Abstract
There is little prospective information on the cardiometabolic risks of testosterone and growth hormone (GH) replacement therapy to youthful levels during aging. We conducted a double-masked, partially placebo controlled study in 112 men 65–90 years-old. Transdermal testosterone (5g-vs-10g/day) using a Leydig Cell Clamp and subcutaneous recombinant GH (rhGH) (0-vs-3-vs-5ug/kg/day) were administered for 16-weeks. Measurements included testosterone and IGF-1 levels, body composition by DEXA, and cardiometabolic risk factors (upper body fat, blood pressure, insulin sensitivity, fasting triglycerides, HDL-cholesterol, and serum adiponectin) at baseline and after 16 weeks of treatment. Some cardiometabolic factors improved (total and trunk fat, triglycerides, HDL-cholesterol) and others worsened (systolic blood pressure, insulin sensitivity index [QUICKI], adiponectin). Cardiometabolic risk composite scores (CRCS) improved (−0.69±1.55, p<0.001). In multivariate analyses, QUICKI, triglycerides, and HDL-cholesterol contributed 33%, 16%, and 14% of the variance in CRCS, respectively. Pathway analyses indicated that changes in fat and lean mass were related to individual cardiometabolic variables and CRCS in a complex manner. Changes in BMI, reflecting composite effects of changes in fat and lean mass, were more robustly associated with cardiometabolic risks than changes in fat mass or LBM individually. In conclusion, testosterone and rhGH administration was associated with diverse changes in individual cardiometabolic risk factors, but in aggregate appeared not to worsen cardiometabolic risk in healthy older men after 4-months. The long term effects of these and similar anabolic therapies on cardiovascular events should be investigated in populations with greater funtional limitations along with important health disabilities including upper body obesity and other cardiometabolic risks.
Keywords: Cardiometabolic Risks, Testosterone, Growth Hormone, BMI, Aging
INTRODUCTION
The obesity epidemic is now a global health problem. In the United States alone, approximately two thirds of American adults (nearly 200 million) are overweight (33.3%) or obese (35.9%) (1). With advancing age, upper body obesity and other cardiometabolic risk factors may worsen with increases in blood pressure, insulin resistance, and abnormalities in lipid metabolism, which together constitute the Metabolic Syndrome, although it has several somewhat different definitions (2, 3).
The aging process per se is associated with the occurrence of increasing cardiometabolic risks for heart attack, stroke, and peripheral vascular disease, as well as with declining testosterone, growth hormone (GH), and insulin-like growth factor-1 (IGF-1) status (4). Indeed, approximately 25–30% of men over 60 years-of-age have low levels of testosterone (4, 5) that may be associated with upper body adiposity, increased cardiovascular disease (CVD) risk, and mortality (6–8), although the directionality of this association remains unclear (9). Declines in GH synthesis and release also occur with aging and have been associated with similar co-morbidities including central adiposity and cardiometabolic risks, even in persons with normal testosterone levels (10–15). Levels of IGF-1, a mediator of several but not all anabolic effects of GH, continue to decline into the 8th and 9th decades and are associated with increases in adiposity (4).
In epidemiologic studies, low total testosterone levels have been associated cross-sectionally with increased cardiometabolic risks (16, 17). However, in longitudinal analyses, sex hormone binding globulin (SHBG) levels, but neither total nor free testosterone levels, were significantly associated with the metabolic syndrome, as demonstrated in the Framingham Heart Study (18). Thus, it remains unclear whether testosterone or SHBG is independently associated with the increased cardiometabolic risks, although declines in both occur with increases in upper body adiposity and aging. Similarly, therapeutic trials of supplementation with testosterone, recombinant human GH (rhGH) or the combination in older persons have resulted in variable effects on cardiometabolic risk factors, with some studies showing improvements in upper body obesity, insulin resistance, dyslipidemia and blood pressure and others worsening or relatively little change in these markers (13, 19–27). Further, many of these studies were relatively small, and the dose and formulation of the endocrine replacements were variable, sometimes resulting in minimal changes in hormone levels or fat mass. Thus, there is little prospective information on how restoring testosterone and rhGH/IGF-1 to physiological levels typical of younger men affects the individual and total constellation of the cardiometabolic risks in older persons.
We herein report for the first time how changes in serum hormone levels (testosterone and IGF-1) and body composition after 16 weeks of treatment in the Hormonal Regulators of Muscle and Metabolism in Aging (HORMA) study were related to changes in individual cardiometabolic risk factors and their composite summation for potential cardiovascular complications. Further, we utilized a pathway statistical strategy to evaluate models to assess predictors and mediators of these outcomes.
METHODS and PROCEDURES
Study Design
The full design of the HORMA Trial, a double-masked investigation of testosterone with or without rhGH supplementation for 16 weeks in men 65–90 years old, was published previously (28). End of treatment measurements were collected at either week 16 (assessment for adverse events and hormone levels) or week 17 (body composition and muscle performance).
Study Participants
Participants provided written informed consent approved by the local IRBs. Eligible men had screening morning total testosterone levels in the lower half (≤ 550ng/dL) of the adult male range and serum IGF-1 in the lower tertile for adults (<130ng/mL), both typical of 65–90 year old-men (28). Other inclusion criteria included PSA ≤4.0μg/L, hematocrit ≤50%, and fasting blood glucose <126 mg/dL (28).
Treatment Regimens
Participants received a GnRH agonist (leuprolide acetate depot, 7.5mg intramuscularly (Tap Pharmaceutical Products Inc., Lare Forest, IL) monthly for 12 weeks to suppress endogenous testosterone production (Leydig cell clamp). Participants were randomized to receive 5g or 10g of 1% testosterone transdermal gel (Abbott Pharmaceuticals Inc., Chicago, IL) each morning and 0, 3 and 5μg/kg of rhGH (Nutropin, Genentech Inc., San Francisco, CA) by subcutaneous injection each evening for 16 weeks.
