327558ijbm0602_4. [DOI] [PubMed] [Google Scholar]
- 34.Reyes Ortiz CA, Freeman JL, Kuo YF, Goodwin JS. The influence of marital status on stage at diagnosis and survival of older persons with melanoma. J Gerontol A Biol Sci Med Sci. 2007;62:892–898. doi: 10.1093/gerona/62.8.892. [DOI] [PubMed] [Google Scholar]
- 35.National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch. Surveillance, Epidemiology, and End Results (SEER) Program SEER*Stat Database: Incidenc–SEER 17 Regs Limited-Use + Hurricane Katrina Impacted Louisiana Cases, Nov 2008 Sub (1973-2006 varying)–Linked to County Attributes–Total U.S., 1969-2006 Counties: National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, April 2009, based on November 2008 submission.
- 36.Frey CM, McMillen MM, Cowan CD, Horm JW, Kessler LG. Representativeness of the surveillance, epidemiology, and end results program data: recent trends in cancer mortality rates. J Natl Cancer Inst. 1992;84:872–877. doi: 10.1093/jnci/84.11.872. [DOI] [PubMed] [Google Scholar]
- 37.Merrill RM, Dearden KA. How representative are the surveillance, epidemiology, and end results (SEER) program cancer data of the United States? Cancer Causes Control. 2004;15:1027–1034. doi: 10.1007/s10552-004-1324-5. [DOI] [PubMed] [Google Scholar]
- 38.Surveillance Epidemiology and End Results (SEER) Program About SEER. Bethesda,MD: National Cancer Institute; 2009. [Google Scholar]
- 39.Reyes-Ortiz CA, Goodwin JS, Freeman JL, Kuo YF. Socioe-conomic status and survival in older patients with melanoma. J Am Geriatr Soc. 2006;54:1758–1764. doi: 10.1111/j.1532-5415.2006.00943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.World Health Organization. International Classification of Diseases for Oncology. 3rd. Geneva, Switzerland: World Health Organization; 2000. [Google Scholar]
- 41.Zell JA, Cinar P, Mobasher M, Ziogas A, Meyskens FL, Anton-Culver H. Survival for patients with invasive cutaneous melanoma among ethnic groups: the effects of socioeco-nomic status and treatment. Journal of clinical oncology: official journal of the Am Soc Clin Oncol. 2008;26:66–75. doi: 10.1200/JCO.2007.12.3604. [DOI] [PubMed] [Google Scholar]
- 42.Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. Am J Epidemiol. 1989;129:125–137. doi: 10.1093/oxfordjournals.aje.a115101. [DOI] [PubMed] [Google Scholar]
- 43.Swetter SM, Layton CJ, Johnson TM, Brooks KR, Miller DR, Geller AC. Gender differences in melanoma awareness and detection practices between middle-aged and older men with melanoma and their female spouses. Arch Dermatol. 2009;145:488–490. doi: 10.1001/archdermatol.2009.42. [DOI] [PubMed] [Google Scholar]
- 44.Balch CM, Soong SJ, Gershenwald JE, et al. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol. 2001;19:3622–3634. doi: 10.1200/JCO.2001.19.16.3622. [DOI] [PubMed] [Google Scholar]
- 45.American Cancer Society, Cancer Facts & Figures 2009. Atlanta, GA: American Cancer Society, Inc.; 2009. [Google Scholar]
- 46.MacKinnon JA, Duncan RC, Huang Y, Lee DJ, Fleming LE, Voti L, et al. Detecting an association between socioe-conomic status and late stage breast cancer using spatial analysis and area-based measures. Cancer Epidemiol Bio-markers Prev. 2007;16:756–762. doi: 10.1158/1055-9965.EPI-06-0392. [DOI] [PubMed] [Google Scholar]
- 47.Fisher J, Engelhardt HS, Stephens JA, et al. Cancer-related disparities among residents of Appalachia Ohio. J Health Dispar Res Pract. 2008;2:61–74. [Google Scholar]
- 48.Hart LG, Larson EH, Lishner DM. Rural definitions for health policy and research. Am J Public Health. 2005;95:1149–1155. doi: 10.2105/AJPH.2004.042432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koh HK, Miller DR, Geller AC, Clapp RW, Mercer MB, Lew RA. Who discovers melanoma? Patterns from a population-based survey. J Am Acad Dermatol. 1992;26:914–919. doi: 10.1016/0190-9622(92)70132-y. [DOI] [PubMed] [Google Scholar]