Outcome Measures
Hormone Assays
Serum samples obtained at baseline and study weeks 16 were batch tested. Testosterone levels were quantified using liquid chromatography-tandem mass spectrometry (29) and SHBG was measured by a fluoremetric assay (interassay CVs were 8.3%, 7.9% and 10.9% in low, medium, and high level pools) (30) at Boston University. IGF-1, insulin and adiponectin levels were determined in the USC Clinical Translational Research Institiute (CTSI) Core Laboratory using an automated chemiluminescent analyzer (Immulite 1000, Siemens Healthcare Diagnostics, Deerfield, IL). Methods for IGF-1 and insulin have been reported previously (29). For adiponectin, the sensitivity of the assay is 0.019ng/mL, and inter-assay CV=5.0% and intra-assay CV=5.4%.
BioNutrition Assessments
Entries in three-day food diaries at baseline and week 16 were reviewed with participants by study nutritionists. Total energy and macronutrient intake were quantified using Nutritionist Pro (Axxya Systems, Stafford, Texas). Total and regional lean tissue and fat mass were quantified by dual energy x-ray absorptiometry (DEXA). Scans were analyzed at the USC DEXA Reading Center by a single DEXA-certified technician and validated by a senior DEXA supervisor.
Statistical Considerations
Change in Study Outcomes
Paired t-tests were used to compare baseline values to the week 16 and 17 post-treatment outcomes. Correlation analyses were used to assess the overall relationship of changes in body composition and cardiometabolic variables. Significance of group specific mean changes for each variable was examined using independent t-tests within groups defined by absolute changes in LBM and fat mass greater (high) or lower (low) than the median changes for each variable. For these multiple comparison analyses, the Bonferroni adjusted p-value was set at 0.0125 (=0.05/4).
Cardiometabolic Variable and the Composite Scores
Vital signs, including controlled measurements of systolic blood pressure, trunk fat, insulin sensitivity, and serum lipids (fasting triglycerides and HDL-C) were available in HORMA. These cardiometabolic variables were used to derive the cardiometabolic risk composite score (CRCS). For insulin sensitivity, the qualitative insulin sensitivity check index (QUICKI) (31) was chosen rather than HOMA-IR because the former was log transformed and HOMA-IR was highly correlated to QUICKI (r = −0.73, p<0.0001). Change in each cardiometabolic variable was given a sub-score of +1 (unfavorable), 0 (no change), or −1 (favorable). Breakpoints were predetermined as those likely to be clinically meaningful for changes in fasting triglycerides, HDL-cholesterol, systolic blood pressure, and trunk fat. For QUICKI, the +1 and −1 boundaries were calculated based on 2-standard errors apart from 0 change. Appendix Table 1 shows the a priori breakpoints for each variable and the distribution of participants having changes at the various thresholds after study therapy. The CRCS was calculated as the summation of the five sub-scores. To understand the relative contribution of the cardiometabolic variable to the CRCS, partial R2 was estimated for each variables using multivariate linear regression.
Appendix Table 1.
Cardiometabolic Parameters and Particpant Allocation To Subscore Categories
| Metabolic Parameter | Criteria | N | Sub-core | Mean ± SD |
|---|---|---|---|---|
| Change in triglycerides mg/dL | Δ > 10 | 26 | +1 | 44.7±35.6 |
| −10 ≤ Δ ≤ 10 | 29 | 0 | −2.2±5.4 | |
| Δ < −10 | 57 | −1 | −55±50 | |
| Change in HDL-C mg/dL | Δ < −5 | 12 | +1 | −8.4±3.4 |
| −5 ≤ Δ ≤ 5 | 52 | 0 | 0.9±2.7 | |
| Δ > 5 | 48 | −1 | 9.3±4 | |
| Change in systolic BP mm Hg | Baseline < 130 & WK16 ≥140 | 17 | +1 | 28.4±7.9 |
| Otherwise | 95 | 0 | 9.5±13.4 | |
| Baseline ≥140 & WK16 <130 | 0 | −1 | None | |
| Change in QUICKIa | Δ < −0.14b | 52 | +1 | −0.743±0.613 |
| −0.0028≤ Δ ≤0.0028 | 17 | 0 | 0.0004±.0018 | |
| Δ > 0.0028b | 32 | −1 | 0.0128±.0068 | |
| Change in trunk fat kg | Δ > 0.25 | 13 | +1 | 0.91±0.72 |
| −0.25 ≤ Δ ≤ 0.25 | 21 | 0 | 0.04±0.12 | |
| Δ < −0.25 | 78 | −1 | −1.43±0.98 |
Qualitative insulin sensitivity check index
Breakpoint is 2 X the standard error from the mean change
Changes in adiponectin levels (total and high molecular weight [HMW]) during study therapy were evaluated. We only report HMW adiponectin since those values were closely related to total adiponectin (r=0.85, p<0.001).
Pathway Analysis
A correlation matrix was generated to examine the association of changes in hormones (testosterone and IGF-1 levels), body composition (total and regional LBM and fat mass, BMI), lipids, blood pressure, measures of insulin sensitivity, and HMW adiponectin (Appendix Table 2). Based on the regression coefficients, pathway analyses using structural equation modeling (32) were conducted to examine the direct and indirect effects of the changes in hormone levels (predictors) on changes in total LBM, total fat, and BMI (mediators), cardiometabolic risk components (outcomes), and CRCS (outcome). Assuming linear relationships, the pathway model was fitted by analyzing the covariance matrix, and the goodness of fit (GOF) of the overall model was assessed using the chi-square test of the null hypothesis: the proposed path model provides an acceptable fit to the data. Two other GOF indices, the non-normed fit index (NNFI) and the comparative fit index (CFI), were also examined (33). In these models, the null hypothesis was accepted when the p value was >0.1 and NNFI and CFI>0.99, suggesting that the model fits the data.
Appendix Table 2.
Inter-relationship of the Change in Cardiometabolic Variables and Composite Risk Scores
| IGF-1 | Free T | Total LBM | Total Fat | Trunk Fat | Extrem. Fat | BMI | QUICKI | Triglycerids | SBP | HDL-Chol | Adiponectin | SHBGf | CRCSg | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tesosterone | 0.18 | 0.92 | 0.39 | −0.39 | −0.28 | −0.47 | 0.03 | −0.07 | −0.11 | −0.13 | −0.17 | −0.48 | 0.06 | 0.16 |
| P value | 0.06 | <.001 | <.001 | <.001 | 0.003 | <.001 | 0.72 | 0.46 | 0.26 | 0.18 | 0.07 | <.001 | 0.56 | 0.10 |
| IGF-1 | 0.25 | 0.40 | −0.33 | −0.31 | −0.27 | 0.10 | −0.02 | −0.08 | 0.06 | −0.14 | −0.15 | 0.03 | 0.12 | |
| P value | .008 | <.001 | <.001 | <.001 | 0.004 | 0.30 | 0.81 | 0.42 | 0.52 | 0.15 | 0.11 | 0.72 | 0.21 | |
| Free T. | 0.44 | −0.37 | −0.28 | −0.44 | 0.11 | −0.12 | −0.09 | −0.12 | −0.12 | −0.41 | 0.06 | 0.19 | ||
| P value | <.001 | <.001 | .003 | <.001 | 0.27 | 0.20 | 0.34 | 0.22 | 0.20 | <.001 | 0.50 | 0.05 | ||
| LBMa | −0.43 | −0.38 | −0.40 | 0.59 | −0.02 | 0.03 | −0.02 | −0.24 | −0.34 | 0.09 | 0.25 | |||
| P value | <.001 | <.001 | <.001 | <.001 | 0.86 | 0.77 | 0.86 | 0.01 | <.001 | 0.34 | .008 | |||
| Total Fat | 0.93 | 0.83 | 0.47 | −0.22 | 0.19 | −0.06 | 0.06 | 0.15 | −0.20 | 0.23 | ||||
| P value | <.001 | <.001 | <.001 | 0.02 | 0.04 | 0.54 | 0.56 | 0.13 | 0.04 | 0.02 | ||||
| Trunk Fat | 0.57 | 0.46 | −0.17 | 0.18 | −0.03 | 0.02 | 0.08 | −0.21 | 0.28 | |||||
| P value | <.001 | <.001 | 0.07 | 0.06 | 0.78 | 0.80 | 0.42 | 0.03 | .003 | |||||
| Extrem. Fat | 0.34 | −0.21 | 0.17 | −0.10 | 0.10 | 0.21 | −0.11 | 0.07 | ||||||
| P value | <.001 | 0.03 | 0.08 | 0.31 | 0.30 | 0.02 | 0.26 | 0.44 | ||||||
| BMIb | −0.21 | 0.19 | −0.07 | −0.19 | −0.20 | −0.09 | 0.45 | |||||||
| P value | 0.03 | 0.04 | 0.47 | 0.05 | 0.03 | 0.26 | <.001 | |||||||
| QUICKIc | −0.31 | 0.11 | −0.05 | −0.02 | 0.15 | −0.49 | ||||||||
| P value | 0.001 | 0.26 | 0.64 | 0.85 | 0.12 | <.001 | ||||||||
| Tryglcerides | −0.14 | 0.05 | 0.13 | −0.07 | 0.34 | |||||||||
| P value | 0.14 | 0.61 | 0.16 | 0.49 | <.001 | |||||||||
| Systolic BPd | −0.02 | 0.07 | −0.19 | 0.21 | ||||||||||
| P value | 0.83 | 0.47 | 0.05 | 0.03 | ||||||||||
| HDL-Chol | 0.17 | 0.14 | −0.41 | |||||||||||
| P value | 0.08 | 0.14 | <.001 | |||||||||||
| Adiponectine | 0.06 | −0.33 | ||||||||||||
| P value | 0.51 | <.001 | ||||||||||||
| SHBG | −0.34 | |||||||||||||
| P value | <.001 |
Lean body mass
Body mass index
Qualitative insulin sensitivity check index
Blood pressure
High molecular weight adiponectin component
Sex hormone binding globulin
Cardiometabolic risk composite score
Body Composition Mediators
Because of the importance of upper body obesity in cardiometabolic risks, the study cohort was divided into two groups of 56 participants with those above and below the baseline median BMI of 27.4 (data not shown). The only cardiometabolic risk factor that responded differently to the anabolic hormone interventions in the two subgroups was trunk fat, which decreased more in the participants with higher than lower BMI (−1.10±1.26kg versus −0.66±1.14kg lost, respectively; p=0.05). Further, outcomes were generally not associated with study drug assignment but were related to a broad range of changes in testosterone and IGF-1 levels regardless of the dose of testosterone or rhGH (29). Thus, we examined outcomes for the study population as a whole (n=112). Finally, since BMI proved to be the central mediator of the pathway analysis and represents a composite change of the changes in total LBM and total fat mass, we sought to understand the contributions of these two body composition variables to the changes in BMI. Linear regression models were used to determine the relative effects (partial R2) of changes in LBM and fat mass together on change in BMI.
All statistical analyses were carried out using Statistical Analysis System 9.2, Cary, NC.
RESULTS
Subject Characteristics
Characteristics of the study participants have been described previously (28). Briefly, of 242 potential participants screened, 122 were randomized and 112 completed 16 weeks of study therapies. Participants were relatively healthy men, 70.8±4.2 years of age with an average BMI of 27.4±3.4 (range 20.4–34.8), similar to the median BMI of 27.8 for US adults in the most recent NHANES (1), and with average Framingham 10-year cardiovascular risk of 13.8±1.2%. Other baseline characteristics of importance to cardiometabolic risks are shown in Table 1.
Table 1.
Baseline Characteristics of the Study Population and Change After Hormone Treatment
| Entire Cohort (N=112) | Baseline | Change after16 weeks | P value |
|---|---|---|---|
| Hormones | |||
| Total testosteronea, ng/dL | 493±170 | 320±478 | <0.001 |
| Free testosteronea, pg/mL | 112±50 | 127±199 | <0.001 |
| IGF-1a, ng/mL | 125±34 | 58±59 | <0.001 |
| Body Composition | |||
| BMIb, kg/m2 | 27.4±3.4 | 0.16±0.64 | 0.01 |
| Total fat, kg | 22.4±7.2 | −1.33±1.74 | <0.001 |
| Total LBMc, kg | 58.2±6.7 | 1.79±1.89 | <0.001 |
| Appendicular LBM, kg | 25.5±3.3 | 0.85±1.15 | <0.001 |
| Bionutrition Descriptors | |||
| Energy intake, kcal/day | 2198±484 | 105±613 | 0.09 |
| Fat intake, g/day | 87.0±42.4 | 3.6±52 | 0.48 |
| Physical activity score of elderly | 147±65 | 5.9±68 | 0.40 |
| Cardiometabolic Components | |||
| Fasting triglycerides, mg/dL | 126±61 | −18.2±57.0 | 0.001 |
| HDL-cholesterol, mg/dL | 43±12 | 3.5±6.7 | <0.001 |
| QUICKIa,d | 0.16±0.02 | −0.004±0.015 | 0.003 |
| Systolic blood pressure, mmHg | 117±14 | 12±14 | <0.001 |
| Trunk fat, kg | 12.7±4.3 | −0.88±1.21 | <0.001 |
| Other Markers of Cardiovascular Risks | |||
| HMWe adiponectina, ng/dL | 1.91±1.09 | −0.18±0.76 | 0.02 |
| LDL-cholesterol, mg/dL | 106±27 | 4±23 | 0.08 |
| SHBG a,f, mg/dL | 54.3±20.8 | −0.1±11.6 | 0.92 |
Results of samples batch tested after completion of the study in the Boston University and University of Southern California research laboratories of the investigators, as described in the Methods
Body mass index
Lean body mass
Qualitative insulin sensitivity check index = 1/[log (If) + log (Gf)], where (If) is the fasting insulin level (μU/ml) and (Gf) is the fasting glucose level
High molecular weight
Sex hormone binding globulin
Cardiometabolic Risk Outcomes
For the entire cohort, total and regional LBM and fat mass improved (Table 1). Some cardiometabolic variables improved (trunk fat, fasting triglycerides, HDL-cholesterol) and others worsened (systolic blood pressure, QUICKI, HMW adiponectin). The improvements in aerobic endurance, skeletal muscle mass and physical strength across the study (28) were not associated with decreases in resting heart rate (a measure of cardiovascular fitness) as expected (data not shown). The cardiometabolic risk composite score (CRCS) calculated from changes for the five cardiometabolic variables based on our apriori assignment of +1, 0, or −1 scores for each component improved and on average CRCS decreased by −0.69±1.55 (p<0.001).
Pathway Analysis and Model
A correlation matrix to investigate the relationship of changes in testosterone and IGF-1 levels, lean and fat mass, lipids, QUICKI, systolic blood pressure, and adiponectin is shown in Appendix Table 2. Based on these associations, several pathway models were considered. The parsinmonius model that best described the data is shown in Figure 1. Changes in testosterone and IGF-1 (pathway predictors) were both significantly associated with changes in total LBM and total fat mass (pathway mediators; no interaction by multiple linear regression) and changes in both body composition parameters were significantly associated with alterations in BMI (second tier mediator). Changes in BMI were highly correlated with four of the cardiometabolic variables (not systolic blood pressure). Overall, the model provided a good fit to the data (chi-square statistic =39.65 with 32 degrees of freedom; p-value=0.17). The other two goodness-of-fit indices (NNFI and CFI) also confirmed this finding (both >0.99).
Figure 1.
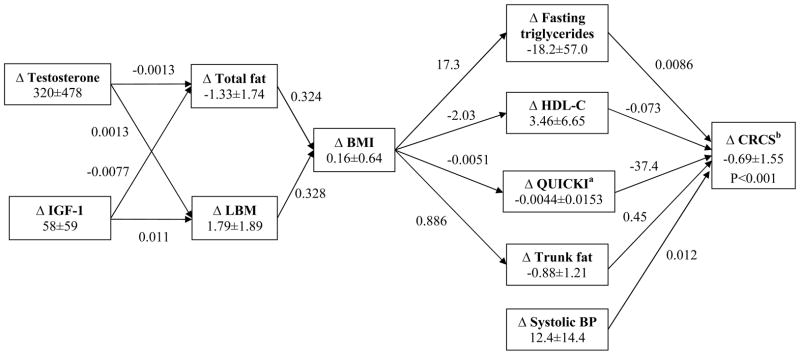
Pathway Model of Predictors and Mediators of Cardiometabolic Variables and their Composite Risk Scores
By linear regression, changes in LBM accounted for 64% and changes in fat mass accoutned for 35% of the changes in BMI. These data along with separate bootstrap analyses (not shown) confirmed that the changes in LBM had a greater effect on BMI than the changes in fat, explaining the average increase in BMI (0.06±0.64kg/m2, Table 1, Figure 1), thereby explaining the overall increase in BMI.
Factors Affecting Cardiometabolic Risk Composite Score and Its Components
To better understand how changes in BMI components (fat mass and LBM) might affect the CRCS and its components, participants with high and low absolute changes (i.e., above and below the median) in LBM and fat mass were compared. The two groups with high changes in fat mass had mean CRCS significantly lower than zero, regardless of whether there were low (mean CRCS at −1.30±1.42; p<0.001) or high changes in LBM (mean CRCS at −1.00±1.43, p<0.001; Figure 2). For the lipid variables, the largest (improvement) and only significant change in fasting triglycerides (−28.2±51.8mg/dL; p=0.003) occurred in participants who had large changes in both fat mass and LBM. By contrast, the largest change (improvement) in HDL-cholesterol occurred in the group with large declines in fat and low changes in LBM (5.7±7.6mg/dL; p=0.003).
Figure 2.
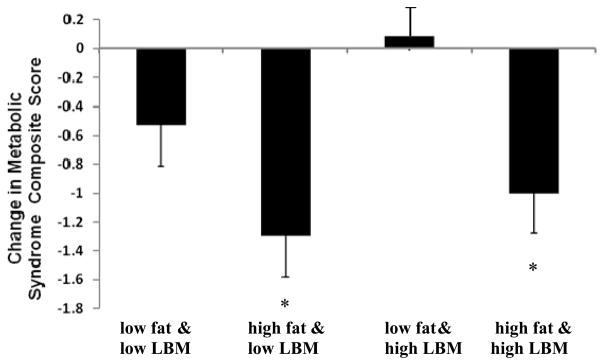
Change in Cardiometabolic Risk Composite Scores Relative to Change in LBM and Fat Mass
Changes in total and trunk fat were positively correlated with changes in fasting triglycerides and QUICKI (Table 2). For triglycerides, QUICKI, and HMW adiponectin, there was no relationship with change in fat for participants who only received testosterone. However, the associations of these three variables with fat changes were of greater magnitude during treatment with testosterone plus rhGH than for the study population as a whole (Table 2). SHBG did not change during the 16 weeks of hormone treatments and was not associated with any of the other cardiometabolic parameters.
Table 2.
Relationship of Change in Fat and Changes in Other Cardiometabolic Risk Variables
| Treatment Assignment | Total Fat | Trunk fat | |||
|---|---|---|---|---|---|
| r d | P value | r | P value | ||
| Entire cohort N=112 | Triglyceridesa | 0.19 | 0.04 | 0.18 | 0.06 |
| HDL-cholesterol | 0.06 | 0.56 | 0.02 | 0.80 | |
| Systolic BP | −0.06 | 0.54 | −0.03 | 0.78 | |
| HMWb adiponectin | 0.15 | 0.13 | 0.08 | 0.42 | |
| QUICKIc | −0.22 | 0.02 | −0.17 | 0.07 | |
|
| |||||
| Testosterone only (rhGH placebo) Cohort N=39 | Triglycerides | 0.06 | 0.70 | 0.03 | 0.84 |
| HDL-cholesterol | 0.11 | 0.52 | 0.10 | 0.53 | |
| Systolic BP | −0.19 | 0.26 | −0.17 | 0.32 | |
| HMW adiponectin | −0.03 | 0.88 | −0.16 | 0.35 | |
| QUICKI | −0.08 | 0.61 | −0.03 | 0.87 | |
|
| |||||
| Testosterone Plus rhGH cohort N=73 | Triglycerides | 0.27 | 0.02 | 0.26 | 0.03 |
| HDL-cholesterol | 0.03 | 0.82 | −0.02 | 0.89 | |
| Systolic BP | −0.01 | 0.92 | 0.02 | 0.87 | |
| HMW adiponectin | 0.27 | 0.02 | 0.22 | 0.06 | |
| QUICKI | −0.29 | 0.01 | −0.24 | 0.04 | |
Variable units per Table 1;
High molecular weight;
Qualitative insulin sensitivity check index
Pearson’s correlation coefficien
In multivariate linear regression modeling to assess the independent effects of the cardiometabolic variables on composite risks (CRCS), QUICKI, fasting triglycerides, and HDL-cholesterol provided the largest contributions (33%, 16%, and 14%, respectively) to the CRCS (Table 3). Of note, HDL-cholesterol, a major contributor to the CRCS in this analysis, was not related directly to either change in LBM or fat mass but presumably through an interaction of the two via BMI (r=−0.19, p=0.05, Appendix Table 2).
Table 3.
Contributions of Cardiometabolic Components to the Composite Risk Score
| Cardiometabolic Component | Partial R2 b | P-value |
|---|---|---|
| Δ in QUICKIa | 33% | <0.001 |
| Δ in fasting triglycerides mg/dL | 16% | <0.001 |
| Δ in HDL-cholesterol mg/dL | 14% | <0.001 |
| Δ in trunk fat kg | 5% | <0.001 |
| Δ in systolic blood pressure mm Hg | 2% | 0.02 |
Δ = change; qualitative insulin sensitivity check index
By multivariate linear regression
DISCUSSION
Strategies that reduce body fat are expected to improve cardiometabolic profiles. Little is known about the cardiometabolic risks of combined anabolic hormone therapy in older persons (23, 26). In the HORMA Trial, whole body fat mass, trunk fat, HDL-cholesterol and triglycerides improved during anabolic therapy with testosterone and rhGH administration for 4- months. However, other markers of cardiovascular risk such as blood pressure, insulin sensitivity, and adiponectin levels worsened. In aggregate, there were modest improvements in the cardiometabolic risk composite scores, which suggested that 4-months of therapy did not adversely affect overall cardiometabolic risks in these relatively healthy, community-dwelling men.
In our pathway model, changes in testosterone and IGF-1 levels induced by study interventions were associated with signficant improvements in body composition but had no direct relationship with changes in cardiometabolic risk variables. Whereas, the improvements in LBM and fat mass during anabolic study interventions resulted in a net increase in BMI, the final mediator in the pathway to cardiometabolic risk factors. Generally, the percentage of fat mass and BMI are highly correlated (r=0.72–0.79) (34–36), but in HORMA changes in LBM accounted for 64% of the variance in BMI. The pathway analyses suggest that changes in both fat mass and lean body mass are related to overall changes in the cardiometabolic risk composite score in a complex manner through their diverse effects on the individual components of the metabolic risk. These observations are consistent with a growing body of data suggesting that anabolic therapies by their effects on multiple body composition components and at different anatomical sites may secondarily affect multiple metabolic pathways (37).
The major contributors to the cardiometabolic risk composite scores were changes in the insulin sensitivity index QUICKI (33%), HDL-cholesterol (14%), and fasting triglycerides (16%). The changes in trunk fat explained only 5% of the variation in cardometabolic risk composite score, but were significantly associated with changes in triglycerides and markers of insulin sensitivity, such as adiponectin levels and the QUICKI index; these associations were especially robust in participants receiving both testosterone and rhGH.
Although loss of upper body fat is usually associated with improvements in adiponectin, in our study absolute decrements in total fat mass were less than the increases in LBM and thus BMI increased. With the increases in LBM and BMI, HMW adiponectin levels declined; levels also fell in participants treated with both hormones as their total fat mass decreased. The explanation for these changes in adiponectin is unclear and again reflect the complex interaction of changes in lean tissue and fat mass relative to different cardiometabolic parameters.
The HORMA trial has many attributes of good clinical trial design: randomization, masked subject allocation and interventions, and a relatively large population sample guided by a priori considerations of effect size and power. In this context, the analytical approach presented here provides a unique perspective on the overall cardiometabolic risk of anabolic therapy using testosterone with and without rhGH.
Our study also has some limitations. The participants were relatively healthy, community dwelling men and these findings may not apply to frail older men with multiple comorbid conditions and/or disabilities, who may be at higher risk of adverse cardiovascular events. Since study participants were not selected for obesity or metabolic condition, the outcomes might be different in a population where all of the participants have, for example, increased BMI (i.e. ≥ 30kg/m2) prior to hormonal treatments. The cardiometabolic variables investigated here represent surrogate markers of cardiometabolic risks, and the effects of hormonal interventions on cardiovascular event rates may not always be concordant with the changes in surrogate markers of cardiometabolic risk. We used trunk fat measured by DEXA as a marker of abdominal adiposity instead of waist circumference or BMI. The 4-month study duration was relatively short; the long term effects of anabolic therapy may differ from those of short term therapy. These analyses represent secondary analyses and need confirmation in prospective randomized trials of longer duration. The metabolic syndrome concept has undergone considerable debate, and several different definitions have been used. Thus, we have focused on individual cardiometabolic risk factors. The value as a composite marker of cardiometabolic risk especially when individual components may show directional divergence has been recognized, and the potential colinearity of its individual components is also well known.
In summary, anabolic therapy using testosterone and rhGH was associated with diverse changes in individual risk factors for metabolic and cardiovascular disease that appeared related to a complex interaction of changes in LBM and fat mass. In aggregate, these therapies appeared not to worsen cardiometabolic risk in relatively healthy, community-dwelling older men. The aging of human populations along with the associated increase in the prevalence of aging-associated functional limitations has provided the impetus for the development of a number of function promoting anabolic therapies. The long term effects of such anabolic therapies, including not only anabolic strategies used in this study but growth hormone releasing peptides and mimetics, selective androgen or estrogen receptor agonists, and antimyostatin strategies on cardiovascular events should be investigated in other aging populations with obesity, metabolic syndrome, sarcopenia or functional limitations, for whom anabolic therapies may be indicated. These individuals with high burden of comorbid conditions may have high baseline risk of cardiovascular disease, and may yet need careful screening and monitoring to mitigate potential cardiovascular risk.
Acknowledgments
We are grateful for the dedication of the study volunteers and other members of the HORMA research team without whom the study would not have been possible.
Funding Support: Primary support for HORMA trial was provided from R01 AG18169 with secondary support from NCRR GCRC M0I RR00043 at USC, the U.S. Department of Agriculture (USDA) ARS Cooperative Agreement 58-1950-9-001, the NCRR GCRC grant M01 RR000054 at Tufts University, the Mass Spectrometry Research Resource at Washington University (RR000954, DK020579, and DK056341), and U01AG14369 and 1R01DK70534 at Boston Medical Center, Boston University of Medicine.
Sponsors' Role:
The primary funding source was the National Institute of Aging. National Center for Research Resources and United States Department of Agriculture provided funding for the General Clinical Research Centers and Tufts University Metabolic Research Unit, repectivcely. Study therapies were provided by Solvay Pharmaceuticals Inc, Genentech Inc, and Tap Pharmaceutical Products Inc; industry sponsors provided no monetary support and no input for the design, methods, subject recruitment, data collection or analysis, and did not review this manuscript.
Footnotes
National Clinical Trials Number: NCT00183040
Author Contributions:
- Study Concept and Design: FRS (PI), SB, RR, KY, and SA (lead statistician) were responsible for the hypotheses, specific aims, and study design.
- Data Acquisition: EFB, KY, CC-S, ETS, RR, and FRS were responsible for data acquisition.
- Data Quality and Analysis: SA and FRS created the manual of operations and procedures, case report forms, an electronic data base for web-based data entry, and the manual and electronic screening of data for outliers, quality control, and audits of all data with verification from source documents, and statistical analyses. C-PC assisted with the pathway analysis.
- Manuscript Preparation: All authors reviewed the data base, analyses and their interpretation, and then reviewed and contributed to the writing of the manuscript.
Scientific Meeting Presentation: Paper was presented in part as a poster at Endo Soc 2011 meeting: He J, Bhasin S, Binder EF, Castaneda-Sceppa C, Yarasheski K, Schroeder ET, Roubenoff R, Chou H-P, Azen SP, Sattler FR. Effects of Testosterone and rhGH on Metabolic Syndrome Components in Older Men: the HORMA Study, Abstract P3-208, Endo 2011, June 4–7, 2011, Boston, MA.
Conflict of Interest Statement: FRS, EFB, and SB have received grant support from Solvay Pharmaceuticals.
References
- 1.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307(5):491–7. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 2.Reaven GM. Syndrome X: 6 years later. J Intern Med Suppl. 1994;736:13–22. [PubMed] [Google Scholar]
- 3.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109(3):433–8. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 4.Abbasi AA, Drinka PJ, Mattson DE, Rudman D. Low circulating levels of insulin-like growth factors and testosterone in chronically institutionalized elderly men. J Am Geriatr Soc. 1993;41(9):975–82. doi: 10.1111/j.1532-5415.1993.tb06764.x. [DOI] [PubMed] [Google Scholar]
- 5.Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86(2):724–31. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- 6.Corona G, Monami M, Rastrelli G, et al. Testosterone and metabolic syndrome: a meta-analysis study. J Sex Med. 2011;8(1):272–83. doi: 10.1111/j.1743-6109.2010.01991.x. [DOI] [PubMed] [Google Scholar]
- 7.Makhsida N, Shah J, Yan G, Fisch H, Shabsigh R. Hypogonadism and metabolic syndrome: implications for testosterone therapy. J Urol. 2005;174(3):827–34. doi: 10.1097/01.ju.0000169490.78443.59. [DOI] [PubMed] [Google Scholar]
- 8.Wang C, Jackson G, Jones TH, et al. Low testosterone associated with obesity and the metabolic syndrome contributes to sexual dysfunction and cardiovascular disease risk in men with type 2 diabetes. Diabetes Care. 2011;34(7):1669–75. doi: 10.2337/dc10-2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mohr BA, Bhasin S, Link CL, O'Donnell AB, McKinlay JB. The effect of changes in adiposity on testosterone levels in older men: longitudinal results from the Massachusetts Male Aging Study. Eur J Endocrinol. 2006;155(3):443–52. doi: 10.1530/eje.1.02241. [DOI] [PubMed] [Google Scholar]
- 10.Rudman D, Kutner MH, Rogers CM, Lubin MF, Fleming GA, Bain RP. Impaired growth hormone secretion in the adult population: relation to age and adiposity. J Clin Invest. 1981;67(5):1361–9. doi: 10.1172/JCI110164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Snel YE, Doerga ME, Brummer RM, Zelissen PM, Koppeschaar HP. Magnetic resonance imaging-assessed adipose tissue and serum lipid and insulin concentrations in growth hormone-deficient adults. Effect of growth hormone replacement. Arterioscler Thromb Vasc Biol. 1995;15(10):1543–8. doi: 10.1161/01.atv.15.10.1543. [DOI] [PubMed] [Google Scholar]
- 12.Iranmanesh A, Lizarralde G, Veldhuis JD. Age and relative adiposity are specific negative determinants of the frequency and amplitude of growth hormone (GH) secretory bursts and the half-life of endogenous GH in healthy men. J Clin Endocrinol Metab. 1991;73(5):1081–8. doi: 10.1210/jcem-73-5-1081. [DOI] [PubMed] [Google Scholar]
- 13.Munzer T, Harman SM, Hees P, et al. Effects of GH and/or sex steroid administration on abdominal subcutaneous and visceral fat in healthy aged women and men. J Clin Endocrinol Metab. 2001;86(8):3604–10. doi: 10.1210/jcem.86.8.7773. [DOI] [PubMed] [Google Scholar]
- 14.Attanasio AF, Mo D, Erfurth EM, et al. Prevalence of metabolic syndrome in adult hypopituitary growth hormone (GH)-deficient patients before and after GH replacement. J Clin Endocrinol Metab. 2010;95(1):74–81. doi: 10.1210/jc.2009-1326. [DOI] [PubMed] [Google Scholar]
- 15.Maison P, Balkau B, Souberbielle JC, et al. Evidence for distinct effects of GH and IGF-I in the metabolic syndrome. Diabet Med. 2007;24(9):1012–8. doi: 10.1111/j.1464-5491.2007.02195.x. [DOI] [PubMed] [Google Scholar]
- 16.Kupelian V, Page ST, Araujo AB, Travison TG, Bremner WJ, McKinlay JB. Low sex hormone-binding globulin, total testosterone, and symptomatic androgen deficiency are associated with development of the metabolic syndrome in nonobese men. J Clin Endocrinol Metab. 2006;91(3):843–50. doi: 10.1210/jc.2005-1326. [DOI] [PubMed] [Google Scholar]
- 17.Brand JS, van der Tweel I, Grobbee DE, Emmelot-Vonk MH, van der Schouw YT. Testosterone, sex hormone-binding globulin and the metabolic syndrome: a systematic review and meta-analysis of observational studies. Int J Epidemiol. 2011;40(1):189–207. doi: 10.1093/ije/dyq158. [DOI] [PubMed] [Google Scholar]
- 18.Bhasin S, Jasjua GK, Pencina M, et al. Sex hormone-binding globulin, but not testosterone, is associated prospectively and independently with incident metabolic syndrome in men: the framingham heart study. Diabetes Care. 2011;34(11):2464–70. doi: 10.2337/dc11-0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rudman D, Feller AG, Cohn L, Shetty KR, Rudman IW, Draper MW. Effects of human growth hormone on body composition in elderly men. Horm Res. 1991;36(Suppl 1):73–81. doi: 10.1159/000182193. [DOI] [PubMed] [Google Scholar]
- 20.Marin P. Testosterone and regional fat distribution. Obes Res. 1995;3(Suppl 4):60, 9S–12S. doi: 10.1002/j.1550-8528.1995.tb00233.x. [DOI] [PubMed] [Google Scholar]
- 21.Johannsson G, Marin P, Lonn L, et al. Growth hormone treatment of abdominally obese men reduces abdominal fat mass, improves glucose and lipoprotein metabolism, and reduces diastolic blood pressure [see comments] J Clin Endocrinol Metab. 1997;82(3):727–34. doi: 10.1210/jcem.82.3.3809. [DOI] [PubMed] [Google Scholar]
- 22.Marcus R, Butterfield G, Holloway L, et al. Effects of short term administration of recombinant human growth hormone to elderly people. J Clin Endocrinol Metab. 1990;70(2):519–27. doi: 10.1210/jcem-70-2-519. [DOI] [PubMed] [Google Scholar]
- 23.Blackman MR, Sorkin JD, Munzer T, et al. Growth hormone and sex steroid administration in healthy aged women and men: a randomized controlled trial. JAMA. 2002;288(18):2282–92. doi: 10.1001/jama.288.18.2282. [DOI] [PubMed] [Google Scholar]
- 24.Nair KS, Rizza RA, O'Brien P, et al. DHEA in elderly women and DHEA or testosterone in elderly men. N Engl J Med. 2006;355(16):1647–59. doi: 10.1056/NEJMoa054629. [DOI] [PubMed] [Google Scholar]
- 25.Emmelot-Vonk MH, Verhaar HJ, et al. Effect of testosterone supplementation on functional mobility, cognition, and other parameters in older men: a randomized controlled trial. JAMA. 2008;299(1):39–52. doi: 10.1001/jama.2007.51. [DOI] [PubMed] [Google Scholar]
- 26.Giannoulis MG, Sonksen PH, Umpleby M, et al. The effects of growth hormone and/or testosterone in healthy elderly men: a randomized controlled trial. J Clin Endocrinol Metab. 2006;91(2):477–84. doi: 10.1210/jc.2005-0957. [DOI] [PubMed] [Google Scholar]
- 27.Singh AB, Hsia S, Alaupovic P, et al. The effects of varying doses of T on insulin sensitivity, plasma lipids, apolipoproteins, and C-reactive protein in healthy young men. J Clin Endocrinol Metab. 2002;87(1):136–43. doi: 10.1210/jcem.87.1.8172. [DOI] [PubMed] [Google Scholar]
- 28.Sattler FR, Castaneda-Sceppa C, Binder EF, et al. Testosterone and growth hormone improve body composition and muscle performance in older men. J Clin Endocrinol Metab. 2009;94(6):1991–2001. doi: 10.1210/jc.2008-2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sattler F, Bhasin S, He J, Chou CP, et al. Testosterone threshold levels and lean tissue mass targets needed to enhance skeletal muscle strength and function: the HORMA trial. J Gerontol A Biol Sci Med Sci. 2011;66(1):122–9. doi: 10.1093/gerona/glq183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhasin S, Woodhouse L, Casaburi R, et al. Older men are as responsive as young men to the anabolic effects of graded doses of testosterone on the skeletal muscle. J Clin Endocrinol Metab. 2005;90(2):678–88. doi: 10.1210/jc.2004-1184. [DOI] [PubMed] [Google Scholar]
- 31.Quon MJ. QUICKI is a useful and accurate index of insulin sensitivity. J Clin Endocrinol Metab. 2002;87(2):949–51. doi: 10.1210/jcem.87.2.8223. [DOI] [PubMed] [Google Scholar]
- 32.Kline RB. Principles and Practice of Structural Equation Modeling. 2. New York: The Guilford Press; 2005. [Google Scholar]
- 33.Bentler PM, Bonnett DG. Significance tests of goodness-of-fit in the analysis of covariance structures. Pyschological Bulletin. 1980;88:586–606. [Google Scholar]
- 34.Flegal KM, Shepherd JA, Looker AC, et al. Comparisons of percentage body fat, body mass index, waist circumference, and waist-stature ratio in adults. Am J Clin Nutr. 2009;89(2):500–8. doi: 10.3945/ajcn.2008.26847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rahman M, Temple JR, Breitkopf CR, Berenson AB. Racial differences in body fat distribution among reproductive-aged women. Metabolism. 2009;58(9):1329–37. doi: 10.1016/j.metabol.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fernandez JR, Heo M, Heymsfield SB, et al. Is percentage body fat differentially related to body mass index in Hispanic Americans, African Americans, and European Americans? Am J Clin Nutr. 2003;77(1):71–5. doi: 10.1093/ajcn/77.1.71. [DOI] [PubMed] [Google Scholar]
- 37.Tu P, Bhasin S, Hruz PW, et al. Genetic disruption of myostatin reduces the development of proatherogenic dyslipidemia and atherogenic lesions in Ldlr null mice. Diabetes. 2009;58(8):1739–48. doi: 10.2337/db09-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]


